Abstract
Post-weaning diarrhea (PWD) is an often disease affecting piglets. It is caused mainly by enterotoxigenic Escherichia coli (ETEC) colonization in pig gut. Antibiotics has been used to prevent, combat and control PWD and its negative impact on the productivity of pig breeding sector. Nonetheless, antibiotics due to their wide antibacterial spectrum also can reach beneficial gut bacteria, such as Lactobacillus. Lately, essential oils (EOs) have emerged as a potential alternative to using antibiotics in animal breeding because of their effect on bacterial growth. Commonly, citrus EOs are by-products of food industry and the availability of these EOs in the worldwide market is huge. Thus, six commercials citrus EOs were evaluated on ETEC strains, as model of pathogenic bacteria, and on Lactobacillus species, as models of beneficial bacteria. In overall, citrus EOs exhibited a selective antibacterial activity with higher effect on pathogenic bacteria (ETECs) than beneficial bacteria (Lactobacillus). Brazilian orange terpenes (BOT) oil presented the highest selective performance and caused higher disturbances on the normal growth kinetic of ETEC than on Lactobacillus rhamnosus. The action was dose-dependent on the maximal culture density (A) and the lag phase duration (λ) of the ETEC. The highest sub-inhibitory concentration (0.925 mg/mL) extended the λ duration to ETEC eight times (14.6 h) and reduced A in 55.9%. For L. rhamnosus, the λ duration was only extended 1.6 times. Despite the fact that limonene was detected as the major compound, the selective antibacterial activity of the citrus EOs could not be exclusively attributed to limonene since the presence of minor compounds could be implicated in conferring this feature.
Subject terms: Natural products, Natural products, Antibiotics, Antibiotics
Introduction
The incidence of post-weaning diarrhea (PWD) is a serious problem in the worldwide pig industry, causing severe economic losses due to increased pig morbidity and mortality, decreased animal growth rate and increased need for medication to treat animals1,2. PWD is caused mainly by enterotoxigenic Escherichia coli (ETEC) that is highly present in the gastrointestinal tract of affected pigs. This pathotype is characterized by production of adhesins, which intermediate bacterial adherence to the intestine1,3, and toxins that lead to hypersecretion of water and electrolytes4,5. The presence of ETEC in the environment is an important transmission factor since they can survive protected in the manure for about 6 months. Furthermore, E. coli multiply rapidly and can reach up to 109 CFU per gram of feces, with the infection produced dependent on the degree of bacterial colonization6.
To control PWD outbreaks caused by enterobacteria as ETECs, antibiotics have frequently been included in the diet of weaned piglets as treatment, preventive measure, or growth promoter. However, continuous antibiotic use has been suggested as one cause of the emergence and worldwide dissemination of resistant bacteria. For example, currently, there is great concern about the emergence of plasmid-mediated colistin resistance (mcr-1) in E. coli, a resistance mechanism to an important antibiotic class in human health, polymyxins, which was largely used in pig production as growth promoter7–9, but banned its use for this purpose by the European Union legislation10 since 2006, and recently, by China11, Japan12 and Brazil legislations13. However, therapeutic use of colistin is still allowed following the current recommendation of the European Medicine Agency, which restrict the rational use of colistin to treat clinical cases in livestock animals14. Moreover, the wide spectrum activity of some antibiotics can affect gut microbiota since they can kill or inhibit both pathogenic and beneficial bacteria. Thus, longtime antibiotic use can provoke decreased microbiota diversity and increased chances of pathogens colonizing the gut2. On the other hand, it is well known that Lactobacillus is the major group of beneficial bacteria presented in pig gut microbiota, which has been identified as an important group of bacteria able to prevent gut diseases15. Possible Lactobacillus mechanisms to fight post-weaning infections in piglets have been described as (i) the direct inhibition of pathogen growth and its virulence by secretion of antimicrobial metabolites as bacteriocins, (ii) the modulation of microbiota composition and its activity and, (iii) the stimulation of the host immune system and improvement of intestinal barrier integrity2. In this scenario, finding an antimicrobial feed additive with a selective antibacterial activity, high spectrum activity on pathogenic bacteria and a reduced or not effect on beneficial bacteria like Lactobacillus would be very desirable. In the last decade, phytogenic compounds like essential oils (EOs) have received more attention as potential alternatives to replace antimicrobial growth promoters (AGP) in animal production due to their known biological properties: antimicrobial, antioxidant and anti-inflammatory16,17. A few studies have reported that some EOs can suppress pathogenic bacteria while stimulating beneficial microorganisms such as Lactobacillus in the pig gut18,19. Specifically, the citrus EOs, which are by-products of orange juice production20, could be an excellent alternative for that purpose since they have shown good potential to fight pathogenic bacteria such as Listeria spp.21, Salmonella spp.22, E. coli, Staphylococcus aureus and Bacillus cereus23. Furthermore, the use of citrus EOs in animal feed could become feasible since there is a huge availability of these oils in the worldwide market. Therefore, the aim of this study was to evaluate the selective antibacterial activity of six commercial citrus EOs on ETEC strains isolated from pig gut and on two Lactobacillus species belonging to ATCC, as well as to determine the chemical composition of these citrus EOs.
Results
Antibacterial activity
Screening by disc diffusion
The antibacterial activity screening of the six citrus EOs on the ETECs and Lactobacillus species is shown in Table 1. The association of inhibition zone diameter (IZD) means of six citrus EOs, when evaluated on ETECs and Lactobacillus species, by principal component analysis (PCA), showed that the first principal component explained 96.62%, and the second component 2.80% of the total variance (Fig. 1). Therefore, a good representation of antibacterial activity of these citrus oils was obtained. The IZD data showed that all the citrus oils had high antibacterial activity on all the ETECs, while low activity on the two Lactobacillus species was observed (Table 1). Consequently, it is possible to highlight these citrus oils as having a selective antibacterial activity. Furthermore, in contrast to the antibiotic colistin, an antibiotic that presented a selective performance (antibacterial activity on ETECs and no activity on Lactobacillus species), the citrus EOs showed superior performance on ETECs. Looking at the PCA (Fig. 1), four of the six citrus EOs, BOT, OOPE, CT and OPO, were the most selective oils since a closer association of their antibacterial activity with ETECs than with Lactobacillus species was observed. IZDs of these oils on ETECs were >18 mm. However, from these four citrus EOs, BOT stood out by exhibiting the best selective antibacterial activity (p < 0.05), since it presented the largest IZDs to all ETECs and considerable low IZDs (p < 0.05) to the two evaluated Lactobacillus species. Therefore, this oil was selected to continue the study.
Table 1.
Antibacterial activity of the citrus essential oils on ETECs strains isolated from pig gut and Lactobacillus strains*.
| Strain | E. coli U7 | E. coli U21 | E. coli U23 | E. coli U25 | L. rhamnosus ATCC 7469 | L. plantarum ATCC 8014 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential oil*** | IZD** | PC** | % I | IZD** | PC** | % I | IZD** | PC** | % I | IZD** | PC** | % I | IZD** | PC** | % I | IZD** | PC** | % I | |
| 1 | BOT | 27.4 ± 0.8ab,A | 17.4 ± 0.2 | 157.4 | 20.1 ± 0.3a,C | 12.4 ± 0.3 | 161.6 | 22.8 ± 0.9a,B | 13.3 ± 0.2 | 171.0 | 25.4 ± 1.5a,AB | 15.6 ± 0.7 | 162.4 | 9.9 ± 0.3bc,A | 6.0 ± 0.0 | 165.7 | 8.8 ± 0.4ab,B | 6.0 ± 0.0 | 161.6 |
| 2 | TLOP | 24.3 ± 1.1bc,A | 15.3 ± 1.8 | 158.7 | 12.5 ± 1.7c,B | 12.4 ± 0.4 | 100.8 | 14.9 ± 0.8b,B | 12.9 ± 0.1 | 115.6 | 22.3 ± 2.5a,A | 16.7 ± 1.2 | 133.9 | 8.5 ± 0.2d,A | 6.0 ± 0.0 | 141.3 | 8.0 ± 0.3b,A | 6.0 ± 0.0 | 133.9 |
| 3 | OPO | 23.8 ± 1.4c,A | 16.1 ± 1.8 | 148.2 | 18.1 ± 0.2b,B | 12.5 ± 0.5 | 144.6 | 20.8 ± 1.6a,AB | 12.8 ± 0.4 | 162.9 | 22.2 ± 1.6a,AB | 16.4 ± 0.3 | 135.6 | 9.1 ± 0.3 cd,A | 6.0 ± 0.0 | 151.8 | 8.4 ± 0.4ab,B | 6.0 ± 0.0 | 140.2 |
| 4 | OPOFF | 13.4 ± 0.9d,A | 17.1 ± 0.5 | 78.3 | 9.3 ± 0.9d,B | 12.7 ± 0.1 | 73.2 | 10.1 ± 0.4c,B | 12.6 ± 0.1 | 80.2 | 13.3 ± 0.9b,A | 15.6 ± 1.2 | 85.4 | 6.0 ± 0.0e,A | 6.0 ± 0.0 | 100.0 | 6.0 ± 0.0c,A | 6.0 ± 0.0 | 100.0 |
| 5 | OOPE | 25.7 ± 0.6abc,A | 16.9 ± 0.6 | 151.1 | 19.2 ± 0.5ab,B | 12.6 ± 0.5 | 152.4 | 22.3 ± 1.5a,AB | 13.0 ± 0.5 | 172.7 | 22.9 ± 1.3a,A | 15.8 ± 0.4 | 144.7 | 10.4 ± 0.5ab,A | 6.0 ± 0.0 | 173.2 | 8.8 ± 0.1ab,B | 6.0 ± 0.0 | 146.8 |
| 6 | CT | 28.6 ± 2.2a,A | 17.7 ± 1.1 | 161.9 | 19.8 ± 0.8ab,B | 12.1 ± 0.6 | 163.0 | 22.1 ± 1.2a,B | 12.8 ± 0.7 | 172.4 | 22.5 ± 0.7a,B | 15.8 ± 0.5 | 142.0 | 10.9 ± 0.5a,A | 6.0 ± 0.0 | 182.4 | 9.1 ± 0.4a,A | 6.0 ± 0.0 | 151.0 |
***OOPE = Orange oil phase essence, OPO = Orange peel oil, BOT = Brazilian orange terpenes, TLOP = Tahiti lime oil phase, OPOFF = Orange peel oil five fold and CT = Citrus terpenes.
**IZD: Inhibition zone diameter; PC: Positive control (Colistin); %I: inhibition of essential oil in relation to colistin.
*Values are means ± Standard Deviation (SD) of triplicate determinations expressed in mm including 6 mm of paper disk.
a,bMean values within a column having different superscripts are significantly different (essential oils) by the least significant difference Tukey test (p < 0.05).
A,BMean values within a row having different superscripts are significantly different (strains) by the least significant difference Tukey test (p < 0.05).
Figure 1.
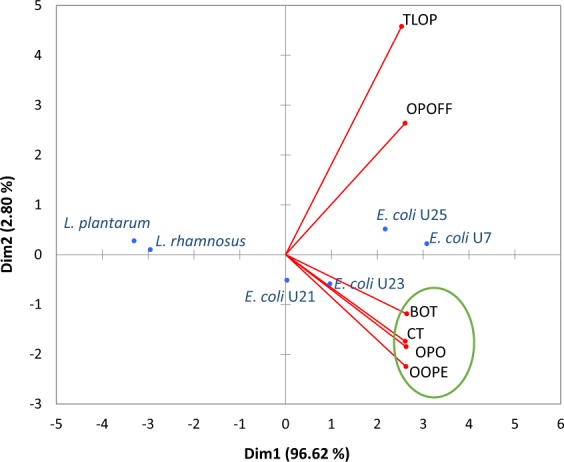
Principal component analysis (PCA) of six citrus EOs based on their antibacterial activity on ETECs strain and Lactobacillus species. OOPE = Orange oil phase essence, OPO = Orange peel oil, BOT = Brazilian orange terpenes, TLOP = Tahiti lime oil phase, OPOFF = Orange peel oil five fold and CT = Citrus terpenes.
Regarding the susceptibility of ETECs, E. coli U7 was the most sensitive strain (p < 0.05) to the activity of the citrus EOs, since the largest IZDs were observed for this ETEC. Conversely, E. coli U21 was the least sensitive or the most resistant ETEC, since were gotten the lowest IZDs (p < 0.05) on this bacterium. In the case of Lactobacillus species, it was observed that L. plantarum was the more resistant beneficial bacterium to the citrus EOs activity, since lowest IZDs were gotten on this bacterium. This behavior of the sensitivity for the bacteria tested is also represented in the PCA (Fig. 1), where the farthest association of E. coli U21 to the EOs is observed, and the closer association of L. rhamnosus than of L. plantarum to EOs can be observed. Therefore, E. coli U21 was selected as the most resistant ETEC and L. rhamnosus selected as the most sensitive beneficial bacterium to the antibacterial activity of the citrus oils.
Determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC)
The MIC and MBC determinations were made only using the most selective EO, which was BOT, the most resistant E. coli strain (E. coli U21), and most sensitive Lactobacillus species (L. rhamnosus), as determined in the screening phase. The MIC and MBC values for BOT, as determined by survival curves and resazurin test, are shown in Table 2. The MIC for E. coli U21 was 1.85 mg/mL (Fig. 2a), which was also the MBC for this bacterium. The MIC for L. rhamnosus was 3.70 mg/mL (Fig. 2b) and the MBC was 7.40 mg/mL (Table 2). Thus, these results reaffirm the selective antibacterial activity of this citrus oil, since to totally inhibit the growth of the beneficial bacterium L. rhamnosus required an EO concentration equivalent to twice the MIC observed for E. coli U21, and to kill it necessitated a concentration of four times the E. coli U21 MIC. Therefore, pathogenic bacterium was more sensitive to BOT than beneficial bacterium.
Table 2.
MIC and MBC for Brazilian orange terpenes (BOT).
| Bacterial strain | Brazilian Orange Terpens | |
|---|---|---|
| MIC (mg/mL)a | MBC (mg/mL) | |
| E. coli U21 | 1.85 | 1.85 |
| L. rhamnosus ATCC 7469 | 3.70 | 7.40 |
aDetermined by survival curves and resazurin test.
Figure 2.
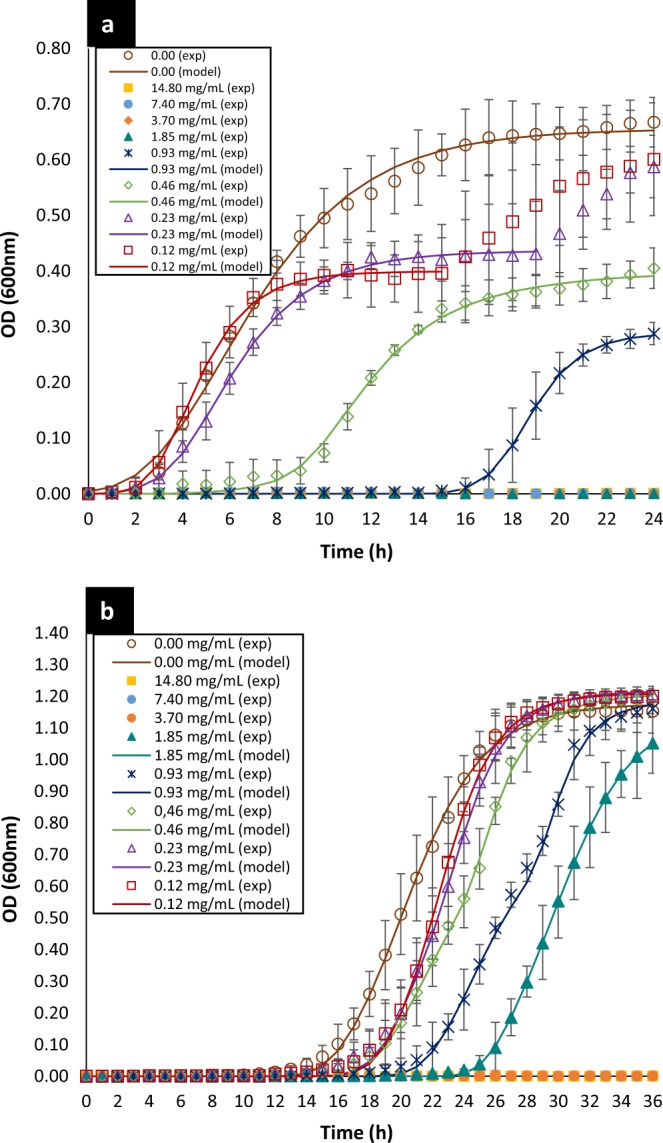
Bacterial growth kinetic as function of BOT concentrations of E. coli U21 (a) and L. rhamnosus (b). The dots are the experimental values; the vertical bars are the standard deviation and the curves are the modified Gompertz model (Eq. (6)). BOT = Brazilian orange terpenes
Bacterial growth modeling and calculation of kinetic parameters
The curves representing the bacterial growth kinetic for E. coli U21 and L. rhamnosus, when they were exposed to different concentrations of the BOT, are shown in Fig. 2. The Gompertz model modified by Zwietering et al.24 was used to fit the data of the bacterial growth (Eq. 6). The parameters obtained of this model for both bacteria are shown in Table 3.
Table 3.
Calculated parameters* of Modified Gomperzt Model (Eq. (6)24) for each evaluated concentration of Brazilian orange terpenes (BOT).
| CEOc (mg/mL) |
E. coli U21 | L. rhamnosus ATCC 7469 | ||||||
|---|---|---|---|---|---|---|---|---|
| A (OD600 nm)a | µmax (h−1)b | λ (h)a | R2 | A (OD600 nm)b | µmax (h−1)b | λ (h)a | R2 | |
| 14.80 | — | — | — | — | — | — | — | — |
| 7.40 | — | — | — | — | — | — | — | — |
| 3.70 | — | — | — | — | — | — | — | — |
| 1.85 | — | — | — | — | 1.143 ± 0.027 | 0.165 ± 0.005 | 26.61 ± 2.19 | 0.99 |
| 0.925 | 0.289 ± 0.016 | 0.078 ± 0.004 | 16.88 ± 0.97 | 0.99 | 1.192 ± 0.047 | 0.215 ± 0.104 | 23.85 ± 3.95 | 0.99 |
| 0.463 | 0.400 ± 0.054 | 0.058 ± 0.018 | 8.16 ± 1.31 | 0.99 | 1.210 ± 0.040 | 0.197 ± 0.089 | 20.24 ± 3.37 | 0.99 |
| 0.231 | 0.437 ± 0.060 | 0.072 ± 0.008 | 3.04 ± 0.57 | 0.98 | 1.216 ± 0.032 | 0.202 ± 0.048 | 19.55 ± 2.34 | 0.99 |
| 0.116 | 0.400 ± 0.053 | 0.097 ± 0.006 | 2.58 ± 0.45 | 0.98 | 1.210 ± 0.028 | 0.204 ± 0.035 | 19.35 ± 1.77 | 0.99 |
| 0.00 | 0.655 ± 0.049 | 0.073 ± 0.012 | 2.30 ± 0.55 | 0.99 | 1.176 ± 0.054 | 0.148 ± 0.008 | 16.61 ± 1.94 | 0.99 |
*A = maximal bacterial culture density (OD600 nm), µmax = the maximum specific growth rate (h−1), λ = the lag phase duration (h).
(−)Undetermined parameters due to total inhibition.
cConcentration of essential oil.
aThere are significant differences in the growth kinetics parameters after exposure to essential oil concentrations (p < 0.05).
bNo significant differences were observed (p < 0.05).
The survival curves or growth kinetics of E. coli U21 (Fig. 2a) showed that the four highest EO concentrations totally inhibited the growth of this bacterium and the growth was only observed for concentrations equal to or under 0.925 mg/mL of BOT oil. In the case of L. rhamnosus (Fig. 2b), bacterial growth was observed up to the concentration of 1.85 mg/mL of BOT and the three highest concentrations caused complete inhibition of this bacteria. Furthermore, we observed that EO concentration was able to provoke higher disturbances on the normal growth kinetic of E. coli U21 than L. rhamnosus. The modified Gompertz model allowed us to evaluate these disturbances with more accuracy through the three main biological parameters that it considers: maximal bacterial culture density (A), maximum specific growth rate (µmax) and lag phase duration or adaptation time (λ).
The values of parameter A were significantly affected (p < 0.05) for E. coli U21, since A was greatly reduced as the EO concentration was increased (Table 3). For instance, A was reduced in 38.9% at the lowest concentration and in 55.9% at the highest subinhibitory concentration (0.925 mg/mL), in contrast with the control (0 mg/mL of BOT). The parameter A had an inverse sigmoidal behavior as function of the EO concentration (Fig. 3a) and the mathematical function that describes this behavior to E. coli U21 is shown in Eq. (1), which had a good fit (R2 = 0.91). Regarding L. rhamnosus, this parameter was not affected by EO concentrations (Table 3), no significant differences were detected (p < 0.05), and it was considered the average in the general model of this bacterium, which was ALR = 1.191 ± 0.028.
| 1 |
Figure 3.
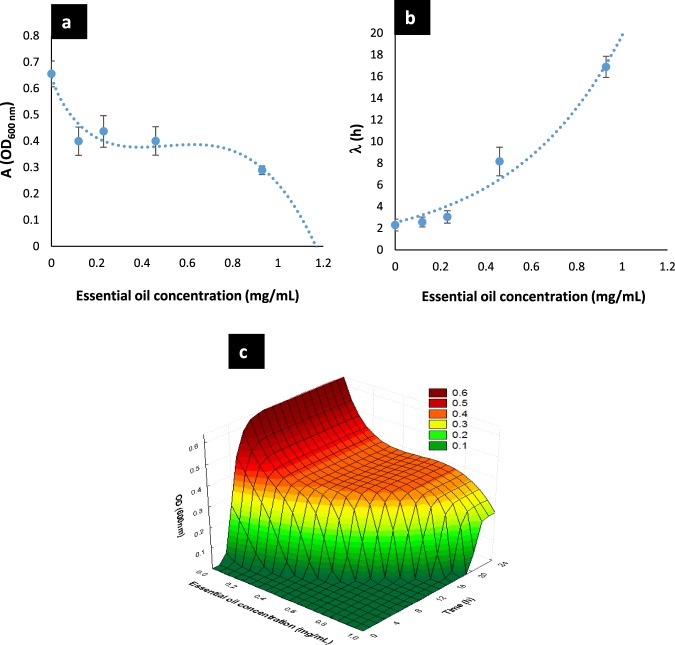
Parameters A (a) and λ (b) of the modified Gompertz model (Eq. (6)) to E. coli U21 as function of the BOT concentration. The dots are the experimental values; the vertical bars are the standard deviation and the curves are the model of Eqs. (1) and (2), respectively. (c) General model that describes the bacterial culture density of E. coli U21 as function of the time of treatment and the BOT concentration applied (Eq. (4)). BOT = Brazilian orange terpenes.
The maximal growth rate, that is, the parameter µmax for both bacteria, E. coli U21 and L. rhamnosus, was not affected by the EO concentrations since no significant differences were detected among the concentrations tested (p < 0.05). Consequently, it was considered an average growth rate of each bacterium for the general model, 0.076 ± 0.014 h−1 to E. coli U21 and 0.189 ± 0.026 h−1 to L. rhamnosus. As observed, the growth rate was quite higher for L. rhamnosus than E. coli U21.
Regarding parameter λ, which is the lag phase duration, we observed that for both bacteria this parameter increased as the EO concentration was increased. For E. coli U21, λ was slightly longer than control at the three lowest EO concentrations, but from 0.463 mg/mL to above, the lag phase was notably increased. For instance, λ increased approximately eight times at the highest subinhibitory concentration of BOT oil (0.925 mg/mL), in contrast with the control (0.00 mg/mL). Therefore, this parameter had an exponential behavior as function of the EO concentration (Fig. 3b) and the mathematical function that describes it is shown in Eq. (2), which had a good fit (R2 = 0.97). For L. rhamnosus, λ only suffered a significant increase, approximately 1.6 times at the highest effective subinhibitory BOT concentration (1.85 mg/mL). The behavior of λ to this beneficial bacterium was lineal as function of the EO concentration (Fig. 4); the mathematical function that describes it is shown in Eq. (3) and it had a good fitting (R2 = 0.93).
| 2 |
| 3 |
Figure 4.
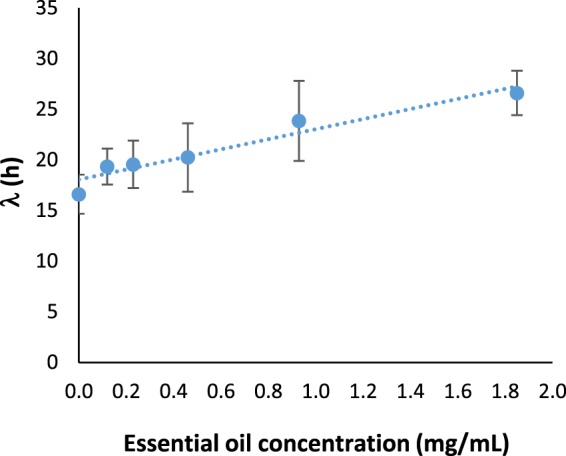
Parameters λ of the modified Gompertz model (Eq. (6)) to L. rhamnosus as function of the BOT concentration. The dots are the experimental values; the vertical bars are the standard deviation and the curves are the model of Eq. (3). BOT = Brazilian orange terpenes.
Finally, the modified Gompertz model to describe the bacterial growth kinetics as function of EO concentration (0.00 < CEO < 14.80 mg/mL) and time of exposure (in hours), is shown in Eq. (4) for E. coli U21, and in Eq. (5) for L. rhamnosus. Additionally, the model for E. coli U21 was plotted in 3D and is shown in Fig. 3c. The surface obtained highlights the shifts in the bacterial growth kinetic of E. coli U21 as function of initial EO concentration.
| 4 |
| 5 |
Therefore, evaluation of growth bacterial kinetic parameters confirmed that the citrus oil, BOT, had a stronger effect on the pathogenic bacterium than on the beneficial bacterium, provoking higher disturbances in its growth kinetics. This proves the selective antibacterial features of the BOT oil.
Chemical composition characterization of essential oils
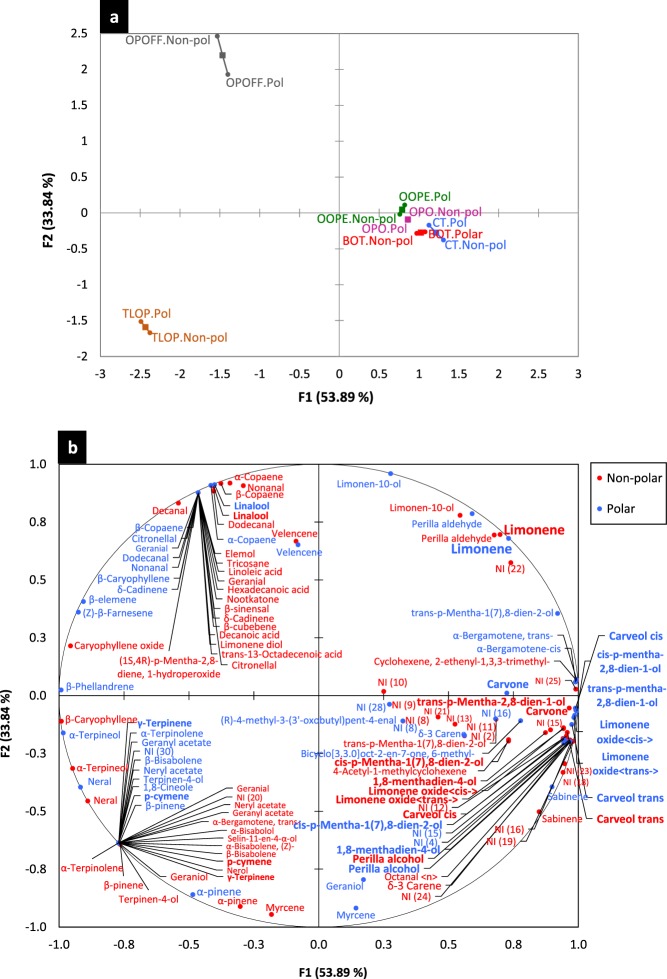
The chemical composition of the six citrus EOs is shown in Table S1 (Supplementary information). Overall, the identification of the chemical composition of each citrus EO by both columns was quite similar, as is observed in the individual factor map of the MFA (Fig. 5a), the polar and non-polar identification points were very close to each other. In addition, a good representation of the chemical composition data was obtained, since the first dimension of the MFA explained 53.89% and the second dimension explained 33.84% of the total variance. For the six citrus oils, limonene was detected as the major compound; however, TLOP had a negative association with this compound in both dimensions (Fig. 5b), since it presented the lowest relative amount of limonene (46.53%/47.46%).
Figure 5.
Multiple factor analysis (MFA) of the chemical composition profile of six citrus commercial EOs. The individual factor map of the overall chemical composition profiles by polar and non-polar identification (a) and biplot of the detailed chemical composition profile (b). OOPE = Orange oil phase essence, OPO = Orange peel oil, BOT = Brazilian orange terpenes, TLOP = Tahiti lime oil phase, OPOFF = Orange peel oil five fold and CT = Citrus terpenes.
Moreover, it was observed that BOT, CT, OOPE and OPO had a positive association with limonene in the two dimensions of the MFA and OPOFF had that positive association in the second dimension, showing all these EOs presented higher amount of limonene (Fig. 5b). However, OPOFF presented a fully different chemical profile than BOT, CT, OOPE and OPO oils (Fig. 5a) and it was the oil that presented the weakest antibacterial activity. BOT, CT, OOPE and OPO oils presented a very close chemical composition profile and they were characterized by having the highest selective antibacterial activity. Therefore, this allows us to suggest that the antibacterial activity of the citrus EOs could not be attributed specifically to limonene. Minor common compounds such as cis-limonene oxide, trans-carveol, carvone, trans-limonene oxide, cis-p-Mentha-2,8-dien-1-ol, trans-p-Mentha-2,8-dien-1-ol, perrilla alcohol, cis-carveol, cis-p-Mentha-1(7),8-dien-2-ol and 1,8-menthadien-4-ol (Fig. 5b) were detected in these four citrus EOs and they were either present exclusively in these four EOs or in higher amounts than TLOP and OPOFF oils. Therefore, these minor compounds could be implicated to confer the selective and highest antibacterial activity of BOT, CT, OOPE and OPO oils.
In addition. we observed that TLOP and OPOFF had a different chemical profile. The TLOP profile is shown in the third quadrant of the MFA (Fig. 5b); besides limonene, this oil had a high amount of p-cymene and γ-terpinene. The OPOFF profile is shown in the second quadrant of the MFA (Fig. 5b), and linalool was detected as second major compound in it.
Discussion
Citrus EOs are one of the main by-products of the orange juice industry and could have several applications besides cosmetology and the food industry. Citrus oils have been reported as having antimicrobial properties20–22,25, but testing their antimicrobial activity on bacteria affecting animal farms has not been greatly mentioned. E. coli causing post-weaning diarrhea in piglets during their early life is a critical problem for the pig industry2. The antibacterial activity of commercial citrus EOs evaluated in this study on E. coli strains isolated from pig gut and on Lactobacillus bacteria showed that, in overall, citrus EOs presented a selective antibacterial activity, having higher activity on pathogenic bacteria E. coli than on beneficial bacteria Lactobacillus spp. The selectivity towards bacterial pathogens by EOs or EO compounds rather than beneficial bacteria have been already reported18,26,27, EOs or single EO compounds were shown to have higher inhibitory effect on E. coli O157:H7, E. coli K88, S. typhimurium than on Lactobacillus and Bifidobacterium spp18. As an alternative to AGPs, the selectivity of citrus oils could be an important feature of antimicrobial spectrum, which commonly has not been considered to conventional AGPs long used, since the aim is to have an effect on the pig gut. Thus, current search for antimicrobial substances as alternatives to AGPs should consider selectivity aspects between pathogenic and beneficial gut bacteria. The lower effect on beneficial bacteria by an AGP and its potential alternatives (such as citrus EOs) would be desirable since beneficial bacteria such as Lactobacillus spp. contribute to fighting pathogen colonization in the gut and preventing gut infections. Thereby, reinforcing gut microbiota and contribute to improve animal health27.
The capability of citrus EOs to inhibit pathogenic bacteria has been well reported in several studies. For instance, tangerine EO (Citrus reticulata) was reported as having an inhibitory effect on S. aureus, Bacillus subtilis, and Pseudomonas aeruginosa28. Another study proved the high effectiveness of the lemon EO (Citrus limon L. Burm) to inhibit several strains of Listeria monocytogenes, S aureus and Salmonella enterica associated with foodborne diseases25 Also, it has been reaffirmed the anti-salmonella22 and anti-listeria21 activities of several commercial citrus oils. Specifically, the antibacterial activity of citrus oils on E. coli has been investigated. Tangerine EO (Citrus reticulata) was shown to be effective to produce an inhibition of 14.6 ± 1.1 mm on E. coli28. A total inhibition of E. coli growth by this oil was found at 1.96 mg/mL29. A close value to this MIC was found for BOT oil in our study (1.85 mg/mL = 0.21%v/v). Other citrus EOs such as bergamot, orange and lemon were also effective to produce inhibitions ≥18 mm on E. coli O157, but full inhibition of the growth of this bacterium was reported at higher MICs than the BOT oil, between 0.5–1.0%v/v30. Similarly, another study reported higher MICs than the BOT to mandarin and lemon EOs on E. coli, 5 and 30 µL/mL, respectively31. In addition, the EO of sweet orange (Citrus sinensis Osbeck) has presented an inhibitory effect on E. coli at 18.8 µL/mL32, also considered as a high MIC in contrast to the BOT oil. Conversely, the non-effectiveness of several citrus oils to fight E. coli affecting animals, such as E. coli associated with poultry colibacillosis, has been reported33. In comparison, the citrus EOs tested in our study were quite effective in treating E. coli affecting pigs.
Furthermore, some studies highlighted that citrus EOs have higher effectiveness to inhibit Gram-positive pathogenic bacteria than Gram-negative pathogenic bacteria30,34. However, in our study, the opposite was observed, since the Gram-negative E. coli was more sensitive than the Gram-positive Lactobacillus spp. to the activity of the citrus oils. The difference in the sensitivity to EOs between these two groups of bacteria has been hypothesized to be the consequence of differences in the cell wall structure, since Gram-positive bacteria lack an outer membrane (OM), which Gram-negative bacteria have. This OM contains lipopolysaccharides (LPS) with polar ends (O-polysaccharides) and transmembrane channels (porins), which permit the passage of hydrophilic solutes and make difficult for hydrophobic compounds to diffuse such as EOs components into the cell. Therefore, this would allow Gram-negative bacteria be more resistant to EOs35. Nonetheless, the antibacterial spectrum of EOs depends on the specificity of the functional groups of EO compounds to single or multiple targets. Some EO compounds have the ability to disintegrate the OM of Gram-negatives as E. coli, release the material associated to this membrane and penetrate the cell, provoking a disruptive effect36. Probably, the compounds present in citrus EOs may have this ability due to their higher effectiveness observed on this Gram-negative bacterium, E. coli. On the other hand, the antibacterial activity of citrus oils on Gram-positive beneficial bacteria has been little reported. Orange, lemon, mandarin and grapefruit EOs had a low inhibitory effect on Lactobacillus sakei and Lactobacillus curvatus, exhibiting the orange oil the lowest effect on these bacteria (12.8 ± 0.5 and 13.5 ± 0.2 mm, respectively)37. The authors demonstrated that the inhibitory effect of these four oils was dose-dependent causing inhibition of those Lactobacillus species only at the highest concentrations tested37. This was also noticed in our study, where the citrus oils exhibited IZDs lower than 11 mm to Lactobacillus species. Moreover, L. rhamnosus was inhibited at a high BOT concentration and killed even at an upper concentration, thus showing BOT had a low antibacterial activity on L. rhamnosus. Although general structures and biosynthesis pathways among Gram-positive bacteria are conserved, some Gram-positive bacteria, such as Lactobacillus spp., could show low sensitivity to antimicrobials, such as EOs, since the cell wall of Gram-positive lactic acid bacteria (LAB) as Lactobacillus spp. possess unique properties that could confer intrinsic resistance to some antimicrobial agents38. For instance, the intrinsic resistance to antibiotics of some Lactobacillus (e.g. to vancomycin) would be related to the fact of having a D-lactate instead of D-alanine as the last amino acid in the peptidic chain of the peptidoglycan layer of their cell wall38,39, which would avoid the antibiotic binds to the peptidic chain and cause the inhibition of these bacteria40.
Additionally, it was observed that BOT oil caused higher disturbances on the growth kinetics of E. coli than L. rhamnosus, significantly affecting its maximal culture density and the lag phase duration. Both parameters were dose-affected and changed as function of the BOT concentration. The higher dose-dependent effect of some EOs and single EO compounds on the growth kinetic of E. coli than on Lactobacillus spp. has already been observed27. Oregano, thyme and rosemary EOs, carvacrol, eugenol and thymol provoked higher reduction on the maximal culture density of E. coli strains than Lactobacillus fermentum and Lactobacillus reuteri with increasing of the concentration of EOs/EO compounds27. Also, the dose-dependent effect of carvacrol to extend the lag phase of E. coli by increasing the concentration of this compound has been proved41. In addition, some combinations of EOs have been reported as more efficient to cause an increase of the E. coli lag phase. For instance, combinations of oregano with basil EOs and oregano with lemon balm EOs were able to significantly increase the E. coli lag phase, approximately 7.4 h and 3.6 h longer, respectively, compared to when oregano EO was used alone42. Regarding L. rhamnosus, in our study, we observed only the lag phase duration was extended as the BOT concentration was increased. This effect on L. rhamnosus has been previously observed with the oil of Melaleuca armillaris, which additionally reduced the growth rate and final culture density with increasing of the EO concentration43. Therefore, this oil had a higher dose-dependent effect on the growth kinetic parameters of L. rhamnosus than the citrus oil (BOT) tested in our study. Moreover, a recent study observed that Eucalyptus globulus and Pimenta pseudocaryophyllus EOs presented a dose-dependent effect on the lag phase of L. rhamnosus as well; however, P. pseudocaryophyllus oil caused higher extension of this parameter in comparison to E. globulus oil at the same sub-MICs44.
Limonene has been shown as a major compound of citrus EO composition and most of their biological activities have been attributed to this compound. All citrus oils evaluated in our study presented limonene as the major compound. However, the mismatching between limonene content and antibacterial activity of these oils suggested that their antibacterial activity cannot be attributed exclusively to limonene. Some studies have already reported the lack of antibacterial activity of limonene individually tested. For instance, the pompia EO (Citrus limon var. pompia), which presented limonene as major compound (28%), at a concentration of 256.3 mg/mL, presented an antibacterial effect on several pathogenic bacteria, but when limonene was evaluated alone, it did not exhibit any antibacterial effect45. Thus, this proved that limonene would not be the compound responsible for the antibacterial activity observed for this oil. Nonetheless, coexisting minor compounds in citrus oils could contribute to conferring the antibacterial property of these oils. In mandarin EO, compounds like octanal, decanal, citral, citronellal, linalool, α-sinensal and thymol were suggested as possible collaborators to the antibacterial activity, when this oil (with 56.8% of limonene) was tested against Gram-negative and Gram-positive bacteria28. Other minor compounds, belonging to oxygenated monoterpenes class, such as 4-terpineol, α-terpineol, cis-geraniol, β-citral, nerol and α-citral, might also be implicated in conferring the antibacterial activity of citrus oils, since they have been detected in high amounts in the composition of the citrus EO that presented high antibacterial activity25. Minor oxygenated monoterpenes compounds (cis-limonene oxide, trans-carveol, carvone, trans-limonene oxide and perrilla alcohol) were also detected in the group of the most selective citrus oils of our study. Possibly, these compounds might play an important role in conferring the selective antibacterial activity of citrus EOs. In addition, an orange cold pressed EO rich in limonene (85.3%) presented an antibacterial activity on E. coli ten times higher than limonene alone, and even minor compounds, such as linalool, pinene and terpineol, presented a higher activity than limonene46. Furthermore, the antibacterial activity of limonene has been shown to be variable and depending on its stereoisomeric form present in the EO. The (−) stereoisomer of limonene could inhibit E. coli at a lower concentration (8 mg/mL) than the (+) stereoisomer (11 mg/mL). On the other hand, limonene alone has been proved to stimulate the growth of beneficial bacteria as L. fermentum, instead of having any inhibitory effect on it27. Likewise, limonene has been reported as not effective to inhibit several Lactobacillus species including L. rhamnosus ATCC 746945, the bacteria also evaluated in our study, and which showed be the more resistant to the antibacterial activity of the citrus oils proved. Therefore, it would be possible to infer that limonene could collaborate with the selective activity of citrus oils when it is present in the gut, promoting the beneficial bacteria while other minor compounds could act in inhibiting pathogenic bacteria. It has been reported that in an EO, major and minor components probably act in synergism to confer the biological properties of the EO47. When an EO compound is proved, individually, its effect may differ from the effect that this compound may have in combination with the other compounds inside the EO. Thus, it would be recommended the use of the whole EO instead of single EO compounds, since every compound inside an EO could exert a different mechanism of action on the bacteria cell48, and this could reduce the chance to bacteria develop easily resistance to the EO. Contrariwise, bacteria could develop a rapid and easy mechanism of resistance to a single EO compound, as in the case of an antibiotic, which consist of a single compound. Several mechanisms of action of EOs have been proposed in the literature. The mechanism of action comprises a serie of events on the bacterial cells. Initially, they can destabilize the cellular architecture, leading to the breakdown of membrane integrity and thus increased permeability of cellular constituents. This disrupts many cellular activities, including energy production, membrane transport, and other metabolic regulatory functions48. In addition, EOs can alter the membrane fatty acids composition, the membrane proton motive force and affect proteins in the cytoplasmatic membrane. Additionally, EOs can interfere with the quorum sensing activity, decreasing the proteolytic activity, biofilm formation, and virulence factors expression and their functions, as well as to affect the metabolome of bacteria35.
In conclusion, our study highlights as an important feature of antimicrobial spectrum the selectivity between pathogenic and beneficial gut bacteria, which should be considered when searching for antimicrobial substances as alternatives to conventional AGPs, since the aim is to have an effect on the pig gut. Our study has proved, by a screening, MIC determination, and growth kinetic parameters evaluation, the selective antibacterial activity of citrus EOs on E. coli and Lactobacillus spp., thus suggesting these EOs as potential alternatives to AGPs. Consequently, based on the selective performance and the huge viability in the global market of citrus EOs, the possible application of these oils in the pig production sector could turn feasible. In addition, chemical composition characterization showed that minor compounds present in these citrus oils would be implicated in conferring their selective activity, instead of limonene, the major present compound, playing this role exclusively. Finally, our results motivate further research to clarify, for instance, the possible mechanism of action that citrus oils would have on pathogenic and beneficial bacteria as well as their direct effect on the pig gut and on the microbiota resident in it.
Material and Methods
Essential oils supply
Six citrus commercial EOs were used in the study. These were by-products from orange juice production and were supplied by a factory from São Paulo State, Brazil. The oils were named by the factory as follows: Orange oil phase essence (OOPE), Orange peel oil (OPO), Brazilian orange terpenes (BOT), Tahiti lime oil phase (TLOP), Orange peel oil five fold (OPOFF) and Citrus terpenes (CT). Once the samples were received, they were kept in amber bottles under refrigeration (4 °C) until use.
Bacterial strains
The evaluated bacterial strains in this research were four ETECs strains and two Lactobacillus species. The ETECs strains were isolated from pig gut and provided by The Swine Heath Laboratory of the Department of Preventive Veterinary Medicine and Animal Health from FMVZ-São Paulo University: E. coli U7 (K88+/LT+/STb+), E. coli U21 (K88+/LT+/STb+/F18+/Sta+), E. coli U23 (LT+/STb+/F18+) and E. coli U25 (LT+/STb+/F18+/Sta+). The two Lactobacillus species were standard cultures from the American Type Culture Collection (ATCC), L. plantarum ATCC 8014 and L. rhamnosus ATCC 7469. ETECs strains were cultivated in Tryptic Soy Agar-Difco (TSA, DifcoTM) at 37 °C for 18–20 h and Lactobacillus species in MRS (Man, Rogosa and Sharpe agar, DifcoTM) agar at 30 °C for 48 h. After activation, the bacteria were sub-cultured in Brain-Heart Infusion broth or MRS both (DifcoTM) supplemented with 15% of glycerol. After incubation, they were stored at −20 °C until their use.
Antibacterial activity
Screening by disc diffusion
All EOs were initially screened by disc diffusion method, following the standard protocol M02-A11 from the Clinical and Laboratory Standards Institute49. EO solutions were prepared at 90% (v/v), using acetone as an emulsifier to improve dispersion. ETECs strains (E. coli U7, U21, U23 and U25) were grown on TSA agar and Lactobacillus species on MRS agar. Isolated colonies of each bacterium were transferred to tubes containing sterile saline solution (0.85%) until reaching an optical density within 0.08 to 0.1 abs, at 625 nm, which corresponds to 0.5 McFarland standard, therefore containing ~1–2 × 108 CFU/mL49. After this, Mueller Hinton (MH) agar plates (E. coli) and MRS agar plates (Lactobacillus spp.) were inoculated and the bacterial inoculum spread. Seven microliters of each EO solution (90% v/v) were placed on 6-mm diameter sterile paper discs (Whatman N° 3), which was transferred to the inoculated agar plates. Three discs with the same EO solution were placed in each plate; one disc of colistin (15 µg/disc) was used as a positive control, and one disc of acetone (10 µL/disc) was used as a negative control since its non-antimicrobial activity was proved. Then, the agar plates were incubated at 37 °C for 24 h (E. coli) and at 30 °C for 48 h (Lactobacillus spp.). Inhibition zone diameters (IZD) were measured after incubation with the aid of a caliper rule. The experiment was carried out in three independent replicates.
The EO presenting the highest IZDs for the four ETECs strains and the lowest IZDs for the two Lactobacillus species was considered as the best selective EO between pathogenic and beneficial bacteria and was thus selected for further investigation. Referring to the bacterial strains, the most resistant ETEC strain and the most sensitive Lactobacillus specie to the activity of EOs were selected to continue with the study.
Determination of minimal inhibitory concentration (MIC)
The determination of the MIC of the selected EO was performed by microdilution assay in a 96-well microplate following the standard protocol M07-A9 from the Clinical and Laboratory Standards Institute, with some modifications50. For the assay, the standard inoculum was prepared in sterile saline (0.85% w/v) from living colonies of the selected bacteria above contained in plates of TSA agar (E. coli) or MRS agar (Lactobacillus spp.) at the optical density equivalent to 0.5 McFarland Standard (0.08–0.13 at 625 nm) as described previously. Subsequently, this inoculum was diluted at 1:100 to obtain an inoculum of 106CFU/mL (final inoculum). The EO stock solution was prepared at 29.6 mg/mL (3.29%) with MH or MRS broth using Tween 80 as emulsifier. From the stock solution, two-fold serial dilutions were made in a range from 14.80 to 0.116 mg/mL along the Y-axis of the microplate. Twenty microliters from the final inoculum were added to each well containing 180 µL of several EO concentrations, being the final volume in each well of 200 µL and bacterial population of approximately 105 CFU/mL. The following controls were used: culture medium control (200 µL of MH or MRS broth); growth control (180 µL of MH or MRS broth +20 µL of inoculum); Tween 80-emulsifier control (200 µL of MH or MRS broth with Tween 80) and growth control containing the emulsifier (180 µL of MH or MRS broth with Tween 80 + 20 µL of inoculum). Finally, microplates were incubated in a microplate reader (VitorTM X3, PerkinElmer) at 37 °C for 24 h for E. coli and at 30 °C for 36 h for Lactobacillus spp.
The MIC was established as the lowest EO concentration that inhibited visible bacterial growth. The existence or not of bacterial growth was evaluated by construction of survival curves and by resazurin test at the end of the incubation period. The lowest concentration that did not produce detectable absorbance values (at 600 nm) until the end of incubation was considered as the MIC obtained by survival curves. For resazurin test, 25 µL of resazurin (R7017; Sigma-Aldrich) solution at 0.0135% m/v were used per well. Thus, after visual inspection the presence of viable cells was evidenced through a change in the resazurin color from blue resazurin to pink resofurin51, after further incubation at 37 °C (E. coli) or 30 °C (Lactobacillus spp.) for 1 h. Assays were carried out in triplicate in three independent replicates.
Bacterial growth modeling and calculation of kinetics parameters
Bacterial growth kinetics (or survival curves) for each tested EO concentration was built from absorbance readings at 600 nm of the wells of the microplate configured as above, carried out every hour during the total incubation period, 24 h for E. coli and 36 h for Lactobacillus spp. Bacterial growth kinetics were modeled using the Gompertz model modified by Zwietering et al.24 (Eq. (6)), since this model considers the three main biological parameters of bacterial growth. The data were fitted to the mathematical model with a confidence level of 95% using the Levenberg–Marquardt algorithm in Statistica 12.0 (StatSoft, Inc., Tulsa, OK, USA) software.
| 6 |
where: y represents the relative population size against time, the A, µmax and λ are the three parameters that described three phases of the bacterial growth curve24. The asymptote A is the maximal bacterial culture density (OD600 nm), µmax represents the maximum specific growth rate (h−1) and it is the tangent of the log phase curve, λ is the lag phase duration (h) and is defined as the x-axis intercept of this tangent. ℯ represents the number e = 2.7183.
Finally, the goodness of fit for the model was measured based on the mean square error (MSE) and on the corrected determination coefficient (corrected R2) for each set of data.
Minimal bactericidal concentration (MBC)
The determination of MBC was performed from wells containing EO concentrations where there was no visible bacterial growth. So, an aliquot of 100 µL was taken from each well and seeded in MH or MRS agar. Plates were incubated for 24 h at 37 °C for E. coli and for 48 h for Lactobacillus spp. The MBC was defined as the lowest concentration of EO able to cause total bacterial death, represented by the visible absence of colonies on the agar plates.
Chemical composition of essential oils
The chemical composition characterization of the EOs was performed by gas chromatography coupled with mass spectrometry (GC/MS) using non-polar and polar columns.
The analysis on non-polar column was carried out using an Agilent Technology gas chromatograph 6890 Plus Series (Santa Clara, CA, USA) coupled to a selective Mass Spectrometry Detector 5973 and an Auto Sampler 7893. A fused-silica capillary column DB-5MS (J&W Scientific, Folsom, CA, USA) of 60 m × 0.25 mm id × 0.25 μm of film thickness coated with 5%-phenyl polydimethylsiloxane was used. The oven temperature was set as follows: initial oven temperature was held at 45 °C for 5 min, then raised to 150 °C at 4 °C/min for 2 min, one more time raised to 250 °C at 5 °C/min, and finally to 300 °C at 10 °C/min, which was kept for 60 min. The injector temperature was 250 °C, 2.0 µL of samples diluted in dichloromethane was injected in the “split” mode at a ratio of 30:1. EIMS, electron energy was 70 eV. The mass detector operated in full scan mode in the range of 40 to 350 m/z. The temperature of the ion source and transfer line was 230 °C and 285 °C, respectively. Helium gas was used as the carrier gas with an inlet pressure of 16.97 psi. The retention index (RI) was calculated for all the volatile compounds using a homologous series of C7–C30 n-alkanes (49451-U Sigma-Aldrich), according the linear equation of Van den Dool and Kratz52.
The analysis on polar column was carried out using an Agilent Technology gas chromatograph 7890a Plus Series (Palo Alto, CA, USA) coupled to a selective Mass Spectrometry Detector 5975 C. A fused-silica capillary column DB-WAX (J&W Scientific, Folsom, CA, USA) of 60 m × 0.25 mm i.d × 0.25 μm of film thickness coated with polyethylene glycol was used. The oven temperature was set as follows: initial oven temperature was held at 50 °C for 5 min, then raised to 150 °C at 4 °C/min for 7 min and finally to 230 °C at 4 °C/min, which was kept for 40 min. The injector temperature was 250 °C, 2.0 µL of samples diluted in dichloromethane was injected in the “split” mode at a ratio of 30:1. EIMS, electron energy was 70 eV. Helium gas was used as the carrier gas with an inlet pressure of 16.91 psi. Mass detector operated in full scan mode in the range of 40 to 350 m/z. The retention index (RI) was calculated for all the volatile compounds as described above.
The identification of the components was performed by comparing their RI and mass spectra with data published in the literature53,54 and in the computer libraries (NIST 107 and WILEY 8).
Data analysis
The IZD data from the initial screening was evaluated by analysis of variance (ANOVA) followed by the Tukey test for pairwise comparison at 5% of significance using XLSTAT (Addinsoft, New York, NY, USA). Based on this, the susceptibility of ETECs strains and Lactobacillus species was determined. Also, principal component analysis (PCA) using the correlation matrix was performed based on IZDs means using XLSTAT (Addinsoft, New York, NY, USA).
Furthermore, an ANOVA to detect significant differences in the growth kinetics parameters A, λ and µmax of E. coli and Lactobacillus spp. after exposure to EO concentrations was performed (p < 0.05) using R software. If significant differences were detected in those parameters, as effect of the EO concentrations, the behavior of each parameter was modeling by nonlinear regression.
In addition, the multiple factor analysis (MFA) was performed on polar and non-polar data (two tables) to describe and contrast the chemical composition profile of the six citrus EOs obtained by GC-MS, with this analysis running in the XLSTAT software.
Supplementary information
Table S1. Chemical composition of the six citrus essential oils*.
Acknowledgements
The authors are grateful to CIENCIACTIVA for Carmen M.S. Ambrosio Ph.D. scholarship (Contract 278-2015-FONDECYT) from the “Consejo Nacional de Ciencia, Tecnología e Inovación Tecnológica” (CONCYTEC - Peru).
Author contributions
C.A. conception and drafting of the work, analysis and interpretation of results, writing and design of this manuscript. N.I. support in laboratory work. A.C.M. assistance in the mathematical modelling and critical manuscript review. E.S. assistance in the statistical analysis. A.M. support with microbiology expertise and critical manuscript review. E.S. support GC-MS analysis expertise, offered facilities to perform GC-MS analysis and critical manuscript review. C.C.C. guidance in all stages of the work and critical manuscript review. E.D.G. conception and drafting of the work, analysis and interpretation of results, guidance in all stages of the work, critical review and final approval of the last version of this manuscript to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carmen M. S. Ambrosio, Email: carmen.milagros8@usp.br
Eduardo M. Da Gloria, Email: emgloria@usp.br
Supplementary information
is available for this paper at 10.1038/s41598-019-54084-3.
References
- 1.Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Heal. Res. Rev. 2005;6:17–39. doi: 10.1079/AHR2005105. [DOI] [PubMed] [Google Scholar]
- 2.Gresse R, et al. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends in Microbiology. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Rhouma M, Fairbrother JM, Beaudry F, Letellier A. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017;59:31. doi: 10.1186/s13028-017-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amezcua R, Friendship RM, Dewey CE, Gyles C, Fairbrother JM. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can. J. Vet. Res. 2002;66:73–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998;11:142–201. doi: 10.1128/CMR.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubreuil, J. D., Isaacson, R. E. & Schifferli, D. M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus7 (2016). [DOI] [PMC free article] [PubMed]
- 7.Liu YY, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Liu J-H. Monitoring Colistin Resistance in Food Animals, An Urgent Threat. Expert Rev. Anti. Infect. Ther. 2018;16:443–446. doi: 10.1080/14787210.2018.1481749. [DOI] [PubMed] [Google Scholar]
- 9.Rhouma M, Beaudry F, Letellier A. Resistance to colistin: what is the fate for this antibiotic in pig production? Int. J. Antimicrob. Agents. 2016;48:119–126. doi: 10.1016/j.ijantimicag.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 10.EU. Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off J Eur Union268, 29–43 (2003).
- 11.Walsh, T. R. & Wu, Y. China bans colistin as a feed additive for animals. The Lancet Infectious Diseases16, 1102–1103 (2016). [DOI] [PubMed]
- 12.Food Safety Commission of Japan. Antimicrobial-resistant Bacteria Arising from the Use of Colistin Sulfate in the Livestock (Antimicrobial-resistant Bacteria). Food Saf.5, 24–28 (2017). [DOI] [PMC free article] [PubMed]
- 13.MAPA. Instrução Normativa 45°, de 22 de novembro de 2016. Diário Oficial da União. 2016;229:6. [Google Scholar]
- 14.European Medicines Agency. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. 56 Available at: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211080.pdf (Accessed: 8th July 2019) (2016).
- 15.Dowarah R, Verma AK, Agarwal N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Animal Nutrition. 2017;3:1–6. doi: 10.1016/j.aninu.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Z, Zhang S, Wang H, Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz-Sanchez S, D’Souza D, Biswas D, Hanning I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015;94:1419–1430. doi: 10.3382/ps/pev014. [DOI] [PubMed] [Google Scholar]
- 18.Si W, et al. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006;100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x. [DOI] [PubMed] [Google Scholar]
- 19.Simitzis PE. Enrichment of Animal Diets with Essential Oils—A Great Perspective on Improving Animal Performance and Quality Characteristics of the Derived Products. Medicines. 2017;4:35. doi: 10.3390/medicines4020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci. Technol. 2008;19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- 21.Friedly, E. C. et al. In vitro antilisterial effects of citrus oil fractions in combination with organic acids. J. Food Sci. 74 (2009). [DOI] [PubMed]
- 22.O’Bryan, C. A., Crandall, P. G., Chalova, V. I. & Ricke, S. C. Orange essential oils antimicrobial activities against Salmonella spp. J. Food Sci. 73 (2008). [DOI] [PubMed]
- 23.Chanthaphon S, Chanthachum S, Hongpattarakere T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. Against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008;30:125–131. [Google Scholar]
- 24.Zwietering, M. H., Jongenburger, I., Rombouts, F. M., Van’ Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1875–1881 (1990). [DOI] [PMC free article] [PubMed]
- 25.Settanni L, et al. Inhibition of foodborne pathogen bacteria by essential oils extracted from citrus fruits cultivated in Sicily. Food Control. 2012;26:326–330. doi: 10.1016/j.foodcont.2012.01.050. [DOI] [Google Scholar]
- 26.Ambrosio CMS, de Alencar SM, de Sousa RLM, Moreno AM, Da Gloria EM. Antimicrobial activity of several essential oils on pathogenic and beneficial bacteria. Ind. Crops Prod. 2017;97:128–136. doi: 10.1016/j.indcrop.2016.11.045. [DOI] [Google Scholar]
- 27.Ouwehand AC, et al. In vitro effects of essential oils on potential pathogens and beneficial members of the normal microbiota. Vet. Med. (Praha). 2010;55:71–78. doi: 10.17221/152/2009-VETMED. [DOI] [Google Scholar]
- 28.Yi F, Jin R, Sun J, Ma B, Bao X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT. 2018;95:346–353. doi: 10.1016/j.lwt.2018.05.011. [DOI] [Google Scholar]
- 29.Mandal Shyamapada, Mandal Manisha. Essential Oils in Food Preservation, Flavor and Safety. 2016. Tangerine (Citrus reticulata L. var.) Oils; pp. 803–811. [Google Scholar]
- 30.Fisher K, Phillips CA. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006;101:1232–1240. doi: 10.1111/j.1365-2672.2006.03035.x. [DOI] [PubMed] [Google Scholar]
- 31.Espina L, et al. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control. 2011;22:896–902. doi: 10.1016/j.foodcont.2010.11.021. [DOI] [Google Scholar]
- 32.Tao N, Liu Y, Zhang M. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck) Int. J. Food Sci. Technol. 2009;44:1281–1285. doi: 10.1111/j.1365-2621.2009.01947.x. [DOI] [Google Scholar]
- 33.Ebani V, et al. Chemical Composition and In Vitro Antimicrobial Efficacy of Sixteen Essential Oils against Escherichia coli and Aspergillus fumigatus Isolated from Poultry. Vet. Sci. 2018;5:62. doi: 10.3390/vetsci5030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Jia-jing, Gao Zhi-peng, Xia Jin-lan, Ritenour Mark A., Li Gao-yang, Shan Yang. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT. 2018;97:825–839. doi: 10.1016/j.lwt.2018.07.060. [DOI] [Google Scholar]
- 35.Nazzaro Filomena, Fratianni Florinda, De Martino Laura, Coppola Raffaele, De Feo Vincenzo. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals. 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helander IM, et al. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 37.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Perez-Álvarez J. J. Food Saf. 2008. Antibacterial activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils; pp. 567–576. [Google Scholar]
- 38.Chapot-Chartier M-P, Kulakauskas S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014;13(Suppl 1):S9. doi: 10.1186/1475-2859-13-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gueimonde M, Sánchez B, G. de los Reyes-Gavilán C, Margolles A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013;4:202. doi: 10.3389/fmicb.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campedelli I, et al. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2018;85:e01738–18. doi: 10.1128/AEM.01738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJA. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 2005;68:919–26. doi: 10.4315/0362-028X-68.5.919. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez J, Barry-Ryan C, Bourke P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008;124:91–97. doi: 10.1016/j.ijfoodmicro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Hayouni EA, Bouix M, Abedrabba M, Leveau J-Y, Hamdi M. Mechanism of action of Melaleuca armillaris (Sol. Ex Gaertu) Sm. essential oil on six LAB strains as assessed by multiparametric flow cytometry and automated microtiter-based assay. Food Chem. 2008;111:707–718. doi: 10.1016/j.foodchem.2008.04.044. [DOI] [Google Scholar]
- 44.Ambrosio CMS, de Alencar SM, Moreno AM, Da Gloria EM. Evaluation of the selective antibacterial activity of Eucalyptus globulus and Pimenta pseudocaryophyllus essential oils individually and in combination on Enterococcus faecalis and Lactobacillus rhamnosus. Can. J. Microbiol. 2018;64:844–855. doi: 10.1139/cjm-2018-0021. [DOI] [PubMed] [Google Scholar]
- 45.Fancello F, et al. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT - Food Sci. Technol. 2016;69:579–585. doi: 10.1016/j.lwt.2016.02.018. [DOI] [Google Scholar]
- 46.Guo Q, et al. Chemical composition and antimicrobial activity of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall) peel essential oils. Food Sci. Nutr. 2018;6:1431–1437. doi: 10.1002/fsn3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils - A review. Food and Chemical Toxicology. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 48.Swamy Mallappa Kumara, Akhtar Mohd Sayeed, Sinniah Uma Rani. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evidence-Based Complementary and Alternative Medicine. 2016;2016:1–21. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Eleventh Edition. Clinical and Laboratory Standars Institute M02-A11, Wayne, PA. 32 (2012).
- 50.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard — Ninth Edition. Clinical and Laboratory Standards Institute M07-A9, Wayne, PA. 32 (2012).
- 51.Pereira V, Dias C, Vasconcelos MC, Rosa E, Saavedra MJ. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa) Ind. Crops Prod. 2014;52:1–7. doi: 10.1016/j.indcrop.2013.09.032. [DOI] [Google Scholar]
- 52.Van Den Dool H, Kratz DP. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 53.Adams, R. P. Identification of essential oils by gas chromatography/mass spectrometry. Carol Stream Allured Publ. Corp. (2007).
- 54.Babushok VI, Linstrom PJ, Zenkevich IG. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Chemical composition of the six citrus essential oils*.