Abstract
Background and Aims
The ability of wheat genotypes to save water by reducing their transpiration rate (TR) at times of the day with high vapour pressure deficit (VPD) has been linked to increasing yields in terminal drought environments. Further, recent evidence shows that reducing nocturnal transpiration (TRN) could amplify water saving. Previous research indicates that such traits involve a root-based hydraulic limitation, but the contribution of hormones, particularly auxin and abscisic acid (ABA), has not been explored to explain the shoot–root link. In this investigation, based on physiological, genetic and molecular evidence gathered on a mapping population, we hypothesized that root auxin accumulation regulates whole-plant water use during both times of the day.
Methods
Eight double-haploid lines were selected from a mapping population descending from two parents with contrasting water-saving strategies and root hydraulic properties. These spanned the entire range of slopes of TR responses to VPD and TRN encountered in the population. We examined daytime/night-time auxin and ABA contents in the roots and the leaves in relation to hydraulic traits that included whole-plant TR, plant hydraulic conductance (KPlant), slopes of TR responses to VPD and leaf-level anatomical traits.
Key Results
Root auxin levels were consistently genotype-dependent in this group irrespective of experiments and times of the day. Daytime root auxin concentrations were found to be strongly and negatively correlated with daytime TR, KPlant and the slope of TR response to VPD. Night-time root auxin levels significantly and negatively correlated with TRN. In addition, daytime and night-time leaf auxin and ABA concentrations did not correlate with any of the examined traits.
Conclusions
The above results indicate that accumulation of auxin in the root system reduces daytime and night-time water use and modulates plant hydraulic properties to enable the expression of water-saving traits that have been associated with enhanced yields under drought.
Keywords: Drought tolerance, water saving, transpiration rate, nocturnal, night-time, auxin, abscisic acid, root hydraulics, vasculature, wheat, yield
INTRODUCTION
Mediterranean-type terminal drought events are the most penalizing water deficit regimes experienced by crops, and their frequency and intensity are expected to increase across the globe as a result of anthropogenic climate change (Berger et al., 2016). Under such conditions, plants typically grow on stored soil moisture, following early-season precipitation which typically triggers planting by the farmer. In those environments, plants will have to achieve a seed-to-seed cycle by relying on an amount of stored soil water that does not evaporate, run off or percolate to deeper, inaccessible soil layers. For subsistence farming, the situation is even more challenging since the crop will have to generate yields that have to be economically viable for the household (Solh and Van Ginkel, 2014).
Water-saving traits have a promising potential for enhancing yields under a terminal drought environment (e.g. Sinclair et al., 2017). Over the last decade, a series of simulation studies using physiologically informed, process-based simulation modelling consistently demonstrated for several crops such as maize [Zea mays (L.)], soybean [Glycine max (L.) Merr.] and lentil [Lens culinaris (Medik.)] that the expression of such traits would translate into an increased probability of significant yield benefits (e.g. Sinclair et al., 2010; Messina et al., 2015; Guiguitant et al., 2017). In those studies, a functional trait that was found consistently to generate yield benefits under terminal drought consisted of decreased transpiration rate (TR) during times of the day where the levels of atmospheric vapour pressure deficit (VPD) would outmatch the ability of the plant to supply water to the transpiring leaf, resulting in a decrease in canopy conductance (Sinclair et al., 2005, 2017).
On wheat [Triticum aestivum (L.)], such a trait has been shown to be consistently expressed among historical genotypes released for the rain-fed production environments in Australia between 1890 and 2008 (Schoppach et al., 2017). Experimentation carried out on an elite, drought-tolerant Australian breeding line ‘RAC875’ showed that this water-saving behaviour stemmed from a lower root hydraulic conductivity that was not exhibited by a drought-sensitive cultivar called 'Kukri' (Schoppach and Sadok 2012; Schoppach et al., 2014a). This difference was traced to an increased resistance to radial, trans-membrane water movement in the roots of RAC875, putatively controlled by a lack of a mercury-sensitive aquaporin population (Schoppach et al., 2014a). In addition, the roots of RAC875 were found to exhibit particularly smaller diameters of central metaxylem (CMX) vessels, below a limit (55 μm) that was found by Richards and Passioura (1989) to be associated the with up to 11 % of yield increases in a breeding programme that specifically targeted decreasing CMX diameters to achieve water saving in the field in Australia.
Currently, there is evidence supporting the idea that whole-plant TR response to VPD involves an interaction between ‘local’ (i.e. leaf-based) and ‘non-local’ (root-based) mechanisms mobilizing long-distance hydraulic signals that involve a combination of root anatomical traits and a dynamic control over radial water flow mediated by aquaporins (Vadez, 2014; Vandeleur et al., 2014; Maurel et al., 2016; Sivasakthi et al., 2017). However, other investigations point to a role played by a shoot-to-root hormonal signal, namely abscisic acid (ABA), in co-ordinating TR with root hydraulic conductivity in response to increasing VPD (Kudoyarova et al., 2011; Veselov et al., 2018).
In an effort to illuminate clues underpinning these links, a high-throughput phenotyping approach for characterizing TR response curves to naturally increasing VPD was undertaken in a double-haploid (DH) mapping population resulting from a cross between RAC875 and Kukri (Schoppach et al., 2016). The genetic analysis revealed a major quantitative trait locus (QTL) controlling these responses, which explained >25 % of the genetic variance, with a peak region that was mapped to nine drought tolerance candidate genes that were found to be expressed in the roots independent of phenology, in an RNA sequencing (RNA-Seq) experiment (Schoppach et al., 2016). In further support of the root-based origin of these responses, the putative functions of several of those genes directly indicated their involvement in root development, root xylem patterning and response to ABA and water stress. However, an unexpected finding of that study was that several of the genes were also auxin related, raising the speculation that root auxin could be directly involved in the variation in TR responses to VPD found in this population.
Considering evidence documenting direct involvement of auxin (1) in root development and vasculature patterning (Fàbregas et al., 2015; Alabdallah et al., 2017); (2) in downregulating aquaporin expression and hydraulic conductivity (Péret et al., 2012); and (3) as a shoot–root signalling molecule (Vandeleur et al., 2014), a first goal of this research was to examine the involvement of root auxin levels in variation of traits associated with TR responses to VPD. To this end, we examined the relationship between root auxin concentrations and variation in whole-plant hydraulic conductance, daytime TR and TR response curves to increasing VPD among a group of eight DH lines from the RAC875 × Kukri cross, which previously expressed variation in canopy conductance spanning the entire range of the population.
A second goal of the investigation was to evaluate two alternative, competing hypotheses for the direct involvement of root auxin in regulating whole-plant hydraulics in wheat. The first alternative hypothesis is based on the idea that leaf auxin could be involved in regulating those hydraulic traits. Considering findings documenting the role played by leaf auxins in leaf vasculature development, particularly xylem (Taneda and Terashima, 2012; Moreno-Piovano et al., 2017), and given that leaf vasculature plays a central role in leaf hydraulic conductance (Caringella et al., 2015), we examined the relationship between leaf auxin concentrations, the above hydraulic traits and a group of six vasculature-related, leaf anatomical traits. The second alternative hypothesis consisted of the involvement of shoot and/or root ABA in regulating those traits. Such a possibility is supported by findings of Kudoyarova et al. (2011) on durum wheat and of Veselov et al. (2018) on barley, indicating that high VPD triggers leaf ABA export to the root to regulate root hydraulic conductivity, as a way of maintaining leaf hydration as VPD increases. In contrast, the findings of Kholová et al. (2010) on pearl millet indicate a localized phenomenon where the accumulation of leaf ABA under high VPD drives a decrease in stomatal conductance and therefore the expression of water saving of drought-tolerant genotypes. To address these hypotheses, another goal of the investigation was to examine the relationships between root and leaf ABA and whole-plant hydraulic conductance, daytime TR and TR response curves to increasing VPD among the same eight lines selected from the RAC875 × Kukri population.
In addition to traits controlling daytime water use, there is indirect evidence to suggest potential yield benefits arising from reduced nocturnal transpiration in crops grown in drought-prone environments. In the case of Australian wheat, Rawson and Clarke (1988) found that night-time transpiration rates (TRN) could be in excess of 0.5 mm per night, being the first to hypothesize potential yield benefits resulting from night-time water saving. This potential was confirmed in a series of studies on crops including wheat (Schoppach et al., 2014b), bean (Resco de Dios et al., 2015) and grapevine (Coupel-Ledru et al., 2016). Under controlled environment conditions, Schoppach et al. (2014b) identified significant genotypic variability in wheat TRN, which was driven by nocturnal VPD, reaching values that were up to 55 % of maximal daytime TR, under high levels of nocturnal VPD (2.1 kPa). Interestingly, those responses were found to mirror daytime TR response curves to VPD, in that the water-saving genotype RAC875 also exhibited a particularly limited TRN under high nocturnal VPD, in contrast to the drought-sensitive cultivar Kukri. More recently, Claverie et al. (2018) found that under water-deficit conditions, TRN in wheat was much less sensitive to progressive soil drying relative to daytime TR, resulting in a progressively higher contribution of TRN to daily water use. In that study, RAC875 was found to exhibit a tighter control of TRN than Kukri during the soil drying sequence, resulting in the expression of what could result in a water-saving behaviour (Claverie et al., 2018).
While the genetic basis of TRN was found to be controlled by numerous QTLs in the RAC875 × Kukri population (Schoppach et al., 2016), the physiological basis driving this variation remains poorly understood. On well-watered wheat, Claverie et al. (2018) found that high TRN levels were associated with changes in root anatomy, particularly smaller endodermis cell size and reduced xylem sap exudation rates, a proxy measure for root pressure. In trees, high TRN levels have been associated with higher nitrogen uptake by the roots to compensate for low nitrogen availability (Rohula et al., 2014), or with the need for higher O2 delivery via the nocturnal xylem sap to maintain functions sustained via dark respiration (Marks and Lechowicz, 2007). However, so far, hormonal involvement in regulating TRN has not been explored. Given the involvement of root auxin in regulating root vasculature development (Fàbregas et al., 2015; Alabdallah et al., 2017), and N foraging by the roots (Song et al., 2013), we examined in this investigation the relationship between root auxin concentration and TRN levels among the same eight DH lines from the RAC875 × Kukri cross. Similar to the first goal of this investigation, we also tested the hypothesis of the involvement of night-time leaf auxin levels and leaf anatomical traits in controlling TRN. Finally, we examined the possibility that night-time leaf or root ABA could be involved in the variation in TRN in this group.
MATERIALS AND METHODS
Genetic material
Eight genotypes were selected from a population of 143 bread wheat [Tricticum aestevum (L.)] DH lines that descended from a cross between the drought-tolerant breeding line RAC875 (RAC655/3/Sr21/4*LANCE//4*BAYONET) and the check, drought-sensitive cultivar Kukri (76ECN44/76ECN36//MADDEN/6*RAC177). The eight genotypes were selected such that they span the entire range of the slopes of TR response curves to increasing VPD that was found for this population (Schoppach et al., 2016; Supplementary Data Fig. S1).
Growth conditions
Four independent experiments were undertaken in this study (Table 1). In all experiments, plants were grown for 34–37 d in a glasshouse at the Université catholique de Louvain, Belgium (50°40’N, 4°36’E). The glasshouse was equipped with a supplementary LED lighting system (Pro650, LumiGrow, Novato, CA, USA), which provided an additional photosynthetic photon flux density (PPFD) of 200 μmol m–2 s–1 to the natural ambient PPFD at canopy level. The lighting system activated each time the incident radiation dropped below 500 W m–2 between 06.00 h and 22.00 h in experiments E1 and E2 and between 08.00 h and 20.00 h in experiment E3. Plants were sown and grown in well-watered garden soil (DCM Corporation, Grobendonk, Belgium) in experiment E1 and in a hydroponic system in experiments E2 and E3 (see below for details).
Table 1.
Summary of the experiments
| Experiment | Sowing date | Measurement date | Measured variable | Plant age (d) | Root medium | Growth period conditions (± s.e.) | |||
|---|---|---|---|---|---|---|---|---|---|
| Daytime | Night-time | ||||||||
| T (°C) | VPD (kPa) | T (°C) | VPD (kPa) | ||||||
| E1 | 28/04/15 | 02/06/15 | Hydraulic conductance | 36 | Potting mix | 27.6 ± 0.4 | 2.6 ± 0.10 | 21.1 ± 0.2 | 1.4 ± 0.03 |
| E2 | 12/05/15 | 17/06/15 | Night-time IAA and ABA, TRN | 37 | Hydroponic | 28.4 ± 0.5 | 2.8 ± 0.12 | 21.5 ± 0.3 | 1.4 ± 0.03 |
| E3.1 | 21/12/16 | 24/01/17 | Night-time IAA and ABA, TRN | 34 | Hydroponic | 25.8 ± 0.1 | 1.6 ± 0.03 | 24.6 ± 0.1 | 1.7 ± 0.02 |
| E3.2 | 21/12/16 | 24/01/17 | Night-time IAA and ABA, TRN | 34 | Hydroponic | 25.8 ± 0.1 | 1.6 ± 0.03 | 24.6 ± 0.1 | 1.7 ± 0.02 |
| E3.3 | 21/12/16 | 24/01/17 | Daytime IAA and ABA, TR | 34 | Hydroponic | 25.8 ± 0.1 | 1.6 ± 0.03 | 24.6 ± 0.1 | 1.7 ± 0.02 |
| E4 | 07/05/14 | 12/06/14 | Leaf anatomical traits | 36 | Potting mix | 28.6 ± 0.5 | 2.2 ± 0.10 | 22.8 ± 0.3 | 1.3 ± 0.03 |
Potted plants were grown as described in Schoppach et al. (2016). Briefly, three seeds per pot were sown at a depth of 2.5 cm in custom-made PVC columns (0.11 m diameter and 0.33 m tall) filled with 1205 ± 5 g of garden soil. Ten days after sowing, each pot was thinned to a single plant. Pots were watered regularly until the measurements (hydraulic conductance, see below) were initiated. Hydroponically grown plants (E2 and E3) were grown as in Schoppach et al. (2014a) with the exception that plant growth took place in the same greenhouse as and under relatively similar conditions to the potted plants (Table 1). Seeds were germinated for 3 d in Petri dishes on a layer of filter paper humidified with ultra-pure water, inside a dark climate cabinet where temperature (T) was maintained at 20 °C. The germinated seeds were subsequently placed on expanded polystyrene plates (30 plants per plate) floating on a nutrient solution that filled 26 L plastic tanks. The solution was regularly aerated by means of an automatic pump system (flow rate: 150 L h–1, for 15 min every hour) and was prepared based on the method described by Rengel and Graham (1996). This solution contained: 2 mm Ca(NO3)2, 0.5 mm MgSO4·7H2O, 1.5 mm KNO3, 0.1 mm KC1, 2 mm MES-KOH, 0.1 mm NH4H2PO4, 10 mm H3BO3, 0.1 mm Na2MoO4, 25 mm K3-[N-(2-hydroxyethyl) ethylenedinitrilotriacetic acid] (HEDTA), 0.1 mm FeHEDTA, 1 mm MnHEDTA, 0.5 mm CuHEDTA, 0.1 mm NiHEDTA and 2 mm ZnHEDTA. During the first 6 d, seedlings were grown in a half-strength nutrient solution. The solutions were replaced every week. The pH of the nutrient solution was checked daily and, when necessary, adjusted to a value of 6 using MES-KOH. During all experiments, T and relative humidity (RH) conditions were continuously recorded every 5 min by pocket sensors connected to USB dataloggers (EL-USB-2-LCD, Lascar Electronics, Whiteparish, UK) placed in 3–5 locations across the setup.
Plant hydraulic conductance
This experiment (E1) was carried out on the potted plants (Table 1). On the day prior to the measurements, three replicate plants per genotype (i.e. a total of 24 pots) were watered to dripping at 16.00 h. They were slightly re-watered again on the following morning around 05.00 h to minimize the occurrence of transient soil moisture deficit during the measurements. Afterwards, the soil in each pot was covered with aluminium foil to nullify direct soil water evaporation. The plants were then placed inside a walk-in growth chamber (PGV36, Conviron, Winnipeg, Manitoba, Canada) and left to acclimate for 6 h under the steady-state conditions PPFD = 480 μmol m–2 s–1, T = 31.9 °C, VPD = 2.8 kPa (Table 2).
Table 2.
Environmental conditions during the measurements
| Experiment | TR measurement period* | Tissue sampling period | ||
|---|---|---|---|---|
| T (°C) | VPD (kPa) | T (°C) | VPD (kPa) | |
| E1 | 31.6 ± 0.1 | 2.7 ± 0.01 | 31.9 ± 0.1 | 2.8 ± 0.02 |
| E2 | 19.9 ± 0.1 | 1.3 ± 0.01 | 19.3 ± 0.1 | 1.2 ± 0.01 |
| E3.1 | 24.7 ± 0.1 | 1.9 ± 0.01 | 24.2 ± 0.1 | 2.0 ± 0.01 |
| E3.2 | 24.5 ± 0.1 | 2.1 ± 0.01 | 24.4 ± 0.1 | 2.1 ± 0.01 |
| E3.3 | 25.6 ± 0.2 | 2.2 ± 0.03 | 24.8 ± 0.1 | 1.4 ± 0.06 |
*Conditions during the 1 h transpiration rate measurement period, preceding tissue sampling for hydraulic conductance measurement (E1) or hormonal dosage (E2 and E3).
Following the acclimation period, whole-plant TR values were determined for all eight lines by performing two weighings separated by 60 min, using an electronic balance with a resolution of 0.01 g (Model Fx-3000i, A&D Co. Ltd, Tokyo, Japan). TR (mg H2O m–2 s–1) was later calculated as the difference in pot mass, normalized by whole-plant leaf area which was measured destructively, using a leaf area meter (LI-3100C, Li-Cor, Lincoln, NE, USA). Leaf water potential (Ψleaf) of the uppermost fully developed leaf was measured using a standard pressure chamber (Scholander 670, OMS Instrument, Albany, NY, USA). Whole-plant conductance (KPlant) was defined as the flux of water through the plant (TR), divided by the water potential gradient between the soil (Ψsoil) and the top leaf (Ψleaf), as follows:
Since the pots were well watered, it was assumed that Ψsoil is approaching 0 MPa. Consequently, the unit of K is: mg H2O leaf area m–2 s–1 MPa–1.
Auxin and abscisic acid measurements
Tissue harvest conditions.
Measurements were carried out on hydroponically grown plants (E2 and E3, Table 1). Prior to measurements, all plants measured in E2 and E3 were carefully removed from the polystyrene plates at the end of the afternoon and transferred into individual 300 mL dark brown glass bottles covered with aluminium foil and filled with a fresh hydroponic solution at 20.00 h. Plants were then placed in the glasshouse at a PPFD of 0 μmol m–2 s–1 under the environmental conditions displayed in Table 2.
Nocturnal auxin (indole-3-acetic acid or IAA) and ABA measurements were made on leaf and root tissues (four replicate plants per genotype) sampled at 04.00 h in experiment E2 and at two time points during the night (E3.1, 02.00 h; and E3.2, 05.00 h) in experiment E3, under conditions where PPFD was zero (Table 2). Daytime measurements of leaf and root IAA and ABA (experiment E3.3) were made on tissues sampled in the morning at 08.00 h, under conditions reported in Table 2. Prior to each one of these samplings, whole-plant TR was determined gravimetrically during the previous hour using the same approach as mentioned earlier. In total, this resulted in 12 and four nocturnal TR (TRN) and daytime TR measurements per genotype, respectively. Temperature and VPD conditions observed during this period are reported in Table 2.
For leaf hormonal dosage, a pre-determined segment located in the middle of the uppermost fully expanded leaf was quickly cut using a sharp scalpel blade and flash-frozen in liquid nitrogen. The leaf area represented by the cut leaf segment was accounted for when normalizing calculating whole-plant transpirational water loss by leaf area. Immediately after leaf harvesting, whole root systems were harvested for hormonal dosage by de-rooting the plant using a sharp blade, and wiping the roots quickly on water-absorbing tissue before placing them inside Falcon tubes which were flash-frozen in liquid nitrogen.
Hormone extraction and dosage.
After harvesting, leaf and root samples were ground in liquid nitrogen, homogenized, packaged in Eppendorf tubes and stored in a –80 °C freezer. Free IAA and ABA were extracted based on the extraction procedure described in Prinsen et al. (2000). Briefly, homogenized plant material (60–80 mg) was extracted overnight in 80 % methanol (10 μl mg–1 f. wt). C613-phenyl-IAA (50 pmol, Cambridge Isotope Laboratories Inc., Andover, MA, USA) and D6-ABA [100 pmol, (±)-3′,5′,5′,7′,7′,7′-d6 ABA, National Research Council Canada, Saskatoon, Canada] were added as internal standards. After centrifugation (20 000 g, 15 min, 4 °C, 5810R, rotor FA-45-30-11 Eppendorf, Hamburg, Germany) the supernatant was passed over a C18 cartridge (500 mg, Varian, Middelburg, The Netherlands) to retain pigments. The effluent was then diluted to 50 % methanol and concentrated on a DEAE-Sephadex (2 mL, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) anion exchange column for the analysis of free IAA and ABA, which are retained on the DEAE. The DEAE cartridge was eluted with 10 mL of 6 % formic acid, and IAA and ABA were concentrated on a C18 cartridge, which was coupled underneath. This C18 cartridge was eluted with 2 × 0.5 mL of diethylether. The ether was evaporated under vacuum and the sample was dissolved in acidified methanol for methylation with diazomethane. After methylation, the samples were dried under a nitrogen stream and samples were further dissolved in 50 μL of 10 % MeOH for analysis.
The concentrations of IAA and ABA were determined following the protocol by Prinsen et al. (1995). Leaf and root IAA and ABA were analysed by UPLC-MS/MS (Acquity TQD, Waters, Manchester, UK). The settings for the analysis were as follows: 6 μL injection by partial loop, column T 30 °C, solvent gradient 0–2 min: 95/5; 10 % MeOH in NH4OAc 1 mm/MeOH; 2–4 min linear gradient until 10/90 10 % MeOH in NH4OAc 1 mm/MeOH; 4–6 min, isocratic 10/90 10 % MeOH in NH4OAc 1 mm/MeOH; MS conditions: polarity MS ES(+), capillary 2 kV, cone 20 V, collision energy: 20 eV, source temperature: 120 °C, desolvation temperature: 450 °C, cone gas flow 50 L h–1, desolvation gas flow: 750 L h–1, collision gas flow: 0.19 mL h–1). The diagnostic ions used for quantification are 190>130 m/z for Me-IAA, 196>136 m/z for Me-C13-IAA, 279>173 m/z for Me-ABA and 285>179 m/z for d6-Me-ABA (dwell time 0.020 s). Methanol and water used for MS are UPLC grade from Biosolve (Valkenswaard, The Netherlands). Data are expressed in pmol g–1 f. wt). Using this method, root ABA concentrations were too low to be detected since they were below the detection threshold of 10 pmol g–1 f. wt (Prinsen et al., 1995).
Leaf anatomical measurements
During experiment E4, 5 cm leaf segments from the eight selected genotypes were examined for leaf anatomical traits. These segments were carefully sectioned from the middle part (approx. 10 cm from the leaf tip) of the top, most fully developed leaf using a scalpel blade and instantaneously fixed with FAA solution (99 % ethanol, demineralized water, 36 % formaldehyde and acetic acid, 45 %/45 %/5 %/5% v/v/v/v) for 1 week. Afterwards, they were transferred into a bleaching solution (99 % ethanol and acetic acid, 70 %/30 % v/v) for 3 d. Samples were then stored for approx. 3 weeks in an ethanol/water solution (70 %/30 % v/v) prior to further examination.
Freehand sections were produced using a sharp razor blade and stained with a safranin solution [0.5 g L–1 ethanol/water (1/19 v/v)] for 10 s before being quickly rinsed with water followed by 100 % ethanol to avoid an overcoloration. Afterwards, thin leaf slices were mounted on microscope blade at an amplification of ×400. Approximately 50 pictures were taken for each leaf in order to cover the entire transversal section of each leaf. Using the Image-J plugin Mosaic-J, images were then sequentially placed, adjusted and merged in order to obtain one complete, large-angle and high-resolution image of the entire leaf transversal section (Supplementary Data Fig. S2).
Those images were then used to quantify the leaf traits reported in Supplementary Data Fig. S2. The following anatomical traits were then determined on each leaf section image using distance and area measurement tools from Image J software: leaf width (LW, mm), major and minor vein densities (VDM and VDm, respectively, mm–1), average distance between veins (DV, μm), average vein section area (VSA, μm2), average metaxylem section area (MXA, μm2) and average leaf thickness (LT, μm). LW was measured as the length of the straight line passing through all the vein centres from one border of the leaf to the other (Supplementary Data Fig. S2). VDM and VDm were calculated as the leaf width divided by the number of major and minor veins, respectively. DV was measured from the centre of the vein to the centre of the following one (irrespective of the type of the vein) and averaged on the whole leaf cross-section. VSA is the area delimited by the mestome sheath. MXA was calculated as the averaged section area of the two main metaxylem vessels in each major vein. LT was determined from the leaf thickness measured at all the veins and between all the veins (Supplementary Data Fig. S2). Abaxial and adaxial stomatal densities (mm–2, AB_SD and AD_SD, respectively) were determined for those genotypes at a similar leaf position in a previous study (Schoppach et al., 2016).
Data and statistical analysis
All statistical analyses [regression analyses, correlation analyses and one-way analyses of variance (ANOVAs)] were carried out using GraphPad PRISM version 7.0b (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Daytime root auxin levels correlate with daytime transpiration and plant hydraulic conductance measured independently
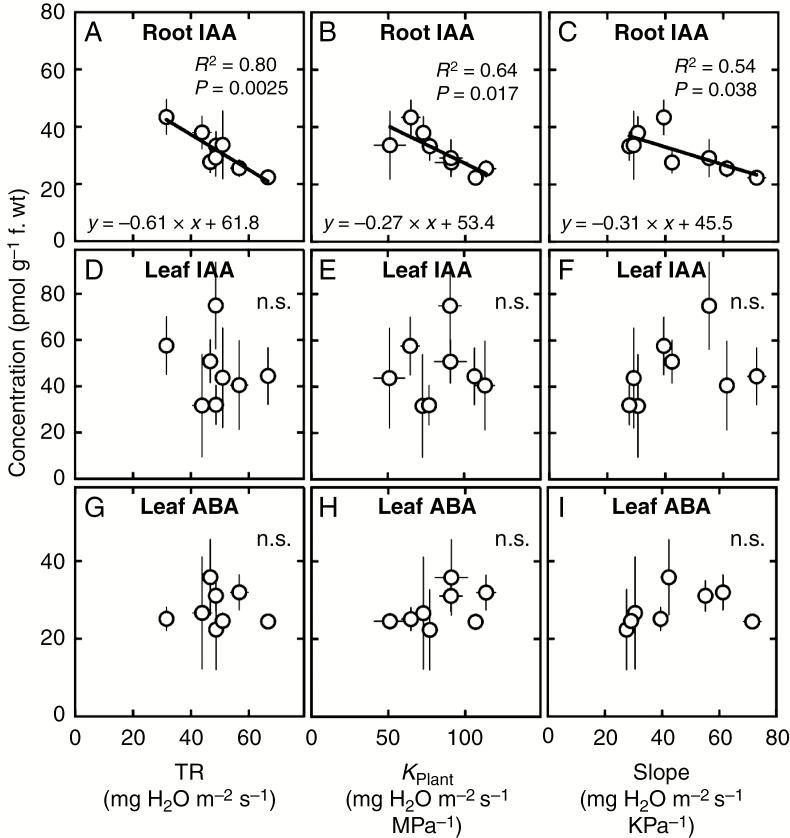
Daytime root IAA concentrations significantly correlated with all three hydraulic traits examined in the study, namely whole-plant daytime TR (Fig. 1A), KPlant (Fig. 1B) and the previously characterized slopes of whole-plant TR responses to increasing VPD (Fig. 1C), determined in Schoppach et al. (2016). In all cases, the correlations were negative, with Pearson’s r-values ranging from –0.75 (root IAA vs. slopes) to –0.9 (root IAA vs. daytime TR).
Fig. 1.
Relationships between root or shoot concentrations in indole acetic acid (IAA) or abscisic acid (ABA) and whole-plant daytime hydraulic traits in wheat. (A–C) Correlations between root IAA and whole-plant transpiration rate (TR), plant hydraulic conductance (KPlant) and the slope of TR response to VPD, respectively. (D–F) and (G–I) Correlations between leaf IAA and leaf ABA concentrations and these same variables, respectively. When significant, statistical data and regression coefficients are indicated. Each data point is the average of three observations (± s.e.) made on eight double haploid wheat lines resulting from the cross between parents RAC875 and Kukri.
The correlation analysis also revealed that KPlant strongly and positively correlated with the independently measured slopes of TR responses to VPD (Pearsons’s r = 0.84, P = 0.008, R2 = 0.72, Fig. 2), indicating that the eight selected genotypes consistently exhibit the same hydraulic properties independent of the experiment.
Fig. 2.
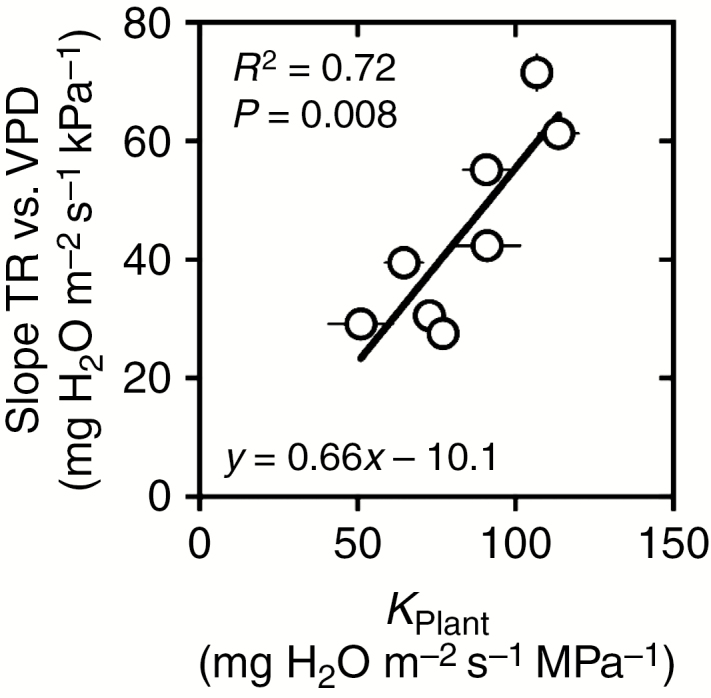
Relationship between the slope of the whole-plant transpiration rate (TR) response curve to increasing vapour pressure deficit (VPD) determined in Schoppach et al. (2016) and plant hydraulic conductance (KPlant) measured independently in experiment E1 on the eight wheat genotypes of the study.
In sharp contrast to daytime root IAA, daytime leaf IAA and leaf ABA concentrations were found not to correlate significantly with daytime whole-plant TR, KPlant or the slopes of whole-plant TR responses to increasing VPD (Fig. 1D–I). Further, daytime leaf IAA and leaf ABA concentrations did not correlate with any of the examined leaf vascular traits.
Night-time root IAA levels are stable across experiments
Regardless of the time of the day, root ABA concentrations were below the detection levels of the method used for quantification (see the Materials and Methods). Irrespective of the genotype, night-time leaf IAA, root IAA and leaf ABA concentrations did not vary significantly between experiments E3.1 and E3.2 (Fig. 3A). Experiment E2 showed significantly lower leaf IAA and higher leaf ABA concentrations (Fig. 3B) but, in contrast, night-time IAA root concentrations were more stable and did not exhibit significant variation across all three experiments (Fig. 3C). Correlation analyses showed that genotypic rankings in night-time leaf IAA and leaf ABA levels were not consistent across experiments, even between E3.1 and E3.2, and none of these concentrations significantly correlated with leaf vasculature measurements.
Fig. 3.
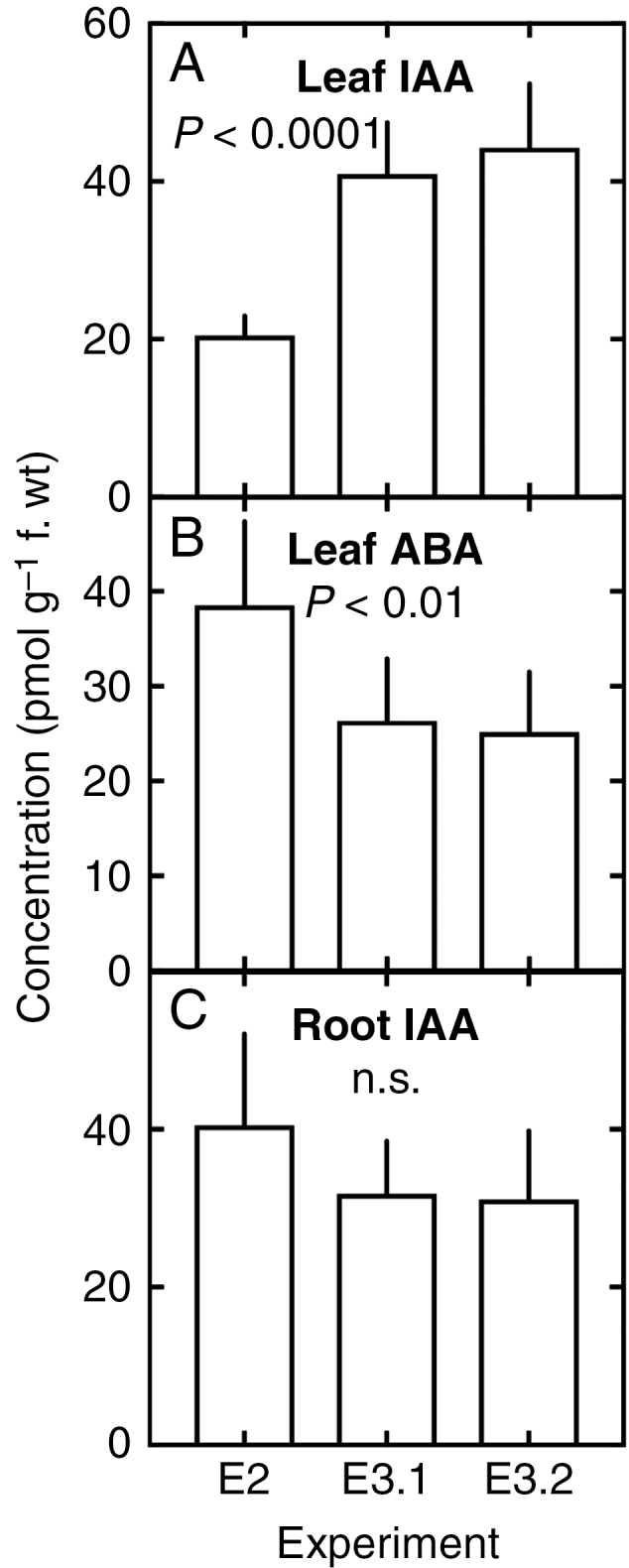
Variation in night-time leaf concentrations of indole acetic acid (IAA; A), night-time abscisic acid (ABA; B) and night-time IAA root concentrations (C) across three experiments (see Tables 1 and 2 for details of environmental conditions).
Root IAA concentrations were highly correlated between E2 and E3.1 (r = 0.97, Pearson’s P < 0.0001) and between E3.1 and E3.2 (Pearson’s r = 0.74, P = 0.037), while the correlation between E2 and E3.2 was significant at P = 0.08 (Pearson’s r = 0.65) as a result of an outlier datum (P < 0.05 if removed). Therefore, night-time root IAA data were pooled across the three experiments for further analysis (see below).
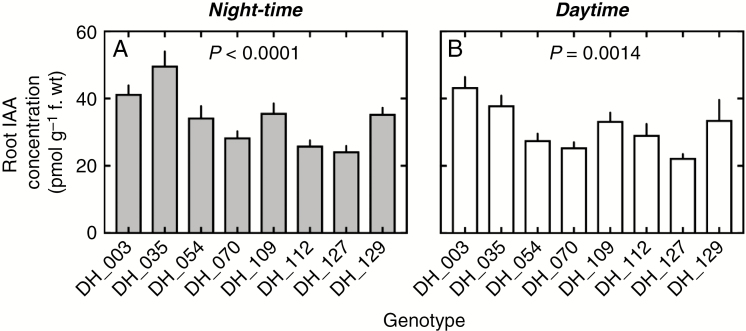
Night-time root IAA is genotype dependent and correlates strongly with daytime root IAA and with nocturnal transpiration rates
As reported in Fig. 4, variation in root IAA was found to be strongly dependent on genotypes during both the night (P < 0.0001, Fig. 4A) and the day (P < 0.005, Fig. 4B). During the night, root IAA concentrations ranged from 24.3 pmol g–1 for genotype DH_127 to more than twice that value, around 49.8 pmol g–1, for genotype DH_035. This variation spanned a similar range during the day, from 22.4 pmol g–1 (genotype DH_127) to 43.4 pmol g–1 (genotype DH_003), with a similar ranking among genotypes (compare Fig. 4A and B). Consistently, root IAA concentrations were found to be strongly and positively correlated among these eight lines (Pearson’s r = 0.83, R2 = 0.68, P = 0.011, Fig. 5).
Fig. 4.
Genetic variability in night-time and daytime indole acetic acid (IAA) concentrations in the roots of the eight wheat genotypes of the study. P-values are significance levels of one-way ANOVAs testing for the genotypic effect of the differences in means between genotypes. The number of observations per genotype ranged from four (daytime) to 12 (night-time).
Fig. 5.
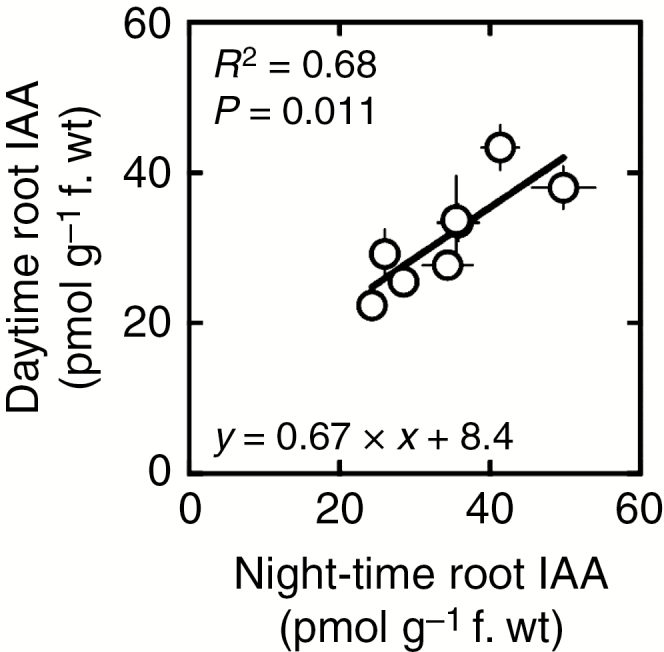
Relationship between nocturnal and daytime indole acetic acid (IAA) concentrations in the roots of the eight studied wheat genotypes. Each data point represents a given genotype. Daytime and night-time values are the average of four and 12 observations, respectively. Statistical data (R2, P-value and regression coefficients) are indicated.
Regardless of the experiment, night-time root IAA concentrations correlated significantly with TRN (Pearson’s r = –0.69, R2 = 0.48, P < 0.0005, Fig. 6). This correlation was confirmed (i.e. statistically significant) when analysing data from experiments E3.1 and E3.2 separately (not shown), while experiment E2 displayed a very similar but non-significant tendency as a result of one single outlier datum (R2 = 0.81 and P < 0.01 if the outlier is not included). Night-time root IAA concentrations did not correlate with any of the leaf anatomical traits examined.
Fig. 6.
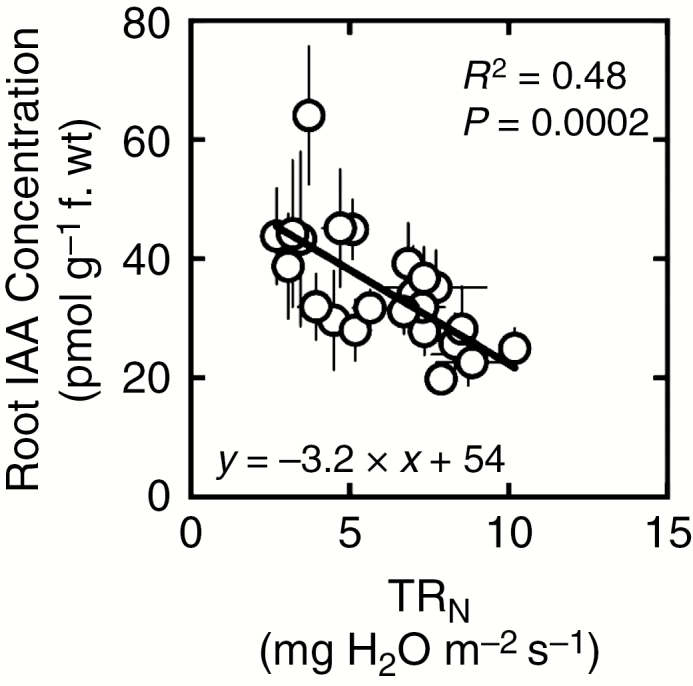
Relationship between whole-plant nocturnal transpiration rate (TRN) and root concentrations of indole acetic acid (IAA) among the eight studied wheat genotypes. Each data point is the average of 3–4 (± s.e.) observations. Data are pooled from three independent experiments (see the Materials and Methods for details). Statistical data for the regression (R2, P-value and regression coefficient) are indicated.
DISCUSSION
Root auxins are potentially involved in regulating daytime whole-plant hydraulics
A first major finding of this research was that root IAA – but not leaf IAA – was potentially involved in controlling daytime water use in wheat, in a way that is consistent with the contrasting root hydraulic properties of the parents of the studied population and with what we already know about the putative roles of auxin in plant hydraulics (see below for details). To our knowledge, this is the first time that auxin is shown to be involved in whole-plant hydraulics, with a role in the expression of a water-saving trait that has been mechanistically linked to improved yields under terminal drought conditions.
In this investigation, this finding stems from the convergence of three independent sources of evidence, highlighting a negative correlation between root IAA and (1) whole-plant TR (Fig. 1A); (2) KPlant (Fig. 1B); and (3) the slope of TR response curves to increasing VPD (Fig. 1C). In all cases, those correlations were of the same sign, collectively indicating a putative involvement of auxin accumulation in the root in the expression of a hydraulic restriction at the canopy level. Such results are in line with Péret et al. (2012) who found that accumulation of root auxin decreases hydraulic conductivity at both the cell and whole-root levels by negatively regulating aquaporin expression, in support of previous findings of Paciorek et al. (2005) who demonstrated that auxin suppress the endocytosis of PIP2 aquaporin in Arabidopsis thaliana.
These findings also strongly support previous results established on the parents of this population which indicated that lower TR under high VPD expressed by the drought-tolerant parent (RAC875) relative to the drought-sensitive parent (Kukri) probably stems from a lower hydraulic conductivity of the roots of the former. Interestingly, this hydraulic limitation was found to be associated with decreased CMX vessel diameters combined with a restriction on the radial, aquaporin-mediated water transport in the root (Schoppach et al., 2014a) – both of which are influenced by auxin. Indeed, local accumulation of root auxin is consistent with its role in repressing root aquaporins (Péret et al., 2012) and in decreasing the vascular cell size (Fàbregas et al., 2015). Furthermore, the involvement of root auxin is consistent with the outcome of the QTL mapping carried out on the DH population from which the eight genotypes of the study were selected, and the independent RNA-Seq analysis which mapped the peak region of the major QTLs for TR responses to VPD to root-specific transcripts with functions suggesting involvement of root auxin (Schoppach et al., 2016).
The lack of correlation between leaf IAA, leaf ABA and the hydraulic variables examined in this study seems to further support the idea of a predominant involvement of auxin-mediated root hydraulic processes in controlling whole-plant water use in wheat, at least in this population. However, our inability to measure root ABA in this study does not allow us to discard the possibility that genotypic variation in root ABA would have contributed to variation in TR response to VPD, as suggested by a previous study on durum wheat (Kudoyarova et al., 2011). Furthermore, our study does not eliminate the possibility that auxin effects were mediated through interaction with other plant hormones such as cytokinin and ethylene, as suggested by studies highlighting such cross-talks (Tanaka et al., 2006; Rowe et al., 2016). Nevertheless, the above findings make it clear that root auxin plays an important role in regulating whole-plant water use and hydraulic properties at least in this population.
A potential role for root auxins in controlling nocturnal transpiration
The second major finding of this investigation was that nocturnal water use is strongly and negatively correlated with auxin accumulation levels in wheat roots (Fig. 6). This finding was consistently observed over three independent experiments. To our knowledge, this is the first time that variation in hormonal levels was associated with changes in nocturnal water use. Importantly, the negative correlation between root auxin concentrations and TRN was consistent with the relationship linking daytime root auxin concentration and daytime water use, indicating that root auxin accumulation tends to reduce transpiration regardless of the time of the day, a hypothesis reinforced by the strong correlation between daytime and night-time auxin levels in the roots (Fig. 5). This in turn may help explain why the drought-tolerant parent RAC875 expressed the water-saving behaviour during both the day and the night, and, more generally, the previously reported strong correlation between daytime and night-time slopes of TR response to VPD on wheat (Schoppach et al., 2014b). Similar to daytime conditions, leaf IAA, leaf ABA and leaf vascular traits were not found to correlate with TRN values, indicating that this TRN variation in this population is largely controlled by roots. However, as previously stated, the additional involvement of root ABA could not be disproved, given that the root ABA concentrations were below the detection levels of the method used for quantification.
The dominating theories explaining the functional relevance of nocturnal water use typically revolve around transport mechanisms, defining a trade-off space where roots are central players. For instance, it is thought that increased TRN would facilitate nitrogen acquisition by roots grown in nitrogen-limited environments (Rohula et al., 2014), and oxygen delivery in the xylem sap to sustain dark respiration-mediated nocturnal carbohydrate export (Marks and Lechowicz, 2007). On the other hand, increases in TRN could also decrease the rate of hydraulic redistribution in the soil, a phenomenon driving the movement of water from moist to dry soil through the root system, which was associated with enhanced daytime transpiration efficiency and whole-season growth (Howard et al., 2009; Neumann et al., 2014). In concert with studies documenting regulatory effects of root auxin on aquaporin-mediated root hydraulic conductivity (Péret et al., 2012), xylem vessel development (Fàbregas et al., 2015) and nitrogen foraging by the roots (Song et al., 2013), our results indicate that the root auxins are potentially involved in the trade-offs associated with TRN.
Root auxins: implications for drought tolerance
The key findings of this research are consistent with a spate of recent publications suggesting the involvement of root auxins in crop drought tolerance. Our own findings on wheat indicate that root auxin accumulation potentially drives the restriction in root hydraulic conductivity that is associated with the expression of the water-saving limitation of whole-plant TR, particularly at times of the day with high evaporative demand (Schoppach et al., 2014a) or at night (Schoppach et al., 2014b; Claverie et al., 2018). Other research suggests yield benefits resulting from auxin accumulation in the roots, although the link with whole-plant hydraulics was not documented. On maize, Li et al. (2018) found that maize mutants overexpressing ZmPIN1a exhibited an increase in auxin export from the shoot to the root where its accumulation in the root tips drove enhanced root development that was associated with increased yields under drought, probably through improved water acquisition. In wheat, the overexpression of the auxin biosynthetic gene TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2.1-3A was associated with enhanced grain yield under various nitrogen supply levels that was likely to be the result of enhanced lateral root branching that promoted nitrogen foraging capabilities. Importantly, this gene was found to be predominantly expressed in the roots, and its overexpression generated elevated auxin accumulation in the primary and lateral root tips (Shao et al., 2017). Finally, on sorghum, multiple QTLs controlling stay-green, a trait that is linked to tolerance to terminal drought, have been found to co-localize with markers associated with an auxin-responsive gene (indole-3-acetic acid-amido synthetase GH3.5; Rama Reddy et al., 2014) or with genes from the PIN family of auxin efflux carriers (Borrell et al. 2015).
Taken together, these results indicate that root auxin accumulation drives yield increases under drought either via a dynamic regulation of root hydraulic conductivity in order to express a water-saving behaviour or via a developmental control of branching to optimize water capture, two strategies that have been proven to be widely effective in drought breeding (e.g. Wasson et al., 2012; Vadez 2014).
Caveats and limits
This study suggests an important role for root IAA in controlling daytime and nocturnal water use, but does not necessarily disprove the hypothesis that root and shoot ABA are also involved in regulating whole-plant TR under increasing VPD as previously found in durum wheat (Kudoyarova et al., 2011). Indeed, in this investigation, root ABA levels were below the detection threshold of the method used for quantification, which is around 10 pmol g–1 f. wt, while in the study of Kudoyarova et al. nominal root ABA concentrations were equivalent to 6.8 pmol g–1 f. wt. However, it is noteworthy that in the study of Kudoyarova et al. (2011), experiments were carried on another species, (durum wheat), on young seedlings (7 d old) and using a different technique (immunoassay). While always possible, an error in the protocol used in this study is unlikely, considering the consistency of lack of detection of root ABA and the consistent levels of root IAA across independent experiments and genotypes.
Another potentially confounding effect in this research was that leaf-level determinations of IAA and ABA concentrations were made on the basis of single leaves, while root concentrations were made on the basis of the bulk root system. While we harvested tissue segments from leaves of similar age and positions in the upper layer of the canopy, it is possible that leaf-to-leaf variation masked correlations between leaf auxin or ABA and the examined traits. That said, whole-root system hormonal dosage does not necessarily guarantee stable, consistent measurements, since roots of different ranks, age and microenvironment are not necessarily expected to exhibit the same hormonal concentrations. In any case, further studies are required to shed light on (1) the exact mechanisms controlling auxin redistribution from the shoot to the root; (2) the interplay between root auxin accumulation and root/shoot hydraulic properties; and (3) the role played by interactions with other hormones such as ABA, ethylene and cytokinin.
Conclusions
This study indicates that variation in whole-plant transpiration responses to evaporative demand, a major trait that drives the expression of water-saving, drought tolerance strategies in wheat, is potentially controlled by auxin levels in the root system. Specifically, root auxin accumulation was found to be negatively correlated with instantaneous TR, the slope of whole-plant TR response to VPD and plant hydraulic conductance. Furthermore, we unravelled a previously undocumented association between root auxin and nocturnal water use in a way that is consistent with its role as a negative regulator of hydraulic conductance.
Those findings shed light on potentially important roles of root auxin in regulating daytime and nocturnal water use that are consistent with previous physiological, genetic and molecular evidence established on the studied population. They are also in line with evidence from other sources documenting a role for root auxins in regulating hydraulic conductivity and enhancing crop yields under water-limited environments. While this study suggests that root auxin levels might be a stable trait that could predict drought tolerance capabilities of wheat genotypes, further investigation is needed to link auxin accumulation and its hydraulic and developmental consequences mechanistically at the local and the whole-plant levels and in relation to other hormones.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: diversity in the slopes of whole-plant transpiration rate response curves to naturally increasing vapour pressure deficit within the population of 143 double haploid lines from the RAC875 × Kukri cross and among the eight lines selected lines. Figure S2: illustration of the examined leaf anatomical traits among the eight lines of the study.
FUNDING
This research was funded in part by the Belgian National Fund for Scientific Research (FNRS, contract no. 1.E038.13), the Minnesota Agricultural Experiment Station (MAES, project no. MIN-13-095) and by the National Science Foundation/Civilian Research & Development Foundation (award no. OISE-16-62788-0).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Elisabeth Majerus for her help in preparing leaf sections, and Coline Kinkin for her help in grinding tissue samples for hormone dosage.
LITERATURE CITED
- Alabdallah O, Ahou A, Mancuso N, et al. . 2017. The Arabidopsis polyamine oxidase/dehydrogenase 5 interferes with cytokinin and auxin signaling pathways to control xylem differentiation. Journal of Experimental Botany 68: 997–1012. [DOI] [PubMed] [Google Scholar]
- Berger J, Palta J, Vadez V. 2016. Review: an integrated framework for crop adaptation to dry environments: responses to transient and terminal drought. Plant Science 253: 58–67. [DOI] [PubMed] [Google Scholar]
- Borrell A, Mullet J, George-Jaeggli B, et al. . 2015. Identifying the function of sorghum’s drought tolerance stay-green QTL. Plant and Animal Genome XXIII Conference, January 10–15, San Diego, CA, USA. [Google Scholar]
- Caringella MA, Bongers FJ, Sack L. 2015. Leaf hydraulic conductance varies with vein anatomy across Arabidopsis thaliana wild-type and leaf vein mutants. Plant, Cell & Environment 38: 2735–2746. [DOI] [PubMed] [Google Scholar]
- Claverie E, Meunier F, Javaux M, Sadok W. 2018. Increased contribution of wheat nocturnal transpiration to daily water use under drought. Physiologia Plantarum 162: 290–300. [DOI] [PubMed] [Google Scholar]
- Coupel-Ledru A, Lebon E, Christophe A, et al. . 2016. Reduced nighttime transpiration is a relevant breeding target for high water-use efficiency in grapevine. Proceedings of the National Academy of Sciences, USA 113: 8963–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fàbregas N, Formosa-Jordan P, Confraria A, et al. . 2015. Auxin influx carriers control vascular patterning and xylem differentiation in Arabidopsis thaliana. PLoS Genetics 11: e1005183. doi: 10.1371/journal.pgen.1005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiguitant J, Marrou H, Vadez V, et al. . 2017. Relevance of limited-transpiration trait for lentil (Lens culinaris Medik.) in South Asia. Field Crops Research 209: 96–107. [Google Scholar]
- Howard AR, Van Iersel MW, Richards JH, Donovan LA. 2009. Night-time transpiration can decrease hydraulic redistribution. Plant, Cell & Environment 32: 1060–1070. [DOI] [PubMed] [Google Scholar]
- Kholová J, Hash CT, Kumar PL, Yadav RS, Kocova M, Vadez V. 2010. Terminal drought-tolerant pearl millet [Pennisetum glaucum (L) R. Br.] have high leaf ABA and limit transpiration at high vapour pressure deficit. Journal of Experimental Botany 61: 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoyarova G, Veselova S, Hartung W, Farhutdinov R, Veselov D, Sharipova G. 2011. Involvement of root ABA and hydraulic conductance in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 233: 87–94. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang X, Zhao Y, et al. . 2018. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnology Journal 16: 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CO, Lechowicz MJ. 2007. The ecological and functional correlates of nocturnal transpiration. Tree Physiology 27: 577–584. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Rodrigues O. 2016. Aquaporins and plant transpiration. Plant, Cell & Environment 39: 2580–2587. [DOI] [PubMed] [Google Scholar]
- Messina CD, Sinclair TR, Hammer GL, et al. . 2015. Limited-transpiration trait may increase maize drought tolerance in the US corn belt. Agronomy Journal 107: 1978–1986. [Google Scholar]
- Moreno-Piovano GS, Moreno JE, Cabello JV, Arce AL, Otegui ME, Chan RL. 2017. A role for LAX2 in regulating xylem development and lateral-vein symmetry in the leaf. Annals of Botany 120: 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann RB, Cardon ZG, Teshera-Levye J, Rockwell FE, Zwieniecki MA, Holbrook NM. 2014. Modelled hydraulic redistribution by sunflower (Helianthus annuus L.) matches observed data only after including night-time transpiration. Plant, Cell & Environment 37: 899–910. [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zažímalová E, Ruthardt N, et al. . 2005. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256. [DOI] [PubMed] [Google Scholar]
- Péret B, Li G, Zhao J, Band LR, et al. . 2012. Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biology 14: 991–998. [DOI] [PubMed] [Google Scholar]
- Prinsen E, Redig P, Strnad M, Galís I, Van Dongen W, Van Onckelen H. 1995. Quantifying phytohormones in transformed plants. In: Gartland KMA, Davey MR, eds. Agrobacterium protocols. Totowa, NJ: Springer, 245–262. [DOI] [PubMed] [Google Scholar]
- Prinsen E, van Laer S, Öden S, van Onckelen H. 2000. Auxin analysis. In: Tucker GA, Robert JA, eds. Plant hormone protocols. Totowa, NJ: Humana Press, 49–65. [DOI] [PubMed] [Google Scholar]
- Rama Reddy NR, Ragimasalawada M, Sabbavarapu MM, Nadoor S, Patil JV. 2014. Detection and validation of stay-green QTL in post-rainy sorghum involving widely adapted cultivar, M35-1 and a popular stay-green genotype B35. BMC Genomics 15: 909. doi: 10.1186/1471-2164-15-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson HM, Clarke JM. 1988. Nocturnal transpiration in wheat. Australian Journal of Plant Physiology 15: 397–406. [Google Scholar]
- Rengel Z, Graham RD. 1996. Uptake of zinc from chelate-buffered nutrient solutions by wheat genotypes differing in zinc efficiency. Journal of Experimental Botany 47: 217–226. [Google Scholar]
- Resco de Dios V, Roy J, Ferrio JP, et al. . 2015. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Scientific Reports 5: 10975. doi: 10.1038/srep10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA, Passioura JB. 1989. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research 40: 943–950. [Google Scholar]
- Rowe JH, Topping JF, Liu J, Lindsey K. 2016. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytologist 211: 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohula G, Kupper P, Räim O, Sellin A, Sõber A. 2014. Patterns of night-time water use are interrelated with leaf nitrogen concentration in shoots of 16 deciduous woody species. Environmental and Experimental Botany 99: 180–188. [Google Scholar]
- Schoppach R, Sadok W. 2012. Differential sensitivities of transpiration to evaporative demand and soil water deficit among wheat elite cultivars indicate different strategies for drought tolerance. Environmental and Experimental Botany 84: 1–10. [Google Scholar]
- Schoppach R, Wauthelet D, Jeanguenin L, Sadok W. 2014a Conservative water use under high evaporative demand associated with smaller root metaxylem and limited trans-membrane water transport in wheat. Functional Plant Biology 41: 257–269. [DOI] [PubMed] [Google Scholar]
- Schoppach R, Claverie E, Sadok W. 2014b Genotype-dependent influence of night-time vapour pressure deficit on night-time transpiration and daytime gas exchange in wheat. Functional Plant Biology 41: 963–971. [DOI] [PubMed] [Google Scholar]
- Schoppach R, Taylor JD, Majerus E, Claverie E, Baumann U, Suchecki R, Fleury D, Sadok W. 2016. High resolution mapping of traits related to whole-plant transpiration under increasing evaporative demand in wheat. Journal of Experimental Botany 67: 2847–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppach R, Fleury D, Sinclair TR, Sadok W. 2017. Transpiration sensitivity to evaporative demand across 120 years of breeding of Australian wheat cultivars. Journal of Agronomy and Crop Science 203: 219–226. [Google Scholar]
- Shao A, Ma W, Zhao X, et al. . 2017. The auxin biosynthetic TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2.1-3A increases grain yield of wheat. Plant Physiology 174: 2274–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Hammer GL, van Oosterom EJ. 2005. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology 32: 945–952. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Messina CD, Beatty A, Samples M. 2010. Assessment across the United States of the benefits of altered soybean drought traits. Agronomy Journal 102: 475–482. [Google Scholar]
- Sinclair TR, Devi J, Shekoofa A, et al. . 2017. Limited-transpiration response to high vapor pressure deficit in crop species. Plant Science 260: 109–118. [DOI] [PubMed] [Google Scholar]
- Sivasakthi K, Tharanya M, Kholová J, Muriuki RW, Thirunalasundari T, Vadez V. 2017. Chickpea genotypes contrasting for vigor and canopy conductance also differ in their dependence on different water transport pathways. Frontiers in Plant Science 8: 1663. doi: 10.3389/fpls.2017.01663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solh M, Van Ginkel M. 2014. Drought preparedness and drought mitigation in the developing world’s drylands. Weather and Climate Extremes 3: 62–66. [Google Scholar]
- Song W, Sun H, Li J, et al. . 2013. Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen. Annals of Botany 112: 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Toshio T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. 2006. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. Journal of Experimental Botany 57: 2259–2266. [DOI] [PubMed] [Google Scholar]
- Taneda H, Terashima I. 2012. Co-ordinated development of the leaf midrib xylem with the lamina in Nicotiana tabacum. Annals of Botany 110: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadez V. 2014. Root hydraulics: the forgotten side of roots in drought adaptation. Field Crops Research 165: 15–24. [Google Scholar]
- Vandeleur RK, Sullivan W, Athman A, et al. . 2014. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant, Cell & Environment 37: 520–538. [DOI] [PubMed] [Google Scholar]
- Veselov DS, Sharipova GV, Veselov SY, Dodd IC, Ivanov I, Kudoyarova GR. 2018. Rapid changes in root HvPIP2;2 aquaporins abundance and ABA concentration are required to enhance root hydraulic conductivity and maintain leaf water potential in response to increased evaporative demand. Functional Plant Biology 45: 143–149. [DOI] [PubMed] [Google Scholar]
- Wasson AP, Richards RA, Chatrath R, et al. . 2012. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63: 3485–3498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.