Abstract
Background:
Acute kidney injury (AKI) and intraventricular hemorrhage (IVH) are common in premature infants. We previously demonstrated that infants with AKI have a higher hazards ratio to develop grade ≥2 IVH when controlling for confounders. However, that single-center study was unable to show an overall association.
Objectives:
To test the hypothesis that infants diagnosed with AKI have an increased risk of IVH independent of variables associated with both AKI and IVH, we performed a study on 825 infants from the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study (a 24-center multinational retrospective cohort).
Method:
A neonatal modified KDIGO definition of AKI was used based on serum creatinine (SCr) and/or urine output criteria. Baseline SCr was defined as the lowest previous value. IVH was diagnosed with head ultrasounds.
Results:
AKI was documented in 183/825 (22.2%) infants and IVH in 118/825 (14.3%). Infants with AKI (n=183) were more likely to have IVH [26.8% (49/183)] than those without AKI (n=642) who had IVH [10.7% (69/642), p<0.0001]. After controlling for 5-minute Apgar score, vasopressive support within 1st week of age, and gestational age, infants with AKI had 1.6 times higher adjusted odds to develop any grade IVH (95% CI 1.04–2.56). Further, infants of gestational age of 22–28 weeks had 1.9 times higher adjusted odds to develop IVH (1.87, 95% CI 1.08–3.23).
Conclusions:
We present the first multicenter evaluation of the association between AKI and IVH in premature infants showing a significant independent association between AKI and IVH. Development of strategies to reduce AKI may also reduce IVH.
Keywords: Acute kidney injury, Intraventricular hemorrhage, Prematurity, AWAKEN
2. Introduction
Over the past decades, remarkable improvements in the care of premature infants have decreased morbidity and mortality. Single-center studies show that premature infants are at increased risk of acute kidney injury (AKI) with an incidence of 12–40% depending on the gestational age (GA) and those with AKI have higher adverse outcomes [1–5]. The first multi-center study on neonatal AKI, The Assessment of Worldwide Acute Kidney Epidemiology in Neonates (AWAKEN), found an AKI incidence of 48% in infants with a GA of 22–29 weeks. Furthermore, those with AKI had a 4-fold higher independent odds of death compared to those without AKI [3]. In recent years the relationship of AKI to distant organ dysfunction, including neurologic outcomes, has become a growing area of investigation [6,7]. Evaluation of the association of AKI with neurologic outcomes including intraventricular hemorrhage (IVH) may lead to strategies to reduce IVH and its effects on neurologic development.
IVH is a major cause of morbidity and mortality and occurs in approximately 20% of very low birth weight (<1500g) infants and 45% of extremely low birth weight (<1000g) infants [8,9]. Due to the immaturity of the germinal matrix, prematurity infants are predisposed to IVH due to disturbances in cerebral blood flow changes [10–12].
The kidney may play an important role in the pathophysiology of IVH by its role in blood pressure regulation, as the kidney receives 10–20% of blood flow [13,14]. Previous work in neonates with perinatal asphyxia has shown an association of AKI with neurologic lesions on brain MRIs and poor neurocognitive outcomes[6,15]. AKI may predispose neonates to IVH via alterations in blood pressure regulation through changes in fluid status and/or to the renin-aldosterone-angiotensin system (RAAS). Alternatively, AKI may lead to IVH via systemic inflammatory dysregulation[14,16]. In our own previous single-center study, we demonstrated that infants with AKI have an over three fold risk to develop grade ≥2 IVH; however, it was limited by its single-center cohort design and study size [17].
To examine the association between AKI and IVH in premature infants we performed a secondary analysis of infants (<33 weeks GA) enrolled in the AWAKEN study. We tested the hypothesis that infants with AKI have an increased risk of IVH independent of variables associated with both AKI and IVH. Further, we aimed to evaluate if a dose-dependent relationship is present with stages of AKI and IVH grades.
3. Materials and Methods
3.a. Study Population
The methodology and protocol for the AWAKEN study have previously been published[18]. Briefly, the AWAKEN study is a cohort study of infants admitted to level II-III neonatal intensive care units from January 1 – March 31, 2014 across 24 institutions[18]. The original AWAKEN cohort excluded infants if: they did not receive intravenous fluids for at least 48 hours, were ≥14 days of age at admission, died within 48hr of admission, had congenital heart disease repaired <7 days of life, lethal anomaly, and/or bilateral severe congenital kidney or urinary tract malformation. Additionally, we excluded infants >33 weeks GA or infants with any CNS anomaly other than IVH.
3.b. Variable Definitions
The current analysis utilized the same AKI definition used in other AWAKEN studies according to the neonatal modified KDIGO definition of AKI based on serum creatinine (SCr) and/or urine output (UOP) criteria[19]. Baseline SCr was defined as the lowest previous value. All SCr values measured were incorporated (the median number of SCr values for each patient was 5). Stage 1 AKI was defined as a rise in SCr ≥0.3mg/dl within 48hr or ≥150% of baseline within seven days; Stage 2 as a rise of 200% from baseline; and Stage 3 as a rise of SCr ≥2.5mg/dl, ≥300% rise from baseline, or recipient of dialysis. UOP data was obtained from days 2–7 of life and criteria was defined as follow: Stage 1 >0.5 to ≤ 1 ml/kg/hr, Stage 2 >0.3 to ≤0.5 ml/kg/hr, and Stage 3 ≤ 0.3 ml/kg/hr. The highest value between SCr and UOP criteria was used to obtain the max AKI stage. IVH diagnosis was extracted from discharge summaries based on the Papille classification system based on head ultrasounds (HUS). SCr, UOP, and, HUSs were performed according to institutional/clinical practices and were obtained from medical records.
3.c. Statistical Analysis
Infant demographics and maternal characteristics were compared between infants with AKI vs. without AKI. Standard descriptive statistics included chi-square for categorical variables, t-test for continuous variables, and a Wilcoxon ranked sums for non-parametric continuous data (Apgar scores). Tables were created comparing IVH grades and AKI stages and analyzed using Cochran-Mantel-Haenszel test. An ordinal logistic regression model was used to estimate odds ratios (ORs) and associated 95% confidence intervals (CIs) for the association between AKI and IVH. Models were adjusted for 5-minute Apgar, vasopressive support anytime during the 1st week of life, and GA. In a secondary analysis, we considered GA a possible modifier of the association between AKI and IVH, creating an ordinal logistic model with an additional interaction term between GA category (<29 weeks and 29–35 weeks) and AKI. The proportionality assumption was checked for each logistic model using a score test, and all models met the assumption of proportionality of the estimated odds ratio. SAS v9.4 (SAS Institute Inc.) was used for all analyses.
4. Results
4.a. Patient Characteristics
The AWAKEN study screened a total of 4273 neonates, 2162 met the AWAKEN inclusion criteria, of which 866 infants were <33 weeks GA. 22 additional infants were excluded (Figure 1). The final study population included 825 neonates (Figure 1). Tables 1 and 2 summarize the baseline characteristics of the study population, dichotomized by discharge diagnosis of AKI and IVH, respectively. The mean GA of the entire cohort was 29.7 (±3.0); the mean birthweight (BW) was 1422.5 (±548.3), and the median Apgar score of 6 (±4,8) at 1 minute and 8 (±7,7) at 5 minutes. The majority of infants were black white and had male predominance.
Figure 1 -.
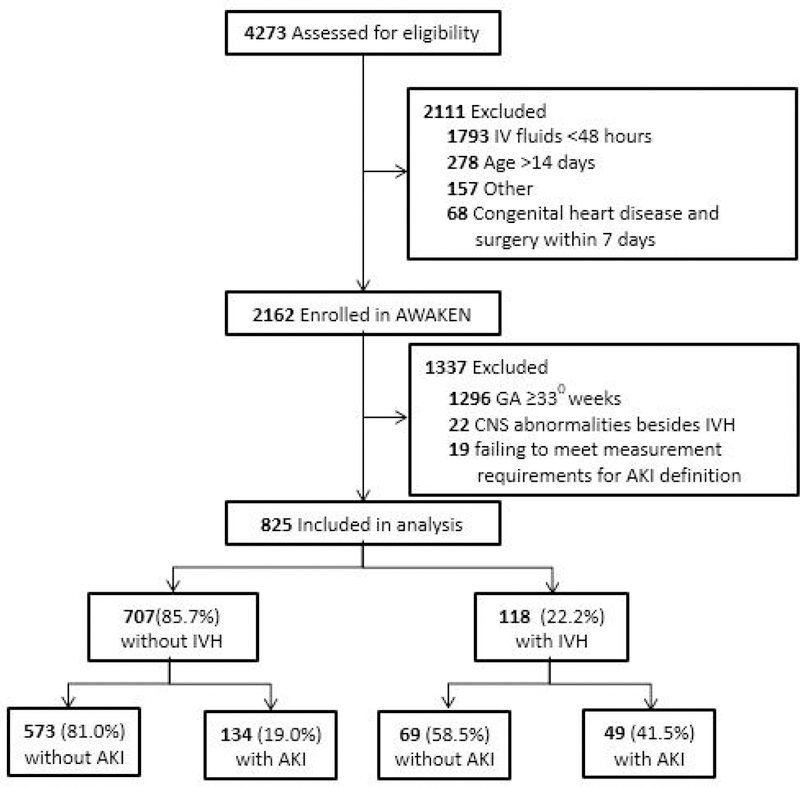
Study Flow Diagram.
Table 1.
Demographic, clinical, and maternal characteristics among neonates with and without acute kidney injury (AKI) diagnosis at discharge
| All Patients (n = 825) |
No AKI (n = 642) |
AKI (n = 183) |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Race° | 0.60 | |||
| White | 440 (53.3%) | 348 (54.2%) | 92 (50.3%) | |
| Black | 184 (22.3%) | 142 (22.1%) | 42 (22.9%) | |
| Other | 201 (24.4%) | 152 (23.7%) | 49 (26.8%) | |
| Gender (1 ambiguous)° | 0.13 | |||
| Male | 442 (53.6%) | 349 (54.4%) | 89 (48.6%) | |
| Female | 382 (46.3%) | 293 (45.6%) | 93 (50.8%) | |
| Gestational Age (mean ± SD) | 29.7 ± 3.0 | 30.2 ± 2.6 | 27.7 ± 3.3 | <0.0001* |
| Birth Weight (mean ± SD) | 1422.5 ± 548.3 | 1503.6 ± 510.9 | 1152.2 ± 582.2 | <0.0001* |
| Apgar (median ± SD) | ||||
| 1-minute | 6 (4,8) | 7 (4, 8) | 5 (2, 7) | <0.0001* |
| 5-minute | 8 (7, 9) | 8 (7, 9) | 7 (5, 8) | <0.0001* |
| Clinical | ||||
| Maternal | ||||
| Hypertension° | 93 (11.3%) | 74 (11.5%) | 19 (10.4%) | 0.67 |
| Pre-eclampsia° | 158 (19.2%) | 123 (19.4%) | 35 (18.3%) | 0.74 |
| Intrauterine Growth Restriction° | 75 (9.1%) | 57 (9.0%) | 18 (9.4%) | 0.86 |
| Prenatal Medications | ||||
| NSAIDs° | 45 (5.5%) | 35 (5.4%) | 10 (5.5%) | 0.99 |
| Steroids° | 578 (70.1%) | 452 (71.3%) | 126 (66.0%) | 0.16 |
| Neonatal | ||||
| Resuscitation | ||||
| O2 and/or Positive Pressure Ventilation° | 642 (77.8%) | 480 (75.7%) | 162 (84.8%) | 0.01* |
| CPR, Epinephrine, and/or Bolus*° | 58 (7.0%) | 34 (5.3%) | 24 (13.1%) | 0.0003* |
| Medications | ||||
| Vasopressor support¥° | 97 (11.7%) | 50 (7.9%) | 47 (24.6%) | <0.0001* |
| Nephrotoxic Medication†° | 681 (82.5%) | 509 (80.3%) | 172 (90.0%) | 0.002* |
| Discharge | ||||
| Necrotizing Enterocolitis (NEC) | <0.0001* | |||
| No NEC | 769 (93.2%) | 610 (96.2%) | 159 (83.2%) | |
| NEC | ||||
| Medical Management | 29 (3.5%) | 17 (2.7%) | 12 (6.3%) | |
| Surgical Management | 27 (3.3%) | 7 (1.1%) | 20 (10.5%) | |
| Respiratory Support at 7 days° | <0.0001* | |||
| None | 381 (46.2%) | 327 (51.6%) | 54 (28.3%) | |
| Non-invasive (Oxyhood, Nasal Canula, CPAP) | 131 (15.9%) | 58 (9.1%) | 73 (38.2%) | |
| Invasive (Conventional Ventilation, HFOV, ECMO) € | 313 (37.9%) | 249 (39.3%) | 64 (33.5%) | |
| Respiratory Support at 28 days° | <0.0001* | |||
| None | 523 (63.4%) | 449 (70.8%) | 74 (38.7%) | |
| Non-invasive (Oxyhood, Nasal Canula, CPAP) | 96 (11.6%) | 42 (6.6%) | 54 (28.3%) | |
| Invasive (Conventional Ventilation, HFOV, ECMO) € | 206 (25.0%) | 143 (23.6%) | 63 (33.0%) |
Bolus: Normal saline or packed red blood cells
19 missing for failing to meet the measurement requirements necessary for AKI definition
Vasopressor Support in 1st week of life: Dopamine, Dobutamine, Milrinone, Norepinephrine, Epinephrine
Nephrotoxic: Acyclovir, Amphotericin B, Aminoglycosides, Piperacillin/Tazobactam, Vancomycin
HFOV: High frequency oscillation ventilation, ECMO: extracorporeal membrane oxygenation
Table 2.
Demographic, clinical, and maternal characteristics among neonates with and without intraventricular hemorrhage (IVH) diagnosis at discharge
| All Patients (n = 825) |
No IVH (n =707) |
IVH (n = 118) |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Race | 0.18 | |||
| White | 440 (53.3%) | 383 (54.2%) | 57 (48.3%) | |
| Black | 184 (22.3%) | 150 (21.2%) | 34 (28.8%) | |
| Other | 201 (24.4%) | 174 (24.6%) | 27 (22.9%) | |
| Gender (1 ambiguous) | 0.79 | |||
| Male | 442 (53.6%) | 376 (53.2%) | 66 (55.9%) | |
| Female | 382 (46.3%) | 330 (46.7%) | 52 (44.1%) | |
| Gestational Age (mean ± SD) | 29.7 ± 3.0 | 30.1 ± 2.7 | 26.9 ± 3.1 | <0.0001* |
| Birth Weight (mean ± SD) | 1422.5 ± 548.3 | 1488.8 ± 538.4 | 1026.0 ± 427.4 | <0.0001* |
| Apgar (median ± SD) | ||||
| 1-minute | 6 (4,8) | 6 (4, 8) | 4 (2, 6) | <0.0001* |
| 5-minute | 8 (7, 9) | 8 (7, 9) | 6 (4, 8) | <0.0001* |
| Clinical | ||||
| Maternal | ||||
| Hypertension | 93 (11.3%) | 82 (11.6%) | 11 (9.3%) | 0.47 |
| Pre-eclampsia | 158 (19.2%) | 148 (20.9%) | 10 (8.5%) | 0.001* |
| Intrauterine growth restriction° | 75 (9.1%) | 65 (9.2%) | 10 (8.5%) | 0.80 |
| Prenatal Medications | ||||
| NSAIDs | 45 (5.5%) | 38 (5.4%) | 7 (5.9%) | 0.81 |
| Steroids | 578 (70.1%) | 494 (69.9%) | 84 (71.2%) | 0.77 |
| Neonatal | ||||
| Resuscitation | ||||
| O2 and/or Positive Pressure Ventilation | 642 (77.8%) | 538 (76.1%) | 104 (88.1%) | 0.004* |
| CPR, Epinephrine, and/or Bolus* | 58 (7.0%) | 34 (4.8%) | 24 (20.3%) | <0.0001* |
| Medications | ||||
| Vasopressor support¥ | 97 (11.7%) | 63 (8.9%) | 34 (28.8%) | <0.0001* |
| Nephrotoxic Medication† | 681 (82.5%) | 572 (80.9%) | 109 (92.4%) | 0.002* |
| Discharge | ||||
| Necrotizing Enterocolitis (NEC) | 0.001* | |||
| No NEC | 769 (93.2%) | 667 (94.3%) | 102 (86.4%) | |
| NEC | ||||
| Medical Management | 29 (3.5%) | 23 (3.3%) | 6 (5.1%) | |
| Surgical Management | 27 (3.3%) | 17 (2.4%) | 10 (8.5%) | |
| Respiratory Support at 7 days | <0.0001* | |||
| None | 381 (46.2%) | 352 (49.8%) | 29 (24.6%) | |
| Non-invasive (Oxyhood, Nasal Canula, CPAP) | 131 (15.9%) | 81 (11.4%) | 50 (42.4%) | |
| Invasive (Conventional Vent, HFOV, ECMO) € | 313 (37.9%) | 274 (38.8%) | 39 (33.0%) | |
| Respiratory Support at 28 days | <0.0001* | |||
| None | 523 (63.4%) | 483 (68.3%) | 40 (33.9%) | |
| Non-invasive (Oxyhood, Nasal Canula, CPAP) | 96 (11.6%) | 63 (8.9%) | 33 (28.0%) | |
| Invasive (Conventional Ventilation, HFOV, ECMO) € | 206 (25.0%) | 161 (22.8%) | 45 (38.1%) |
Bolus: Normal saline or packed red blood cells
Vasopressor Support in 1st week of life: Dopamine, Dobutamine, Milrinone, Norepinephrine, Epinephrine
Nephrotoxic: Acyclovir, Amphotericin B, Aminoglycosides, Piperacillin/Tazobactam, Vancomycin
HFOV: High frequency oscillation ventilation, ECMO: extracorporeal membrane oxygenation
4.b. Acute Kidney Injury
AKI was documented in 183/825 (22.2%) infants (n=90 Stage 1, n=39 Stage 2, and n=54 Stage 3). There was no difference in race/,gender, or maternal characteristics between infants with and without AKI (Table 1). Significant differences between infants with AKI and without AKI are shown in Table 1, (all p<0.05).
4.c. Intraventricular Hemorrhage
IVH was found in 14.3% (118/820) of infants (n=49 Grade 1, n=29 for Grade 2, n=16 for Grade 3, and n=24 for Grade 4). There was no difference in race/,gender, or maternal characteristics between infants with and without IVH; the exception being infants born to mothers with pre-eclampsia were less likely to have IVH (Table 2). Significant differences between infants with and without IVH are shown in Table 2 (all p<0.05).
4.d. The Association of Acute Kidney Injury and Intraventricular Hemorrhage
The association between IVH grades and AKI stages shows that the overwhelming number of infants in the cohort had neither AKI nor IVH (69.5%). Those with AKI (n=183) were more likely to have IVH than those without AKI (n=642) who had IVH [26.8% (49/183) vs 10.7% (69/642), p<0.0001] (Table 3). Among those that did have AKI and IVH, those with AKI Stage 1 had the greatest number of infants with either IVH grade 1 or 4 [8.9% (8/90) and 10.0% (9/90), respectively]. Infants with AKI stage ≥2 had higher number of infants with IVH grade 2 and 4 [8/93 (8.6%) and 9/93 (9.7%), respectively]. However, no dose-dependency could be demonstrated when comparing AKI 1 vs AKI ≥2 (Table 3).
Table 3.
Association between IVH grades and AKI stages
| AKI (n=183)† | |||||
|---|---|---|---|---|---|
| No AKI (n=642) | AKI Stage 1 (n=90) |
AKI Stage ≥2 (n=93) |
|||
| IVH (n=118) | p-value | p-value | |||
| None | 573 (89.3%) | <0.0001* | 68 (75.6%) | 66 (71.0%) | 0.46** |
| Grade 1 | 34 (5.3%) | 8 (8.9%) | 7 (7.5%) | ||
| Grade 2 | 19 (3.0%) | 2 (2.2%) | 8 (8.6%) | ||
| Grade 3 | 10 (1.6%) | 3 (3.3%) | 3 (3.2%) | ||
| Grade 4 | 6 (0.9%) | 9 (10.0%) | 9 (9.7%) | ||
AKI definition based on serum creatinine criteria and/or urine output (UOP) criteria
No AKI vs Any AKI
AKI Stage 1 vs AKI Stage ≥2
The results of the ordinal logistic model for the study population overall suggests that infants with AKI were over 3 times as likely to have a higher grade IVH than those infants without AKI (OR 3.26, 95% CI 2.18–4.88) (Table 4). This associated remained after adjusting for 5-minute Apgar, vasopressive support within the first week of life, and GA (OR 1.63, 95% CI 1.04–2.56). Though the increased association was only significant for those infants with a GA of 22–28 weeks (adjusted OR 1.87, 95% CI 1.08–3.23), the association between AKI and IVH was not statistically different by GA (p=0.4031), suggesting no effect modification by GA.
Table 4.
Crude and adjusted*odds ratios (ORs) and associated confidence intervals (CIs) for the association between acute kidney injury (AKI) and intraventricular hemorrhage (IVH) grade, overall and stratified by gestational age (GA)
| IVH Grade |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
p-value |
|---|---|---|---|
| OVERALL | 3.26 (2.18–4.88) | 1.63 (1.04–2.56) | 0.0347 |
| GESTATIONAL AGE | |||
| 22–28 weeks | 2.28 (1.35–3.85) | 1.87 (1.08–3.23) | 0.0260 |
| 29–35 weeks | 1.74 (0.79–3.80) | 1.22 (0.53–2.82) | 0.6431 |
Odds Ratios (ORs) were estimated from an ordered logistic regression and adjusted for 5-minute Apgar, vasopressive support within 1st week of life, and (for overall model only) gestational age
5. Discussion/Conclusion
It has been shown that AKI occurs commonly in critically ill neonates and is associated with adverse outcomes. In this secondary analysis from the AWAKEN study, we demonstrate an independent association between AKI and IVH in premature neonates. After controlling for multiple confounders, preterm infants with AKI, compared to those without AKI, had 1.6 increased odds of IVH.
This study expands our understanding of the association between AKI and IVH previously presented in a single-center cohort which demonstrated that infants with AKI had higher hazard ratios of grade 2 and 3 IVH [17]. However, it was unable to demonstrate an overall association for lower grades of IVH. In contrast, this current analysis, utilizing a larger sample size, found that even low grades of AKI are associated with any degree of IVH. To our knowledge, this is the first study to demonstrate this relationship.
Premature infants are at risk for the development of both AKI and IVH, with multiple overlapping risk factors including lower GA, BW, and Apgar scores[5,15]. It is currently unclear if AKI causes IVH, or if this association is due to a concurrent physiologic insult. However, multiple potential mechanisms exist to support a direct effect of AKI on IVH. As the kidney has an important role in blood pressure regulation, infants with an acute kidney injury may be predisposed to develop IVH [11,13,14,20]. Because IVH commonly originates in the germinal matrix, an area with high cerebral vascular density, disturbances in cerebral blood flow are commonly the trigger [10–12]. The majority of IVH events occur during the first six hours after birth-and are thought to be due to lower cerebral blood flow [21,22]. Perhaps more importantly, fluctuating cerebral blood-flow velocity, when compared to stable blood flow, increase the incidence and severity of IVH [11,20]. It is possible that these acute cerebral blood pressure fluctuations could be due to poor renal blood pressure regulation, which was not examined in these studies. Additionally, data demonstrates that changes in blood pressure alters renin production in the kidney. Post-mortem studies from premature infants with twin-to-twin transfusion syndrome have shown that infants have hypo- and hyperperfusion of the “donor” and “recipient” twins, respectively, with correlating differences in renin-staining kidney cells[16]. These alterations in renin production have a cascade effect in the RAAS, causing peripheral vasoconstriction via angiotensin II, which ultimately leads to alterations in blood flow.
The association between AKI and IVH may be mediated by an inflammatory cascade reaction following an AKI. Known modulators of cerebral blood flow include prostaglandins and the cyclooxygenase 2 (COX-2) system, the latter of which is induced by various factors including hypotension and other inflammatory markers [23]. Using a murine model, Liu et al. observed that AKI secondary to renal ischemic injury had a significant inflammatory effect on the brain. Increases in brain vascular permeability and elevations of cerebral and systemic pro-inflammatory markers, including kidney IL-1B and IL-6, were found in the brain secondary to AKI[14,24]. The group tested the same hypothesis in a liver ischemic injury model but did not find the same brain inflammatory markers. These studies support the brain-kidney crosstalk that could contribute to IVH in the presence of AKI.
In light of this discussion, the current results should be viewed with certain strengths and limitations. First, the secondary analysis was performed from a large, multinational study which allowed controlling for multiple confounders. Additionally, it allowed increased generalizability in comparison to our previous single-center study. Despite these strengths, we acknowledge potential limitations. First, because the physiology of IVH is so closely related to the immature cerebral vasculature it is very tightly associated with GA and was considered both an effect modifier and confounding factor. For this reason, GA was included in the overall model only. Second, the number and timing of the SCr, UOP, and HUS measurements were not protocol-based and instead relied on clinical discretion which varied by study center. Third, the outcome definition of IVH relied on radiologist interpretation of HUS. Since there was no single radiologist, HUS could be interpreted differently and that would affect the diagnosed IVH grade resulting in our reported associations being biased towards the null and not support an association. The associations may still be biased by residual confounding caused by unknown factors present in infants with IVH, particularly for higher IVH grades. Particularly, perinatal events post-natal resuscitation efforts that were not included in the cohort dataset. Lastly, although we used the most contemporary definition of AKI in the neonate, it remains a debated definition which should be validated and possibly refined.
With this investigation, we present the first multicenter evaluation of the association between AKI and IVH in premature infants. It can be debated that those infants who are severely ill are predisposed to both AKI and IVH. Nevertheless, this study demonstrates at minimum a significant relationship between AKI and IVH. More comprehensive studies are needed to clarify the timing of AKI and IVH to examine causality. As the majority of IVHs occurs during the first 3–5 days of life, a prospective study evaluating daily SCr and UOP in addition to non-invasive evaluation of cerebral and renal blood flow measures (e.g. NIRS technology) and protocolized assessments of HUSs could provide detailed, real-time data on these physiologic changes. Lastly, detailed investigation of the RAAS during AKI should be considered. These studies could yield potential strategies to reduce or prevent AKI and subsequently decrease the burden of IVH in this vulnerable population.
6.1. Acknowledgements
The authors would like to thank Krysta Smith (Department of Pediatrics, University of Alabama at Birmingham) for help with technical editing and proofreading of this manuscript. The authors would also like to thank the outstanding work of the following clinical research personnel and colleagues for their involvement in AWAKEN:
Ariana Aimani, Samantha Kronish, Ana Palijan, MD, Michael Pizzi — Montreal Children’s Hospital, McGill University Health Centre, Montreal, Quebec, Canada; Laila Ajour, BS, Julia Wrona, BS — University of Colorado, Children’s Hospital Colorado, Aurora, Colorado, USA; Melissa Bowman, RN — University of Rochester, Rochester, New York, USA; Teresa Cano, RN, Marta G. Galarza, MD, Wendy Glaberson, MD, Aura Arenas Morales, MD, Denisse Cristina Pareja Valarezo, MD — Holtz Children’s Hospital, University of Miami, Miami, Florida, USA; Sarah Cashman, BS, Madeleine Stead, BS — University of Iowa Children’s Hospital, Iowa City, Iowa, USA; Jonathan Davis, MD, Julie Nicoletta, MD, Joshua Dowers, MD — Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts, USA; Alanna DeMello — British Columbia Children’s Hospital, Vancouver, British Columbia, Canada; Lynn Dill, RN — University of Alabama at Birmingham, Birmingham, Alabama, USA; Ellen Guthrie, RN — MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio, USA, Nicholas L. Harris, BS, Susan M. Hieber, MSQM — C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, Michigan, USA; Katherine Huang, Rosa Waters — University of Virginia Children’s Hospital, Charlottesville, Virginia, USA; Judd Jacobs, Ryan Knox, BS, Hilary Pitner, MS, Tara Terrell — Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Nilima Jawale, MD — Maimonides Medical Center, Brooklyn, New York, USA; Emily Kane — Australian National University, Canberra, Australia; Vijay Kher, DM, Puneet Sodhi, MBBS — Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Grace Mele — New York College of Osteopathic Medicine, Westbury, New York, USA; Patricia Mele, DNP — Stony Brook Children’s Hospital, Stony Brook, New York, USA; Charity Njoku, Tennille Paulsen, Sadia Brook, New York, USA; Charity Njoku, Tennille Paulsen, Sadia Zubair — Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA; Emily Pao — University of Washington, Seattle Children’s Hospital, Seattle, Washington, USA; Becky Selman RN, Michele Spear, CCRC — University of New Mexico Health Sciences Center Albuquerque, New Mexico, USA; Melissa Vega, PA-C — The Children’s Hospital at Montefiore, Bronx, New York, USA); Leslie Walther RN — Washington University, St. Louis, Missouri, USA.
For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: David J Askenazi serves on the speaker board for Baxter (Baxter, USA), and the Acute Kidney Injury (AKI) Foundation (Cincinnati,OH, USA); he also receives grant funding for studies not related to this manuscript from National Institutes of Health — National Institutes of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK, R01 DK103608 and NIH-FDA (R01 FD005092). Jennifer G Jetton is supported by the University of Iowa Institute for Clinical and Translational Sciences NIH U54TR001356. Juan C. Kupferman is on the speaker’s Bureau and consultant for Alexion Pharmaceuticals. Robert Woroniecki is supported by the Department of Pediatrics at Stony Brook Children’s hospital (NY, USA). AWAKEN investigators at the Canberra Hospital were supported by the Canberra Hospital Private Practice fund, and investigators at University of Virginia Children’s Hospital were supported by a 100 Women Who Care Grant (100 Women Charitable Foundation, CA, USA).
6.4. Funding Sources
Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the AWAKEN Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) provided support for web meetings, for the NKC steering committee annual meeting at the University of Alabama at Birmingham (UAB), as well as support for some of the AWAKEN investigators at UAB (LBJ, RJG). PICAN is part of the Department of Pediatrics at the University of Alabama at Birmingham (UAB), and is funded by Children’s of Alabama Hospital, the Department of Pediatrics, UAB School of Medicine, and UAB’s Center for Clinical and Translational Sciences (CCTS, NIH grant UL1TR001417). Finally, the AWAKEN study at the University of New Mexico was supported by the Clinical and Translational Science Center (CTSC, NIH grant UL1TR001449) and by the University of Iowa Institute for Clinical and Translational Science (U54TR001356).
Funding sources for this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Statement of Ethics
The University of Alabama at Birmingham Institutional Review Board (IRB) approved this collaborative study, and each center received approval from their respective IRBs.
Disclosure Statement
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication.
7. References
- 1.Viswanathan S, Manyam B, Azhibekov T, Mhanna MJ: Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol 2012;27:303–311. [DOI] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N: Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol 2009;24:991–997. [DOI] [PubMed] [Google Scholar]
- 3.Jetton J, Boohaker L, K Sethi S, Wazir S, Rohatgi S, Soranno D, Chishti A, Woroniecki R, Mammen C, Swanson J, Sridhar S, Wong C, Kupferman J, Griffin R, Askenazi D: Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. The Lancet Child & Adolescent Health 2017;1:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmody JB, Swanson JR, Rhone ET, Charlton JR: Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 2014;9:2036–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL: Neonatal Acute Kidney Injury. Pediatrics 2015;136:e463–473. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar S, Askenazi DJ, Jordan BK, Bhagat I, Bapuraj JR, Dechert RE, Selewski DT: Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res 2014;75:431–435. [DOI] [PubMed] [Google Scholar]
- 7.Pickering JW, Blunt IRH, Than MP: Acute Kidney Injury and mortality prognosis in Acute Coronary Syndrome patients: A meta-analysis. Nephrology (Carlton) 2018;23:237–246. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD, Network EKSNIoCHaHDNR: Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M: Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 2005;115:997–1003. [DOI] [PubMed] [Google Scholar]
- 10.Volpe JJ: Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol 1998;5:135–151. [DOI] [PubMed] [Google Scholar]
- 11.Perlman JM, Goodman S, Kreusser KL, Volpe JJ: Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med 1985;312:1353–1357. [DOI] [PubMed] [Google Scholar]
- 12.Ballabh P: Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol 2014;41:47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yap SC: Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence.; in HT L (ed), Anesthesiology, 2012, 116, pp 1139–1148. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Chien CC, Grigoryev DN, Gandolfo MT, Colvin RB, Rabb H: Effect of T cells on vascular permeability in early ischemic acute kidney injury in mice. Microvasc Res 2009;77:340–347. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM: Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol 2009;26:419–424. [DOI] [PubMed] [Google Scholar]
- 16.Mahieu-Caputo D, Dommergues M, Delezoide AL, Lacoste M, Cai Y, Narcy F, Jolly D, Gonzales M, Dumez Y, Gubler MC: Twin-to-twin transfusion syndrome. Role of the fetal renin-angiotensin system.Am J Pathol 2000;156:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoops C, Sims B, Griffin R, Askenazi DJ: Neonatal Acute Kidney Injury and the Risk of Intraventricular Hemorrhage in the Very Low Birth Weight Infant. Neonatology 2016;110:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, Selewski DT, Abitbol CL, Kaskel FJ, Mhanna MJ, Ambalavanan N, Charlton JR, Collaborative NK: Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a Retrospective Cohort Study. Front Pediatr 2016;4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jetton JG, Askenazi DJ: Update on acute kidney injury in the neonate. Curr Opin Pediatr 2012;24:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlman JM, McMenamin JB, Volpe JJ: Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med 1983;309:204–209. [DOI] [PubMed] [Google Scholar]
- 21.Al-Abdi SY, Al-Aamri MA: A Systematic Review and Meta-analysis of the Timing of Early Intraventricular Hemorrhage in Preterm Neonates: Clinical and Research Implications. J Clin Neonatol 2014;3:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ment LR, Duncan CC, Ehrenkranz RA, Lange RC, Taylor KJ, Kleinman CS, Scott DT, Sivo J, Gettner P: Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J Pediatr 1984;104:419–425. [DOI] [PubMed] [Google Scholar]
- 23.McCrea HJ, Ment LR: The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol 2008;35:777–792, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M: Acute kidney injury leads to inflammation and functional changes in the brain; in Y L (ed), Journal of American Society of Nephrology, 2008, 19, pp 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]


