Abstract
BACKGROUND
Fecal metabolites are associated with gut visceral sensitivity, mucosal immune function and intestinal barrier function, all of which have critical roles in the pathogenesis of irritable bowel syndrome (IBS). However, the metabolic profile and pathophysiology of IBS are still unclear. We hypothesized that altered profiles of fecal metabolites might be involved in the pathogenesis of IBS with predominant diarrhea (IBS-D).
AIM
To investigate the fecal metabolite composition and the role of metabolites in IBS-D pathophysiology.
METHODS
Thirty IBS-D patients and 15 age- and sex-matched healthy controls (HCs) underwent clinical and psychological assessments, including the IBS Symptom Severity System (IBS-SSS), an Italian modified version of the Bowel Disease Questionnaire, the Bristol Stool Form Scale (BSFS), the Hospital Anxiety and Depression Scale, and the Visceral Sensitivity Index. Visceral sensitivity to rectal distension was tested using high-resolution manometry system by the same investigator. Fecal metabolites, including amino acids and organic acids, were measured by targeted metabolomics approaches. Correlation analyses between these parameters were performed.
RESULTS
The patients presented with increased stool water content, more psychological symptoms and increased visceral hypersensitivity compared with the controls. In fecal metabolites, His [IBS-D: 0.0642 (0.0388, 0.1484), HC: 0.2636 (0.0780, 0.3966), P = 0.012], Ala [IBS-D: 0.5095 (0.2826, 0.9183), HC: 1.0118 (0.6135, 1.4335), P = 0.041], Tyr [IBS-D: 0.1024 (0.0173, 0.4527), HC: 0.5665 (0.2436, 1.3447), P = 0.018], Phe [IBS-D: 0.1511 (0.0775, 0.3248), HC: 0.3967 (0.1388, 0.7550), P = 0.028], and Trp [IBS-D: 0.0323 (0.0001, 0.0826), HC: 0.0834 (0.0170, 0.1759), P = 0.046] were decreased in IBS-D patients, but isohexanoate [IBS-D: 0.0127 (0.0060, 0.0246), HC: 0.0070 (0.0023, 0.0106), P = 0.028] was significantly increased. Only Tyr was mildly correlated with BSFS scores in all subjects (r = -0.347, P = 0.019). A possible potential biomarker panel was identified to correlate with IBS-SSS score (R2Adjusted = 0.693, P < 0.001). In this regression model, the levels of Tyr, Val, hexanoate, fumarate, and pyruvate were significantly associated with the symptom severity of IBS-D. Furthermore, visceral sensation, including abdominal pain and visceral hypersensitivity, was correlated with isovalerate, valerate and isohexanoate.
CONCLUSION
Altered profiles of fecal metabolites may be one of the origins or exacerbating factors of symptoms in IBS-D via increasing visceral sensitivity.
Keywords: Fecal metabolite, Irritable bowel syndrome, Amino acids, Organic acids, Short chain fatty acids, Visceral hypersensitivity
Core tip: We comprehensively assessed the clinical and psychological characteristics of irritable bowel syndrome with predominant diarrhea (IBS-D) , visceral sensitivity, and fecal metabolites. As expected, the data confirmed that metabolite compositions were different in subjects with or without IBS-D and the levels of some metabolites were significantly correlated with IBS Symptom Severity System score, visceral sensitivity, and the severity or frequency of abdominal pain. Furthermore, a potential biomarker panel was identified to correlate with the symptom severity of IBS-D. These preliminary findings provide some clues for IBS-D pathogenesis and for the search for biomarkers in symptom severity.
INTRODUCTION
Irritable bowel syndrome (IBS) is a highly prevalent functional bowel disorder, and is characterized by chronic abdominal pain related to disordered bowel habits without evidence of organic diseases[1,2]. Patients with IBS often have psychological disturbances and gastrointestinal symptoms, all of which decline health-related quality of life (QOL)[3,4]. As defined by the Rome IV criteria, IBS is classified into four subtypes: IBS with predominant diarrhea (IBS-D), IBS with predominant constipation, IBS with mixed bowel habits, and IBS unclassified. Among these subtypes, IBS-D accounts for 39.0%-61.9% of IBS cases[5,6] and especially impairs QOL[7]. Although the pathogenesis is complicated, dysregulation of the brain-gut axis, increased gut mucosal immune activation, increased intestinal permeability, and visceral hypersensitivity may be involved[8,9].
The gastrointestinal tract represents the largest surface to the outside world and is a sensory organ[10]. Metabolites in the intestine, as sensory information, are communicated with the gastrointestinal innervation, enteroendocrine hormonal signaling system, immune system, and the local tissue defense system[11,12]. Previous studies have demonstrated that dietary amino acids, which regulate the expression of anti-inflammatory cytokines and pro-inflammatory cytokines, are associated with intestinal inflammation[13-19]. Moreover, amino acids played significant roles in regulating the expression of tight junction proteins, oxidative stress and the apoptosis of enterocytes[19-24], all of which were critical factors for the functions of the intestinal barrier[25,26]. However, the role of amino acids in IBS-D pathogenesis is still unclear.
An increasing number of studies have indicated that organic acids, especially short chain fatty acids (SCFA), are one of the origins of symptoms in IBS with immunological and regulatory functions[27-31]. A recent study suggested that fecal microbial metabolites induced the release of serotonin (5-HT) from enterochromaffin (EC) cells and were involved in visceral hypersensitivity through modulating sensory neurons[32]. Bellono et al[32] also reported that EC cells were polymodal chemosensors and were activated by multiple metabolites. In addition, targeted metabolomics, the measurement of defined groups of metabolites, provided a particularly powerful tool for synchronously quantifying multiple metabolites.
Other studies reported that metabolite compositions were different in patients with IBS from those in healthy controls (HCs)[33-35]. Only a few articles reported that some fecal metabolites were correlated with symptoms in IBS[30,36]. Nevertheless, these studies only measured several kinds of SCFA in fecal samples and did not extensively investigate the relationships between metabolites and IBS symptoms as well as visceral sensitivity.
The aims of this study were to compare fecal metabolites in subjects with or without IBS-D and to explore the associations of metabolites with clinical and experimental parameters.
MATERIALS AND METHODS
Subjects
Based on the sample size of other studies[30,35], 30 patients were recruited from outpatient clinics at a teaching hospital in Beijing, China, together with 15 age- and sex-matched HCs. Patients were eligible for the study if they were 18 years or older and met the Rome IV criteria for IBS-D. HCs were recruited from asymptomatic individuals who had undergone colorectal cancer screening or polyposis follow-up and had negative results. Subjects were excluded if they had a history of major abdominal surgery, organic diseases such as celiac disease, other painful disorders such as dysmenorrhea or psychiatric disorders or were pregnant, lactating or unable to come off any of the following medications: probiotics, antibiotics, analgesics, stool bulking agents, lactulose, prokinetics, histamine antagonists, mast cell stabilizers or antidepressants within 2 wk.
All subjects gave written informed consent before participation. The study protocol was approved by the Ethics Committee of the China-Japan Friendship Hospital (No. 2015-33).
Procedure
Sixty-one patients with IBS-D-like symptoms were referred to the gastroenterological department. Nineteen patients (31.1%) refused to participate, and 12 patients were excluded for severe mental disorder (two patients), colon mucosal inflammation (five patients), a history of abdominal surgery (two patients), lactose intolerance (two patients) and poor compliance (one patient). Finally, 30 IBS-D patients and 15 HCs were included in this study.
After enrollment, the clinical and psychological states were assessed. Then, visceral sensitivity was measured after obtaining stool samples. Each fecal sample was collected with sterile plastic tube after defecation. Samples were frozen in liquid nitrogen immediately and stored at -80 °C.
Clinical and psychological assessments
The degree of symptoms was assessed using the previously validated IBS Symptom Severity System (IBS-SSS)[37]. The total score ranged from 0 to 500 and was summed from five individual scores: Abdominal pain (severity and days of pain), abdominal distension, dissatisfaction with bowel habits and life interference. IBS-D severity was then classified as mild (75-174), moderate (175-299) or severe (300-500).
Each IBS-D patient was invited to complete an Italian modified version of the bowel disease questionnaire (BDQ) to evaluate the severity of abdominal pain during the prior 2 wk[38]. Symptom scores ranged from 0 to 4 according to the influence of symptoms on patients’ daily activities: 0, absent; 1, mild (not influencing daily activities); 2, relevant (diverting from daily activities but not urging modification); 3, severe (influencing daily activities markedly enough to urge modifications); and 4, extremely severe (precluding daily activities). Symptom frequency was also graded 0-4 (0, absent; 1, up to 1 d/wk; 2, 2 or 3 d/wk; 3, 4-6 d/wk; and 4, daily).
The Bristol Stool Form Scale (BSFS), a 7-point scale, was used to measure stool form. The Hospital Anxiety and Depression Scale, which consists of 14 items (7 anxiety items and 7 depression items), was used to evaluate the severity of anxiety and depression symptoms[39,40].
The validated Visceral Sensitivity Index (VSI) scale is a measure of anxiety specifically related to gastrointestinal sensations and symptoms[41]. It consists of 15 items with scores ranging from 0 (no gastrointestinal-specific anxiety or visceral hypersensitivity) to 5 (severe gastrointestinal-specific anxiety and visceral hypersensitivity)[42].
Visceral sensitivity test
After glycerin enema and digital rectal examination, a ManoScan™ high-resolution anorectal catheter (ANN1522) (Given Imaging, Los Angeles, CA, United States) with a latex balloon at the tip was inserted into the rectum. After three min for adapting to the catheter, the balloon was manually inflated with air at a speed of 2 mL/s, and thresholds for initial perception, urgency, and discomfort/pain were recorded[43]. A ManoScan 360™ high-resolution manometry system (Model A100) and ManoView™ AR Analysis Software 2.1 were used in the test.
Selection of fecal metabolites
Thirty-one metabolites were selected based on extensive literature search, using the following criteria: (1) Metabolites are different in subjects with or without IBS or potentially discriminate between subjects with healthy gut function and IBS patients; (2) Metabolites are related to one or more gut health parameters[44]; and (3) Metabolites can be measured in fecal samples by a targeted metabolomics method. Amino acids are associated with intestinal inflammation and barrier[45]. Organic acids, which are produced by gut microbiota, are able to modulate multiple pathological and physiological processes[30]. Based on this, lactate, pyruvate, fumarate, SCFA (i.e., acetate, propionate, butyrate, valerate, hexanoate, isobutyrate, isovalerate, and isohexanoate), and 20 amino acids making up the proteins were selected for this study.
Measurement of biomarkers
Organic acid analysis: A total of 750 μL methanol was added to fecal samples (50-150 mg), and the sample was ground for 1 min, homogenized for 30 min and centrifuged at 10000 rpm for 10 min. The fecal supernatant was transferred into a tube, and 50 mg Na2SO4 was added. After one night at 4 °C, the aliquot was centrifuged at 14000 rpm for 20 min, and the supernatant was retained for subsequent analyses. Similar to previous reports[46], fecal SCFA was measured by gas chromatography-mass spectrometry after derivatization reaction. Liquid chromatography-mass spectrometry was employed to quantify the levels of other organic acids, i.e., lactate, pyruvate, and fumarate, as described previously[47].
Amino acid analysis: After freeze-drying, 5 mg fecal sample was added to 1 ml purified water and incubated on a shaking table for 60 min at 37 °C. The supernatant was prepared by centrifugation at 12000 rpm for 5 min. Ultra-performance liquid chromatographic analysis was performed to quantify the levels of amino acids as described by Boogers et al[48].
Statistical analysis
SPSS (IBM-SPSS Statistics, Chicago, IL, United States) version 21.0 was used for statistical analysis. The Gibbs sampler based left - censored missing value imputation approach (GSimp) was used to measure the metabolite levels that were below the detection limit[49]. Normally distributed data are presented as the mean ± SD, and abnormally distributed data are expressed as the median (Q1, Q3). Levels of individual metabolites were compared between groups using the Mann-Whitney U test. Linear correlations between multiple metabolites and IBS-SSS score, maximum tolerable threshold or BDQ scores were analyzed using multiple linear regression (stepwise). Spearman’s correlation analysis was used for correlations between individual metabolites and BDQ scores. A P value ≤ 0.05 was considered to be significant.
RESULTS
Characteristics of study subjects
Thirty IBS-D patients (22 males and 8 females) and 15 HCs (11 males and 4 females) were enrolled in this study (Table 1). There were no significant differences between the groups in age (P = 0.299), sex (P = 1.00), or body mass index (P = 0.219).
Table 1.
Demographics, clinical characteristics and psychological states of study subjects
| Features | IBS-D patients | Controls | P value |
| n | 30 | 15 | NA |
| Age (yr) | 30.0 (28.0, 41.3) | 28.0 (25.0, 37.0) | 0.299 |
| Gender (male:female) | 22:8 | 11:4 | 1.000 |
| Body mass index (kg/m2) | 23.8 ± 3.9 | 22.4 ± 2.8 | 0.219 |
| Duration of disease (yr) | 7.5 (1.5, 10.1) | NA | NA |
| IBS-SSS | 261.7 ± 40.4 | NA | NA |
| Severity of abdominal pain | 1.0 (1.0, 2.0)b | 0.0 (0.0, 0.0) | < 0.001 |
| Frequency of abdominal pain | 2.0 (1.0, 4.0)b | 0.0 (0.0, 0.0) | < 0.001 |
| BSFS score | 6.0 (6.0,6.0)b | 4.0 (4.0, 4.0) | < 0.001 |
| HADS anxiety score | 6.0 (3.0, 9.0)b | 2.0 (1.0, 4.0) | 0.002 |
| HADS depression score | 4.0 (1.0, 7.3) | 2.0 (2.0, 5.0) | 0.350 |
| VSI score | 34.0 (17.0, 45.3)b | 5.0 (2.0, 15.0) | < 0.001 |
P < 0.01 vs controls. The data are presented as the mean ± SD or the median (Q1, Q3). IBS-D: Irritable bowel syndrome with predominant diarrhea; IBS-SSS: IBS symptom severity scale; BSFS: Bristol stool form scale; HADS: Hospital anxiety and depression scale; VSI: Visceral sensitivity index; NA: Not applicable.
In IBS-D patients, the duration of disease ranged from 0.5 to 20.5 years (median 7.5 years). According to the IBS-SSS scores (261.7 ± 40.4), 23 patients (76.7%) had moderate and 7 (23.3%) had severe IBS-D. The scores of the BSFS [IBS-D: 6.0 (6.0, 6.0), HC: 4.0 (4.0, 4.0)], anxiety [IBS-D: 6.0 (3.0, 9.0), HC: 2.0 (1.0, 4.0)] and VSI [IBS-D: 34.0 (17.0, 45.3), HC: 5.0 (2.0, 15.0)] were significantly higher in IBS-D patients than in HCs. The score of depression showed a tendency to increase in IBS-D patients but failed to reach a significant level.
Visceral sensitivity test
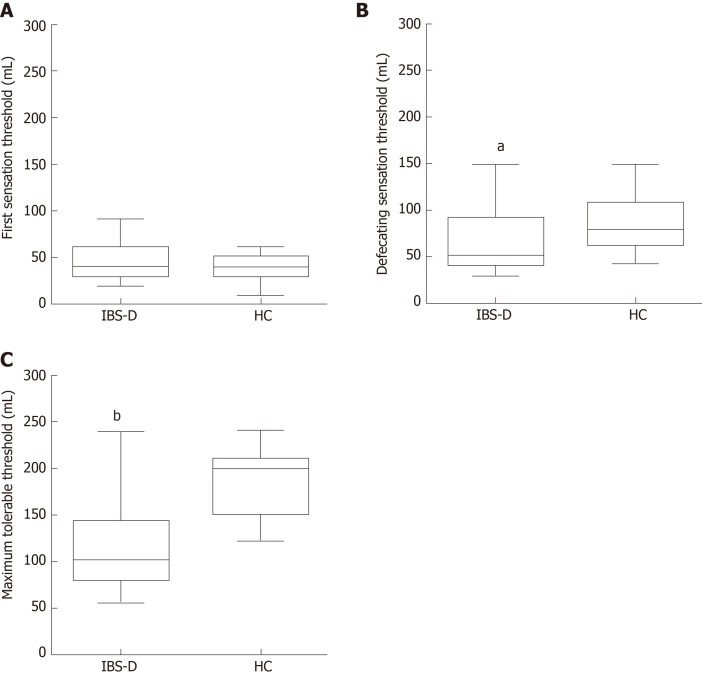
Three parameters for assessing visceral sensitivity, including the first sensation threshold, defecating sensation threshold, and maximum tolerable threshold to rectal distension stimulation, were measured. Scores of the defecating sensation threshold [IBS-D: 50.0 (40.0, 90.0), HC: 80.0 (60.0, 110.0); P = 0.032] and maximum tolerable threshold [IBS-D: 100.0 (80.0, 142.5), HC: 200.0 (150.0, 210.0); P < 0.001] were significantly lower in the patients than those in the HCs (Figure 1). There was no difference between the two groups in the first sensation threshold [IBS-D: 40.0 (30.0, 60.0), HC: 40.0 (30.0, 50.0); P = 0.315].
Figure 1.
Visceral sensation thresholds to rectal distension stimulation. A: The first sensation threshold showed no significant difference between the two groups (P = 0.315). B: The score of the defecating sensation threshold was significantly decreased in the patients (P = 0.032). C: The maximum tolerable threshold was also lower in patients with irritable bowel syndrome with predominant diarrhea than in controls (P < 0.001). Box means the interquartile range; line inside the boxes indicates the median; the two whiskers indicate the 5th percentile and 95th percentile of the data. aP < 0.05 vs healthy control (HC); bP < 0.01 vs HC. IBS-D: Irritable bowel syndrome with predominant diarrhea; HC: Healthy control.
Amino acids
In the control group, Gln was detected in only one subject. Cys was detected in six IBS-D patients and in two controls. The prevalence of Gly, Glu, Thr, Ala, Val, and Leu detection was 100%, and the prevalence of detection of other amino acids ranged from 66.7% to 96.7%. There was no difference in the amount of amino acids in subjects with or without IBS-D. Differences were observed for individual amino acids including His [IBS-D: 0.0642 (0.0388, 0.1484), HC: 0.2636 (0.0780, 0.3966), P = 0.012], Ala [IBS-D: 0.5095 (0.2826, 0.9183), HC: 1.0118 (0.6135, 1.4335), P = 0.041], Tyr [IBS-D: 0.1024 (0.0173, 0.4527), HC: 0.5665 (0.2436, 1.3447), P = 0.018], Phe [IBS-D: 0.1511 (0.0775, 0.3248), HC: 0.3967 (0.1388, 0.7550), P = 0.028], and Trp [IBS-D: 0.0323 (0.0001, 0.0826), HC: 0.0834 (0.0170, 0.1759), P = 0.046] (Table 2). Only Tyr was mildly correlated with BSFS scores in all subjects (r = -0.347, P = 0.019) (Table 3). There were no statistically significant associations between BSFS scores and the levels of His, Ala, Tyr, Phe, or Trp in IBS-D group or HC group.
Table 2.
Levels of partial fecal metabolites in irritable bowel syndrome with predominant diarrhea patients and controls
| Metabolites | Prevalence of detection | IBS-D patients (μmol/g) | Controls (μmol/g) | P value |
| His | 93.3% | 0.0642 (0.0388, 0.1484)a | 0.2636 (0.0780, 0.3966) | 0.012 |
| Ala | 100.0% | 0.5095 (0.2826, 0.9183)a | 1.0118 (0.6135, 1.4335) | 0.041 |
| Tyr | 88.9% | 0.1024 (0.0173, 0.4527)a | 0.5665 (0.2436, 1.3447) | 0.018 |
| Phe | 97.8% | 0.1511 (0.0775, 0.3248)a | 0.3967 (0.1388, 0.7550) | 0.028 |
| Trp | 77.8% | 0.0323 (0.0001, 0.0826)a | 0.0834 (0.0170, 0.1759) | 0.046 |
| Val | 100.0% | 0.2710 (0.1208, 0.5729) | 0.3661 (0.2960, 1.0122) | 0.079 |
| Asp | 97.8% | 0.3086 (0.1797, 0.6904) | 0.4875 (0.1939, 0.6591) | 0.647 |
| Fumarate | 100.0% | 0.0042 (0.0026, 0.0108) | 0.0019 (0.0014, 0.0078) | 0.051 |
| Pyruvate | 100.0% | 0.0067 (0.0023, 0.0719) | 0.0071 (0.0022, 0.0882) | 0.718 |
| Butyrate | 100.0% | 4.3819 (2.4060, 9.0920) | 5.8539 (1.5193, 12.8616) | 0.613 |
| Isobutyrate | 100.0% | 6.8609 (0.5068, 8.9326) | 2.1277 (0.5470, 10.4635) | 0.962 |
| Valerate | 100.0% | 0.2024 (0.0331, 0.8245) | 0.2059 (0.0497, 1.2489) | 0.485 |
| Isovalerate | 100.0% | 0.1218 (0.0495, 0.3227) | 0.1661 (0.0416, 0.2581) | 0.866 |
| Hexanoate | 100.0% | 0.0254 (0.0189, 0.0513) | 0.0383 (0.0153, 0.0539) | 0.613 |
| Isohexanoate | 100.0% | 0.0127 (0.0060, 0.0246)a | 0.0070 (0.0023, 0.0106) | 0.028 |
| Amino acids | NA | 7.2873 (3.4177, 10.7709) | 8.0603 (6.1393, 12.3225) | 0.336 |
| SCFA | NA | 25.8082 (18.5826, 38.5067) | 26.8452 (5.7182, 70.6763) | 0.904 |
| Organic acids | NA | 26.6708 (20.0617, 38.5860) | 26.9449 (5.8047, 71.4057) | 0.866 |
P < 0.05 vs controls. The data are presented as the median (Q1, Q3). IBS-D: Irritable bowel syndrome with predominant diarrhea; SCFA: Short chain fatty acids; NA: Not applicable.
Table 3.
Correlation of bristol stool form scale scores with the levels of His, Ala, Tyr, Phe, or Trp
| Metabolites |
IBS-D |
Controls |
All subjects |
|||
| r | P value | r | P value | r | P value | |
| His | 0.057 | 0.764 | 0.423 | 0.117 | -0.279 | 0.064 |
| Ala | -0.026 | 0.892 | 0.338 | 0.218 | -0.252 | 0.095 |
| Tyr | -0.208 | 0.271 | 0.423 | 0.117 | -0.3471 | 0.019 |
| Phe | 0.002 | 0.994 | 0.465 | 0.081 | -0.250 | 0.098 |
| Trp | -0.114 | 0.548 | 0.423 | 0.117 | -0.268 | 0.075 |
There was association between parameters (P < 0.05). IBS-D: Irritable bowel syndrome with predominant diarrhea.
Organic acids
Propionate was detected in only four IBS-D patients and four controls. Acetate was not detected in five controls, and the prevalence of detection of other organic acids was 100%. Only isohexanoate [IBS-D: 0.0127 (0.0060, 0.0246), HC: 0.0070 (0.0023, 0.0106), P = 0.028] was elevated in the IBS-D patients (Table 2). The level of isobutyrate was higher in the IBS-D group than in the controls, but the difference did not reach statistical significance. There were no differences in the amounts of SCFA or organic acids in subjects with or without IBS-D (Table 2).
Correlation of fecal microbial metabolites with IBS-SSS score
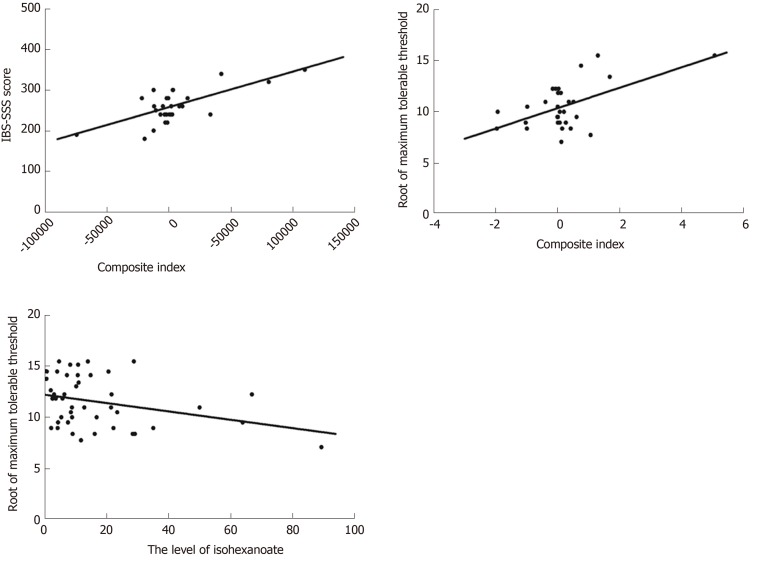
A statistically significant regression equation was found (R2Adjusted = 0.693, F = 11.893, P < 0.001). The levels of fumarate and Val had a significant negative association with IBS-SSS score but hexanoate, pyruvate and Tyr had a positive association (Table 4, Figure 2A). In collinearity diagnostics, the eigenvalues and condition indices ranged from 0.071 to 3.897 and from 1.000 to 7.394, respectively (Table 5). The residual obeyed a normal distribution.
Table 4.
Multiple linear regression model for irritable bowel syndrome symptom severity scale score
| Metabolites |
Unstandardized coefficients |
Standard-ized coefficie-nts β | t | P value |
Collinearity statistics |
||
| b | SE | Tolerance | VIF | ||||
| Hexanoate1 | 116.194 | 29.961 | 0.420 | 3.878 | 0.001 | 0.903 | 1.107 |
| Fumarate1 | -1733.224 | 490.683 | -0.383 | -3.532 | 0.002 | 0.902 | 1.108 |
| Pyruvate1 | 140.381 | 28.619 | 0.528 | 4.905 | < 0.001 | 0.915 | 1.092 |
| Asp | -15.303 | 7.943 | -0.221 | -1.927 | 0.066 | 0.807 | 1.240 |
| Tyr1 | 60.960 | 15.019 | 0.763 | 4.059 | < 0.001 | 0.300 | 3.333 |
| Val1 | -82.922 | 26.139 | -0.626 | -3.172 | 0.004 | 0.272 | 3.671 |
Metabolite which was correlated with irritable bowel syndrome symptom severity scale score (P< 0.01); SE: Standard error; VIF: Variance inflation factor.
Figure 2.
Correlation of fecal microbial metabolites with irritable bowel syndrome symptom severity scale score or maximum tolerable threshold. A: Correlation of fecal microbial metabolites with irritable bowel syndrome symptom severity scale score in irritable bowel syndrome with predominant diarrhea (IBS-D) patients (R2Adjusted = 0.693, P < 0.001). The composite index was the sum of score based on linear combination of metabolites using the coefficients listed in Table 4. B: Correlation of metabolites with maximum tolerable threshold in IBS-D patients (R2Adjusted = 0.255, P = 0.007); The composite index was the sum of scores based on linear combination of isovalerate and valerate using the coefficients listed in Table 6. C: Correlation of metabolites with maximum tolerable threshold in all subjects (R2Adjusted = 0.079, P = 0.034).
Table 5.
Collinearity diagnostics of models
| Models | Dimensions |
Collinearity diagnostics |
|
| Eigenvalues | Condition indices | ||
| Multiple linear regression model for IBS-SSS score in IBS-D group | 1 | 3.897 | 1.000 |
| 2 | 1.008 | 1.966 | |
| 3 | 0.751 | 2.278 | |
| 4 | 0.643 | 2.462 | |
| 5 | 0.407 | 3.093 | |
| 6 | 0.222 | 4.186 | |
| 7 | 0.071 | 7.394 | |
| Multiple linear regression model for maximum tolerable threshold in IBS-D group | 1 | 2.286 | 1.000 |
| 2 | 0.675 | 1.841 | |
| 3 | 0.040 | 7.580 | |
IBS-D: Irritable bowel syndrome with predominant diarrhea; IBS-SSS: Irritable bowel syndrome symptom severity scale score.
Correlation of metabolites with maximum tolerable threshold to rectal distension stimulation
After taking a root of the maximum tolerable threshold, a multiple linear regression model using the stepwise method was applied. In the control group, no metabolites were included in the regression model. In IBS-D patients, associations with isovalerate and valerate for the maximum tolerable threshold (R2Adjusted = 0.255, F = 5.957, P = 0.007) are shown in Table 6 and Figure 2B. The eigenvalues and condition indices ranged from 0.040 to 2.286 and from 1.000 to 7.580, respectively (Table 5). A lower maximum tolerable threshold was associated with increased isohexanoate in all subjects (R2Adjusted = 0.079, F = 4.789, P =0.034) (Table 6, Figure 2C). The residual also obeyed a normal distribution.
Table 6.
Multiple linear regression model for maximum tolerable threshold
| Groups | Metabolites |
Unstandardized coefficients |
Standar-dized coeffici-ents β | t | P value |
Collinearity statistics |
||
| b | SE | Tolerance | VIF | |||||
| IBS-D | Isovalerate2 | 5.451 | 1.824 | 1.525 | 2.989 | 0.006 | 0.099 | 10.132 |
| Valerate1 | -2.171 | 0.946 | -1.171 | -2.295 | 0.030 | 0.099 | 10.132 | |
| All subjects | Isohexanoate1 | -40.910 | 18.695 | -0.317 | -2.188 | 0.034 | NA | NA |
Metabolite which was correlated with maximum tolerable threshold (P < 0.05).
Metabolite which was correlated with maximum tolerable threshold (P < 0.01); SE: Standard error; VIF: Variance inflation factor; IBS-D: Irritable bowel syndrome with predominant diarrhea; NA: Not applicable.
Correlation of BDQ scores with isovalerate, valerate or isohexanoate
In HCs, there was no statistically significant association between BDQ scores and isovalerate, valerate or isohexanoate levels. In the IBS-D group, Spearman’s rank correlation showed that the level of isovalerate was positively correlated with the severity or frequency of abdominal pain. In addition, isohexanoate was significantly correlated with the frequency of abdominal pain in IBS-D patients (Table 7). In all subjects, only isohexanoate was significantly associated with the severity or frequency of abdominal pain (Table 7).
Table 7.
Correlation of bowel disease questionnaire scores with isovalerate or isohexanoate
| Groups | Metabolites |
Frequency of abdominal pain |
Severity of abdominal pain |
||
| r | P value | r | P value | ||
| IBS-D | Isovalerate | 0.4752 | 0.008 | 0.3771 | 0.040 |
| Isohexanoate | 0.4141 | 0.023 | 0.097 | 0.612 | |
| All subjects | Isovalerate | 0.200 | 0.188 | 0.143 | 0.350 |
| Isohexanoate | 0.4732 | 0.001 | 0.3711 | 0.012 | |
There was association between parameters (P < 0.05).
There was association between parameters (P < 0.01). IBS-D: Irritable bowel syndrome with predominant diarrhea.
DISCUSSION
Overwhelming evidence has shown that luminal contents play important roles in gastrointestinal function[10,32]. Although the effects of fecal metabolites in the pathogenesis of IBS are being discovered, there has been evidence that some metabolites in feces are correlated with symptoms in IBS patients[30,36]. We comprehensively assessed the clinical and psychological characteristics of IBS-D patients, visceral sensitivity and fecal metabolites. As expected, these data confirmed the difference in fecal metabolite compositions between IBS-D patients and HCs, and the levels of some metabolites were significantly correlated with IBS-SSS score, visceral sensitivity and the severity or frequency of abdominal pain.
More male patients were recruited in our study, although the morbidity rate of IBS is significantly higher in females. The reasons were as follows: on the one hand, more female patients refused to participate due to reluctance to visceral sensitivity test or distrust. On the other hand, women with a history of dysmenorrheal or cesarean section were excluded.
His, Ala, Tyr, Phe, and Trp were decreased in IBS-D patients compared to controls and were almost unassociated with stool form. Furthermore, the amount of amino acids was not different in subjects with or without IBS. These findings revealed that differences in individual amino acids would be mainly due to differences in the amino acid composition rather than stool water content.
Amino acids in the intestine have critical roles in the expression of tight junction proteins and are closely connected with the apoptosis or proliferation of gastrointestinal epithelial cells[19-22,24,50], all of which are important factors for the functions of the intestinal barrier. Trp enhanced the expression of tight junction proteins (occluden-1, occluden-2, occludin, claudin-3, and claudin-4) in the intestine and was beneficial for mucosal growth or maintenance[51,52]. Increased intestinal permeability is one of the pathophysiological changes in IBS.
Furthermore, increased gut mucosal immune activation was also involved in IBS. Aromatic amino acids, including Trp, Phe, and Tyr, attenuated gastrointestinal inflammation[53]. Inflammatory mediators are also one of the main etiological factors in ulcerative colitis. A previous study demonstrated that Trp and His were significantly lower in ulcerative colitis patients than in controls[54]. However, the functions of amino acids in IBS patients are still unclear. Further study is required to analyze the correlation between decreased individual amino acids and increased intestinal permeability or gut mucosal immune activation in IBS.
The association between the gut microbiota and IBS has been studied extensively. One study transplanting fecal microbiota from IBS-D patients into mice demonstrated that the microbiota played significant roles in gut function, and supported the point that bacteria would contribute to IBS[55]. The findings indicate differences in microbiota between IBS and healthy volunteers, but the results have been inconsistent[56,57]. After dietary treatment, the symptoms of IBS were controlled, but the phylotype of the intestinal microbiota remained unchanged[58]. As important as the phylotype, the function of the microbiota might add mechanistic insight[27,28,30]. The function could be measured as metabolites in the feces. SCFA, as a large group of fecal microbial metabolites, have been described as the link between the host and the microbes[29,31,59].
In this study, the association between SCFA (isohexanoate, isovalerate or valerate) and visceral sensation (abdominal pain or visceral hypersensitivity) indicates that increased chemical stimuli could be one of the origins or exacerbating factors in IBS-D. However, in IBS patients, the chemical stimuli and chemosensitivity are largely unknown. The gastrointestinal tract receives sensory innervation[10]. Some sensory neurons terminate close to the mucosal epithelium and sense chemical stimuli by connecting to enteroendocrine cells or immune cells[8,11,32,60,61]. Previous studies have shown that butyrate is increased in IBS-D patients and induces colonic hypersensitivity in rats[62,63]. However, other studies observed the conflicting results that the level of butyrate was decreased in patients or was not significantly different between patients and controls[36,59]. At the same time, there was no statistically significant association between butyrate and visceral sensation in this and other studies[36]. A recent study suggested that SCFA, especially isovalerate and isobutyrate, was involved in visceral hypersensitivity[32], which contributed to abdominal pain. Therefore, our current results are basically in accordance with the recent concept of visceral sensation.
The current methods of symptom-based diagnosis and the assessment of IBS severity are inherently limiting[64]. There is a pressing need for biomarkers of symptom severity and treatment response in IBS[65]. In the present study, a biomarker panel was identified to correlate with the symptom severity of IBS (R2Adjusted = 0.693, P < 0.001). This indicates a potential biomarker to objectively quantify symptom severity and assess responsiveness to treatments. However, this hypothesis needs to be verified further.
This preliminary study has some limitations. First, because only IBS-D patients were included in this study, the conclusions may not be generalized to other IBS subtypes. Second, the sample size is relatively small, which may undermine the reliability of the conclusions. Further research with a larger number of subjects should be conducted to verify our conclusions. Third, our conclusions were based on an observational study, which cannot make cause and effect inferences. Therefore, conclusions need to be further verified by high-grade evidence. Fourth, isovalerate was simultaneously positively correlated with the maximum tolerable threshold and the severity or frequency of abdominal pain. The reason for this conflict is still unclear. Finally, diet was not standardized during the study period. Habitual diet determines the repertoire of microbial metabolites[66], but food components also plays a significant role in the pathophysiology of IBS[67-69]. Although diet was not standardized in this and other studies[30,34,35], it should be noted that some food components may only relate to fecal metabolite composition but not IBS pathophysiology. It is necessary to standardize diet in the future study.
In summary, this study provides new evidence that metabolite compositions are different in subjects with or without IBS-D and altered profiles of fecal metabolites may be one of the origins or exacerbating factors in IBS-D. Furthermore, a significant correlation was observed between metabolites and symptom severity in the IBS-D group. This preliminary study lays a basis to further explore the role of fecal metabolites in IBS-D pathogenesis and to search for fecal biomarkers in symptom severity.
ARTICLE HIGHLIGHTS
Research background
Irritable bowel syndrome (IBS) is a highly prevalent functional bowel disorder and the pathogenesis is complicated. Fecal metabolites are associated with gut visceral sensitivity, mucosal immune function and intestinal barrier function, all of which have critical roles in the pathogenesis of IBS.
Research motivation
A few articles reported that patients diagnosed with IBS had metabolite compositions that were different from those of healthy controls. However, the role of metabolites in IBS pathophysiology is unclear. The study of fecal metabolites might add insight to investigate IBS.
Research objectives
IBS with predominant diarrhea (IBS-D) is the major subtype of IBS. The aims of this study were to compare fecal metabolites in subjects with or without IBS-D and to explore the associations of metabolites with clinical and experimental parameters.
Research methods
Participants underwent clinical and psychological assessments, including the IBS Symptom Severity System, an Italian modified version of the Bowel Disease Questionnaire, the Bristol Stool Form Scale, the Hospital Anxiety and Depression Scale, and the VSI, along with visceral sensitivity testing. Fecal metabolites were measured by targeted metabolomics approaches. Correlation analyses between these parameters were performed. SPSS (IBM-SPSS Statistics, Chicago, IL, United States) version 21.0 was used for statistical analysis.
Research results
The patients presented with more psychological symptoms and increased visceral hypersensitivity compared with the controls. In fecal metabolites, His, Ala, Tyr, Phe, and Trp were decreased in IBS-D patients, but isohexanoate was increased. Abdominal pain or visceral hypersensitivity was correlated with isovalerate, valerate and isohexanoate. Furthermore, a significant correlation was observed between metabolites and symptom severity.
Research conclusions
This study presented evidence that metabolite compositions were different in subjects with or without IBS-D and some metabolites might be one of the origins or exacerbating factors of symptoms via increasing visceral sensitivity. This study also demonstrated a possible potential biomarker panel of symptom severity. The authors believe that this study provides some clues for IBS-D pathogenesis and for the search for biomarkers in symptom severity.
Research perspectives
This preliminary study investigated the possible role of fecal metabolites in IBS pathophysiology. In the future, we will focus on the following aspects. First, as the present study did not standardize diet and could not make cause and effect inferences, conclusions need to be further verified by additional well-designed clinical and basic studies. Second, the present study focused on IBS-D patients, and the conclusions may not be generalized to other IBS subtypes. We will investigate the role of fecal metabolites in pathophysiology of other IBS subtypes in future studies.
ACKNOWLEDGEMENTS
We thank Dr. Du SY for enrollment of participants.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of China-Japan Friendship Hospital, Beijing, China.
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: All authors report no conflicts of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the first author at zhangwenxue048@163.com.
STROBE statement: The authors have read the STROBE Statement, and the manuscript was prepared and revised according to the STROBE Statement.
Manuscript source: Unsolicited manuscript
Peer-review started: September 19, 2019
First decision: October 14, 2019
Article in press: November 1, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carroccio A, Kamiya T, O'Malley D S-Editor: Tang JZ L-Editor: Ma JY E-Editor: Ma YJ
Contributor Information
Wen-Xue Zhang, Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China; Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Yu Zhang, Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China; Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Geng Qin, Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China; Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Kai-Min Li, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, China.
Wei Wei, Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China; Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Su-Yun Li, Department of Epidemiology and Health Statistics, School of Public Health, Qingdao University, Qingdao 266071, Shandong Province, China.
Shu-Kun Yao, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China. shukunyao@126.com.
References
- 1.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;150:1393–1407.e5. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Amouretti M, Le Pen C, Gaudin AF, Bommelaer G, Frexinos J, Ruszniewski P, Poynard T, Maurel F, Priol G, El Hasnaoui A. Impact of irritable bowel syndrome (IBS) on health-related quality of life (HRQOL) Gastroenterol Clin Biol. 2006;30:241–246. doi: 10.1016/s0399-8320(06)73160-8. [DOI] [PubMed] [Google Scholar]
- 4.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 5.Bai T, Xia J, Jiang Y, Cao H, Zhao Y, Zhang L, Wang H, Song J, Hou X. Comparison of the Rome IV and Rome III criteria for IBS diagnosis: A cross-sectional survey. J Gastroenterol Hepatol. 2017;32:1018–1025. doi: 10.1111/jgh.13642. [DOI] [PubMed] [Google Scholar]
- 6.Ersryd A, Posserud I, Abrahamsson H, Simrén M. Subtyping the irritable bowel syndrome by predominant bowel habit: Rome II versus Rome III. Aliment Pharmacol Ther. 2007;26:953–961. doi: 10.1111/j.1365-2036.2007.03422.x. [DOI] [PubMed] [Google Scholar]
- 7.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukudo S, Nomura T, Muranaka M, Taguchi F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J Clin Gastroenterol. 1993;17:133–141. doi: 10.1097/00004836-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kassam Z, Collins SM, Moayyedi P. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2013;368:577–578. doi: 10.1056/NEJMc1214185. [DOI] [PubMed] [Google Scholar]
- 10.Furness JB RL, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–741. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 11.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Tan B, Huang B, Li J, Wang J, Liao P, Guan G, Ji P, Yin Y. Involvement of calcium-sensing receptor activation in the alleviation of intestinal inflammation in a piglet model by dietary aromatic amino acid supplementation. Br J Nutr. 2018;120:1321–1331. doi: 10.1017/S0007114518002891. [DOI] [PubMed] [Google Scholar]
- 14.Mine Y, Zhang H. Calcium-sensing receptor (CaSR)-mediated anti-inflammatory effects of L-amino acids in intestinal epithelial cells. J Agric Food Chem. 2015;63:9987–9995. doi: 10.1021/acs.jafc.5b03749. [DOI] [PubMed] [Google Scholar]
- 15.Nagarjun S, Dhadde SB, Veerapur VP, Thippeswamy BS, Chandakavathe BN. Ameliorative effect of chromium-d-phenylalanine complex on indomethacin-induced inflammatory bowel disease in rats. Biomed Pharmacother. 2017;89:1061–1066. doi: 10.1016/j.biopha.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, Machiah DK, Lawson B, Hakimpour P, Wang YC, Li S, Sharma P, Kaufman RJ, Martinez J, Pulendran B. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531:523–527. doi: 10.1038/nature17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren W, Zou L, Li N, Wang Y, Liu G, Peng Y, Ding J, Cai L, Yin Y, Wu G. Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice. Br J Nutr. 2013;109:867–872. doi: 10.1017/S0007114512002681. [DOI] [PubMed] [Google Scholar]
- 18.Revelo XS, Winer S, Winer DA. Starving Intestinal Inflammation with the Amino Acid Sensor GCN2. Cell Metab. 2016;23:763–765. doi: 10.1016/j.cmet.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Song Zh, Tong G, Xiao K, Jiao le F, Ke Yl, Hu Ch. L-cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016;22:152–161. doi: 10.1177/1753425916632303. [DOI] [PubMed] [Google Scholar]
- 20.Jiao N, Wu Z, Ji Y, Wang B, Dai Z, Wu G. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J Nutr. 2015;145:2258–2264. doi: 10.3945/jn.115.217661. [DOI] [PubMed] [Google Scholar]
- 21.Vargas Robles H, Castro Ochoa KF, Nava P, Silva Olivares A, Shibayama M, Schnoor M. Analyzing Beneficial Effects of Nutritional Supplements on Intestinal Epithelial Barrier Functions During Experimental Colitis. J Vis Exp. 2017;119:e55095. doi: 10.3791/55095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wils-Plotz EL, Jenkins MC, Dilger RN. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult Sci. 2013;92:735–745. doi: 10.3382/ps.2012-02755. [DOI] [PubMed] [Google Scholar]
- 23.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Li W, Sun Y, Han F, Hu CA, Wu Z. Amino acid deprivation disrupts barrier function and induces protective autophagy in intestinal porcine epithelial cells. Amino Acids. 2015;47:2177–2184. doi: 10.1007/s00726-014-1844-6. [DOI] [PubMed] [Google Scholar]
- 25.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3:e977176. doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM, Mercenier A, Nauta A, Garcia-Rodenas CL. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan N, Pluznick JL. From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol. 2014;307:C979–C985. doi: 10.1152/ajpcell.00228.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 30.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 31.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O'Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011;60:817–827. doi: 10.1099/jmm.0.028126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar V, Homer D, Rigsbee L, Khamis HJ, Michail S, Raymer M, Reo NV, Paliy O. The networks of human gut microbe-metabolite associations are different between health and irritable bowel syndrome. ISME J. 2015;9:1899–1903. doi: 10.1038/ismej.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankar V, Reo NV, Paliy O. Simultaneous fecal microbial and metabolite profiling enables accurate classification of pediatric irritable bowel syndrome. Microbiome. 2015;3:73. doi: 10.1186/s40168-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mujagic Z, Tigchelaar EF, Zhernakova A, Ludwig T, Ramiro-Garcia J, Baranska A, Swertz MA, Masclee AA, Wijmenga C, van Schooten FJ, Smolinska A, Jonkers DM. A novel biomarker panel for irritable bowel syndrome and the application in the general population. Sci Rep. 2016;6:26420. doi: 10.1038/srep26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 38.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 39.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 40.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 41.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 42.Saigo T, Tayama J, Hamaguchi T, Nakaya N, Tomiie T, Bernick PJ, Kanazawa M, Labus JS, Naliboff BD, Shirabe S, Fukudo S. Gastrointestinal specific anxiety in irritable bowel syndrome: validation of the Japanese version of the visceral sensitivity index for university students. Biopsychosoc Med. 2014;8:10. doi: 10.1186/1751-0759-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Qin G, Liu DR, Wang Y, Yao SK. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2019;25:269–281. doi: 10.3748/wjg.v25.i2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff SC. 'Gut health': a new objective in medicine? BMC Med. 2011;9:24. doi: 10.1186/1741-7015-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He F, Wu C, Li P, Li N, Zhang D, Zhu Q, Ren W, Peng Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. Biomed Res Int. 2018;2018:1–13. doi: 10.1155/2018/9171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng X, Qiu Y, Zhong W, Baxter S, Su M, Li Q, Xie G, Ore BM, Qiao S, Spencer MD, Zeisel SH, Zhou Z, Zhao A, Jia W. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics. 2013;9:818–827. doi: 10.1007/s11306-013-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiori J, Amadesi E, Fanelli F, Tropeano CV, Rugolo M, Gotti R. Cellular and mitochondrial determination of low molecular mass organic acids by LC-MS/MS. J Pharm Biomed Anal. 2018;150:33–38. doi: 10.1016/j.jpba.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 48.Boogers I, Plugge W, Stokkermans YQ, Duchateau AL. Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J Chromatogr A. 2008;1189:406–409. doi: 10.1016/j.chroma.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 49.Wei R, Wang J, Jia E, Chen T, Ni Y, Jia W. GSimp: A Gibbs sampler based left-censored missing value imputation approach for metabolomics studies. PLoS Comput Biol. 2018;14:e1005973. doi: 10.1371/journal.pcbi.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demehri FR, Barrett M, Ralls MW, Miyasaka EA, Feng Y, Teitelbaum DH. Intestinal epithelial cell apoptosis and loss of barrier function in the setting of altered microbiota with enteral nutrient deprivation. Front Cell Infect Microbiol. 2013;3:105. doi: 10.3389/fcimb.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Mi S, Ruan Z, Li J, Shu X, Yao K, Jiang M, Deng Z. Dietary Tryptophan Enhanced the Expression of Tight Junction Protein ZO-1 in Intestine. J Food Sci. 2017;82:562–567. doi: 10.1111/1750-3841.13603. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Ji Y, Wu G, Sun K, Sun Y, Li W, Wang B, He B, Zhang Q, Dai Z, Wu Z. l-Tryptophan Activates Mammalian Target of Rapamycin and Enhances Expression of Tight Junction Proteins in Intestinal Porcine Epithelial Cells. J Nutr. 2015;145:1156–1162. doi: 10.3945/jn.114.209817. [DOI] [PubMed] [Google Scholar]
- 53.Tan B, Huang B, Wang J, Guang GP, Yang CB, Yin YL. Aromatic amino acids alleviate intestinal inflammation in piglets through calcium-sensing receptor activation. J. ournal of Animal Science. 2017;95:201–202. [Google Scholar]
- 54.Xin L, Li N, Zhu W, Wu K, Zhang L, Zhai J, Wang Y, Zhu J, Wang X, Shi Y, Li N, Sha L, Wu Z, Zhang P, Wang X. An analysis of amino acid metabolic profile and its clinical significance in ulcerative colitis. Zhonghua Neike Zazhi. 2015;54:210–213. [PubMed] [Google Scholar]
- 55.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pigrau Pastor M, Sidani S, Pinto-Sanchez MI, Philip V, McLean PG, Hagelsieb MG, Surette MG, Bergonzelli GE, Verdu EF, Britz-McKibbin P, Neufeld JD, Collins SM, Bercik P. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 56.Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318–331. doi: 10.5009/gnl14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033–1042. doi: 10.1111/apt.12728. [DOI] [PubMed] [Google Scholar]
- 58.Harvie RM, Chisholm AW, Bisanz JE, Burton JP, Herbison P, Schultz K, Schultz M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol. 2017;23:4632–4643. doi: 10.3748/wjg.v23.i25.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Aerssens J, Hillsley K, Peeters PJ, de Hoogt R, Stanisz A, Lin JH, Van den Wyngaert I, Göhlmann HW, Grundy D, Stead RH, Coulie B. Alterations in the brain-gut axis underlying visceral chemosensitivity in Nippostrongylus brasiliensis-infected mice. Gastroenterology. 2007;132:1375–1387. doi: 10.1053/j.gastro.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mortensen PB, Andersen JR, Arffmann S, Krag E. Short-chain fatty acids and the irritable bowel syndrome: the effect of wheat bran. Scand J Gastroenterol. 1987;22:185–192. doi: 10.3109/00365528708991878. [DOI] [PubMed] [Google Scholar]
- 63.Xu D, Wu X, Grabauskas G, Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62:1466–1474. doi: 10.1136/gutjnl-2012-302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144–158. doi: 10.1111/j.1365-2036.2010.04328.x. [DOI] [PubMed] [Google Scholar]
- 65.Chang L, Di Lorenzo C, Farrugia G, Hamilton FA, Mawe GM, Pasricha PJ, Wiley JW. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology. 2018;154:723–735. doi: 10.1053/j.gastro.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 66.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O'Toole PW, Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 67.De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169–178. doi: 10.1136/gutjnl-2015-309757. [DOI] [PubMed] [Google Scholar]
- 68.Gibson PR, Varney J, Malakar S, Muir JG. Food components and irritable bowel syndrome. Gastroenterology. 2015;148:1158–74.e4. doi: 10.1053/j.gastro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, Franciosi E, Scholz M, Tuohy KM, Lindsay JO, Irving PM, Whelan K. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology. 2017;153:936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]