Abstract
Background and Aims
Cell wall disassembly occurs naturally in plants by the action of several glycosyl-hydrolases during different developmental processes such as lysigenous and constitutive aerenchyma formation in sugarcane roots. Wall degradation has been reported in aerenchyma development in different species, but little is known about the action of glycosyl-hydrolases in this process.
Methods
In this work, gene expression, protein levels and enzymatic activity of cell wall hydrolases were assessed. Since aerenchyma formation is constitutive in sugarcane roots, they were assessed in segments corresponding to the first 5 cm from the root tip where aerenchyma develops.
Key Results
Our results indicate that the wall degradation starts with a partial attack on pectins (by acetyl esterases, endopolygalacturonases, β-galactosidases and α-arabinofuranosidases) followed by the action of β-glucan-/callose-hydrolysing enzymes. At the same time, there are modifications in arabinoxylan (by α-arabinofuranosidases), xyloglucan (by XTH), xyloglucan–cellulose interactions (by expansins) and partial hydrolysis of cellulose. Saccharification revealed that access to the cell wall varies among segments, consistent with an increase in recalcitrance and composite formation during aerenchyma development.
Conclusion
Our findings corroborate the hypothesis that hydrolases are synchronically synthesized, leading to cell wall modifications that are modulated by the fine structure of cell wall polymers during aerenchyma formation in the cortex of sugarcane roots.
Keywords: Aerenchyma, cell wall composite, glycosyl-hydrolases, proteomics, Saccharum, saccharification
INTRODUCTION
Throughout plant development, several cell wall hydrolytic mechanisms culminate in structural and biochemical alterations in different organs (Grandis et al., 2014). The main alterations occur during xylem formation (Mittler and Lam, 1995), leaf senescence, reproductive organ development (DeLong et al., 1993; Pennell and Lamb, 1997), storage mobilization (Buckeridge et al., 1992; Buckeridge, 2010), fruit ripening (Harker et al., 1997; Brummell, 2006) and lysigenous aerenchyma formation (Visser et al., 2000; Evans, 2003; Leite et al., 2017; Tavares et al., 2018). These processes lead to the establishment of specialized tissues in distinct organs, including leaves, petioles, stems and roots (Visser et al., 2000; Takahashi et al., 2014; Kotula et al., 2017). Aerenchyma can occur in both mature and newly developing roots (Visser and Voesenck, 2004) and is formed by longitudinally interconnected intercellular spaces, which facilitate gas diffusion through the plant tissue (Armstrong, 1979). Air spaces are either formed constitutively or induced by the environment, and both mechanisms require programmed cell death followed by cell wall degradation. Aerenchyma formation has been described in essential crops such as barley (Arikado and Adachi, 1955), wheat (Trought and Drew, 1980), maize (He et al., 1996a; Gunawardena et al., 2001a), rice (Justin and Armstrong, 1991) and sugarcane (Tetsushi and Karim, 2007; Begum et al., 2013; Leite et al., 2017). This process has been considered fundamental for the survival of most of these crop species against the adverse effects of flooding, as it contributes to internal aeration helping to minimize harvest losses (Voesenek and Bailey-Serres, 2015; Wright et al., 2017; Yamaguchi et al., 2018). For rice and sugarcane, aerenchyma formation is constitutive, while in the other species it is induced by abiotic factors (e.g. flooding and exogenous ethylene). Interestingly, the use of the ethylene inhibitor 1-methylcyclopropene (1-MCP) is not sufficient to stop aerenchyma development in sugarcane roots (Tavares et al., 2018). The vast majority of studies on lysigenous aerenchyma address its induction by flooding at specific time points, neglecting root developmental stages (Amstrong, 1979; Justin and Armstrong, 1987; Colmer, 2003; Kotula et al., 2017) and cell wall modifications during development. Also, there is limited literature describing the correlations between cell wall composition and glycosyl-hydrolases during constitutive aerenchyma formation (Shiono et al., 2008; Tavares et al., 2019).
Similar to maize, sugarcane cell walls are described as type II, with the main hemicelluloses composed of mixed-linkage glucan (β-glucan), arabinoxylan and a small amount of xyloglucan. In association with cellulose, this matrix is embedded in a reduced pectic domain consisting of homogalacturonan (HG) and rhamnogalacturonan I (RGI) (Carpita, 1996; De Souza et al., 2013). In sugarcane, hemicelluloses are quantitatively the main component of the cell wall (approx. 60 %), followed by cellulose (30 %), pectins (10 %) and lignin (6 %) (De Souza et al., 2013; Leite et al., 2017). The disassembly of these complex polysaccharides requires specific glycosyl-hydrolases, such as those belonging to the plant glycosyl-hydrolase family 17 (GH17). This extensive protein family contains two distinct enzymes with high structural similarity: (1–3)-β-d-glucanases and (1-3,1-4)-β-d-glucanases (Lashbrook et al., 1994), both abundant in growing cell walls (Cosgrove, 1999) and capable of specifically hydrolysing callose and β-glucan, respectively (Minic and Jouanin, 2006). Three classes of plant enzymes are known to degrade xylan, namely endo-xylanases, β-d-xylosidases and α-l-arabinofuranosidases (Sunna and Antranikian, 1997; Wu et al., 2002; Suzuki et al., 2002; Lee et al., 2001). Modification of xyloglucan is catalysed by xyloglucan endotransglycosylase/hydrolases (XTHs) (Fry et al., 1992), β-d-galactosidase (Edwards et al., 1988), α-l-fucosidase and α-d-xylosidase (Sampedro et al., 2001). Although the degradation of pectins is more complex, involving a larger number of enzymes due to the greater diversity of polysaccharides and their respective covalent bonds in comparison with hemicelluloses, they are also classified into three classes: endo- and exo-polygalacturonases, pectin methyl-esterases and pectate lyases (Seymour and Gross, 1996; Willats et al., 2001; Marín-Rodríguez et al., 2002). Only two enzymes are known to participate in the hydrolysis of RGI, α-l-arabinosidases/β-xylosidases and β-l-galactosidase, which degrade arabinan and galactan, respectively (Li et al., 2001; Esteban et al., 2003; Lee et al., 2003).
Although there is a broad diversity in cell wall enzymes, only a few of them such as cellulases (He et al., 1994, 1996a, b; Bragina et al., 2001; Rahji et al., 2011), XTHs (Saab and Sachs, 1996; Rahji et al., 2011) and endo-polygalacturonases (EPGs; Rahji et al., 2011) were found to have enzymatic activity or transcripts that suggest their presence in the cortex of maize roots during aerenchyma development, whereas the cell walls have been described as fully degraded (He et al., 1994, 1996a; Saab and Sachs, 1996; Bragina et al., 2001; Evans, 2003).
Interestingly, it has recently been shown that cell wall hydrolysis is not completed during aerenchyma formation in sugarcane, but rather is restricted to the degradation of β-glucan and some pectins at the middle lamella (Leite et al., 2017). After pectin hydrolysis from the triangular junctions in the middle lamella, cell separation occurs concomitantly with an increase in the content of arabinoxylan, xyloglucan and cellulose that results in a polymer composite around the air spaces (Leite et al., 2017). Due to this pattern, we became interested in investigating which glycosyl-hydrolases take part in this process and how they act to promote polysaccharide modification during sugarcane root development and aerenchyma formation.
For that, in the present work, we followed gene expression patterns, protein levels and enzyme activities during the process of aerenchyma formation in the cortex of sugarcane roots. The analysis of the parallel events lends support to the hypothesis that glycosyl-hydrolases display a sequence of actions on the cell wall polysaccharides that lead to the formation of a composite made of modified walls in the aerenchyma.
MATERIALS AND METHODS
Sampling of sugarcane roots
Sugarcane culms (‘SP80-3280’) were harvested in Piracicaba, São Paulo, Brazil. Culms were cut with a bandsaw to obtain cuttings with only one bud each. The stalk cuttings were planted in trays containing vermiculite and, after bud outgrowth and early development, the juvenile plants approx. 20 cm tall were transferred to plastic pots (15 L) containing vermiculite and NPK fertilizer (30:20:30). Roots from 3-month-old plants were sampled (between 10.00 and 12.00 h local time) and processed following Leite et al. (2017). The first 5 cm of tiller roots (Supplementary data Fig. S1) were collected and sub-divided into five 1 cm sections from the apex containing the meristem (S1) towards the base (S5) (Fig. 1A). These segments correspond to a range from 0 to 40 % (from meristem to base) of aerenchyma in the root cortex (Leite et al., 2017). Sectioned segments were frozen in liquid nitrogen and stored at –80 °C for enzymatic assays, RNA extraction and proteomics. For saccharification, samples were freeze-dried in a lyophilizer.
Fig. 1.
Aerenchyma development in sugarcane roots. (A) Cross-sections of historesin-embedded root segments. S1 to S5 correspond to segments from the apex to base (1 cm each). Scale bar = 500 μm. (B) Aerenchyma area represented as the percentage of gas spaces in the root cortex. Bars indicate data distribution (n = 8).
Microscopy analysis
Aerenchyma quantification (n = 8) was performed as described in Leite et al. (2017) and expressed as the percentage of cortical cross-sectional area occupied by gas spaces.
The activity of cell wall-degrading enzymes
Frozen ground root material (500 mg) was homogenized in 1 mL of McIlvaine buffer (McIlvaine, 1921) pH 5.0 with polyvinylpolypyrrolidone (PVPP) and phenylmethylsulphonyl fluoride (PMSF), followed by centrifugation (12 000 g, 30 min, at 4 °C). Aliquots of 50 μL of the supernatant were screened for activities against 50 μL of p-nitrophenyl-linked substrates for 20 min or 1 h, depending on the substrate, at 40 °C. Reactions were stopped with 1 mL of 50 mM CaCO3. The following substrates were used: 25 mm 4-nitrophenyl-β-d-galactopyranoside, 25 mm 4-nitrophenyl-β-d-cellobioside, 25 mm 4-nitrophenyl-β-d-xylopyranoside, 25 mm 4-nitrophenyl-β-d-glucopyranoside and 10 mm 4-nitrophenyl-α-l-arabinopyranoside. The absorbance was read at 405 nm and converted to enzyme activity (Alcântara et al., 1999). Total protein quantification was performed according to the Bradford assay (Bradford, 1976) using bovine serum albumin (BSA) as standard, and results are expressed as μmol mg–1 protein min–1. All chemicals were purchased from Sigma Aldrich Merck®.
Quantitative real-time reverse transcription–PCR (qRT–PCR)
Total RNA (200 mg of frozen ground root material) was extracted with 1.5 mL of Trizol (Invitrogen®) following the manufacturer’s instructions. RNA quality and concentration were assessed by gel electrophoresis and Nanodrop (Thermo Fisher Scientific®). cDNA was synthesized from 5 μg of total RNA with Superscript III.
Target glycosyl-hydrolases for primer design were selected among those analysed by Lima et al. (2001) and putative orthologues of upregulated genes during aerenchyma formation in maize (Rajhi et al., 2011). All sequences used for primer design were downloaded from the Sugarcane expressed sequence tag (EST) databank (http://sucest-fun.org/;Vettore et al., 2001). Four references genes were selected from their unaltered expression in all samples and used for calculating transcript levels: ubiquitin-conjugating enzyme (UB) (SCCCLR1048F12.g), 60S ribosomal protein (60S) (SCJFRZ2009G01.g), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Iskandar et al., 2004) and ubiquitin-conjugating enzyme E2 (UBE2) (SCBGLR1002D06.g). Primers were designed using Primer 3 (http://frodo.wi.mit.edu/primer3/) and are listed in Supplementary data Table S1. qRT–PCRs were carried out in triplicate, and gene expression ratios were calculated with reference primers selected by GeNorm-Plus tool using the qBase-Plus 2.0 software (Hellemans et al., 2007).
Protein extraction and digestion for proteomics analysis
Sequential protein extraction modified from Borderies et al. (2003) and Feiz et al. (2006) was performed to identify cell wall proteins from sugarcane root segments (n = 5). Root tissues (500 mg) were extracted with 50 mm sodium acetate buffer pH 6.5 containing 1 mm PMSF and 1 μL of protease inhibitor cocktail (Sigma Aldrich®). Salt solutions (0.15 m NaCl, 1 m NaCl, 0.2 m CaCl2, 50 mm EDTA and 3 m LiCl) were sequentially added twice to fractionate protein samples, yielding 1 mL of five different protein extracts. Extracts were incubated at 5 °C for 5 min under constant stirring and samples were centrifuged at 20 000 g for 30 min at 4 °C.
Protein extracts were dialysed using cellulose membranes (cut-off 12 kDa). The dialysis occurred in distilled water at 5 °C for 16 h and samples were dried in a vacuum concentrator. For total protein quantification, samples were resuspended in 40 μL of the running SDS–PAGE buffer (50 mm Tris pH 6.8, 8 % glycerol, 20 % SDS and 4 % bromophenol with β-mercaptoethanol) except from samples containing LiCl and EDTA that were resuspended in 30 μL of buffer containing 6 m urea, 2 m thiourea and 50 mm ammonium bicarbonate. Protein concentration was determined using the Bradford assay (Bradford, 1976).
Protein extracts from fractions 0.15 m NaCl, 1 m NaCl and 0.2 m CaCl2 were subjected to electrophoresis (12% SDS–PAGE) (Laemmli, 1970). After staining with colloidal Coomassie (Neuhoff et al., 1985), bands were separated into three (<250 to >72 kDa, <72 to >28 kDa and <28 kDa; containing 90 μg of protein) or two (<250 to >36 kDa and <36 kDa, containing 6 μg of protein) bands each cut into 1 × 1 mm pieces for NaCl and CaCl2, respectively. LiCl and EDTA fractions (containing 1.7 μg of protein) had low protein concentration and were pooled and analysed together in solution.
All protein samples were submitted to reduction, alkylation and trypsin digestion either in gel (extract with 0.1 m, 1 m NaCl and 0.2 m CaCl2) (Shevchenko et al., 2006) or in solution (EDTA and LiCl) (Borderies et al., 2003). Sample desalting was performed sequentially in homemade microcolumns packed with octadecyl C18 47 mm (disk solid phase extraction 3M®) and Oligo R3® (Applied Biosystems®), and the eluted peptides were dried in a sample vacuum concentrator for the dimethyl labelling procedure.
Multiplex peptide stable isotope dimethyl labelling
Labelling was optimized for 25 μg of protein and performed as described by Boersema et al. (2009) using three different isotopes: 28 Da, light (S1 and S5); 32 Da, intermediate (S2 and S4); and 36 Da, heavy (S3). For quantitative analysis, samples were divided into two sets (set 1, segments S1, S2 and S3; set 2, segments S3, S4 and S5) and S3 was used for normalization in each set (Supplementary data Fig. S2).
Identification and quantification of proteins by peptide fragmentation
Liquid chromatography–tanden mass spectrometry (LC-MS/MS) analysis was performed on an LTQ-Orbitrap mass spectrometer (Thermo®), equipped with a nanospray ion source (NSI ion source, Thermo®). Peptide separation was carried out using an Ultimate 3000 nano-LC system (Dionex-Thermo®) equipped with a reverse phase high-performance liquid chromatography (RP-HPLC) column (75 μm × 15 cm) packed in-house with C18 resin (Magic C18 AQ 3 μm; Michrom BioResources) using a linear gradient from 95 % solvent A (0.1 % formic acid) and 5 % solvent B (98 % acetonitrile, 0.1 % formic acid) to 55 % solvent B over 160 min at a flow rate of 0.3 μL min–1. The data acquisition mode was set to obtain a high-resolution MS scan in the Fourier transformation at a resolution of 30 000 full widths at half-maximum (at m/z 400) followed by MS/MS events in the linear ion trap of the five most intense ions. To increase the efficiency of MS/MS events, the charged state screening modus was enabled to exclude unassigned and singly charged ions. Collision-induced dissociation was triggered when the precursor exceeded 500 ion counts. The dynamic exclusion duration was set to 15 s. The ion accumulation time was set to 300 ms (MS) and 50 ms (MS/MS).
Data in.RAW format were exported directly from Xcalibur, and searched using the Maxquant platform (http://www.maxquant.org/) against a decoy database of the predicted sugarcane proteome from SUCEST (http://sucest-fun.org/) and annotated using homology search with Blast2Go (https://www.blast2go.com). The search criteria were set as follows: full tryptic specificity (cleavage after lysine or arginine residues) (two missed cleavages were allowed); carbamidomethylation of cysteine residues as fixed modification; oxidation of methionine as variable modification; mass tolerance of 6 ppm for precursor ions and 0.5 Da for fragment ions; peptide false discovery rate of 5 % on the peptide level and validated using the number of protein sequence hits using a reverse database. Results were imported into Perseus software version 1.5.0.0 (http://www.perseus-framework.org/) for quantification of protein abundance and statistical analysis (Cox and Mann, 2008). Blast2GO gene ontology was used for classifying protein functions based on biological processes, cellular component and molecular function. Furthermore, enzymes were categorized according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) and EC number.
Saccharification analysis in root segments
In order to evaluate the enzyme accessibility in cell walls from each root segment, a saccharification assay was performed in two pre-treatment conditions: (1) 0.5 n NaOH (only for saccharifying cellulose); and (2) boiling biomass in water (for saccharifying more soluble cell wall components, such as β-glucan). Both pre-treatments were performed at 90 °C for 30 min, in each root segment.
After pre-treatment, the biomass was washed six times with 500 μL of sodium acetate buffer before the enzymatic hydrolysis. Samples were incubated with an enzyme cocktail at 50 °C, and quantification of released sugars was performed automatically by a robotic workstation as described in Gomez et al. (2010).
Data analysis
Enzyme activity, gene expression and biomass saccharification results were analysed using JMP 5.0.1 software (Copyright© 1989–2002 SAS Institute Inc.). The homogeneity of variance was tested and a parametric one-way analysis of variance (ANOVA) test followed by Tukey test (P <0.05) was applied.
Proteome analysis identified a total of 1082 proteins in sugarcane roots using SUCEST (data not shown). Presence and absence of proteins within the root segments were normalized according to S3 (Supplementary data Fig. S2). Proteins not present in at least three experimental replicates were considered as absent, reducing the number of proteins to 525 (data not shown). Only those related to cell wall degradation enzymes were considered for the purposes of this study. The relative quantification of these proteins was determined based on the ratio between subsequent root segments (S1/S2, S2/S3, S3/S4 and S4/S5) (Table 1), following root development. Five biological replicates for each root segment were analysed and subjected to quantification using the MaxQuant software v. 1.3.0.5 supported by Andromeda (Cox et al., 2011) as the database search engine for peptide identification. Data were evaluated and statistics calculated using the Perseus software (version 1.6.0.2, Max Planck Institute of Biochemistry, Martinsried, Germany). MaxQuant data were filtered for reverse identifications (false positives), contaminants and ‘only identified by site’. In order to obtain gradient ratios among segments in agreement with the root development, the S2/S1 and S3/S2 ratios given by the Perseus software were reversed (1/x) to obtain S1/S2 and S2/S3 ratios. Normalized ratios were used for relative quantification among segment ratios, calculated as follows: S1/S2, S2/S3, 3S/S4 and S4/S5 (quantification ratio of a given protein between n segment in relation to its abundance in the segment n – 1). One-way ANOVA was employed to identify significant ratios between segments (P < 0.05).
Table 1.
Normalized ratio values of cell wall proteins detected in sugarcane roots related to wall modifications/degradation during aerenchyma formation. S1 to S5 correspond to root segments from apex to base (1 cm each). Values are medians (n = 5), and significant differences (ANOVA; obtained with Maxquant and Perseus softwares) are shown in bold (P < 0.05). Gene Ontology (GO) terms were obtained with Blast2GO. Negative and positive values represent protein decrease and increase, respectively, from segment a to b (a/b).
| ID | SAS - Sucest | Protein identification (Databank) | Segment differences ratio | ANOVA P-value | GO biological process | GO cell component | GO molecular function | KEGG information | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1/2 | S2/3 | S3/4 | S4/5 | ||||||||
| 1 | SCQSRT2034G08.g | Alpha-galactosidase (UNIPROT) | 0.243 | 0.329 | 0.272 | –0.432 | 0.353 | fintra-Golgi vesicle-mediated transport; root hair elongation; starch biosynthetic process | apoplast; plant-type cell wall; vacuole | alpha- galactosidase activity | alpha-galactosidase [EC:3.2.1.22]; E3.2.1.22B |
| 2 | SCQSRT1034D03.g | Alpha-l-arabinofuranosidase (UNIPROT) | 0.708 | 0.439 | 0.198 | –0.101 | 0.000 | glucuronoxylan metabolic process; l-arabinose metabolic process; xylan biosynthetic process; xylan catabolic process | apoplast; plant-type cell wall; vacuole | alpha-N-arabinofuranosidase activity; xylan 1,4-beta-xylosidase activity | alpha-N-arabinofuranosidase [EC:3.2.1.55]; E3.2.1.55 |
| 3 | SCEQRT1025C10.g | Beta-1,3-glucanase glycosyl hydrolases family 17(UNIPROT) | –0.782 | –0.282 | –0.052 | –0.083 | 0.000 | anatomical structure form. involved in morphogenesis; carbohydrate metabolic process; organ development; organelle organization; post-embryonic development; | anchored to plasma membrane; plant-type cell wall | hydrolase activity, hydrolysing O-glycosyl compounds | |
| 4 | SCEZHR1088G11.g | Beta-1,3-glucanase glycosyl hydrolases family 17(UNIPROT) | 0.532 | –0.111 | –0.107 | –0.157 | 0.004 | asymmetric cell division; auxin polar transport; carbohy drate metabolic process; cellular response to organic substance; cellular response to oxygen-containing compound; regulation of cell size; regulation of meristem growth; root morphogenesis | anchored to plasma membrane; endoplasmic reticulum; nucleus; plant-type cell wall; plasmodesma | glucan endo-1,3-beta-d-glucosidase activity | |
| 5 | SCJFRT1008G05.g | Beta-1,3-glucanase glycosyl hydrolases family 17(UNIPROT) | –0.034 | –0.498 | –0.288 | –0.100 | 0.020 | carbohydrate metabolic process; regulation of innate immune response; regulation of programmed cell death; response to bacterium; response to cold | anchored to plasma membrane; apoplast; plant-type cell wall; plasmodesma; vacuole | beta-glucosidase activity; cellulase activity; protein binding | |
| 6 | SCRFAM2071E02.g | Beta-1,3-glucanase glycosyl hydrolases family 17(UNIPROT) | –0.144 | –0.167 | –0.043 | –0.234 | 0.241 | carbohydrate metabolic process; cell communication; regulation of cellular process; regulation of gene expression, epigenetic; response to organic substance; response to oxygen-containing compound | anchored to plasma membrane; cell wall; cytoplasmic part; nucleus; plasmodesma | hydrolase activity, hydrolysing O-glycosyl compounds | |
| 7 | SCRFLR1055B07.g | Beta-1,3-glucanase glycosyl hydrolases family 17(UNIPROT) | –0.431 | 0.022 | –0.160 | –0.157 | 0.278 | cytokinin-mediated signalling pathway | plasma membrane | ||
| 8 | SCRURT2006B10.g | Beta-1,3-glucanase glycosyl hydrolases family 17(UNIPROT) | –0.037 | –0.079 | –0.174 | –0.195 | 0.605 | carbohydrate metabolic process; gene silencing; multicellular organismal development; organelle organization | anchored to membrane; cell wall; plasma membrane; plasmodesma | hydrolase activity, hydrolysing O-glycosyl compounds | |
| 9 | SCCCCL4001H11.g | Beta-galactosidase (UNIPROT) | 0.707 | 0.258 | 0.230 | –0.047 | 0.000 | alcohol biosynthetic process; carbohydrate metabolic process; catabolic process; cell wall biogenesis; developmental cell growth; developmental growth involved in morphogenesis; hydrogen peroxide metabolic process; plant-type cell wall modification; regulation of biological process | apoplast; cytosol; plant-type cell wall; plasmodesma; vacuolar membrane | beta-galactosidase activity; carbohydrate binding | |
| 10 | SCCCCL6004H07.g | Beta-galactosidase (UNIPROT) | 0.179 | –0.674 | 0.678 | 0.018 | 0.000 | biological regulation; carbohydrate metabolic process; cellular biosynthetic process; cellular process involved in reproduction; plant-type cell wall organization | apoplast; plant-type cell wall; plasmodesma; vacuolar membrane | beta-galactosidase activity | |
| 11 | SCEPRT2044F09.g | Beta-galactosidase (UNIPROT) | 0.101 | –0.457 | 0.650 | –0.160 | 0.000 | carbohydrate metabolic process; cellular cation homeostasis; cellular modified amino acid biosynthetic process; divalent metal ion transport; hydrogen peroxide catabolic process; plant-type cell wall biogenesis; plant-type cell wall modification; polyamine catabolic process; regulation of meristem growth; response to karrikin; root hair elongation | apoplast; cytosol; plant-type cell wall; plasmodesma; vacuolar membrane | beta-galactosidase activity | |
| 12 | SCCCCL4009F05.g | Beta-xylosidase/alpha-l-arabinofuranosidase (GH family 3) (UNIPROT) | 0.536 | –0.011 | –0.043 | –0.044 | 0.000 | arabinan catabolic process; response to arsenic-containing substance; seed coat development; xylan catabolic process | apoplast; chloroplast; plant-type cell wall; plasmodesma; vacuolar membrane | alpha-N-arabinofuranosidase activity; xylan 1,4-beta-xylosidase activity | beta-d-xylosidase 4 [EC:3.2.1.37]; XYL4 |
| 13 | SCQSAM1030G04.g | Beta-xylosidase/alpha-l-arabinofuranosidase (GH family 3) (UNIPROT) | –0.420 | –0.108 | –0.039 | –0.095 | 0.279 | arabinan catabolic process; response to arsenic-containing substance; seed coat development; xylan catabolic process | apoplast; chloroplast; plant-type cell wall; plasmodesma; vacuolar membrane | alpha-N-arabinofuranosidase activity; xylan 1,4-beta-xylosidase activity | |
| 14 | SCCCLR2C01H02.g | Endo-1,3;1,4-beta-d-glucanase (UNIPROT) | –0.208 | –0.001 | –0.328 | 0.304 | 0.083 | glycolysis; Golgi organization; hyperosmotic response; response to cadmium ion; response to salt stress; response to temperature stimulus; water transport | apoplast; cytosol; nucleus; plasma membrane | hydrolase activity | carboxymethylene butenolidase [EC:3.1.1.45]; E3.1.1.45 |
| 15 | SCVPRT2081E09.g | Endo-1,3(4)-beta-glucanase 1; Endo-1,4-beta-glucanase 1; Endo-1,3-beta-glucanase 1; Laminarinase-1 (UNIPROT) | 0.409 | 0.243 | 0.186 | –0.118 | 0.052 | amino acid import; cell wall macromolecule catabolic process; endoplasmic reticulum unfolded protein response; ER to Golgi vesicle-mediated transport; N-terminal protein myristoylation | glucan endo-1,3-beta-glucanase activity, C-3 substituted reducing group; glucan endo-1,4-beta-glucanase activity, C-3 substituted reducing group | ||
| 16 | SCCCCL4006H09.g | Expansin (UNIPROT) | –0.387 | 0.077 | –0.251 | –0.163 | 0.746 | cell growth; developmental growth involved in morphogenesis; plant-type cell wall modification; sexual reproduction; trichoblast differentiation | extracellular region; Golgi apparatus; plant-type cell wall; plasmodesma | ||
| 17 | SCSGLV1004F11.g | Expansin (UNIPROT) | 0.057 | –0.243 | 0.019 | 0.203 | 0.195 | organelle organization; plant-type cell wall loosening; plant-type cell wall modification involved in multidimensional cell growth; regulation of stomatal movement; response to gibberellin stimulus; response to red light; syncytium formation; unidimensional cell growth | chloroplast; cytosol; extracellular region; plant-type cell wall; plasmodesma | structural constituent of cell wall | |
| 18 | SCEPLR1051B04.g | Fasciclin-like arabinogalactan protein (UNIPROT) | 0.172 | –0.034 | 0.155 | –0.046 | 0.265 | alcohol biosynthetic process; cell growth; cellular component organization; plant-type cell wall organization or biogenesis; regulation of hormone levels; shoot system development; single-organism transport; steroid biosynthetic process; trichoblast differentiation | anchored to plasma membrane; cell wall; vacuolar membrane | ||
| 19 | SCQGRT1040D08.g | Fasciclin-like arabinogalactan protein (UNIPROT) | –1.209 | –0.195 | –1.255 | 0.267 | 0.471 | alcohol biosynthetic process; biological regulation; secondary cell wall biogenesis; steroid biosynthetic process; trichoblast differentiation | anchored to plasma membrane; Golgi apparatus; plant-type cell wall; plasmodesma | ||
| 20 | SCQSLR1040G04.g | Fasciclin-like arabinogalactan protein (UNIPROT) | 0.510 | –0.365 | –0.159 | –0.138 | 0.007 | acetyl-CoA metabolic process; brassinosteroid biosynthetic process; cell growth; plant-type cell wall organization; regulation of meristem growth; root development; secondary cell wall biogenesis; sterol biosynthetic process | anchored to plasma membrane; cell wall; cytoplasmic part; intracellular membrane-bounded organelle | ||
| 21 | SCRFLR1034D04.g | Fasciclin-like arabinogalactan protein (UNIPROT) | 0.514 | –0.074 | –0.472 | –0.133 | 0.002 | brassinosteroid biosynthetic process; cell growth; secondary cell wall biogenesis; trichoblast differentiation | anchored to plasma membrane; Golgi apparatus; plant-type cell wall; plasmodesma | ||
| 22 | SCVPRZ2036F10.g | Fasciclin-like arabinogalactan protein (UNIPROT) | 0.402 | 0.045 | –0.158 | 0.027 | 0.198 | acetyl-CoA metabolic process; brassinosteroid biosynthetic process;cell growth; plant-type cell wall organization; regulation of meristem growth; root development; secondary cell wall biogenesis; sterol biosynthetic process | anchored to plasma membrane; cell wall; cytoplasmic part; intracellular membrane-bounded organelle | ||
| 23 | SCAGLR1021F11.g | Glycosyl hydrolase family 1 (UNIPROT) | –0.584 | –0.474 | –0.282 | –0.177 | 0.000 | carbohydrate metabolics;cellular response to cold; defence response by callose deposition in cell wall; defence response to fungus; induced systemic resistance; negative regulation of defence response; response to salt stress; response to symbiotic fungus | apoplast; chloroplast envelope; cytosolic ribosome; endoplasmic reticulum; ER body; Golgi apparatus; membrane; nucleus; peroxisome; plant-type cell wall; plasmodesma; vacuole | beta-mannosidase activity; cellobiose glucosidase activity; copper ion binding; fucosidase activity; phosphatidic acid binding; protease binding | beta-glucosidase [EC:3.2.1.21]; E3.2.1.21 |
| 24 | SCCCCL3001B10.b | Glycosyl hydrolase family 1 (UNIPROT) | 0.492 | 0.077 | 0.032 | –0.276 | 0.000 | carbohydrate metabolic process; cellular response to ethylene stimulus; cellular response to iron ion; cellular response to nitric oxide; lignin biosynthetic process; pollen tube growth; regulation of cellular process; response to carbohydrate stimulus; response to other organism; response to salt stress | apoplast; chloroplast; cytosolic ribosome; Golgi apparatus; plant-type cell wall; plasmodesma | beta-mannosidase activity; cellobiose glucosidase activity; coniferin beta-glucosidase activity | beta-glucosidase [EC:3.2.1.21]; bglB |
| 25 | SCEQHR1082B01.g | Glycosyl hydrolase family 1 (UNIPROT) | –0.681 | 0.463 | 0.341 | 0.167 | 0.000 | carbohydrate metabolic process; glucosinolate metabolic process; pollen tube growth; response to other organism; response to salt stress | apoplast; chloroplast; cytosolic ribosome; Golgi apparatus; membrane; plant-type cell wall; plasmodesma | beta-mannosidase activity; cellobiose glucosidase activity; esculin beta-glucosidase activity; thioglucosidase activity | beta-glucosidase [EC:3.2.1.21]; E3.2.1.21 |
| 26 | SCEQLB1066E08.g | Glycosyl hydrolase family 1 (UNIPROT) | 0.337 | –0.035 | –0.125 | 0.131 | 0.192 | carbohydrate metabolic process; cellular component organization; cellular response to ethylene stimulus; lignin biosynthetic process; response to carbohydrate stimulus | apoplast; chloroplast; cytosolic ribosome; Golgi apparatus; plant-type cell wall; plasmodesma | beta-mannosidase activity; cellobiose glucosidase activity | beta-glucosidase [EC:3.2.1. 21];bglB |
| 27 | SCJFLR1017E03.g | Glycosyl hydrolase family 1 (UNIPROT) | 0.300 | –0.093 | –0.129 | –0.083 | 0.168 | carbohydrate metabolic process; cellular response to ethylene stimulus; lignin biosynthetic process | apoplast; chloroplast; cytosolic ribosome; Golgi apparatus; membrane; plant-type cell wall; plasmodesma | beta-mannosidase activity; cellobiose glucosidase activity; coniferin beta-glucosidase activity; esculin beta-glucosidase activity | |
| 28 | SCAGRT2037A11.g | Glycosyl hydrolase family 3 protein (xylosidase) (UNIPROT) | 0.705 | 0.240 | –0.133 | 0.128 | 0.038 | arabinan catabolic process; regulation of meristem growth; response to arsenic-containing substance; seed coat development; xylan catabolic process | apoplast; chloroplast; plant-type cell wall; plasmodesma; vacuolar membrane | alpha-N-arabinofuranosidase activity; xylan 1,4-beta-xylosidase activity | |
| 29 | SCCCRT1002G03.g | Glycosyl hydrolase family 3 protein (xylosidase) (UNIPROT) | 0.475 | 0.252 | –0.179 | –0.136 | 0.009 | arabinan catabolic process; response to chemical stimulus; seed coat development; xylan catabolic process | apoplast; chloroplast; plant-type cell wall; plasmodesma; vacuolar membrane | alpha-N-arabinofuranosidase activity; xylan 1,4-beta-xylosidase activity | beta-d-xylosidase 4 [EC:3.2.1.37]; XYL4 |
| 30 | SCCCSB1003H06.g | Glycosyl hydrolase family 3 protein (xylosidase) (UNIPROT) | 0.605 | –0.276 | 0.005 | –0.138 | 0.001 | arabinan catabolic process; regulation of meristem growth; response to arsenic-containing substance; seed coat development; xylan catabolic process | apoplast; chloroplast; plant-type cell wall; plasmodesma; vacuolar membrane | alpha-N-arabinofuranosidase activity; xylan 1,4-beta-xylosidase activity | |
| 31 | SCEZRZ3015A12.g | Glycosyl hydrolase family 3 protein (xylosidase) (UNIPROT) | 0.590 | –0.249 | 0.055 | –0.091 | 0.001 | carbohydrate metabolic process | hydrolase activity, hydrolysing O-glycosyl compounds | beta-d-xylosidase 4 [EC:3.2.1.37]; XYL4 | |
| 32 | SCEQLR1093F09.g | Glycosyl hydrolase family 3 protein (UNIPROT) | 0.220 | 0.123 | –0.013 | –0.432 | 0.001 | carbohydrate metabolic process | hydrolase activity, hydrolysing O-glycosyl compounds | beta-glucosidase [EC:3.2.1.21]; bglX | |
| 33 | SCEZLB1007A09.g | Glycosyl hydrolase family 3 protein (UNIPROT) | 0.272 | –0.014 | 0.235 | 0.108 | 0.500 | carbohydrate metabolic process; cell wall organization or biogenesis; cellular | anchored to membrane; cytosol; extracellular region; plant-type cell wall; plasma membrane; plasmodesma; vacuole | xylan 1,4-beta-xylosidase activity | beta-glucosidase [EC:3.2.1.21]; bglX |
| 34 | SCCCCL2001B11.b | Glycosyl hydrolases family 18 (probable chitinase or xylanase inibitor) (UNIPROT) | 0.311 | –0.021 | 0.275 | –0.039 | 0.019 | carbohydrate metabolic process | hydrolase activity, hydrolysing O-glycosyl compounds | chitinase [EC:3.2.1.14]; E3.2.1.14 | |
| 35 | SCCCCL4003F09.g | Glycosyl hydrolases family 18 (probable chitinase or xylanase inibitor) (UNIPROT) | 0.235 | –0.004 | 0.164 | –0.231 | 0.662 | carbohydrate metabolic process; cellular process; response to abiotic stimulus; response to stress; single-organism process | hydrolase activity, hydrolysing O-glycosyl compounds | chitinase [EC:3.2.1.14]; E3.2.1.14 | |
| 36 | SCJLRT1020A08.g | Probable alpha-glucosidase (UNIPROT) | 0.331 | –0.098 | 0.310 | –0.259 | 0.613 | plant-type cell wall biogenesis; plant-type cell wall organization; xylan catabolic process; xyloglucan metabolic process | apoplast; chloroplast; cytosol; plant-type cell wall; plasmodesma; vacuole | alpha-N-arabinofurano sidase activity; xylan 1, 4-beta-xylosidase activity; xyloglucan 1, 6-alpha-xylosidase activity | alpha-glucosidase [EC:3.2.1.20]; malZ |
From the above-mentioned ratios of significant proteins, another normalization was performed to allow visualization of protein abundance among segments. Negative ratio values express that proteins decrease in relation to the previous segment, whereas positive values express that they increase from the previous segments (Table 1). To be able to assess changes over the five segments, data were submitted to the following equation: the difference between segments = (R – 1) × –1, where R is the ratio between two segments considering protein concentration in S1 equal to 0. A representative heat map was generated considering S1 as the reference for all segments.
RESULTS
Cell wall enzyme activities during aerenchyma formation
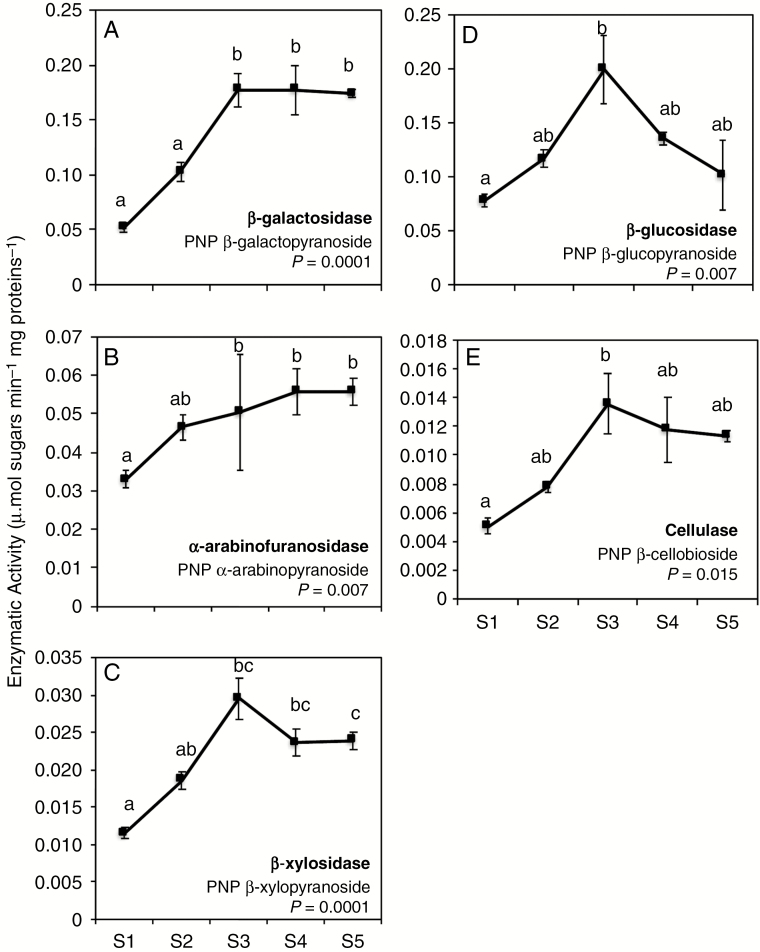
The percentage of aerenchyma occupying the root cortex cross-section was determined within the first 5 cm of the root tip (Fig. 1). The aerenchyma formation is not observed in S1 and S2, and starts from S3 onwards (varying between 10 and 30 % from S3 to S5) (Fig. 1B). The total activity of cell wall-related enzymes on different p-nitrophenyl-linked substrates was assessed to evaluate their contribution to aerenchyma formation among segments. β-Galactosidase, probably responsible for pectin de-branching, displayed a linear increase in activity up to S3, where it was kept constant until S5 (Fig. 2A). α-Arabinofuranosidase activity was found to increase gradually along the segments, reaching its maximum activity in S4 and S5 (Fig. 2B). Depending on the isoform, the enzyme might be associated with degradation of neutral ramifications of pectins or arabinose branches in other polysaccharides such as arabinoxylan. This is the case of β-xylosidases that can modify pectins and/or specifically cleave the linkages at the main chain of xylan. The activity of β-xylosidases increased continuously from S1 to S3, where it reached its maximum (Fig. 2C).
Fig. 2.
Enzymatic activity (μmol sugar min–1 mg protein–1) and the putative corresponding enzymes that act on nucleotide-sugars in five segments of sugarcane roots. (A) 4-Nitrophenyl-β-d-galactopyranoside, (B) 4-nitrophenyl-α-d-arabinopyranoside, (C) 4-nitrophenyl-β-xylopyranoside, (D) 4-nitrophenyl-β-1,4 glucopyranoside and (E) 4-nitrophenyl-β-d-cellobioside. Values are means (n = 5) ± s.e., and significant differences (ANOVA-Tukey) are show by letters (P < 0.05).
The activities of enzymes related to cellulose degradation, such as exo-enzymes (e.g. β-glucosidase) or endo-1,4-β-glucanase (cellulase), were detected in all root segments, peaking on S3 (Fig. 2D, E). This suggests that different cellulases may act simultaneously in distinct regions of cellulose.
Other enzymes probably contribute to the hydrolysis of hemicelluloses, such as xyloglucanases and mannanases. In vitro assays using isolated complex polysaccharides (e.g. xyloglucan, galactomannan and arabinoxylan) did not present any activity with the tested substrates (data not shown).
Gene expression of glycosyl-hydrolases
Changes in transcripts related to cell wall modifications along segments were also assessed in this study. For pectins, two EPGs, two acetyl-esterases and one β-galactosidase were evaluated. EPG1 (SCEZRT2016H09.g) and EPG2 (SCAGRT2038D08.g) had different expression patterns among segments (Fig. 3A, B). While EPG1 transcripts increased from S3 towards S5, EPG2 accumulated transiently in S2 and S3. Transcripts corresponding to pectin acetyl-esterases, enzymes that are responsible for de-acetylation of pectins, peaked in S2 and S5 (acetyl-esterase 1; SCQGAM1045B09.g) and showed a trend of increasing towards S3, although no statistical difference was observed (pectin acetyl-esterase 2; SCJLFL3013D11.g) (Fig. 3C, D). The transcripts of these glycosyl-hydrolases suggest that pectins could be initially de-acetylated before the attack of other endo-hydrolases when aerenchyma formation is not yet visible. β-Galactosidase (SCEPRT2044F09.g), probably responsible for removing pectin side chains, displayed an increase in S2 and S5 (Fig. 3E).
Fig. 3.
Relative expression of genes coding for cell wall enzymes related to modification in polysaccharides from the cell wall during aerenchyma formation in sugarcane roots. S1 to S5 correspond to segments from apex to base (1 cm each). Values are given as calibrated normalized relative quantities (CNRQs). Pectinases: (A) endo-polygalacturanase 1 (SCEZRT2016H09.g); (B) endo-polygalacturanase 2 (SCAGRT2038D08.g); (C) pectin acetyl esterase 1 (SCQGAM1045B09.g); (D) pectin acetyl esterase 2 (SCJLFL3013D11.g). Rhamnogalacturonan I: (E) β-galactosidase (SCEPRT2044F09.g) and possibly acts on arabinoxylan as (F) α-arabinofuranosidase (SCQSRT1034D03.g) and (G) β-xylosidase (SCCCRT1002G03.g). Xyloglucans: (H) α-xylosidase (SCJLRT1020A08.g), (I–L) four xyloglucan endo-transglycosylase/hydrolases (XTHs; SCEQRT2101A02.g, SCQSRT2034B07.g, SCACSB1036D01.g and SCBGLR1023F11, respectively). Expansins: (M) SCQSRT1036C01.g.; (N) SCVPRT2077B02.g.; (O) SCRURT2011A12.g; and (P) SCVPRT2075H10.g. β-1,3-Glucanases (callose degradation): (Q) SCJLRT1023E06.g and (R) SCCCRT2004C04.g. Genes related to cellulose modifications: (S) β-1,4-glucosidase (SCVPRT2073A02.g) and (T) cellulase (SCEQRT1024F11.g). Values are means (n = 5) ± s.e.. and significant differences (ANOVA-Tukey) are show by letters (P < 0.05). **Non-homogeneous data.
Two groups of transcripts related to xylan-degrading enzymes, xylosidases (Fig. 3G) and α-arabinofuranosidases (Fig. 3F), were identified. The first group is responsible for specifically cleaving bonds in the xylan main chain, whereas the second group removes arabinose side chains of arabinoxylan. The level of transcripts related to an α-arabinofuranosidase (SCQSRT1034D03.g) that can either cleave arabinose bonds from arabinoxylans or hydrolyse arabinans from RGI, was higher in S3, S4 and S5, peaking at S4 (Fig. 3F). Higher expression of β-xylosidase (SCCCRT1002G03.g) was found in all segments except S1 (Fig. 3G).
Differential expression profiles of genes coding for expansins, XTHs and the α-xylosidase (Fig. 3H–P), were observed in this study. They could be related to changes in the xyloglucan interaction with cellulose (expansins) and xyloglucan fine structure (XTH). Increased α-xylosidase (SCJLRT1020A08.g) levels were found after S1 (Fig. 3H), suggesting a possible de-branching of xylose in the main xyloglucan chain. For enzymes directly acting on the xyloglucan backbone, XTH1 (SCEQRT2101A02.g) and XTH2 (SCQSRT2034B07.g) presented similar patterns, decreasing sharply on S2 and kept constant among the other segments (Fig. 3I, J). The alignment suggests that these are not the same gene (data not shown). XTH3 (SCACSB1036D01.g) transcript does not change among segments, suggesting that this specific enzyme may not be related to root aerenchyma formation (Fig. 3K). XTH4 (SCBGLR1023F11) decreased on S2 and increased again towards S5 (Fig. 3L). Four expansin transcripts, EXP1 (SCQSRT1036C01.g), EXP2 (SCVPRT2077B02.g), EXP3 (SCRURT2011A12.g) and EXP4 (SCVPRT2075H10.g), encoding proteins that are likely to participate in growth processes related to cell growth and xyloglucan–cellulose interactions, showed the same pattern along the root, with higher levels in S1 and S5 (Fig. 3M–P). Multiple alignment analyses suggested that these genes do not encode the same protein (data not shown).
β-1,3-Glucosidase (SCJLRT1023E06.g), responsible for β-glucan and callose hydrolysis, increased in S2 and remained constant until S5 (Fig. 3Q, R). Regarding modifications on cellulose, there was a peak in the levels of transcripts corresponding to a cellulase (SCEQRT1024F11.g) and one β-1,4-glucosidase (SCVPRT2073A02.g) on S2 compared with the other segments (Fig. 3T, S).
Glycosyl-hydrolase proteomic profile
The sequential protein extraction using salts together with gel fractionation allowed the identification of 39 proteins, including glycosyl-hydrolases and expansins, associated with cell wall degradation that most probably play a role during aerenchyma development in sugarcane roots. A polygalacturonase (SCCCFL4119D10.g), a polygalacturonase inhibitor (SCVPLR2019B03.g) and a pectin methylesterase inhibitor or pectin-esterase (SCVPRZ2040D09.g) that are likely to be related to pectin hydrolysis were identified, but not quantified due to the normalization cut-offs. Twenty proteins displayed significant alterations in their amounts among segment ratios and were considered as a resource to investigate aerenchyma formation. All quantified proteins are listed in Table 1, and those proteins displaying significant alterations were further classified according to the related cell wall polysaccharides in a heat map (Fig. 4).
Fig. 4.
Heat map of proteins related to cell wall polysaccharides in sugarcane root segments. S1 to S5 correspond to root segments from apex to base (1 cm each). Values are median (n = 5), and quantification is given relative to S1. Green intensity is according to protein content. SAS in bold are proteins in which the corresponding gene expression was also measured. Protein Table IDs correspond to the proteins listed in Table 1. All proteins were significantly altered among segments (ANOVA), except for proteins in italic. *Follows the expected pattern of aerenchyma formation.
Six proteins belonging to the GH17 family were quantified (Table 1), and only half of them were found to have significant differences among the root segments. They include three β-1,3 glucanases, likely to act on callose and β-1,3-glucan (Fig. 4). Proteins SCEQRT1025C10.g (ID3) and SCEZHR1088G11.g (ID4) had lower abundance in S1 and increased in S2 (Fig. 4), but the former (ID3) showed higher protein abundance towards S5, whereas ID4 decreased after S2. The third identified β-1,3-glucanase, SCJFRT1008G05.g (ID5), starts to accumulate in S3 and reaches a plateau in S4–S5 (Table 1; Fig. 4).
Two endo-1,3;1,4-β-glucanases, SCVPRT2081E09.g (ID15) and SCCCLR2C01H02.g (ID14), corresponding to lichenases that degrade β-glucan were identified (Fig. 4). ID15 changed along the root, showing higher abundance in S2 and peaking in S4, similar to the β-1,3-glucanase ID3 (Fig. 4). Changes in protein levels of ID14 were not statistically significant (P = 0.083) (Table 1).
From the three β-galactosidases identified (likely to be responsible for removing pectin side chains), SCCCCL4001H11.g (ID9) increased linearly along segments, whereas SCCCCL6004H07.g (ID10) and SCEPRT2044F09.g (ID11) were markedly reduced in S3 (Table 1; Fig. 4).
The α-arabinofuranosidase SCQSRT1034D03.g (ID1) showed an increase from S1 to S5 (Table 1; Fig. 4). Eight GH3 proteins related to β-xylosidases were quantified (Table 1), but only six showed significant differences among the segments (Fig. 4). All GH3 proteins exhibited a very similar profile, showing increased abundance in the transition from S1 to S2. Only SCAGRT2037A11.g (ID28) presented a pattern of increased amount along the root, with higher amplitude compared with other enzymes (Table 1; Fig. 4). ID1 and ID28 proteins correspond to the same EST transcripts analysed (Fig. 3), indicating a similar pattern between transcript and protein levels.
A putative xylanase inhibitor from the GH18 family SCCCCL2001B11.b (ID33) that may be acting as an inhibitor of β-xylosidases, thus controlling the modifications of arabinoxylans, was found to have a bimodal pattern of variation among segments. The proportional level of this protein increased in S2 and S4 and was reduced in S3 and S5 (Table 1).
From the GH1 family (possibly related to β-glucosidase activity, attacking or modifying cellulose polymer), five proteins were detected in proteomic analysis. However, only three were statistically different among segments (Table 1). Cellulases SCAGLR1021F11.g (ID23) and SCCCCL3001B10.b (ID24) increased in all segments in comparison with S1, the former increasing more gradually. Conversely, for SCEQHR1082B01.g (ID25), another cellulase quantified, S2 and S3 ratios were reduced compared with S1, increasing in S4 and S5 (Table 1; Fig. 4). Expansins SCCCCL4006H09.g (ID16) and SCSGLV1004F11.g (ID17) showed a decreasing trend along the segments.
Taken together, the results of the proteomic profile corroborate the gene expression data for GH1 proteins, revealing higher protein and transcript levels in S2. Moreover, the activity of glycosyl-hydrolases gradually increases towards S3 (Fig. 2), where aerenchyma formation becomes visible (Fig. 1).
Lignin-related gene expression and protein level during aerenchyma formation
Transcripts of genes that encode laccase 1 (SCACSB1039F01.g) and laccase 2 (SCCCSD1090D07.g) showed an increase mainly in S2, and the former had a higher amplitude (Fig. 5A, B). Furthermore, laccase 2 also increased in S3, decreasing sharply on the following segments (Fig. 5B). Only one laccase protein presented significant amounts among segments (SCMCRT2087D02.g), increasing towards S5 (Fig. 5C). These data suggest that lignin synthesis takes place mainly between S2 and S3. This polymer can reduce glycosyl-hydrolase activities due to the increased recalcitrance of tissues after S3.
Fig. 5.
Cell wall enzymes related to lignin synthesis during aerenchyma formation in sugarcane roots. S1 to S5 correspond to root segments from apex to base (1 cm each). Expression profile of genes coding for (A) laccase 1 (SCACSB1039F01.g) and (B) laccase 2 (SCCCSD1090D07.g). Values are means (n = 5) ± s.e., and significant differences (ANOVA-Tukey) are show by letters (P < 0.05). (C) Relative protein quantification of a laccase (SCMCRT2087D02.g) significantly altered among segments (ANOVA). Values are median (n = 5), and quantitation is given relative to S1. Data were extracted from Table 1.
Saccharification potential during aerenchyma formation
During the aerenchyma formation, cell walls are targeted for modification via the action of glycosyl-hydrolases on polysaccharides. A saccharification assay using the different segments of sugarcane roots as substrates was performed to gauge the susceptibility of polysaccharides to degrading enzymes, using two different pre-treatments. Root segments pre-treated with NaOH had higher saccharification levels compared with hot water (Fig. 6). However, in the hot water pre-treatment, S1 saccharification levels were higher than those observed for the same root segment treated with NaOH. This could be due to the higher solubility of polysaccharides present in the apical root region segments.
Fig. 6.
Saccharification of sugarcane roots under pre-treatments with NaOH and water. Pre-treatments in (A) NaOH and (B) water. S1 to S5 correspond to root segments from apex to base (1 cm each). Values are means (n = 5) ± s.e., and significant differences (ANOVA-Tukey) are show by letters (P < 0.003 and P < 0.002 for NaOH and water, respectively).
DISCUSSION
The aerenchyma formation in sugarcane roots is lysigenous and constitutive, and occurs centripetally in the cortex (Leite et al., 2017) starting from the second and third centimetre from the root apex and ending on the fifth centimetre (Fig. 1B). Although the formation of lysigenous aerenchyma is widely described as a process leading to complete degradation of the cell wall (Horton and Osborne, 1967; Kawase, 1974, 1979; Drew et al., 1979; Gunawardena et al., 2001b; Subbaiah and Sachs, 2003; Morgan and Drew, 2004), the combination of cell wall fractionation, mono- and oligosaccharides, glycome profiling and microscopy techniques revealed changes in cell wall composition and architecture incompatible with full degradation, at least in sugarcane (Leite et al., 2017).
From these results, we hypothesized that there would be a concerted action of glycosyl-hydrolases on the cell wall to facilitate access to the polysaccharides. This would lead to pectin degradation of the middle lamella followed by a rearrangement in the cell wall, as observed by Leite et al. (2017). Regarding the pectin degradation, there are several lines of evidence that support this idea, including modification of pectins at very early stages of cell death in maize roots (Gunawardena et al., 2001b) and removal of pectins from triangular junctions along the formation of root aerenchyma in sugarcane (Leite et al., 2017). In agreement with these studies, the transcripts, proteins and enzymatic activity of glycosyl-hydrolases related to pectin degradation such as acetyl esterease, EPG, β-galactosidase and α-arabinofuranosidase displayed a linear increase from S2 to S5 (Figs 2–4). Although several pectinases are synthesized at the same time in S2 and S3, it may be speculated that degradation of pectins occurs through the selective action of pectin esterases (it is not yet known whether they are acetyl- or methyl-esterases) on the main chain of endopolygalacturonan/rhamnogalacturonan opening sites for the attack of EPG. We found evidence that a polygalacturonase inhibitor may regulate the latter.
According to Leite et al. (2017), pectin degradation is detected mainly at triangular junctions among the cortex cells. These authors also found a dramatic decrease in the levels of galactose, denoting galactan degradation. Our findings that β-galactosidase and α-arabinofuranosidase vary during the same period confirm these observations. Additional information regarding hormone action (Tavares et al., 2018) and the fact that the transcription factor scRAV1 controls the same EPG gene (scEPG1) during early stages of aerenchyma formation in sugarcane (Tavares et al., 2019) suggests that the pectin degradation appears to be finely controlled at several different levels during aerenchyma formation.
The high solubility of β-glucan suggests that it could be a transition polysaccharide, synthesized in elongating tissues and removed when cell elongation decreases (Carpita, 1996; Kim et al., 2000). Degradation of β-glucan at the storage walls of lupin seeds has been shown to contribute to loosening, allowing cell expansion of cotyledon cells (Buckeridge et al., 2004). In sugarcane, the aerenchyma formation in its roots seems to be complete only after the removal of β-glucan, and variations in this polymer strongly correlate with cell expansion and aerenchyma establishment (Leite et al., 2017). Our observed profile of gene expression and protein levels of endo-1,3(1,4)-β-glucanases (lichenase; GH17) suggest that they are all involved in the degradation of β-glucan between S2 and S3 (Fig. 4; Table 1). These results show that there is a concomitant attack on pectins and β-glucan, during aerenchyma development, the latter possibly controlling cell expansion of the cortex cells. The hypothesis that these polymers interfere with cell wall porosity, thus interfering with the access of enzymes to the cell wall polymers, cannot be discarded.
Besides the involvement with β-glucan degradation, other members of the GH17 family such as the β-1,3-glucanases can also be involved in callose degradation (Worrall et al., 1992). This enzyme is an abundant protein found during development, fertilization and mobilization of reserves in endosperms (Stone and Clarke, 1992), and in response to the attack of pathogens (Bucher et al., 2001). Interestingly, callose deposition is essential for restricting traffic of intracellular substances through the plasmodesmata (Radfor and White, 2001; Zavaliev et al., 2011), and enzymes of the GH17 family have been shown to participate actively in intercellular trafficking in arabidopsis (Levy et al., 2007; Simpson et al., 2009). The fact that we found this protein following the same pattern during aerenchyma formation (Fig. 4; Table 2) is consistent with the hypothesis that communication among the cells forming the aerenchyma is increasing.
Table 2.
Summary of the results obtained in this work, including the main modifications in cell wall polysaccharides during aerenchyma formation and related cell wall glycosyl-hydrolases
| Enzyme | Gene expression | Protein | Enzyme activity | Cell wall modification* | |
|---|---|---|---|---|---|
| Pectins | Endo-polygacturanase and pectin acetyl esterase | Increases from S2 to S5/peaks S2 and S3/peaks S5 | Identified | ND | Middle lamella modification, arabinoxylan debranching, arabinan degradation and galactan degradation |
| α-Arabinofuranosidase | Increases from S2/peaks at S4 | Increases continuously towards S5 | Increases continuously towards S5 | ||
| β-Galactosidase | Peaks at S2 and S5 | Increases from S2 | Levels off from S3 towards | ||
| Hemicelluloses | β-Xylosidase | Peaks at S2 | Levels off from S2 onwards | Peaks at S3 and levels off | Some arabinoxylan modification, β-glucan degradation and xyloglucan modification? |
| Endo-1,3 (1,4)-β-glucanase | ND | levels off at (from) S2 onwards | ND | ||
| GH 17; 1,3 β-glucanase | Levels off from S2 onwards | Levels off at (from) S2 onwards | ND | ||
| α-Xylosidase | Increases from S1 with peaks in S2 and S4 | ND | ND | ||
| XTH; xyloglucan endo-transglycosylase/hydrolase | High in S1, increases linearly from S2 onwards | ND | ND | ||
| Expansins | Does not vary significantly | ND | |||
| Cellulose | β-glucosidase and cellulase | Peaks at S2 | Increases continuously or decrease at S5 | Peaks at S3 | Some cellulose modification? |
| Lignin | Laccases | Peaks at S2 | Increases continuously | ND | No change |
*According to Leite et al et al. (2017).
ND, not detected.
Arabinoxylans are the main hemicelluloses found in sugarcane cell walls (De Souza et al., 2013), and differences in their fine structure can influence the mode of action of enzymes (Buckeridge and De Souza, 2014). Reduction of substitutions with acetyl esters, ferulic acid, arabinose and glucuronic acid decreases the accessibility of enzymes to the polysaccharides (Mortimer et al., 2010). Thus, possible modifications in arabinoxylan branches suggested by the detection of specific glycosyl-hydrolases can be directly correlated with the degradation and structural changes of the walls, contributing to the formation of aerenchyma in sugarcane roots. Transcripts encoding β-xylosidases (Fig. 3G) and quantification of related proteins involved in arabinoxylan modification/degradation such as α-arabinofuranosidases from the GH3 family (Table 1; Fig. 4) were found in all segments, with accumulation starting from S2. Enzymes of the GH3 family are capable of hydrolysing both arabinosyl and xylosyl branches present on hemicelluloses and pectins, being considered as bifunctional enzymes (Montes et al., 2008). An increase in transcripts, protein levels and activities of α-arabinofuranosidase were mainly observed in S3–S4 (Figs 2B, 4 and 3F, respectively). These findings point out modifications such as debranching of arabinoxylan during root development that seem to start at the early stages of aerenchyma formation. Corroborating this idea, there is an increase in the content of xylans – possibly debranched arabinoxylans – tightly bound to the wall along the root segments (Leite et al., 2017).
Similarly to the findings by Tavares et al. (2018), the expression of most expansins followed a similar pattern, i.e. higher levels in the meristematic region (S1) with a marked decrease in S2 and S3, followed by recovery in S5 at similar levels to S1 (Fig. 3M–P). During aerenchyma establishment, expansins are necessary for cell wall loosening and expansion. In other processes such as fruit ripening, upregulation of genes encoding expansins in response to ethylene is known to promote the extensibility of the wall (Rose et al., 2000; Marowa et al., 2016). Cell wall expansion was detected in S1 and S2 by microscopy and was followed by slightly increased deposition of xyloglucan and arabinoxylans from S3 to S5 in the cortex of sugarcane roots (Leite et al., 2017).
Recent findings reported that the balance between ethylene and auxin possibly regulates aerenchyma formation via control of expression of genes related to pectin hydrolysis, expansins and XTHs (Tavares et al., 2018). Here we found that genes encoding several XTHs displayed different transcriptional patterns. Two of them accumulated in S1 (Fig. 3I–L). A possible explanation for the observation of high levels of these XTH transcripts accumulated in S1 could be related to the fact that this segment contains a meristematic region, displaying intense cell division, differentiation and cell expansion. The finding that their levels decline in S2 suggests that they are not directly related to the aerenchyma formation. On the other hand, another XTH transcript with high similarity to the sequence identified in the aerenchyma of maize roots (Rajhi et al., 2011) displayed an increase in S4 and S5 (Fig. 3I–L), compatible with aerenchyma development.
Transcripts of cellulase and β-1,4-glucosidase showed accumulation in S2 (Fig. 3S, T), and total activity of these enzymes increased from S2 to S3 (Fig. 2D, E). Also, in the proteomic analysis, members of glycosyl-hydrolases of the GH1 family, including β-glucosidases, were identified (Table 1; Fig. 4). However, those changes did not result in decreased cellulose contents. In fact, the proportion of cellulose increased significantly along the segments in comparison with other cell wall components (Leite et al., 2017), an indication that this effect is not only due to the development of the vascular cylinder. The presence of these enzymes may indicate cellulose rearrangements in the walls during aerenchyma development. Kawase (1974, 1979), for example, observed an increase in cell wall plasticity when cortical cells of sunflower stamen were treated with cellulase. The nature and possible function of such rearrangement remain to be elucidated.
Although genes encoding laccases were highly expressed in S2 (Fig. 5), the proportion of lignin does not change among segments (Leite et al., 2017). A possible explanation would be that the observed increase in laccase activity may lead to lignan formation, which was not evaluated during this study.
In order to probe the access of hydrolases to the cell wall of sugarcane roots after the aerenchyma formation, saccharification was performed after two different pre-treatments (NaOH and water; Fig. 6). We found that the retrieval of some of the more NaOH-soluble hemicelluloses (possibly xylans and xyloglucans) from the wall led to an increase in saccharification along the root segments (S2–S4), associated with aerenchyma development (Fig. 6A). When aerenchyma is complete (S5), recalcitrance increases, probably due to the formation of a composite, as observed by Leite et al. (2017).
Our results strongly support the hypothesis that cell wall enzymes related to aerenchyma formation are synchronically synthesized at S2–S3 leading to cell wall modifications that seem to be determined by the fine structure of the cell wall polymers. As a result, interactions among polymers give rise to a composite that is recalcitrant to hydrolysis and impermeable to gasses.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: overview of sugarcane plants and roots used in the experiments. Figure S2: experimental design of proteomics labelling, quantification and data analysis in sugarcane root segments. Table S1: oligonucleotide primer sequences used for qRT–PCR in this study.
FUNDING
This work was supported by the National Institute of Science and Technology of Bioethanol (FAPESP 2008/57908-6 and 2014/50884-5) and (CNPq 574002/2008-1 and 465319/2014-9), Centro de Processos Biológicos e Industriais para Biocombustíveis (CeProBIO FAPESP 2009/52840-7 and CNPq 490022/2009-0) and Fapesp research grant (2010/17104-5). A.G. is grateful for PhD fellowships (FAPESP 2010/17070-3 and 2013/0159-0).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Gate-Lab (IB-USP) led by Professor Dr Marie-Anne Van Sluys for help with gene expression analysis, Dr Adriana Paes Leme (LNBio/CNPEM) for help with data analysis from proteomics, Dr Amanda R. Piovezani (IB-USP) for helping with data analysis, Dr Felice Cervone for infrastructure at the University of Rome, and Professor Dr Glaucia Mendes de Souza for kindy providing sugarcane reference primers and SUCEST database access.
LITERATURE CITED
- Alcântara PHN, Dietrich SMC, Buckeridge MS. 1999. Xyloglucan mobilization and purification of a (XLLG/XLXG) specific β-galactosidase from cotyledons of Copaifera langsdorffii. Plant Physiological Biochemistry 37: 653–663. [Google Scholar]
- Arikado H, Adachi Y. 1955. Anatomical and ecological responses of barley and some forage crops to the flooding treatment. Bulletin Faculty Agriculture, Mie University Tsu Mie 11: 1–29. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7: 225–332. [Google Scholar]
- Begum MK, Alam MR, Islam MS. 2013. Adaptive mechanisms of sugarcane genotypes under flood stress condition. World Journal of Agricultural Sciences 1: 056–064. [Google Scholar]
- Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJR. 2009. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nature Protocols 4: 484–494. [DOI] [PubMed] [Google Scholar]
- Borderies G, Jamet E, Lafitte C, et al. 2003. Proteomics of loosely bound cell wall proteins of Arabidopsis thaliana cell suspension cultures: a critical analysis. Electrophoresis 24: 3421–3432. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bragina TV, Martinovich LI, Rodionova NA, Bezborodov AM, Grineva GM. 2001. Ethylene-induced activation of xylanase in adventitious roots of maize as a response to the stress effect of root submersion. Applied Biochemistry and Microbiology 37: 618–621. [PubMed] [Google Scholar]
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology 33: 103–119. [DOI] [PubMed] [Google Scholar]
- Bucher GL, Tarina C, Heinlein M, Di Serio F, Meins F Jr, Iglesias VA. 2001. Local expression of enzymatically active class I beta-1,3-glucanase enhances symptoms of TMV infection in tobacco. The Plant Journal 28: 361–369. [DOI] [PubMed] [Google Scholar]
- Buckeridge MS. 2010. Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. Plant Physiology 154: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckeridge MS, De Souza AP. 2014. Breaking the glycomic code of cell wall polysaccharides may improve second-generation bioenergy production from biomass. BioEnergy Research 7: 1065–1073. [Google Scholar]
- Buckeridge MS, Rocha DC, Reid JSG, Dietrich SMC. 1992. Xyloglucan structure and post-germinative metabolism in seeds of Copaifera langsdorffıi from savanna and forest populations. Physiologia Plantarum 86: 145–151. [Google Scholar]
- Buckeridge MS, Rayon C, Urbanowicz B, Tiné MAS, Carpita NC. 2004. Mixed linkage (1→3),(1→4)-β-d-glucans of grasses. Cereal Chemistry 81: 115–127. [Google Scholar]
- Carpita NC. 1996. Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology 47: 445–476. [DOI] [PubMed] [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26: 17–36. [Google Scholar]
- Cosgrove DJ. 1999. Enzymes and other agents that enhance cell wall extensibility. Annual Review of Plant Physiology and Plant Molecular Biology 50: 391–417. [DOI] [PubMed] [Google Scholar]
- Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnology 26: 1367–72. [DOI] [PubMed] [Google Scholar]
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. 2011. Andromeda: a peptide search engine integrated into the MaxQuant environment. Journal of Proteome Research 10: 1794–1805. [DOI] [PubMed] [Google Scholar]
- De Souza AP, Leite DCC, Pattathil S, Hahn MG, Buckeridge MS. 2013. Composition and structure of sugarcane cell wall polysaccharides: implications for second-generation bioethanol. BioEnergy Research 6: 564–579. [Google Scholar]
- Delong A, Calderon-Urrea A, Dellaporta SL. 1993. Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage-specific floral organ abortion. Cell 74: 757–768. [DOI] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard S. 1979. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147: 83–88. [DOI] [PubMed] [Google Scholar]
- Edwards M, Bowman YJ, Dea IC, Reid JS. 1988. A beta-d-galactosidase from nasturtium (Tropaeolum majus L.) cotyledons. Purification, properties, and demonstration that xyloglucan is the natural substrate. Journal of Biological Chemistry 263: 4333–4337. [PubMed] [Google Scholar]
- Esteban R, Dopico B, Munoz FJ, Romo S, Martin I, Labrador E. 2003. Cloning of a Cicer arietinum beta-galactosidase with pectin-degrading function. Plant & Cell Physiology 44: 718–725. [DOI] [PubMed] [Google Scholar]
- Evans DE. 2003. Aerenchyma formation. New Phytologist 161: 35–49. [Google Scholar]
- Feiz L, Irshad M, Pont-Lezica RF, Canut H, Jamet E. 2006. Evaluation of cell wall preparations for proteomics: a new procedure for purifying cell walls from Arabidopsis hypocotyls. Plant Methods 2: 10. doi: 10.1186/1746-4811-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. 1992. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochemical Journal 282: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gòmez LD, Whitehead C, Barakate A, Halpin C, McQueen-Mason SJ. 2010. Automated saccharification assay for determination of digestibility in plant materials. Biotechnology for Biofuels 3: 23. doi: 10.1186/1754-6834-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis A, de Souza AP, Tavares EQP, Buckeridge MS. 2014. Using natural plant cell wall degradation mechanisms to improve second generation bioethanol. In: McCann MC, Buckeridge M, Carpita N, eds. Plants and bioenergy. Advances in Plant Biology, Vol. 4 New York: Springer, 211–230. [Google Scholar]
- Gunawardena A, Lan H, Pearce DM, Jackson MB, Hawes CR, Evans DE. 2001a Characterization of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212: 205–214. [DOI] [PubMed] [Google Scholar]
- Gunawardena A, Pearce DM, Jackson MB, Hawes CR, Evans DE. 2001b Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant, Cell & Environment 24: 1369–1375. [Google Scholar]
- Harker FR, Redgwell RJ, Hallet IC, Murray SH, Carter G. 1997. Texture of fresh fruit. Horticultural Review 20: 121–124. [Google Scholar]
- He CJ, Drew MC, Morgan PW. 1994. Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiology 105: 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. 1996a Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiology 112: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Pinlayson SA, Drew MC, Jordan WR, Morgan PW. 1996b Ethylene biosynthesis during aerenchyma formation in roots of Zea mays subjected to mechanical impedance and hypoxia. Plant Physiology 112: 1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RF, Osborne DJ. 1967. Senescence, abscission and cellulase activity in Phaseolus vulgaris. Nature 214: 1086–1088. [Google Scholar]
- Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM. 2004. Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Molecular Biology Reporter 22: 325–337. [Google Scholar]
- Justin SHFW, Armstrong W. 1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106: 465–495 [Google Scholar]
- Justin SHFW, Armstrong W. 1991. Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytologist 118: 49–62. [Google Scholar]
- Kawase M. 1974. Aerenchyma development is often associated with a radial enlargement of cortical cells. Physiologia Plantarum 31: 29–38. [Google Scholar]
- Kawase M. 1979. Role of cellulase in aerenchyma development in sunflower. American Jounal of Botany 66: 183–190. [Google Scholar]
- Kim JB, Olek AT, Carpita NC. 2000. Cell wall and membrane-associated exo-beta-d-glucanases from developing maize seedlings. Plant Physiology 123: 471–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula L, Schreiber L, Colmer TD, Nakazono M. 2017. Anatomical and biochemical characterisation of a barrier to radial O2 loss in adventitious roots of two contrasting Hordeum marinum accessions. Functional Plant Biology 44: 845–857. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lashbrook CC, Gonzalez-Bosch C, Bennett AB. 1994. Two divergent endo-beta-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. The Plant Cell 6: 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Burton RA, Hrmova M, Fincher GB. 2001. Barley arabinoxylan arabinofuranosidases: purification, characterization and determination of primary structures from cDNA clones. Biochemical Journal 356: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Hrmova M, Burton RA, Lahnstein J, Fincher GB. 2003. Bifunctional family 3 glycoside hydrolases from barley with α-l-arabinofuranosidase and β-d-xylosidase activity. Journal of Biolical Chemistry 278: 5377–5387. [DOI] [PubMed] [Google Scholar]
- Leite DCC, Grandis, Tavares EQP, et al. 2017. Cell wall changes during the formation of aerenchyma in sugarcane roots. Annals of Botany 120: 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Erlanger M, Rosenthal M, Epel BL. 2007. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. The Plant Journal 49: 669–682. [DOI] [PubMed] [Google Scholar]
- Li SC, Han JW, Chen KC, Chen CS. 2001. Purification and characterization of isoforms of beta-galactosidases in mung bean seedlings. Phytochemistry 57: 349–359. [DOI] [PubMed] [Google Scholar]
- Lima DU, Santos HP, Tiné MA, Molle FD, Buckeridge MS. 2001. Patterns of expression of cell wall related genes in sugarcane. Genetics and Molecular Biology 24: 191–198. [Google Scholar]
- Marín-Rodríguez MC, Orchard J, Seymour GB. 2002. Pectate lyases, cell wall degradation and fruit softening. Journal of Experimental Botany 53: 2115–2119. [DOI] [PubMed] [Google Scholar]
- Marowa P, Ding A, Kong Y. 2016. Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Reports 35: 949–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvaine TC. 1921. A buffer solution for colorimetric comparison. Journal of Biology Chemistry 49: 183–186. [Google Scholar]
- Minic Z, Jouanin L. 2006. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiology and Biochemistry 44: 435–449. [DOI] [PubMed] [Google Scholar]
- Mittler R, Lam E. 1995. In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiology 108: 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes RAC, Ranocha P, Martinez Y, et al. 2008. Cell wall modifications in Arabidopsis plants with altered α-l-arabinofuranosidase activity. Plant Physiology 147: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW, Drew MC. 2004. Plant cell death and cell differentiation. In: Noodén LD, ed. Plant cell death processes. Amsterdam: Elsevier, 19–36. [Google Scholar]
- Mortimer JC, Miles GP, Brown DM, et al. 2010. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proceedings of the National Academy of Sciences, USA 107: 17409–17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V, Stamm R, Eibl H, et al. 1985. Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis 6: 427–448. [Google Scholar]
- Pennell RI, Lamb C. 1997. Programmed cell death in plants. The Plant Cell 9: 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford JE, White RG. 2001. Effecting of tissue-preparation-induced callose synthesis on estimates plasmodesma size limits. Protoplasma 216: 47–55. [DOI] [PubMed] [Google Scholar]
- Rahji I, Yamauchi T, Takahashi H, et al. 2011. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytologist 190: 351–368. [DOI] [PubMed] [Google Scholar]
- Rose JKC, Cosgrove DJ, Albersheim P, Darvill AG, Bennett AB. 2000. Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiology 123: 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sachs MM. 1996. A flooding-induced xyloglucan endotransglycosylase homolog in maize is responsive to ethylene and associated with aerenchyma. Plant Physiology 112: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Sieiro C, Revilla G, Gonzalez-Villa T, Zarra I. 2001. Cloning and expression pattern of a gene encoding an alpha-xylosidase active against xyloglucan oligosaccharides from Arabidopsis. Plant Physiology 126: 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GB, Gross KC. 1996. Cell wall disassembly and fruit softening. Postharvest News and Information 7: 5N–52N. [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. 2006. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols 6: 2856–2860. [DOI] [PubMed] [Google Scholar]
- Shiono K, Takahashi H, Colmer TD, Nakazono M. 2008. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Science 175: 52–58. [Google Scholar]
- Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. 2009. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. The Plant Cell 21: 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BA, Clarke AE. 1992. Chemistry and biology of (1,3)-beta-glucans. Victoria, Australia: La Trobe University Press. [Google Scholar]
- Subbaiah CC, Sachs MM. 2003. Molecular and cellular adaptations of maize to flooding stress. Annals of Botany 90: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunna A, Antranikian G. 1997. Xylanolytic enzymes from fungi and bacteria. Critical Reviews in Biotechnology 17: 39–67. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kato A, Nagata N, Komeda Y. 2002. A xylanase, AtXyn1, is predominantly expressed in vascular bundles, and four putative xylanase genes were identified in the Arabidopsis genome. Plant & Cell Physiology 43: 759–767. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yamauchi T, Colmer TD, Nakazono M. 2014. Aerenchyma formation in plants. Plant Cell Monographs 21: 247–265. [Google Scholar]
- Tavares EQP, Grandis A, Lembke CG, et al. 2018. Roles of auxin and ethylene in aerenchyma formation in sugarcane roots. Plant Signaling & Behavior 13: e1422464. doi: 10.1080/15592324.2017.1422464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares EQP, De Souza AP, Romim GH, et al. 2019. The control of endopolygalacturonase expression by sugarcane RAV transcription factor during aerenchyma formation. Journal of Experimental Botany 70: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsushi H, Karim A. 2007. Flooding tolerance of sugarcane in relation to growth. South Pacific Studies 28: 9–21. [Google Scholar]
- Trought MCT, Drew MC. 1980. The development of waterlogging damage in young wheat plants in anaerobic solution cultures. Journal of Experimental Botany 31: 1573–1580. [Google Scholar]
- Vettore AL, Silva FR, Kemper EL, Arruda P. 2001. The libraries that made SUCEST. Genetics and Molecular Biology 24: 1–4. [Google Scholar]
- Visser EJW, Voesenek LACJ. 2004. Acclimation to soil flooding – sensing and signal-transduction. Plant and Soil 254: 197–214. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. 2000. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell & Environment 23: 1237–1245. [Google Scholar]
- Voesenek LACJ, Bailey-Serres 2015. Flood adaptive traits and processes: an overview. New Phytologist 206: 57–73. [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP. 2001. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology 47: 9–27. [PubMed] [Google Scholar]
- Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R. 1992. Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. The Plant Cell 4: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AJ, de Kroon H, Visser EJ, et al. 2017. Plants are less negatively affected by flooding when growing in species-rich plant communities. New Phytologist 213: 645–656. [DOI] [PubMed] [Google Scholar]
- Wu SSH, Suen DF, Chang HC, Huang AHC. 2002. Maize tapetum xylanase is synthesized as a precursor, processed and activated by a serine protease, and deposited on the pollen. Journal of Biological Chemistry 277: 49055–49064. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Colmer TD, Pedersen O, Nakazono M. 2018. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology 176: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliev R, Ueki S, Epel BL. 2011. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 248:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.