Abstract
Pemphigoid diseases are a group of autoimmune blistering skin diseases defined by an immune response against certain components of the dermal-epidermal adhesion complex. They are prototypical, autoantibody-driven, organ-specific diseases with the emergence of inflammatory skin lesions dependent on the recruitment of immune cells, particularly granulocytes, into the skin. During an acute flare of disease, inflammatory skin lesions typically progressing from erythema through urticarial plaques to subepidermal blisters erosions erupt and, finally, completely resolve, thus illustrating that resolution of inflammation is continuously executed in pemphigoid disease patients and can be directly monitored on the skin. Despite these superb conditions for examining resolution in pemphigoid diseases as paradigm diseases for antibody-induced tissue inflammation, the mechanisms of resolution in pemphigoid are underinvestigated and still largely elusive. In the last decade, mouse models for pemphigoid diseases were developed, which have been instrumental to identify several key pathways for the initiation of inflammation in these diseases. More recently, also protective pathways, specifically IL-10 and C5aR2 signalling on the molecular level and Tregs on the cellular level, counteracting skin inflammation have been highlighted and may contribute to the continuous execution of resolution in pemphigoid diseases. The upstream orchestrators of this process are currently under investigation. Pemphigoid disease patients, particularly bullous pemphigoid patients, who are predominantly above 75 years of age, often succumb to the side effects of the immunosuppressive therapeutics nowadays still required to suppress the disease. Pemphigoid disease patients may therefore represent a group of patients benefiting most substantially from the introduction of non-immunosuppressive, proresolving therapeutics into the treatment regimens for their disease.
Keywords: Autoimmunity, Bullous pemphigoid, Pathophysiology, IL-17, Complement, Blistering, Resolution
Definition of pemphigoid diseases
Organ-specific, IgG- and/or IgA-mediated immune responses are one of the most common pathomechanisms in the pathogenesis of autoimmune diseases. In humans, there are examples for this aberrant type of immune response leading to disease in almost any organ [1]. This also includes the skin where an IgG- and/or IgA-mediated immune response directed to specific components of the dermal-epidermal adhesion complex is the defining, common pathomechanistic principle of pemphigoid diseases, a group of autoimmune blistering skin diseases, which characteristically features the formation of subepidermal, dense blisters of the skin.
Seven different disease entities, namely bullous pemphigoid (BP), pemphigoid gestationis, mucous membrane pemphigoid, linear IgA disease, lichen planus pemphigoides, anti-p200 pemphigoid, and epidermolysis bullosa acquisita (EBA), belong to the group of pemphigoid disease entities. Although the diseases share many aspects of their histopathological and clinical presentation, they also partially differ in many aspects, including in their autoantigens (Table 1) and in the long-term course of disease (reviewed in [2–4]).
Table 1.
Pemphigoid diseases and their respective associated autoantibodies
| Pemphigoid disease | Autoantigens |
|---|---|
| Bullous pemphigoid (BP) |
BP180 NC16A domain (type XVII collagen) BP230 |
| Pemphigoid gestationis |
BP180 NC16A domain (type XVII collagen) BP230 |
| Lichen planus pemphigoides |
BP180 NC16A domain (type XVII collagen) BP230 |
| Epidermolysis bullosa acquisita (EBA) | Type VII collagen |
| Anti-p200 pemphigoid | p200 protein/laminin γ1 |
| Mucous membrane pemphigoid (MMP) | BP180, laminin 332, BP230a, α6β4 integrin, type VII collagen |
| Linear IgA disease | LAD-1, BP230a |
aIn nearly all cases, autoantibodies against one of the other target antigens is found
The by far most common and best examined pemphigoid disease is BP. We will therefore focus our brief overview on the clinical features and pathophysiology pemphigoid disease on BP. More detailed information on other pemphigoid diseases are available in numerous excellent reviews published on the clinical features and management of pemphigoid diseases in the last years [1–4].
Bullous pemphigoid
BP is the most frequent autoimmune blistering skin disease with a prevalence of approximately 21,000 patients, i.e. 260/million, in Germany [5] and an incidence varying between populations from 10 to 25 patients/million/year in Central Europe and the USA [6–10]. Registry data from the UK and Sweden even reveal incidences of about 70/million/year [11, 12]. BP is, notably, a disease of the elderly with most patients affected in the 8th and 9th decennium with a mean age at the time of diagnosis between 75 and 80 years [5, 10, 12]. Accordingly, incidences steeply rise with age to more than 200/million/year in individuals older than 80 years [6–8, 11].
BP is a chronic disease usually exhibiting an undulating, remitting-relapsing course. However, in the pre-corticosteroid era, about 70% of patients already succumbed to the first flare of disease [13]. Since the introduction of various immunosuppressants into the treatment of pemphigoid diseases, acute flares of disease can be usually therapeutically suppressed but disease relapses in 40% of patients within 6 months after the discontinuation of immunosuppressive therapy [14] and the 1-year mortality of BP patients after the first flare is approximately 20–40% and, thus, 2–3-fold that of age- and sex-matched peers [4, 14, 15]. The cause for the increased mortality of BP patients even under treatment is not entirely clear. Many patients, however, succumb to infections, which are certainly promoted by the immunosuppressive drugs employed for the treatment of BP. It appears evident that with the vast majority of BP patients being elderly above 80 years, the collective of BP patients is exceptionally susceptible to the unwanted side effects of immunosuppressive drugs.
BP is defined by the formation of autoantibodies against the hemidesmosomal protein BP180, also termed type XVII collagen (Col17) [2–4, 16]. About half of BP patients, additionally, form autoantibodies against BP230, another hemidesmosomal protein, probably due to epitope spreading, but the pathogenic significance of anti-BP230 autoantibodies is still not fully elucidated [17–19]. Like in all pemphigoid diseases, the deposition of autoantibodies at the dermal-epidermal junction (DEJ) alone does not precipitate inflammatory skin lesions. The latter require the marked recruitment of immune cells, particularly of granulocytes, into the dermis. Apart from old age, the greatest risk factors appear to be debilitating neurological disorders that affect a third to half of the BP patients and usually precede the autoimmune skin disease [20–23], and the use of diuretics, psycholeptics, and dipeptidyl-peptidase IV inhibitors (gliptins) [20, 24–28].
The clinical presentation of BP is variable but in its typical and most common clinical presentation, it exhibits the eruption of inflammatory skin lesions with the individual skin lesion progressing from erythema through urticarial plaques, blisters, and erosions to uninflamed and scarlessly re-epithelizing skin [2, 29] (Fig. 1). Thus, herein, the latter stage represents a state of resolving or resolved skin inflammation on track to reach the reconstitutio ad integrum. The different stages of lesion progression often co-exist in the individual patient during an acute flare of disease. This description of the inflammatory skin lesions in BP already implicates that, although autoantibodies usually deposit at the dermal-epidermal junction throughout the entire skin, in most cases, skin lesions do not involve the entire skin at the same time but only erupt in certain areas scattered all over the body but often sparing the face. The reasons for this selective eruption of skin lesions are still elusive. Of interest, data from the pre-corticosteroid area indicate that one third of BP patients will recover from the disease within 5 years without specific treatment [13]. Collectively, these clinical observations, however, strongly advocate the existence of endogenous, proresolving, and anti-inflammatory mechanisms first counteracting the emergence of skin inflammation and later, terminating (“resolving”) it, where it finally could erupt.
Fig. 1.
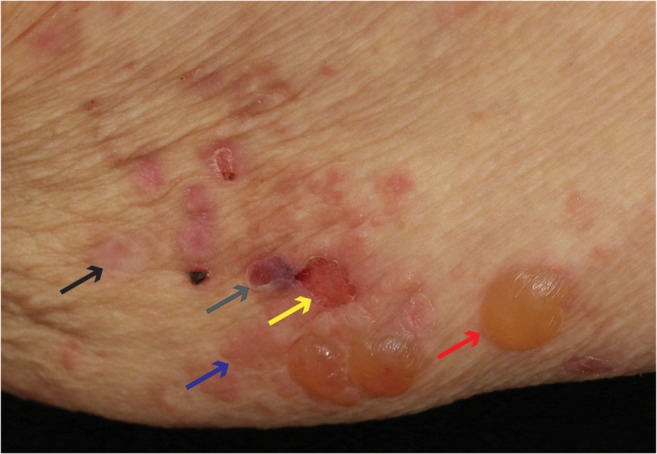
Typical co-existence of inflammatory skin lesions in different stages of development, including an emerging skin lesions in the shape of an urticarial plaque (blue arrow), a skin blister (red arrow), an erosion (yellow arrow), a resolving/healing erosion (grey arrow), and a resolved/healed skin lesions (black arrow) on the medial aspect of the upper arm of a BP patient during an acute flare
Diagnosis is based on (i) a compatible clinical picture, (ii) linear deposits of IgG and/or complement C3 at the dermal-epidermal junction by direct immunofluorescence (IF) microscopy of a perilesional skin biopsy, and (iii) the detection of serum autoantibodies against BP180 and/or BP230 [15, 30–36].
Treatment of moderate and severe BP relies on the long-term use of systemic corticosteroids usually combined with potentially corticosteroid-sparing immunomodulants such as doxycycline and dapsone or immunosuppressants such as methotrexate, azathioprine, and mycophenolate [15, 36–39]. In severe or recalcitrant patients, adjuvant rituximab, immunoadsorption, or high-dose intravenous immunoglobulins can be employed [15, 37, 40–43].
While several mouse models of pemphigoid diseases have been instrumental in uncovering some of the key proinflammatory pathways driving the effector phase of these diseases, research into these protective mechanisms is still in its infancy. The few insights gained to this point into these protective mechanisms will be summarized following a brief update on the pathophysiology of BP.
Pathophysiology
Pathology in BP is driven by autoantibodies against the two hemidesmosomal proteins BP180 (also termed type XVII collagen, Col17) and BP230 (reviewed in [2–4, 16, 44]). In addition, to anti-Col17 IgG about 40 and 60% of BP patients develop IgE and IgA anti-Col17 reactivity, respectively [18, 45–51]. BP230 is recognized by 50–70% of BP sera [32, 34, 52–56].
While in BP, a large body of evidence has been assembled to describe the pathogenic importance of autoantibodies and various mechanisms that mediate tissue destruction of anti-Col17 IgG (detailed below), data about the cellular immune response, undoubtedly essential in every autoimmune disease, are rather scare [57]. T and B cell reactivity against the NH2-terminal portion of the BP180 ectodomain is associated with severe BP, while the central portion is more frequently recognized in patients with limited disease. In contrast, combined T and B cell response against the COOH- and NH2-terminal globular domains of BP230 were found in less than 50% [58]. The response to the Col17 ectodomain is restricted to the DQβ1*0301 allele [59, 60]. Autoreactive T cells in BP patients release a Th1/Th2 mixed cytokine profile [58, 59]. While the number of circulating CD4+CD25+FoxP3+ regulatory T cells, natural killer T cells, and natural killer cells are normal, γδ T cell numbers were reported to be reduced in BP patients [61, 62].
A plethora of data has been published about the pathogenic relevance of anti-Col17 antibodies, while only conflicting reports were available about the pathogenicity of anti-BP230 IgG. Recently, however, the injection of monoclonal BP230-specific IgG in neonatal mice induced macroscopic and microscopic blistering suggesting their pathogenic potential [63, 64].
Our knowledge about the events that lead to subepidermal blistering upon binding of anti-Col17 antibodies to their cell surface receptor on keratinocytes is derived from the observation that serum levels of Col17NC16A-specific IgG antibodies correlate with the disease activity in BP patients [65–70] as well as various experimental models. Latter models include the incubation of cultured human keratinocytes with Col17-specific IgG/IgE, the treatment of cryosections of human skin with Col17-specific IgG followed by incubation with normal human leukocytes, and, importantly, various mouse models of BP and BP-like inflammatory EBA [71–77] (reviewed in [78–81]).
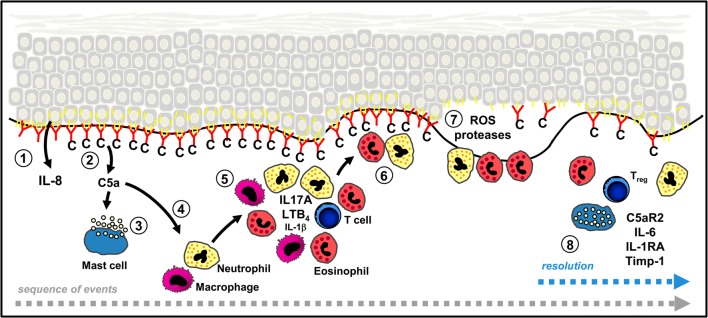
Based on these models, the following sequence of events has appeared (Fig. 2). While most effects depend on the Fc-portion of autoantibodies, also, very early in the diseases course, Fc-independent effects have been described including the release of IL-6 and IL-8 from keratinocytes following binding of anti-Col17 IgG or IgE [72, 82] as well as internalization of Col17 and weakening of keratinocyte attachment in response to anti-Col 17 IgG [83–87].
Fig. 2.
Sequence of events leading to dermal-epidermal separation and potentially proresolving pathways in bullous pemphigoid (BP). Binding of autoantibodies (red) against type XVII collagen (Col17, yellow) initiates Fc-independent events, e.g. the release of IL-8 from basal keratinocytes (1). Complement is activated at the dermal-epidermal junction (DEJ) and C5a released (2). Mast cells degranulate after binding of C5A or anti-Col17 IgE (3) and inflammatory cells attracted by C5a appear in the upper dermis (4, 5). Their secretion of additional inflammatory mediators such as IL-17A, LTB4, and to a lesser extent, of IL-1β further increases and maintains the inflammatory reaction. Subsequently, neutrophils and eosinophils line along the DEJ (6) and release reactive oxygen species and specific proteases that ultimately induce dermal-epidermal separation (7). In animal models of BP and BP-like epidermolysis bullosa acquisita, the anti-inflammatory effect of regulatory T cells (Treg), C5aR2, and IL-6 (via IL-1 receptor antagonist, IL-1RA, and tissue inhibitor of metalloproteinase-1, Timp-1) was shown and point to potentially proresolving mechanisms in BP. Modified from [4] and [3]
The earliest Fc-dependent effect of autoantibodies is most likely the activation of complement at the dermal-epidermal junction, an event observed in the skin of nearly all BP patients. Complement activation leads to the influx of inflammatory cells such as neutrophils, eosinophils, and macrophages [75, 88–90]. Complement-derived anaphylatoxins such as C5a have strong chemotactic effects on these cells. Here, both the classical and the alternative pathway of complement activation are important and most effects are most likely exerted via the C5aR1 on leucocytes [88, 89, 91]. In addition, the degranulation of mast cells, one of the earliest events observed in BP lesions, may be exerted via C5aR1 on mast cells [92]. An even more striking usage of C5aR1 was observed in mouse models of BP-like inflammatory EBA and anti-laminin 332 mucous membrane pemphigoid [93–96].
In addition to mast cells [90, 92], neutrophils [97–101], eosinophils [102], and macrophages [90] were shown to contribute to subsequent tissue destruction. So far, only few individual inflammatory mediators with a striking effect on the autoantibody-mediated tissue destruction have been identified in addition to C5a, including LTB4 and IL-17A.
Leukotriene B4 (LTB4) is another chemoattractant critically involved in the regulation of neutrophil influx into the skin. Thus, deficiency in 5-lipoxygenase, the rate-limiting enzyme in the biosynthesis of LTB4, or in the LTB4 receptor BLT1 confers dramatic resistance to the recruitment of neutrophils into the skin and, consequently, to the emergence of skin lesions in both the antibody transfer BP-like EBA and BP mouse model [103, 104]. Furthermore, scavenging of LTB4 by the drugs Coversin and PAS-L-Coversin significantly attenuates disease [105]. In line with this critical role of LTB4 in the first stages of lesion development, LTB4 levels in the start increasing briefly after the injection of anti-Col7 antibodies into the skin [103]. Tissue resident cells, such as macrophages, are presumably the initial cellular sources of LTB4. This notion, however, still requires clarification. Once recruited into the skin, neutrophils themselves are an abundant source of LTB4 and amplify their recruitment into the skin in a manner similar to what was previously demonstrated for autoantibody-induced inflammation in other organs [104, 106–108].
CD4-positive cells were identified as the major source of IL-17A both in the blood and early skin lesions of BP patients compared to age- and sex-matched controls. mRNA analysis of early skin lesions revealed IL17A and related cytokines and chemokines to be significantly upregulated. IL17A-deficient mice were greatly protected by the otherwise pathogenic effect of anti-Col17 IgG compared to wild-type animals, and anti-Col17 IgG-injected mice developed significantly fewer clinical lesions when treated with an anti-IL17 A antibody compared to isotype control antibody-treated mice [109]. In addition, Antonicelli and coworkers showed that (i) IL-17 serum levels are lower in patients in remission compared to the time when treatment was initiated, (ii) IL-17A is involved in the formation of neutrophil extracellular traps in the BP skin lesions, and (iii) IL-17 induce the release of neutrophil elastase and matrix metalloproteinase-9 from normal human polymorphonuclear cells [110–112].
C5a and LTB4 may thus induce the influx of inflammatory cells in the upper dermis, while IL-17 may orchestrate the inflammatory reaction in the skin that finally leads to blister formation.
In early BP lesions, neutrophils and eosinophils are found to line up along the dermal-epidermal junction. Reactive oxygen species and specific proteases such as matrix metalloproteinase-9 and neutrophil elastase were shown to be released form infiltrating leucocytes and lead to dermal-epidermal splitting [71, 113, 114]. Although the proteolytic activity most likely not specifically targets individual basement membrane proteins, matrix metalloproteinase-9 and neutrophil elastase were found in blister fluid and lesional biopsies of BP patients and were capable to degrade Col17 [115–117]. In fact, the importance of individual proteases was quite well studied in the neonatal mouse model of BP [98–101, 118, 119]. It appears that in the early stages of blistering, matrix metalloproteinase-9 is mainly activated by plasmin, which is formed by activation of plasminogen by tissue plasminogen activator and/or urokinase plasminogen activator. Plasmin and the mast cell-specific serine protease-4 can activate matrix metalloproteinase-9 which then inactivates α1-proteinase inhibitor, the physiological inhibitor of neutrophil elastase. The unrestrained activity of neutrophil elastase is then responsible for the degradation of structural proteins of the dermal-epidermal junction including Col17 [98–101, 118, 119]. This cascade of events is further amplified and perpetuated by the activation of the coagulation cascade by eosinophils, which further promotes the recruitment of eosinophils into the dermis [44, 120, 121].
In summary, some aspects of BP physiology, such as the sequence of events leading to blistering, including the requirement of autoantibodies and the infiltration of inflammatory cells, have been relatively well defined. Further studies will focus on the trigger factors that induce the generation of anti-Col17 and anti-BP230 antibodies in BP and on the identification of pharmacological inhibitors of key inflammatory mediators and pathways.
Resolution
Some mediators have been described that are present in the blood and/or skin of BP patients and were shown to exert anti-inflammatory properties when their functional relevance was explored in mouse models of BP or BP-like EBA. Below we summarize the current knowledge about the so far identified anti-inflammatory factors in BP including C5aR2, IL-6, and IL-10, and discuss them as effector molecules in the resolution of proresolving potential.
Complement activation
Both, the alternative and, to even a larger extent, the classical pathway were shown to be important for blister formation. In the neonatal mouse model of BP, activation of the classical pathway was even reported to be a requisite for dermal-epidermal separation in this model. Mice deficient in C5 or C1q did not develop blisters upon injection of anti-Col17 IgG due to the lack of neutrophil recruitment to the skin [88, 89, 122]. The quasi dogma of complement dependency of BP was later questioned when the passive transfer of F(ab’)2 fragments of human Col17-specific IgG or anti-Col17 IgG4 led to skin fragility when injected in Col17-humanized mice. In the same model, the induction of skin fragility upon injection of Col17-specific IgG in C3-deficient mice pointed in the same direction [122–124]. In the antibody transfer model of BP employing adult mice, the injection of anti-Col17 IgG in C5-deficient mice halved the extent of skin lesions as compared to wild-type mice independent of the IgG dose [91]. In line, when C5aR1-deficient mice were injected with anti-Col17 IgG, significantly less skin lesions arose compared to wild-type animals; however, C5aR1-deficient mice still developed about two third of the extent of lesions induced in wild-type mice [91]. These results indicate that although complement activation is an important factor to recruit leucocytes to the upper dermis, BP lesions may develop independently of complement activation.
Interestingly, when Col17-specific IgG was injected in C5aR2-deficient mice, these mice developed significantly more skin lesions compared to wild-type animals. While C5aR1 clearly is a proinflammatory mediator, the role of C5aR2 in inflammation appears to be multifaceted and may depend on the individual disease and the stage of inflammation [125]. In various disorders, such as sepsis, immune-complex-mediated lung injury, and allergic contact dermatitis, like in BP, C5aR2 has a protective role [125]. In line, migration of bone marrow-derived C5aR1-deficient neutrophils towards recombinant C5a was significantly lower compared to neutrophils from C57BL/6 wild-type mice, while migration of neutrophils from C5aR2-deficient animals was similar to neutrophils from wild-type mice [91]. Current research within the Clinical Research Unit 303 Pemphigoid Diseases aims at further delineating the anti-inflammatory and potentially proresolving role of C5aR2 in BP.
Interleukin-6
IL-6 is a pleiotropic cytokine that has been identified as key proinflammatory mediator in several diseases including rheumatoid arthritis, Castleman’s disease, Takayasu arteritis, and giant cell arteritis [126]. However, proinflammatory effects of IL-6 have been described, e.g. in animal models of endotoxic lung disease and endotoxemia [127]. The classical signalling pathway is initiated by binding of IL-6 to IL-6R and a second transmembrane protein, gp130, which serves as a signal transducer of IL-6. IL-6 can also bind to soluble IL-6Rs (sIL-6Rs forming an IL-6-sIL-6R complex that can bind to membrane-bound gp130 on cells that do not express IL-6R on the surface, a process known as trans-signalling) [126, 128]. In a mouse model of BP-like EBA, we have shown that blockade of IL-6 led to significantly increased skin lesions via classical IL-6 signalling most likely by the inhibition of IL-1R antagonist [129]. More specifically, while patients as well as anti-Col7 IgG-injected mice revealed elevated levels of IL-6 in blister fluids (BP patients only), sera and skin, IL-6-deficient mice or mice treated with an blocking anti-IL-6 antibody developed significantly higher disease activity compared to wild-type animals [129, 130]. In contrast, treatment with recombinant IL-6 dose-dependently impaired the induction of experimental BP-like EBA and led to increased expression of IL-1R antagonist in skin and serum [129]. Co-injection of mice with anti-Col7 IgG and the IL-1R antagonist anakinra significantly reduced the induction of skin lesions [129]. Latter data were later corroborated by the finding that after disease induction by the immunization of susceptible SJL/J mice with recombinant Col7, anakinra prevented disease progression compared to phosphate buffer saline (PBS)-treated mice [131]. In line, IL-1R-deficient or IL-1β-deficient mice were significantly less susceptible to the skin lesion-inducing effect of anti-Col7 IgG [131]. In addition to interfering with IL-1 homeostasis, IL-6 may exert its anti-inflammatory role in experimental BP-like EBA by the observed increased dermal expression of tissue inhibitor of metalloproteinase-1 (Timp-1; a physiological inhibitor of metalloproteinase) in IL-6-treated mice [129]. Metalloproteinase-9 has previously been shown to be essential for blister formation in the neonatal mouse model of BP (see above) [100].
Tregs and Interleukin-10
On the cellular level, Tregs have been highlighted to promote the timely resolution of skin lesions in the antibody transfer BP-like EBA mouse model. Thus, depletion of Tregs in DEREG significantly aggravated and prolonged disease [132]. Supporting also a similar role of Tregs in the human situation, Tregs are also present in lesional skin of BP patients. Notably, their density in lesional BP skin is, however, significant lower than in psoriasis and atopic dermatitis suggesting a relative deficiency in Tregs in BP [133], which may contribute to the persistence of skin inflammation in pemphigoid diseases. The mechanisms Tregs apply to counteract skin inflammation are still largely elusive, but there is first evidence for a significant role of IL-10 in this process. Thus, the induction of IL-10+ plasma cells efficiently suppressed skin inflammation in the antibody transfer BP-like EBA mouse model, among other, by inducing the release of IL-10 from Tregs in the skin [134]. Intriguingly, a series of in vitro experiments suggested that IL-10 may suppress disease in pemphigoid diseases by directly inhibiting the effect of C5a on neutrophils [134].
Proresolving lipid mediators
Multiple lines of evidence predominantly generated in mouse models of acute peritonitis, pouchitis, ischemia, or colitis point at a central role of proresolving lipid mediators as orchestrators of resolution [135–137]. The validity of this principle for the resolution of autoantibody-induced tissue inflammation is, however, still uncertain [135], and the role of proresolving lipid mediators in pemphigoid diseases has not been investigated either. However, profiling the lipidome in emerging skin lesions in the antibody transfer mouse model of BP-like EBA, we detected 12/15-lipoxygenase-derived proresolving lipid mediators [103], suggesting that 12/15-lipoxygenase is already active in the initiation phase of skin lesions and might counteract their emergence from the very beginning. Whether the enzyme is still active during the resolution of skin lesions is currently under investigation.
Concluding remarks
In recent years, a number of anti-inflammatory pathways counteracting skin inflammation in pemphigoid diseases have been uncovered. These pathways may also play a role in the resolution of skin inflammation in pemphigoid diseases which still requires detailed investigation. With single skin lesions in BP continuously emerging and completely resolving, BP appears as excellent model to dissect the mechanisms of resolution in autoantibody-induced tissue inflammation and injury. In that line, the disease may potentially also respond particularly well to proresolving therapeutic strategies, and, with pemphigoid disease patients often succumbing to the side effects of immunosuppressive drugs, these diseases may be among those benefiting the most from the introduction of proresolving therapies into treatment regimens.
Funding information
This work was supported by the Schleswig-Holstein Cluster of Excellence Precision Medicine in Chronic Inflammation (EXC 2167-390884018) and the Clinical Research Unit 303 Pemphigoid Diseases (Sa1960/5-1, SCHM1686/7-2).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603. doi: 10.3389/fimmu.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol. 2018;54(1):26–51. doi: 10.1007/s12016-017-8633-4. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt E, Groves R. Immunobullous diseases. In: Griffith C, Barker J, Chalmers BT, Creamer D, editors. Rook’s textbook of dermatology, part 3, chapter 50. 9. Chichester: Wiley-Blackwell; 2016. pp. 1–56. [Google Scholar]
- 4.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 5.Hubner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases in Germany. J Invest Dermatol. 2016;136(12):2495–2498. doi: 10.1016/j.jid.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Bertram F, Brocker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges. 2009;7(5):434–440. doi: 10.1111/j.1610-0387.2008.06976.x. [DOI] [PubMed] [Google Scholar]
- 7.Gudi VS, White MI, Cruickshank N, Herriot R, Edwards SL, Nimmo F, et al. Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east Scotland. Br J Dermatol. 2005;153(2):424–427. doi: 10.1111/j.1365-2133.2005.06662.x. [DOI] [PubMed] [Google Scholar]
- 8.Marazza G, Pham HC, Scharer L, Pedrazzetti PP, Hunziker T, Trueb RM, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol. 2009;161(4):861–868. doi: 10.1111/j.1365-2133.2009.09300.x. [DOI] [PubMed] [Google Scholar]
- 9.Brick KE, Weaver CH, Lohse CM, Pittelkow MR, Lehman JS, Camilleri MJ, et al. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. 2014;71(1):92–99. doi: 10.1016/j.jaad.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joly P, Baricault S, Sparsa A, Bernard P, Bedane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132(8):1998–2004. doi: 10.1038/jid.2012.35. [DOI] [PubMed] [Google Scholar]
- 11.Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorslund K, Seifert O, Nilzen K, Gronhagen C. Incidence of bullous pemphigoid in Sweden 2005-2012: a nationwide population-based cohort study of 3761 patients. Arch Dermatol Res. 2017;309(9):721–727. doi: 10.1007/s00403-017-1778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lever WF. Pemphigus. Medicine. 1953;32:1–123. doi: 10.1097/00005792-195302000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346(5):321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 15.Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172(4):867–877. doi: 10.1111/bjd.13717. [DOI] [PubMed] [Google Scholar]
- 16.Bagci IS, Horvath ON, Ruzicka T, Sardy M. Bullous pemphigoid. Autoimmun Rev. 2017;16(5):445–455. doi: 10.1016/j.autrev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Delaporte E, Dubost-Brama A, Ghohestani R, Nicolas JF, Neyrinck JL, Bergoend H, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157(8):3642–3647. [PubMed] [Google Scholar]
- 18.Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol. 2017;177(1):141–151. doi: 10.1111/bjd.15114. [DOI] [PubMed] [Google Scholar]
- 19.Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, et al. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2008;49(2):153–161. doi: 10.1016/j.jdermsci.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131(3):637–643. doi: 10.1038/jid.2010.301. [DOI] [PubMed] [Google Scholar]
- 21.Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J Invest Dermatol. 2010;131(3):631–636. doi: 10.1038/jid.2010.357. [DOI] [PubMed] [Google Scholar]
- 22.Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case-control study. Arch Dermatol. 2010;146(11):1251–1254. doi: 10.1001/archdermatol.2010.322. [DOI] [PubMed] [Google Scholar]
- 23.Forsti AK, Huilaja L, Schmidt E, Tasanen K. Neurological and psychiatric associations in bullous pemphigoid-more than skin deep? Exp Dermatol. 2017;26(12):1228–1234. doi: 10.1111/exd.13401. [DOI] [PubMed] [Google Scholar]
- 24.Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, et al. Drugs associated with bullous pemphigoid. A case-control study. Arch Dermatol. 1996;132(3):272–276. [PubMed] [Google Scholar]
- 25.Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149(1):58–62. doi: 10.1001/2013.jamadermatol.376. [DOI] [PubMed] [Google Scholar]
- 26.Kridin K, Cohen AD (2018) Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid: a systematic review and meta-analysis. J Am Acad Dermatol [DOI] [PubMed]
- 27.Varpuluoma O, Forsti AK, Jokelainen J, Turpeinen M, Timonen M, Huilaja L, et al. Vildagliptin significantly increases the risk of bullous pemphigoid: a Finnish nationwide registry study. J Invest Dermatol. 2018;138(7):1659–1661. doi: 10.1016/j.jid.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Benzaquen M, Borradori L, Berbis P, Cazzaniga S, Valero R, Richard MA, Feldmeyer L. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2018;78(6):1090–1096. doi: 10.1016/j.jaad.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt E, della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Dermatol Clin. 2011;29(3):427–438. doi: 10.1016/j.det.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10(2):84–89. doi: 10.1016/j.autrev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt E, Zillikens D. The diagnosis and treatment of autoimmune blistering skin diseases. Deutsches Arzteblatt international. 2011;108(23):399–405. doi: 10.3238/arztebl.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blocker IM, Dahnrich C, Probst C, Komorowski L, Saschenbrecker S, Schlumberger W, et al. Epitope mapping of BP230 leading to a novel enzyme-linked immunosorbent assay for autoantibodies in bullous pemphigoid. Br J Dermatol. 2012;166(5):964–970. doi: 10.1111/j.1365-2133.2012.10820.x. [DOI] [PubMed] [Google Scholar]
- 33.Sitaru C, Dahnrich C, Probst C, Komorowski L, Blocker I, Schmidt E, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16(9):770–777. doi: 10.1111/j.1600-0625.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 34.Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. 2011;147(3):286–291. doi: 10.1001/archdermatol.2011.23. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt E, Goebeler M, Hertl M, Sardy M, Sitaru C, Eming R, et al. S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015;13(7):713–727. doi: 10.1111/ddg.12612. [DOI] [PubMed] [Google Scholar]
- 36.van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges. 2018;16(9):1077–1091. doi: 10.1111/ddg.13637. [DOI] [PubMed] [Google Scholar]
- 37.Eming R, Sticherling M, Hofmann SC, Hunzelmann N, Kern JS, Kramer H, et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015;13(8):833–844. doi: 10.1111/ddg.12606. [DOI] [PubMed] [Google Scholar]
- 38.Sticherling M, Franke A, Aberer E, Glaser R, Hertl M, Pfeiffer C, et al. An open, multicentre, randomized clinical study in patients with bullous pemphigoid comparing methylprednisolone and azathioprine with methylprednisolone and dapsone. Br J Dermatol. 2017;177(5):1299–1305. doi: 10.1111/bjd.15649. [DOI] [PubMed] [Google Scholar]
- 39.Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. 2017;389(10079):1630–1638. doi: 10.1016/S0140-6736(17)30560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubner F, Kasperkiewicz M, Knuth-Rehr D, Shimanovich I, Hubner J, Sufke S, et al. Adjuvant treatment of severe/refractory bullous pemphigoid with protein A immunoadsorption. J Dtsch Dermatol Ges. 2018;16(9):1109–1118. doi: 10.1111/ddg.13642. [DOI] [PubMed] [Google Scholar]
- 41.Kasperkiewicz M, Schulze F, Meier M, van Beek N, Nitschke M, Zillikens D, et al. Treatment of bullous pemphigoid with adjuvant immunoadsorption: a case series. J Am Acad Dermatol. 2014;71(5):1018–1020. doi: 10.1016/j.jaad.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Kasperkiewicz M, Shimanovich I, Ludwig RJ, Rose C, Zillikens D, Schmidt E. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65(3):552–558. doi: 10.1016/j.jaad.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Lamberts A, Euverman HI, Terra JB, Jonkman MF, Horvath B. Effectiveness and safety of rituximab in recalcitrant pemphigoid diseases. Front Immunol. 2018;9:248. doi: 10.3389/fimmu.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Marzano AV. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. 2019;10:1506. doi: 10.3389/fimmu.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42(4):577–583. [PubMed] [Google Scholar]
- 46.Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. 2008;144(1):41–48. doi: 10.1001/archdermatol.2007.9. [DOI] [PubMed] [Google Scholar]
- 47.Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods. 2009;346(1–2):18–25. doi: 10.1016/j.jim.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kromminga A, Scheckenbach C, Georgi M, Hagel C, Arndt R, Christophers E, et al. Patients with bullous pemphigoid and linear IgA disease show a dual IgA and IgG autoimmune response to BP180. J Autoimmun. 2000;15(3):293–300. doi: 10.1006/jaut.2000.0437. [DOI] [PubMed] [Google Scholar]
- 49.Christophoridis S, Budinger L, Borradori L, Hunziker T, Merk HF, Hertl M. IgG, IgA and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. Br J Dermatol. 2000;143(2):349–355. doi: 10.1046/j.1365-2133.2000.03661.x. [DOI] [PubMed] [Google Scholar]
- 50.van Beek N, Luttmann N, Huebner F, Recke A, Karl I, Schulze FS, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. 2017;153(1):30–38. doi: 10.1001/jamadermatol.2016.3357. [DOI] [PubMed] [Google Scholar]
- 51.van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. 2016;12(3):267–277. doi: 10.1586/1744666X.2016.1123092. [DOI] [PubMed] [Google Scholar]
- 52.Kromminga A, Sitaru C, Hagel C, Herzog S, Zillikens D. Development of an ELISA for the detection of autoantibodies to BP230. Clin Immunol. 2004;111(1):146–152. doi: 10.1016/j.clim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Thoma-Uszynski S, Uter W, Schwietzke S, Hofmann SC, Hunziker T, Bernard P, et al. BP230- and BP180-specific auto-antibodies in bullous pemphigoid. J Invest Dermatol. 2004;122(6):1413–1422. doi: 10.1111/j.0022-202X.2004.22603.x. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci. 2006;41(1):21–30. doi: 10.1016/j.jdermsci.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Tampoia M, Lattanzi V, Zucano A, Villalta D, Filotico R, Fontana A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15–20. doi: 10.1111/j.1749-6632.2009.04630.x. [DOI] [PubMed] [Google Scholar]
- 56.Roussel A, Benichou J, Randriamanantany ZA, Gilbert D, Drenovska K, Houivet E, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147(3):293–298. doi: 10.1001/archdermatol.2011.21. [DOI] [PubMed] [Google Scholar]
- 57.Hertl M, Eming R, Veldman C. T cell control in autoimmune bullous skin disorders. J Clin Invest. 2006;116(5):1159–1166. doi: 10.1172/JCI28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thoma-Uszynski S, Uter W, Schwietzke S, Schuler G, Borradori L, Hertl M. Autoreactive T and B cells from bullous pemphigoid (BP) patients recognize epitopes clustered in distinct regions of BP180 and BP230. J Immunol. 2006;176(3):2015–2023. doi: 10.4049/jimmunol.176.3.2015. [DOI] [PubMed] [Google Scholar]
- 59.Budinger L, Borradori L, Yee C, Eming R, Ferencik S, Grosse-Wilde H, et al. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest. 1998;102(12):2082–2089. doi: 10.1172/JCI3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin MS, Fu CL, Giudice GJ, Olague-Marchan M, Lazaro AM, Stastny P, et al. Epitopes targeted by bullous pemphigoid T lymphocytes and autoantibodies map to the same sites on the bullous pemphigoid 180 ectodomain. J Invest Dermatol. 2000;115(6):955–961. doi: 10.1046/j.1523-1747.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 61.Rensing-Ehl A, Gaus B, Bruckner-Tuderman L, Martin SF. Frequency, function and CLA expression of CD4+CD25+FOXP3+ regulatory T cells in bullous pemphigoid. Exp Dermatol. 2007;16(1):13–21. doi: 10.1111/j.1600-0625.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 62.Oswald E, Fisch P, Jakob T, Bruckner-Tuderman L, Martin SF, Rensing-Ehl A. Reduced numbers of circulating gammadelta T cells in patients with bullous pemphigoid. Exp Dermatol. 2009;18(11):991–993. doi: 10.1111/j.1600-0625.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 63.Haeberle S, Wei X, Bieber K, Goletz S, Ludwig RJ, Schmidt E, et al. Regulatory T-cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease. J Allergy Clin Immunol. 2018;142:1831–1842.e7. doi: 10.1016/j.jaci.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Muramatsu K, Ujiie H, Kobayashi I, Nishie W, Izumi K, Ito T, et al. Regulatory T-cell dysfunction induces autoantibodies to bullous pemphigoid antigens in mice and human subjects. J Allergy Clin Immunol. 2018;142(6):1818–30 e6. doi: 10.1016/j.jaci.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136(2):174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 66.Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura KC, Shimizu H. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. 2005;37(3):145–149. doi: 10.1016/j.jdermsci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30(3):224–232. doi: 10.1016/s0923-1811(02)00109-3. [DOI] [PubMed] [Google Scholar]
- 68.Feng S, Wu Q, Jin P, Lin L, Zhou W, Sang H, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47(3):225–228. doi: 10.1111/j.1365-4632.2008.03473.x. [DOI] [PubMed] [Google Scholar]
- 69.Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008;128(3):415–426. doi: 10.1016/j.clim.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Amo Y, Ohkawa T, Tatsuta M, Hamada Y, Fujimura T, Katsuoka K, et al. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2001;26(1):14–18. doi: 10.1016/s0923-1811(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 71.Sitaru C, Schmidt E, Petermann S, Munteanu LS, Brocker EB, Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118(4):664–671. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 72.Messingham KN, Srikantha R, DeGueme AM, Fairley JA. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187(1):553–560. doi: 10.4049/jimmunol.1001753. [DOI] [PubMed] [Google Scholar]
- 73.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92(5):2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, et al. Humanization of autoantigen. Nat Med. 2007;13(3):378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 75.Schulze FS, Beckmann T, Nimmerjahn F, Ishiko A, Collin M, Kohl J, et al. Fcgamma receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am J Pathol. 2014;184(8):2185–2196. doi: 10.1016/j.ajpath.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Sitaru C, Chiriac MT, Mihai S, Buning J, Gebert A, Ishiko A, et al. Induction of complement-fixing autoantibodies against type VII collagen results in subepidermal blistering in mice. J Immunol. 2006;177(5):3461–3468. doi: 10.4049/jimmunol.177.5.3461. [DOI] [PubMed] [Google Scholar]
- 77.Sitaru C, Mihai S, Otto C, Chiriac MT, Hausser I, Dotterweich B, et al. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115(4):870–878. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasperkiewicz M, Sadik CD, Bieber K, Ibrahim SM, Manz RA, Schmidt E, et al. Epidermolysis bullosa Acquisita: from pathophysiology to novel therapeutic options. J Invest Dermatol. 2016;136(1):24–33. doi: 10.1038/JID.2015.356. [DOI] [PubMed] [Google Scholar]
- 79.Ludwig RJ. Clinical presentation, pathogenesis, diagnosis, and treatment of epidermolysis bullosa acquisita. ISRN Dermatol. 2013;2013:812029. doi: 10.1155/2013/812029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bieber K, Koga H, Nishie W. In vitro and in vivo models to investigate the pathomechanisms and novel treatments for pemphigoid diseases. Exp Dermatol. 2017;26:1163–1170. doi: 10.1111/exd.13415. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Li L, Xia Y. BP180 is critical in the autoimmunity of bullous pemphigoid. Front Immunol. 2017;8:1752. doi: 10.3389/fimmu.2017.01752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt E, Reimer S, Kruse N, Jainta S, Brocker EB, Marinkovich MP, et al. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol. 2000;115(5):842–848. doi: 10.1046/j.1523-1747.2000.00141.x. [DOI] [PubMed] [Google Scholar]
- 83.Iwata H, Kamio N, Aoyama Y, Yamamoto Y, Hirako Y, Owaribe K, et al. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (type XVII collagen) and weakens cell attachment. J Invest Dermatol. 2009;129(4):919–926. doi: 10.1038/jid.2008.305. [DOI] [PubMed] [Google Scholar]
- 84.Hiroyasu S, Ozawa T, Kobayashi H, Ishii M, Aoyama Y, Kitajima Y, et al. Bullous pemphigoid IgG induces BP180 internalization via a macropinocytic pathway. Am J Pathol. 2013;182(3):828–840. doi: 10.1016/j.ajpath.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitajima Y, Nojiri M, Yamada T, Hirako Y, Owaribe K. Internalization of the 180 kDa bullous pemphigoid antigen as immune complexes in basal keratinocytes: an important early event in blister formation in bullous pemphigoid. Br J Dermatol. 1998;138(1):71–76. doi: 10.1046/j.1365-2133.1998.02028.x. [DOI] [PubMed] [Google Scholar]
- 86.Wada M, Nishie W, Ujiie H, Izumi K, Iwata H, Natsuga K, Nakamura H, Kitagawa Y, Shimizu H. Epitope-dependent pathogenicity of antibodies targeting a major bullous pemphigoid autoantigen collagen XVII/BP180. J Invest Dermatol. 2016;136(5):938–946. doi: 10.1016/j.jid.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 87.Iwata H, Kamaguchi M, Ujiie H, Nishimura M, Izumi K, Natsuga K, et al. Macropinocytosis of type XVII collagen induced by bullous pemphigoid IgG is regulated via protein kinase C. Lab Investig. 2016;96(12):1301–1310. doi: 10.1038/labinvest.2016.108. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, et al. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95(4):1539–1544. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nelson KC, Zhao M, Schroeder PR, Li N, Wetsel RA, Diaz LA, et al. Role of different pathways of the complement cascade in experimental bullous pemphigoid. J Clin Invest. 2006;116(11):2892–2900. doi: 10.1172/JCI17891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen R, Fairley JA, Zhao ML, Giudice GJ, Zillikens D, Diaz LA, et al. Macrophages, but not T and B lymphocytes, are critical for subepidermal blister formation in experimental bullous pemphigoid: macrophage-mediated neutrophil infiltration depends on mast cell activation. J Immunol. 2002;169(7):3987–3992. doi: 10.4049/jimmunol.169.7.3987. [DOI] [PubMed] [Google Scholar]
- 91.Karsten CM, Beckmann T, Holtsche MM, Tillmann J, Tofern S, Schulze FS, et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front Immunol. 2018;9:488. doi: 10.3389/fimmu.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heimbach L, Li Z, Berkowitz P, Zhao M, Li N, Rubenstein DS, et al. The C5a receptor on mast cells is critical for the autoimmune skin-blistering disease bullous pemphigoid. J Biol Chem. 2011;286(17):15003–15009. doi: 10.1074/jbc.M111.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18(9):1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mihai S, Chiriac MT, Takahashi K, Thurman JM, Holers VM, Zillikens D, et al. The alternative pathway of complement activation is critical for blister induction in experimental epidermolysis bullosa acquisita. J Immunol. 2007;178(10):6514–6521. doi: 10.4049/jimmunol.178.10.6514. [DOI] [PubMed] [Google Scholar]
- 95.Mihai S, Hirose M, Wang Y, Thurman JM, Holers VM, Morgan BP, et al. Specific inhibition of complement activation significantly ameliorates autoimmune blistering disease in mice. Front Immunol. 2018;9:535. doi: 10.3389/fimmu.2018.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heppe EN, Tofern S, Schulze FS, Ishiko A, Shimizu A, Sina C, et al. Experimental laminin 332 mucous membrane pemphigoid critically involves C5aR1 and reflects clinical and Immunopathological characteristics of the human disease. J Invest Dermatol. 2017;137(8):1709–1718. doi: 10.1016/j.jid.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100(5):1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Z, Li N, Diaz LA, Shipley M, Senior RM, Werb Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J Clin Invest. 2005;115(4):879–887. doi: 10.1172/JCI23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Z, Shapiro SD, Zhou X, Twining SS, Senior RM, Giudice GJ, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. 2000;105(1):113–123. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, et al. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188(3):475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, et al. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102(5):647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 102.Lin L, Hwang BJ, Culton DA, Li N, Burette S, Koller BH, et al. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J Invest Dermatol. 2018;138(5):1032–1043. doi: 10.1016/j.jid.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sezin T, Krajewski M, Wutkowski A, Mousavi S, Chakievska L, Bieber K, et al. The leukotriene B4 and its receptor BLT1 act as critical drivers of neutrophil recruitment in murine bullous pemphigoid-like epidermolysis bullosa acquisita. J Invest Dermatol. 2017;137(5):1104–1113. doi: 10.1016/j.jid.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 104.Sadik CD, Miyabe Y, Sezin T, Luster AD. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin Immunol. 2018;37:21–29. doi: 10.1016/j.smim.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Sezin T, Murthy S, Attah C, Seutter M, Holtsche MM, Hammers CM et al (2019) Dual inhibition of complement factor 5 and leukotriene B4 synergistically suppresses murine pemphigoid disease. JCI Insight 4(15) [DOI] [PMC free article] [PubMed]
- 106.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proc Natl Acad Sci U S A. 2012;109(46):E3177–E3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91(2):207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chakievska L, Holtsche MM, Kunstner A, Goletz S, Petersen BS, Thaci D, et al. IL-17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J Autoimmun. 2019;96:104–112. doi: 10.1016/j.jaut.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Le Jan S, Plee J, Vallerand D, Dupont A, Delanez E, Durlach A, et al. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol. 2014;134(12):2908–2917. doi: 10.1038/jid.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Plee J, Le Jan S, Giustiniani J, Barbe C, Joly P, Bedane C, et al. Integrating longitudinal serum IL-17 and IL-23 follow-up, along with autoantibodies variation, contributes to predict bullous pemphigoid outcome. Sci Rep. 2015;5:18001. doi: 10.1038/srep18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giusti D, Bini E, Terryn C, Didier K, Le Jan S, Gatouillat G, et al. NET formation in bullous pemphigoid patients with relapse is modulated by IL-17 and IL-23 interplay. Front Immunol. 2019;10:701. doi: 10.3389/fimmu.2019.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Brocker EB, Opdenakker G, et al. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol. 2004;204(5):519–527. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 114.Yu X, Holdorf K, Kasper B, Zillikens D, Ludwig RJ, Petersen F. FcgammaRIIA and FcgammaRIIIB are required for autoantibody-induced tissue damage in experimental human models of bullous pemphigoid. J Invest Dermatol. 2010;130(12):2841–2844. doi: 10.1038/jid.2010.230. [DOI] [PubMed] [Google Scholar]
- 115.Stahle-Backdahl M, Inoue M, Guidice GJ, Parks WC. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J Clin Invest. 1994;93(5):2022–2030. doi: 10.1172/JCI117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Niimi Y, Pawankar R, Kawana S. Increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and matrix metalloproteinase-13 in lesional skin of bullous pemphigoid. Int Arch Allergy Immunol. 2006;139(2):104–113. doi: 10.1159/000090385. [DOI] [PubMed] [Google Scholar]
- 117.Verraes S, Hornebeck W, Polette M, Borradori L, Bernard P. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J Invest Dermatol. 2001;117(5):1091–1096. doi: 10.1046/j.0022-202x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- 118.Lin L, Bankaitis E, Heimbach L, Li N, Abrink M, Pejler G, et al. Dual targets for mouse mast cell protease-4 in mediating tissue damage in experimental bullous pemphigoid. J Biol Chem. 2011;286(43):37358–37367. doi: 10.1074/jbc.M111.272401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin L, Betsuyaku T, Heimbach L, Li N, Rubenstein D, Shapiro SD, et al. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biol. 2012;31(1):38–44. doi: 10.1016/j.matbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marzano AV, Tedeschi A, Berti E, Fanoni D, Crosti C, Cugno M. Activation of coagulation in bullous pemphigoid and other eosinophil-related inflammatory skin diseases. Clin Exp Immunol. 2011;165(1):44–50. doi: 10.1111/j.1365-2249.2011.04391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cugno M, Marzano AV, Bucciarelli P, Balice Y, Cianchini G, Quaglino P, et al. Increased risk of venous thromboembolism in patients with bullous pemphigoid. The INVENTEP (INcidence of VENous ThromboEmbolism in bullous Pemphigoid) study. Thromb Haemost. 2016;115(1):193–199. doi: 10.1160/TH15-04-0309. [DOI] [PubMed] [Google Scholar]
- 122.Iwata H, Ujiie H. Complement-independent blistering mechanisms in bullous pemphigoid. Exp Dermatol. 2017;26(12):1235–1239. doi: 10.1111/exd.13367. [DOI] [PubMed] [Google Scholar]
- 123.Natsuga K, Nishie W, Shinkuma S, Ujiie H, Nishimura M, Sawamura D, et al. Antibodies to pathogenic epitopes on type XVII collagen cause skin fragility in a complement-dependent and -independent manner. J Immunol. 2012;188(11):5792–5799. doi: 10.4049/jimmunol.1003402. [DOI] [PubMed] [Google Scholar]
- 124.Ujiie H, Sasaoka T, Izumi K, Nishie W, Shinkuma S, Natsuga K, et al. Bullous pemphigoid autoantibodies directly induce blister formation without complement activation. J Immunol. 2014;193(9):4415–4428. doi: 10.4049/jimmunol.1400095. [DOI] [PubMed] [Google Scholar]
- 125.Li XX, Lee JD, Kemper C, Woodruff TM. The complement receptor C5aR2: a powerful modulator of innate and adaptive immunity. J Immunol. 2019;202(12):3339–3348. doi: 10.4049/jimmunol.1900371. [DOI] [PubMed] [Google Scholar]
- 126.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 127.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50(4):812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 129.Samavedam UK, Kalies K, Scheller J, Sadeghi H, Gupta Y, Jonkman MF, et al. Recombinant IL-6 treatment protects mice from organ specific autoimmune disease by IL-6 classical signalling-dependent IL-1ra induction. J Autoimmun. 2012;40:74–85. doi: 10.1016/j.jaut.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Schmidt E, Bastian B, Dummer R, Tony HP, Brocker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. 1996;288(7):353–357. doi: 10.1007/BF02507102. [DOI] [PubMed] [Google Scholar]
- 131.Sadeghi H, Lockmann A, Hund AC, Samavedam UK, Pipi E, Vafia K, et al. Caspase-1-independent IL-1 release mediates blister formation in autoantibody-induced tissue injury through modulation of endothelial adhesion molecules. J Immunol. 2015;194(8):3656–3663. doi: 10.4049/jimmunol.1402688. [DOI] [PubMed] [Google Scholar]
- 132.Bieber K, Sun S, Witte M, Kasprick A, Beltsiou F, Behnen M, et al. Regulatory T cells suppress inflammation and blistering in pemphigoid diseases. Front Immunol. 2017;8:1628. doi: 10.3389/fimmu.2017.01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Antiga E, Quaglino P, Volpi W, Pierini I, Del Bianco E, Bianchi B, et al. Regulatory T cells in skin lesions and blood of patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2014;28(2):222–230. doi: 10.1111/jdv.12091. [DOI] [PubMed] [Google Scholar]
- 134.Kulkarni U, Karsten CM, Kohler T, Hammerschmidt S, Bommert K, Tiburzy B, et al. IL-10 mediates plasmacytosis-associated immunodeficiency by inhibiting complement-mediated neutrophil migration. J Allergy Clin Immunol. 2016;137(5):1487–97 e6. doi: 10.1016/j.jaci.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 135.Sugimoto MA, Vago JP, Perretti M, Teixeira MM. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019;40(3):212–227. doi: 10.1016/j.it.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Headland SE, Norling LV. The resolution of inflammation: principles and challenges. Semin Immunol. 2015;27(3):149–160. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 137.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]