Abstract
Background and Aims
INDETERMINATE DOMAIN 10 (IDD10) is a key transcription factor gene that activates the expression of a large number of NH4+-responsive genes including AMMONIUM TRANSPORTER 1;2 (AMT1;2). Primary root growth of rice (Oryza sativa) idd10 mutants is hypersensitive to NH4+. The involvement of CALCINEURIN B-LIKE INTERACTING PROTEIN KINASE (CIPK) genes in the action of IDD10 on NH4+-mediated root growth was investigated.
Methods
Quantitative reverse transcription–PCR was used to analyse NH4+- and IDD10-dependent expression of CIPK genes. IDD10-regulated CIPK target genes were identified using electrophoretic mobility shift assays, chromatin immunoprecipitation and transient transcription assays. Root growth rate, ammonium content and 15N uptake of cipk mutants were measured to determine their sensitivity to NH4+ and to compare these phenotypes with those of idd10. The genetic relationship between CIPK9 OX and idd10 was investigated by crosses between the CIPK9 and IDD10 lines.
Key Results
AMT1;2 was overexpressed in idd10 to determine whether NH4+-hypersensitive root growth of idd10 resulted from limitations in NH4+ uptake or from low cellular levels of NH4+. High NH4+ levels in idd10/AMT1;2 OX did not rescue the root growth defect. Next, the involvement of CIPK genes in NH4+-dependent root growth and interactions between IDD10 and CIPK genes was investigated. Molecular analysis revealed that IDD10 directly activated transcription of CIPK9 and CIPK14. Expression of CIPK8, 9, 14/15 and 23 was sensitive to exogenous NH4+. Further studies revealed that cipk9 and idd10 had almost identical NH4+-sensitive root phenotypes, including low efficiency of 15NH4+ uptake. Analysis of plants containing both idd10 and CIPK9 OX showed that CIPK9 OX could rescue the NH4+-dependent root growth defects of idd10.
Conclusions
CIPK9 was involved in NH4+-dependent root growth and appeared to act downstream of IDD10. This information will be useful in future explorations of NH4+ signalling in plants.
Keywords: Rice (Oryza sativa), ammonium, root growth, CIPK9, IDD10, transcription factor
INTRODUCTION
Nitrate (NO3–) and ammonium (NH4+) are the major forms of nitrogen (N) in higher plants. Nitrogen is an important macro-element required for the synthesis of cellular molecules such as amino acids and nucleotides. Reduction of NO3– to NH4+ consumes 12–26 % of photosynthetically generated reductant (Patterson et al., 2010), which makes NH4+ an energetically favourable N source. At high concentrations, however, NH4+ is toxic to many plant species (Britto and Kronzucker, 2002). Rice grown in paddy fields uses NH4+ as its main N source. Previous studies on the effects of NH4+ on rice seedling roots showed that high NH4+ concentrations induce primary root coiling under light conditions, and that inhibition of NH4+ assimilation rescues NH4+-induced root coiling (Hirano et al., 2008; Shimizu et al., 2009); however, the details of this mechanism remain elusive. We showed previously that INDETERMINATE DOMAIN 10 (IDD10) regulates NH4+-mediated gene expression and root growth in rice (Oryza sativa) (Xuan et al., 2013); idd10 mutants are sensitive to NH4+ and exhibit short primary roots.
A comparison of the effects of NO3– and NH4+ on the arabidopsis (Arabidopsis thaliana) transcriptome found that approx. 60 % of genes displayed a common response to NO3– and NH4+. In addition, application of methionine sulphoximine (MSX), an inhibitor of glutamine synthetase, did not block all NH4+-responsive genes, indicating that NH4+ itself is a signalling molecule (Patterson et al., 2010). Researchers have studied global gene expression changes under N starvation conditions to explore N signalling in rice (Lian et al., 2006; Cai et al., 2012; W. Yang et al., 2015) and a broad range of early responses have been analysed under different concentrations of NH4+ (Xuan et al., 2013; S.Y. Yang et al., 2015). Rice genes responding to cellular N status are involved in diverse aspects of metabolism and signalling, including N and carbon metabolism, stress responses and hormonal signalling, suggesting that NH4+ is a signalling molecule that regulates diverse biological pathways in plants.
Expression of the CALCINEURIN B-LIKE INTERACTING PROTEIN KINASE 23 (CIPK23) gene is sensitive to exogenous NO3– content; moreover, in arabidopsis, CIPK23 interacts with and phosphorylates NO3–TRANSPORTER 1.1 (NRT1.1), thus modulating its affinity for its substrate (Ho et al., 2009). Furthermore, when the exogenous NH4+ concentration is high, the T460 residue in AMMONIUM TRANSPORTER 1;1 (AMT1;1) is phosphorylated (Lanquar et al., 2009). This reaction is also mediated by CIPK23 in arabidopsis (Straub et al., 2017); furthermore, CIPK23 directly regulates the potassium transporter, AKT1, via phosphorylation in both arabidopsis and rice (Xu et al., 2006; Li et al., 2014). Another member of this kinase gene family, CIPK8, regulates the low-affinity phase N response (Hu et al., 2009). In arabidopsis, CIPK9 regulates potassium homeostasis under low potassium conditions (Pandey et al., 2007; Liu et al., 2012). Transcriptomic and phosphoproteomic studies have revealed that transcription and phosphorylation of CIPKs are upregulated during NH4+ response (Patterson et al., 2010; Engelsberger and Schulze, 2012), indicating that CIPK and nutrient signalling are closely connected in plants.
We explored NH4+-dependent root growth using idd10 mutants, whose roots are hypersensitive to NH4+. The relationship between the cellular NH4+ level and IDD10 was examined by overexpressing AMT1;2 in idd10 plants. Involvement of CIPK genes in NH4+-dependent root growth and the interaction between IDD10 and CIPK genes was analysed using quantitative reverse transcription–PCR (qRT–PCR), chromatin immunoprecipitation (ChIP), electrophoretic mobility shift assay (EMSA) and transient transcription assays. Ammonium-dependent root growth was compared between cipk9 and idd10 mutants, and, in addition, the genetic relationship between CIPK9 and IDD10 was examined. These approaches enabled elucidation of the interaction and relationship between IDD10 and CIPK9 in determining NH4+-dependent root growth. This constitutes an important contribution to the current understanding of NH4+ signalling in plants.
MATERIALS AND METHODS
Plant materials and growth conditions
To examine the effects of NH4+ on gene expression, germinated seeds were grown in dH2O in a greenhouse for 14 d to deplete nutrients in the endosperm. Seedlings were then grown for an additional 3 d in N-free nutrient (–N) solution (Abiko et al., 2005) before being transferred to the same nutrient solution containing 0.5 mm (NH4)2SO4. The N-free nutrient solution contained 7 μm Na2HPO4·12H2O, 16 μm KCl, 7 μm CaCl2·2H2O, 15 μm MgCl2·6H2O, 36 μm FeSO4·7H2O, 9 μm MnSO4·7H2O, 45 μm H3BO4, 3 μm ZnSO4·7H2O, 0.2 μm CuSO4·7H2O, 0.05 μm Na2MoO4·2H2O. Whole roots were harvested 0, 1, 3 and 6 h after supplementation with (NH4)2SO4.
To measure plant growth and ammonium contents, germinated seeds were cultured for 4 d in modified half-strength Kimura B (KB) solutions containing NH4+ or NO3– as the sole N source. The original half-strength KB nutrient solution contained macronutrients [0.18 mm (NH4)2SO4, 0.27 mm MgSO4·7H2O, 0.09 mM KNO3, 0.18 mM CaNO3·4H2O and 0.09 mm KH2PO4] and micronutrients [20 μm Na2EDTA-Fe(II)·3H2O, 9.0 μm MnCl2·4H2O, 46 μm H3BO3, 9.0 μm Na2MoO4·4H2O, 0.7 μm ZnSO4·7H2O and 0.3 μm CuSO4·5H2O] at pH 5.8. For the modified KB nutrient, 0.18 mm (NH4)2SO4 and 0.09 mm KNO3 were replaced with appropriate concentrations of (NH4)2SO4 or KNO3, and 0.18 mm CaNO3·4H2O was replaced with 0.18 mm CaCl2. Detailed information on the constituents of solutions is given in Supplementary data Table S1.
To analyse the effects of MSX on cipk9 phenotypes, germinated seeds were cultured for 4 d in modified half-strength KB solutions containing an N source and MSX as follows: Solution 1, both NH4+ and NO3–; Solution 2, 0.5 mm (NH4)2SO4; Solution 3, 0.5 mm KNO3; Solution 4, 1 μm MSX; and Solution 5, 0.5 mm (NH4)2SO4 and 1 μm MSX. Detailed information on the constituents of solutions is given in Supplementary data Table S2.
To examine the effect of potassium ions (K+) on cipk9 and idd10 phenotypes, germinated seeds were cultured in modified half-strength KB solutions containing K+ and NH4+ as follows: Solution A, 10 mM K+ and no NH4+; Solution B, 10 mm K+ and 0.5 mm NH4+; Solution C, no K+ and 0.5 mm NH4+; and Solution D, no K+ and no NH4+. Detailed information on the constituents of solutions is given in Supplementary data Table S3.
To test N-dependent root growth and chlorophyll biosynthesis, 0.1, 0.25, 0.5 and 1 mm NO3– or NH4+ were added to the N-free nutrient solution (Supplementary data Table S1). Primary root length was measured after 4 d of growth. The NH4+ content was also measured in roots from 4-day-old plants grown in a solution containing 0.5 mm NO3– or (NH4)2SO4. Detailed information on the constituents of solutions is given in Supplementary data Table S1.
CIPK lines containing T-DNA insertions were identified using the RiceGE database (http://signal.salk.edu/cgi-bin/RiceGE);cipk8 (PFG_1C-09231.R), cipk14 (PFG_1A-20931.L) and cipk23 (PFG_3D-01239.L) were identified in this way. In addition, cipk9 was isolated from the Ds-tagging mutant pool (Chin et al., 1999).
RNA extraction and qRT–PCR analysis
Total cellular RNA was isolated using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) and treated with RQ-RNase free DNase (Promega, Madison, WI, USA). A reverse transcriptase RNase H (Toyobo, http://www.toyobo-global.com/) transcription kit was used to synthesize cDNA according to the manufacturer’s instructions (Promega). qRT–PCR products were quantified using Illumina Eco 3.0 software (Illumina, San Diego, CA, USA), and values were normalized against UBIQUITIN, OsUBC3 (Yang et al., 2017; Hsieh et al., 2018), UBQ1 (Wang et al., 2018), UBQ5 (Wu et al., 2017) and 18S rRNA (Jain et al., 2006; Ishikawa-Sakurai et al., 2014) levels in the same samples. All primers used for qRT–PCR are presented in Supplementary data Table S4.
Chromatin immunoprecipitation assay
Samples of 8 g of calli from transgenic rice plants expressing IDD10-GFP were prepared for ChIP analysis. Pre-absorption with a pre-immune serum was performed before immunoprecipitation with an anti-GFP (green fluorescent protein) monoclonal antibody (Clontech). Immunoprecipitates were analysed using qPCR. Each input DNA level was used as a control for normalizing the levels of immunoprecipitated DNA in the qPCR assay (Je et al., 2010). All primers used for ChIP-PCR are listed in Supplementary data Table S5.
Electrophoretic mobility shift assay
N-terminal IDD10 cDNA sequences (67–254 amino acids) were sub-cloned into the pGEX5×-1 expression vector to produce the pGEX5×-1-IDD10 plasmid. Escherichia coli strain BL21 DE3 was transformed with pGEX5×-1-IDD10 to enable production of IDD10 recombinant protein.
A single colony of BL21 E. coli harbouring pGEX5×-1-IDD10 plasmid was inoculated into liquid LB medium containing ampicillin at 100 μg ml–1 and 0.5 mm isopropyl-β-d-thiogalactopyranoside (IPTG) was added when the optical density (OD) reached 0.2. After 4 h of induction at 28 °C, cells were harvested by centrifugation at 4000 rpm for 5 min at 4 °C. The supernatant was then removed and the cells were resuspended in 1× phosphate-buffered saline (PBS) solution, and were subsequently sonicated for 15 min (three times, 5 min each). After sonication, the cell lysate was centrifuged at 12 000 rpm for 20 min at 4 °C, and the supernatant was then reacted with glutathione S-transferase (GST) beads for purification (Xuan et al., 2013). The purified GST–IDD10 protein was examined using SDS–PAGE (Supplementary data Fig. S1).
For EMSA, a standard binding reaction was performed in a total volume of 20 μL by incubating 1 μg of purified protein with 40 000 cpm of 32P-labelled DNA probe and 1 μg of poly(dI–dC) in reaction buffer [25 mm HEPES-KOH, pH 7.5, 100 mm KCl, 0.1 mm EDTA, 10 % (v/v) glycerol, 1 mm dithiothreitol (DTT)] at room temperature for 30 min. The binding reaction products were resolved on an 8 % polyacrylamide gel run in 0.5× TBE buffer (Je et al., 2010). For probe labelling, 30 nucleotide DNA fragments were synthesized and end-labelled with [γ-32P]ATP using T4 polynucleotide kinase (NEB, Ipswich, MA, USA). The primers used to produce the EMSA probes are listed in Supplementary data Table S5.
Transcriptional activity analysis
Constructs containing an effector (35S:IDD10), reporters (P2, P3, mP2 and mP3) and an internal control (35S:LUC) were used to co-transform arabidopsis protoplasts (Yamaguchi et al., 2010). To generate p35S:IDD10, the GAL4BD region of the p35S:GAL4BD vector was replaced by IDD10 cDNA using the BamHI and SacI restriction sites. To obtain reporter vectors, 1.5 kb portions of the CIPK9 or CIPK14 promoter sequences were amplified using PCR. Expression of β-glucuronidase (GUS) was normalized against luciferase expression (Xuan et al., 2013). Polyethylene glycol (PEG)-mediated transformation and activity assays were performed as described previously (Yoo et al., 2007). The primers used to amplify the CIPK9 and CIPK14 promoter fragments are listed in Supplementary data Table S5.
Yeast one-hybrid analysis
The 1.5 kb regions from the CIPK9 and CIPK14 promoters were cloned into the pHISi vector, and the open reading frame (ORF) sequence of IDD10 was cloned into the pGAD424 vector. The appropriate pCIPK-His plasmid was transformed into yeast strain YM4271. Transformants containing each CIPK promoter were used as competent cells and transformed with pGAD424-IDD10 or pGAD424 empty vector. The growth of yeast cells on synthetic dropout media (-Leu and -Ura or -Leu and -Ura-His) was monitored.
Generation of transgenic plants
To generate plants overexpressing AMT1;2 and CIPK9, the AMT1;2 and CIPK9 ORFs were cloned into the BamHI and HindIII restriction sites of the pGA1611 binary vector to produce pGA1611-AMT1;2 and pGA1611-CIPK9, in which AMT1;2 and CIPK9 were expressed under the control of the UBIQUITIN promoter. Rice calli were transformed with pGA1611-AMT1;2 and pGA1611-CIPK9 via Agrobacterium-mediated transformation (Chin et al., 1999).
Determination of ammonium content
Enzymatic determination of NH4+ content in roots was performed using the F-kit (Roche), according to the manufacturer’s instructions (Oliveira et al., 2002).
GUS assay
Seven-day-old seedlings of CIPK9::Ds (cipk9 heterozygotes) were collected in 15 mL Falcon tubes containing GUS staining solution. All tubes were wrapped in foil and kept at 37 °C for 2 d (Chin et al., 1999).
Localization of CIPK9 in plants
The CIPK9 ORF was cloned into the pABindGFP destination plasmid (Bleckmann et al., 2010) to produce CIPK9:GFP, in which the CIPK9 ORF, fused to the GFP sequence at the N-terminus, was expressed under the control of the 35S promoter. This vector was expressed in Nicotiana benthamiana leaves via Agrobacterium-mediated transient expression (Kim et al., 2009). GFP fluorescence was detected using an Olympus confocal laser scanning microscope (Fluoview FV 1000, http://www.olympus-global.com/).
Tissue culture regeneration
To produce plantlets from calli, four different tissue culture media (NB, N6-7-CH, N6S3-CH-I and N6S3-CH-II) were used. The regeneration methodology was as described previously (Kim et al., 2004).
Southern blot analysis
Samples of 5 μg of genomic DNA were digested with EcoRI restriction endonucleases and separated on a 0.8 % agarose gel, followed by transfer to a nylon membrane. The blots were hybridized to probes in hybridization buffer containing 6× SSC, 5× Denhardt’s, 0.5 % SDS, 50 nm Tris (pH 8.0), 10 nm EDTA, 0.1 mg mL–1 heat-denatured salmon sperm DNA and 5 % dextran sulphate. Final washes of the filters were carried out in 0.2× SSC and 0.1 % SDS for 15 min at 65 °C. The membranes were exposed to X-rays.
15N uptake analysis
For hydroponic culture, rice seeds were surface-sterilized using 0.1 % Previcur N (Bayer, Monheim, Germany) for 15 min and germinated on paper towels at 37 °C for 3 d. Seedlings were transferred to, and pre-cultured in, deionized water for 2 weeks, and then grown in an N-free nutrient solution (Sonoda et al., 2003) for 3 d before 15NH4+ uptake analysis. Plants were grown in a climate-controlled chamber under a 14 h/37 °C and 10 h/30 °C day–night cycle at 60 % relative humidity.
Roots were washed in 1 mm CaSO4 for 1 min, and incubated for 6 min in N-free nutrient solution (pH 5.8) containing 200 μm15NH4+ (95 atom % 15N) as the sole N source. Roots were rinsed in 1 mm CaSO4 for 1 min before harvest to remove the tracer from the apoplast. Freeze-dried 1.5 mg samples were subjected to 15N analysis using isotope ratio mass spectrometry (DELTAplus XP, Thermo-Finnigan).
Statistical analyses
Statistical calculations were performed using Prism 5 (GraphPad, San Diego, CA, USA). Comparisons between groups were made using one-way analysis of variance (ANOVA; Brady et al., 2015), followed by Bonferroni’s correction for multiple comparisons. Differences in P-values < 0.05 were considered statistically significant. All data are expressed as the mean ± s.e.
RESULTS
Overexpression of AMT1;2 does not rescue idd10 root growth inhibition
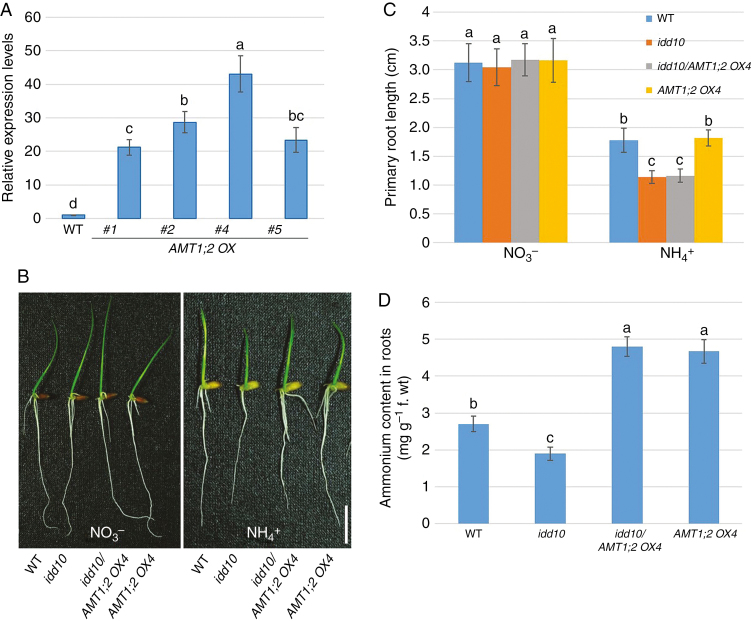
IDD10 directly activates expression of AMT1;2, and NH4+ accumulates to lower cellular levels in idd10 mutants than in wild-type plants (Xuan et al., 2013). To investigate whether limited NH4+ uptake or low levels of cellular NH4+ were responsible for growth retardation of idd10 roots, several lines overexpressing AMT1;2 (AMT1;2 OX) were generated and crossed with idd10 mutants. Analysis by qRT–PCR showed that AMT1;2 levels in the roots of plants from the overexpression lines (#1, #2, #4 and #5) were >20-fold higher than in wild-type roots (Fig. 1A). Line AMT1;2 OX4, which showed the highest expression of AMT1;2 mRNA, was selected for crossing with idd10 to examine the effect of combining the idd10 mutation with AMT1;2 overexpression.
Fig. 1.
Root growth and cellular NH4+ content of wild-type, AMT1;2 OX, idd10 and idd10/AMT1;2 OX4 plants. (A) AMT1;2 expression levels in the wild type (WT) and four AMT1;2 overexpression lines (AMT1;2 OX; #1, #2, #4 and #5) were monitored using qRT–PCR. AMT1;2 mRNA levels in the samples were normalized against expression of UBIQUITIN. Data are means ± S.E. (n = 3). (B) Wild-type, idd10, idd10/AMT1;2 OX4 and AMT1;2 OX4 were grown for 4 d in half-strength KB nutrient solution containing either NO3– or NH4+ as the sole N source. Scale bar = 1 cm. (C) Primary root length measurements of the plants shown in (B). Data are means ± s.e. (n > 10 plants per line). (D) Cellular NH4+ levels in wild-type and mutant roots grown for 4 d in a nutrient solution containing NH4+ as the sole N source. Details of all nutrient media are in Supplementary data Table S1. Data are means ± s.e. (n > 10 plants per line); different letters indicate significant differences between results (P < 0.05).
The four genotypes (wild type, idd10, AMT1;2 OX4 and idd10/AMT1;2 OX4) produced by crosses between AMT1;2 OX4 and idd10 lines were analysed using qRT–PCR to determine expression of AMT1;2 and IDD10 in 7-day-old roots (Supplementary data Fig. S2). Roots of idd10/AMT1;2 OX4 and AMT1;2 OX4 plants expressed similar levels of AMT1;2 mRNA; however, IDD10 expression was completely inhibited in both idd10/AMT1;2 OX4 and idd10 roots.
The growth responses of wild-type seedlings to nutrient solutions containing different concentrations (0.1, 0.25, 0.5 and 1 mm) of NO3– or NH4+ were determined. Concentrations of 1 mm NO3– or NH4+ produced the highest chlorophyll contents in leaves (Supplementary data Fig. S3A). Primary roots grown in NO3– were longer than primary roots grown in the same concentrations of NH4+ (Supplementary data Fig. S3B).
To evaluate the effect of AMT1;2 overexpression on idd10 root growth, wild-type, idd10, idd10/AMT1;2 OX4 and AMT1;2 OX4 roots were grown in nutrient solutions containing 1 mm NO3– or NH4+. Nitrogen-dependent root growth was measured in 7-day-old roots after incubation in the nutrient solutions for 4 d (Fig. 1B, C). A nutrient solution containing 1 mm KNO3 produced no differences in primary root growth between wild-type, idd10, idd10/AMT1;2 OX4 and AMT1;2 OX lines. Following 4 d of growth in a solution containing 1 mm NH4+, primary root lengths of idd10/AMT1;2 OX4 and idd10 were similar, and shorter than roots of wild-type and AMT1;2 OX4 seedlings. The growth of wild-type and AMT1;2 OX4 primary roots was similar in the solution containing 1 mm NH4+.
Cellular NH4+ content was determined in the roots of all four genotypes (Fig. 1D). Ammonium content was lowest in idd10 roots, but accumulated to a similar level in idd10/AMT1;2 OX4 and AMT1;2 OX4 roots; this level was higher than that found in wild-type roots. These results suggest that enhancing NH4+ uptake and cellular NH4+ levels does not rescue the growth defect of idd10 roots.
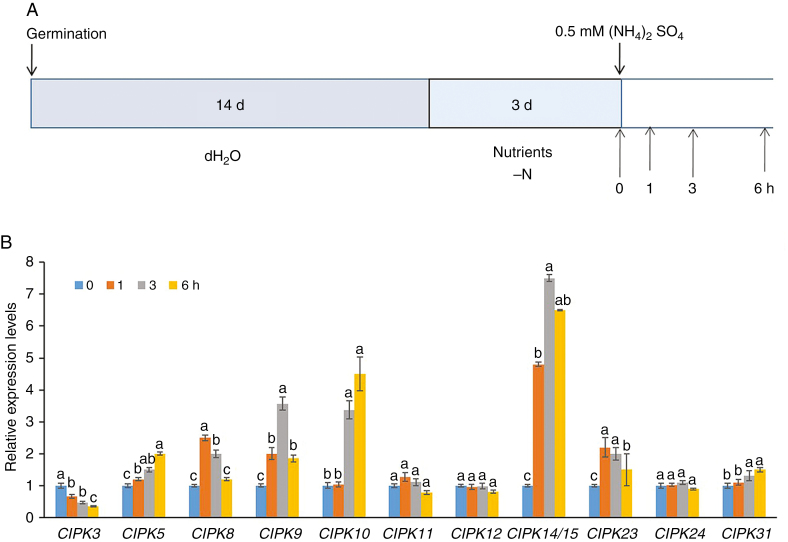
Expression of CIPK genes is sensitive to ammonium
To determine the genetic factors responsible for the defective root growth of idd10, NH4+-responsive regulatory genes, identified in an earlier analysis of transcriptomic data (Xuan et al., 2013), were inspected. The expression of 12 CIPK genes out of a 31 member family in rice was noted to be sensitive to NH4+. To analyse CIPK gene expression in response to NH4+ further, 17-day-old wild-type plants grown without N were treated with 0.5 mm (NH4)2SO4 for 0, 1, 3 and 6 h (Fig. 2A) and RNA was extracted from samples of whole roots. Expression of the 12 CIPK genes (CIPK3, 5, 8, 9, 10, 11, 12, 14/15, 23, 24 and 31) was analysed using qRT–PCR to determine their responses to NH4+; CIPK expression was normalized against expression of the internal control genes UBIQUITIN (Fig. 2B), OsUBC3, UBQ1, UBQ5 and 18S rRNA (Supplementary data Fig. S4A–D, respectively). Induction of CIPK14 and 15 had to be calculated together as CIPK14/15 due to extremely high homology (96 % identity) between their nucleotide sequences (Kurusu et al., 2010).
Fig. 2.
Expression of CIPK genes in response to ammonium treatment. (A) Schematic showing NH4+ treatments and time points for sampling expression of NH4+-responsive CIPK genes. Wild-type seedlings were grown for 14 d in dH2O, followed by 3 d in nutrient medium without N. The 17-day-old plants were then transferred to medium supplemented with 0.5 mm (NH4)2SO4 for 0, 1, 3 and 6 h. Details of the nutrient medium are described in the Materials and Methods. (B) Responses of 12 CIPK genes (CIPK3, 5, 8, 9, 10, 11, 12, 14/15, 23, 24 and 31) to NH4+ treatment. Expression levels of CIPK genes were determined using qRT–PCR. mRNA levels in the samples were normalized against those of UBIQUITIN mRNA. Data are means ± s.e. (n = 3); different letters indicate significant differences between results (P < 0.05).
Ammonium repressed expression of CIPK3 in roots but induced expression of CIPK5, CIPK8, CIPK9, CIPK10, CIPK14/15, CIPK23 and CIPK31; transcript levels of CIPK11, CIPK12 and CIPK24 were not altered by NH4+. CIPK8, CIPK9 and CIPK23 were induced >2-fold, while CIPK14/15 showed a >10-fold increase (Fig. 2B).
In addition, NH4+-dependent expression of CBL genes was analysed (Supplementary data Fig. S5). Of the ten CBL genes, CBL1, CBL2, CBL3 and CBL6 were consistently induced by NH4+ supplementation; expression of CBL2 and CBL3 increased >2-fold. Expression of the other CBL genes was not altered by NH4+.
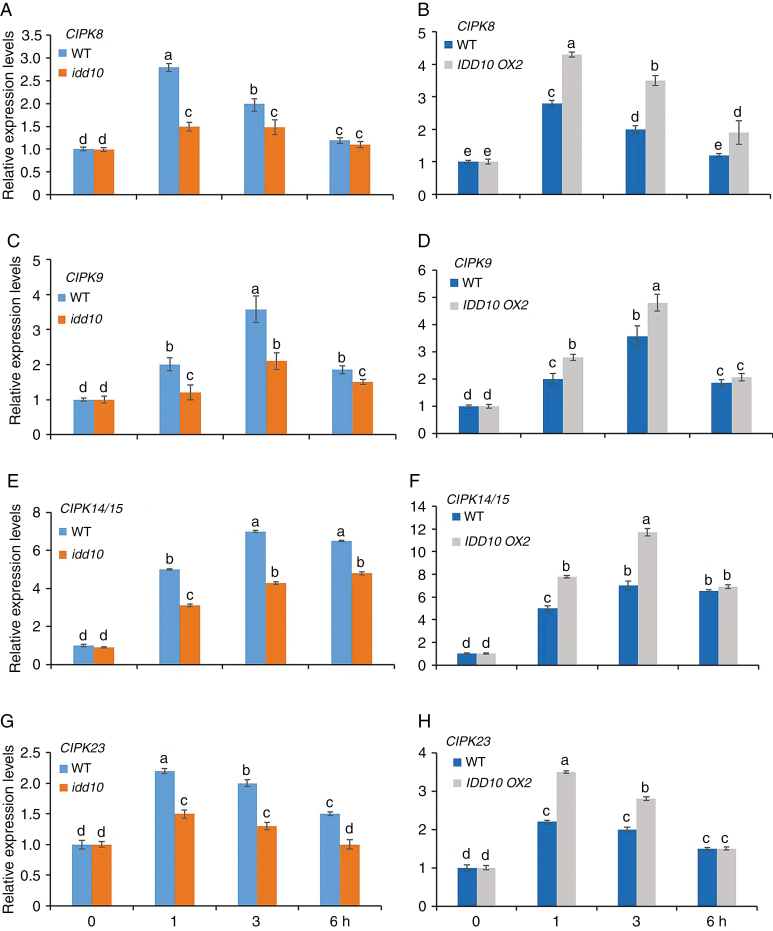
Five CIPK genes are positively regulated by IDD10 in an NH4+-dependent manner
To examine whether IDD10 was involved in NH4+-responsive CIPK expression, the expression levels of the five CIPK genes (CIPK8, CIPK9, CIPK14/15 and CIPK23) that were induced >2-fold by NH4+ treatment were examined in the roots of idd10 and IDD10 OX (OX2) plants and their wild-type siblings. Seventeen-day-old wild-type seedlings were treated with 0.5 mm (NH4)2SO4 for 0, 1, 3 and 6 h. Expression levels of the five genes were determined using qRT–PCR; UBIQUITIN (Fig. 3) and OsUBC3 (Supplementary data Fig. S6) were used as internal normalization controls.
Fig. 3.
Responses of five CIPK genes in roots of idd10 and IDD10 OX2 plants to exposure to NH4+. (A, C, E and G) Expression of CIPK8, 9, 14/15 and 23 in idd10 and wild-type plants. (B, D, F and H) Expression of CIPK8, 9, 14/15 and 23 in IDD10 OX2 and wild-type plants. Levels of CIPK mRNA expression were determined using qRT–PCR. Sample mRNA levels were normalized against those of UBIQUITIN mRNA. Seedling growth conditions and treatments were as shown in Fig. 2A; total roots were sampled 0, 1, 3 and 6 h after the addition of NH4+. Data are means ± s.e. (n = 3); different letters indicate significant differences between results (P < 0.05).
There were no differences in expression levels of any of the five CIPK genes between idd10 and its wild-type siblings (Fig. 3A, C, E, G) or between OX2 and its wild-type siblings (Fig. 3B, D, F, H) before NH4+ treatment. After NH4+ treatment, lower levels of induction of CIPK genes were observed in idd10 than in wild-type plants; in contrast, induction of CIPK genes was higher in OX2 than in wild-type plants following treatment. These results suggest that IDD10 exerts positive effects on NH4+-mediated induction of CIPK8, CIPK9, CIPK14/15 and CIPK23.
CIPK9 is a direct target of IDD10 and its mutation results in sensitivity to ammonium
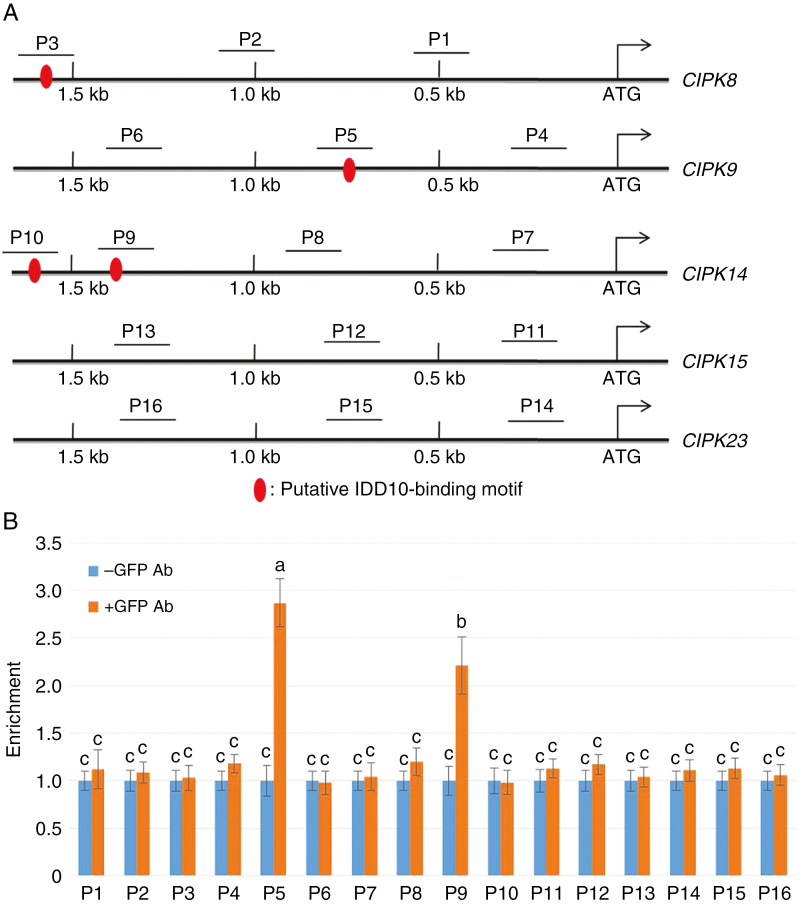
As IDD10 influenced NH4+-mediated induction of five CIPK genes, the possibility that they were directly activated by IDD10 was examined by analysing 1.5 kb of their promoter sequences. The putative IDD10-binding motif (TTTGTCG/C) (Xuan et al., 2013) was found in the CIPK8, CIPK9 and CIPK14 promoters, but not in the CIPK15 and CIPK23 promoters (Fig. 4A). The IDD family protein JKD activates the SCR and MGP promoters even though they lack the putative IDD-binding motif, suggesting that IDD proteins have diverse binding modes (Ogasawara et al., 2011).
Fig. 4.
Binding affinity of IDD10 for the promoters of CIPK9 and CIPK14. (A) Schematic diagram showing the locations of putative IDD10-binding motifs in 1.5 kb regions of the promoters of five different CIPK genes. The areas amplified by the probes P1–P16 in the ChIP assay are underlined. (B) Results of the ChIP assay. DNA was immunoprecipitated and amplified to determine the extent to which IDD10 bound regions P1–P16 within a 1.5 kb portion of each CIPK promoter. The relative ratios of immunoprecipitated DNA to input DNA were determined using qPCR. Input DNA was used to normalize the data. DNA immunoprecipitated without addition of GFP antibody (Ab) was used as a control. Data are means ± s.e. (n = 3); different letters indicate significant differences between results (P < 0.05).
To determine whether IDD10 binds directly to the CIPK8, CIPK9, CIPK14, CIPK15 and CIPK23 promoters, ChIP assays were performed using IDD10-GFP transgenic plants. Immunoprecipitates obtained from IDD10-GFP transgenic plants in the presence or absence of a GFP antibody were analysed using qRT–PCR. In total, 16 primer sets were used to amplify 16 promoter regions (P1–P16) from the five CIPK genes; these included four regions containing putative IDD10-binding motifs. All the promoter regions were located within 1.5 kb from the ATG start sites of the genes. qRT–PCR analysis detected significantly higher amounts of DNA from the P5 and P9 regions following immunoprecipitation with a GFP antibody compared with controls without the antibody (Fig. 4B). These two regions corresponded to the sections of the CIPK9 and CIPK14 promoters containing the putative IDD10-binding motifs.
A yeast one-hybrid assay was also used to confirm whether IDD10 activated the CIPK promoters. The 1.5 kb regions from the CIPK8, CIPK9, CIPK14, CIPK15 and CIPK23 promoters were examined for transcriptional activation in the yeast one-hybrid system. IDD10 was able to activate the CIPK9 and CIPK14 promoters in yeast (Supplementary data Fig. S7), consistent with the results obtained from the ChIP assays.
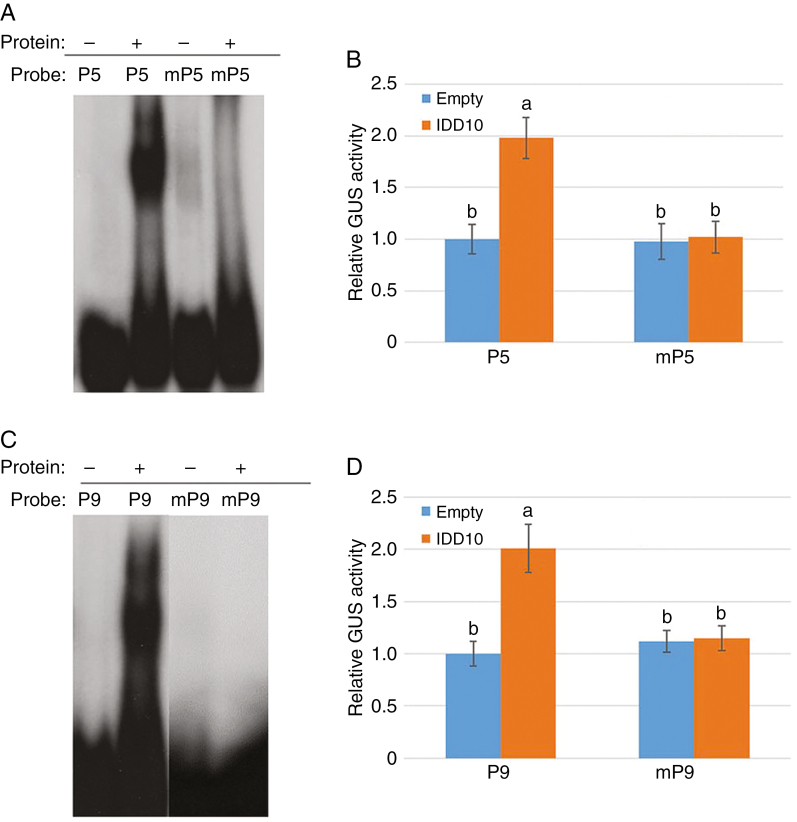
To verify that IDD10 activated CIPK9 and CIPK14 via IDD-binding motifs, EMSA and an in vivo transcription activation assay were performed. Mutant P5 and P9 (mP5 and mP9) regions were created by changing the binding motif sequence in P5 from TTTGTCG to TTTTTTT and the sequence in P9 from AAACAGG to AAAAAAA. EMSA was used to determine the binding affinities of IDD10 for the wild-type and mutated P5 and P9 regions. The assays showed that IDD10 bound to P5 and P9, but not to mP5 and mP9 (Fig. 5A, C).
Fig. 5.
Activation of CIPK9 and CIPK14 mediated by binding of IDD10 to IDD-binding motifs. (A) Binding affinities of IDD10 for the native IDD10-binding motif (TTTGTCG; P5) from the CIPK9 promoter and a mutated motif (TTTTTTT; mP5). (B) Activation of the native CIPK9 promoter (P5) and the mutated promoter (mP5) in protoplasts co-transfected with IDD10. (C) Binding affinities of IDD10 for the native IDD10-binding motif (AAACAGG; P9) from the CIPK14 promoter and a mutated motif (AAAAAAA; mP9). (D) Activation of the native CIPK14 promoter (P9) and the mutated promoter (mP9) in protoplasts co-transfected with IDD10. Binding affinities (A and C) of IDD10 for native (P5 and P9) and mutated (mP5 and mP9) putative IDD10-binding motifs were determined using EMSA. Transient expression assays (B and D) were performed in arabidopsis protoplasts co-transfected with IDD10 and vectors expressing GUS under the control of a 1.5 kb promoter from CIPK9 (B) or CIPK14 (D) containing either native or mutated IDD-binding motif sequences; co-transfection with empty vector was used as a control. Luciferase expression driven by the 35S promoter was used as an internal control for normalizing GUS expression data. Data in (B) and (D) are means ± s.e. (n = 3); different letters indicate significant differences between results (P < 0.05).
To confirm that these cis-elements were responsible for transcriptional activation of the CIPK9 and CIPK14 promoters by IDD10 in vivo, transient expression assays were performed using an arabidopsis protoplast system (Fig. 5B, D). Protoplast cells were co-transformed with the 35S:IDD10 plasmid and a vector expressing GUS under the control of either the wild-type CIPK9 (P5) and CIPK14 (P9) promoters or promoters containing the mutated motifs (mP5 and mP9); 35S:LUC was used as an internal control to normalize transformation efficiency in each assay. GUS activity was approx. 2-fold higher in protoplasts expressing IDD10 under the P5 and P9 promoters than in protoplasts expressing the reporter alone or containing either mutated promoter. This indicates that IDD10 activates expression of CIPK9 and CIPK14 directly by binding to their promoters.
CIPK9 and CIPK23 mutants show NH4+-sensitive phenotypes
To determine whether CIPK mutants exhibited NH4+-sensitive root growth, cipk8, cipk14 and cipk23 T-DNA insertion mutants and a cipk9 Ds gene trap mutant (Chin et al., 1999) were isolated as described in the Materials and Methods (Fig. 6A). Expression of each CIPK gene was examined in the roots of 7-day-old wild-type and cipk mutant seedlings (Supplementary data Fig. S8). Expression of CIPK8, CIPK9 and CIPK23 was completely suppressed in their respective mutants. A low level of CIPK14 mRNA was detected in cipk14 mutants; this may result from the high sequence homology between CIPK14 and CIPK15 (Kurusu et al., 2010) (Supplementary data Fig. S8).
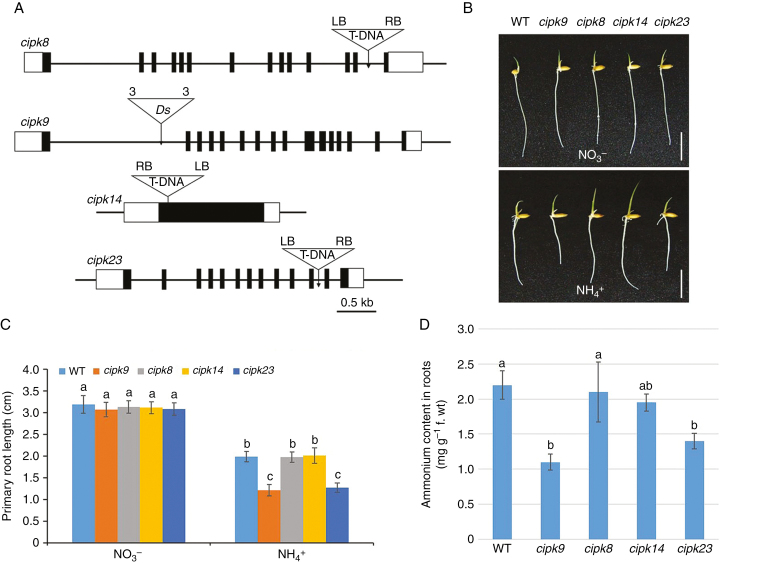
Fig. 6.
Gene structure, root growth and cellular NH4+ content of cipk mutants. (A) Schematic diagrams showing gene structures of the cipk insertion mutants, cipk8, cipk9, cipk14 and cipk23. Black boxes, exons; white boxes, untranslated regions. Triangles indicate T-DNA or Ds insertion sites; ‘3’ and ‘5’ indicate the 3’ and 5’ ends of Ds, respectively; RB, right border of T-DNA; LB, left border of T-DNA. Scale bar = 0.5 kb. (B) Appearances of wild-type (WT), cipk8, cipk9, cipk14 and cipk23 seedlings after 4 d growth in nutrient solution containing either NO3– or NH4+ as the sole N source (Supplementary data Table S1). Scale bar = 1 cm. (C) Primary root lengths of wild-type, cipk8, cipk9, cipk14 and cipk23 seedlings grown as in (B). (D) Cellular NH4+ levels in roots of each line after 4 d growth in nutrient solution containing NH4+ as the sole N source. Data in (C) and (D) are means ± s.e. (n > 10 plants per line); different letters indicate significant differences between results (P < 0.05).
The primary root lengths of cipk mutants were determined following growth in nutrient solutions containing 1 mm NO3– or NH4+ (Fig. 6B, C). No significant differences between wild-type and cipk mutants were observed after growth in NO3– solution. In contrast, the primary roots of cipk9 and cipk23 were significantly shorter than wild-type roots after 4 d of growth in NH4+ solution. Cellular NH4+ content was measured in roots of 4-day-old wild-type, cipk8, 9, 14 and 23 plants grown in nutrient solution containing NH4+ (Fig. 6D). cipk9 and cipk23 plants contained lower levels of cellular NH4+ than wild-type plants, but the NH4+ content of cipk8 and cipk14 was similar to that of wild-type roots. Together, these results show that CIPK9 and CIPK23 exhibit NH4+-sensitive phenotypes.
Comparative analysis of NH4+-sensitive root growth in cipk9 and idd10
Although it is likely that IDD10 influences the effect of NH4+ on the expression and phenotypes of both CIPK9 and CIPK23, only CIPK9 was found to be a direct target of IDD10. The expression pattern of CIPK9 was therefore analysed in greater detail to compare its effect on NH4+-dependent root phenotypes with that of IDD10.
The pattern of CIPK9 expression was examined by a qRT–PCR analysis of different tissues from wild-type plants and by GUS staining the cipk9::Ds insertion mutant (Chin et al., 1999) in planta (Supplementary data Fig. S9). Both analyses showed that CIPK9 was expressed in all tissues. To determine the subcellular localization of CIPK9, CIPK9–GFP was transiently expressed in N. benthamiana leaves using Agrobacterium-mediated transformation. Strong GFP signals were detected in both the nuclei and cytosol (Fig. 7B).
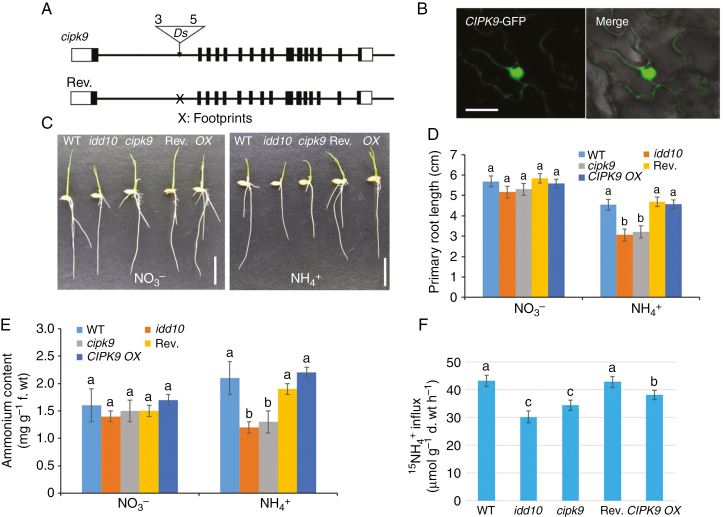
Fig. 7.
Gene structures of cipk9 and its revertant, sub-cellular localization of CIPK9, and N-responsive root growth, NH4+ content and 15NH4+ uptake in cipk9 and idd10 mutants. (A) Schematic diagrams showing the genomic structures of the cipk9::Ds insertion mutant and its revertant. Black boxes, exons; white boxes, untranslated regions. The triangle indicates the Ds insertion site; ‘3’ and ‘5’ indicate the 3′ and 5′ ends of Ds, respectively. Ds was excised from revertant plants leaving a footprint ‘X’ in the first intron. (B) Transient expression of CIPK9–GFP in Nicotiana benthamiana leaves. Left panel, confocal microscopy of green fluorescent protein (GFP); right panel, overlay of light microscopy image with GFP signal from the same tissue. Scale bars = 20 μm. (C) Appearance of wild-type (WT), idd10, cipk9, CIPK9 revertant (Rev.) and CIPK9 overexpressing (OX) seedlings after 4 d growth in nutrient solutions with either NO3– or NH4+ as the sole N source (Supplementary data Table S1). Scale bar = 1 cm. (D) Measurements of primary root lengths of plants grown as shown in (C) (n > 10 plants per line). (E) Endogenous NH4+ levels in roots of seedlings grown for 4 d in a nutrient solution with either NO3– or NH4+ as the sole N source (n > 10 plants per line). (F) Analysis of 15NH4+ influx into roots of wild-type, idd10, cipk9, Rev. and CIPK9 OX plants. Ammonium uptake into rice roots was determined following exposure to 200 μm15N-labelled NH4+. Data shown in (D) and (E) are means ± s.e.; data shown in (F) are means ± s.d. (n > 10 plants per line). In all cases, different letters indicate significant differences between results (P < 0.05).
To analyse the hypersensitivity of root growth of cipk9 mutants to NH4+ further, CIPK9 revertants (Rev.) and overexpression lines (OX) were generated. Six revertants were obtained from cipk9 seeds via tissue culture regeneration (Fig. 7A). Southern blot hybridization and sequencing analysis of revertants confirmed excision of the Ds element from its original insertion site in the CIPK9 locus (Supplementary data Fig. S10). In the overexpression lines, CIPK9 cDNA was expressed under the control of the UBIQUITIN promoter.
Root growth, ammonium content and NH4+ uptake in CIPK9 Rev., CIPK9 OX, cipk9, idd10 and wild-type plants were measured and compared. No notable differences in root growth were observed between these five lines during culture in nutrient solution with NO3– as the sole N source (Fig. 7C, D). When NH4+ was the sole N source, the primary root length of Rev. plants resembled that of wild-type and OX plants; in contrast, the primary roots of cipk9 and idd10 mutants, although similar in length to each other, were shorter than those of the other genotypes (Fig. 7C, D). The internal NH4+ content of seedlings grown in nutrient solution containing NH4+ was measured and compared (Fig. 7E). The levels of NH4+ that accumulated in the roots of cipk9 and idd10 mutants were similar, and lower than levels in the other genotypes. There were no significant differences between NH4+ levels in wild-type, Rev. and CIPK9 OX seedlings. To determine whether CIPK9 affected NH4+ uptake, short-term influx of 15N-labelled ammonium into roots of 17-day-old wild-type, idd10, cipk9, Rev. and CIPK9 OX plants was measured (Fig. 7F). Roots were exposed to 200 μm15NH4+ for 6 min; the 15N influx was measured as μmol g–1 root d. wt h–1 (Yuan et al., 2007, 2013). 15N-labelled ammonium influx into cipk9 and idd10 mutants was similar and lower than influx into wild-type, Rev. and CIPK9 OX roots. Influx into CIPK9 OX roots was slightly lower than that into wild-type or Rev. roots.
As idd10 and cipk9 mutants exhibited the same root phenotypes, the possibility that expression of AMT1;2 was altered in cipk9 mutants was examined (Supplementary data Fig. S11). qRT–PCR was performed to measure expression of AMT1;2 mRNA in wild-type, idd10, cipk9, Rev. and OX plants. Similar levels of AMT1;2 mRNA were observed in cipk9 and CIPK9 OX seedlings, indicating that CIPK9 did not affect steady-state levels of AMT1;2 mRNA.
Overall, cipk9 and idd10 mutants showed an extremely similar pattern of sensitivity to NH4+, as measured by growth rate, cellular NH4+ level and NH4+ uptake in roots. It is therefore likely that IDD10 and its target gene CIPK9 are involved in the same signalling pathway mediating root growth in response to NH4+.
Overexpression of CIPK9 rescues NH4+-sensitive root growth in idd10
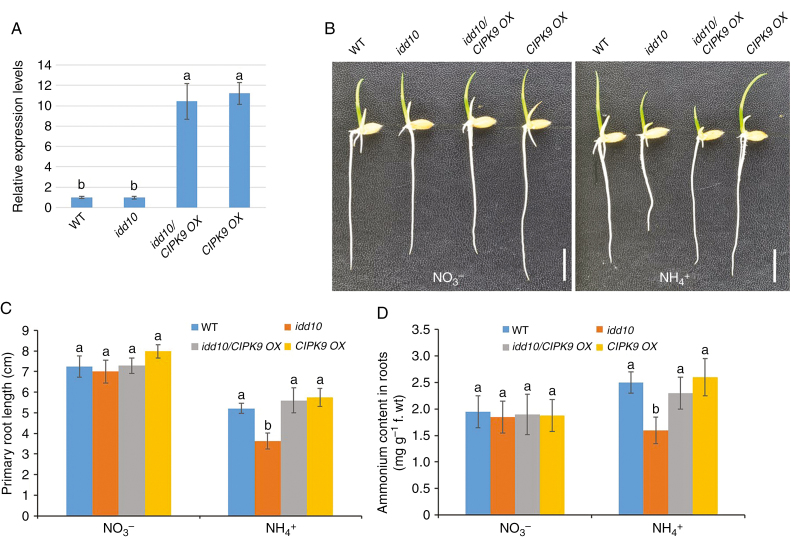
To understand the genetic relationship between IDD10 and CIPK9, CIPK9 OX plants were crossed with idd10 mutants. Ammonium-responsive root growth and cellular NH4+ content were examined in wild-type, idd10, CIPK9 OX and idd10/CIPK9 OX plants segregating from the same cross. A qRT–PCR analysis showed that levels of CIPK9 mRNA were approximately ten times higher in idd10/CIPK9 OX and CIPK9 OX roots than in wild-type and idd10 roots (Fig. 8A). No significant differences in root length between genotypes were observed when plants were grown in nutrient solution containing NO3– (Fig. 8B, C). When grown in nutrient solution containing NH4+, however, idd10/CIPK9 OX produced longer roots than idd10 plants, while wild-type, idd10/CIPK9 OX and CIPK9 OX plants exhibited primary roots of similar lengths (Fig. 8B, C). The cellular NH4+ content was measured in roots of 7-day-old seedlings grown for 4 d in nutrient solution containing NH4+ (Fig. 8D). Seedlings with the idd10/CIPK9 OX genotype accumulated a higher level of cellular NH4+ than did idd10 seedlings. Wild-type, idd10/CIPK9 OX and CIPK9 OX seedlings all contained similar amounts of cellular NH4+. These data strongly suggest that CIPK9 acts downstream of IDD10 in the signalling pathway mediating NH4+-sensitive root growth.
Fig. 8.
CIPK9 expression, primary root growth and cellular NH4+ levels in plants produced by crosses between IDD10 and CIPK9 OX. (A) Levels of CIPK9 expression in wild-type (WT), IDD10, idd10/CIPK9 OX and CIPK9 OX determined using qRT–PCR. Levels of CIPK9 mRNA were normalized against the level of UBIQUITIN mRNA in the same samples. Data are means ± S.E. (n = 3); different letters indicate significant differences between results (P < 0.05). (B) Appearances of wild-type (WT), idd10, idd10/CIPK9 OX and CIPK9 OX seedlings after growth for 4 d in a nutrient solution with either NO3– or NH4+ as the sole N source (Supplementary data Table S1). Scale bar = 1 cm. (C) Measurements of primary root length of plants grown as in (B). (D) Levels of cellular NH4+ levels in roots of seedlings grown for 4 d in a nutrient solution with either NO3– or NH4+ as the sole N source. Data shown in (C) and (D) are means ± s.e. (n >10 plants per line); different letters indicate significant differences between results (P < 0.05).
DISCUSSION
Exposure to high concentrations of exogenous NH4+ inhibits the elongation of primary roots in rice (Hirano et al., 2008; Shimizu et al., 2009). Root elongation of idd10 mutants is inhibited, however, even at NH4+ levels that are optimal for growth of normal rice seedlings (Xuan et al., 2013). The molecular mechanisms underlying the hypersensitivity to NH4+ of root growth in idd10 mutants were therefore explored.
The hypothesis that root growth retardation of idd10 resulted from limited intracellular NH4+ was tested first. This hypothesis was formed from the following observations: first, that IDD10 regulates the expression of N uptake genes; secondly, that idd10 plants contain low levels of intracellular NH4+; and, finally, that treatment with the glutamine synthetase inhibitor MSX, which suppresses NH4+-mediated inhibition of root growth, leads to an increase in intracellular levels of NH4+ in both wild-type and idd10 plants (Hirano et al., 2008; Shimizu et al., 2009; Xuan et al., 2013). Our results demonstrated, however, that the level of internal NH4+ was not related to the NH4+-dependent growth defect in idd10 roots.
This prompted us to examine regulatory factors whose expression was influenced by both NH4+ and IDD10. A second hypothesis was proposed that growth retardation in idd10 mutants resulted from disruption of the signalling mechanisms underlying NH4+ tolerance. CIPK proteins regulate potassium, NH4+ and NO3– uptake and signalling in plants (Xu et al., 2006; Pandey et al., 2007; Ho et al., 2009; Hu et al., 2009; Liu et al., 2012; Li et al., 2014; Straub et al., 2017). We showed that five CIPK genes and four CBL genes were induced by NH4+, with two genes (CIPK9 and CIPK14) being directly regulated by IDD10. Ammonium-mediated expression levels of CIPK15 and CIPK23 varied with expression of IDD10 even though no putative IDD10-binding motifs were detected in their promoters. Moreover, the roots of cipk9 and cipk23 seedlings showed NH4+-sensitive phenotypes, as both mutants had short roots that accumulated low cellular NH4+ (Fig. 6). These data strongly imply that the CBL–CIPK cassette plays a role in NH4+ signalling, that IDD10 directly regulates the expression of some CIPK genes, and that IDD10 and CIPK genes interact to regulate NH4+ signalling.
The most notable finding was that disrupting CIPK9, a direct target of IDD10, produced almost identical root phenotypes to disruption of IDD10. Moreover, roots of cipk9 and idd10 mutants showed the same response to MSX (Supplementary data Fig. S12); NH4+-dependent retardation of root elongation could be rescued in both mutants by MSX treatment. The genetic interaction between CIPK9 and IDD10 was examined by overexpressing CIPK9 in the idd10 mutant background. The genetic data implied that either CIPK9 acted downstream of IDD10 in the signalling pathway or that CIPK9 was required for the action of IDD10 on ammonium-mediated root growth. All our data indicate that CIPK9 and IDD10 are involved in the same mechanisms regulating ammonium-mediated root growth.
It is well known that a high concentration of exogenous ammonium has a detrimental effect on plant growth and development (for reviews, see Britto and Kronzucker, 2002; Esteban et al., 2016). Severe modifications in root architecture, including primary root systems, are widely recognized as an initial phenotypic symptom of NH4+ toxicity. Although we have demonstrated that IDD10 and CIPK9 work co-operatively to determine root growth in response to NH4+, the underlying cause of growth defects in their mutants in the presence of NH4+ remains unknown. The mutants showed lower NH4+ uptake activity and accumulated lower levels of cellular NH4+ than wild-type plants. This observation contrasts with the general notion that a high level of cellular ammonium is one of the major causes of NH4+ toxicity in higher plants.
Many physiological and molecular causes of NH4+-associated defects in roots have been suggested and discussed (for reviews, see Britto and Kronzucker, 2002; Esteban et al., 2016). Given that low levels of cellular NH4+ accumulated in roots of idd10 and cipk9 mutants, increased NH4+ efflux resulting in excessive energy consumption might be one of the causes underlying their NH4+-associated phenotypes. Although the futile transmembrane NH4+ cycle hypothesis, which states that NH4+ exclusion from the cytoplasm eventually leads to growth retardation (Britto and Kronzucker, 2002), has been questioned (Esteban et al., 2016), it would be worth examining whether NH4+ efflux increases in cipk9 plants.
Another factor influencing NH4+ flux is the cellular K+ concentration in roots. In arabidopsis, addition of potassium partially suppresses the retardation of root growth induced by NH4+ toxicity (Cao et al., 1993). To examine whether potassium modulated the NH4+-associated root phenotypes of cipk9 and idd10, the effect of potassium on growth of cipk9, idd10, CIPK9 OX and idd10/CIPK9 OX roots was examined (Supplementary data Figs. S13 and S14). Potassium supplementation had no effect on root growth inhibition induced by NH4+ in either mutant.
Further study is required to understand the molecular nature of NH4+-associated root growth defects. Signalling responses to NH4+ have been extensively reported (for a review, see Esteban et al., 2016). As IDD10 and CIPK9 encode a transcriptional regulator and a component of Ca2+ signalling modules, respectively, it is likely that the phenotypes observed in their mutants result from disruption of the signalling pathways involved in NH4+ tolerance. It is worth noting that 15NH4+ influx in CIPK9 OX was lower than that in wild-type plants but higher than that in cipk9. This implies that overaccumulation of CIPK9 might have a dominant negative effect in the regulation of downstream signalling. In other words, the role of CIPK9 might be fine-tuning 15NH4+ flux in rice root cells. A recent study of arabidopsis showed that CIPK23 inhibits the activities of ammonium transporters by direct phosphorylation (Straub et al., 2017). Ammonium uptake increased in arabidopsis cipk23 mutants, but the opposite observation was made in cipk9 mutants in this study. Although it is possible that CIPK9 performs enzymatic modification of ammonium transporters, the functional relationships between CIPKs and AMTs in rice may differ from those in arabidopsis.
Conclusion
This study demonstrated that CIPK9 is a regulator of NH4+-dependent root growth in rice. Extensive analyses of transcriptomes and metabolomes are required to understand the molecular and physiological roles of IDD10 and CIPK9 in regulating root growth in response to NH4+. These results provide an important foundation for a more extensive understanding of the regulatory basis of NH4+ signalling in rice plants.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: modified half-strength KB nutrient solutions (pH 5.8). Table S2: modified half-strength KB with MSX (Fig. S12). Table S3: modified half-strength KB to examine K+ effect (Figs S13 and 14). Table S4: sequences of primers used for qRT–PCR. Table S5: sequences of primers used in EMSA, ChIP and transient transfection assays. Figure S1: induction and purification of recombinant IDD10 protein. Figure S2: expression levels of (A) AMT1;2 mRNA and (B) IDD10 mRNA in idd10, AMT1;2 OX4 and idd10/AMT1;2 OX4 roots. Figure S3: chlorophyll content in shoots and primary root growth following exposure to different concentrations of NO3– or NH4+. Figure S4: responses of 12 CIPK genes (CIPK3, 5, 8, 9, 10, 11, 12, 14/15, 23, 24 and 31) to NH4+. Figure S5: responses of ten CBL genes to NH4+ treatment. Figure S6: responses of five CIPK genes in roots of idd10 and IDD10 OX2 plants to exposure to NH4+. Figure S7: activation of CIPK promoters by IDD10. Figure S8: expression of CIPK genes in their respective insertion mutants. Figure S9: CIPK9 expression patterns. Figure S10: selection and analysis of revertants from plants regenerated from CIPK9::Ds via tissue culture. Figure S11: levels of AMT1;2 mRNA expression in wild-type (WT), idd10, cipk9, Rev. and CIPK9 OX plants. Figure S12: effect of MSX treatment on root growth of idd10 and cipk9. Figure S13: effect of K+ on NH4+-sensitive root growth of idd10 and cipk9. Figure S14: effect of K+ on NH4+-sensitive root growth of the idd10/CIPK9 OX genotype.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03030555), from the Next-Generation BioGreen 21 Program (PJ01326601), the Rural Development Administration, Republic of Korea, and from Natural Science Foundation of Liaoning Province (20170540812).
LITERATURE CITED
- Abiko T, Obara M, Ushioda A, Hayakawa T, Hodges M, Yamaya T. 2005. Localization of NAD-isocitrate dehydrogenase and glutamate dehydrogenase in rice roots: candidates for providing carbon skeletons to NADH-glutamate synthase. Plant & Cell Physiology 46: 1724–1734. [DOI] [PubMed] [Google Scholar]
- Bleckmann A, Weidtkamp-Peters S, Seidel CA, Simon R. 2010. Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiology 152: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Burow M, Busch W, et al. . 2015. Reassess the t test: interact with all your data via ANOVA. The Plant Cell 27: 2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159: 567–584. [Google Scholar]
- Cai H, Lu Y, Xie W, Zhu T, Lian X. 2012. Transcriptome response to nitrogen starvation in rice. Journal of Bioscience 37: 731–747. [DOI] [PubMed] [Google Scholar]
- Cao Y, Glass AD, Crawford NM. 1993. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiology 102: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin HG, Choe MS, Lee SH, et al. . 1999. Molecular analysis of rice plants harboring an Ac/Ds transposable element‐mediated gene trapping system. The Plant Journal 19: 615–623. [DOI] [PubMed] [Google Scholar]
- Engelsberger WR, Schulze WX. 2012. Nitrate and ammonium lead to distinct global dynamic phosphorylation patterns when resupplied to nitrogen‐starved Arabidopsis seedlings. The Plant Journal 69: 978–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R, Ariz I, Cruz C, Moran JF. 2016. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Science 248: 92–101. [DOI] [PubMed] [Google Scholar]
- Hirano T, Satoh Y, Ohki A, Takada R, Arai T, Michiyama H. 2008. Inhibition of ammonium assimilation restores elongation of seminal rice roots repressed by high levels of exogenous ammonium. Physiologia Plantarum 134: 183–190. [DOI] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. 2009. CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194. [DOI] [PubMed] [Google Scholar]
- Hsieh PH, Kan CC, Wu HY, Yang HC, Hsieh MH. 2018. Early molecular events associated with nitrogen deficiency in rice seedling roots. Scientific Reports 8: 12207. doi: 10.1038/s41598-018-30632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HC, Wang YY, Tsay YF. 2009. AtCIPK8, a CBL‐interacting protein kinase, regulates the low‐affinity phase of the primary nitrate response. The Plant Journal 57: 264–278. [DOI] [PubMed] [Google Scholar]
- Ishikawa-Sakurai J, Hayashi H, Murai-Hatana M. 2014. Nitrogen availability affects hydraulic conductivity of rice roots possibly through changes in aquaporin gene expression. Plant and Soil 379: 289–300. [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. 2006. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochemical and Biophysical Research Communications 345: 646–651. [DOI] [PubMed] [Google Scholar]
- Je BI, Piao HL, Park SJ, et al. . 2010. RAV-Like1 maintains brassinosteroid homeostasis via the coordinated activation of BRI1 and biosynthetic genes in rice. The Plant Cell 22: 1777–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CM, Piao HL, Park SJ, et al. . 2004. Rapid, large‐scale generation of Ds transposant lines and analysis of the Ds insertion sites in rice. The Plant Journal 39: 252–263. [DOI] [PubMed] [Google Scholar]
- Kim JG, Li X, Roden JA, et al. . 2009. Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. The Plant Cell 21: 1305–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Hamada J, Nokajima H, et al. . 2010. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiology 153: 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V, Loqué D, Hörmann F, et al. . 2009. Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. The Plant Cell 21: 3610–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Long Y, Qi GN, Xu ZJ, Wu WH, Wang Y. 2014. The OsAKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1–CIPK23 complex. The Plant Cell 26: 3387–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Wang S, Zhang J, et al. . 2006. Expression profiles of 10,422 genes at early stage of low nitrogen stress in rice assayed using a cDNA microarray. Plant Molecular Biology 60: 617–631. [DOI] [PubMed] [Google Scholar]
- Liu LL, Ren HM, Chen LQ, Wang Y, Wu WH. 2012. A protein kinase CIPK9 interacts with calcium sensor CBL3 and regulates K+ homeostasis under low-K+ stress in Arabidopsis. Plant Physiology 161: 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara H, Kaimi R, Colasanti J, Kozaki A. 2011. Activity of transcription factor JACKDAW is essential for SHR/SCR-dependent activation of SCARECROW and MAGPIE and is modulated by reciprocal interactions with MAGPIE, SCARECROW and SHORT ROOT. Plant Molecular Biology 77: 489–499. [DOI] [PubMed] [Google Scholar]
- Oliveira IC, Brears T, Knight TJ, Clark A, Coruzzi GM. 2002. Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiology 129: 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GK, Cheong YH, Kim BG, Grant JJ, Li L, Luan S. 2007. CIPK9: a calcium sensor-interacting protein kinase required for low-potassium tolerance in Arabidopsis. Cell Research 17: 411. [DOI] [PubMed] [Google Scholar]
- Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA. 2010. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant, Cell & Environment 33: 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Tanabata T, Xie X, et al. . 2009. Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiologia Plantarum 137: 289–297. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Ikeda A, Saiki S, Yamaya T, Yamaguchi J. 2003. Feedback regulation of the ammonium transporter gene family AMT1 by glutamine in rice. Plant & Cell Physiology 44: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Straub T, Ludewig U, Neuhaeuser B. 2017. The kinase CIPK23 inhibits ammonium transport in Arabidopsis thaliana. The Plant Cell 29: 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hu B, Yuan D, et al. . 2018. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. The Plant Cell 30: 638–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yang W, Wei J, Yoon H, An G. 2017. Transcription factor OsDOF18 controls ammonium uptake by inducing ammonium transporters in rice roots. Molecules and Cells 40: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. 2006. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360. [DOI] [PubMed] [Google Scholar]
- Xuan YH, Priatama RA, Huang J, et al. . 2013. Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytologist 197: 791–804. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ohtani M, Mitsuda N, et al. . 2010. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. The Plant Cell 22: 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Hao DL, Song ZZ, Yang GZ, Wang L, Su YH. 2015. RNA-Seq analysis of differentially expressed genes in rice under varied nitrogen supplies. Gene 555: 305–317. [DOI] [PubMed] [Google Scholar]
- Yang W, Yoon J, Choi H, Fan Y, Chen R, An G. 2015. Transcriptome analysis of nitrogen-starvation-responsive genes in rice. BMC Plant Biology 15: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- Yang HC, Kan CC, Hung TH, et al. . 2017. Identification of early ammonium nitrate-responsive genes in rice roots. Scientific Reports 7: 16885. doi: 10.1038/s41598-017-17173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Loque D, Ye F, Frommer WB, von Wiren N. 2007. Nitrogen-dependent posttranscriptional regulation of the ammonium transporter AtAMT1;1. Plant Physiology 143: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Gu R, Xuan Y, et al. . 2013. Allosteric regulation of transport activity by heterotrimerization of Arabidopsis ammonium transporter complexes in vivo. The Plant Cell 25: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.