Abstract
Background:
Although antimicrobial susceptibility testing (AST) frequently guides cystic fibrosis (CF) pulmonary exacerbation (PEx) management, its clinical utility is unclear. This study examined associations between AST and antimicrobial switching during PEx treatment and time and occurrence of next PEx as treatment outcomes.
Methods:
This retrospective cohort study utilized Pediatric Health Information System data. Children and adolescents aged 1–18 years admitted for a PEx from 2011–2016 were studied. Antimicrobial switching was defined as any intra-admission change in intravenous (IV), oral, and/or inhaled antimicrobials. Time to next PEx was defined as the time between index PEx hospital discharge and subsequent hospital admission requiring IV antimicrobials. Odds of antimicrobial switching ≥5 days after treatment initiation were determined by generalized linear mixed models, and associations between AST and time to next PEx were studied using Kaplan-Meier curves and Cox proportional hazards regression.
Results:
AST occurred in 2,518 (39%) of 6,451 PEx at 36 hospitals and was associated with increased odds of antimicrobial switching (OR 1.33, 95% CI 1.16–1.52; p=0.001) and increased hazard of future PEx (HR 1.32, 95% CI 1.16–1.50; p<0.001). However, antimicrobial switching was not associated with a longer time to next PEx.
Conclusions:
AST was associated with both increased probability of antimicrobial regimen change and increased PEx hazard. There was no evidence that antimicrobial regimen change was associated with clinical benefit as assessed by time to next PEx. However, these results indicate residual indication bias remained after adjustment for available disease covariates. Additional studies of the clinical value of AST are warranted.
Keywords: Antimicrobial Susceptibility Testing, Pediatrics, Pulmonary Exacerbations, Antimicrobial Switching
Introduction
Significant morbidity and mortality are associated with pulmonary exacerbations (PEx) in people with cystic fibrosis (CF)1, 2. Common PEx treatment strategies include increasing airway clearance and oral, inhaled and/or intravenous (IV) antimicrobial treatments. Traditionally, PEx antimicrobial selection is guided by surveillance respiratory culture results and antimicrobial susceptibility testing (AST)—such as Kirby-Bauer disk diffusion testing—to identify pathogen-specific antimicrobial susceptibility patterns and to aid clinicians in choosing appropriate antimicrobial therapy. Although an evidence-based AST performance guideline exists3 and CF treatment guidelines support AST use to guide antimicrobial treatment4, 5, the clinical utility of AST in the management of CF-related infections, including PEx management, is unclear.
Data from single center retrospective analyses have failed to illustrate benefits of AST for several CF-related clinical outcomes, including lung function, body-mass index (BMI), and time to next PEx6, 7. A randomized trial comparing CF patients treated with IV antimicrobials chosen either from conventional sputum bacterial isolate susceptibility testing or multiple combination bactericidal testing found no difference in clinical outcomes between the two groups6. In addition, a quality improvement project implemented to reduce AST did not worsen several measured clinical parameters, including change in lung function, weight, and IV antimicrobial therapy duration9. These aforementioned studies, however, were limited by concerns related to sample size and generalizability.
We sought to evaluate the use of AST in a pediatric CF cohort using a large national administrative dataset. The study aims were 1) to describe the frequency of AST conducted during admission for pediatric CF-related PEx, 2) to determine if AST conducted during admission for PEx treatment is associated with an increased probability of changes in antimicrobial treatment regimen (i.e., switching) beyond 5 days of treatment, and 3) to determine if AST conducted during admission is associated with better clinical outcomes demonstrated by an increased time to next PEx requiring IV antimicrobials. We hypothesized that antimicrobial switching would occur in a greater proportion of PEx with AST compared to PEx without AST, and that AST would be associated with a longer time to next PEx requiring IV antimicrobials. Our goal from this study is to aid CF clinicians and microbiology laboratory personnel in the appropriate use of AST in clinical practice and to inform future AST guidelines.
Methods:
This retrospective cohort study utilized data from the Pediatric Health Information System (PHIS; Children’s Hospital Association [CHA], Lenexa, KS), an administrative database that includes inpatient, ambulatory surgery, emergency department and observation unit clinical and resource utilization data from 49 U.S. children’s hospitals10. PHIS data have been used extensively for studies related to resource utilization variation, cost estimation/analysis, and clinical effectiveness and includes International Classification of Diseases (ICD) and Clinical Transaction Classification (CTC) diagnostic codes to indicate individual diseases, medications, and procedures. PHIS data can thus be used to determine if AST was performed during a PEx; however, neither AST results (i.e. specific resistance patterns) nor related culture results are available for review within the PHIS database.
Children and adolescents aged 1–18 years at time of discharge were included if they fit 1 of 2 possible CF PEx definitions. The first definition comprised an ICD-9 code for CF with pulmonary manifestations and IV antimicrobial use within the first 24 hours of admission. The second definition included any other CF-related ICD-9 code (CF with gastrointestinal manifestations, CF without mention of meconium ileus, or CF with other manifestations) with one of several respiratory disease-specific ICD-9 codes (acute upper respiratory tract infections of multiple or unspecific sites, acute bronchitis or bronchiolitis, pneumonia and influenza, bronchitis and chronic bronchitis, or bronchiectasis) and IV antimicrobials within the first 24 hours of admission. These two definitions were chosen to be consistent with a previous analysis of PHIS data that characterized the management of inpatient pediatric CF-related PEx11.
Any PEx that occurred from January 1st, 2011 through December 31st, 2016 and had documented antimicrobial use each hospital day (with the exception of discharge date) qualified for analysis. To determine if AST occurred during a PEx, we used the PHIS CTC code 361500, “Sensitivity/Susceptibility Testing” (which includes disk diffusion and Kirby-Bauer testing). A PEx in which AST occurred at any point from admission to discharge was included in the AST PEx group. We performed a single-center chart review to determine the sensitivity and specificity of the CTC code “Sensitivity/Susceptibility Testing” within the PHIS database.
Length of stay was limited to 7–21 days to account for the fact that antimicrobial susceptibilities typically take at least 5–6 days after specimen collection to become available (L. Hoffman and X. Qin, personal communication, October 26th, 2017), and to exclude hospitalizations not thought to be representative of the majority of inpatient pediatric PEx. People with CF were excluded if they had a history of malignancy or organ transplant. PEx were excluded if an intensive care unit stay occurred or if one of the following ICD-9 diagnostic codes were utilized: complications of transplant or other postoperative complication, acute pancreatitis, or mechanical complication of gastrostomy, colostomy, enterostomy tube, insulin pump, or vascular device. In addition, hospitals were excluded if they provided incomplete oral medication information or systematic coding discrepancies making data unreliable, or if they were not CF Foundation-accredited at time of PEx (CF Foundation-accredited centers are more likely aware of existing CF Foundation AST and PEx guidelines).
An antimicrobial switch was defined as any change in IV, oral, and/or inhaled antimicrobials after hospitalization day 5 up to the day prior to discharge. Hospitalization day 5 was chosen to both avoid tolerability-based early regimen changes (i.e., allergic reactions) and to define baseline antimicrobial therapy before AST results were likely to be known. The day prior to hospital discharge (rather than the discharge day itself) was chosen because parenteral antimicrobials might be switched to oral to facilitate outpatient therapy. At Seattle Children’s Hospital, the following sequence of events occurs following the collection of a respiratory culture: Day 1-respiratory sample arrives, is processed, cultures set up and incubated; Day 3-CF isolates sub-cultured; Day 4-species are identified and AST performed; Day 5/6—antimicrobial susceptibilities are available for faster (day 5) or slower (day 6) growers (L. Hoffman and Xuan Qin, personal communication, October 26th, 2017).
Time to next PEx requiring IV antimicrobials was defined as the time between the index PEx hospital discharge and a subsequent hospital admission requiring IV antimicrobials. The index PEx was defined as the first PEx that met inclusion and exclusion criteria recorded during the study period. We included only one PEx per patient to ensure that patients with multiple PEx would not contribute more PEx episodes and bias the results towards a shorter time to next PEx requiring IV antimicrobials. Patients for whom a subsequent PEx was not observed prior to December 31, 2016 were censored on December 31, 2016.
Characteristics of the study cohorts were summarized using descriptive statistics. Continuous variables were compared using a two-sample t test or Wilcoxon rank sum test, whereas categorical variables were compared by Pearson’s χ2 tests.
To test the hypothesis that AST was associated with increased antibiotic switching, AST was entered as a predictor into a generalized linear mixed model predicting antibiotic switching with a nested random effect of participant within hospital. A compound symmetry covariance structure was used to model structural clustering and the associated expected correlation. Covariates included sociodemographic measures (age group, gender, race/ethnicity, and insurance). We chose to include additional clinical variables felt to be related to disease severity; these included number of PEx recorded in the prior 12 months, administration of any IV aminoglycoside antimicrobial (amikacin, gentamicin, kanamycin, or tobramycin) during a PEx (as a surrogate for presence of Pseudomonas aeruginosa (Pa)), and administration of pancreatic enzymes, insulin, and ursodiol as markers of pancreatic insufficiency, CF-related diabetes, and CF-related liver disease, respectively. PEx year was also included as a categorical covariate in the model to adjust for temporal patterns.
The primary analysis included PEx from all hospitals meeting inclusion and exclusion criteria. To distinguish between hospitals that use AST routinely versus hospitals that more frequently order AST by indication only, we conducted a sensitivity analysis that stratified results into three AST tertiles (lowest, middle, and highest AST use). These tertiles were also included as covariates in the generalized linear mixed model. Hospitals with an antimicrobial switch rate of <10% were excluded to address concerns that antimicrobial recording at these hospitals might be incomplete.
Time from index to subsequent PEx requiring IV antimicrobials was visualized by Kaplan-Meier curves, stratified by (1) AST and (2) antibiotic switching. AST and antibiotic switching were entered into a Cox proportional hazards regression model predicting next PEx, adjusted for the aforementioned covariates as well as hospital length of stay as a surrogate for index PEx severity. Hospital and year of index PEx discharge were also included as fixed effects to adjust for variation across hospitals and over time, respectively. Analyses were conducted at the=0.05 level of significance using SAS (version 9.4, SAS Institute, Cary, NC). This study was approved by the Seattle Children’s Hospital Institutional Review Board (STUDY00000759).
Results:
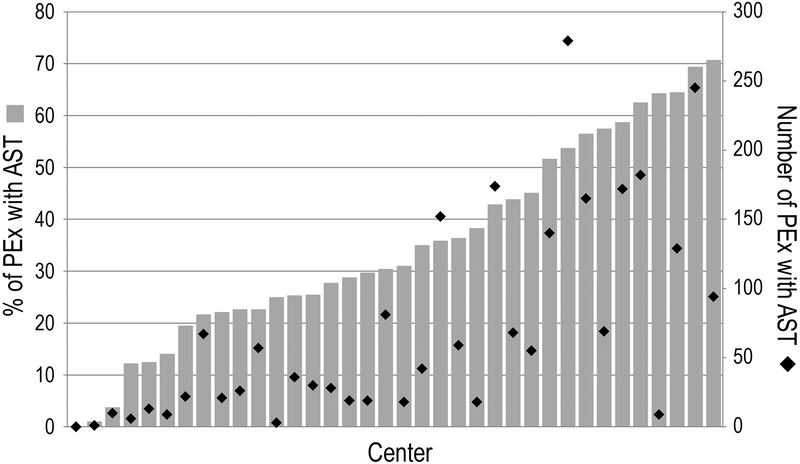
From January 1st, 2011 through December 31st, 2016, AST was associated with 2,518 (39%) of 6,451 included PEx at 36 PHIS hospitals. The mean AST frequency among all hospitals was 35% of PEx (Figure 1). Three hospitals performed AST in <10% of PEx, while AST occurred in >50% of PEx at 10 PHIS hospitals (28%). A single-center chart review performed at Seattle Children’s Hospital found the sensitivity and specificity of the AST CTC code 361500 (“Sensitivity/Susceptibility Testing”) to be 94.7% and 100%, respectively.
Figure 1:
Histogram illustrating frequency of AST and number of PEx with AST among PHIS hospitals
Characteristics of the study cohorts are described in Table 1. Children and adolescents with CF who had AST associated with admission for PEx treatment were older (66% ≥12 years vs 50% ≥12 years for those without AST associated with their treatment), more likely female (57% vs 52%), and more likely to have public insurance (56% vs 49%). In addition, children and adolescents with CF who had AST were more likely to use insulin (21% vs 15%) and ursodiol (21% vs 16%) (markers of CF-related diabetes and CF-related liver disease, respectively), and were more likely to have had at least 2 PEx requiring IV antimicrobials in the prior 12 months (36% vs 25%). Antimicrobial switching after 5 days occurred in 58% of PEx with AST compared to 51% of PEx without AST.
Table 1:
Characteristics of the Study Cohort
| Demographic Variable Overall N=6,451 | PEx with AST N=2,518 | PEx without AST N=3,933 | p-value |
|---|---|---|---|
| Age | <0.0001 | ||
| 0–5 years | 201 (8%) | 687 (17%) | |
| 6–11 years | 650 (26%) | 1286 (33%) | |
| 12–18 years | 1667 (66%) | 1960 (50%) | |
| Gender (Female) | 1440 (57%) | 2053 (52%) | 0.0001 |
| Race/Ethnicity | <0.0001 | ||
| Caucasian | 1760 (70%) | 2916 (74%) | |
| Hispanic/Latino | 553 (22%) | 697 (18%) | |
| African American | 101 (4%) | 152 (4%) | |
| Other | 45 (2%) | 117 (3%) | |
| Unknown | 59 (2%) | 51 (1%) | |
| Insurance Status | <0.0001 | ||
| Private | 971 (39%) | 1741 (44%) | |
| Public | 1398 (56%) | 1912 (49%) | |
| Other/Unknown | 149 (6%) | 280 (7%) | |
| Pancreatic Enzyme Use (Yes) | 2396 (95%) | 3723 (95%) | 0.4 |
| Ursodiol Use (Yes) | 524 (21%) | 612 (16%) | <0.0001 |
| Insulin Use (Yes) | 537 (21%) | 578 (15%) | <0.0001 |
| IV Aminoglycoside Use (Yes) | 1755 (70%) | 2662 (60%) | 0.09 |
| Antibiotic Count* | <0.0001 | ||
| 1 | 129 (5%) | 371 (10%) | |
| 2 | 842 (33%) | 1579 (40%) | |
| ≥3 | 1547 (61%) | 1983 (50%) | |
| # of PEx in Prior 12 Months | <0.0001 | ||
| 0 | 960 (38%) | 1955 (50%) | |
| 1 | 656 (26%) | 1000 (25%) | |
| ≥2 | 902 (36%) | 978 (25%) | |
| Length of Stay | <0.0001 | ||
| 7–10 days | 848 (34%) | 1591 (40%) | |
| 11–15 days | 1307 (52%) | 1998 (51%) | |
| 16–21 days | 363 (14%) | 344 (9%) | |
| Antibiotic Switching (Yes) | 1467 (58%) | 1996 (51%) | <0.0001 |
Number of oral, inhaled and/or IV antibiotics administered on hospitalization day 5
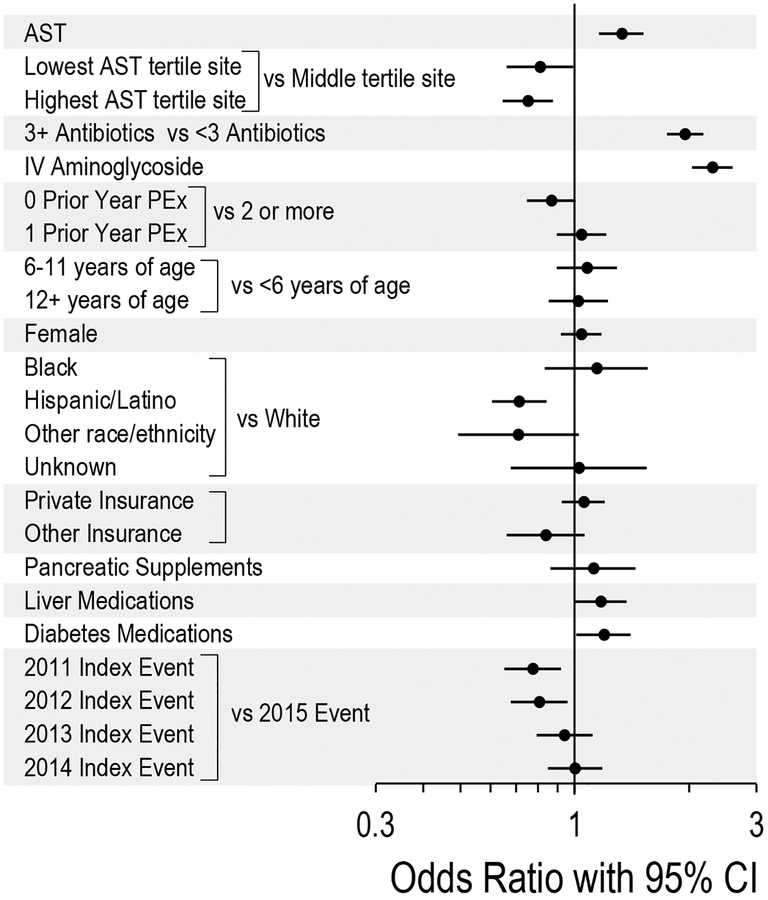
AST during PEx treatment was associated with increased odds of antimicrobial switching (odds ratio (OR) 1.33 95% confidence interval (CI) 1.16–1.52, p<0.001; Figure 2). Other variables associated with antimicrobial switching included insulin use (OR 1.19, 95% CI 1.01–1.41, p=0.04), IV aminoglycoside use (OR 2.30, 95% CI 2.04–2.60, p<0.0001), and number of oral, inhaled and/or IV antimicrobials administered on hospitalization day 5 (3 vs <3) (OR 1.95, 95% CI 1.75–2.18, p<0.0001). Among AST tertiles, AST during PEx treatment remained associated with increased odds of antimicrobial switching in the lowest and highest tertiles (OR 1.58, 95% CI 1.17–2.13, p=0.003 and OR 1.20, 95% CI 1.02–1.42, p=0.03, respectively), but not the middle tertile (OR 1.16, 95% CI 0.93–1.44, p=0.2; Supplemental Figures 1 and 2).
Figure 2:
Forest plot illustrating the odds of antibiotic switching for study variables
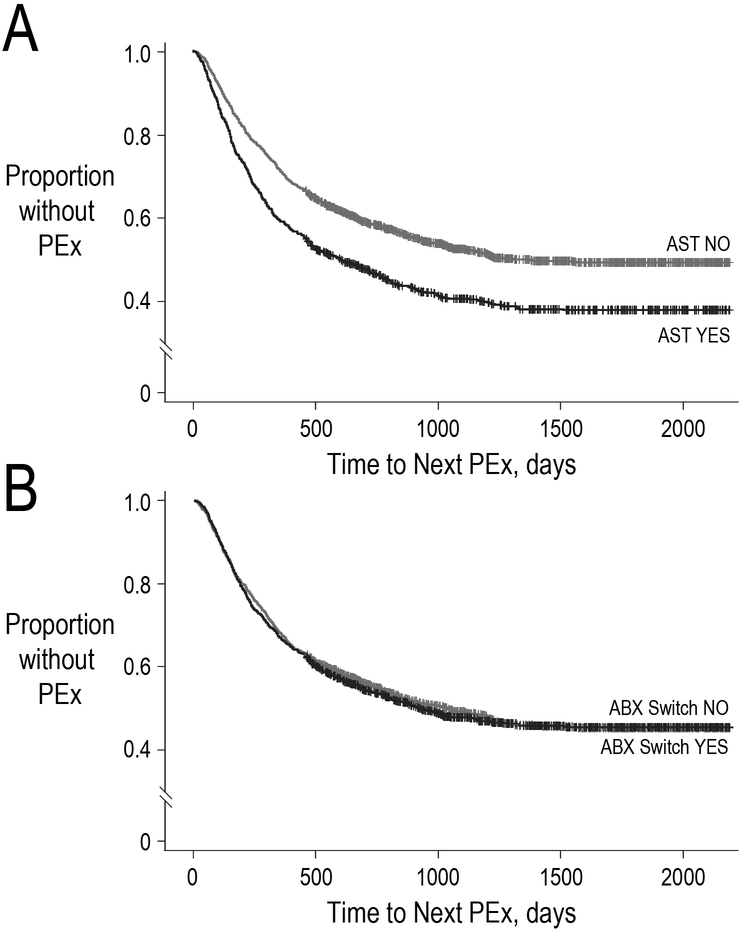
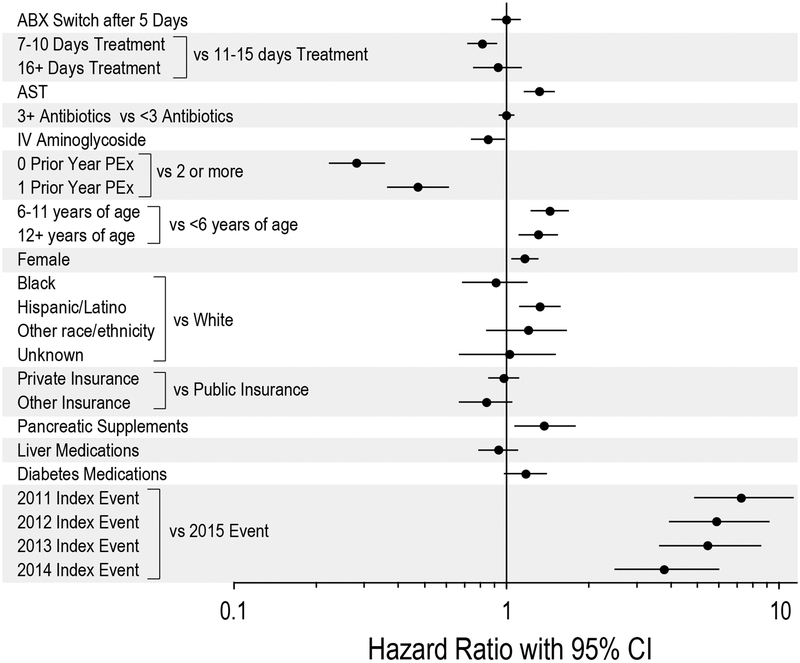
AST at admission was associated with a shorter time to next PEx requiring IV antimicrobials (HR 1.32, 95% CI 1.16–1.50, p<0.0001; Figure 3a), while antimicrobial switching was not associated with a difference in hazard of next PEx requiring IV antimicrobials (HR 1.00, 95% CI 0.88–1.13, p=1.00; Figure 3b and 4). The median time to next PEx requiring IV antimicrobials was 257 days (IQR 120–478) among PEx with AST compared to 1,255 days (792–1,800) for PEx without AST. Other variables associated with an increased hazard of PEx requiring IV antimicrobials were age (12–18 years and 6–11 years vs 0–5 years), female sex, Hispanic/Latino vs Caucasian race/ethnicity, use of pancreatic enzymes, and PEx requiring IV antimicrobials in the prior 12 months. In contrast, treatment with IV aminoglycosides and shorter treatment durations (7–10 days vs 11–15 days) were associated with a reduced hazard of PEx. There was a strong association between PEx hazard and the year in which the index PEx was recorded, with earlier index PEx associated with higher subsequent PEx hazard (Figure 4). When stratified by AST tertile, AST remained associated with a shorter time to next PEx requiring IV antimicrobials in the highest AST tertile (HR 1.49, 95% CI 1.23–1.80, p<0.0001; Supplemental Figure 3), while antimicrobial switching remained unassociated with time to next PEx requiring IV antimicrobials in all three tertiles (Supplemental Figure 4).
Figure 3.
a: Kaplan-Meier curve illustrating the time to next PEx requiring IV antimicrobials with respect to antibiotic switching
b: Kaplan-Meier curve illustrating the time to next PEx requiring IV antimicrobials with respect to AST
Figure 4:
Forest plot illustrating time to next PEx requiring IV antimicrobials for study variables
Discussion:
Using the PHIS database to evaluate CF-related PEx, we found that AST use during inpatient pediatric PEx was associated with higher odds of antimicrobial switching compared to PEx in which AST was not ordered. In addition, contrary to our hypothesis, AST use was not associated with better outcomes (longer time to next PEx requiring IV antimicrobials). Our single center validation of the AST code found high sensitivity and specificity (94.7% and 100%, respectively), indicating that this PHIS code could reliably identify AST use during PEx treatment. Further, the strong association observed between AST and antimicrobial switching in our primary and sensitivity analyses in the lowest and highest AST tertiles suggests that the CTC code for AST is likely to be accurate in our analyses.
AST use was associated with a 33% higher odds of antimicrobial switching during PEx treatment, suggesting that AST results influenced clinician antimicrobial prescribing patterns. Potential reasons for antimicrobial switching during PEx treatment include sensitivity/intolerance to prescribed antimicrobials, development of unwanted antimicrobial side effects (e.g., acute kidney injury, liver toxicity), lack of expected improvement in symptoms, lung function, or weight gain, and discordance of antimicrobial regimens with AST results. We purposely analyzed antimicrobial switching occurring after 5 days to decrease contribution from patient intolerance of antimicrobials and to allow sufficient time for AST results to become available to treating clinicians. Interestingly, antimicrobial switching was also high among PEx without AST, particularly among hospitals in the lowest and middle AST tertiles (probability of switching 53% (95% CI 46–61%) and 55% (95% CI 45–65%), respectively); it is likely that an element of antimicrobial switching across the population irrespective of AST was related to lack of expected improvement from original antimicrobial treatments after 5 days, and it may even be that lack of response was greater in those in which AST was ordered, as they tended to have more advanced disease as indicated by IV aminoglycoside and insulin use (Figure 2). However, antimicrobial switching was not associated with a treatment benefit as measured by time to next PEx requiring IV antimicrobials.
Our results indicate that AST in the primary analysis and in the highest AST tertile was associated with a shorter rather than longer time to next PEx requiring IV antimicrobials (probably due to confounding where individuals with more advanced disease were more likely to have AST performed) and that AST was not associated with improved clinical outcomes. As noted, sicker patients admitted for PEx treatment are more likely to have AST performed. However, even after adjustment for disease severity using medication data as surrogates for pancreatic status, Pa colonization, CF-related diabetes, and CF-related liver disease, as well as number of PEx in the prior 12 months (a known risk factor that predicts future PEx13), AST was still not associated with improved outcomes with respect to time to next PEx, raising questions about the utility of this test. It is important to note that AST is generally performed only on specific bacterial species considered to be “standard CF pathogens”, and the absence of AST in this study may indicate an absence of these organisms, another potential source of confounding. This highlights an important limitation of using PHIS data in this manner, as it lacks airway microbiology and other markers of disease stage and severity such as lung function and BMI.
A recent collaborative effort by the Antimicrobial Resistance in Cystic Fibrosis International Working group, using the PICO (patient, intervention, comparator, outcome) format, undertook a systematic review that evaluated the relationships between AST results and clinical response14. Similar to our results, these investigators found that among twenty separate studies (13 of which were related to PEx) included in their analysis, little evidence existed that AST predicted clinical outcomes of CF antimicrobial treatment. Our analysis takes this line of investigation a step further by studying possible effects on treatment of ordering AST at the time of the PEx.
Although AST is recommended in CF PEx treatment guidelines, it can be burdensome, resource- and time-intensive, and may not be uniformly available. An adult CF clinic in Leeds, England found that reducing the number of antimicrobial susceptibility tests by 56% resulted in saving an estimated 170 hours of laboratory staff time per year.9 Interestingly, our data suggest a modest trend towards increasing use of AST among the institutions studied over this time period (Figure 2). At Seattle Children’s Hospital, depending on the organisms isolated and the location where the respiratory culture is collected (outpatient vs inpatient), the cost of AST ranges from $78-$370/test. Over a 12-month period (November 1st, 2017 through October 31st, 2018) at Seattle Children’s Hospital, 1,455 susceptibility tests were performed on isolates from 733 CF respiratory cultures at a total cost of >$580,000 (J. Stapp, personal communication, November 1st, 2018). Future cost-effectiveness analyses utilizing multicenter AST cost data can additionally evaluate the potential financial impact of the use of AST in clinical practice.
Strengths of this study include the large number of PEx available for analysis and the inclusion of large pediatric hospitals from across the country to increase its generalizability. This study does have several important limitations. Since PHIS is an administrative dataset, the reason for admission (planned ‘tune-up’ or acute illness), motivation for performing AST and reasons for antimicrobial switching remain unknown. We were unable to validate the AST code at other institutions, and thus it is plausible that some PEx with AST were missed, which would bias the results evaluating the relationship between AST and antimicrobial switching towards the null. Within PHIS, there is no way to associate AST with a specific culture (respiratory, urine, blood, etc.), and thus it is possible that, for a few PEx, AST was performed on non-respiratory cultures. Since PHIS only captures in-hospital data, we were unable to determine whether patients had AST performed on respiratory cultures collected just prior to admission that might impact whether AST was performed during a PEx. The CF AST literature has a heavy emphasis on Pa treatment, but in our analysis, it was not clear which organisms were of greatest concern for a given PEx. Lastly, as mentioned previously, PHIS does not capture lung function, airway microbiology, CFTR genotype, or data on growth parameters—important markers of disease stage and severity. However, in collaboration with Children’s Hospital Association and the CF Foundation Patient Registry (CFFPR) team, we recently successfully linked the CFFPR with the PHIS database15. This newly-created CFFPR-PHIS linked dataset will allow for further analyses evaluating AST and additional relevant clinical outcomes, including change in lung function pre- to post-PEx and return to lung function baseline.
In conclusion, this study provides further evidence that AST (and in particular AST ordered during hospital admission) might not be helpful in PEx antimicrobial treatment. We found that PEx in which AST was ordered had significantly higher odds of antimicrobial switching after 5 days of treatment, but antimicrobial switching was not associated with improved outcomes as assessed by time to next PEx requiring IV antimicrobials. AST is resource-intensive and may lead to unnecessary antimicrobial use; additional studies evaluating its clinical impact in CF are warranted, particularly in the current era of worsening global antimicrobial resistance.
Supplementary Material
Supplemental Figure 1: Probability of antibiotic switching and odds ratio for antibiotic switching based on AST for all hospitals and stratified by AST tertile group
Supplemental Figure 2: Forest plot illustrating the odds of antibiotic switching for study variable stratified by AST tertile group
Supplemental Figure 3: Kaplan-Meier curves illustrating the time to next PEx requiring IV antimicrobials with respect to antibiotic switching stratified by AST tertile group
Supplemental Figure 4: Kaplan-Meier curves illustrating the time to next Pex requiring IV antimicrobials with respect to AST stratified by AST tertile group
Acknowledgements:
We would like to thank Jenny Stapp and Xuan Qin from the Seattle Children’s Microbiology Laboratory for input related to the logistics and cost of antimicrobial susceptibility testing.
Funding: None
Footnotes
Data presented at: North American Cystic Fibrosis Conference, October 18th-20th, 2018 Denver CO
Conflicts of Interest Statement:
Drs. Cogen, Gibson and Ms. Whitlock have no conflicts of interest to disclose.
Dr. Hoffman consults on behalf of the CF Foundation regarding incorporating AST into research studies; he does not receive personal fees.
Dr. VanDevanter reports personal fees from AbbVie, Albumedix, AN2, Aradigm, Calithera, Cystic Fibrosis Foundation, Chiesi USA, Concert, CURx, Eloxx, Enbiotix, Genentech, Horizon, IBF, ICON Clinical Sciences, Ionis, Kala, Life Science Strategies, OrbiMed, Protalix, PTC, Pulmocide, Raptor, Recida, Respirion, Savara, VAST, and Vertex, outside the submitted work.
References:
- 1).Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. European Respiratory Journal 2012; 40: 61–66. [DOI] [PubMed] [Google Scholar]
- 2).Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. American Journal of Respiratory and Critical Care Medicine 2010; 182: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, 27th ed, Wayne, Pennsylvania: 2017. [Google Scholar]
- 4).Treatment of Pulmonary Exacerbation of Cystic Fibrosis. Clinical Practice Guidelines for Cystic Fibrosis. Bethesda, MD: Cystic Fibrosis Foundation; 1997. [Google Scholar]
- 5).UK Cystic Fibrosis TrustWorking Group. Antibiotic Treatment for Cystic Fibrosis. 3rd ed; May 2009. Available at https://www.cysticfibrosis.org.uk/~/media/documents/the-work-we-do/care/consensus-docs-with-new-address/anitbiotic-treatment.ashx?la=en.
- 6).Smith AL, Fiel SB, Mayer-Hamblett N, Ramsey B, Burns JL. Susceptibility Testing of Pseudomonas aeruginosa Isolates and Clinical Response to Parenteral Antibiotic bAdministration: Lack of Association in Cystic Fibrosis. Chest 2003; 123: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 7).Hurley MN, Amin Ariff AH, Bertenshaw C, Bhatt J Smyth AR. Results of Antibiotic Susceptibility Testing Do Not Influence clinical Outcome in Children with Cystic Fibrosis. Journal of Cystic Fibrosis 2012; 11: 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Aaron SD, Vandemheen KL, Ferris W, Fergusson D, Tullis E, Haase D, Berthiaume Y, Brown N, Wilcox P, Yozghatlian V, Bye P, Bell S, Chan F, Rose B, Jeanneret A, Stephenson A, Noseworth M, Freitag A, Paterson N, Doucette S, Harbour C, Ruel M, MacDonald N. Combination Antibiotic Susceptibility Testing to Treat Exacerbations of Cystic Fibrosis Associated with Multiresistant Bacteria: A Randomized, Double-Blind, Controlled Clinical Trial. Lancet 2005; 366: 463–471. [DOI] [PubMed] [Google Scholar]
- 9).Etherington C, Hall M, Conway S, Peckham D, Denton M. Clinical Impact of Reducing Routine Susceptibility Testing in Chronic Pseudomonas aeruginosa Infections in Cystic Fibrosis. Journal of Antimicrobial Chemotherapy 2008; 61: 425–427. [DOI] [PubMed] [Google Scholar]
- 10).Data Source: Pediatric Health Information Systems Database (PHIS). Children’s Hospital Association, Lenexa, KS. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Pediatric-Health-Information-System
- 11).Cogen JD, Oron AP, Gibson RL, Hoffman LR, Kronman MP, Ong T, Rosenfeld M. Characterization of Inpatient Cystic Fibrosis Pulmonary Exacerbations. Pediatrics 2017; 139(2): e20162642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Flume PA, Waters VJ, Bell SC, Van Devanter DR, Elborn JS. Antimicrobial Resistance in Cystic Fibrosis: Does it Matter? Journal of Cystic Fibrosis 2018; 17(6): 687–689. [DOI] [PubMed] [Google Scholar]
- 13).VanDevanter DR, Morris NJ, Konstan MW. IV-treated pulmonary exacerbations in the prior year: An important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016. May; 15(3):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Somayaji R, Parkins MD, Shah A, Martiniano SL, Tunney MM, Kahle JS, Waters VJ, Elborn JS, Bell SC, Flume PA, VanDevanter DR. Antimicrobial Susceptibility Testing (AST) and Associated Clinical Outcomes in Individuals with Cystic Fibrosis: A Systematic Review. J Cyst Fibros. 2019. March;18(2): 236–243. [DOI] [PubMed] [Google Scholar]
- 15).Cogen JD, Hall M, Loeffler DR, Gove N, Onchiri F, Sawicki GS, Fink AK. Linkage of the CF Foundation Patient Registry with the Pediatric Health Information System Database. Pediatr Pulmonol. 2019. March 18. doi: 10.1002/ppul.24272. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Probability of antibiotic switching and odds ratio for antibiotic switching based on AST for all hospitals and stratified by AST tertile group
Supplemental Figure 2: Forest plot illustrating the odds of antibiotic switching for study variable stratified by AST tertile group
Supplemental Figure 3: Kaplan-Meier curves illustrating the time to next PEx requiring IV antimicrobials with respect to antibiotic switching stratified by AST tertile group
Supplemental Figure 4: Kaplan-Meier curves illustrating the time to next Pex requiring IV antimicrobials with respect to AST stratified by AST tertile group