Abstract
BACKGROUND
Serum amyloid A (SAA) is an acute phase protein mainly synthesized by the liver. SAA induces inflammatory phenotype and promotes cell proliferation in activated hepatic stellate cells, the major scar forming cells in the liver. However, few studies have reported on the serum levels of SAA in human liver disease and its clinical significance in various liver diseases.
AIM
To investigate the serum levels of SAA in patients with different liver diseases and analyze the factors associated with the alteration of SAA levels in chronic hepatitis B (CHB) patients.
METHODS
Two hundred and seventy-eight patients with different liver diseases and 117 healthy controls were included in this study. The patients included 205 with CHB, 22 with active autoimmune liver disease (AILD), 21 with nonalcoholic steatohepatitis (NASH), 14 with drug-induced liver injury (DILI), and 16 with pyogenic liver abscess. Serum levels of SAA and other clinical parameters were collected for the analysis of the factors associated with SAA level. Mann-Whitney U test was used to compare the serum SAA levels of patients with various liver diseases with those of healthy controls. Bonferroni test was applied for post hoc comparisons to control the probability of type 1 error (alpha = 0.05/6 = 0.008). For statistical tests of other variables, P < 0.05 was considered statistically significant. Statistically significant factors determined by single factor analysis were further analyzed by binary multivariate logistic regression analysis.
RESULTS
All patients with active liver diseases had higher serum SAA levels than healthy controls and the inactive CHB patients, with the highest SAA level found in patients with pyogenic liver abscess (398.4 ± 246.8 mg/L). Patients with active AILD (19.73 ± 24.81 mg/L) or DILI (8.036 ± 5.685 mg/L) showed higher SAA levels than those with active CHB (6.621 ± 6.776 mg/L) and NASH (6.624 ± 4.891 mg/L). Single (P < 0.001) and multivariate logistic regression analyses (P = 0.039) for the CHB patients suggested that patients with active CHB were associated with an SAA serum level higher than 6.4 mg/L. Serum levels of SAA and CRP (C-reactive protein) were positively correlated in patients with CHB (P < 0.001), pyogenic liver abscess (P = 0.045), and active AILD (P = 0.02). Serum levels of SAA (0.80-871.0 mg/L) had a broader fluctuation range than CRP (0.30-271.3 mg/L).
CONCLUSION
Serum level of SAA is a sensitive biomarker for inflammatory activity of pyogenic liver abscess. It may also be a weak marker reflecting milder inflammatory status in the liver of patients with CHB and other active liver diseases.
Keywords: Serum amyloid A, Liver diseases, Pyogenic liver abscess, Chronic hepatitis B, Inflammation
Core tip: Serum amyloid A (SAA) is an acute phase protein known to have diagnostic and prognostic value in many diseases. However, the SAA level and its clinical significance in various liver diseases have not been reported. Our study found that serum level of SAA is a sensitive biomarker for inflammatory activity of pyogenic liver abscess, and to a less extent, to reflect mild inflammatory status in autoimmune liver diseases, drug-induced liver injury, chronic active hepatitis B, and nonalcoholic steatohepatitis. Serum level of SAA can be confounded by various inflammatory diseases, thus it is not a specific indicator for certain diseases.
INTRODUCTION
Serum amyloid A (SAA) is an acute phase protein mainly synthesized by the liver[1]. SAA1 and SAA2 are the major isoforms of acute phase SAA (A-SAA) in humans, which act on various receptors and signal pathways that participate in the pathological process of various diseases, with inflammatory, immune regulatory, and anti-microbial effects[1]. The increase of SAA level is not only a consequence of inflammation or tissue injury stimulated by inflammatory cytokines and mediators[2-4], but also acts as a promoting factor by itself to intensify disease processes. SAA was found to activate formyl peptide receptor 2 (FPR2)[5] and Toll like receptors (TLRs)[6,7] to exert chemotactic effects, and also to promote the synthesis and secretion of other inflammatory factors[1]. The serum level of SAA fluctuates with the severity of inflammation in many diseases such as amyloidosis[8], chronic obstructive pulmonary disease[9,10], rheumatoid arthritis[3,11], atherosclerosis[12], inflammatory bowel disease[13], and certain neoplastic diseases[14,15], in which SAA has important diagnostic and prognostic value. Up-regulation of local SAA1 and SAA2 expression in injured tissues and cells stimulated by inflammatory mediators was detected in chronic lung diseases, accompanied by accumulation of macrophages[9,10].
Long-term inflammatory stimulation occurs in the liver as a consequence of a variety of disease etiologies including chronic hepatitis B (CHB), and this can lead to the formation of diffuse liver fibrosis via the activation of hepatic stellate cells (HSCs). Activated HSCs are the major scar forming cells in the inflamed liver, and they express a number of fibrogenic signaling pathways which, if unchecked, may further lead to liver cirrhosis and decompensated cirrhosis with fatal complications. The activation of HSCs is therefore a central event of liver fibrogenesis and a driver of cirrhosis. Apart from the important fibrogenic activity of HSCs, they have emerged as key effectors of the liver’s inflammatory response by regulating leukocyte trafficking and Kupffer cell recruitment and activation via secretion of cytokines and chemokines. It has been demonstrated that SAA may effectively activate c-Jun N-terminal kinase (JNK), Erk, Akt, IκB kinase, and NF-κB in primary human and rat HSCs. These provide mechanisms by which SAA promotes HSC proliferation and stimulates the production of inflammatory factors such as MCP-1, RANTES, and MMP9. The transcription of SAA mRNA has been shown to be significantly elevated in mouse models of liver fibrosis induced by carbon tetrachloride injection and bile duct ligation[16]. However, few studies have reported on the levels of SAA in human liver disease and the clinical significance of SAA in various liver diseases. In this study, serum levels of SAA in patients with common liver diseases were examined. The factors associated with the alteration of SAA levels in chronic liver diseases were analyzed.
MATERIALS AND METHODS
Study population
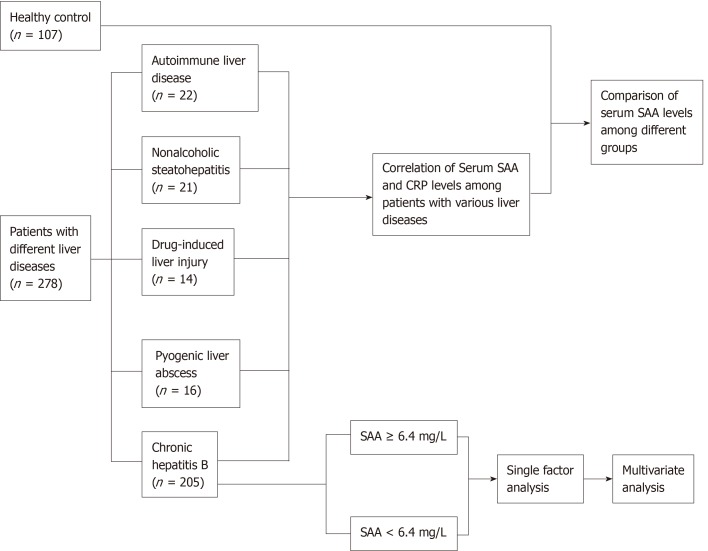
This research was approved by the ethics committee of Zhongshan Hospital Affiliated to Fudan University. The study protocol conformed to the provisions of the Declaration of Helsinki. Informed consent was obtained from all subjects. A total of 278 patients with different liver diseases and 117 healthy controls from Zhongshan Hospital Affiliated to Fudan University were enrolled in this study. The patients include 205 with chronic hepatitis B (146 with inactive hepatitis and 59 with active hepatitis), 22 with active autoimmune liver disease (AILD; 13 with primary biliary cholangitis, 6 with autoimmune hepatitis, and 3 with overlap syndromes), 21 with nonalcoholic steatohepatitis (NASH), 14 with drug-induced liver injury (DILI), and 16 with pyogenic liver abscess. The flow chart of the study is shown in Figure 1.
Figure 1.
Flow chart of the study. SAA: Serum amyloid A; CRP: C-reactive protein.
The diagnosis of liver diseases and their active status were referred to most recent AASLD guidelines (https://www.aasld.org/publications/practice-guidelines). The diagnostic criteria for active hepatitis B included: (1) HBsAg present for no less than 6 months; (2) Serum HBV DNA > 20000 IU/mL in HBeAg-positive CHB and > 2000 IU/mL in HBeAg-negative CHB; and (3) Intermittently or persistently elevated ALT and/or AST levels, or liver biopsy results showing chronic hepatitis with moderate or severe necroinflammation. The diagnostic criteria for inactive hepatitis B were: (1) HBsAg present for no less than 6 months; (2) Serum HBV DNA < 2000 IU/mL; (3) Persistently normal ALT and/or AST levels; and (4) Liver biopsy or noninvasive test results showing absence of significant necroinflammation. Individuals in the healthy control group were people who came to the hospital for medical examination with negative findings.
The exclusion criteria were: (1) Patients with chronic liver disease caused by viruses other than HBV and other liver diseases such as genetic liver diseases (e.g., hepatolenticular degeneration and hereditary hyperbilirubinemia) and parasitic liver diseases; (2) Patients who have received immunomodulatory therapy in the past three months; (3) Patients with advanced malignant tumors except liver cancer; and (4) Patients with two or more comorbidities that may affect the serum level of SAA, including systematic inflammation, diabetes, infectious ascites, etc.
Data collection
Peripheral blood tests and other related examinations were provided by the Laboratory Department of Zhongshan Hospital. Serum SAA level was detected by the scattering turbidimetry method (OQMP11 Germany/Siemens). The human serum samples were stored at 4 °C and tested within 24 hours after collection. The upper normal limits of SAA and CRP in the clinical laboratory of Zhongshan Hospital were 6.4 mg/L and 3 mg/L, respectively. Other clinical parameters including liver function test and serum CRP level were collected for the analysis of the factors associated with SAA levels.
Statistical analysis
Statistical analyses were performed using SPSS22.0 software. Continuous normal distribution data were analyzed by the t-test to determine the difference between two groups. Continuous non-normal distribution data were analyzed by the Mann-Whitney U-test, or the data were grouped based on the normal reference value and compared by the chi-square test. Enumeration data were analyzed by the chi-square test. The Spearman’s rank correlation test was used to determine the correlation between two groups of consecutive non-normal distribution data. Statistically significant factors determined by single factor analysis were further analyzed by binary multivariate logistic regression analysis. The Mann-Whitney U test was used to compare the serum levels of SAA between various liver disease groups and the healthy control group. Bonferroni method was applied for post hoc comparisons to control the probability of type 1 error (alpha = 0.05/6 = 0.008). For statistical tests of other variables, P < 0.05 was considered statistically significant.
RESULTS
Analysis of serum levels of SAA in different groups of subjects
Serum SAA levels in patients with various liver diseases and healthy controls are shown in Table 1. All patients except those with inactive CHB had higher serum SAA levels than healthy controls. Specifically, patients with pyogenic liver abscess had the highest SAA level (mean value: 398.4 ± 246.8 mg/L; median value: 413.5 mg/L). Serum SAA levels in patients with AILD, DILI, and pyogenic liver abscess were higher than those in patients with active CHB. No difference in SAA levels was found between patients with NASH and active CHB. No difference between the healthy control and the inactive CHB patient groups was found by univariate and covariance (ANCOVA) analyses after adjusting baseline data of gender, age, and CRP level (data not shown).
Table 1.
Serum levels of serum amyloid A in different groups of subjects
| Subjects (n) | mean ± SD, (mg/L) | Median (minimum-maximum), (mg/L) | Z1 (compared with healthy controls/active CHB) | P1 (compared with healthy controls/ active CHB) |
| Healthy controls (117) | 2.902 ± 1.801 | 2.250 (0.797-9.040) | - | - |
| Inactive CHB (146) | 2.936 ± 3.092 | 2.350 (0.800-29.90) | -1.129/-7.281 | 0.259/< 0.001 |
| Active CHB (59) | 6.621 ± 6.776 | 4.000 (1.700-39.90) | -5.980 | < 0.001 |
| NASH (21) | 6.624 ± 4.891 | 5.500 (2.800-23.00) | -4.867 | < 0.001 |
| Drug-induced liver injury (14) | 8.036 ± 5.685 | 6.800 (3.400-26.20) | -4.992 | < 0.001 |
| Autoimmune liver disease (22) | 19.73 ± 24.81 | 13.70 (3.300-108.0) | -6.870 | < 0.001 |
| Pyogenic liver abscess (16) | 398.4 ± 246.8 | 413.5 (62.20-871.0) | -6.474 | < 0.001 |
Mann-Whitney U test was used to compare the serum amyloid A levels between various liver disease groups and the healthy control group, and Bonferroni test was applied for post hoc comparisons to control the probability of type 1 error (alpha = 0.05/6 = 0.008). CHB: Chronic hepatitis B; NASH: Nonalcoholic steatohepatitis.
Differences in serum levels of SAA and their association factors in patients with CHB
Two hundred and five patients with CHB (59 active hepatitis and 146 inactive hepatitis) were divided into SAA ≥ 6.4 mg/L and SAA < 6.4 mg/L groups. Single factor analysis showed that patients with active CHB (χ2 = 16.78, P < 0.001, OR = 5.881), ALP ≥ 135 U/L (χ2 = 4.592, P = 0.032, OR = 4.093), and CRP ≥ 3 mg/L (χ2 = 17.01, P < 0.001, OR = 6.993) were associated with SAA levels higher than 6.4 mg/L (Table 2). Other liver function parameters, hepatitis B virus markers, and blood routine test results were not statistically different between the SAA ≥ 6.4 mg/L group and SAA < 6.4 mg/L group.
Table 2.
Single factor analysis of serum levels of serum amyloid A in patients with chronic hepatitis B
| Factor |
SAA < 6.4 mg/L |
SAA ≥ 6.4 mg/L |
χ2/t value1 | P1 value | OR (95%CI) |
| Positive cases/total cases (%) | Positive cases/total cases (%) | ||||
| Female | 39/182 (21.4%) | 6/23 (26.1%) | 0.259 | 0.611 | 1.294 (0.478-3.503) |
| Age (yr) | 47.87 ± 13.17 | 49.26 ± 14.476 | -0.471 | 0.638 | - |
| ALT ≥ 40 U/L | 53/178 (29.8%) | 7/22 (31.8%) | 0.039 | 0.844 | 1.101 (0.424-2.854) |
| AST ≥ 35 U/L | 68/178 (38.2%) | 7/22 (31.8%) | 0.340 | 0.560 | 0.755 (0.293-1.946) |
| γ-GT ≥ 45 U/L | 60/178 (33.7%) | 9/22 (40.9%) | 0.449 | 0.503 | 1.362 (0.551-3.365) |
| ALP ≥ 135 U/L | 12/179 (6.70%) | 5/22 (22.7%) | 4.592 | 0.032 | 4.093 (1.288-13.011) |
| Elevated ALP or γ-GT level | 63/178 (35.4%) | 10/22 (45.5%) | 0.855 | 0.355 | 1.521 (0.622-3.718) |
| AFP ≥ 20 ng/mL | 16/173 (9.2%) | 3/22 (13.6%) | 0.074 | 0.786 | 1.549 (0.413-5.810) |
| A/G ≤ 1.2 | 32/177 (18.1%) | 7/22 (31.8%) | 1.553 | 0.213 | 2.115 (0.797-5.608) |
| Active CHB | 44/182 (24.2%) | 15/23 (65.2%) | 16.78 | < 0.001 | 5.881 (2.337-14.797) |
| Child-Pugh grade B or C | 19/182 (10.4%) | 6/23 (26.1%) | 3.322 | 0.068 | 1.212 (0.946-1.552) |
| Complicated by UGIB | 5/152 (3.29%) | 1/20 (5.00%) | 0.000 | 1.000 | 1.547 (0.172-13.959) |
| Complicated by HE | 4/152 (2.63%) | 1/20 (5.00%) | 0.000 | 1.000 | 1.947 (0.207-18.343) |
| Complicated by ascites | 19/152 (12.5%) | 6/20 (30.0%) | 3.062 | 0.080 | 3.000 (1.029-8.749) |
| Complicated by UGIB or HE or ascites | 23/152 (15.1%) | 6/20 (30.0%) | 1.828 | 0.176 | 2.404 (0.838-6.898) |
| Complicated by HCC | 28/182 (15.4%) | 3/23 (13.0%) | 0.000 | 1.000 | 0.825 (0.230-2.963) |
| CRP ≥ 3 mg/L | 5/35 (14.3%) | 7/7 (100%) | 17.01 | < 0.001 | 6.993 (3.106-15.873) |
| HBsAg | 160/168 (95.2%) | 20/22 (90.9%) | 0.121 | 0.728 | 0.500 (0.099-2.521) |
| HBV DNA ≥ 2000 IU/mL | 43/177 (24.3%) | 4/21 (19.0%) | 0.285 | 0.593 | 0.733 (0.234-2.297) |
| Received antiviral therapy | 87/162 (53.7%) | 13/22 (59.1%) | 0.227 | 0.634 | 1.245 (0.504-3.076) |
Continuous normal distribution data were analyzed by the t-test to determine the difference between two groups. Enumeration data were analyzed by the chi-square test to analyze the difference between groups. ALT: Alanine transaminase; AST: Aspartate transaminase; γ-GT: γ-glutamyltransferase; ALP: Alkaline phosphatase; AFP: Alpha-fetoprotein; A/G: Albumin/globulin ratio; HCC: Hepatocellular carcinoma; CRP: C-reactive protein; UGIB: Upper gastrointestinal bleeding; HE: Hepatic encephalopathy; SAA: Serum amyloid A; CHB: Chronic hepatitis B.
Active CHB (P = 0.039, OR = 6.222) was the independent factor associated with SAA serum levels higher than 6.4 mg/L by binary multivariate logistic regression analysis (Table 3).
Table 3.
Multivariate analysis of serum levels of serum amyloid A in patients with chronic hepatitis B
| Factor | Regression coefficient | P value | OR | 95%CI |
| Active CHB | 1.828 | 0.039 | 6.222 | 1.095-35.36 |
CHB: Chronic hepatitis B.
The disease history of the CHB patients ranged from 1 year to 40 years. No significant difference was found in serum SAA levels between cirrhotic and non-cirrhotic patients (P = 0.537), or between patients with or without receiving oral antiviral drugs (P = 0.634). Eighteen of the 59 active hepatitis patients and 82 of the 146 inactive hepatitis patients were receiving antiviral therapy. The types and proportions of antiviral drugs used by the patients were enticavir (ETV) monotherapy (58%), adefovir (ADV) monotherapy (12%), ETV and ADV combination therapy (2%), lamivudine monotherapy (6%), lamivudine and ADV combination therapy (5%), telbivudine (12%), telbivudine and ADV combination therapy (2%), and TDF monotherapy (3%). Among patients who were receiving antiviral therapy, patients with inactive hepatitis (n = 82) had significantly lower blood SAA levels than those with active hepatitis (n = 18) (Z value = -4.077, P = 0.000) (Table 4).
Table 4.
Comparison of serum amyloid A levels between active and inactive chronic hepatitis B patients with oral antiviral therapy
| Status of patients with antiviral therapy | n | mean ± SD of SAA (mg/L) | Average rank of SAA | Z1 value | P1 value |
| Active CHB | 18 | 6.289 ± 6.042 | 74.92 | -4.077 | 0.000 |
| Inactive CHB | 82 | 3.379 ± 4.726 | 44.46 |
The Mann-Whitney U test was used to compare the serum amyloid A levels between various liver disease groups. SAA: Serum amyloid A; CHB: Chronic hepatitis B.
Correlation between serum levels of SAA and CRP in patients with CHB, autoimmune liver disease, and pyogenic liver abscess
Spearman's rank correlation test revealed that serum levels of SAA and CRP were positively correlated in patients with CHB (r = 0.620, P < 0.001), AILD (r = 0.504, P = 0.020), and pyogenic liver abscess (r = 0.508, P = 0.045). Serum levels of SAA (0.80-871.0 mg/L) displayed a broader fluctuation range than CRP (0.30-271.3 mg/L) (Table 5). We found no significant correlation between CRP and SAA levels in patients with DILI.
Table 5.
Correlation of serum amyloid A and C-reactive protein levels among patients with various liver diseases
| Disease |
SAA (mg/L) |
CRP (mg/L) |
1Spearman’s rank correlation coeffi-cient | P value | ||
| mean ± SD | Median (minimum-maximum) | mean ± SD | Median (minimum-maximum) | |||
| CHB | 3.984 ± 4.743 | 3.250 (0.80-39.90) | 4.398 ± 6.522 | 1.500 (0.30-25.40) | 0.620 | < 0.001 |
| Autoimmune liver disease | 19.73 ± 24.81 | 13.70 (3.30-108.0) | 9.633 ± 5.977 | 9.100 (1.20-19.60) | 0.504 | 0.020 |
| Pyogenic liver abscess | 398.4 ± 246.8 | 413.5 (62.20-871.0) | 138.8 ± 57.46 | 141.1 (22.90-271.3) | 0.508 | 0.045 |
| Drug-induced liver injury | 8.036 ± 1.519 | 6.800 (3.40-26.20) | 6.490 ± 2.616 | 2.850 (0.30-25.30) | 0.219 | 0.544 |
The Spearman’s rank correlation test was used to determine correlation between groups. SAA: Serum amyloid A; CRP: C-reactive protein.
DISCUSSION
SAA and liver diseases
In this study, the serum levels of SAA in patients with different liver diseases were investigated and the association factors were analyzed. Patients with pyogenic liver abscess, active AILD, DILI, NASH, and active CHB were found to have higher serum SAA levels than patients with inactive CHB and healthy controls. The results extend the diagnostic and prognostic value of SAA as a sensitive inflammatory marker in liver diseases, especially liver abscess.
Patients with pyogenic liver abscess had the highest blood levels of SAA, which can be hundred times higher than those of inactive CHB and healthy controls. This may be due to a systemic release of inflammatory factors in the context of bacterial infection, which in turn may stimulate the production of SAA, forming a positive feedback loop to amplify inflammation.
The performance of SAA in reflecting liver inflammation and fibrogenesis was also tested in non-abscess liver diseases with milder inflammatory status such as CHB. By single factor analysis of 205 patients with CHB, it was observed that serum SAA levels were significantly higher in patients with active CHB than in those with inactive CHB. Patients with active CHB have intermittently or persistently elevated ALT and/or AST levels, which are markers reflecting hepatocyte and biliary destruction. Elevated serum levels of ALP and/or γ-GT usually indicate impaired biliary drainage. Studies have shown that ALP has a higher specificity than γ-GT in the diagnosis of cholestasis[17]. In the present study, the elevation of ALP level, rather than γ-GT, was associated with higher SAA levels by single factor analysis. The association between SAA and cholestasis and the underlying mechanisms warrant further investigation.
Serum SAA level has been found to be an indicator of disease activity in autoimmune diseases such as rheumatoid arthritis[18]. SAA participates in the disease's progression by promoting formation of synovial pannus and inducing local synthesis of cytokines and chemokines[11]. In this study, serum levels of SAA in patients with autoimmune liver disease were also remarkably increased, indicating that SAA may also exert pro-inflammatory properties and be involved in the pathological process of autoimmune responses in the liver.
It can also be concluded that SAA lacks specificity in the judgment of disease activity. The analysis of serum SAA level can be complicated by other local or systemic inflammatory diseases such as pyogenic liver abscess and inflammatory bowel disease. It is therefore necessary to consider the patient's disease status systemically when using SAA as a parameter of the disease activity of aforementioned liver diseases.
SAA and CRP
Similar to SAA, CRP is also an acute phase protein produced by the liver, with a half-life of 46.4 ± 21.7 h compared with 34.9 ± 28.7 h for SAA[19]. IL-1, IL-6, and TNF-α stimulate the production of SAA as well as CRP in the liver[20]. CRP is not sensitive in the detection of liver injury and dysfunction in clinical practice. Comparative studies have demonstrated that SAA has a higher sensitivity and specificity, as well as a broader range of serum level than CRP in viral inflammation[21], infection after kidney transplantation[22], acute appendicitis, and inflammatory bowel disease[23]. The present study demonstrated that serum levels of SAA were positively correlated with CRP levels in patients with CHB, AILD, and pyogenic liver abscess. SAA has a broader range of serum levels than CRP in liver diseases and thus is a more sensitive and better indicator to capture mild inflammation[21].
There may be functional similarity and difference between CRP and SAA in liver diseases. Many studies have reported the pro-inflammatory effects of CRP[24,25]. Intraperitoneal injection of CRP in rats resulted in a significant increase in superoxide anion, NF-κB activity, and the release of biomarkers of inflammation from macrophages[26]. In vitro, the expression of intercellular cell adhesion molecule-1 (ICAM-1) remarkably increased in human umbilical vein endothelial cells and human coronary artery endothelial cells stimulated by CRP. CRP also promotes MCP-1 production. Similarly, SAA promotes the synthesis of inflammatory factors, and strengthens the inflammatory response as aforementioned, but with an additional property to stimulate HSC activities during liver injury and hepatitis. SAA may be a potential fibrogenic factor that dynamically changes with liver fibrogenesis.
SAA and active CHB
By single and multivariate logistic regression analyses, it was revealed that active CHB was the independent factor associated with SAA serum levels higher than 6.4 mg/L among CHB patients. Oral antiviral drug treatment itself was not associated with significant changes of SAA level. Among patients who were receiving antiviral drugs, those with inactive hepatitis had lower blood SAA levels than patients with active hepatitis, albeit their mean level of SAA (6.289 ± 6.042 mg/L) was under the upper normal limit. This may reflect a confounded status of insufficient or ineffective antiviral therapy in these active CHB patients, and is in line with the result that patients with active CHB had higher levels of SAA than those with inactive CHB. Piotti et al[27] showed immunopositivity for SAA protein in the liver biopsy specimens of hepatitis C and B patients with active chronic hepatitis and early fibrosis. The functional impact of SAA in the progression of chronic hepatitis B and various other liver diseases warrants further studies.
Single factor analysis in this study showed that SAA level ≥ 6.4 mg/L was not associated with albumin/globulin ratio, globulin level, aspartate aminotransferase-to-platelet ratio index score, Child-Pugh score/grade, or major complications of cirrhosis such as upper gastrointestinal bleeding, hepatic encephalopathy, or ascites in CHB patients. These results are in line with the inflammatory property of SAA, and thus the increase coordinates with active hepatitis, rather than cirrhosis.
Although there was no statistical difference of SAA levels in patients with ascites alone compared with patients without ascites by continuous calibration chi-square test (P = 0.080), the OR value of SAA levels above 6.4 mg/L in patients with ascites alone was found to be 3.000 (95%CI: 1.029-8.749; Table 2). This may be due to the slight inflammatory state in patients with ascites, or the small sample size. Follow-up studies may expand the research by enrolling patients with spontaneous bacterial peritonitis.
The positive rates and quantitative values of HBV markers were not associated with SAA levels. There was also no difference of SAA levels in patients with or without receiving antiviral therapy. HBV markers are important in reflecting viral infection status and viral replication activity, whereas the carrying and amplification of HBV alone may not induce immune response that causes liver injury and inflammation.
Summary
Serum level of SAA is a sensitive biomarker for the inflammatory activity of pyogenic liver abscess, and to a lesser extent can reflect the mild inflammatory status of AILD, DILI, CHB, and NASH. SAA lacks specificity in the judgment of inflammatory diseases. It is therefore necessary to comprehensively consider the patient's systemic status before making judgment.
ARTICLE HIGHLIGHTS
Research background
Serum amyloid A (SAA) is an acute phase protein mainly synthesized by the liver, which participates in the pathological process of a variety of inflammatory diseases. Immune and inflammatory responses participate in chronic liver injuries and fibrogenesis of various liver diseases including chronic hepatitis B (CHB) via activating hepatic stellate cells (HSCs), the key fibrogenic cells in the liver. It has been demonstrated that SAA induces inflammatory phenotype and promotes cell proliferation in activated HSCs. However, few studies have been reported on the serum levels of SAA in human liver diseases. It is of interest to investigate the diagnostic value and clinical significance of serum SAA level in patients with inflammatory liver diseases.
Research motivation
Few studies have been reported on the serum level of SAA and its clinical significance in human liver diseases. Yet many studies have demonstrated that the increase of serum SAA level is not only a consequence of inflammation or tissue injury, but also a promoting factor by itself to intensify the disease process. It has been reported that the transcription of SAA in the liver was significantly elevated in mouse models of liver fibrogenesis. SAA induces inflammatory phenotype and promotes cell proliferation in activated HSCs. The present study aimed at investigating the clinical significance of serum SAA levels in various liver diseases. The results will help to delineate whether SAA level may serve as an indicator of liver inflammation and fibrogenesis, and the potential impact of SAA on the diseases’ progression. The upstream regulation of SAA expression in various liver diseases will also be of great interest.
Research objectives
The main objective of this study was to investigate the serum levels of SAA in patients with various liver diseases, especially chronic hepatitis B, and analyze the factors associated with the alteration of SAA levels.
Research methods
Two hundred and seventy-eight patients with different liver diseases and 117 healthy controls were enrolled in this study. The patients included 205 with chronic hepatitis B (CHB), 22 with active autoimmune liver disease (AILD), 21 with nonalcoholic steatohepatitis (NASH), 14 with drug-induced liver injury (DILI), and 16 with pyogenic liver abscess. The Mann-Whitney U test was used to compare the serum SAA levels of patients with various liver diseases and those of healthy controls. Bonferroni test was applied for post hoc comparisons to control the probability of type 1 error. Serum levels of SAA and other clinical parameters were collected for the analysis of the factors associated with SAA levels. The 205 patients with CHB (59 active hepatitis and 146 inactive hepatitis) were divided into SAA ≥ 6.4 mg/L and SAA < 6.4 mg/L groups. The t-test or Chi-square test was used to perform single factor analysis of serum levels of SAA in patients with CHB. Then multivariate analysis was used to determine the independent risk factors for high serum levels of SAA in patients with CHB. Finally, the Spearman’s rank correlation test was used to determine correlation of SAA levels and CRP among patients with different liver diseases.
Research results
All patients except those with inactive CHB had higher serum SAA levels than healthy controls. Specifically, patients with pyogenic liver abscess had the highest SAA level (mean value: 398.4 ± 246.8 mg/L; median value: 413.5 mg/L). The serum SAA levels in patients with AILD, DILI, and pyogenic liver abscess were higher than those in patients with active CHB. Active CHB (χ2 = 16.78, P < 0.001, OR = 5.881), ALP ≥ 135 U/L (χ2 = 4.592, P = 0.032, OR = 4.093), and CRP ≥ 3 mg/L (χ2 = 17.01, P < 0.001, OR = 6.993) were associated with SAA levels higher than 6.4 mg/L. Active CHB (P = 0.039, OR = 6.222) was the independent factor associated with SAA serum levels higher than 6.4 mg/L by binary multivariate logistic regression analysis. Serum levels of SAA and CRP were positively correlated in patients with CHB (r = 0.620, P < 0.001), AILD (r = 0.504, P = 0.020), and pyogenic liver abscess (r = 0.508, P = 0.045). Serum levels of SAA (0.80-871.0 mg/L) displayed a broader fluctuation range than CRP (0.30-271.3 mg/L).
Research conclusions
It was found in the present study that serum level of SAA is a sensitive biomarker for the inflammatory activity of pyogenic liver abscess, and to a lesser extent can reflect the mild inflammatory status of AILD, DILI, CHB, and NASH. SAA lacks specificity in the judgment of inflammatory diseases. It is therefore necessary to comprehensively consider the patient's systemic status before making judgment.
Research perspectives
Previous studies and our data have showed that SAA participates in inflammatory response in human liver diseases. It may own an additional property to stimulate HSC activities during chronic liver injury and hepatitis. We speculate that SAA may be a potential fibrogenic factor that dynamically changes during liver fibrogenesis. The value of SAA detection in monitoring the prognosis of liver abscess and other liver diseases, the potential impact and underlying mechanisms of SAA on liver diseases’ progression, the upstream regulation of SAA expression in various liver diseases, and whether SAA may become a treatment target for inflammatory liver diseases warrant further investigation.
ACKNOWLEDGEMENTS
The authors thank Professor Derek A. Mann of Newcastle University, England for language modification of this manuscript.
Footnotes
Institutional review board statement: This research was approved by Ethics Committee of Zhongshan Hospital Affiliated to Fudan University.
Informed consent statement: Informed consent was obtained from all subjects.
Conflict-of-interest statement: All authors declare that they have no conflicts of interest to disclose.
Data sharing statement: Data are available from the corresponding author at guo.jinsheng@zs-hospital.sh.cn.
Manuscript source: Unsolicited manuscript
Peer-review started: June 26, 2019
First decision: August 28, 2019
Article in press: November 7, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Gencdal G S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Zi-Ying Yuan, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China; Shanghai Institute of Liver Diseases, Shanghai 200032, China; Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China.
Xing-Xin Zhang, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China; Shanghai Institute of Liver Diseases, Shanghai 200032, China.
Yu-Jing Wu, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China; Shanghai Institute of Liver Diseases, Shanghai 200032, China.
Zhi-Ping Zeng, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China; Shanghai Institute of Liver Diseases, Shanghai 200032, China.
Wei-Min She, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China; Shanghai Institute of Liver Diseases, Shanghai 200032, China.
Shi-Yao Chen, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China; Shanghai Institute of Liver Diseases, Shanghai 200032, China.
Yuan-Qing Zhang, The First Affiliated Hospital, Yunnan Institute of Digestive Disease, Kunming Medical University, Kunming 650000, Yunnan Province, China.
Jin-Sheng Guo, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai 200032, China. guo.jinsheng@zs-hospital.sh.cn; Shanghai Institute of Liver Diseases, Shanghai 200032, China.
References
- 1.Sun L, Ye RD. Serum amyloid A1: Structure, function and gene polymorphism. Gene. 2016;583:48–57. doi: 10.1016/j.gene.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci USA. 1994;91:3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Local expression of the serum amyloid A and formyl peptide receptor-like 1 genes in synovial tissue is associated with matrix metalloproteinase production in patients with inflammatory arthritis. Arthritis Rheum. 2004;50:1788–1799. doi: 10.1002/art.20301. [DOI] [PubMed] [Google Scholar]
- 4.Sung HJ, Ahn JM, Yoon YH, Rhim TY, Park CS, Park JY, Lee SY, Kim JW, Cho JY. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J Proteome Res. 2011;10:1383–1395. doi: 10.1021/pr101154j. [DOI] [PubMed] [Google Scholar]
- 5.Gouwy M, De Buck M, Pörtner N, Opdenakker G, Proost P, Struyf S, Van Damme J. Serum amyloid A chemoattracts immature dendritic cells and indirectly provokes monocyte chemotaxis by induction of cooperating CC and CXC chemokines. Eur J Immunol. 2015;45:101–112. doi: 10.1002/eji.201444818. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010;3:21. doi: 10.1186/1755-1536-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandri S, Rodriguez D, Gomes E, Monteiro HP, Russo M, Campa A. Is serum amyloid A an endogenous TLR4 agonist? J Leukoc Biol. 2008;83:1174–1180. doi: 10.1189/jlb.0407203. [DOI] [PubMed] [Google Scholar]
- 8.Westermark GT, Fändrich M, Westermark P. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol. 2015;10:321–344. doi: 10.1146/annurev-pathol-020712-163913. [DOI] [PubMed] [Google Scholar]
- 9.Anthony D, Seow HJ, Uddin M, Thompson M, Dousha L, Vlahos R, Irving LB, Levy BD, Anderson GP, Bozinovski S. Serum amyloid A promotes lung neutrophilia by increasing IL-17A levels in the mucosa and γδ T cells. Am J Respir Crit Care Med. 2013;188:179–186. doi: 10.1164/rccm.201211-2139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozinovski S, Hutchinson A, Thompson M, Macgregor L, Black J, Giannakis E, Karlsson AS, Silvestrini R, Smallwood D, Vlahos R, Irving LB, Anderson GP. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]
- 11.Connolly M, Marrelli A, Blades M, McCormick J, Maderna P, Godson C, Mullan R, FitzGerald O, Bresnihan B, Pitzalis C, Veale DJ, Fearon U. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2010;184:6427–6437. doi: 10.4049/jimmunol.0902941. [DOI] [PubMed] [Google Scholar]
- 12.Fyfe AI, Rothenberg LS, DeBeer FC, Cantor RM, Rotter JI, Lusis AJ. Association between serum amyloid A proteins and coronary artery disease: evidence from two distinct arteriosclerotic processes. Circulation. 1997;96:2914–2919. doi: 10.1161/01.cir.96.9.2914. [DOI] [PubMed] [Google Scholar]
- 13.Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90–107. [PubMed] [Google Scholar]
- 14.Biran H, Friedman N, Neumann L, Pras M, Shainkin-Kestenbaum R. Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol. 1986;39:794–797. doi: 10.1136/jcp.39.7.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, Black TA, Chien AL, Majmundar KS, Thompson JC, Yee SS, O'Hara MH, Aggarwal C, Xin D, Shaked A, Gao M, Liu D, Borad MJ, Ramanathan RK, Carpenter EL, Ji A, de Beer MC, de Beer FC, Webb NR, Beatty GL. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249–252. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegmund SV, Schlosser M, Schildberg FA, Seki E, De Minicis S, Uchinami H, Kuntzen C, Knolle PA, Strassburg CP, Schwabe RF. Serum Amyloid A Induces Inflammation, Proliferation and Cell Death in Activated Hepatic Stellate Cells. PLoS One. 2016;11:e0150893. doi: 10.1371/journal.pone.0150893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Shen C, Sun XG, Liu N, Mu Y, Hong CC, Wei W, Zheng F. Increased serum amyloid A and its association with autoantibodies, acute phase reactants and disease activity in patients with rheumatoid arthritis. Mol Med Rep. 2015;11:1528–1534. doi: 10.3892/mmr.2014.2804. [DOI] [PubMed] [Google Scholar]
- 19.Takata S, Wada H, Tamura M, Koide T, Higaki M, Mikura SI, Yasutake T, Hirao S, Nakamura M, Honda K, Nagatomo T, Tanaka Y, Sohara E, Watanabe M, Yokoyama T, Saraya T, Kurai D, Ishii H, Goto H. Kinetics of c-reactive protein (CRP) and serum amyloid A protein (SAA) in patients with community-acquired pneumonia (CAP), as presented with biologic half-life times. Biomarkers. 2011;16:530–535. doi: 10.3109/1354750X.2011.607189. [DOI] [PubMed] [Google Scholar]
- 20.Yeh ET. CRP as a mediator of disease. Circulation. 2004;109:II11–II14. doi: 10.1161/01.CIR.0000129507.12719.80. [DOI] [PubMed] [Google Scholar]
- 21.Wu TL I, Chen Tsai, Chang PY, Tsao KC, Sun CF, Wu LL, Wu JT. Establishment of an in-house ELISA and the reference range for serum amyloid A (SAA): complementarity between SAA and C-reactive protein as markers of inflammation. Clin Chim Acta. 2007;376:72–76. doi: 10.1016/j.cca.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999;37:381–388. doi: 10.1515/CCLM.1999.063. [DOI] [PubMed] [Google Scholar]
- 23.Yarur AJ, Quintero MA, Jain A, Czul F, Barkin JS, Abreu MT. Serum Amyloid A as a Surrogate Marker for Mucosal and Histologic Inflammation in Patients with Crohn's Disease. Inflamm Bowel Dis. 2017;23:158–164. doi: 10.1097/MIB.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem. 2015;396:1181–1197. doi: 10.1515/hsz-2015-0149. [DOI] [PubMed] [Google Scholar]
- 25.Kushner I, Agrawal A. CRP can play both pro-inflammatory and anti-inflammatory roles. Mol Immunol. 2007;44:670–671. doi: 10.1016/j.molimm.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jialal I, Devaraj S, Smith G, Lam KS, Kumaresan PR. A novel peptide inhibitor attenuates C-reactive protein's pro-inflammatory effects in-vivo. Int J Cardiol. 2013;168:3909–3912. doi: 10.1016/j.ijcard.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piotti KC, Yantiss RK, Chen Z, Jessurun J. Serum amyloid A immunohistochemical staining patterns in hepatitis. Histopathology. 2016;69:937–942. doi: 10.1111/his.13016. [DOI] [PubMed] [Google Scholar]