Abstract
Background and Aims
Root hairs are single-cell extensions of the epidermis that face into the soil and increase the root–soil contact surface. Root hairs enlarge the rhizosphere radially and are very important for taking up water and sparingly soluble nutrients, such as the poorly soil-mobile phosphate. In order to quantify the importance of root hairs for maize, a mutant and the corresponding wild type were compared.
Methods
The rth2 maize mutant with very short root hairs was assayed for growth and phosphorus (P) acquisition in a slightly alkaline soil with low P and limited water supply in the absence of mycorrhization and with ample P supply.
Key Results
Root and shoot growth was additively impaired under P deficiency and drought. Internal P concentrations declined with reduced water and P supply, whereas micronutrients (iron, zinc) were little affected. The very short root hairs in rth2 did not affect internal P concentrations, but the P content of juvenile plants was halved under combined stress. The rth2 plants had more fine roots and increased specific root length, but P mobilization traits (root organic carbon and phosphatase exudation) differed little.
Conclusions
The results confirm the importance of root hairs for maize P uptake and content, but not for internal P concentrations. Furthermore, the performance of root hair mutants may be biased by secondary effects, such as altered root growth.
Keywords: Macronutrients, micronutrients, phosphate, fine roots, water, rhizosphere
INTRODUCTION
In low-phosphate soils and under dry conditions, root hairs comprise a cheap, ‘cost’-effective strategy to increase plant uptake of the sparingly soluble phosphate and water from soils (Jungk, 2001; Lynch and Ho, 2005; Brown et al., 2013). Water enters roots primarily in the root hair zone and is directly taken up via root hairs (Cailloux, 1972). The water (and nutrient) taken up via root hairs may, however, make only a limited contribution to transpiration during the day (Segal et al., 2008).
The contribution of long and dense root hairs to depleting Olsen P from soil was initially estimated with diverse wheat and barley genotypes (Gahoonia et al., 1997). A spontaneous barley mutant without root hairs (brb) depleted less P from the soil and was less competent on low-P soil compared with the wild type (Gahoonia et al., 2001). When P-limited barley was exposed to drought, deficiency symptoms and growth retardation were most severe in plants lacking root hairs, confirming the synergism in nutrient and water uptake in this species (Brown et al., 2012). Interestingly, at low P supply, root-hair-deficient barley mutants tended to have elevated internal P concentration, although this effect was not significant (Brown et al., 2012). Furthermore, the brb barley root-hair-deficient mutant was impaired in water uptake from drying soil, at least at high evaporative demand (Carminati et al., 2017) but less at low evaporative demand (Dodd and Diatloff, 2016).
In addition to root hairs, symbiotic vesicular arbuscular mycorrhiza help to acquire P and water via extension of the below-ground uptake surface. Mycorrhiza may compensate for root hairs, if successfully established. Mycorrhization reduced root hair density and length in wild-type maize by ~40 % (Kothari et al., 1990). Furthermore, seedling root hairs are thought to help mechanical root anchorage during establishment and root tip penetration into compacted soil (Bengough et al., 2011). Finally, root hairs are crucial for the interaction with rhizobacteria and may serve as entry points for endophytes (Prieto et al., 2011).
The patterning of root hairs in the rhizodermis and root-hair-specific gene expression are best characterized in Arabidopsis (Lan et al., 2013) and experiments with this species and mutants have suggested that root hairs provide a competitive advantage in mixed cultures in low-P soil (Bates and Lynch, 2001). As P in soil is hardly mobile and typically only enriched in the upper soil layers, root hairs are especially effective in soil fractions close to the surface. Thus, their functional contribution cannot be viewed independently of other root architectural and morphological features (Lynch, 2013).
The maize crop is especially sensitive to P deficiency in juvenile phases, after internal stores have been used up (Nadeem et al., 2011). The internal P stores are large enough to provide sufficient P for several days, but a proteomic study revealed that phosphate uptake transporters were already more abundant in root hairs from just germinated seedlings exposed to a nutrient solution lacking P (Li et al., 2015).
We hypothesized that the loss of root hairs increases the severity of P deficiency in maize and strongly represses plant growth under drought, especially when combined with low P availability. The root-hair-defective maize mutant rth2 (Wen and Schnable, 1994) was used to estimate the importance of maize root hairs under low P availability and drought. Importantly, a soil mix was used in which mycorrhization was essentially absent (Neumann, 2007), as mycorrhization might compensate for the loss of root hairs (Kothari et al., 1990). We observed that juvenile shoot P concentrations were strongly decreased by the stresses, but were unaffected by the very short root hairs in rth2, irrespective of water limitation or P level. However, plant growth and plant P content (the product of concentration and biomass) were severely compromised by very short root hairs under combined drought and low P. Interestingly, rth2 roots also had more fine roots.
MATERIALS AND METHODS
Plant material
We used the maize inbred line B73 as wild type and the rth2 mutant, which was backcrossed to B73 more than seven times and is therefore nearly isogenic (Wen and Schnable, 1994). The seeds were surface-sterilized by rinsing them for 2 min in a 10 % H2O2 solution and were then placed in a 10 mm CaSO4 solution for 24 h. Seeds were put between foam sheets soaked in a 3 mm CaSO4 solution for 4 d to germinate and were then transferred gently to soil or nutrient solutions.
Growth experiments in soil
Plants were grown in 5-L ceramic, cylindrical Mitscherlich pots (diameter 20 cm, height 18 cm) in the greenhouse (48°42′41.04″ N, 9°12′34.20″ E) in a warm spring and were harvested after 44 d. For the first experiments, a long-term-stored nutrient-poor subsoil (pH 7.6, Corg <0.3.%, CaCO3 30 %) with the following mineral concentrations was used (mg kg−1): 7.9 total P; 59.9 Ca; 11.3 Mg; 15 Mn; 7.8 Fe; 0.6 Zn; 0.2 B; and 0.7 Cu (VDLUFA, 1997). This subsoil was mixed with quartz sand (17 % w/w) and fertilized with (mg kg−1 soil): 200 NH4NO3; 200 K2SO4; 100 MgSO4; 1.9 Fe-Sequestrene; 2.6 ZnSO4; 1 CuSO4; and 37.5 (−P) or 150 (+P) Ca(H2PO4)2. The rationale for using this carbonate-rich subsoil mix was its very low basal nutrient content and especially low P availability. Carbonate-rich soils may limit plant P availability and growth even when the soil pH is adjusted by liming to an optimal pH of 6–7 (Rothwell et al., 2015).
Plants were additionally fertilized at day 17 with 100 mg nitrogen kg−1 substrate and 2.6 mg Zn kg−1 at day 27. In this subsoil mix, mycorrhization was previously checked in hundreds of samples over several years and was essentially absent across a wide array of plant species, including maize (Neumann, 2007). The absence of mycorrhiza was confirmed in our samples by using trypan blue stain (Neumann, 2007).
Each pot contained one plant and 6 kg of substrate. For the well-watered control (W+), the 15 % initial soil water content (total water-holding capacity 30 %) was raised at day 11 to 20 % water. For the drought treatments, the plants were gradually exposed to less water to allow adaptation to the dry conditions. These plants were treated like the controls until day 19, when water was reduced to 15 % and at day 27 further to 12 %. The soil moisture level was gravimetrically measured and adjusted on a daily basis.
For later experiments a nutrient-rich peat soil (Einheitserde type T, Einheitserde- und Humuswerke, Sinntal-Jossa, Germany) was mixed with 15 % of the loamy loess subsoil and 10 % sand in 5-L Mitscherlich pots, to obtain a substrate with high P availability. Soil P was 130 mg kg−1, pH 6, and plants on this substrate were additionally fertilized twice with 100 mg kg−1 P (as Ca(H2PO4)2) in the last two growing weeks.
Growth experiments in nutrient solution
Hydroponic plants were grown for 6 weeks in a climate chamber at 24 °C, 60 % humidity and photosynthetically active photon flux density of 400 µmol m−2 s−1 for 14 h. Hydroponics started with six seedlings per pot in pots containing 2.8 L of a diluted maize nutrient solution, containing 0.1 mm K2SO4, 0.12 mm MgCl2, 0.5 mm Ca(NO3)2, 200 µm KH2PO4, 0.2 µm H3BO3, 0.1 µm MnSO4, 0.1 µm ZnSO4, 0.04 µm CuSO4 and 2 nm (NH4)6Mo7O24. Three days later the seedlings were separated into two seedlings per pot and the macronutrient concentrations were increased to 0.5 mm K2SO4, 0.6 mm MgCl2, 2.5 mm Ca(NO3)2 and 0.1 mm KH2PO4. The KH2PO4 concentration was progressively raised to 0.2 mm and finally to 0.5 mm in week 4, while micronutrients were 1 µm H3BO3, 0.5 µm MnSO4, 0.5 µm ZnSO4, 0.2 µm CuSO4, 0.01 µm (NH4)6Mo7O24 and 100 µm Fe-Sequestrene, which was raised to 200 µm at the first nutrient solution change and to its final amount of 300 µm at the second solution change. The first nutrient solution change was done after 1 week and from then on every 3 d until harvest.
Elemental analysis
Element concentrations of P, Zn and Fe were determined from oven-dried (60 °C) shoot material that was ground to fine powder. Half a gram of shoot dry matter was incubated with 5 mL of HNO3, 4 mL of H2O2 and 2 mL of distilled water in a microwave (MLS Maxi 44, Germany) at a maximum of 210 °C and 1400 W for 65 min. This solution was adjusted to 20 mL and filtered over activated charcoal and through 90-µm mesh filter paper. Concentrations of Zn and Fe were determined by atomic absorption spectroscopy (AAS, ATI Unicam Solaar 939; Thermo Electron, USA). Before measuring Fe concentrations, caesium chloride and lanthanum chloride buffer (Merck, No. 116755) was added at 1:50 ratio to eliminate spectral interferences. Phosphorus was measured spectrophotometrically via orthophosphate determination after addition of molybdate–vanadate reagent (Gericke and Kurmies, 1952).
Root analyses
Root dry biomass, specific root length (total root length per unit dry mass) and root diameter fractions were measured after washing the soil from the roots. To determine root traits, the roots were scanned and digitized at a resolution of 600 dpi and analysed with WinRHIZO (Regent Instruments Inc., Canada) software. Mycorrhization of root samples in the carbonate-rich soil–sand mix was checked with classical trypan blue staining methods and confirmed to be essentially absent (Neumann, 2007).
Exudate analyses and phosphatase activity
Exudates were collected from 5-week-old hydroponically grown plants that were P-starved for 2 d before sampling. Collection was for 1 h in 1-L pots containing sufficient 1 mm CaSO4 solution that all roots were completely covered by the buffer solution. The total carbon in exudates was quantified with a total organic carbon analyser (Elementar). Phosphatase activities were photometrically quantified in 1250-µL extracts shaken for 1 h at 27 °C at pH 5.3 using p-nitrophenyl phosphate (Tabatabai and Bremner, 1969).
Statistical analyses
A three-way ANOVA and pairwise Tukey tests were used to investigate genotype ζ environment interactions between wild type, rth2 mutant and different treatments. Data are given as mean ± s.d.
RESULTS
Maize biomass under drought, low P and combined stress
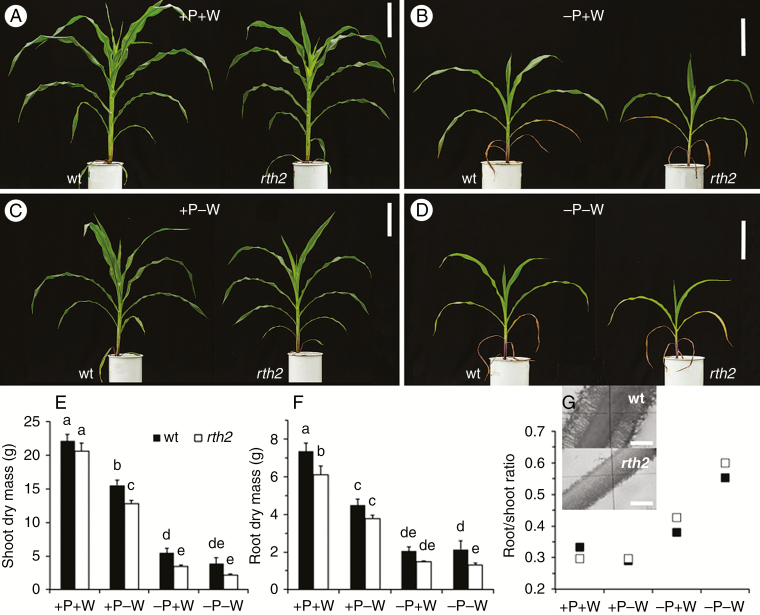
Maize plants were grown with two different water and P supply levels under greenhouse conditions for 6 weeks. Representative maize plants are shown in Fig. 1A–D and indicate that mutant shoots grew similarly under optimal conditions, but were smaller with increasing stress compared with the wild type. The unstressed control plants were largest, while drought (−W) reduced the wild-type and rth2 mutant shoot biomass by 30 and 38 %, respectively. The root dry biomass in −W was 39 and 38 % lower than in controls, respectively.
Fig. 1.
Phenotype and biomass of 5-week-old maize plants. (A–D) Representative shoots (wt, wild type, left; rth2, root hair defective2, right) with (A) optimal P nutrition and water (+P+W), (B) low P but water (−P+W), (C) optimal P but low water (+P−W) and (D) low P and low water supply (−P−W). Scale bars = 20 cm. (E) Dry shoot, (F) root biomass and (G) root/shoot ratio under different P and water levels. The inset in G shows primary roots of both genotypes with root hairs at day 7 on filter paper. Significant differences (P < 0.01) are indicated by different letters above columns.
The low-P (−P) treatment severely decreased wild-type shoot biomass by 75 % and that of the mutant by 83 %, while root dry mass was reduced in the wild type and mutant by 72 and 76 %, respectively. Shoot size and dry shoot and root biomass of the rth2 mutant tended to be always smaller than in the wild type, although this was not always statistically significant in all treatments (Fig. 1E, F). The combined stress did not further reduce shoot and root biomass. The root/shoot ratio was little affected by drought as the only stress, but this ratio was higher in −P, especially when plants were additionally exposed to drought (Fig. 1G). The root/shoot ratio was higher in the mutant than in the wild type in −P, potentially indicating more investment in root growth in the mutant (Fig. 1G).
The rth2 mutant was not completely devoid of root hairs, but short root hairs with similar density were detected, although their length was reduced by >80 % in the primary root just after germination (inset in Fig. 1G). This decrease in total root hair length was somewhat less at later growth stages and in seminal roots, but very short root hairs were consistently observed on all rth2 root types. While wild-type root hairs at harvest in soil were on average 0.84 mm in length, the root hairs of the rth2 mutant were only ~0.19 mm long (Weber et al., 2018).
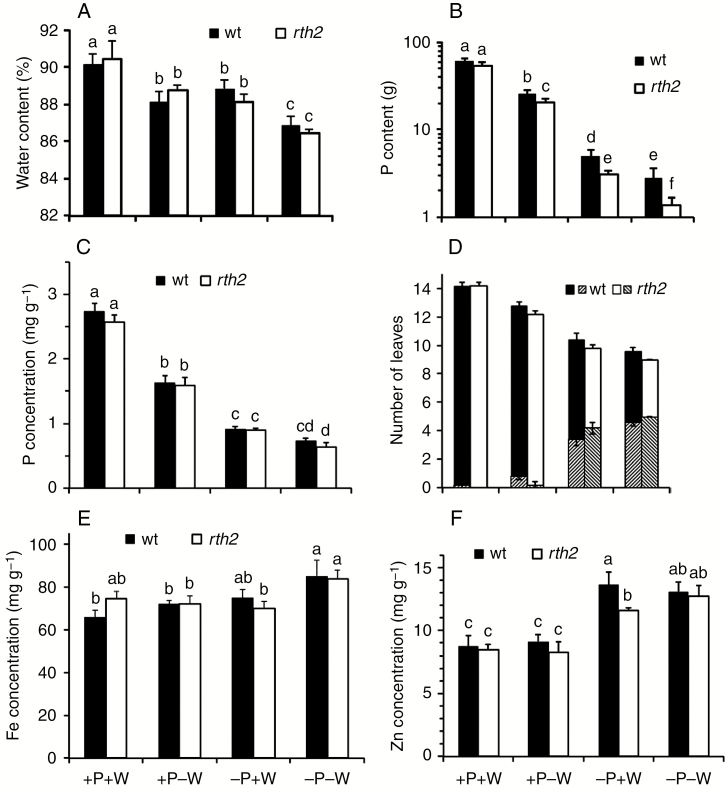
Effect of water supply, P level and root hairs on shoot water content and nutrients
Both genotypes contained ~90 % water in their fresh shoot biomass under control conditions (Fig. 2A), but both the wild type and the mutant had marginally reduced water content, at about 88–89 %, in the fresh shoot biomass under drought. The same lower water content was measured in −P, while the water content was further reduced in −P−W to ~86 % in both genotypes (Fig. 2A).
Fig. 2.
Water and P contents, nutrient concentrations and visible deficiency symptoms with different P and water supply. (A) Water content (%) at harvest. (B) Shoot P content (note logarithmic scale) and (C) shoot P concentrations of wild type (wt) and rth2. (D) Total numbers of leaves and (hatching) number of leaves that were >50 % chlorotic or necrotic. (E) Shoot Fe and (F) shoot Zn concentrations. Significant differences (P < 0.01) are indicated by different letters.
Shoot P content per plant (product of P concentration and dry biomass) followed the same trend as shoot biomass (Figs 1E and 2B). Except for the well-supplied condition, the mutant had a lower shoot P content, which is commonly taken as evidence for the importance of root hairs in taking up P (Zhu et al., 2010; Brown et al., 2012). This increased P content in the wild type relative to the mutant was only due to higher biomass of these plants, as the wild-type and mutant shoot P concentrations were indistinguishable in each condition (Fig. 2C). By contrast, the shoot P concentration of both genotypes in –W was below the sufficiency threshold (around 2.5 mg g−1). The P concentration was further drastically lowered in –P and was lowest in the −P−W condition. Wild-type and rth2 plants were progressively delayed in development under increasing stress, as both genotypes had fewer leaves. In −P, these plants also showed necrotic old leaves, the typical visible signs of P starvation (Fig. 2D). The severity of this symptom was greater in the mutant, suggesting that although shoot P concentration was identical to that in the wild type, the mutant suffered more under the −P condition.
Since root hairs are also considered important for the uptake of sparingly soluble micronutrients, such as Fe and Zn, the concentrations of these elements were also measured. While the Fe concentrations were increased in the most severe stress condition, the Zn concentration was higher in −P. However, except for the Zn concentration in –P with sufficient water supply, the mutant and wild-type element concentrations matched and were independent of root hairs (Fig. 2E, F).
The relationship and interaction of individual factors (P supply, water supply and genotype) with plant biomass, root/shoot ratio and nutrient concentrations are summarized in Table 1. The P supply significantly affected root and shoot dry biomass, root/shoot ratio and P, Zn and Fe concentrations. Drought also affected all these parameters, except for Zn concentration. Genotype, i.e. whether long root hairs were present or not, significantly affected plant biomass but not internal nutrient concentrations (Table 1). Most interestingly, interactions between factors were only significant for the combination of limited water and P supply, which affected root and shoot biomass, root/shoot ratio and P concentrations. Phenotypic plasticity for these factors, also called genotype × environment interaction, however, was entirely absent (Table 1).
Table 1.
Factorial analysis (three-way ANOVA)
| DM shoot | DM root | R/S ratio | P (g g–1) | Zn (g g–1) | Fe (g g–1) | |
|---|---|---|---|---|---|---|
| P | * | * | * | * | * | * |
| W | * | * | * | * | ns | * |
| G | * | * | ns | ns | ns | ns |
| P × W | * | * | * | * | ns | ns |
| P × G | ns | ns | ns | ns | ns | ns |
| W × G | ns | ns | ns | ns | ns | ns |
| P × W × G | ns | ns | ns | ns | ns | ns |
Main effects and significant interactions (P < 0.05) are indicated with an asterisk. ns, interaction not significant.
P, phosphate supply; W, water supply; G, genotype; DM, dry mass; R/S, root shoot ratio.
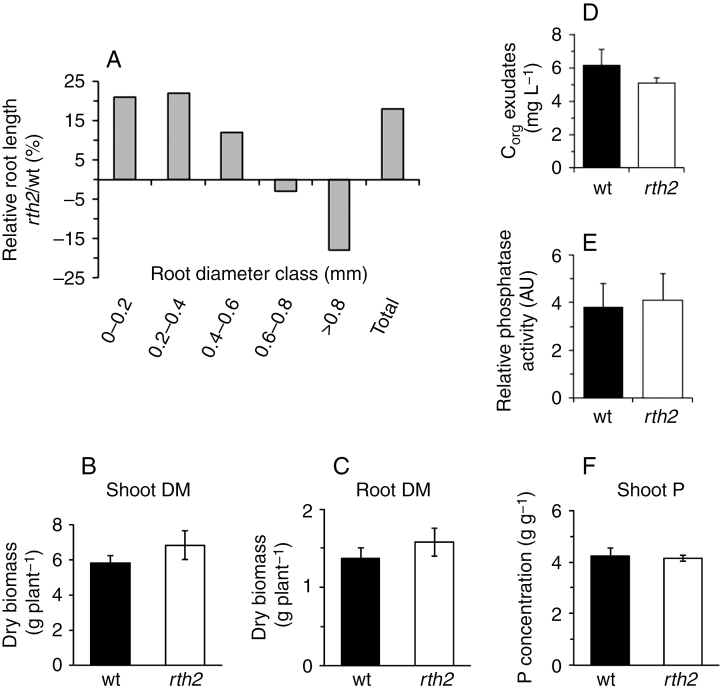
Very short root hairs are associated with more fine roots
We then quantified root length in different diameter classes, to test whether the mutant had altered root morphology, in addition to root hair phenotype. Most of the roots were fine roots of diameter <0.2 mm, which represent lateral roots (Tai et al., 2016). Despite substantial variation amongst individual plants, rth2 clearly tended to have more lateral roots, independent of the P supply (Figs 2B and 3A). We did not detect differences between the tested conditions, probably because the variance among the samples was relatively large. Therefore only the common trend for all roots per genotype is shown. Thinner root fractions in rth2 were observed despite the overall reduced root dry biomass (Fig. 1F), suggesting a morphological switch to the production of thinner roots and an increase in specific root length (length per unit root dry mass).
Fig. 3.
Compensation for root hair loss by fine roots. (A) Relative amounts of roots of different diameters in mutant (rth2) and wild type (wt), averaged across all treatments in soil. (B) Shoot and (C) root dry biomass (DM) in full-nutrient hydroponics. (D) Corg released and (E) phosphatase activity for hydroponic, P-starved roots. AU, arbitrary units. (F) Shoot P concentrations in hydroponics. Black bars, wild type; white bars, rth2. Mean ± s.d. is shown for all values.
In a second experiment the growth of wild-type and rth2 plants was compared in another, slightly acidic, but nutrient-rich soil substrate with contrasting texture and sufficient moisture, as well as in hydroponics, since root hairs may represent an extra metabolic cost to the plant in P-rich, moist conditions. In the nutrient-rich substrate, we failed to identify significant genotypic differences between the shoot and root biomass, as well as in the shoot P concentration after 5 weeks, in agreement with the +P+W conditions in the first experiment. Furthermore, in hydroponics, where both genotypes had the same, unrestricted access to water and otherwise immobile nutrients, the rth2 mutant accumulated slightly, but significantly, higher shoot and root biomass (Fig. 3B, C).
Furthermore, root hairs participate in releasing protons, sugars and organic anions from roots, to feed the soil microflora and mobilize P from sparingly soluble soil fractions (Holz et al., 2018). Short-term carbon release from the roots into the nutrient solution, which accounts for the sum of all organic solutes released, was therefore quantified in hydroponically grown plants after 2 d of P starvation. However, the released total Corg was similar in the plants without root hairs (Fig. 3D). Likewise, the (low) phosphatase activity of P-starved roots was also similar in the two genotypes (Fig. 3E), suggesting minor effects of the very short root hairs on rhizosphere P mobilization processes, at least when plants were grown in hydroponics. High shoot P concentrations occurred after 5 weeks of growth under luxury P supply in hydroponics, as expected, with no differences between mutant and the wild type (Fig. 3F).
DISCUSSION
This study confirms the importance of root hairs for water and P uptake in dry conditions and when P bioavailability is low in maize, similar to results in other species (Jungk, 2001; Lynch and Ho, 2005; Brown et al., 2013). The bald root barley (brb) line, a root-hairless mutant (Gahoonia et al., 2001), has frequently been used to analyse the function of crop root hairs (Zuchi et al., 2011; Holz et al., 2018). However, the genetic cause of the brb phenotype is unknown and it was recently reported that in this mutant other plant properties are also affected, besides the formation of root hairs. Indeed, brb shows increased root growth in dry soil, which compensated for the surface loss of root hairs (Dodd and Diatloff, 2016). This was apparently P-independent and young (non-tillering) plants of this mutant had shoot growth similar to that of the wild type, in both low- and high-P soil (Dodd and Diatloff, 2016). A somewhat different genetic compensation of root growth was found for the maize rth2 mutant. The fine root fraction was rather constitutively increased, independently of the stress, but the rth2 mutant had less total root biomass (Fig. 1). Although we cannot rule out that the genetic defect in rth2 also affects other processes in addition to root hairs, our observation that P concentrations in the mutant are not affected suggests that the metabolism may be little different in the mutant. Importantly, mycorrhization was absent in our set-up, excluding the possibility that fungal symbioses compensated or aggravated the lack of root hairs (Zhu et al., 2010; Brown et al., 2012).
Under P limitation, as well as under drought stress in the carbonate-rich soil, the rth2 mutant performed worse than the wild type. Thus, growth of rth2 was most drastically impaired under combined stress with low P and drought (Fig. 1), in agreement with the substantial importance of root hairs for P and water uptake in maize under low availability. Indeed, the longer root hairs and root hairs that were induced by low P conferred a yield advantage on maize plants grown in low-P environments (Zhu et al., 2010). The limited P and water availability in our experiments strongly reduced internal shoot P concentrations below the critical value of 2.5 mg g−1 and induced P deficiency symptoms in the plants, such as darker coloration of young leaves and necrotic old leaves (Jones et al., 1991; Bergmann, 1993). The P content per plant (the product of concentration and dry biomass per plant) was higher for the wild type compared with the mutant in each condition, indicating how much the root hairs contributed to P uptake: +14 % in +P+W, +24 % in +P-W, +61 % in −P+W and +103 % in −P−W. The shoot P concentration, typically used by farmers, researchers and the plant itself to estimate crop nutritional status, did not indicate that rth2 was more deficient than the wild type. Indeed, the inverse of the P concentration is proportional to P utilization in plant tissue, i.e. how much biomass is generated at a given tissue P supply. This was apparently unchanged in the mutant, indicating that at least this aspect of metabolism was similar in rth2 and the wild type, at least when the wild type and mutant were exposed to the same external P concentration. The P content, by contrast, a measure of how much total P was acquired at a given external supply, indicated the massive growth benefit conferred by the root hairs (Fig. 2), especially under combined stress. Non-significant differences in internal P concentrations among barley root hair mutants and wild type (higher P concentrations in mutants) were also reported in barley, but these did not drop below 2 mg g−1 (Brown et al., 2012).
At higher soil P levels, a large collection of modern maize hybrids differed substantially in their juvenile P concentrations, but this poorly predicted (rp = 0.04) their final yield (Melchinger et al., 2016). Here, the nutrient concentrations indicated that the plants were apparently already P-deficient under drought conditions (although water was probably the growth-limiting factor), in agreement with the idea that deficiencies of soil-immobile nutrients are a rapid consequence of low soil moisture. The poorly soluble micronutrient Zn was also low (deficiency threshold ~10–20 ppm), although substantial Zn fertilizer was added to the alkaline soil. Zinc was increased under the –P condition, which is not uncommon, as Zn is frequently co-mobilized by rhizosphere processes induced by –P (Neumann and Römheld, 2002). Under −P with sufficient water, such rhizosphere processes might be less efficient in the root hair mutant (as the rhizosphere did not extend as deep into the soil as in the wild type), so in low P the Zn concentration was higher in the wild type than in the mutant (Fig. 2). Iron is taken up in maize by an independent phytosiderophore complexation mechanism (Marschner and Römheld, 1984), potentially explaining why root hairs did not affect internal Fe concentrations across environments (Fig. 2).
Plant strategies to adapt to low soil phosphorus bioavailability generally can be divided into ‘foraging’ (by adapting root architecture and morphology) and ‘mobilizing’ (by solubilization of fixed P via rhizosphere processes) strategies (Hinsinger, 2001; Lynch, 2011). Maize is a crop that mainly responds to insufficient P with root architectural changes (Lyu et al., 2016), while this species is thought to invest relatively little in rhizosphere processes, such as proton and organic acid release, secretion of phosphohydrolyses, interaction with rhizosphere microbes, etc. However, substantial organic metabolites are released in hydroponics in a nutrient deficiency-specific way; these comprise amino acids, sugars and organic anions (Carvalhais et al., 2011). The very short root hairs in rth2 were apparently not associated with major differences in some rhizosphere-related processes, such as the sum of organic molecules released and phosphatase activity (Fig. 3).
The mutant apparently had, in addition to the root hair phenotype, altered root architecture. Based on previous analyses of the maize root system, thick roots of diameter >0.8 mm can be classified as primary and shoot-borne roots, while the category of 0.6–0.8 mm comprises the seminal roots and the 0.4- to 0.6-mm class contains a mixture of seminal and lateral roots. Roots thinner than 0.4 mm are lateral roots (Tai et al., 2016), indicating an increased amount of laterals in rth2. Interestingly, genotype × environment interaction was apparently low or absent, suggesting that the effects of the very short root hairs were similar in each environment. Thus, the root architecture changes were seemingly not a response to short root hairs under P-limiting conditions. Importantly, the altered root architecture in rth2 and brb in barley (Dodd and Diatloff, 2016) may also have influenced P uptake, so mechanistic conclusions on root hair function from these mutants must be drawn with care.
The importance of root hairs has been questioned in some rice varieties recently, where root hairs improved P efficiency only in some genotypes (Nestler and Wissuwa, 2016). In a population of native Arabidopsis, root hair density and length responded surprisingly heterogeneously to differential P supply and some genotypes reduced hair length or density when locally lacking P in agar plates (Stetter et al., 2015). However, predictions of root hair behaviour in real soils from root hair density and length on agar plates must be made with great care, as enormous variability in root hair traits was found in single genotypes between synthetic growth substrates and real soils (Nestler et al., 2016). Surprisingly, the mutant performed better than the wild type in well-supplied hydroponics, which may reflect a substantial energetic cost of building root hairs in conditions where they are not required.
Conclusions
Long root hairs were substantially important for growth and P content of maize under drought and low P conditions, but they were dispensable for internal P concentrations and even slightly detrimental in well-supplied conditions. Because the reduction in root hair length in rth2 was associated with secondary effects on root architecture, future experiments on the relevance of root hairs should involve several independent mutants (especially those with complete absence of root hairs). The collection of root hair mutants in maize is growing and it will be interesting to see whether other maize root hair mutants are associated with secondary root phenotypes (Hochholdinger et al., 2018).
ACKNOWLEDGEMENTS
We thank H. Ochott and C. Haake for help with nutrient analyses. U.L. conceived and designed the research. F.K., F.V. and H.B. conducted the experiments. F.K., F.V., B.N., G.N., F.H. and X.L. analysed the data. U.L. wrote the manuscript. All authors read and approved the manuscript. The authors declare that they have no conflict of interest.
LITERATURE CITED
- Bates TR, Lynch JP. 2001. Root hairs confer a competitive advantage under low phosphorus availability. Plant and Soil 236: 243–250. [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA. 2011. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 62: 59–68. [DOI] [PubMed] [Google Scholar]
- Bergmann W. 1993. Ernährungsstörungen bei Kulturpflanzen. Jena: Gustav Fischer. [Google Scholar]
- Brown LK, George TS, Thompson JA, et al. 2012. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Annals of Botany 110: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, George TS, Dupuy LX, White PJ. 2013. A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Annals of Botany 112: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailloux M. 1972. Metabolism and the absorption of water by root hairs. Canadian Journal of Botany 50: 557–573. [Google Scholar]
- Carminati A, Passioura JB, Zarebanadkouki M, et al. 2017. Root hairs enable high transpiration rates in drying soils. New Phytology 216: 771–781. [DOI] [PubMed] [Google Scholar]
- Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wiren N. 2011. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. Journal of Plant Nutrition and Soil Science 174: 3–11. [Google Scholar]
- Dodd IC, Diatloff E. 2016. Enhanced root growth of the brb (bald root barley) mutant in drying soil allows similar shoot physiological responses to soil water deficit as wild type plants. Functional Plant Biology 43: 199–206. [DOI] [PubMed] [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE. 1997. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant and Soil 191: 181–188. [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. 2001. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant and Soil 235: 211–219. [Google Scholar]
- Gericke S, Kurmies B. 1952. Die kolorimetrische Phosphorsäurebestimmung mit Ammonium–Vandadat–Molybdat und ihre Anwendung in der Pflanzenanalyse. Zeitschrift für Pflanzenernährung, Düngung und Bodenkunde 59: 235–247. [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237: 173–195. [Google Scholar]
- Hochholdinger F, Yu P, Marcon C. 2018. Genetic control of root system development in maize. Trends in Plant Science 23: 79–88. [DOI] [PubMed] [Google Scholar]
- Holz M, Zarebanadkouki M, Kuzyakov Y, Pausch J, Carminati A. 2018. Root hairs increase rhizosphere extension and carbon input to soil. Annals of Botany 121: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JB, Wolf B, Mills HA. 1991. Plant analysis handbook: a practical sampling, preparation, analysis, and interpretation guide. Athens, GA: Micro-Macro Publishing. [Google Scholar]
- Jungk A. 2001. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science 164: 121–129. [Google Scholar]
- Kothari SK, Marschner H, George E. 1990. Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytology 116: 303–311. [Google Scholar]
- Lan P, Li W, Lin WD, Santi S, Schmidt W. 2013. Mapping gene activity of Arabidopsis root hairs. Genome Biology 14: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Phillip D, Neuhauser B, Schulze WX, Ludewig U. 2015. protein dynamics in young maize root hairs in response to macro- and micronutrient deprivation. Journal of Proteome Research 14: 3362–3371. [DOI] [PubMed] [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Ho MD. 2005. Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil 269: 45–56. [Google Scholar]
- Lyu Y, Tang H, Li H, et al. 2016. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Frontiers in Plant Science 7: 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H, Römheld V. 1984. Strategies of plants for acquisition of iron. Plant and Soil 165: 261–274. [Google Scholar]
- Melchinger AE, Utz FH, Bay A, Mirdita V, Ludewig U. 2016. Silage yield and quality traits in elite maize hybrids and their relationship to elemental concentrations in juvenile plants. Plant Breeding 135: 55–62. [Google Scholar]
- Nadeem M, Mollier A, Morel C, Vives A, Prud’homme L, Pellerin S. 2011. Relative contribution of seed phosphorus reserves and exogenous phosphorus uptake to maize (Zea mays L.) nutrition during early growth stages. Plant and Soil 346: 231–244. [Google Scholar]
- Nestler J, Wissuwa M. 2016. Superior root hair formation confers root efficiency in some, but not all, rice genotypes upon P deficiency. Frontiers in Plant Science 7: 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler J, Keyes SD, Wissuwa M. 2016. Root hair formation in rice (Oryza sativa L.) differs between root types and is altered in artificial growth conditions. Journal of Experimental Botany 67: 3699–3708. [DOI] [PubMed] [Google Scholar]
- Neumann E. 2007. Mycorrhiza technology for sustainable agriculture – results and ideas. Berlin: Mensch & Buch. [Google Scholar]
- Neumann G, Römheld V. 2002. Root-induced changes in the availability of nutrients in the rhizosphere. In: Waisel Y, Eshel A, Kafkafi U, eds. In: Plant roots. The hidden half, 3rd edn New York: Marcel Dekker, 617–649. [Google Scholar]
- Prieto P, Schiliro E, Maldonado-Gonzalez MM, Valderrama R, Barroso-Albarracin JB, Mercado-Blanco J. 2011. Root hairs play a key role in the endophytic colonization of olive roots by Pseudomonas spp. with biocontrol activity. Microbial Ecology 62: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell SA, Elphinstone ED, Dodd IC. 2015. Liming can decrease legume crop yield and leaf gas exchange by enhancing root to shoot ABA signalling. Journal of Experimental Botany 66: 2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E, Kushnir T, Mualem Y, Shani U. 2008. Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone Journal 7: 1027–1034. [Google Scholar]
- Stetter MG, Schmid K, Ludewig U. 2015. Uncovering genes and ploidy involved in the high diversity in root hair density, length and response to local scarce phosphate in Arabidopsis thaliana. PLoS ONE 10: e0120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai MA, Bremner JM. 1969. Use of p-nitrophenyl phosphate for assy of soil phosphatase activity. Soil Biology and Biochemistry 1: 301–307. [Google Scholar]
- Tai H, Lu X, Opitz N, et al. 2016. Transcriptomic and anatomical complexity of primary, seminal, and crown roots highlight root type-specific functional diversity in maize (Zea mays L.). Journal of Experimental Botany 67: 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VDLUFA 1997. Method book III. Darmstadt: VDLUFA. [Google Scholar]
- Weber NF, Herrmann I, Hochholdinger F, Ludewig U, Neumann G. 2018. PGPR-induced growth stimulation and nutrient acquisition in maize: do root hairs matter? Scientia Agriculturae Bohemica 49: 164–172. [Google Scholar]
- Wen TJ, Schnable PS. 1994. Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. American Journal of Botany 81: 833–842. [Google Scholar]
- Zhu J, Zhang C, Lynch JP. 2010. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology 37: 313–322. [Google Scholar]
- Zuchi S, Cesco S, Gottardi S, Pinton R, Romheld V, Astolfi S. 2011. The root-hairless barley mutant brb used as model for assessment of role of root hairs in iron accumulation. Plant Physiology and Biochemistry 49: 506–512. [DOI] [PubMed] [Google Scholar]