Abstract
Exposure to prolonged or high intensity noise increases the risk for permanent hearing impairment. Over several decades, researchers characterized the nature of harmful noise exposures and worked to establish guidelines for effective protection. Recent laboratory studies, primarily conducted in rodent models, indicate that the auditory system may be more vulnerable to noise-induced hearing loss (NIHL) than previously thought, driving renewed inquiries into the harmful effects of noise in humans. To bridge the translational gaps between rodents and humans, nonhuman primates (NHPs) may serve as key animal models. The phylogenetic proximity of NHPs to humans underlies tremendous similarity in many features of the auditory system (genomic, anatomical, physiological, behavioral), all of which are important considerations in the assessment and treatment of NIHL. This review summarizes the literature pertaining to NHPs as models of hearing and noise-induced hearing loss, discusses factors relevant to the translation of diagnostics and therapeutics from animals to humans, and concludes with some of the practical considerations involved in conducting NHP research.
I. INTRODUCTION
Auditory research has greatly benefitted from basic and applied research involving a broad range of species. At every level of analysis, from molecular to cellular to systems, the vast majority of what we know about the structure and function of the auditory system has been gleaned from studies conducted in selected animal models. Each model offers inherent advantages for the exploration of particular features but may have limited utility for the study of others. The tremendous depth and breadth of our understanding, both current and future, is the product of this diverse collective.
It is well-established that single or multiple exposures to loud noise can elevate auditory thresholds, and it is hypothesized that acoustic trauma can induce hypersensitivity and tinnitus. Noise-induced threshold shifts can be temporary (temporary threshold shift, TTS) or permanent (permanent threshold shift, PTS). Early research indicated that PTS is caused primarily by outer hair cell (OHC) loss, and that nerve fiber loss was secondary to the loss of inner hair cells (IHCs), whereas TTS was not associated with permanent cochlear pathology (Liberman and Dodds, 1984; Moody et al., 1978; reviewed in McGill and Schuknecht, 1976; Saunders et al., 1985). These conclusions have been augmented by recent studies in rodents showing that IHC ribbon synapses and afferent nerve fibers are more sensitive to acoustic trauma than previously thought (Kujawa and Liberman, 2009). Ribbon synapses are rapidly and permanently lost following exposure to noise sufficiently loud enough to induce TTS, followed by delayed loss of spiral ganglion cells (Fernandez et al., 2015). Furthermore, exposures sufficient to kill OHCs are accompanied by significant losses of afferent nerve fibers on IHCs that survive the exposure (Valero et al., 2017).
As these discoveries expand our understanding of NIHL, they also raise issues relevant to human health and lifestyle. First, the vulnerability of humans to all forms of NIHL is uncertain. Most of the recent discoveries were derived from rodent studies, where histological verification of cochlear pathology is easily achieved. Comparable studies in humans are limited by practical and ethical concerns. Second, susceptibility to NIHL appears to vary widely between individuals and species. TTS and PTS are induced at lower sound pressure levels (SPLs) in rodents, compared to humans and nonhuman primates (e.g., Luz and Lipscomb, 1973; Valero et al., 2017). The dose-response defining the risk factors for developing NIHL along the TTS-PTS continuum is incomplete, as the parameter space is quite large, including variables such as age, sex, circadian rhythms, and spectrotemporal characteristics of the noise (see Topics 1 and 2, this issue). Third, reliable and sensitive diagnostic metrics are needed to identify synaptopathy and other types of peripheral and central pathology associated with noise exposure. The pure tone audiogram and other classic audiologic assessment tools are generally insensitive to the presence of synaptopathy in TTS. Finally, the treatment of NIHL by emerging pharmacologic and genomic techniques under development in rodent models raise questions about translation to humans (see Cousins, this issue).
Nonhuman primates (NHPs) may be a key translational model to help address many of these issues. NHPs occupy a unique niche in biomedical research due to their phylogenetic proximity to humans, and because the physiological processes and phenotypic outcomes associated with human disorders are often closely mirrored in monkey models. Old-world monkeys, such as rhesus macaques, cynomolgus macaques, and baboons, as well as New-World monkeys, such as marmosets and squirrel monkeys, have served as invaluable models in a wide array of biomedical studies, including within the auditory research field. These model systems may be key to better defining regulations for workplace noise exposure and for translating therapeutics to the clinic.
In this review, we summarize literature pertaining to the use of NHPs as models of hearing and noise-induced hearing loss. Because macaque monkeys are currently the most thoroughly studied NHP with respect to noise trauma, studies of this species are emphasized. We also discuss factors relevant to the translation of therapeutic strategies from animals to humans, including potential advantages of NHPs as an intermediate model. The article concludes with some of the practical considerations involved in conducting NHP research.
II. NONHUMAN PRIMATES AS A MODEL OF AUDITION
A. Phylogeny
The primary rationale for the inclusion of NHPs in basic and applied biomedical research is their phylogenetic proximity to humans, and Old-World monkeys are more closely related to humans than are New-World monkeys. Macaque monkeys, for example, diverged from humans approximately 25 × 106 years ago and share 93.5% genetic sequence similarity with humans. By comparison, rodents diverged from humans about 70 × 106 years ago and retain about 85% sequence homology (Kumar and Hedges, 1998; Rhesus Macaque Genome Sequencing and Analysis Consortium et al., 2007). Consequently, NHPs exhibit greater similarity to human physiology, neurobiology, and susceptibility to infectious and metabolic diseases. These features support the inclusion of NHPs in biomedical research, where the goal is to maximize success and minimize risk in a wide array of human applications (e.g., cardiology, cognition, genetics, HIV/AIDS, immunology, neurology, pharmacology, reproduction, respiratory disease, movement disorders, and vaccines against Ebola and Zika viruses) (Phillips et al., 2014; Wichmann et al., 2018; Espeland et al., 2018; Heppner et al., 2017).
Within the field of auditory research, the genomic conservation between macaques and humans will likely facilitate our understanding of how gene expression, and the regulation thereof, contribute to the varying vulnerability between individuals to acoustic trauma (e.g., (Barden et al., 2012; Burns et al., 2015; Cai et al., 2015; Lavinsky et al., 2016; Mutai et al., 2018), age-related hearing loss (Bowl and Brown, 2018; Hoffmann et al., 2016), as well as one's responsivity to therapeutics. While emerging genomic studies of the auditory periphery of NHP and human cochleas highlight some similarities in cochlear gene expression (Mutai et al., 2018; Schrauwen et al., 2016), comparable studies of the central auditory system are lacking.
B. Behavioral training and psychoacoustic testing
One of the most notable advantages of the NHP model relative to rodents is its ability to quickly learn complex tasks and perform these tasks with great accuracy for long durations of time. Within a few weeks to months of training, primates can perform behavioral tasks in daily sessions lasting up to several hours. Various training methods have been employed with great success, including positive reinforcement with fluid or food rewards or shock avoidance paradigms. Because primates are highly motivated by positive reinforcement, this more ethically favorable technique is most commonly used today. Furthermore, technological advances that allow for cage-side subject training and testing (depending on the study constraints) increase subject comfort (Berger et al., 2018; Calapai et al., 2017). Behavioral studies considerably strengthen the translational power of the primate model, as the same tasks can be utilized in both human and nonhuman studies, allowing for direct cross-species comparisons. Here, we describe behavioral studies of NHP hearing across the hierarchy of auditory perception, including investigations of auditory detection, discrimination, identification, and comprehension.
The first behavioral investigations of NHP auditory function characterized hearing sensitivity by assessing tone detection in quiet. Audiograms have been measured in NHPs under a variety of pathologic states, including noise-induced hearing loss (as discussed in detail below) and age-related hearing loss (Bennett et al., 1983). Previously published reviews have extensively discussed normative behavioral audiograms in nearly 30 different NHP species, including Coleman (2009) and Coleman and Colbert (2010), as well as more recent additions by Osmanski and Wang (2011) and Dylla et al. (2013).
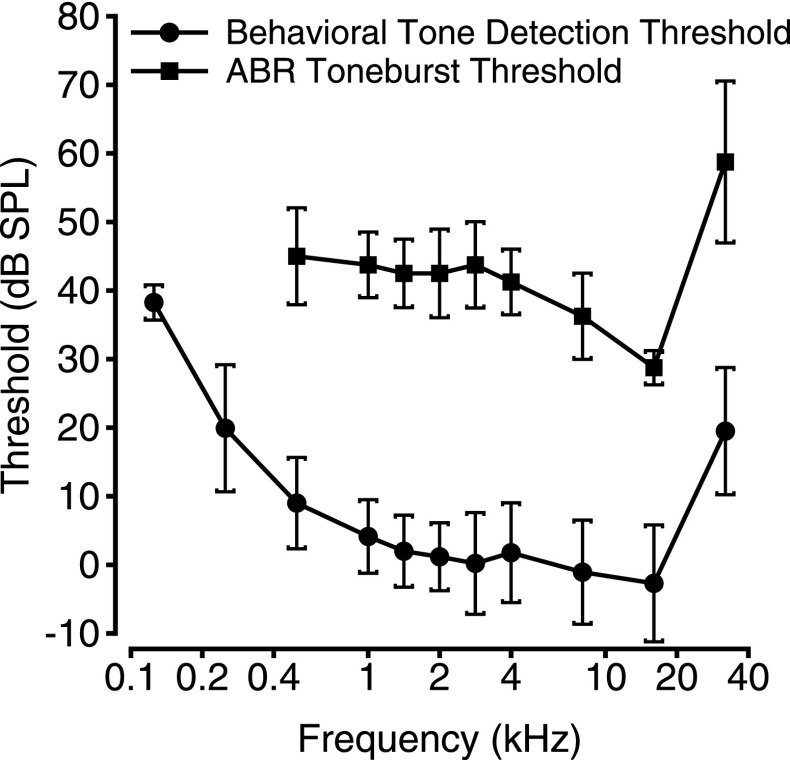
Briefly, primates have varying audible frequency ranges, but generally cover frequencies between 40 and 40 000 Hz (Coleman, 2009), approximately one octave higher than the 20 to 20 000 Hz range of humans (Hawkins and Stevens, 1950; Sivian and White, 1933; further species comparisons in Heffner and Heffner, 2007). NHP audiograms generally resemble those of humans, though with slightly poorer low frequency hearing and an extended high frequency hearing range (see Heffner, 2004). Humans and macaques have a U-shaped audiogram with an area of greatest sensitivity that approaches values of 0 dB SPL (humans: 500–4000 Hz, e.g. Hawkins and Stevens, 1950; Sivian and White, 1933; rhesus macaques: 1000–16 000 Hz, Fig. 1; Pfingst et al., 1978; Dylla et al., 2013), surrounded by a shallow low frequency tail and a steep high frequency tail. Several species of New-World primates, including marmosets, owl monkeys, and squirrel monkeys, have W-shaped audiograms, in which a less sensitive frequency region is flanked by a lower- and higher-frequency region of increased sensitivity (marmosets: Seiden, 1957; Osmanski and Wang, 2011; owl monkeys: Beecher, 1974a; squirrel monkeys: Beecher, 1974b). However, this should not be mistaken as a phenomenon specific to New-World primates, as W-shaped audiograms have also been observed in baboons (Hienz et al., 1982) and chimpanzees (Kojima, 1990).
FIG. 1.
Mean behavioral (n = 10 rhesus macaques) and auditory brainstem response (ABR; n = 8 ears from 4 rhesus macaques) thresholds as a function of stimulus frequency. Error bars illustrate one standard deviation from the mean.
In addition to tone detection in quiet, the macaque psychoacoustics literature is rich with iterations of tone detection experiments in quiet and in background noise to probe more complex auditory processing (e.g., Dylla et al., 2013; Gourevitch, 1970). Our understanding of basic auditory processing has been refined by these assessments, and these assays will be important in future studies to define the functional consequences of acoustic trauma on auditory perception and to inform diagnostics. For example, loudness perception has been estimated in NHPs by examining the relationship between signal intensity and reaction time latency (Gates et al., 1963; Stebbins, 1966; Stebbins and Miller, 1964). Such studies have provided evidence that NHPs experience loudness recruitment during temporary and permanent noise-induced hearing loss (see Sec. III for further details), which is consistent with reports in humans (Moore, 1996).
Primate frequency selectivity has been measured behaviorally via psychophysical tuning curves (Serafin et al., 1982) and tone detection in narrowband noise (Gourevitch, 1970) or notched-noise (Burton et al., 2018). These behavioral studies, as well as a pair of studies using otoacoustic emissions (OAEs) to probe frequency selectivity (Joris et al., 2011; Verschooten et al., 2018), have demonstrated slightly broader frequency selectivity in normal hearing macaques relative to normal hearing humans. There are no known studies to date that have assessed the effects of noise trauma or aging on frequency selectivity in NHPs.
Temporal resolution has been assessed in behavioral tasks such as amplitude-modulation detection (Moody, 1994; O'Connor et al., 2011), tone detection in amplitude-modulated noise (Bohlen et al., 2014; Dylla et al., 2013), and tone detection in gated and inversely-gated noise (Rocchi et al., 2017). While some studies have suggested that temporal resolution in macaques is poorer than in humans (O'Connor et al., 1999; O'Connor et al., 2011), data from the authors' laboratory show comparable temporal resolution in normal hearing macaques (Dylla et al., 2013). Furthermore, spatial release from masking in macaques appears to be similar to humans (Rocchi et al., 2017).
NHPs are also able to perform a variety of auditory discrimination tasks that may inform the consequences of acoustic trauma, but they are too extensive to review thoroughly here. Acoustic parameters to discriminate include: tone frequency (Moody et al., 1971; Osmanski et al., 2016; Pfingst, 1993; Prosen et al., 1990; Recanzone et al., 1991; Sinnott et al., 1985; Stebbins, 1973; Wienicke et al., 2001), tone intensity (Pfingst, 1993; Sinnott and Brown, 1993a,b; Sinnott et al., 1985; Stebbins, 1973), amplitude-modulation frequency (Moody, 1994), monaural phase (Moody et al., 1998), stimulus rise time (Prosen and Moody, 1995), stimulus location (Brown et al., 1978; Brown et al., 1978, 1980; Heffner and Heffner, 1990; Heffner and Masterton, 1975; May et al., 1986), and harmonic complex composition (Le Prell et al., 2001; Tomlinson and Schwarz, 1988). Monkeys have also been trained to discriminate conspecific vocalizations (Heffner and Heffner, 1984; Hopp et al., 1992; Le Prell and Moody, 1997; May et al., 1989; Petersen et al., 1978; Zoloth et al., 1979) as well as human speech sounds (Sinnott et al., 1976; Sinnott, 1989; Sinnott et al., 2006; Sommers et al., 1992).
Auditory stimulus identification and comprehension are more challenging to probe in nonhuman animals. In perhaps one of the first studies of its kind in the auditory domain, Hocherman et al. (1976) trained rhesus macaques to perform an audiovisual selective attention task, where subjects moved the lever to the left or right according to the type of auditory or visual stimulus presented. Researchers continue to push the envelope with regards to task complexity. In recent studies, NHPs have been trained to perform tasks such as a “delayed match to sample” task to assess auditory working memory (Ng et al., 2014; Scott et al., 2012). Another task assesses short-term memory, as well as decision-making, by asking subjects to discriminate acoustic flutter stimuli with long inter-stimulus intervals (Lemus et al., 2009). Even more complex behaviors include the discrimination of auditory illusory percepts to investigate auditory feature-ground grouping (Petkov et al., 2003), stream segregation (Christison-Lagay and Cohen, 2014; Lakatos et al., 2013) or feature-specific discrimination (Downer et al., 2017) to probe selective auditory attention, and sequence content identification (i.e., does the sequence contain more high or low frequency tones) to investigate perceptual decision-making (Tsunada et al., 2016).
While it is not trivial to train primates on behavioral tasks, the data provide an invaluable link to the following complementary approaches for studying auditory function in primates as well as illuminate the translatability of the NHP model to humans.
C. Noninvasive electrophysiology
Behavioral assessments of hearing and hearing loss may be augmented by a number of noninvasive techniques to probe the integrity of specific structures in the auditory pathway. Several clinical audiology measures have been modified for use in animals, including the auditory brainstem response (ABR), electrocochleography (ECochG), OAEs, and immittance testing. These noninvasive diagnostic tests can be performed identically in well-trained or anesthetized animal models and human patient populations, linking invasive observations in animal models, such as histology and/or invasive physiology, to the noninvasive metrics in humans. In particular, these metrics are essential for the differential diagnosis of auditory pathologies, especially when behavioral data are difficult to obtain, as in children and some animal species.
ABRs are evoked potentials measured at the scalp in response to repeated presentations of short-duration stimuli. This test evaluates the integrity and synchrony of the auditory system from cochlea to brainstem. The ABR waveform is characterized by four to five peaks that are time-locked to the stimulus onset and represent the summed response of progressively more central generators in the auditory periphery and brainstem. The generator of Wave I is clearly the auditory nerve, regardless of species, but macaque ABRs have prominent Waves I, II, and IV, which are likely homologous to the classical human Waves I, III, and V (Allen and Starr, 1978; Kraus et al., 1985; Lasky et al., 1995; Alegre et al., 2001). Similar waveform discrepancies have been noted in squirrel monkeys (Pineda et al., 1989) and marmosets (Harada and Tokuriki, 1997).
ABRs have been used in macaques to assess hearing status and auditory system integrity following acoustic trauma (Hauser et al., 2018; Valero et al., 2017), to assess the effects of the normal aging process (Torre and Fowler, 2000; Fowler et al., 2002; Fowler et al., 2010; Ng et al., 2015), to assess whether caloric restriction can ameliorate the aging effects on the auditory system (Fowler et al., 2002; Fowler et al., 2010), and to assess the effects of AIDS (Raymond et al., 1998; Riazi et al., 2009), prosthetic implantation (Dai et al., 2011), lead exposure (Lasky et al., 2001), or ototoxic drug administration (Shepherd et al., 1994) on hearing status. Furthermore, Dai et al. (2017) used ABRs to demonstrate the safety of intracochlear injections using saline, which provided a promising foundation for direct drug delivery to the ear. ABRs are typically used to estimate hearing thresholds (as illustrated for rhesus macaques in Fig. 1), although ABR thresholds tend to be higher than behavioral thresholds across many species, including NHPs (Lasky et al., 1999; see Fig. 1). Suprathreshold ABR measurements may be more informative for identifying the loss of IHC synapses (see below).
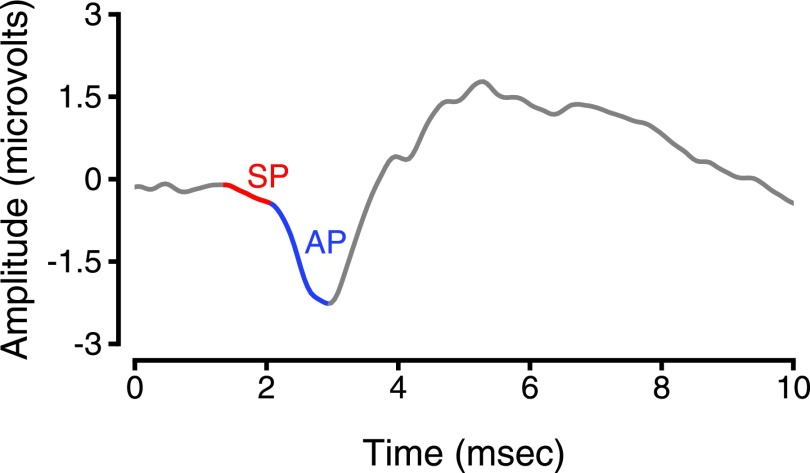
ECochG is conceptually similar to the ABR, except the recording electrode is placed on or near the tympanic membrane instead of the ear lobe or mastoid. This nearer-field electrode placement improves the isolation of the summating potential (SP) and Wave I. ECochG is primarily used clinically in the diagnosis of Meniere's disease. However, it has recently regained popularity as a possible diagnostic for synaptopathy (Liberman et al., 2016). ECochG has been reliably obtained in macaques, showing similar morphology to humans (see Fig. 2; also Pugh et al., 1973).
FIG. 2.
Electrocochleography tracing measured from a rhesus macaque monkey using a TM-trode. SP = summating potential. AP = action potential.
Otoacoustic emissions (OAEs), which are spontaneous or sound-evoked sounds originating from nonlinearities in OHC electromotility, can be measured non-invasively from the external auditory canal. As such, OAEs are used to evaluate OHC health, and this metric is an important differential diagnostic tool when paired with ABRs, particularly in cases of auditory neuropathy, in which ears with normal OAEs have grossly abnormal ABR waveform morphology (Starr et al., 1996). Several varieties of OAEs have been reported for macaques, including: spontaneous (SOAEs: Martin et al., 1985, 1988; Lonsbury-Martin et al., 1988; Lonsbury-Martin and Martin, 1988), stimulus-frequency (SFOAEs: Martin et al., 1988; Lonsbury-Martin and Martin, 1988; Joris et al., 2011), transient-evoked (TEOAEs: Martin et al., 1988; Lasky et al., 2000), and distortion product (DPOAEs: Martin et al., 1988; Lasky et al., 1995; Park et al., 1995; Lasky et al., 1999; McFadden et al., 2006; Dai et al., 2011; Dai et al., 2017; Valero et al., 2017). The prevalence of SOAEs is lower in macaques than humans, though much higher than other laboratory species (Lonsbury-Martin and Martin, 1988). DPOAEs amplitudes are similar to those observed for humans using similar stimulus parameters, suggesting similar peripheral generation mechanisms (Martin et al., 1988; Lasky et al., 1995; Park et al., 1995; Lasky et al., 2000).
Acoustic immittance testing can be used to evaluate patency of the middle ear (tympanometry) and integrity of the acoustic reflex pathways (middle ear muscle reflex, medial olivocochlear reflex). Tympanometry has been evaluated in normal hearing and pathologic macaques and squirrel monkeys (Igarashi et al., 1979; Jerger et al., 1978b,a; Lasky et al., 2000; Bachmann, 1996). Macaques have smaller ear canal volumes and reduced compliance compared to humans (Bachmann, 1996; Lasky et al., 2000). Stapedius reflexes have been evaluated in squirrel monkeys (Igarashi et al., 1979; Jerger et al., 1978b, 1978a; Thompson et al., 1984) and macaques (Mangham and Miller, 1976). Immittance testing is a reliable diagnostic tool for differentiating between conductive and sensorineural hearing losses (Jerger et al., 1978b,a), and is a promising metric for the diagnosis of synaptopathy (Valero et al., 2016; Valero et al., 2018; Wojtczak et al., 2017; Bharadwaj et al., 2019).
III. AUDITORY DYSFUNCTION FOLLOWING NOISE EXPOSURE
A. Background
The primary motivations for studies of noise-induced hearing loss have historically centered on establishing safety standards and damage-risk criteria for industrial workers and military personnel. More recently, motivations have extended to include the development of diagnostics and potential therapeutics for prevention or recovery from acoustic trauma. As is the case for all human pathologies, humans are the most relevant model system for assessing vulnerability to noise-induced hearing loss. However, the availability of post-mortem cochlear tissue is necessarily opportunistic in human research, and the likelihood of a concomitant audiogram and noise-exposure history being available is low.
Controlled noise exposure studies carried out on young adult humans in the mid-20th century helped to characterize the relationship between signal duration and intensity to the severity of TTS and rate of TTS recovery (e.g., Davis et al., 1950; Ward et al., 1959; Ward, 1960; Klein and Mills, 1981; Mills et al., 1981) and the results of such studies are reviewed elsewhere (Dobie and Humes, 2017). These studies were informative for setting damage-risk criteria, but the lack of structure-function correlations in this experimental design, due to the inability to non-invasively biopsy or image cochleas, was limiting. Furthermore, ethical considerations caused these studies to quickly fall out of favor due to the potential for permanent cochlear damage. In more recent years, human noise exposure studies have re-emerged in the context of drug development (Grinn et al., 2017; Le Prell et al., 2012; Spankovich et al., 2014), but exposures are carefully designed to minimize the risk for permanent damage and to maintain ethical standards (Maison and Rauch, 2017).
As human noise-exposure studies declined, researchers turned to laboratory animals, such as rats and chinchillas, to address the persistent questions concerning noise-induced hearing loss. Chinchillas were particularly favored, as their hearing range and cochlear length are similar to humans, and their docile nature facilitates awake non-invasive procedures and operant conditioning behavioral paradigms. However, concerns were raised regarding species-specific differences in susceptibility to damage by acoustic overexposure (e.g., Drescher and Eldredge, 1974; Hunter-Duvar and Bredberg, 1974; Luz and Lipscomb, 1973), suggesting limited translatability for establishing damage-risk criteria. This instigated the onset of several series of experiments in nonhuman primates.
These studies aimed to describe the relationships between:
-
(1)
Noise exposure stimulus parameters and cochlear pathology at the gross anatomical level, in terms of both severity and location of cochlear damage.
-
(2)
Noise exposure stimulus parameters and the magnitude of TTS and PTS, as assessed by behavioral audiograms.
-
(3)
Initial severity, growth, and recovery rate of TTS and any eventual PTS.
What follows is a detailed review of the existing literature on nonhuman primates and noise-induced hearing loss. While many aspects of the experimental design varied across studies, this review will be divided into sections based on the type of exposure stimulus: octave band and broadband noise, pure tones, and impulse noise. Studies of noise-induced hearing loss in NHPs are listed with experimental details in Table I. This review is intended to be comprehensive to the best knowledge of the authors. The relative paucity of NHP studies should be apparent from the table.
TABLE I.
Nonhuman primate studies of noise exposures, hearing impairment, and cochlear pathology. Studies are listed chronologically. NBN = narrowband noise; OBN = octave band noise; BBN = broadband noise; CF = center frequency; BW = bandwidth; ABR = auditory brainstem response; DPOAE = distortion product otoacoustic emission; OHC = outer hair cell; IHC = inner hair cell; LF = low frequency; AP = action potential from auditory nerve. Literature searches for this review were completed in PubMed using keywords such as: nonhuman primate, monkey, macaque, noise, exposure, impulse, hearing loss, cochlea, hair cell, sensorineural, threshold shift.
| Citation | Species | Exposure Stimulus | Exposure Level | Exposure Duration | Multiple Exposures? | Behavioral Audiogram? | ABR/DPOAE/Immittance? | Cochlear Histology? | Additional Details |
|---|---|---|---|---|---|---|---|---|---|
| Martin et al. (1962) | Rhesus macaques | Machine gun impulse | 165 dB SPL | 1x | No | Yes | No | No | Single subject; mild TTS in mid/high frequencies only; full recovery within 72 h |
| Romba and Gates (1964) | Rhesus macaques | Machine gun impulses | 154-166 dB SPL | 1x | Yes (8–12) | Yes | No | No | TTS recovery and PTS accumulation varies extensively across subjects and exposures |
| Harris (1967) | Rhesus macaques | Pure tones (2-kHz) | 90-120 dB SPL | 30–60 min | Yes (4<) | Yes | No | No | TTS and PTS accumulate across exposures |
| Luz and Hodge (1971) | Rhesus macaques | Impulse noise | 168 dB SPL | 2x | Yes (2) | Yes | No | No | TTS severity and recovery |
| Tank noise | 110 dB SPL | 12 min | Yes (3) | ||||||
| Hunter-Duvar and Elliott (1972) | Squirrel monkeys | Pure tones (1- or 2-kHz) | 120 dB SPL | 5–15 min, 20 min–12 h | Yes (1–7) | Yes | No | Yes | TTS and PTS do not correlate with OHC or IHC loss |
| Hunter-Duvar and Elliott (1973) | Squirrel monkeys | Pure tones (1- or 2-kHz) | 130 or 140 dB SPL | 3 or 4 h | No | Yes | No | Yes | PTS does not correlate with OHC or IHC loss |
| Luz et al. (1973) Jordan et al. (1973) Pinheiro et al. (1973) | Rhesus macaques | Impulse noise | 168 dB SPL | 2x | Yes (3–18) | Yes | No | Yes | TTS and PTS accumulation across exposures; OHC and IHC counts; improved LF sensitivity in some subjects |
| Tank noise | 110 dB SPL | 12 min | Yes (2) | ||||||
| Pugh et al. (1974) | Pigtail macaques, squirrel monkeys | OBN (8-kHz CF) | 114 dB SPL | 30 min | Yes (not specified) | Yes | Yes | No | Simultaneously recorded AP from chronically implanted electrode; smaller neural TTS than behavioral TTS |
| Scheib et al. (1975a) | Rhesus macaques | OBN (2-kHz CF) | 90 dB SPL | 36 days | No | Yes | No | No | TTS growth over duration of exposure |
| Scheib et al. (1975b) | Rhesus macaques | OBN (2-kHz CF) | 90 dB SPL | 90 days | No | Yes | No | Yes | TTS growth and accumulation to PTS; no relation to OHC/IHC loss |
| Hawkins et al. (1976) | Rhesus, pigtail, and crab-eating macaques, baboon | OBN (0.5-, 2-, 4-, or 8-kHz CF) or BBN (100-Hz to 10-kHz) | 120 dB SPL | 8 h | Yes (20) | Yes | No | Yes | TTS and PTS accumulation over time; weakly correlated with OHC and IHC loss; BBN causes more damage than OBN |
| Jerger et al. (1978b) | Squirrel monkey | BBN | 108–118 dB SPL | 1-2 h | Yes (1–5) | No | Yes | Yes | Tympanometry and acoustic reflexes pre- and post-noise exposure; reflexes predict severity and extent of cochlear damage |
| Nielsen et al. (1978) | Squirrel monkeys | NBN (375-750-Hz) | 95 or 105 dB SPL | 1, 2, 4, 8, 16, 24, or 48 h | Yes (7<) | Yes | No | No | TTS growth increases with longer exposure times; TTS recovery is biphasic |
| Moody et al. (1978) | Rhesus, pigtail, and crab-eating macaques, baboon | OBN (0.5-, 2-, 4-, or 8-kHz CF) or BBN (100-Hz to 10-kHz) | 120 dB SPL | 8 h | Yes (20) | Yes | No | Yes | Extension of Hawkins et al. (1976); TTS does not increase with continued exposure; weak correlation between PTS and OHC/IHC loss; Stebbins et al. (1979) references this data in species comparison |
| OBN (2-kHz CF) | 120 dB SPL | 40 hr | No | Yes | Yes | ||||
| Nielsen et al. (1978) | Squirrel monkeys | NBN (375-750-Hz) | 95 or 105 dB SPL | 1, 2, 4, 8, 16, 24, or 48 h | Yes (7<) | Yes | No | No | TTS growth increases with longer exposure times; TTS recovery is biphasic |
| Pugh et al. (1979) | Pigtail macaques | OBN (8-kHz CF) | 108 dB SPL | 1 h | No | Yes | Yes | Yes | Loudness recruitment during TTS and PTS; similar estimates via reaction time task and chronic electrocochleography |
| OBN (8-kHz CF) | 118 dB SPL | 8 h | Yes (20) | ||||||
| Moody et al. (1980) | Rhesus macaques | OBN (2-kHz CF) | 100 dB SPL | 1 or 2 h | No | Yes | No | No | Response latency as a function of tone intensity during TTS recovery; compared to effects of ethanol administration |
| Lonsbury-Martin and Martin (1981) | Rhesus macaques | Pure tones (many different CFs) | 100 dB SPL | 3 min | Yes (not specified) | Yes | No | No | TTS and neuronal adaptation recovery times; recorded neurons in cochlear nucleus and inferior colliculus |
| Nielsen et al. (1984) | Squirrel monkeys | OBN (500-Hz CF) | 95 dB SPL | 2, 4, 8, 12, 16, 24, 36, 48, 60, 72, or 96 h | Yes (7<) | Yes | No | No | Continuous vs. interrupted exposures; TTS growth is faster for continuous than interrupted noise |
| Lonsbury-Martin et al. (1987) | Rhesus macaques | Pure tones (many different CFs) | 100 dB SPL | 3 min | Yes (not specified; 5.5–14.4 h total) | Yes | No | Yes | Total of 5–14 h of exposure; mild PTS accumulation from TTS; no relationship between PTS and OHC/IHC loss |
| Valero et al. (2017) | Rhesus macaques | NBN (2-kHz CF, 50-Hz BW) | 108, 120, 140, 146 dB SPL | 4 h | Yes (1–5) | No | Yes | Yes | ABR and DPOAE characterization of TTS and PTS; OHC, IHC, and IHC ribbon synapse counts |
| Hauser et al. (2018) | Rhesus and bonnet macaques | NBN (2-kHz CF, 50-Hz BW) | 140, 146 dB SPL | 4 h | No | Yes | Yes | Yes | Tone detection in quiet, steady state noise, and amplitude modulated noise following PTS; correlated with OHC/IHC/synapse loss |
B. Octave band and broadband noise exposures
Researchers at the University of Michigan were among the first to study cochlear pathology in NHPs. Their initial focus on antibiotic ototoxicity identified severe cochlear lesions characterized by complete IHC and OHC loss and the presence of phalangeal scars following aminoglycoside use (e.g., Stebbins et al., 1969). The lesions progressed from base to apex with increasing treatment duration. Behavioral pure-tone audiograms were correlated with the anatomical findings, with threshold shifts of 60+ dB resulting from the cochlear lesions. Steep cutoffs and a high degree of symmetry across ears were noted, both anatomically and behaviorally. Overall, these findings provided some of the first direct scientific evidence for the place theory of hearing, which was relatively new at the time (Davis, 1957).
Following this and other studies on ototoxicity, several groups took on investigations of noise-induced hearing loss, due to its broader relevance and greater prevalence. Modeling noise exposure conditions against typical work-related noise conditions, the Michigan group created permanent hearing loss with long, repeated exposures to 120 dB SPL noise (presented for 8 h per day for 20 days). In these classical studies, the exposures consisted of either broadband or octave band noise with varying center frequencies (Hawkins et al., 1976; Moody et al., 1978).
In agreement with the prior ototoxicity studies, the basal cochlea seemed uniquely vulnerable to damage. The basal-most hook region of the cochlea was particularly vulnerable, showing complete ablation in nearly all noise-exposed subjects (Hawkins et al., 1976). This extreme basal loss of all OHCs and IHCs was thus termed a juxtafenestral (“near the window”) lesion (Hawkins et al., 1976). Beyond the base, noise-induced damage was observed tonotopically along the cochlear length, according to the frequency spectrum of noise to which the subject was exposed. These tonotopic lesions were broader and less severe than the juxtafenestral lesions. OHC loss was more severe than IHC loss, suggesting greater vulnerability of OHCs than IHCs to noise-induced damage. Higher center frequency noise bands (e.g., 2-, 4-, or 8-kHz) were more effective at generating noise-induced hearing loss than lower center frequency noise bands (e.g., 0.5- or 1-kHz), and broadband noise caused more severe hearing loss and greater hair cell loss than any of the octave band noises. However, Hawkins et al. (1976) noted “a ‘central tendency’, reminiscent of the familiar 4-kHz dip in the audiograms of patients with noise-induced hearing loss,” referencing the tendency of the mid-cochlear (and more basal) regions to be more vulnerable to noise damage, regardless of the spectrum of the noise exposure.
Behavioral audiograms revealed that these macaquess experienced TTS of up to 60–85 dB and PTS typically peaking around 40–55 dB. Both TTS and PTS were highly symmetric within a given subject. While TTS did not increase throughout the course of exposure, PTS accumulated over time, with greater losses observed after longer exposure durations (Hawkins et al., 1976; Moody et al., 1978; also demonstrated in chinchillas: Clark and Bohne, 1978).
Moody et al. (1978) concluded that their data supported a strong relationship between cochlear pathology and audiometric threshold, again furnishing the place theory of hearing, but a closer examination of the data suggest a weak relationship with several exceptions. Importantly, some subjects had significant PTS accompanied by minimal hair cell loss along the entire cochlear length (Moody et al., 1978). The authors suggested that some hair cells, though still present, must have experienced extensive damage without being lost. In a subsequent publication, Stebbins et al. (1979) argued that these data supported the notion of two distinct receptor cell types in the cochlea. Through investigations of behavioral thresholds and cochlear damage following ototoxic treatment in chinchillas, Ryan and Dallos (1975) concluded that OHCs were necessary for normal hearing detection and that OHCs facilitate normal IHC function. Still regarded today as largely true, Stebbins et al. (1979) provided the critical cross-species validation by comparing across datasets in chinchillas, guinea pigs, patas monkeys, and macaques.
The same researchers at University of Michigan also studied TTS in macaques using 2-kHz octave band noise continuously presented at 90 dB SPL for 36–90 days (Scheib et al., 1975a; Scheib et al., 1975b). The minimal descriptions available from these studies suggest that TTS accumulated to an initial plateau of approximately 20 dB over the first 7–12 h of exposure (sometimes described as an “asymptotic threshold shift”; Clark and Bohne, 1978). Thresholds continued to increase, though much more slowly, over the next 5–7 days until leveling to a second plateau, approximately 10 dB higher than the initial TTS. Considerable inter-subject variability was noted. One subject had much larger threshold shifts (60 dB) than the other three, and when thresholds were measured 72 h following termination of the noise exposure, sensitivity had fully recovered in two subjects and the remaining two had PTS of 15–25 dB. Thus, a stimulus that initially caused only a TTS eventually caused a PTS in 50% of the NHPs. All subjects had scattered hair cell loss that was not predicted by the TTS or PTS. These studies demonstrate the extent to which susceptibility can vary, even in a small cohort of NHP subjects.
Pugh et al. (1973), also at the University of Michigan, conducted chronic intracochlear recording in NHPs to investigate the relationship between noise-induced changes to the auditory nerve action potential (AP) and behavior (Pugh et al., 1974; Pugh et al., 1979). Following exposure to 8-kHz octave band noise at 114 dB SPL for 30 min, pigtail macaques and squirrel monkeys exhibited a mild TTS that was larger in behavioral than neural measures (Pugh et al., 1974). Furthermore, although relatively small reductions in the suprathreshold AP amplitude were observed in these monkeys, an increase in the slope of the AP input/output function was interpreted as evidence of loudness recruitment (Pugh et al., 1974).
To test this more directly, Pugh et al. (1979) estimated loudness recruitment by examining the effects of stimulus intensity on behavioral reaction times and AP latency in monkeys before and after noise exposure. Subjects were exposed to 8-kHz octave band noise at 108 dB SPL for 1 h to induce TTS and were later exposed to the same noise at 118 dB SPL for 8 h daily for 20 days to induce PTS. Indeed, reaction times for low-intensity sounds were much longer following noise exposure (during TTS and following PTS) and the slope of the reaction-time vs stimulus intensity function was increased, while reaction times for high intensity tones were unchanged. The AP latency vs stimulus intensity functions showed comparable results, and the OHC loss observed in the PTS ears was interpreted as evidence that loudness recruitment may be related to OHC function.
In relation to their previous work investigating loudness recruitment in normal hearing macaques (Stebbins, 1966; Stebbins and Miller, 1964), Moody et al. (1980) used a more acute model of TTS to investigate changes in the latency-intensity function. Macaques were exposed to 1 or 2 h of 2-kHz octave band noise at 100 dB SPL. As thresholds recovered over the next 48 h, reaction times were recorded across tone levels. Consistent with the findings of Pugh et al. (1979), these results suggested that the subjects had loudness recruitment during TTS recovery, as evidenced by the increased slope of the latency-intensity functions. Once hearing sensitivity recovered to pre-exposure levels, the latency-intensity functions also returned to normal. These investigations of loudness recruitment were some of the only early nonhuman primate studies of noise-induced hearing loss (including TTS or PTS) to examine perceptual changes beyond basic hearing sensitivity.
Concurrently, researchers at Henry Ford Hospital in Detroit, Michigan began investigating the time course of TTS in squirrel monkeys exposed to 500-Hz octave band noise. The subjects underwent several exposures of varying durations across several days to weeks. Hearing sensitivity was assessed behaviorally at 750 Hz only. Nielsen et al. (1978) observed 5–10 dB of initial TTS growth during the first 1–8 h of noise exposure, followed by a continuous increase in TTS severity with increasing exposure time (up to 48-h duration). This lack of asymptotic threshold shift contrasts the findings described above (Hawkins et al., 1976; Moody et al., 1978; Scheib et al., 1975a; Scheib et al., 1975b). Following cessation of the noise exposure, TTS recovered in a biphasic manner: an initial fast phase (<15 min) followed by a slow phase (up to 48 h). Higher intensity exposures caused more severe TTS and longer recovery times. The results of these studies were remarkably similar to human studies of TTS growth and recovery. In a follow-up study, Nielsen et al. (1984) observed faster TTS growth in subjects with continuous—as opposed to interrupted—noise exposures. Despite large variability in severity of TTS and TTS growth rate, all subjects recovered back to normal hearing sensitivity within a few days after exposure.
A separate group studied changes in middle ear acoustic immittance following PTS caused by broadband noise exposure in squirrel monkeys (Jerger et al., 1978b). Tympanometry and acoustic reflexes (elicited by 0.5, 1, 2, and 4 kHz tonebursts and broadband noise) were measured before and after exposure to 108–118 dB SPL noise for 1–2 h over the course of multiple days. While tympanometry showed excellent middle ear compliance pre- and post-exposure, suggesting that there were no permanent effects on the integrity of the tympanic membrane and ossicular chain, the acoustic reflex thresholds predicted the severity and extent of cochlear hair cell loss.
C. High intensity pure tone exposures
Pre-dating the use of noise as an exposure stimulus, researchers utilized high intensity pure tone exposure to examine TTS growth and recovery and the accumulation to PTS in macaques (Harris, 1967). Harris (1967) was particularly interested in predicting susceptibility to PTS from TTS, so he employed a cross-species approach of humans, rats, and macaques (though it is important to note that exposure stimuli and conditions were quite varied across experiments and species). Macaques were exposed to 2-kHz tones for 30–60 min, with tone levels increasing from 90 to 120 dB SPL over several sessions. Higher exposure levels caused greater TTS and a mild PTS (<30 dB) accumulated across several exposures for 75% of the macaques (Harris, 1967). The one NHP subject that did not develop PTS was also notably more resistant to TTS than the others. No other obvious trends between TTS and PTS were observed for the remaining subjects. Humans and rats exhibited similarly weak TTS-PTS relationships (Harris, 1967).
In contrast to octave band or broadband noise, pure tones generate narrower activation patterns in the cochlea. Therefore, exposure to high intensity tones should lead to narrower cochlear lesions and a more limited spectrum of threshold elevation. In two seminal studies, Hunter-Duvar and Elliott (1972, 1973) monaurally exposed squirrel monkeys to 1- or 2-kHz pure tones at 120, 130, or 140 dB SPL. Behavioral audiograms were measured prior to obtaining cytocochleograms for the exposed and unexposed ears. Shorter duration exposures (5–15 min) elicited up to 30 dB TTS, with no differences in IHC or OHC counts between the exposed and unexposed ear. Longer duration (2–4 h) and higher intensity exposures ultimately generated PTS. However, severity and extent of PTS varied extensively across subjects, ranging from 20 to 50 dB peak loss anywhere between 1- and 6-kHz.
The impact of these experiments, however, comes from the fact that Hunter-Duvar and Elliott did not observe any measurable relationship between hair cell loss and PTS. For example, one subject presented with a 50 dB PTS following a three-hour exposure to a 1-kHz tone at 140 dB SPL but had normal hair cell counts bilaterally. Additionally, a different subject had less than 20 dB PTS following a four-hour exposure to a 140 dB SPL 1-kHz tone but exhibited complete loss of OHCs and some IHC loss along the entire basal half of the overexposed cochlea. No subjects showed narrow, tonotopically localized cochlear lesions, as might be predicted by cochlear mechanics. Instead, either unilateral basal cochlear lesions of varying extent were observed or no observable damage was present at all. Pure juxtafenestral lesions were not observed in any of the subjects.
A few years later, Lonsbury-Martin and Martin (1981) used short (3 min) 100 dB SPL pure tone exposures to create mild, quickly reversible monaural TTS in macaque monkeys. Behavioral thresholds typically recovered within 15–20 min post-exposure. High frequency tones elicited more severe TTS and longer recovery times than low frequency tones. Single unit recordings in the cochlear nucleus and inferior colliculus of the awake subjects revealed that neurons in the CN and IC typically exhibited larger threshold shifts and took longer to recover to baseline levels when compared with behavioral thresholds.
In a follow-up study, Lonsbury-Martin et al. (1987) conducted repeated monaural pure tone exposures over the course of 12–18 months, using similar stimulus conditions as the 1981 study. After 12 months of 100 dB SPL pure tone exposures accumulating to a total of 5.5 h, one subject had a narrow cochlear lesion (complete loss of IHCs and OHCs) in the mid-basal cochlea, but did not have any measurable PTS. The two macaques that underwent 18 months of 100 dB SPL pure tone exposures accumulating to 13–14 h had up to 10–15 dB PTS between 8 and 16 kHz. Cytocochleograms revealed a narrow cochlear lesion in one subject and normal cochlear anatomy in the other. Once again, these data suggest that sounds that initially only cause a TTS can accumulate to create PTS, but the underlying cochlear pathology is not well predicted by audiometric threshold shifts.
D. Impulse noise exposures
Due to the Department of Defense's vested interest in noise-induced hearing loss, many experimental paradigms are intended to model noise exposure conditions experienced by military personnel. High intensity impulse noises have been used in studies of humans and animals to probe the effect of blast exposures on hearing sensitivity. In fact, the earliest studies of noise-induced hearing loss in NHPs were completed in rhesus macaques by Romba and colleagues in the early 1960s using highly realistic military exposure conditions (Martin et al., 1962; Romba, 1962; Romba and Gates, 1964). Subjects were seated in a tank and exposed to machine gun blasts, which were approximately 165 dB SPL. Audiograms were obtained immediately following blast exposure and repeated over the course of 72 h. TTS was greatest (up to 20 dB) at 2- and 4-kHz, less severe at 6-, 8-, and 12-kHz, and not present below 1-kHz. Following multiple exposures, some subjects acquired PTS while others did not. TTS recovery and PTS accumulation varied extensively across subjects (Romba and Gates, 1964), suggesting large individual differences in susceptibility to NIHL.
Luz and Hodge (1971) also undertook experiments to probe the effect of blast exposures on hearing sensitivity in rhesus macaques and humans, specifically inquiring about TTS recovery patterns following exposure to blasts and to continuous broadband tank noise (110 dB SPL, 12-min duration). Following exposure to two 168 dB SPL impulses, subjects had TTS ranging from 5 to 40 dB that recovered to baseline sensitivity in as little as 20 min in some subjects or up to 32 h in others. Recovery patterns suggested two independent pathophysiological processes with different time constants (consistent with the observations of Nielsen et al., 1978; Nielsen et al., 1984), resulting in five distinct TTS recovery pattern classifications. Subjects underwent several impulse noise exposures and several continuous noise exposures. Severity of TTS and recovery pattern varied extensively by subject, test frequency, exposure type, and exposure number. In comparison to young adults exposed to gun shots in the laboratory, macaques had more severe TTS and slower recovery times. However, monkey and human shared the same recovery patterns, suggesting similar pathophysiology across species (Luz and Hodge, 1971).
Following their initial study, Luz et al. (1973) continued exposing the macaques to the impulse and continuous noises in order to generate PTS, with four weeks between exposures in order to reach maximal hearing recovery. As seen in nearly all studies described thus far, the magnitude and recovery pattern of TTS, the magnitude and bandwidth of PTS, and overall individual susceptibility was highly variable across subjects. However, a few unique findings are worth mentioning in greater detail here. First, seven of the nine subjects showed less severe TTS following their second noise exposure than following their first noise exposure. This and similar findings have been posited as a “toughening of the ears,” or an increased resistance or tolerance of damage within the cochlea. Taken together with the notion that TTS-related noise damage can accumulate to generate PTS, one can certainly appreciate the complexity of noise-induced cochlear pathology. Second, the majority of subjects required many noise exposures to induce even a mild, high frequency PTS (e.g., a series of 10 or 20 impulse noises resulted in 10–25 dB PTS). Macaques seem quite robust to blast exposure, albeit more susceptible than humans. Third, most subjects exhibited improved low frequency hearing sensitivity following noise exposure in the presence of high frequency PTS. The reason for this improved sensitivity is unknown, but has been reported by others (Moody et al., 1978).
Jordan et al. (1973) completed cytocochleograms on the Luz et al. (1973) macaque cohort. The extent and severity of hair cell loss was highly variable across subjects, ranging from normal IHC counts with a few missing OHCs and auditory nerve fibers to large basal wipeouts to isolated mid-cochlear OHC losses. All subjects exhibited juxtafenestral lesions of differing extents. Furthermore, hair cell damage was not well predicted by the pure tone audiogram (Pinheiro et al., 1973). Jordan et al. (1973) noted that hair cells adjacent to areas of loss were often swollen or damaged, suggesting ultrastructural damage and possible malfunction. At the level of the hair-cell and audiogram, cochlear pathology resulting from impulse noise exposure does not seem to differ from the damage resulting from continuous noise or pure tone exposures in NHPs.
E. Recent nonhuman primate studies of noise-induced hearing loss
In the 30+ years since the last studies of NIHL in NHPs, many methodological improvements have emerged including advanced behavioral assays, novel histological preparations including immunohistochemistry, higher-resolution imaging methods, and improved electrophysiological measures. Due to these advances, it is appropriate to re-visit the classical studies of macaque noise-induced hearing loss in order to gain a more complete understanding of the relationship between noise exposure, cochlear pathology, auditory pathway integrity, and behavioral manifestations.
The first application of these comprehensive and updated methodological approaches in NHPs was completed by the present authors (Valero et al., 2017). In this study, cochlear function was assessed by ABRs and DPOAEs in macaques following exposure to narrowband noise at SPLs ranging from 108 to 146 dB SPL. Histopathological assessments of hair cell and synapse survival revealed a vulnerability of IHC ribbon synapses even in cases of TTS and in the absence of IHC or OHC loss. Furthermore, this study revealed that in instances of severe PTS accompanied by IHC and OHC loss, the loss of ribbon synapses can be quite robust in the IHCs that survived the noise exposure and recovery time.
In a companion study designed to establish perceptual consequences of cochlear histopathology in NHPs, and for comparison with the human literature, noise-exposed macaques were trained to detect tones in quiet and in the presence of various background noises before and after a four hour narrowband noise exposure that caused PTS (Hauser et al., 2018). Following noise exposure, subjects had PTS of 40–60 dB across a narrow range of tone frequencies in quiet. Macaques with PTS had a slower increase in detection thresholds in increasing levels of broadband background noise maskers (i.e., a lower threshold shift rate), and a reduced release from masking in the presence of amplitude-modulated masking noise, to a degree that correlated with the magnitude of PTS across test frequencies. Additionally, threshold shift rate was significantly correlated with IHC, OHC, and synapse loss observed in a cohort of NHPs that underwent an identical noise exposure (from Valero et al., 2017). We are continuing studies of these and other noise exposed animals in order to investigate changes in auditory perception following TTS and PTS. These data serve as one of the first direct corroborations of complex auditory perception (beyond a behavioral audiogram) and cochlear histopathology following noise-induced hearing loss for any species.
F. Summary of NHP noise exposure studies
Despite the relative paucity of primate studies of noise-induced hearing loss, several noteworthy conclusions, including conspecific trends, can be gleaned from this literature:
-
(1)
Higher intensity and longer duration stimuli generate more severe cochlear damage, starting with OHC damage/loss, followed by IHC loss.
-
(2)
A stimulus that initially causes a TTS can, with repeated exposures, eventually cause a PTS.
-
(3)
The basal-most region of the cochlea is more susceptible to noise-induced damage than the apical regions, regardless of the characteristics of the exposure stimulus.
-
(4)
Severity of TTS can predict the likelihood, but not the severity, of PTS.
-
(5)
The relationship between severity of cochlear damage and the magnitude of TTS or PTS remains unclear.
-
(6)
The lack of relationship between severity of cochlear damage and degree of TTS or PTS may be due to ultrastructural damage that is not visible in light microscopy. These pathophysiological processes may also account for the different configurations of TTS recovery over time.
-
(7)
NHPs are more resistant to noise-induced damage than other laboratory species, but more susceptible than humans (Luz and Hodge, 1971; Luz and Lipscomb, 1973; Stebbins et al., 1979; Valero et al., 2017).
IV. NONHUMAN PRIMATES AS A MODEL FOR DEVELOPMENT AND VALIDATION OF THERAPEUTICS FOR NOISE-INDUCED HEARING LOSS
A. Overview
The discovery and validation of therapeutic approaches to treat medical conditions, such as hearing loss, is an extremely long process fraught with numerous challenges. Only a tiny fraction of the promising therapeutics that reach clinical trials are effective, let alone ultimately approved, by the FDA (Garner, 2014). Long before the commencement of clinical trials, prospective treatments are developed and validated in small animal models, typically mice and other rodents.
Intermediate species (e.g., canines, felines, NHPs) are used when deemed appropriate. As a recent example, Voretigene became the first FDA-approved gene therapy for correction of a specific gene mutation in the U.S. (Petersen-Jones and Komáromy, 2015; Russell et al., 2017). In earlier stages of development, the procedure was refined and vetted in rodents, then applied to a large-animal canine model for further validation (Acland et al., 2001). This was a suitable choice as the mutation naturally occurs in some dogs. By virtue of their close phylogenetic relationship to humans, the use of NHPs as an intermediate animal model may be an appropriate choice to increase confidence in the application and translation of foundational discoveries made in other species. Indeed, as mentioned above, NHPs have been chosen for development of diagnostics and therapeutics where phylogenetic similarity was an important factor (e.g., cardiology, cognition, genetics, HIV/AIDS, immunology, pharmacology, reproduction, respiratory disease) (Phillips et al., 2014).
Ideally, species selected as models would provide information that translates directly to humans with high sensitivity and specificity in a manner that is cost-effective. Unfortunately, the path is rarely this direct. In theory, translational challenges from rodents to humans should be minimal for highly conserved biological targets (e.g., hair cells), and the necessity of a large-animal intermediate could potentially be minimal. In practice, unforeseen factors combine to impede progress (Perlman, 2016), as successful outcomes may also depend on interactions with other factors, such as body size, inflammatory response, metabolic rate, hormonal composition, biocompatibility, etc.
The development of pharmacologic and gene therapies for acquired and hereditary forms of hearing loss has rapidly progressed over the last decade, but most therapies remain at a relatively early stage. The vast majority involves rodent models, and none of the datasets derived from systematic testing in a large animal intermediate have been publicly disclosed. Here we consider a few of the many factors that may significantly impact the development of effective therapeutics, including species differences that may pose challenges to translation.
B. Species differences in susceptibility to NIHL
As briefly mentioned above, susceptibility to NIHL (PTS, TTS) and related conditions (e.g., hyperacusis, tinnitus) appears to differ significantly between individuals and species (Dobie and Humes, 2017; Henderson et al., 1993; Knipper et al., 2013; Luz and Hodge, 1971; Luz and Lipscomb, 1973; Sliwinska-Kowalska and Pawelczyk, 2013; Stebbins et al., 1979; Valero et al., 2017), including strains of inbred mice used in research (Myint et al., 2016). Controlled studies in NHP and humans are relatively rare (or prohibited), and often have lower subject numbers, variable or unknown noise exposures, and less control of contributing factors such as exposure history, lifestyle, sex, age and genetics.
An important observation is that the exposures sufficient to generate TTS and PTS are lower overall in rodents than NHPs and humans (see Table I; also discussed in Dobie and Humes, 2017; Valero et al., 2017; Yankaskas et al., 2017). The range of SPLs that cause cochlear damage in mice, ranging from synaptopathy to hair cell loss, is relatively small when compared to NHPs and humans. In mice, a single exposure to octave-band noise of 97–98 dB SPL causes TTS, accompanied by a narrow-band synaptopathic lesion, while an increase to 116 dB SPL can rupture the reticular lamina, leading to large wipeout regions in the organ of Corti (Wang et al., 2002). In macaque monkeys, the range of exposures over which these effects have been observed spans 108–146 dB SPL (Valero et al., 2017). Cochlear synaptopathy of approximately 30% accompanied a single TTS-inducing 108-dB exposure to narrowband noise, whereas a single 146-dB exposure caused PTS, substantial synaptopathy (up to ∼80% in a given region), and hair cell loss. Comparable PTS data and hair-cell counts have been reported in other NHP studies and humans (see Table I).
C. Factors influencing therapeutic efficacy
The mouse model is invaluable for early-stage development and validation of potential therapeutics, particularly when a transgenic model can add value to mechanistic questions. However, mice and humans often respond differently to the same treatments (Perlman, 2016), and there are obvious anatomical differences that may limit the translation of a given approach (see Secs. IV C 2 and IV C 3). Therefore, an intermediate translational model will likely be essential when developing drugs and the delivery approach for humans. For some treatments, intermediate testing in NHPs may be an effective strategy to optimize effectiveness and reduce risk, with respect to the biological target, design of the therapeutic agent, delivery route, therapeutic window, and other (perhaps unforeseen) factors. A few of these are highlighted here.
1. Genetics
Similarities and differences in gene expression and regulation between species are certain to be important factors with respect to hearing and hearing loss. Several studies have linked genomic variations to significant differences in anatomy and physiology, as well as to hearing loss (Dou et al., 2003; Hosoya et al., 2016a; Hosoya et al., 2016b; Köppl et al., 2018; Makishima et al., 2005; Matsuzaki et al., 2018; Plum et al., 2001; Suzuki et al., 2007; Van Laer et al., 2006, 2005; Wang et al., 2018). Transcriptome profiling has been productively applied to the cochlea and portions of the central pathways of humans and mice (Burns et al., 2015; Cai et al., 2015; Guo et al., 2016; Hackett et al., 2015; Schrauwen et al., 2016), while studies of the impact of NIHL on gene expression are beginning to emerge (Frenzilli et al., 2017; Lavinsky et al., 2016; Manohar et al., 2019; Manohar et al., 2016; Sun et al., 2008). To date, none include NHPs, although improved diagnostic and treatment efficacy could potentially be fostered by studies in species with closer phylogenetic and developmental similarity to humans. This may be especially relevant for applications involving gene therapy (Ahmed et al., 2017; Gao et al., 2018), where genomic and gestational differences between species are significant factors in treatment efficacy (Wang et al., 2018).
2. Inner ear anatomy
Fortunately, the major structures in the cochlea (hair cells, supporting cells, neuronal types) are highly conserved across species, implying relative uniformity with respect to biological targets. However, the dimensions of most structures and fluid filled compartments (i.e., hair cells, supporting cells, stereocilia, round window, oval window, scala tympani, scala media, scala vestibuli, cochlear aqueduct, endolymphatic duct, round window membrane, etc.) vary significantly between species and in a manner that could impact one or more aspects of drug delivery (Glueckert et al., 2018). For example, differences in fluid volume and flow in the perilymphatic or endolymphatic spaces may contribute to pharmacokinetic variability (Salt and Hirose, 2018). The volume of the macaque inner ear is about 24 times greater than mouse, and the human cochlea is about three times larger than macaques (Dai et al., 2017; Ekdale, 2013; Kirk and Gosselin-Ildari, 2009). Basilar membrane lengths range from a mean of 6.8 mm in mice, 12.1 mm in rats, 20.5 mm in guinea pigs, 22.5 mm in cats, 27 mm in macaques, 29 mm in baboons (Wright et al., 1987; Felix, 2002), compared to a mean of 35 mm in humans (Kirk and Gosselin-Ildari, 2009). NHPs have one row of inner hair cells and three rows of outer hair cells, with ectopic or supernumerary hair cells frequently noted (Valero et al., 2017), consistent with reports in humans (Rask-Andersen et al., 2017).
An important feature related to labyrinthine volume concerns the patency and dimensions of the cochlear aqueduct, which is longer and narrower in NHPs and humans (Gopen et al., 1997). This channel links the scala tympani with the subarachnoid space in the brain and is a potential route by which drugs delivered to the scala tympani could exit the cochlea or mix with incoming CSF. Rodents and primates appear to differ with respect to CSF influx and efflux through this channel. These and numerous other factors (not discussed here) can significantly alter the pharmacokinetics of drugs delivered to the perilymph, and differentially impact basal and apical regions (Salt and Hirose, 2018; Salt et al., 2016). Comparable principles impact pharmacokinetics in the middle ear, as well. Accordingly, species differences are important considerations, and while modeling may be a useful guide, direct testing in large animal models may be needed to validate predictions and/or refine the models.
3. Innervation of the cochlea
Afferent and efferent innervation appears to be fairly well conserved between species, although intensive studies in NHPs are lacking. Branching of type 1 radial afferent fibers has been noted in NHPs (Kimura, 1975), as well as rats (Perkins and Morest, 1975), guinea pigs (Fernandez, 1951), cats (Liberman, 1982; Perkins and Morest, 1975), and humans (Nadol, 1983). Additionally, human spiral ganglion cell somata are primarily unmyelinated (Nadol, 1988; Ota and Kimura, 1980; Rattay et al., 2013), unlike most other laboratory species (Rattay et al., 2013). It is unknown whether NHP spiral ganglion cell somata are myelinated.
While there are very limited data on NHP auditory nerve fiber (ANF) physiology (Katsuki et al., 1962; Nomoto et al., 1964; Nomoto, 1980; Joris et al., 2011), all studies seem to stray from the properties observed in other laboratory species. For example, macaques do exhibit a bimodal population distribution of ANF spontaneous rates similar to that observed in other mammals (Nomoto et al., 1964; Joris et al., 2011). However, there is no clear evidence for a relationship between spontaneous rate and threshold at the ANF's characteristic frequency in macaques (CF; an ANF's most sensitive frequency; Joris et al., 2011; Nomoto et al., 1964). The relationship between CF threshold and spontaneous rates of auditory nerve fibers is one of the most important features of the findings in other mammalian species (e.g., Liberman, 1978). These results suggest that one of the primary organizational principles of the auditory periphery may be different in primates relative to other mammals, causing concern for translatability (Hickox et al., 2017). This is especially relevant to pathologies like synaptopathy, which preferentially affects low spontaneous rate ANFs in rodents (Furman et al., 2013; Song et al., 2016).
4. Delivery route
The effective delivery of therapeutic agents to the inner ear is an active area of exploration. Major factors include the route of delivery and composition of the therapeutic. Both factors may be significantly impacted by species specific features, with implications for translation to humans. Promising delivery routes include transtympanic injection into the middle ear space (tympanum), direct injection into perilymphatic space through the round window membrane, cochleostomy of the basal or apical turns, and injection into the posterior semicircular canal (Akil and Lustig, 2019; El Kechai et al., 2015; Isgrig and Chien, 2019; Lichtenhan et al., 2016; Suzuki et al., 2017). Each has advantages and disadvantages, including the risk of unintended middle or inner ear damage. In addition, efficacy appears to depend on interactions between the delivery route and the biological target (cell type), the therapeutic agent, and various subject characteristics (Salt and Plontke, 2018). A few examples follow.
5. Therapeutic window
Although afferent synapses on IHCs are immediately lost following acoustic overexposure, the terminal dendrites retract slowly and the neuronal cell bodies can remain in the spiral ganglion for months to years (Fernandez et al., 2015). This offers a long window during which a therapeutic agent might encourage the reinnervation of IHCs by cochlear nerve fibers. Treatments under evaluation for NIHL typically involve delivery of the therapeutic agent (e.g., a viral vector encoding a gene of interest or a small pharmacologic molecule) within a window of hours to weeks after the exposure (Du et al., 2018; Sly et al., 2016; Suzuki et al., 2016), or even prior to exposure (Chen et al., 2018). The optimal therapeutic window for humans is unknown, therefore preliminary studies in NHPs may improve predictions.
6. Properties of therapeutic agents
A thorough discussion of the factors related to design of potential therapeutic agents is well beyond the scope of this review, however a few relevant observations are highlighted here.
For genetic and acquired hearing loss, viral mediated gene delivery for cell-type-specific targeting currently offers the most promise for effective treatments (Ahmed et al., 2017; Akil and Lustig, 2019; Chien et al., 2015; Fukui and Raphael, 2013; Géléoc and Holt, 2014; Holt and Vandenberghe, 2012; Zheng and Zuo, 2017). Adeno-associated viruses (AAV) are the most promising vectors for gene transfer. Scores of serotypes, identified from screens in NHP and human tissue (Gao et al., 2004; Gao et al., 2002), are now known, but transduction appears to vary by cell type (Kim et al., 2019). In addition, tropism patterns have not been determined for most serotypes, and could certainly vary by species and biological target. Fortunately, the conservation of cellular and molecular features between species appears to be quite high, suggesting that cell-type specific therapies vetted in rodents may also be effective in primates, including humans. Indeed, recent findings indicate that the synthetic AAV vector Anc80 can efficiently transduce cochlear hair cells in macaques in a dose-dependent manner (Francis et al., 2019). However, differences in the expression and regulation of some genes and proteins can be substantial, and these should be carefully considered in the design of therapeutics.
For the treatment of NIHL or other conditions by pharmacologic agents (e.g., anti-inflammatories, neurotrophins, antibiotics), translational efficacy also depends on myriad factors, many of which remain incompletely defined. The resultant impact on pharmacokinetics appears to depend on interactions between the anatomical features briefly highlighted above and the delivery method, dosage, and physical properties of the compound (Salt and Plontke, 2018). Species differences are well characterized for very few of the compounds currently in clinical use, thus it remains to be determined how predictive these data will be for novel formulations.
7. Conclusion
Overall, the data highlighted in this section reveal that multiple interdependent factors contribute to treatment efficacy. The differences between species in this respect are not merely a matter of scaling but involve complex interactions between factors that cannot be reliably predicted from modeling alone. Direct testing in animal models and humans will be needed to augment predictions, and given the sizable differences between mice and humans, we suggest that NHPs are an ideal intermediate species for improving the efficacy and safety of this process.
V. PRACTICAL CONSIDERATIONS
The paper thus far highlights the importance of the NHP model to investigate noise induced hearing loss, both the basic aspects as well as the clinical translational and therapeutic aspects. While there are many possible opportunities to important and fruitful research plans, there are a few practical matters to consider. As opposed to rats and mice, the care and use of NHPs is regulated by the United States Department of Agriculture (USDA), and are under much stricter oversight from the Institutional Animal Care and Use Committee and veterinary staff. The institutional laboratory animal veterinary staff then must include expertise in primate medicine to assure and provide adequate veterinary oversight of the animals in the research program. In addition, the program needs to ensure the provision of species-specific environmental enrichment to adhere to the USDA policies as expressed in their document, Guide for the Care and Use of Laboratory Animals. Making sure that such requirements are met requires additional staff with specialized training.
A second consideration is space. Macaques are larger than the traditional laboratory animal species used (mice, rats, gerbils, guinea pigs, cats, etc.), and this necessitates greater housing room. As with other species, the space requirement varies with the body weight of the animal; the smallest primates require the least space per animal. Minimum space requirements range from about 2.1 sq. ft/animal for the smallest animals (<1.5 kg) to >25 sq. ft. for animals over 30 kg. These are much larger compared to the range for mice (6 – >15 sq. in/animal), rats (17–70 sq. in.), and guinea pigs (60–100 sq. in./animal). The minimum space requirement for the smallest primates are about four times the space requirement for the largest rats and three times the caging size requirements for the largest guinea pigs. Further, the social nature of nonhuman primates requires that they are socially housed in pairs or groups. Additionally, primates are required to have enough vertical space to permit standing vertically on two legs, to swing from the cage ceiling without hitting the floor, and to make brachiating movements. These constraints increase the space requirements to house and maintain these valuable animals.
The third consideration is the monetary costs for acquiring and maintaining primates. These costs include purchasing, shipping, and housing. A survey of nonhuman primate vendors revealed that the purchase costs were species dependent and far higher than that of common rodents. In comparison to the cost of a mouse or a rat, squirrel monkeys cost about 100–130 times as much, marmosets cost about 140–200 times, and macaques range from 200 to 300 times the cost. The shipping costs depend on the distance between the institutions and the vendor, ranging from $4000 to $12 000 per batch of primates. Housing costs were extrapolated from the 2017 Yale University survey on housing costs, with information collected from 57 institutions, with an annual increase of about 3%. These costs depended on the primate species and institution (public vs private, location within the United States of America). Housing or per-diem costs range from about 12 times the cost of a mouse cage (typically 3–5 mice) to 25 times the cost of a cage of mice, depending on the location. While it is true that most NHP labs utilize fewer subjects and maintain the same colony for many years, costs remain significantly greater than those incurred by rodent research programs. Such high costs necessarily constrain the funds that can be devoted to non-animal costs given the limited funds provided by funding agencies to perform the studies that have highly variable effects, as discussed above.
VI. FUTURE DIRECTIONS
Given the relatively sparse literature on nonhuman primates and NIHL, the opportunities are vast, and the primate is an excellent candidate to fill the gaps in our knowledge. We propose some broad classes of studies that would be essential to further our understanding of the mechanisms of NIHL, their perceptual effects, and treatment option to ultimately reverse the effects of the noise exposures. In spite of the considerations discussed above, these essential experiments would advance our knowledge of basic mechanisms and enhance the translatability of the growing rodent and human literatures on noise-induced pathologies.
-
(1)
Genomics. Although the human genome is more similar to NHPs than mice and other species (Breschi et al., 2017; Marques-Bonet et al., 2009), they are not identical, and the differences in structure and function can be significant in ways that limit translation (Bailey, 2005). For many genes, structural and functional conservation is quite high, suggesting a better prognosis for translation, while for others, species differences are substantial, even in homologous structures (Bernard et al., 2012; Chen et al., 2016; Konopka and Geschwind, 2010; Mashiko et al., 2012; Mitchell and Silver, 2018; Sousa et al., 2017; Zeng et al., 2012). For this reason, predictions about functional outcomes for a specific biological target (e.g., hair cells, auditory nerve) must be determined in a cell- or tissue-specific manner for each species. To improve predictions and outcomes, genomic and proteomic profiling of peripheral and central auditory structures should be pursued in NHPs and humans for comparison with other models.
-
(2)
Inner ear anatomy and physiology. Descriptions of the structural and functional features of the inner ear and major cochlear structures have not been systematically carried out for NHPs, and existing data may lack essential details. Advanced understanding of key features (e.g., dimensions of fluid compartments, cell types, innervation, membrane permeability, fluid dynamics) could greatly enhance functional modeling and therapeutic design (i.e., pharmacological, gene therapy). Further, characterizing the physiological encoding schemes and their changes with the structural damage caused by noise exposure will also aid in identifying physiological and behavioral assays for differential diagnosis of specific cochlear pathologies.
-
(3)
Clinically viable assessment tools. Development of sensitive new tools to augment routine audiological assessments are needed to identify different forms of auditory pathology caused by overexposure to noise (e.g., synaptopathy with and without hair cell loss), and perhaps distinguish those patterns from hearing loss caused by other factors (e.g., aging, hereditary factors, ototoxicity). The same tools could be used to assess recovery from NIHL, or other pathology, as therapeutic tools move toward clinical trials in humans. Research involving NHPs will be invaluable in this regard, as assessment tools can be developed and subsequently validated by histological analyses of the cochlea, auditory nerve, and central pathways, with support from direct recordings from these structures (see Valero et al., 2017).
-
(4)
Individual variability. It is often noted that two subjects with identical noise exposure histories can have very different cochlear pathology and performance in perceptual tasks. This difference in susceptibility to noise exposure has been attributed in the literature to “tough” and “tender” ears (Cody and Robertson, 1983; Maison and Liberman, 2000). It is not a big stretch to extend the individual variability to treatment effectiveness as well. Coupled with the large inter- and intra-species genetic variability that is observed in primates (including humans, reviewed briefly above), individual variability should be systematically investigated. These investigations may ultimately shed light on efficacious treatment options to combat NIHL.
ACKNOWLEDGMENTS
The authors would like to acknowledge the anonymous reviewers for their review of this manuscript, Amy Stahl for compiling the data for Fig. 1, and Chase Mackey for comments on an earlier version of the manuscript. J.B. was supported by Grant No. NIH T32 MH 064913-16 (PI: Danny Winder), and T.A.H. and R.R. were partially supported by Grant No. NIH R01 DC 015988 (MPI: R.R. and B. Shinn-Cunningham).
References
- 1. Acland, G. M. , Aguirre, G. D. , Ray, J. , Zhang, Q. , Aleman, T. S. , Cideciyan, A. V. , Pearce-Kelling, S. E. , Anand, V. , Zheng, Y. , Maguire, A. M. , Jacobson, S. G. , Hauswirth, W. W. , and Bennett, J. (2001). “ Gene therapy restores vision in a canine model of childhood blindness,” Nature Genetics 28(1), 92–95. 10.1038/ng0501-92 [DOI] [PubMed] [Google Scholar]
- 2. Ahmed, H. , Shubina-Oleinik, O. , and Holt, J. R. (2017). “ Emerging gene therapies for genetic hearing loss,” JARO 18(5), 649–670. 10.1007/s10162-017-0634-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akil, O. , and Lustig, L. (2019). “ AAV-mediated gene delivery to the inner ear,” in Adeno-Associated Virus Vectors: Design and Delivery, edited by Castle M. J. ( Springer, New York: ), pp. 271–282. [DOI] [PubMed] [Google Scholar]
- 4. Alegre, M. , Gurtubay, I. G. , Iriarte, J. , Ciordia, E. , Manrique, M. , and Artieda, J. (2001). “ Brainstem auditory evoked potentials (BAEPs) in the cynomolgus macaque monkey Equivalence with human BAEPs and proposal of a new nomenclature,” Hear. Res. 151, 115–120. 10.1016/S0378-5955(00)00215-X [DOI] [PubMed] [Google Scholar]
- 5. Allen, A. R. , and Starr, A. (1978). “ Auditory brain stem potentials in monkey (M. Mulatta) and manm,” Electroencephalogr. Clin. Neurophysiol. 45(1), 53–63. 10.1016/0013-4694(78)90341-3 [DOI] [PubMed] [Google Scholar]
- 6. Bachmann, K. R. (1996). “ A study of the effect of clinical doses of furosemide on DPOAEs and ABRs in a non-human primate,” Ph.D. thesis, Vanderbilt University, Nashville, TN. [Google Scholar]
- 7. Bailey, J. (2005). “ Non-human primates in medical research and drug development: A critical review,” Biogenic Amines 19(4), 235–255. 10.1163/156939105774647385 [DOI] [Google Scholar]
- 8. Barden, E. K. , Rellinger, E. A. , Ortmann, A. J. , and Ohlemiller, K. K. (2012). “ Inheritance patterns of noise vulnerability and ‘protectability’ in (C57BL/6J × CBA/J) F1 hybrid mice,” J. Am. Acad. Audiol. 23(5), 332–340. 10.3766/jaaa.23.5.4 [DOI] [PubMed] [Google Scholar]
- 9. Beecher, M. D. (1974a). “ Hearing in the owl monkey (Aotus trivirgatus): I. Auditory sensitivity,” J. Compar. Physiol. Psychol. 86(5), 898–901. 10.1037/h0036416 [DOI] [PubMed] [Google Scholar]
- 10. Beecher, M. D. (1974b). “ Pure-tone thresholds of the squirrel monkey (Saimiri sciureus),” J. Acoust. Soc. Am. 55(1), 196–198. 10.1121/1.1928152 [DOI] [PubMed] [Google Scholar]
- 11. Bennett, C. L. , Davis, R. T. , and Miller, J. M. (1983). “ Demonstration of presbycusis across repeated measures in a nonhuman primate species,” Behav. Neurosci. 97(4), 602–607. 10.1037/0735-7044.97.4.602 [DOI] [PubMed] [Google Scholar]
- 12. Berger, M. , Calapai, A. , Stephan, V. , Niessing, M. , Burchardt, L. , Gail, A. , and Treue, S. (2018). “ Standardized automated training of rhesus monkeys for neuroscience research in their housing environment,” J. Neurophysiol. 119(3), 796–807. 10.1152/jn.00614.2017 [DOI] [PubMed] [Google Scholar]
- 13. Bernard, A. , Lubbers, L. S. , Tanis, K. Q. , Luo, R. , Podtelezhnikov, A. A. , Finney, E. M. , McWhorter, M. M. , Serikawa, K. , Lemon, T. , Morgan, R. , and Copeland, C. (2012). “ Transcriptional architecture of the primate neocortex,” Neuron 73(6), 1083–1099. 10.1016/j.neuron.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bharadwaj, H. M. , Mai, A. R. , Simpson, J. M. , Choi, I. , Heinz, M. G. , and Shinn-Cunningham, B. G. (2019). “ Non-invasive assays of cochlear synaptopathy—Candidates and considerations,” Neuroscience 407, 53. 10.1016/j.neuroscience.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohlen, P. , Dylla, M. , Timms, C. , and Ramachandran, R. (2014). “ Detection of modulated tones in modulated noise by non-human primates,” J. Assoc. Res. Otolaryngol. 15(5), 801–821. 10.1007/s10162-014-0467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowl, M. R. , and Brown, S. D. M. (2018). “ Genetic landscape of auditory dysfunction,” Human Mol. Genetics 27(R2), R130–R135. 10.1093/hmg/ddy158 [DOI] [PubMed] [Google Scholar]
- 17. Breschi, A. , Gingeras, T. R. , and Guigó, R. (2017). “ Comparative transcriptomics in human and mouse,” Nat. Rev. Genetics 18(7), 425–440. 10.1038/nrg.2017.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown, C. , Beecher, M. , Moody, D. , and Stebbins, W. (1978). “ Localization of primate calls by old world monkeys,” Science 201(4357), 753–754. 10.1126/science.97785 [DOI] [PubMed] [Google Scholar]
- 19. Brown, C. H. , Beecher, M. D. , Moody, D. B. , and Stebbins, W. C. (1978). “ Localization of pure tones by Old World monkeys,” J. Acoust. Soc. Am. 63(5), 1484–1492. 10.1121/1.381842 [DOI] [Google Scholar]
- 20. Brown, C. H. , Beecher, M. D. , Moody, D. B. , and Stebbins, W. C. (1980). “ Localization of noise bands by Old World monkeys,” J. Acoust. Soc. Am. 68(1), 127–132. 10.1121/1.384638 [DOI] [PubMed] [Google Scholar]
- 21. Burns, J. C. , Kelly, M. C. , Hoa, M. , Morell, R. J. , and Kelley, M. W. (2015). “ Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear,” Nat. Commun. 6, 8557. 10.1038/ncomms9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burton, J. A. , Dylla, M. E. , and Ramachandran, R. (2018). “ Frequency selectivity in macaque monkeys measured using a notched-noise method,” Hear. Res. 357, 73–80. 10.1016/j.heares.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai, T. , Jen, H.-I. , Kang, H. , Klisch, T. J. , Zoghbi, H. Y. , and Groves, A. K. (2015). “ Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor,” J. Neurosci. 35(14), 5870–5883. 10.1523/JNEUROSCI.5083-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calapai, A. , Berger, M. , Niessing, M. , Heisig, K. , Brockhausen, R. , Treue, S. , and Gail, A. (2017). “ A cage-based training, cognitive testing and enrichment system optimized for rhesus macaques in neuroscience research,” Behav. Res. Methods 49(1), 35–45. 10.3758/s13428-016-0707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen, W. , Xia, X. , Song, N. , Wang, Y. , Zhu, H. , Deng, W. , Kong, Q. , Pan, X. , and Qin, C. (2016). “ Cross-species analysis of gene expression and function in prefrontal cortex, hippocampus and striatum,” PLoS One 11(10), e0164295. 10.1371/journal.pone.0164295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen, H. , Xing, Y. , Xia, L. , Chen, Z. , Yin, S. , and Wang, J. (2018). “ AAV-mediated NT-3 overexpression protects cochleae against noise-induced synaptopathy,” Gene Therapy 25(4), 251–259. 10.1038/s41434-018-0012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chien, W. W. , Monzack, E. L. , McDougald, D. S. , and Cunningham, L. L. (2015). “ Gene therapy for sensorineural hearing loss,” Ear Hear. 36(1), 1–7. 10.1097/AUD.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 28. Christison-Lagay, K. L. , and Cohen, Y. E. (2014). “ Behavioral correlates of auditory streaming in rhesus macaques,” Hear. Res. 309, 17–25. 10.1016/j.heares.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clark, W. W. , and Bohne, B. A. (1978). “ Animal model for the 4-kHz tonal dip,” Ann. Otol. Rhinol. Laryngol. 87, 1–16. 10.1177/00034894780870S401 [DOI] [PubMed] [Google Scholar]
- 30. Cody, A. R. , and Robertson, D. (1983). “ Variability of noise-induced damage in the guinea pig cochlea: Electrophysiological and morphological correlates after strictly controlled exposures,” Hear. Res. 9, 55–70. 10.1016/0378-5955(83)90134-X [DOI] [PubMed] [Google Scholar]
- 31. Coleman, M. N. (2009). “ What do primates hear? A meta-analysis of all known nonhuman primate behavioral audiograms,” Int. J. Primatol. 30, 55–91. 10.1007/s10764-008-9330-1 [DOI] [Google Scholar]
- 32. Coleman, M. N. , and Colbert, M. W. (2010). “ Correlations between auditory structures and hearing sensitivity in non-human primates,” J. Morphol. 271, 511–532. 10.1002/jmor.10814 [DOI] [PubMed] [Google Scholar]
- 33. Dai, C. , Fridman, G. Y. , and Della Santina, C. C. (2011). “ Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys,” Hear. Res. 277(1–2), 204–210. 10.1016/j.heares.2010.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dai, C. , Lehar, M. , Sun, D. Q. , Rvt, L. S. , Carey, J. P. , MacLachlan, T. , Brough, D. , Staecker, H. , Della Santina, A. M. , Hullar, T. E. , and Della Santina, C. C. (2017). “ Rhesus cochlear and vestibular functions are preserved after inner ear injection of saline volume sufficient for gene therapy delivery,” J. Assoc. Res. Otolaryngol. 18(4), 601–617. 10.1007/s10162-017-0628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis, H. (1957). “ Biophysics and physiology of the inner ear,” Physiol. Rev. 37(1), 1–49. 10.1152/physrev.1957.37.1.1 [DOI] [PubMed] [Google Scholar]
- 36. Davis, H. , Morgan, C. T. , Hawkins, J. E. , Galambos, R. , and Smith, F. W. (1950). “ Temporary deafness following exposure to loud tones and noise,” Acta Oto-Laryngol. 88, 19–21. [PubMed] [Google Scholar]
- 37. Dobie, R. A. , and Humes, L. E. (2017). “ Commentary on the regulatory implications of noise-induced cochlear neuropathy,” Int. J. Audiol. 56, 74–78. 10.1080/14992027.2016.1255359 [DOI] [PubMed] [Google Scholar]
- 38. Dou, H. , Finberg, K. , Cardell, E. L. , Lifton, R. , and Choo, D. (2003). “ Mice lacking the B1 subunit of H+-ATPase have normal hearing,” Hear. Res. 180(1–2), 76–84. 10.1016/S0378-5955(03)00108-4 [DOI] [PubMed] [Google Scholar]
- 39. Downer, J. D. , Rapone, B. , Verhein, J. , O'Connor, K. N. , and Sutter, M. L. (2017). “ Feature-selective attention adaptively shifts noise correlations in primary auditory cortex,” J. Neurosci. 37(21), 5378–5392. 10.1523/JNEUROSCI.3169-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drescher, D. G. , and Eldredge, D. H. (1974). “ Species differences in cochlear fatigue related to acoustics of outer and middle ears of guinea pig and chinchilla,” J. Acoust. Soc. Am. 56(3), 929–934. 10.1121/1.1903350 [DOI] [PubMed] [Google Scholar]
- 41. Du, X. , Cai, Q. , West, M. B. , Youm, I. , Huang, X. , Li, W. , Cheng, W. , Nakmali, D. , Ewert, D. L. , and Kopke, R. D. (2018). “ Regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs,” Mol. Therapy 26(5), 1313–1326. 10.1016/j.ymthe.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dylla, M. , Hrnicek, A. , Rice, C. , and Ramachandran, R. (2013). “ Detection of tones and their modification by noise in nonhuman primates,” J. Assoc. Res. Otolaryngol. 14(4), 547–560. 10.1007/s10162-013-0384-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ekdale, E. G. (2013). “ Comparative anatomy of the bony labyrinth (inner ear) of placental mammals,” PLoS One 8(6), e66624. 10.1371/journal.pone.0066624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El Kechai, N. , Agnely, F. , Mamelle, E. , Nguyen, Y. , Ferrary, E. , and Bochot, A. (2015). “ Recent advances in local drug delivery to the inner ear,” Int. J. Pharm. 494(1), 83–101. 10.1016/j.ijpharm.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 45. Espeland, E. M. , Tsai, C.-W. , Larsen, J. , and Disbrow, G. L. (2018). “ Safeguarding against ebola: Vaccines and therapeutics to be stockpiled for future outbreaks,” PLoS Negl. Tropical Dis. 12(4), e0006275. 10.1371/journal.pntd.0006275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Felix, H. (2002). “ Anatomical differences in the peripheral auditory system of mammals and man,” Adv. Otorhinolaryngol. 59, 1–10. [DOI] [PubMed] [Google Scholar]
- 47. Fernandez, C. (1951). “ The innervation of the cochlea (guinea pig),” Laryngoscope 61(12), 1152–1172. [DOI] [PubMed] [Google Scholar]
- 48. Fernandez, K. A. , Jeffers, P. W. C. , Lall, K. , Liberman, M. C. , and Kujawa, S. G. (2015). “ Aging after noise exposure: Acceleration of cochlear synaptopathy in ‘recovered’ ears,” J. Neurosci. 35(19), 7509–7520. 10.1523/JNEUROSCI.5138-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fowler, C. G. , Chiasson, K. B. , Leslie, T. H. , Thomas, D. , Beasley, T. M. , Kemnitz, J. W. , and Weindruch, R. (2010). “ Auditory function in rhesus monkeys: Effects of aging and caloric restriction in the Wisconsin monkeys five years later,” Hear. Res. 261(1–2), 75–81. 10.1016/j.heares.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fowler, C. G. , Torre, P. , and Kemnitz, J. W. (2002). “ Effects of caloric restriction and aging on the auditory function of rhesus monkeys (Macaca mulatta): The University of Wisconsin study,” Hear. Res. 169, 24–35. 10.1016/S0378-5955(02)00335-0 [DOI] [PubMed] [Google Scholar]
- 51. Francis, S. P. , McKenna, M. J. , Ng, R. , Gao, Y. , Qu, E. , Vandenberghe, L. H. , Sewell, W. J. , Simons, E. J. , and Valero, M. D. (2019). “ The adeno-associated viral Anc80 vector efficiently transduces hair cells in cynomolgus macaques (M. Fascicularis): Development of a non-human primate (NHP) model for cochlear gene therapy,” Mol. Therapy 27, 116–117. [Google Scholar]
- 52. Frenzilli, G. , Ryskalin, L. , Ferrucci, M. , Cantafora, E. , Chelazzi, S. , Giorgi, F. S. , Lenzi, P. , Scarcelli, V. , Frati, A. , Biagioni, F. , Gambardella, S. , Falleni, A. , and Fornai, F. (2017). “ Loud noise exposure produces DNA, neurotransmitter and morphological damage within specific brain areas,” Front. Neuroanat. 11, 49. 10.3389/fnana.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fukui, H. , and Raphael, Y. (2013). “ Gene therapy for the inner ear,” Hear. Res. 297, 99–105. 10.1016/j.heares.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Furman, A. C. , Kujawa, S. G. , and Liberman, M. C. (2013). “ Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates,” J. Neurophysiol. 110(3), 577–586. 10.1152/jn.00164.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao, G.-P. , Alvira, M. R. , Wang, L. , Calcedo, R. , Johnston, J. , and Wilson, J. M. (2002). “ Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy,” Proc. Natl. Acad. Sci. 99(18), 11854–11859. 10.1073/pnas.182412299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gao, X. , Tao, Y. , Lamas, V. , Huang, M. , Yeh, W.-H. , Pan, B. , Hu, Y.-J. , Hu, J. H. , Thompson, D. B. , Shu, Y. , Li, Y. , Wang, H. , Yang, S. , Xu, Q. , Polley, D. B. , Charles Liberman, M. , Kong, W.-J. , Holt, J. R. , Chen, Z.-Y. , and Liu, D. R. (2018). “ Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents,” Nature 553(7687), 217–221. 10.1038/nature25164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gao, G. , Vandenberghe, L. H. , Alvira, M. R. , Lu, Y. , Calcedo, R. , Zhou, X. , and Wilson, J. M. (2004). “ Clades of adeno-associated viruses are widely disseminated in human tissues,” J. Virol. 78(12), 6381–6388. 10.1128/JVI.78.12.6381-6388.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garner, J. P. (2014). “ The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it?,” ILAR J. 55(3), 438–456. 10.1093/ilar/ilu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gates, H. W. , Romba, J. J. , and Martin, P. (1963). “ Response latencies in the rhesus monkey as a function of tone intensity,” U.S. Army Human Engineering Laboratories, Technical Memorandum No. 3-63, U.S. Army Research Laboratory, Adelphi, MD, pp. 1–15.
- 60. Géléoc, G. S. G. , and Holt, J. R. (2014). “ Sound strategies for hearing restoration,” Science 344(6184), 1241062. 10.1126/science.1241062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Glueckert, R. , Johnson Chacko, L. , Rask-Andersen, H. , Liu, W. , Handschuh, S. , and Schrott-Fischer, A. (2018). “ Anatomical basis of drug delivery to the inner ear,” Hear. Res. 368, 10–27. 10.1016/j.heares.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 62. Gopen, Q. , Rosowski, J. J. , and Merchant, S. N. (1997). “ Anatomy of the normal human cochlear aqueduct with functional implications,” Hear. Res. 107(1–2), 9–22. 10.1016/S0378-5955(97)00017-8 [DOI] [PubMed] [Google Scholar]
- 63. Gourevitch, G. (1970). “ Detectability of tones in quiet and in noise by rats and monkeys,” in Animal Psychoacoustics: The Design and Conduct of Sensory Experiments, edited by Stebbins W. C. ( Appleton-Century-Crofts, New York: ), pp. 67–97. [Google Scholar]
- 64. Grinn, S. K. , Wiseman, K. B. , Baker, J. A. , and Le Prell, C. G. (2017). “ Hidden hearing loss? No effect of common recreational noise exposure on cochlear nerve response amplitude in humans,” Front. Neurosci. 11, 465. 10.3389/fnins.2017.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guo, Y. , Zhang, P. , Sheng, Q. , Zhao, S. , and Hackett, T. A. (2016). “ lncRNA expression in the auditory forebrain during postnatal development,” Gene 593(1), 201–216. 10.1016/j.gene.2016.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hackett, T. A. , Guo, Y. , Clause, A. , Hackett, N. J. , Garbett, K. , Zhang, P. , Polley, D. B. , and Mirnics, K. (2015). “ Transcriptional maturation of the mouse auditory forebrain,” BMC Genomics 16(1), 606. 10.1186/s12864-015-1709-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harada, T. , and Tokuriki, M. (1997). “ Brain-stem auditory evoked potentials in the common marmoset (Callithrix jacchus),” Electroencephalogr. Clin. Neurophysiol. 104(1), 43–50. 10.1016/S0168-5597(96)96015-3 [DOI] [PubMed] [Google Scholar]
- 68. Harris, J. D. (1967). “ Relations among aftereffects of acoustic stimulation,” J. Acoust. Soc. Am. 42(6), 1306–1324. 10.1121/1.1910720 [DOI] [PubMed] [Google Scholar]
- 69. Hauser, S. N. , Burton, J. A. , Mercer, E. T. , and Ramachandran, R. (2018). “ Effects of noise overexposure on tone detection in noise in nonhuman primates,” Hear. Res. 357, 33–45. 10.1016/j.heares.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hawkins, J. E. , Johnsson, L.-G. , Stebbins, W. C. , Moody, D. B. , and Coombs, S. L. (1976). “ Hearing loss and cochlear pathology in monkeys after noise exposure,” Acta Otolaryngol. 81(3–6), 337–343. 10.3109/00016487609119971 [DOI] [PubMed] [Google Scholar]
- 71. Hawkins, J. E. , and Stevens, S. S. (1950). “ The masking of pure tones and of speech by white noise,” J. Acoust. Soc. Am. 22(1), 6–13. 10.1121/1.1906581 [DOI] [Google Scholar]
- 72. Heffner, R. S. (2004). “ Primate hearing from a mammalian perspective,” Anat. Rec. 281(1), 1111–1122. 10.1002/ar.a.20117 [DOI] [PubMed] [Google Scholar]
- 73. Heffner, H. , and Heffner, R. (1984). “ Temporal lobe lesions and perception of species-specific vocalizations by macaques,” Science 226(4670), 75–76. 10.1126/science.6474192 [DOI] [PubMed] [Google Scholar]
- 74. Heffner, H. E. , and Heffner, R. S. (1990). “ Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques,” J. Neurophysiol. 64(3), 915–931. 10.1152/jn.1990.64.3.915 [DOI] [PubMed] [Google Scholar]
- 75. Heffner, H. E. , and Heffner, R. S. (2007). “ Hearing ranges of laboratory animals,” J. Am. Assoc. Lab. Animal Sci. 46(1), 20–22. [PubMed] [Google Scholar]
- 76. Heffner, H. , and Masterton, B. (1975). “ Contribution of auditory cortex to sound localization in the monkey (Macaca mulatta),” J. Neurophysiol. 38(6), 1340–1358. 10.1152/jn.1975.38.6.1340 [DOI] [PubMed] [Google Scholar]
- 77. Henderson, D. , Subramaniam, M. , and Boettcher, F. (1993). “ Individual susceptibility to noise-induced hearing loss,” Ear Hear. 14(3), 152–168. 10.1097/00003446-199306000-00002 [DOI] [PubMed] [Google Scholar]
- 78. Heppner, D. G. , Kemp, T. L. , Martin, B. K. , Ramsey, W. J. , Nichols, R. , Dasen, E. J. , Link, C. J. , Das, R. , Xu, C. J. , Sheldon, E. A. , Nowak, T. A. , Monath, T. P. , and Adams, M. (2017). “ Safety and immunogenicity of the rVSVΔG-ZEBOV-GP ebola virus vaccine candidate in healthy adults: A phase 1B randomised, multicentre, double-blind, placebo-controlled, dose-response study,” Lancet Infect. Dis. 17(8), 854–866. 10.1016/S1473-3099(17)30313-4 [DOI] [PubMed] [Google Scholar]
- 79. Hickox, A. E. , Larsen, E. , Heinz, M. G. , Shinobu, L. , and Whitton, J. P. (2017). “ Translational issues in cochlear synaptopathy,” Hear. Res. 349, 164–171. 10.1016/j.heares.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hienz, R. D. , Turkkan, J. S. , and Harris, A. H. (1982). “ Pure tone thresholds in the yellow baboon (Papio cynocephalus),” Hear. Res. 8(1), 71–75. 10.1016/0378-5955(82)90035-1 [DOI] [PubMed] [Google Scholar]
- 81. Hocherman, S. , Benson, D. A. , Goldstein, M. H. , Heffner, H. E. , and Hienz, R. D. (1976). “ Evoked unit activity in auditory cortex of monkeys performing a selective attention task,” Brain Res. 117(1), 51–68. 10.1016/0006-8993(76)90555-2 [DOI] [PubMed] [Google Scholar]
- 82. Hoffmann, T. J. , Keats, B. J. , Yoshikawa, N. , Schaefer, C. , Risch, N. , and Lustig, L. R. (2016). “ A large genome-wide association study of age-related hearing impairment using electronic health records,” PLoS Gen. 12(10), e1006371. 10.1371/journal.pgen.1006371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Holt, J. R. , and Vandenberghe, L. H. (2012). “ Gene therapy for deaf mice goes viral,” Mol. Therapy 20(10), 1836–1837. 10.1038/mt.2012.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hopp, S. L. , Sinnott, J. M. , Owren, M. J. , and Petersen, M. R. (1992). “ Differential sensitivity of Japanese macaques (macaca fuscata) and humans (homo sapiens) to peak position along a synthetic coo call continuum,” J. Compar. Psychol. 106(2), 128–136. 10.1037/0735-7036.106.2.128 [DOI] [PubMed] [Google Scholar]
- 85. Hosoya, M. , Fujioka, M. , Kobayashi, R. , Okano, H. , and Ogawa, K. (2016a). “ Overlapping expression of anion exchangers in the cochlea of a non-human primate suggests functional compensation,” Neurosci. Res. 110, 1–10. 10.1016/j.neures.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 86. Hosoya, M. , Fujioka, M. , Ogawa, K. , and Okano, H. (2016b). “ Distinct expression patterns of causative genes responsible for hereditary progressive hearing loss in non-human primate cochlea,” Sci. Rep. 6(1), 22250. 10.1038/srep22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hunter-Duvar, I. M. , and Bredberg, G. (1974). “ Effects of intense auditory stimulation: Hearing losses and inner ear changes in the chinchilla,” J. Acoust. Soc. Am. 55(4), 795–801. 10.1121/1.1914602 [DOI] [PubMed] [Google Scholar]
- 88. Hunter-Duvar, I. M. , and Elliott, D. N. (1972). “ Effects of intense auditory stimulation: Hearing losses and inner ear changes in the squirrel monkey,” J. Acoust. Soc. Am. 52(4B), 1181–1192. 10.1121/1.1913230 [DOI] [PubMed] [Google Scholar]
- 89. Hunter-Duvar, I. M. , and Elliott, D. N. (1973). “ Effects of intense auditory stimulation: Hearing losses and inner ear changes in the squirrel monkey. II,” J. Acoust. Soc. Am. 54(5), 1179–1183. 10.1121/1.1914364 [DOI] [PubMed] [Google Scholar]
- 90. Igarashi, M. , Mauldin, L. , and Jerger, J. (1979). “ Impedance Audiometry in the Squirrel monkey: Effect of Transection of Crossed Olivocochlear Bundle,” Arch. Otolaryngol. 105(5), 258–259. 10.1001/archotol.1979.00790170028007 [DOI] [PubMed] [Google Scholar]
- 91. Isgrig, K. , and Chien, W. W. (2019). “ Surgical methods for inner ear gene delivery in neonatal mouse,” in Viral Vectors for Gene Therapy: Methods and Protocols, edited by Manfredsson F. P. and Benskey M. J. ( Springer, New York: ), pp. 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jerger, J. , Mauldin, L. , and Igarashi, M. (1978a). “ Impedance audiometry in the squirrel monkey: Effect of middle ear surgery,” Arch. Otolaryngol. Head Neck Surg. 104(4), 214–224. 10.1001/archotol.1978.00790040036008 [DOI] [PubMed] [Google Scholar]
- 93. Jerger, J. , Mauldin, L. , and Igarashi, M. (1978b). “ Impedance audiometry in the squirrel monkey: Sensorineural losses,” Arch. Otolaryngol. Head Neck Surg. 104(10), 559–563. 10.1001/archotol.1978.00790100013003 [DOI] [PubMed] [Google Scholar]
- 94. Jordan, V. M. , Pinheiro, M. L. , Chiba, K. , and Jimenez, A. (1973). “ Cochlear pathology in monkeys exposed to impulse noise,” Acta Otolaryngol. 76, 16–30. 10.3109/00016487309125497 [DOI] [PubMed] [Google Scholar]
- 95. Joris, P. X. , Bergevin, C. , Kalluri, R. , Laughlin, M. M. , Michelet, P. , van der Heijden, M. , and Shera, C. A. (2011). “ Frequency selectivity in Old-World monkeys corroborates sharp cochlear tuning in humans,” Proc. Natl. Acad. Sci. U.S.A. 108(42), 17516–17520. 10.1073/pnas.1105867108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Katsuki, Y. , Suga, N. , and Kanno, Y. (1962). “ Neural mechanism of the peripheral and central auditory system in monkeys,” J. Acoust. Soc. Am. 34(9B), 1396–1410. 10.1121/1.1918357 [DOI] [Google Scholar]
- 97. Kim, M.-A. , Ryu, N. , Kim, H.-M. , Kim, Y.-R. , Lee, B. , Kwon, T.-J. , Bok, J. , and Kim, U.-K. (2019). “ Targeted gene delivery into the mammalian inner ear using synthetic serotypes of adeno-associated virus vectors,” Mol. Therapy 13, 197–204. 10.1016/j.omtm.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kimura, R. S. (1975). “ The ultrastructure of the organ of Corti,” Int. Rev. Cytol. 42, 173–222. 10.1016/S0074-7696(08)60981-X [DOI] [PubMed] [Google Scholar]
- 99. Kirk, E. C. , and Gosselin-Ildari, A. D. (2009). “ Cochlear labyrinth volume and hearing abilities in primates,” Anat. Rec. 292(6), spc1–spc1. 10.1002/ar.20923 [DOI] [PubMed] [Google Scholar]
- 100. Klein, A. J. , and Mills, J. H. (1981). “ Physiological and psychophysical measures from humans with temporary threshold shift,” J. Acoust. Soc. Am. 70(4), 1045–1053. 10.1121/1.386955 [DOI] [PubMed] [Google Scholar]
- 101. Knipper, M. , Van Dijk, P. , Nunes, I. , Rüttiger, L. , and Zimmermann, U. (2013). “ Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis,” Prog. Neurobiol. 111, 17–33. 10.1016/j.pneurobio.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 102. Kojima, S. (1990). “ Comparison of auditory functions in the chimpanzee and human,” Folia Primatol. 55(2), 62–72. 10.1159/000156501 [DOI] [PubMed] [Google Scholar]
- 103. Konopka, G. , and Geschwind, D. H. (2010). “ Human brain evolution: Harnessing the genomics (r)evolution to link genes, cognition, and behavior,” Neuron 68(2), 231–244. 10.1016/j.neuron.2010.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Köppl, C. , Wilms, V. , Russell, I. J. , and Nothwang, H. G. (2018). “ Evolution of endolymph secretion and endolymphatic potential generation in the vertebrate inner ear,” Brain Behav. Evol. 92(1–2), 1–31. 10.1159/000494050 [DOI] [PubMed] [Google Scholar]
- 105. Kraus, N. , Smith, D. I. , Reed, N. L. , Willott, J. , and Erwin, J. (1985). “ Auditory brainstem and middle latency responses in non-human primates,” Hear. Res. 17(3), 219–226. 10.1016/0378-5955(85)90066-8 [DOI] [PubMed] [Google Scholar]
- 106. Kujawa, S. G. , and Liberman, M. C. (2009). “ Adding insult to injury: Cochlear nerve degeneration after ‘temporary’ noise-induced hearing loss,” J. Neurosci. 29(45), 14077–14085. 10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kumar, S. , and Hedges, S. B. (1998). “ A molecular timescale for vertebrate evolution,” Nature 392(6679), 917–920. 10.1038/31927 [DOI] [PubMed] [Google Scholar]
- 108. Lakatos, P. , Musacchia, G. , O'Connell, M. N. , Falchier, A. Y. , Javitt, D. C. , and Schroeder, C. E. (2013). “ The spectrotemporal filter mechanism of auditory selective attention,” Neuron 77(4), 750–761. 10.1016/j.neuron.2012.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lasky, R. E. , Beach, K. E. , and Laughlin, N. K. (2000). “ Immittance and otoacoustic emissions in rhesus monkeys and humans,” Int. J. Audiol. 39(2), 61–69. 10.3109/00206090009073055 [DOI] [PubMed] [Google Scholar]
- 110. Lasky, R. E. , Luck, M. L. , Torre, P. , and Laughlin, N. (2001). “ The effects of early lead exposure on auditory function in rhesus monkeys,” Neurotoxicol. Teratol. 23(6), 639–649. 10.1016/S0892-0362(01)00175-1 [DOI] [PubMed] [Google Scholar]
- 111. Lasky, R. E. , Maier, M. M. , Snodgrass, E. B. , Laughlin, N. K. , and Hecox, K. E. (1995). “ Auditory evoked brainstem and middle latency responses in Macaca mulatta and humans,” Hear. Res. 89(1–2), 212–225. 10.1016/0378-5955(95)00140-7 [DOI] [PubMed] [Google Scholar]
- 112. Lasky, R. E. , Snodgrass, E. B. , Laughlin, N. K. , and Hecox, K. E. (1995). “ Distortion product otoacoustic emissions in Macaca mulatta and humans,” Hear. Res. 89(1–2), 35–51. 10.1016/0378-5955(95)00120-1 [DOI] [PubMed] [Google Scholar]
- 113. Lasky, R. E. , Soto, A. A. , Luck, M. L. , and Laughlin, N. K. (1999). “ Otoacoustic emission, evoked potential, and behavioral auditory thresholds in the rhesus monkey (Macaca mulatta),” Hear. Res. 136(1–2), 35–43. 10.1016/S0378-5955(99)00100-8 [DOI] [PubMed] [Google Scholar]
- 114. Lavinsky, J. , Ge, M. , Crow, A. L. , Pan, C. , Wang, J. , Salehi, P. , Myint, A. , Eskin, E. , Allayee, H. , Lusis, A. J. , and Friedman, R. A. (2016). “ The genetic architecture of noise-induced hearing loss: Evidence for a gene-by-environment interaction,” G3(Bethesda) 6(10), 3219–3228. 10.1534/g3.116.032516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Le Prell, C. G. , Dell, S. , Hensley, B. , Hall, J. W. , Campbell, K. C. M. , Antonelli, P. J. , Green, G. E. , Miller, J. M. , and Guire, K. (2012). “ Digital music exposure reliably induces temporary threshold shift in normal-hearing human subjects,” Ear Hear. 33(6), 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Le Prell, C. G. , and Moody, D. B. (1997). “ Perceptual salience of acoustic features of Japanese monkey coo calls,” J. Compar. Psychol. 111(3), 261–274. 10.1037/0735-7036.111.3.261 [DOI] [PubMed] [Google Scholar]
- 117. Le Prell, C. G. , Niemiec, A. J. , and Moody, D. B. (2001). “ Macaque thresholds for detecting increases in intensity: Effects of formant structure,” Hear. Res. 162, 29–42. 10.1016/S0378-5955(01)00357-4 [DOI] [PubMed] [Google Scholar]
- 118. Lemus, L. , Hernández, A. , and Romo, R. (2009). “ Neural codes for perceptual discrimination of acoustic flutter in the primate auditory cortex,” Proc. Natl. Acad. Sci. U.S.A. 106(23), 9471–9476. 10.1073/pnas.0904066106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liberman, M. C. (1978). “ Auditory-nerve response from cats raised in a low-noise chamber,” J. Acoust. Soc. Am. 63(2), 442–455. 10.1121/1.381736 [DOI] [PubMed] [Google Scholar]
- 120. Liberman, M. (1982). “ Single-neuron labeling in the cat auditory nerve,” Science 216(4551), 1239–1241. 10.1126/science.7079757 [DOI] [PubMed] [Google Scholar]
- 121. Liberman, M. C. , and Dodds, L. W. (1984). “ Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves,” Hear. Res. 16(1), 55–74. 10.1016/0378-5955(84)90025-X [DOI] [PubMed] [Google Scholar]
- 122. Liberman, M. C. , Epstein, M. J. , Cleveland, S. S. , Wang, H. , and Maison, S. F. (2016). “ Toward a differential diagnosis of hidden hearing loss in humans,” PLoS ONE 11(9), e0162726. 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lichtenhan, J. T. , Hartsock, J. , Dornhoffer, J. R. , Donovan, K. M. , and Salt, A. N. (2016). “ Drug delivery into the cochlear apex: Improved control to sequentially affect finely spaced regions along the entire length of the cochlear spiral,” J. Neurosci. Methods 273, 201–209. 10.1016/j.jneumeth.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lonsbury-Martin, B. L. , and Martin, G. K. (1981). “ Effects of moderately intense sound on auditory sensitivity in rhesus monkeys: Behavioral and neural observations,” J. Neurophysiol. 46(3), 563–586. 10.1152/jn.1981.46.3.563 [DOI] [PubMed] [Google Scholar]
- 125. Lonsbury-Martin, B. L. , and Martin, G. K. (1988). “ Incidence of spontaneous otoacoustic emissions in macaque monkeys: A replication,” Hear. Res. 34(3), 313–317. 10.1016/0378-5955(88)90011-1 [DOI] [PubMed] [Google Scholar]
- 126. Lonsbury-Martin, B. L. , Martin, G. K. , and Bohne, B. A. (1987). “ Repeated TTS exposures in monkeys: Alterations in hearing, cochlear structure, and single-unit thresholds,” J. Acoust. Soc. Am. 81(5), 1507–1518. 10.1121/1.394503 [DOI] [PubMed] [Google Scholar]
- 127. Lonsbury-Martin, B. L. , Martin, G. K. , Probst, R. , and Coats, A. C. (1988). “ Spontaneous otoacoustic emissions in a nonhuman primate. II. Cochlear anatomy,” Hear. Res. 33(1), 69–93. 10.1016/0378-5955(88)90021-4 [DOI] [PubMed] [Google Scholar]
- 128. Luz, G. A. , and Hodge, D. C. (1971). “ Recovery from impulse-noise induced TTS in monkeys and men: A descriptive model,” J. Acoust. Soc. Am. 49(6B), 1770–1777. 10.1121/1.1912580 [DOI] [PubMed] [Google Scholar]
- 129. Luz, G. A. , and Lipscomb, D. M. (1973). “ Susceptibility to damage from impulse noise: Chinchilla versus man or monkey,” J. Acoust. Soc. Am. 54(6), 1750–1754. 10.1121/1.1914475 [DOI] [PubMed] [Google Scholar]
- 130. Luz, G. A. , Mosko, J. D. , Fletcher, J. L. , and Fravel, W. J. (1973). “ The relation between temporary threshold shift and permanent threshold shift in rhesus monkeys exposed to impulse noise,” Acta Otolaryngol. 76, 5–15. 10.3109/00016487309125496 [DOI] [PubMed] [Google Scholar]
- 131. Maison, S. F. , and Liberman, M. C. (2000). “ Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength,” J. Neurosci. 20(12), 4701–4707. 10.1523/JNEUROSCI.20-12-04701.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Maison, S. F. , and Rauch, S. D. (2017). “ Ethical considerations in noise-induced hearing loss research,” Lancet 390(10098), 920–922. 10.1016/S0140-6736(17)31875-5 [DOI] [PubMed] [Google Scholar]
- 133. Makishima, T. , Rodriguez, C. I. , Robertson, N. G. , Morton, C. C. , Stewart, C. L. , and Griffith, A. J. (2005). “ Targeted disruption of mouse Coch provides functional evidence that DFNA9 hearing loss is not a COCH haploinsufficiency disorder,” Hum. Genet. 118(1), 29–34. 10.1007/s00439-005-0001-4 [DOI] [PubMed] [Google Scholar]
- 134. Mangham, C. A. , and Miller, J. M. (1976). “ Effects of an experimental acoustic neurinoma on stapedius reflex activity,” J. Acoust. Soc. Am. 60(S1), S104–S105. 10.1121/1.2003060 [DOI] [Google Scholar]
- 135. Manohar, S. , Jamesdaniel, S. , Ding, D. , Salvi, R. , Seigel, G. M. , and Roth, J. A. (2016). “ Quantitative PCR analysis and protein distribution of drug transporter genes in the rat cochlea,” Hear. Res. 332, 46–54. 10.1016/j.heares.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 136. Manohar, S. , Ramchander, P. V. , Salvi, R. , and Seigel, G. M. (2019). “ Synaptic reorganization response in the cochlear nucleus following intense noise exposure,” Neuroscience 399, 184–198. 10.1016/j.neuroscience.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Marques-Bonet, T. , Ryder, O. A. , and Eichler, E. E. (2009). “ Sequencing primate genomes: What have we learned?,” Ann. Rev. Genom. Human Genet. 10(1), 355–386. 10.1146/annurev.genom.9.081307.164420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Martin, G. K. , Lonsbury-Martin, B. L. , Probst, R. , and Coats, A. C. (1985). “ Spontaneous otoacoustic emissions in the nonhuman primate: A survey,” Hear. Res. 20(1), 91–95. 10.1016/0378-5955(85)90062-0 [DOI] [PubMed] [Google Scholar]
- 139. Martin, G. K. , Lonsbury-Martin, B. L. , Probst, R. , and Coats, A. C. (1988). “ Spontaneous otoacoustic emissions in a nonhuman primate. I. Basic features and relations to other emissions,” Hear. Res. 33(1), 49–68. 10.1016/0378-5955(88)90020-2 [DOI] [PubMed] [Google Scholar]
- 140. Martin, P. , Romba, J. J. , and Gates, H. W. (1962). “ A method for the study of hearing loss and recovery in rhesus monkeys,” U.S. Army Human Engineering Laboratories, Technical Memorandum 11-62, U.S. Army Research Laboratory, Adelphi, MD, pp. 1–31.
- 141. Mashiko, H. , Yoshida, A. C. , Kikuchi, S. S. , Niimi, K. , Takahashi, E. , Aruga, J. , Okano, H. , and Shimogori, T. (2012). “ Comparative anatomy of marmoset and mouse cortex from genomic expression,” J. Neurosci. 32(15), 5039–5053. 10.1523/JNEUROSCI.4788-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Matsuzaki, S. , Hosoya, M. , Okano, H. , Fujioka, M. , and Ogawa, K. (2018). “ Expression pattern of EYA4 in the common marmoset (Callithrix jacchus) cochlea,” Neurosci. Lett. 662, 185–188. 10.1016/j.neulet.2017.10.030 [DOI] [PubMed] [Google Scholar]
- 143. May, B. , Moody, D. B. , and Stebbins, W. C. (1989). “ Categorical perception of conspecific communication sounds by Japanese macaques,” J. Acoust. Soc. Am. 85(2), 837–847. 10.1121/1.397555 [DOI] [PubMed] [Google Scholar]
- 144. May, B. , Moody, D. B. , Stebbins, W. C. , and Norat, M. A. (1986). “ Sound localization of frequency-modulated sinusoids by Old World monkeys,” J. Acoust. Soc. Am. 80(3), 776–782. 10.1121/1.393952 [DOI] [PubMed] [Google Scholar]
- 145. McFadden, D. , Pasanen, E. G. , Raper, J. , Lange, H. S. , and Wallen, K. (2006). “ Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta),” Horom. Behav. 50(2), 274–284. 10.1016/j.yhbeh.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 146. McGill, T. J. I. , and Schuknecht, H. F. (1976). “ Human cochlear changes in noise induced hearing loss,” Laryngoscope 86(9), 1293–1302. 10.1288/00005537-197609000-00001 [DOI] [PubMed] [Google Scholar]
- 147. Mills, J. H. , Adkins, W. Y. , and Gilbert, R. M. (1981). “ Temporary threshold shifts produced by wideband noisem,” J. Acoust. Soc. Am. 70(2), 390–396. 10.1121/1.386774 [DOI] [PubMed] [Google Scholar]
- 148. Mitchell, C. , and Silver, D. L. (2018). “ Enhancing our brains: Genomic mechanisms underlying cortical evolution,” Sem. Cell Dev. Biol. 76, 23–32. 10.1016/j.semcdb.2017.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Moody, D. B. (1994). “ Detection and discrimination of amplitude-modulated signals by macaque monkeys,” The J. Acoust. Soc. Am. 95(6), 3499–3510. 10.1121/1.409967 [DOI] [PubMed] [Google Scholar]
- 150. Moody, D. B. , Le Prell, C. G. , and Niemiec, A. J. (1998). “ Monaural phase discrimination by macaque monkeys: Use of multiple cues,” J. Acoust. Soc. Am. 103(5), 2618–2623. 10.1121/1.422782 [DOI] [PubMed] [Google Scholar]
- 151. Moody, D. B. , Stebbins, W. C. , Hawkins, J. E. , and Johnsson, L.-G. (1978). “ Hearing loss and cochlear pathology in the monkey (Macaca) following exposure to high levels of noise,” Arch. Otorhinolaryngol. 220(1–2), 47–72. 10.1007/BF00456301 [DOI] [PubMed] [Google Scholar]
- 152. Moody, D. B. , Stebbins, W. C. , and Iglauer, C. (1971). “ Auditory generalization gradients for response latency in the monkey,” J. Exp. Anal. Behav. 16(1), 105–111. 10.1901/jeab.1971.16-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Moody, D. B. , Winger, G. , Woods, J. H. , and Stebbins, W. C. (1980). “ Effect of ethanol and of noise on reaction time in the monkey: Variation with stimulus level,” Psychopharmacology 69(1), 45–51. 10.1007/BF00426520 [DOI] [PubMed] [Google Scholar]
- 154. Moore, B. C. J. (1996). “ Perceptual consequences of cochlear hearing loss and their implications for the design of hearing aids,” Ear Hear. 17(2), 133–161. 10.1097/00003446-199604000-00007 [DOI] [PubMed] [Google Scholar]
- 155. Mutai, H. , Miya, F. , Shibata, H. , Yasutomi, Y. , Tsunoda, T. , and Matsunaga, T. (2018). “ Gene expression dataset for whole cochlea of Macaca fascicularis,” Sci. Rep. 8(1), 15554. 10.1038/s41598-018-33985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Myint, A. , White, C. H. , Ohmen, J. D. , Li, X. , Wang, J. , Lavinsky, J. , Salehi, P. , Crow, A. L. , Ohyama, T. , and Friedman, R. A. (2016). “ Large-scale phenotyping of noise-induced hearing loss in 100 strains of mice,” Hear. Res. 332, 113–120. 10.1016/j.heares.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nadol, J. B. (1983). “ Serial section reconstruction of the neural poles of hair cells in the human organ of Corti. I. Inner hair cells,” Laryngoscope 93(5), 599–614. 10.1002/lary.1983.93.5.599 [DOI] [PubMed] [Google Scholar]
- 158. Nadol, J. B. (1988). “ Comparative anatomy of the cochlea and auditory nerve in mammals,” Hear. Res. 34(3), 253–266. 10.1016/0378-5955(88)90006-8 [DOI] [PubMed] [Google Scholar]
- 159. Ng, C.-W. , Navarro, X. , Engle, J. R. , and Recanzone, G. H. (2015). “ Age-related changes of auditory brainstem responses in nonhuman primates,” J. Neurophysiol. 114(1), 455–467. 10.1152/jn.00663.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ng, C.-W. , Plakke, B. , and Poremba, A. (2014). “ Neural correlates of auditory recognition memory in the primate dorsal temporal pole,” J. Neurophysiol. 111(3), 455–469. 10.1152/jn.00401.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Nielsen, D. W. , Burnham, J. , and Talley, C. (1978). “ Squirrel monkey temporary threshold shift from 48-h exposures to low-frequency noise,” J. Acoust. Soc. Am. 64(2), 478–484. 10.1121/1.382020 [DOI] [PubMed] [Google Scholar]
- 162. Nielsen, D. W. , Franseen, L. , and Fowler, D. (1984). “ The effects of interruption on squirrel monkey temporary threshold shift to a 96-hour noise exposure,” Int. J. Audiol. 23(3), 297–308. 10.3109/00206098409072841 [DOI] [PubMed] [Google Scholar]
- 163. Nomoto, M. (1980). “ Representation of cochlear innervation patterns in single auditory nerve fiber responses,” Jpn. J. Physiol. 30(1), 31–40. 10.2170/jjphysiol.30.31 [DOI] [PubMed] [Google Scholar]
- 164. Nomoto, M. , Suga, N. , and Katsuki, Y. (1964). “ Discharge pattern and inhibition of primary auditory nerve fibers in the monkey,” J. Neurophysiol. 27(5), 768–787. 10.1152/jn.1964.27.5.768 [DOI] [PubMed] [Google Scholar]
- 165. O'Connor, K. N. , Barruel, P. , Hajalilou, R. , and Sutter, M. L. (1999). “ Auditory temporal integration in the rhesus macaque (Macaca mulatta),” J. Acoust. Soc. Am. 106(2), 954–965. 10.1121/1.427108 [DOI] [PubMed] [Google Scholar]
- 166. O'Connor, K. N. , Johnson, J. S. , Niwa, M. , Noriega, N. C. , Marshall, E. A. , and Sutter, M. L. (2011). “ Amplitude modulation detection as a function of modulation frequency and stimulus duration: Comparisons between macaques and humans,” Hear. Res. 277(1–2), 37–43. 10.1016/j.heares.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Osmanski, M. S. , Song, X. , Guo, Y. , and Wang, X. (2016). “ Frequency discrimination in the common marmoset (Callithrix jacchus),” Hear. Res. 341, 1–8. 10.1016/j.heares.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Osmanski, M. S. , and Wang, X. (2011). “ Measurement of absolute auditory thresholds in the common marmoset (Callithrix jacchus),” Hear. Res. 277(1–2), 127–133. 10.1016/j.heares.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ota, C. Y. , and Kimura, R. S. (1980). “ Ultrastructural study of the human spiral ganglion,” Acta Otolaryngol. 89, 53–62. 10.3109/00016488009127108 [DOI] [PubMed] [Google Scholar]
- 170. Park, J. Y. , Clark, W. W. , Coticchia, J. M. , Esselman, G. H. , and Fredrickson, J. M. (1995). “ Distortion product otoacoustic emissions in rhesus (Macaca mulatta) monkey ears: Normative findings,” Hear. Res. 86(1–2), 147–162. 10.1016/0378-5955(95)00065-C [DOI] [PubMed] [Google Scholar]
- 171. Perkins, R. E. , and Morest, D. K. (1975). “ A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarski optics,” J. Compar. Neurol. 163(2), 129–158. 10.1002/cne.901630202 [DOI] [PubMed] [Google Scholar]
- 172. Perlman, R. L. (2016). “ Mouse models of human disease,” Evol. Med. Pub. Health 2016(1), 170–176. 10.1093/emph/eow014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Petersen, M. , Beecher, M. , Zoloth, Moody, D. , and Stebbins, W. (1978). “ Neural lateralization of species-specific vocalizations by Japanese macaques (Macaca fuscata),” Science 202(4365), 324–327. 10.1126/science.99817 [DOI] [PubMed] [Google Scholar]
- 174. Petersen-Jones, S. M. , and Komáromy, A. M. (2015). “ Dog models for blinding inherited retinal dystrophies,” Human Gene Therapy Clin. Dev. 26(1), 15–26. 10.1089/humc.2014.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Petkov, C. I. , O'Connor, K. N. , and Sutter, M. L. (2003). “ Illusory sound perception in macaque monkeys,” J. Neurosci. 23(27), 9155–9161. 10.1523/JNEUROSCI.23-27-09155.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Pfingst, B. E. (1993). “ Comparison of spectral and nonspectral frequency difference limens for human and nonhuman primates,” J. Acoust. Soc. Am. 93(4), 2124–2129. 10.1121/1.406673 [DOI] [PubMed] [Google Scholar]
- 177. Pfingst, B. E. , Laycock, J. , Flammino, F. , Lonsbury-Martin, B. , and Martin, G. (1978). “ Pure tone thresholds for the rhesus monkey,” Hear. Res. 1, 43–47. 10.1016/0378-5955(78)90008-4 [DOI] [PubMed] [Google Scholar]
- 178. Phillips, K. A. , Bales, K. L. , Capitanio, J. P. , Conley, A. , Czoty, P. W. , Hart, B. A. , Hopkins, W. D. , Hu, S. L. , Miller, L. A. , Nader, M. A. , Nathanielsz, P. W. , Rogers, J. , Shively, C. A. , and Voytko, M. L. (2014). “ Why primate models matter,” Am. J. Primatol. 76(9), 801–827. 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Pineda, J. A. , Holmes, T. C. , Swick, D. , and Foote, S. L. (1989). “ Brain-stem auditory evoked potentials in squirrel monkey (Saimiri sciureus),” Electroencephalogr. Clin. Neurophysiol. 73, 12. [DOI] [PubMed] [Google Scholar]
- 180. Pinheiro, M. , Jordan, V. , and Luz, G. A. (1973). “ The relationship between permanent threshold shift and the loss of hair cells in monkeys exposed to impulse noise,” Acta Otolaryngol. 76, 31–40. 10.3109/00016487309125498 [DOI] [PubMed] [Google Scholar]
- 181. Plum, A. , Winterhager, E. , Pesch, J. , Lautermann, J. , Hallas, G. , Rosentreter, B. , Traub, O. , Herberhold, C. , and Willecke, K. (2001). “ Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation,” Dev. Biol. 231(2), 334–347. 10.1006/dbio.2000.0148 [DOI] [PubMed] [Google Scholar]
- 182. Prosen, C. A. , and Moody, D. B. (1995). “ Rise-time difference thresholds in the monkey,” J. Acoust. Soc. Am. 97(1), 697–700. 10.1121/1.413060 [DOI] [PubMed] [Google Scholar]
- 183. Prosen, C. A. , Moody, D. B. , Sommers, M. S. , and Stebbins, W. C. (1990). “ Frequency discrimination in the monkey,” J. Acoust. Soc. Am. 88(5), 2152–2158. 10.1121/1.400112 [DOI] [PubMed] [Google Scholar]
- 184. Pugh, J. E. , Horwitz, M. R. , and Anderson, D. J. (1974). “ Cochlear electrical activity in noise-induced hearing loss: Behavioral and electrophysiological studies in primates,” Arch. Otolaryngol. 100, 36–40. 10.1001/archotol.1974.00780040040008 [DOI] [PubMed] [Google Scholar]
- 185. Pugh, J. E. , Horwitz, M. R. , Anderson, D. J. , and Singleton, E. F. (1973). “ A chronic implant for recording of cochlear potentials in primates,” Am. J. Phys. Anthropol. 38(2), 351–355. 10.1002/ajpa.1330380232 [DOI] [PubMed] [Google Scholar]
- 186. Pugh, J. E. , Moody, D. B. , and Anderson, D. J. (1979). “ Electrocochleography and experimentally induced loudness recruitment,” Arch. Otorhinolaryngol. 224(3–4), 241–255. 10.1007/BF01108782 [DOI] [PubMed] [Google Scholar]
- 187. Rask-Andersen, H. , Li, H. , Löwenheim, H. , Müller, M. , Pfaller, K. , Schrott-Fischer, A. , and Glueckert, R. (2017). “ Supernumerary human hair cells—Signs of regeneration or impaired development? A field emission scanning electron microscopy study,” Upsala J. Med. Sci. 122(1), 11–19. 10.1080/03009734.2016.1271843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rattay, F. , Potrusil, T. , Wenger, C. , Wise, A. K. , Glueckert, R. , and Schrott-Fischer, A. (2013). “ Impact of morphometry, myelinization and synaptic current strength on spike conduction in human and cat spiral ganglion neurons,” PLoS ONE 8(11), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Raymond, L. A. , Wallace, D. , Berman, N. E. , Marcario, J. , Foresman, L. , Joag, S. V. , Raghavan, R. , Narayan, O. , and Cheney, P. D. (1998). “ Auditory brainstem responses in a rhesus macaque model of neuro-AIDS,” J. Neurovirol. 4(5), 512–520. 10.3109/13550289809113495 [DOI] [PubMed] [Google Scholar]
- 190. Recanzone, G. H. , Jenkins, W. M. , Hradek, G. T. , and Merzenich, M. M. (1991). “ A behavioral frequency discrimination paradigm for use in adult primates,” Behav. Res. Methods Inst. Comput. 23(3), 357–369. 10.3758/BF03203397 [DOI] [Google Scholar]
- 191.Rhesus Macaque Genome Sequencing and Analysis Consortium, Gibbs, R. A. , Rogers, J. , Katze, M. G. , Bumgarner, R. , Weinstock, G. M. , Mardis, E. R. , Remington, K. A. , Strausberg, R. L. , Venter, J. C. , Wilson, R. K. , Batzer, M. A. , Bustamante, C. D. , Eichler, E. E. , Hahn, M. W. , Hardison, R. C. , Makova, K. D. , Miller, W. , Milosavljevic, A. , Palermo, R. E. , Siepel, A. , Sikela, J. M. , Attaway, T. , Bell, S. , Bernard, K. E. , Buhay, C. J. , Chandrabose, M. N. , Dao, M. , Davis, C. , Delehaunty, K. D. , Ding, Y. , Dinh, H. H. , Dugan-Rocha, S. , Fulton, L. A. , Gabisi, R. A. , Garner, T. T. , Godfrey, J. , Hawes, A. C. , Hernandez, J. , Hines, S. , Holder, M. , Hume, J. , Jhangiani, S. N. , Joshi, V. , Khan, Z. M. , Kirkness, E. F. , Cree, A. , Fowler, R. G. , Lee, S. , Lewis, L. R. , Li, Z. , Liu, Y. S. , Moore, S. M. , Muzny, D. , Nazareth, L. V. , Ngo, D. N. , Okwuonu, G. O. , Pai, G. , Parker, D. , Paul, H. A. , Pfannkoch, C. , Pohl, C. S. , Rogers, Y. H. , Ruiz, S. J. , Sabo, A. , Santibanez, J. , Schneider, B. W. , Smith, S. M. , Sodergren, E. , Svatek, A. F. , Utterback, T. R. , Vattathil, S. , Warren, W. , White, C. S. , Chinwalla, A. T. , Feng, Y. , Halpern, A. L. , Hillier, L. W. , Huang, X. , Minx, P. , Nelson, J. O. , Pepin, K. H. , Qin, X. , Sutton, G. G. , Venter, E. , Walenz, B. P. , Wallis, J. W. , Worley, K. C. , Yang, S. P. , Jones, S. M. , Marra, M. A. , Rocchi, M. , Schein, J. E. , Baertsch, R. , Clarke, L. , Csürös, M. , Glasscock, J. , Harris, R. A. , Havlak, P. , Jackson, A. R. , Jiang, H. , Liu, Y. , Messina, D. N. , Shen, Y. , Song, H. X. , Wylie, T. , Zhang, L. , Birney, E. , Han, K. , Konkel, M. K. , Lee, J. , Smit, A. F. , Ullmer, B. , Wang, H. , Xing, J. , Burhans, R. , Cheng, Z. , Karro, J. E. , Ma, J. , Raney, B. , She, X. , Cox, M. J. , Demuth, J. P. , Dumas, L. J. , Han, S. G. , Hopkins, J. , Karimpour-Fard, A. , Kim, Y. H. , Pollack, J. R. , Vinar, T. , Addo-Quaye, C. , Degenhardt, J. , Denby, A. , Hubisz, M. J. , Indap, A. , Kosiol, C. , Lahn, B. T. , Lawson, H. A. , Marklein, A. , Nielsen, R. , Vallender, E. J. , Clark, A. G. , Ferguson, B. , Hernandez, R. D. , Hirani, K. , Kehrer-Sawatzki, H. , Kolb, J. , Patil, S. , Pu, L. L. , Ren, Y. , Smith, D. G. , Wheeler, D. A. , Schenck, I. , Ball, E. V. , Chen, R. , Cooper, D. N. , Giardine, B. , Hsu, F. , Kent, W. J. , Lesk, A. , Nelson, D. L. , O'brien, W. E. , Prüfer, K. , Stenson, P. D. , Wallace, J. C. , Ke, H. , Liu, X. M. , Wang, P. , Xiang, A. P. , Yang, F. , Barber, G. P. , Haussler, D. , Karolchik, D. , Kern, A. D. , Kuhn, R. M. , Smith, K. E. , and Zwieg, A. S. (2007). “ Evolutionary and biomedical insights from the rhesus macaque genome,” Science 316(5822), 222–234. 10.1126/science.1139247 [DOI] [PubMed] [Google Scholar]
- 192. Riazi, M. , Marcario, J. K. , Samson, F. K. , Kenjale, H. , Adany, I. , Staggs, V. , Ledford, E. , Marquis, J. , Narayan, O. , and Cheney, P. D. (2009). “ Rhesus macaque model of chronic opiate dependence and neuro-AIDS: Longitudinal assessment of auditory brainstem responses and visual evoked potentials,” J. Neuroimm. Pharmacol. 4(2), 260–275. 10.1007/s11481-009-9149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rocchi, F. , Dylla, M. E. , Bohlen, P. A. , and Ramachandran, R. (2017). “ Spatial and temporal disparity in signals and maskers affects signal detection in non-human primates,” Hear. Res. 344, 1–12. 10.1016/j.heares.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Romba, J. J. (1962). “ The rhesus monkey in hearing loss research,” in Proceedings of the Eighth Annual Army Human Factors Engineering Conference (October 16–19, 1962, Fort Benning, GA), pp. 163–171. [Google Scholar]
- 195. Romba, J. J. , and Gates, H. W. (1964). “ Hearing loss in the rhesus monkey after repeated exposures to identical noises,” U.S. Army Human Engineering Laboratories, Technical Memorandum 3-64, U.S. Army Research Laboratory, Adelphi, MD, pp. 1–11.
- 196. Russell, S. , Bennett, J. , Wellman, J. A. , Chung, D. C. , Yu, Z.-F. , Tillman, A. , Wittes, J. , Pappas, J. , Elci, O. , McCague, S. , Cross, D. , Marshall, K. A. , Walshire, J. , Kehoe, T. L. , Reichert, H. , Davis, M. , Raffini, L. , George, L. A. , Hudson, F. P. , Dingfield, L. , Zhu, X. , Haller, J. A. , Sohn, H. , Mahajan, V. B. , Pfeiffer, W. , Weckmann, M. , Johnson, C. , Gewaily, D. , Drack, A. , Stone, E. , Wachtel, K. , Simonelli, F. , Leroy, B. P. , Wright, J. F. , High, K. A. , and Maguire, A. M. (2017). “ Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial,” Lancet 390(10097), 849–860. 10.1016/S0140-6736(17)31868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ryan, A. , and Dallos, P. (1975). “ Effect of absence of cochlear outer hair cells on behavioural auditory threshold,” Nature 253, 44–46. 10.1038/253044a0 [DOI] [PubMed] [Google Scholar]
- 198. Salt, Alec N. , and Hirose, K. (2018). “ Communication pathways to and from the inner ear and their contributions to drug delivery,” Hear. Res. 362, 25–37. 10.1016/j.heares.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Salt, Alec N. , and Plontke, S. K. (2018). “ Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications,” Hear. Res. 368, 28–40. 10.1016/j.heares.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Salt, A. N. , Hartsock, J. J. , Gill, R. M. , King, E. , Kraus, F. B. , and Plontke, S. K. (2016). “ Perilymph pharmacokinetics of locally-applied gentamicin in the guinea pig,” Hear. Res. 342, 101–111. 10.1016/j.heares.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Saunders, J. C. , Dear, S. P. , and Schneider, M. E. (1985). “ The anatomical consequences of acoustic injury: A review and tutorial,” J. Acoust. Soc. Am. 78(3), 833–860. 10.1121/1.392915 [DOI] [PubMed] [Google Scholar]
- 202. Scheib, B. T. , Stebbins, W. C. , and Moody, D. B. (1975a). “ Temporary threshold shift in nonhuman primates resulting from chronic exposure to a 2-kHz octave band of noise,” J. Acoust. Soc. Am. S1, S41–S41. 10.1121/1.1995227 [DOI] [Google Scholar]
- 203. Scheib, B. T. , Stebbins, W. C. , Moody, D. B. , Johnsson, L.-G. , and Muraski, A. A. (1975b). “ Auditory threshold shift in nonhuman primates chronically exposed to low-level noise,” J. Acoust. Soc. Am. 58(S1), S89–S90. 10.1121/1.2002379 [DOI] [Google Scholar]
- 204. Schrauwen, I. , Hasin-Brumshtein, Y. , Corneveaux, J. J. , Ohmen, J. , White, C. , Allen, A. N. , Lusis, A. J. , Van Camp, G. , Huentelman, M. J. , and Friedman, R. A. (2016). “ A comprehensive catalogue of the coding and non-coding transcripts of the human inner ear,” Hear. Res. 333, 266–274. 10.1016/j.heares.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Scott, B. H. , Mishkin, M. , and Yin, P. (2012). “ Monkeys have a limited form of short-term memory in audition,” Proc. Natl. Acad. Sci. U.S.A. 109(30), 12237–12241. 10.1073/pnas.1209685109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Seiden, H. R. (1957). Auditory Acuity of the Marmoset Monkey (Hapale Jacchus) ( Princeton University Press, Princeton, NJ: ). [Google Scholar]
- 207. Serafin, J. V. , Moody, D. B. , and Stebbins, W. C. (1982). “ Frequency selectivity of the monkey's auditory system: Psychophysical tuning curves,” J. Acoust. Soc. Am. 71(6), 1513–1518. 10.1121/1.387851 [DOI] [PubMed] [Google Scholar]
- 208. Shepherd, R. K. , Xu, S. A. , and Clark, G. M. (1994). “ Partial hearing loss in the macaque following the co-administration of kanamycin and ethacrynic acid,” Hear. Res. 72(1–2), 89–98. 10.1016/0378-5955(94)90209-7 [DOI] [PubMed] [Google Scholar]
- 209. Sinnott, J. M. (1989). “ Detection and discrimination of synthetic English vowels by Old World monkeys (Cercopithecus, Macaca) and humans,” J. Acoust. Soc. Am. 86(2), 557–565. 10.1121/1.398235 [DOI] [PubMed] [Google Scholar]
- 210. Sinnott, J. M. , Beecher, M. D. , Moody, D. B. , and Stebbins, W. C. (1976). “ Speech sound discrimination by monkeys and humans,” J. Acoust. Soc. Am. 60(3), 687–695. 10.1121/1.381140 [DOI] [PubMed] [Google Scholar]
- 211. Sinnott, J. M. , and Brown, C. H. (1993a). “ Effects of varying signal and noise levels on pure-tone frequency discrimination in humans and monkeys,” J. Acoust. Soc. Am. 93(3), 1535–1540. 10.1121/1.406811 [DOI] [PubMed] [Google Scholar]
- 212. Sinnott, J. M. , and Brown, C. H. (1993b). “ Effects of varying signal duration on pure-tone frequency discrimination in humans and monkeys,” J. Acoust. Soc. Am. 93(3), 1541–1546. 10.1121/1.406812 [DOI] [PubMed] [Google Scholar]
- 213. Sinnott, J. M. , Petersen, M. R. , and Hopp, S. L. (1985). “ Frequency and intensity discrimination in humans and monkeys,” J. Acoust. Soc. Am. 78(6), 1977–1985. 10.1121/1.392654 [DOI] [PubMed] [Google Scholar]
- 214. Sinnott, J. M. , Powell, L. A. , and Camchong, J. (2006). “ Using monkeys to explore perceptual ‘loss’ versus ‘learning’ models in English and Spanish voice-onset-time perception,” J. Acoust. Soc. Am. 119(3), 1585–1596. 10.1121/1.2162440 [DOI] [PubMed] [Google Scholar]
- 215. Sivian, L. J. , and White, S. D. (1933). “ On minimum audible sound fields,” J. Acoust. Soc. Am. 4, 288–321. 10.1121/1.1915608 [DOI] [Google Scholar]
- 216. Sliwinska-Kowalska, M. , and Pawelczyk, M. (2013). “ Contribution of genetic factors to noise-induced hearing loss: A human studies review,” Mutat. Res. Rev. Mutat. Res. 752(1), 61–65. 10.1016/j.mrrev.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 217. Sly, D. J. , Campbell, L. , Uschakov, A. , Saief, S. T. , Lam, M. , and O'Leary, S. J. (2016). “ Applying neurotrophins to the round window rescues auditory function and reduces inner hair cell synaptopathy after noise-induced hearing loss,” Otol. Neurotol. 37(9), 1223–1230. 10.1097/MAO.0000000000001191 [DOI] [PubMed] [Google Scholar]
- 218. Sommers, M. S. , Moody, D. B. , Prosen, C. A. , and Stebbins, W. C. (1992). “ Formant frequency discrimination by Japanese macaques (Macaca fuscata),” J. Acoust. Soc. Am. 91(6), 3499–3510. 10.1121/1.402839 [DOI] [PubMed] [Google Scholar]
- 219. Song, Q. , Shen, P. , Li, X. , Shi, L. , Liu, L. , Wang, J. , Yu, Z. , Stephen, K. , Aiken, S. , Yin, S. , and Wang, J. (2016). “ Coding deficits in hidden hearing loss induced by noise: The nature and impacts,” Sci. Rep. 6(1), 25200. 10.1038/srep25200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Sousa, A. M. M. , Meyer, K. A. , Santpere, G. , Gulden, F. O. , and Sestan, N. (2017). “ Evolution of the human nervous system function, structure, and development,” Cell 170(2), 226–247. 10.1016/j.cell.2017.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Spankovich, C. , Griffiths, S. K. , Lobariñas, E. , Morgenstein, K. E. , de la Calle, S. , Ledon, V. , Guercio, D. , and Le Prell, C. G. (2014). “ Temporary threshold shift after impulse-noise during video game play: Laboratory data,” Int. J. Audiol. 53, S53–S65. 10.3109/14992027.2013.865844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Starr, A. , Picton, T. W. , Sininger, Y. , Hood, L. J. , and Berlin, C. I. (1996). “ Auditory neuropathy,” Brain 119, 741–753. 10.1093/brain/119.3.741 [DOI] [PubMed] [Google Scholar]
- 223. Stebbins, W. C. (1966). “ Auditory reaction time and the derivation of equal loudness contours for the monkey,” J. Exp. Anal. Behav. 9(2), 135–142. 10.1901/jeab.1966.9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Stebbins, W. C. (1973). “ Hearing of old world monkeys (Cercopithecinae),” Am. J. Phys. Anthropol. 38(2), 357–364. 10.1002/ajpa.1330380233 [DOI] [PubMed] [Google Scholar]
- 225. Stebbins, W. C. , Hawkins, J. E. , Johnsson, L.-G. , and Moody, D. B. (1979). “ Hearing thresholds with outer and inner hair cell loss,” Am. J. Otolaryngol. 1(1), 15–27. 10.1016/S0196-0709(79)80004-6 [DOI] [PubMed] [Google Scholar]
- 226. Stebbins, W. C. , and Miller, J. M. (1964). “ Reaction time as a function of stimulus intensity for the monkey,” J. Exp. Anal. Behav. 7(4), 309–312. 10.1901/jeab.1964.7-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Stebbins, W. C. , Miller, J. M. , Johnsson, L.-G. , and Hawkins, J. E. (1969). “ Ototoxic hearing loss and cochlear pathology in the monkey,” Ann. Otol. Rhinol. Laryngol. 78(5), 1007–1025. 10.1177/000348946907800508 [DOI] [PubMed] [Google Scholar]
- 228. Sun, W. , Zhang, L. , Lu, J. , Yang, G. , Laundrie, E. , and Salvi, R. (2008). “ Noise exposure induced enhancement of auditory cortex response and changes in gene expression,” Neuroscience 156(2), 374–380. 10.1016/j.neuroscience.2008.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Suzuki, J. , Corfas, G. , and Liberman, M. C. (2016). “ Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure,” Sci. Rep. 6(1), 24907. 10.1038/srep24907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Suzuki, J. , Hashimoto, K. , Xiao, R. , Vandenberghe, L. H. , and Liberman, M. C. (2017). “ Cochlear gene therapy with ancestral AAV in adult mice: Complete transduction of inner hair cells without cochlear dysfunction,” Sci. Rep. 7(1), 45524. 10.1038/srep45524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Suzuki, S. , Suzuki, N. , Mori, J.-I. , Oshima, A. , Usami, S. , and Hashizume, K. (2007). “ Micro-crystallin as an intracellular 3,5,3′-triiodothyronine holder in vivo,” Mol. Endocrinol. (Baltimore, MD) 21(4), 885–894. 10.1210/me.2006-0403 [DOI] [PubMed] [Google Scholar]
- 232. Thompson, G. C. , Stach, B. A. , and Jerger, J. F. (1984). “ Effect of ketamine on the stapedius reflex in the squirrel monkey,” Arch. Otolaryngol. Head Neck Surg. 110(1), 22–24. 10.1001/archotol.1984.00800270026007 [DOI] [PubMed] [Google Scholar]
- 233. Tomlinson, R. W. W. , and Schwarz, D. W. F. (1988). “ Perception of the missing fundamental in nonhuman primates,” J. Acoust. Soc. Am. 84(2), 560–565. 10.1121/1.396833 [DOI] [PubMed] [Google Scholar]
- 234. Torre, P. , and Fowler, C. G. (2000). “ Age-related changes in auditory function of rhesus monkeys (Macaca mulatta),” Hear. Res. 142(1–2), 131–140. 10.1016/S0378-5955(00)00025-3 [DOI] [PubMed] [Google Scholar]
- 235. Tsunada, J. , Liu, A. S. K. , Gold, J. I. , and Cohen, Y. E. (2016). “ Causal contribution of primate auditory cortex to auditory perceptual decision-making,” Nat. Neurosci. 19(1), 135–142. 10.1038/nn.4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Valero, M. D. , Burton, J. A. , Hauser, S. N. , Hackett, T. A. , Ramachandran, R. , and Liberman, M. C. (2017). “ Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta),” Hear. Res. 353, 213–223. 10.1016/j.heares.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Valero, M. D. , Hancock, K. E. , and Liberman, M. C. (2016). “ The middle ear muscle reflex in the diagnosis of cochlear neuropathy,” Hear. Res. 332, 29–38. 10.1016/j.heares.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Valero, M. D. , Hancock, K. E. , Maison, S. F. , and Liberman, M. C. (2018). “ Effects of cochlear synaptopathy on middle-ear muscle reflexes in unanesthetized mice,” Hear. Res. 363, 109–118. 10.1016/j.heares.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Van Laer, L. , Carlsson, P.-I. , Ottschytsch, N. , Bondeson, M.-L. , Konings, A. , Vandevelde, A. , Dieltjens, N. , Fransen, E. , Snyders, D. , Borg, E. , Raes, A. , and Camp, G. V. (2006). “ The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss,” Hum. Mutat. 27(8), 786–795. 10.1002/humu.20360 [DOI] [PubMed] [Google Scholar]
- 240. Van Laer, L. , Pfister, M. , Thys, S. , Vrijens, K. , Mueller, M. , Umans, L. , Serneels, L. , Van Nassauw, L. , Kooy, F. , Smith, R. J. , Timmermans, J. P. , Van Leuven, F. , and Van Camp, G. (2005). “ Mice lacking Dfna5 show a diverging number of cochlear fourth row outer hair cells,” Neurobiol. Dis. 19(3), 386–399. 10.1016/j.nbd.2005.01.019 [DOI] [PubMed] [Google Scholar]
- 241. Verschooten, E. , Desloovere, C. , and Joris, P. X. (2018). “ High-resolution frequency tuning but not temporal coding in the human cochlea,” PLoS Biol. 16(10), e2005164. 10.1371/journal.pbio.2005164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wang, H. , Zhao, H. , Huang, X. , Sun, K. , and Feng, J. (2018). “ Comparative cochlear transcriptomics of echolocating bats provides new insights into different nervous activities of CF bat species,” Sci. Rep. 8(1), 15934. 10.1038/s41598-018-34333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Wang, L. , Kempton, J. B. , and Brigande, J. V. (2018). “ Gene therapy in mouse models of deafness and balance dysfunction,” Front. Mol. Neurosci. 11, 300. 10.3389/fnmol.2018.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wang, Y. , Hirose, K. , and Liberman, M. C. (2002). “ Dynamics of noise-induced cellular injury and repair in the mouse cochlea,” J. Assoc. Res. Otolaryngol. 3(3), 248–268. 10.1007/s101620020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Ward, W. D. (1960). “ Recovery from high values of temporary threshold shift,” J. Acoust. Soc. Am. 32(4), 497–500. 10.1121/1.1908111 [DOI] [Google Scholar]
- 246. Ward, W. D. , Glorig, A. , and Sklar, D. L. (1959). “ Temporary threshold shift from octave-band noise: Applications to damage-risk criteria,” J. Acoust. Soc. Am. 31(4), 522–528. 10.1121/1.1907746 [DOI] [Google Scholar]
- 247. Wichmann, T. , Bergman, H. , and DeLong, M. R. (2018). “ Basal ganglia, movement disorders and deep brain stimulation: Advances made through non-human primate research,” J. Neural Trans. (Vienna, Austria: 1996) 125(3), 419–430. 10.1007/s00702-017-1736-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wienicke, A. , Hausler, U. , and Jurgens, U. (2001). “ Auditory frequency discrimination in the squirrel monkey,” J. Comp. Physiol. A 187(3), 189–195. 10.1007/s003590100189 [DOI] [PubMed] [Google Scholar]
- 249. Wojtczak, M. , Beim, J. A. , and Oxenham, A. J. (2017). “ Weak middle-ear-muscle reflex in humans with noise-induced tinnitus and normal hearing may reflect cochlear synaptopathy,” ENeuro 4(6), ENEURO.0363-17.2017. 10.1523/ENEURO.0363-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Wright, C. G. , Halama, A. R. M. D. , and Meyerhoff, W. L. M. D. (1987). “ Ototoxicity of an ototopical preparation in a primate. [Editorial],” J. Otol. 8(1), 56–60. [PubMed] [Google Scholar]
- 251. Yankaskas, K. , Hammill, T. , Packer, M. , and Zuo, J. (2017). “ Editorial: Auditory injury—A military perspective,” Hear. Res. 349, 1–3. 10.1016/j.heares.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 252. Zeng, H. , Shen, E. H. , Hohmann, J. G. , Oh, W. S. , Bernard, A. , Royall, J. J. , Glattfelder, K. J. , Sunkin, S. M. , Morris, J. A. , Guillozet-Bongaarts, A. L. , Smith, K. A. , Ebbert, A. J. , Swanson, B. , Kuan, L. , Page, D. T. , Overly, C. C. , Lein, E. S. , Hawrylycz, M. J. , Hof, P. R. , Hyde, T. M. , Kleinman, J. E. , and Jones, A. R. (2012). “ Large-scale cellular-resolution gene profiling in human neocortex reveals species-specific molecular signatures,” Cell 149(2), 483–496. 10.1016/j.cell.2012.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zheng, F. , and Zuo, J. (2017). “ Cochlear hair cell regeneration after noise-induced hearing loss: Does regeneration follow development?,” Hear. Res. 349, 182–196. 10.1016/j.heares.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Zoloth, S. , Petersen, M. , Beecher, M. , Green, S. , Marler, P. , Moody, D. , and Stebbins, W. (1979). “ Species-specific perceptual processing of vocal sounds by monkeys,” Science 204(4395), 870–873. [DOI] [PubMed] [Google Scholar]