Abstract
The advent and wide-spread adoption of electric lighting over the past century has profoundly affected the circadian organization of physiology and behavior for many individuals in industrialized nations; electric lighting in homes, work environments, and public areas has extended daytime activities into the evening, thus, increasing night-time exposure to light. Although initially assumed to be innocuous, chronic exposure to light at night (LAN) is now associated with increased incidence of cancer, metabolic disorders, and affective problems in humans. However, little is known about potential acute effects of LAN. To determine whether acute exposure to low level LAN alters brain function, adult male and female mice were housed in either light days and dark nights (LD; 14h of 150 lux:10 h of 0 lux) or light days and low level light at night (LAN; 14h of 150 lux:10 h of 5 lux). Mice exposed to LAN on three consecutive nights increased depressive-like responses compared to mice housed in dark nights. Additionally, female mice exposed to LAN increased central tendency in the open field. LAN was associated with reduced hippocampal vascular endothelial growth factor-A (VEGF-A) in both male and female mice, as well as increased VEGFR1 and interleukin-1β mRNA expression in females, and reduced brain derived neurotrophic factor mRNA in males. Further, LAN significantly altered circadian rhythms (activity and temperature) and circadian gene expression in female and male mice respectively. Altogether, this study demonstrates that acute exposure to LAN alters brain physiology and can be detrimental to wellbeing in otherwise healthy individuals.
INTRODUCTION
Circadian rhythms are adaptations to the predictable daily cycles of alternating light and darkness under which life on Earth evolved. Indeed, the synchronization of vital biological and behavioral processes is crucial for achieving and maintaining optimal health and wellness. Among humans, light is a powerful zeitgeber (“time giver” or environmental cue), entraining endogenous circadian rhythms to the solar day. Consequently, disruption of circadian rhythms has become increasingly common in industrialized countries over the past century as the use of artificial lighting and electric devices at night have been widely adopted. Indeed, prior to the invention of electric lights, most humans were exposed to minimal light at night. For example, a full moon on a cloudless night typically provides <1 lux of light1. By comparison, nighttime light intensities on urban streets in industrialized countries are typically ~5–15 lux, and as a result of modernization it is estimated that greater than 80% of the World’s population and 99% of individuals in the United States and Europe live among light pollution2. Additionally, there has been a profound shift in indoor nighttime light exposure; whereas a single candle flame casts ~11 lux of light on an object one foot away, nighttime lighting in a typical, modern living room is between 100–300 lux1. Many humans are exposed to additional sources of light at night, including appliances, television, computer screens, smart phones, and tablets. Thus, during the past 130 years, there has been a dramatic increase in exposure of people to light at night (LAN), in terms of both the intensity of the light and the duration of exposure past sunset.
Although widely assumed to be innocuous, the detrimental consequences of chronic exposure to LAN are becoming increasingly apparent3. Indeed, long-term exposure to artificial LAN is linked to increased risk of cancer (breast and prostate), metabolic disorders, cardiovascular disease, and psychiatric disorders in humans4–10. The reported clinical effects are remarkably consistent across studies and several aspects of these conditions have been recapitulated in rodent models of chronic LAN exposure11–17.The majority of these studies report adverse health outcomes precipitated by weeks to years of LAN exposure. Given the unexplained rise in major depressive disorder (MDD) among people living in industrialized countries and the enormous escalation in night time light exposure that has occurred over the past century, it is important to understand the extent to which LAN and mood are linked3. Previous studies examining the effects of chronic (3–8 weeks) LAN exposure on affective behavior in rodents have demonstrated that LAN alters hippocampal neuroplasticity by reducing spine density and dendritic length, thus provoking a depressive-like response15,16,18,19. Additionally, these studies have suggested that increased neuroinflammation and reduced hippocampal neurotrophin expression underlie these changes in hippocampal circuitry. Increased neuroinflammation and reduced hippocampal neurotrophin expression have long been associated with depressive-like behavior20–23. Specifically, brain-derived neurotrophic factor (BDNF), insulin-like growth factor (IGF), and vascular endothelial growth factor (VEGF) modulate neuronal plasticity23. Indeed, infusion or overexpression of BDNF increased neuronal complexity and neurogenesis within the hippocampus24,25 and upregulation of BDNF has been shown to be necessary for the action of anti-depressants26,27. Further, administration of anti-depressants is associated with increases in IGF and VEGF within the hippocampus and similar effects on neurogenesis and neuronal plasticity have been reported28–33. Additionally, increased neuroinflammation reduces neurogenesis and neuronal complexity within the hippocampus34–36. Thus, the current experiment sought to examine the acute effects (up to 4 nights) of 5 lux of LAN on the expression of depressive-like behavior in male and female mice, as well as measures of neuroinflammation and neurotrophin expression within the hippocampus. Specifically, we hypothesized that altered neurotrophin expression and/or neuroinflammation are associated with depressive-like behavior in mice exposed to acute low level light at night.
MATERIALS AND METHODS
Mice and Lighting Conditions.
The experiments used adult (> 8weeks) female and male Swiss Webster mice (Charles River Laboratories, MA). Upon arrival in the vivarium, the mice were acclimated to LD conditions (14 h 150 lux light: 10 h dark 0 lux) for one week prior to any experimental manipulation. A subset of mice was randomly selected by block randomization in excel, and then transferred to the low level light at night (LAN) condition (14 h 150 lux light: 10 h dark 5 lux) while the remainder remained in LD. Sample sizes were determined based on previous studies examining the effects of chronic LAN exposure. Low level light at night was supplied using standard LUMA5 LED light strips (Hitlights Inc; 1.5W/ft, 5000K “cool white”, 1200 lumens). Cages were placed equidistant from the light strip and light levels were measured inside each cage, from the center, with the light meter facing upward to ensure ~5 lux of light exposure. Behavioral testing occurred during the light phase following three nights of LAN and consisted of five cohorts of mice (female LD n=31, female LAN n=31; male LD n=31, male LAN n=31). Tissue collection occurred in separate cohorts during the subsequent light phase and included processing for ELISA (VEGF-A; female LD n=15, female LAN n=15; male LD n=15, male LAN n=15) and RT-qPCR (female LD n=15, female LAN n=15; male LD n=15, male LAN n=15). An additional cohort of mice were implanted with a wireless telemetry device (G2 E-Mitter; Star Life Science, Oakmont, PA) to assess activity and body temperature rhythms throughout the experiment (female LD n=7, female LAN n=8; male LD n=9, male LAN n=8). Further, a final cohort of mice was used to assess circadian gene expression within the hippocampus, as well as serum corticosterone concentrations at 4 time points throughout the day; mice were placed in their randomly assigned lighting conditions and tissue was collected at six hour intervals starting at ZT14 on day three and concluding at ZT8 on day four (female LD n=8, female LAN n=8; male LD n=8, male LAN n=8 per time point). Throughout the experiment, the mice had ad libitum access to food and filtered water. All experiments were performed in accordance with NIH Animal Welfare guidelines and were approved The Ohio State University and West Virginia University Institutional Animal Care and Use Committee. Behavioral testing was scored by an independent blinded investigator. Additionally, investigators processing tissues for ELISA and PCR were blinded to groups until data analysis.
Behavioral Testing.
Behavioral testing (16 mice/group) was performed during the light phase from ZT5-ZT8 following the third night of LAN exposure so that mice from all of the groups would be tested under the same lighting parameters. The tasks were performed in a fixed consecutive order (open field, elevated plus maze, then forced swim test). Approximately 30 min elapsed between tests. The behavioral testing occurred for four cohorts tested on separate days with equal representation from all experimental groups. The individual collecting and scoring the behavioral data was unaware of group assignment.
The mice were tested for novelty-induced locomotor activity in the open field, the elevated plus maze to assess anxiety-like behavior, and the forced swim test to assess depressive-like behavior. The open field test (30 min) was performed in an enclosed polypropylene insert (36 cm × 36 cm × 36 cm) with two rows of infrared sensors to detect horizontal and vertical movement (Photobeam Activity System, San Diego Instruments Inc., CA). Total activity, center versus peripheral activity, and number of rears were recorded. Next, the mice were tested in a 5-min elevated plus maze (EPM) task; it uses a plus-shaped apparatus, elevated a meter above the floor, with two enclosed arms and two open arms (112 cm each). The mouse was placed in the center of the apparatus and the number of entries and time spent on each arm type was recorded. Lastly, mice were tested in the forced swim test (FST); mice were placed for 5 min in a 5000 ml beaker filled with 3500 ml of water maintained at 27±1 °C. Latency to float, time spent floating, and number of floating bouts were determined. Behavior in the EPM and FST was recorded and later scored using The Observer XT 8.0 software (Noldus, Leesburg, VA). Additionally, a fifth cohort of mice (female LD n=15, female LAN n=15; male LD n=15, male LAN n=15) underwent sucrose preference testing to further assess depressive-like behavior. Sucrose preference testing was similar to previously described12. To allow for habituation to the modified water bottles, mice were presented with two modified water bottles each containing tap water from ZT14-ZT19 for three consecutive nights prior to placing mice in LAN. At the start of the third night of LAN (ZT14) mice were presented with two modified water bottles for 5 hr, one containing a 3% sucrose solution and the other containing tap water. The modified water bottles were weighed prior to and after the 5 hr testing period. Sucrose preference was determined using the following formula (amount of sucrose consumed/total amount of liquid consumption *100). Notably, sucrose preference occurred during the dark phase as mice typically only consume water and food during the dark phase.
VEGF ELISA.
Protein Extraction and ELISA followed a protocol previously described37,38. Once extracted hippocampi were placed in a 1.5 ml tube containing a solution of RIPA buffer (Thermo Fisher Scientific, Waltham, MA) and Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA) at a concentration of 1ml/100mg of tissue. Next hippocampi were homogenized via sonication and allowed to sit on ice for 30 min. After 30 min, samples were centrifuged at 13,300 rpm for 15 min at 4°C. The supernatant was removed and placed in a new 1.5 ml tube. Next, a Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA) was performed according to the manufacturer’s instructions to determine the protein concentration in each sample and to ensure for equal amounts of protein load during the subsequent ELISA. To determine the concentration of VEGF-A in each hippocampus (350μg of protein), a mouse VEGF ELISA (MMV00, R&D Systems, Minneapolis, MN) was performed according to the manufacturer’s instructions (assay range 7.8–500 pg/ml); samples were run in duplicate. The plates were read using a SPECTRAmax Plus plate reader (Molecular Devices, San Jose, CA).
Serum Corticosterone.
Serum corticosterone was determined using an enzyme immunoassay kit (K014-H5, Arbor Assays, Ann Arbor, MI) according to the manufactures instructions. Serum samples were diluted (1/100) in assay buffer. Plates were read using a SPECTRAmax Plus plate reader (Molecular Devices, San Jose, CA) at 450nm and concentrations were determined using a 4-parameter logistic curve.
RNA extraction, cDNA, and qRT-PCR.
RNA extraction, cDNA, and qRT-PCR followed a protocol previously described38. RNA was extracted from hippocampi using Trizol Reagent according to the manufacturer’s instructions (Ambion, Waltham, MA). RNA quantity and quality was assessed using a spectrophotometer (Nanodrop One, Wilmington, DE) and then cDNA was synthesized using M-MLV reverse transcriptase and diluted 1:10 for subsequent analysis via PCR. For qRT-PCR, 4μl (40μg) of diluted cDNA was combined with 16μl of master mix solution containing: Taqman Fast Advanced Master Mix (Life Technologies, Carlsbad, CA), an inventoried probe from Applied Biosystems (Life Technologies, Carlsbad, CA) (Table 1), a primer-limited probe for the endogenous control GAPDH, and water. Each sample was run in duplicate. The 2-step real-time PCR cycling conditions used were 95 °C for 20 s, 40 cycles of 95 °C for 3 s, and then 60 °C for 30 s. Gene expression was quantified using the Pfaffl Method39.
Table 1:
Gene names and assay ids for TaqMan primer/probes used throughout the experiment
| Gene Name | Assay ID |
|---|---|
| il-1β | Mm00434228_m1 |
| tnfα | Mm00443258_m1 |
| bdnf | Mm01334044_m1 |
| igf1 | Mm00439560_m1 |
| flt1 (vegfr1) | Mm00438980_m1 |
| kdr (vegfr2) | Mm01222421_m1 |
| clock | Mm00455950_m1 |
| arntl (bmal1) | Mm00500226_m1 |
| per1 | Mm00501813_m1 |
| per2 | Mm00478099_m1 |
| cry1 | Mm00514392_m1 |
| cry2 | Mm01331539_m1 |
| nr3c1 (GR) | Mm00433832_m1 |
| nr3c2 (MR) | Mm01241596_m1 |
| nr1d1 (rev-erbα) | Mm00520708_m1 |
| Gapdh | Mm99999915_g1 |
Telemetry Implantation.
Following a one week recovery period from shipping, mice were deeply anesthetized and implanted with a wireless telemetry device (G2 E-Mitter; Star Life Science, Oakmont, PA). A small incision (~8 mm) was made in the abdominal flank and peritoneum to allow for insertion of the E-Mitter into the abdominal cavity. Following insertion, the peritoneum and skin were sutured to insure containment within the abdominal cavity. After surgery mice were singly housed and allowed one week to recover before being placed in the appropriate lighting conditions. To assess body temperature and activity counts, mouse cages were placed on top of receiver boards (ER-4000) on static racks in the appropriate lights conditions. These boards relayed the data to a computer running VitalView Telemetry Software version 5.1 (Star Life Science, Oakmont, PA). Recording began at ZT6 on day 1 and concluded at ZT6 on day 4 of the experiment.
Statistical Analyses.
Outliers were removed a priori. An outlier was defined as having a within-group Z score greater than 2; no more than one outlier was removed from each group. The behavioral and tissue data (Figs 1–3; 4 D–F and J–L) were analyzed using a 2-Way ANOVA, with sex and lighting condition as independent factors. Post-hoc comparisons were made using Tukey’s multiple comparisons test. Gene expression data were collected at 4 time points throughout the day (Fig. 5); the data were analyzed using a 3-Way ANOVA with sex, time, and lighting condition as independent factors. Post-hoc comparisons were made using Tukey’s multiple comparisons test. Notably, each time point was treated as a separate family and multiple comparisons were only made within one timepoint. Differences were considered statistically significant when p<0.05. Data were tested for homoscedasticity using Spearman’s test for heteroscedasticity and normality was tested using the Shapiro-Wilk test. If data failed to meet the assumptions of an ANOVA, then data were first log2 transformed then square root transformed. If data failed to meet the assumptions of an ANOVA after being log2 and square root transformed, then multiple t-tests were run with a Bonferroni correction applied (0.05/number of t-tests run). For data analyzed using Bonferroni correction, p<0.008 was considered statistically significant (0.05/6 = 0.008). All statistical analyses were performed using GraphPad Prism 8.0 software. The data are graphed as mean ± SEM.
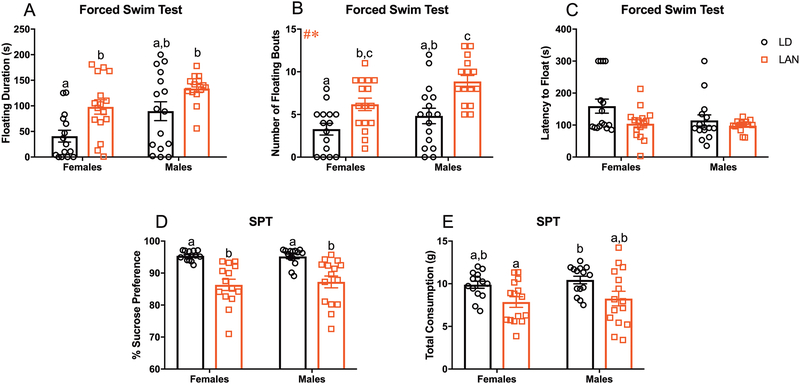
Figure 1:
Acute LAN Increases Depressive-like Behavior (A-C) Average time spent floating, number of floating bouts, and latency to float during forced swim test. (A) LAN significantly increased floating duration in females relative to LD (t= 3.133; p<0.008; Bonferroni correction)(F LD n=15; M LD n= 16; F LAN n=16; M LAN n=14). (B) Main effect of lighting and sex on the number of floating bouts (F1,58=20.63, p<0.001; F1,58=7.568, p<0.01); increased number of floating bouts in males and females housed in LAN relative to sex matched controls. (F LD n=15; M LD n= 16; F LAN n=16; M LAN n=15) (C) No group differences in latency to float (p>0.008 for all t-tests; Bonferroni correction) (F LD n=15; M LD n= 15; F LAN n=16; M LAN n=14). (D-E) Percent sucrose preference and total liquid consumption during sucrose preference testing. (D) LAN significantly decreased sucrose preference in males and female mice relative to their sex matched controls (males, t=3.893; p<0.001; females, t=5.043; p<0.0001; Bonferroni correction)) (F LD n=14; M LD n= 14; F LAN n=14; M LAN n=15) (E) LAN reduced total liquid consumption in females relative to males house in normal lighting condition (t=3.320; p<0.008; Bonferroni correction). No differences in total liquid consumption were detected within sexes (p>0.008 for all t-tests; Bonferroni correction) (F LD n=14; M LD n= 14; F LAN n=15; M LAN n=15) Error bars represent SEM; # main effect of lighting, * main effect of sex, + sex by lighting interaction; (B) two-way ANOVA; Tukey’s multiple comparisons test (A and C-E) multiple t-tests between each group with Bonferroni correction. Bars that do not share a letter represent Tukey’s multiple comparisons at p<0.05 or Bonferroni correction at p<0.008.
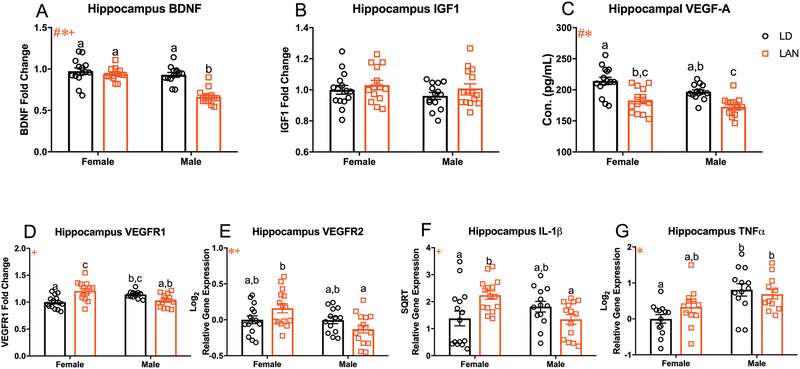
Figure 3:
Acute LAN Reduces Neurotrophic Signaling and Increases Neuroinflammation (A-C) Measure of neurotrophins in the hippocampus. (A) Main effect of lighting (F1,51=26.66, p<0.001), sex (F1,51=29.52, p<0.001), and sex by lighting interaction (F1,51=15.57, p<0.001) on bdnf expression in the hippocampus; LAN reduced bdnf expression in males relative to same sex controls. (F LD n=14; M LD n= 13; F LAN n=14; M LAN n=14) (B) No group differences in igf1 expression in the hippocampus. (F LD n=15; M LD n= 14; F LAN n=14; M LAN n=15) (C) Main effect of lighting (F1,53=34.32, p<0.001) and sex (F1,53=8.392, p<0.01) on concentration of VEGF-A in the hippocampus; decreased VEGF-A in males and females housed in LAN relative to sex matched controls. (F LD n=15; M LD n= 14; F LAN n=14; M LAN n=14) (D) Sex by lighting interaction in vegfr1 expression (F1,49=21.03, p<0.001); LAN increased the expression of vegfr1 in females relative to LD. (F LD n=14; M LD n= 13; F LAN n=13; M LAN n=13) (E) Main effect of sex (F1,54=7.078, p<0.05) and a sex by lighting interaction (F1,54=6.528, p<0.05) on vegfr2 expression in the hippocampus. (F LD n=15; M LD n= 13; F LAN n=15; M LAN n=15) (F) Sex by lighting interaction in il-1β expression in the hippocampus (F1,53=10.31, p<0.01); increased il-1β in females housed in LAN relative to LD. (F LD n=15; M LD n= 13; F LAN n=15; M LAN n=14) (G) Main effect of sex (F1,46=15.87, p<0.001) in the expression of tnf-α in the hippocampus. (F LD n=13; M LD n= 13; F LAN n=12; M LAN n=12) Error bars represent SEM; # main effect of lighting, * main effect of sex, + sex by lighting interaction; bars that do not share a letter represent multiple comparisons at p<0.05, two-way ANOVA; Tukey’s multiple comparisons test.
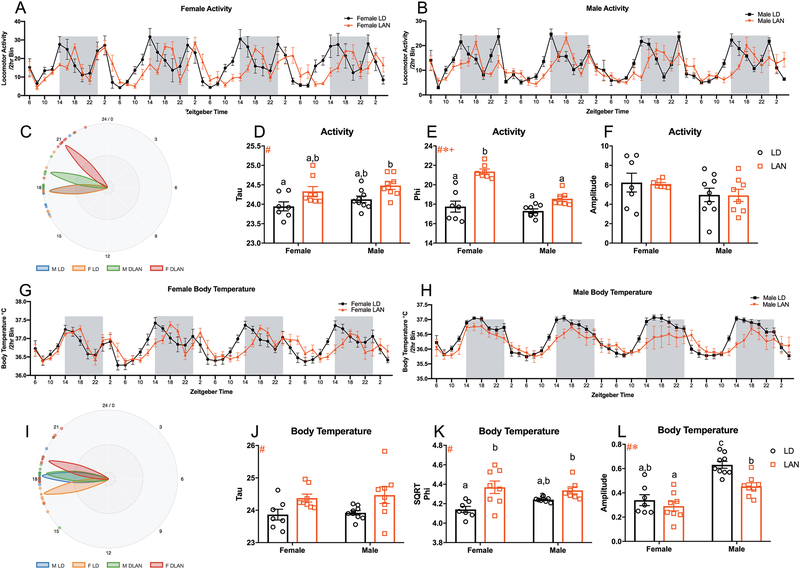
Figure 4:
Acute LAN Alters Activity and Body Temperature Rhythms (A-B) Activity rhythms in female and male mice exposed to LAN; 2 hr bins beginning at ZT6 on Day 1 and ending at ZT4 on Day 4. (C) Graphic illustrating the phi (phase) of activity rhythms for each group from CT0 (D) Main effect of lighting (F1,28=12.41, p<0.01) on tau (period) of activity rhythms. (F LD n=7; M LD n= 9; F LAN n=8; M LAN n=8) (E) Main effect of lighting (F1,25=48.16, p<0.0001), sex (F1,25=21.51, p<0.0001), and sex by lighting interaction (F1,25=11.24, p<0.01) on phi of activity rhythms; LAN increased phi in females relative to same sex controls. (F LD n=7; M LD n= 8; F LAN n=7; M LAN n=7) (F) No group differences in the amplitude of activity rhythms (F LD n=7; M LD n= 9; F LAN n=7; M LAN n=8) (G-H) Body temperature rhythms in female and male mice exposed to LAN; 2 hr bins beginning at ZT6 on Day 1 and ending at ZT4 on Day 4. (I) Graphic illustrating the phi (phase) of body temperature rhythms for each group from CT0 (J) Main effect of lighting (F1,28=12.41, p<0.01) on tau (period) of body temperature rhythms. (F LD n=7; M LD n= 9; F LAN n=8; M LAN n=8) (K) Main effect of lighting (F1,26=15.59, p<0.001) on phi (phase) of body temperature rhythms; female mice exposed to LAN demonstrate increased phi compared to female mice housed in LD. (F LD n=7; M LD n= 8; F LAN n=8; M LAN n=7) (L) Main effect of lighting (F1,28=10.23, p<0.01) and sex (F1,28=41.16, p<0.0001) on the amplitude of body temperature rhythms; LAN reduced the amplitude of body temperature rhythms in male mice relative to sex matched controls. (F LD n=7; M LD n= 9; F LAN n=8; M LAN n=8) Error bars represent SEM; # main effect of lighting, * main effect of sex, + sex by lighting interaction; bars that do not share a letter represent multiple comparisons at p<0.05, two-way ANOVA; Tukey’s multiple comparisons test.
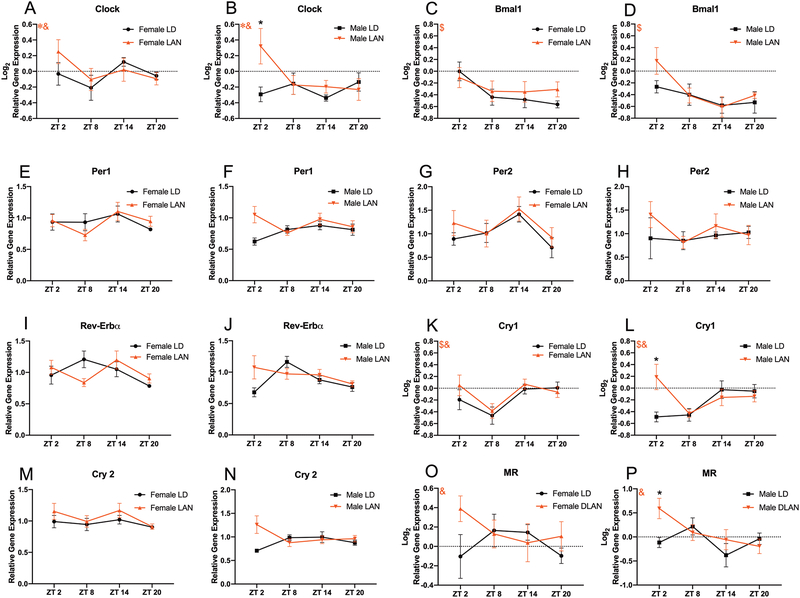
Figure 5:
Acute LAN Alters Hippocampal Circadian Gene Expression in Male Mice (A-N) Expression of circadian genes with the hippocampus throughout the day. (A-B) Main effect of sex (F1,105=4.486, p<0.05) and time by lighting interaction (F3,105=3.018, p<0.05) on the expression of clock within the hippocampus; male mice exposed to LAN demonstrated increased clock expression at ZT 2 relative to their sex matched controls. (F LD n=7–8; M LD n= 7–8; F LAN n=7–8; M LAN n=7–8 per time point) (C-D) Main effect of time (F3,108=15.07, p<0.001) on the expression of bmal1 within the hippocampus. (F LD n=7–8; M LD n= 7–8; F LAN n=7–8; M LAN n=7–8 per time point). (E-J) No groups differences in the expression of per1, per2, or rev-erbα (p>0.008 for all t-tests; Bonferroni correction) (F LD n=7–8; M LD n= 7–8; F LAN n=7–8; M LAN n=7–8 per time point). (K-L) Main effect of time (F3,106=8.378, p<0.0001) and time by lighting interaction (F3,106=3.480, p<0.05) on the expression of cry1 within the hippocampus; male mice exposed to LAN demonstrated increased cry1 expression at ZT 2 relative to their sex matched controls. (F LD n=7–8; M LD n= 7–8; F LAN n=7–8; M LAN n=7–8 per time point) (M-N) No groups differences in the expression of cry2 (p>0.008 for all t-tests; Bonferroni correction) (F LD n=7–8; M LD n= 7–8; F LAN n=6–8; M LAN n=7–8 per time point) (O-P) Time by lighting interaction (F3,104=2.983, p<0.05) on the expression of hippocampal nr3c2 (MR); male mice exposed to LAN increased MR expression at ZT2 compared to LD males. Error bars represent SEM; # main effect of lighting, * main effect of sex, + sex by lighting interaction, $ main effect of time, & time by lighting interaction; (A-D, K-L, and O-P) three-way ANOVA; Tukey’s multiple comparisons test (E-J and M-N) multiple t-tests between each group with Bonferroni correction. * represent Tukey’s multiple comparisons at p<0.05
RESULTS
Acute Exposure to Low Level LAN Alters Behavior.
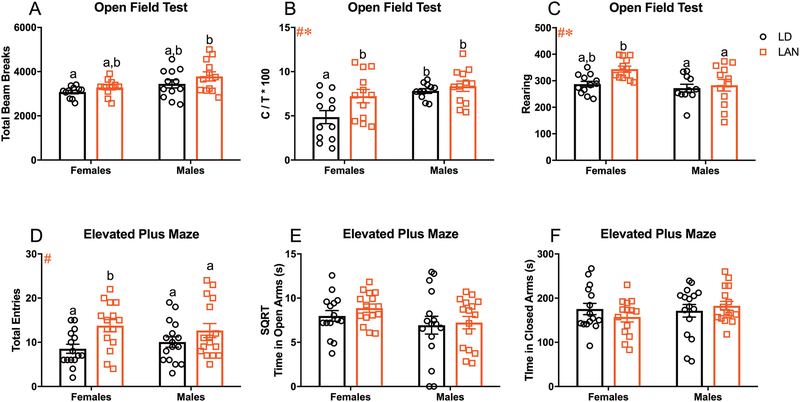
Because long term (3–4 weeks) exposure to LAN has previously been demonstrated to have detrimental effects on behavior12–15; we first sought to determine whether acute (3 nights) LAN exposure was sufficient to alter behavior in mice. Among females, LAN significantly increased floating duration relative to LD (t= 3.133; p<0.008; Bonferroni correction). In contrast, among males time spent floating was not significant different between groups (t=2.114; p=0.04; Bonferroni correction). There was a significant effect of both sex and lighting on number of floating bouts (F1,58=7.568, p<0.01; F1,58=20.63, p<0.001; Figure 1B) in the FST; specifically, LAN increased the number of floating bouts in males (p<0.01; Tukey’s multiple comparisons) and females (p<0.05; Tukey’s multiple comparisons) relative to their sex matched controls. However, there was no significant effect of LAN on the latency to float (p>0.008 for all t-tests; Bonferroni correction; Figure 1C). An additional cohort of mice underwent sucrose preference testing to further assess the effects of LAN on depressive-like behavior. LAN reduced sucrose preference in both females (t=5.043; p<0.0001; Bonferroni correction; Figure 1D) and males (t=3.893; p<0.001; Bonferroni correction; Figure 1D) relative to their sex matched controls. Additionally, there was a significant reduction in total consumption of liquid during sucrose preference testing in females exposed to LAN compared to males housed in normal lighting conditions (t=3.320; p<0.008; Bonferroni correction; Figure 1E). However, there were no significant differences among mice exposed to LAN compared to their sex match controls (p>0.008 for all t-tests; Bonferroni correction; Figure 1E). Together, these data demonstrate increased depressive-like behavior following acute exposure to LAN. In the open field, there was a significant increase in total locomotor activity of males housed in LAN compared to females housed in normal lighting conditions (t=3.051, p<0.008; Figure 2A). However, there were no significant differences in total locomotor activity among mice exposed to LAN compared to their sex match controls (p>0.008 for all t-tests; Bonferroni correction; Figure 2A). Additionally, there was a main effect of sex in central tendency, with males increasing time spent in the center (F1,43=10.42, p<0.01; Figure 2B), and rearing behavior, with females rearing more frequently than males (F1,43= 6.062, p<0.05; Figure 2C). Further, there was a significant effect of lighting on central tendency, with LAN increasing the time spent in the center of the open field (F=1,43=5.338, p<0.05; Figure 2B), and number of rears, with LAN increasing the number of rears (F=1,43=4.783, p<0.05; Figure 2C). Females exposed to LAN significantly increased the amount of time spent in the center of the open field relative to females housed in normal lighting conditions (p<0.05; Tukey’s multiple comparisons), demonstrating an anxiolytic effect of LAN in females. However, there were no significant differences among males. In the EPM, there was a significant effect of lighting on total number of arm entries (F1,58=8.709, p<0.01; Figure 2D); specifically, LAN significantly increased the number of arm entries among females (p<0.05; Tukey’s multiple comparisons). However, there were no group differences in time spent in the open or closed arms (p>0.05 Figure 2E and F).
Figure 2:
Anxiolytic Effect of LAN in Female Mice (A-C) Total beam break, central tendency, and rearing during open field testing. (A) Male mice exposed to LAN demonstrated a significant increase in total beam breaks compared to females housed in LD (t=3.051; p<0.01; Bonferroni correction. No differences in total beam breaks were detected within sexes (p>0.008 for all t-tests; Bonferroni correction) (F LD n=11; M LD n= 12; F LAN n=11; M LAN n=12) (B) Main effect of lighting (F=1,43=5.338, p<0.05) and sex (F1,43=10.42, p<0.01) on central tendency; females housed in LAN increased the time spent in the center relative to females housed in LD. (F LD n=12; M LD n= 11; F LAN n=12; M LAN n=12) (C) Main effect of lighting (F=1,43=4.783, p<0.05) and sex (F1,43= 6.062, p<0.05) on number of rears. (F LD n=12; M LD n= 11; F LAN n=12; M LAN n=12) (D-F) Total entries, time spent in the open arm, and time spent in the closed arm during elevated plus maze. (D) Main effect of lighting on total arm entries (F1,58=8.709, p<0.01); increased number of total arm entries in females relative to LD. (F LD n=15; M LD n= 16; F LAN n=15; M LAN n=16) (E and F) No group differences on time spent in the open or closed arms. (E) (F LD n=15; M LD n= 16; F LAN n=15; M LAN n=16) (F) (F LD n=15; M LD n= 16; F LAN n=14; M LAN n=16) Error bars represent SEM; # main effect of lighting, * main effect of sex, + sex by lighting interaction; (B-F) two-way ANOVA; Tukey’s multiple comparisons test (A) multiple t-tests between each group with Bonferroni correction. Bars that do not share a letter represent Tukey’s multiple comparisons at p<0.05 or Bonferroni correction at p<0.008.
Acute Exposure to Low Level LAN Reduces Hippocampal VEGF and BDNF and Increases IL-1β.
Considering that acute (3 nights) LAN exposure increased depressive-like behavior in mice, we next sought to examine the effects of acute LAN on the expression of neurotrophic factors and proinflammatory cytokines. There was a significant effect of lighting (F1,51=26.66, p<0.001; Figure 3A), sex (F1,51=29.52, p<0.001; Figure 3A), and a sex by lighting interaction (F1,51=15.57, p<0.001; Figure 3A) on the expression of brain derived neurotrophic factor (bdnf); specifically, LAN reduced hippocampal bdnf expression in males relative to all other groups (p<0.001; Tukey’s multiple comparisons). There were no group differences on the expression of insulin growth factor-1 (igf1) in the hippocampus (p>.05; Figure 3B). Additionally, there was a significant effect of lighting (F1,53=34.32, p<0.001; Figure 3C), and sex (F1,53=8.392, p<0.01; Figure 3C), on VEGF-A protein concentration in the hippocampus; specifically, LAN significantly reduced hippocampal VEGF-A protein concertation in both females and males relative to their same sex controls (p<0.001 and p<0.01, respectively; Tukey’s multiple comparisons). Further, there was an interaction between sex and lighting condition in the expression of VEGF Receptor 1 (vegfr1) (F1,49=21.03, p<0.001, Figure 3D) such that among females, but not males, LAN significantly increases expression of vegfr1 (p<.001; Tukey’s multiple comparisons). In contrast, there was a significant effect of sex (F1,54=7.078, p<0.05, Figure 3E) and a sex by lighting interaction (F1,54=6.528, p<0.05, Figure 3E) on vegfr2 gene expression. Additionally, two proinflammatory cytokines were also examined in the hippocampus; there was no main effect of either lighting or sex on interleukin-1β mRNA (il-1β), but there was a significant interaction (F1,53=10.31, p<0.01, Figure 3F) between these variables; specifically, among females LAN significantly increased il-1β expression relative to LD (p<0.05; Tukey’s multiple comparisons). Although there was no significant effect of lighting on tumor necrosis factor alpha (tnf-α), there was a significant main effect of sex (F1,46=15.87, p<0.001, Figure 3G), such that the concentration of tnf- α was significantly greater among males than females.
Acute Exposure to Low Level LAN Alters Activity Rhythms, Body Temperature Rhythms, and Circadian Gene Expression within the Hippocampus.
Considering that chronic exposure (2–4 weeks) to low level light at night is associated with changes in circadian rhythms and circadian gene expression15,16,40,41, we next sought to assess the effects of acute exposure to low level light at night on body temperature and activity rhythms, as well as circadian gene expression within the hippocampus. To determine the effects of LAN on body temperature and activity rhythms an mFourfit analysis was performed to calculate tau (τ; period), phi (Φ; phase), and amplitude of each group using BioDare2 analysis software42. There was a significant effect of lighting on period (τ), with LAN increasing τ length in activity rhythms (F1,28=12.41, p<0.01, Figure 4A–B and D). However, there were no significant differences in τ among mice exposed to LAN compared to their sex match controls (p>0.05; Tukey’s multiple comparisons; Figure 4A–B and D). Additionally, there was a significant effect of lighting (F1,25=48.16, p<0.0001; Figure 4A–B and E), sex (F1,25=21.51, p<0.0001; Figure 4A–B and E), and a sex by lighting interaction (F1,25=11.24, p<0.01; Figure 4A–B and E) on the phase (Φ) of activity rhythms; specifically, LAN delayed the acrophase of activity rhythms in female mice compared to all other groups (p<0.0001;Tukey’s multiple comparisons; Figure 4E). However, there were no group differences in the amplitude of activity rhythms (p>0.05; Figure 4A–B and F). Additionally, there was a significant effect of lighting on the period of body temperature rhythms (F1,28=10.24, p<0.01; Figure 4G–H and J), with mice exposed to LAN displaying longer periods and delayed phase of peak amplitude (F1,26=15.59, p<0.001; Figure 4G–H and K); specifically, female mice housed in LAN displayed significant delay in the acrophase of body temperature rhythms compared to females housed in normal lighting conditions (p<0.05; Tukey’s multiple comparisons; Figure 4K). There were no differences in the τ of body temperature rhythms within groups (p>0.05; Tukey’s multiple comparisons; Figure 4J). Further, the amplitude of body temperature rhythms demonstrated a significant effect of sex (F1,28=41.16; p<0.0001; Figure 4G–H and L), with males displaying higher amplitudes, and lighting (F1,28=10.23; p<0.01; Figure 4G–H and L), with LAN reducing the amplitude of body temperature rhythms; specifically, male and female mice housed in LAN demonstrated reduced amplitude in body temperature rhythms compared to male mice housed in normal lighting conditions (p<0.05; Tukey’s multiple comparisons; Figure 4L).
Next, we sought to assess the effects of acute LAN exposure on the expression of circadian genes within the hippocampus “around the clock”. We chose to assess circadian gene expression within the hippocampus exclusively as the hippocampus was the site of reduced neurotrophin expression (males and females) and increased neuroinflammation (females) following exposure to LAN. There was significant effect of sex (F1,105=4.486; p<0.05; Figure 5A–B) on expression of clock within the hippocampus, with females displaying increased expression of clock, and a time × lighting interaction (F3,105=3.018; p<0.05; Figure 5A–B); specifically, males exposed to acute LAN demonstrated increased clock expression at ZT2 relative to males housed in normal lighting conditions (p<0.01; Tukey’s multiple comparisons; Figure 5B). Additionally, there was a significant effect of time (F3,108=6.917; p<0.001; Figure 5C–D) on the expression of bmal1 (arntl) within the hippocampus. However, there were no significant differences within groups (p>0.05; Tukey’s multiple comparisons; Figure 5C–D). Further, there was a significant effect of time (F3,106=8.378; p<0.0001; Figure 5K–L) and a time × lighting interaction (F3,106=3.480; p<0.05; Figure 5K–L) on the expression of cry1 within the hippocampus; specifically, males housed in LAN demonstrated increased cry1 expression at ZT2 compared to males housed in normal lighting conditions (p<0.01; Tukey’s multiple comparisons; Figure 5L). However there were no differences between groups in the expression of per1, per2, rev-erbα, or cry2 (p>0.008 for all t-tests; Bonferroni correction; Figure 5E–J and M–N). A summary of hippocampal clock gene changes are included in Table 2. Additionally, we sought to assess the effects of acute LAN exposure on the hypothalamic-pituitary-adrenal axis by measuring corticosterone concentrations within the serum, as well as the expression of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) within the hippocampus every six hours. There were no differences in serum corticosterone concentrations or hippocampal GR expression between groups (p>0.008 for all t-tests; Bonferroni correction; data not shown). However, there was a significant time × lighting interaction on the expression of hippocampal MR (F3,104=2.983; p<0.05; Figure 5O–P); specifically males housed in LAN increased MR expression at ZT2 compared to LD males (p<0.05; Tukey’s multiple comparisons).
Table 2:
Summary of alterations in hippocampal clock gene expression throughout the day. * Significant difference between F LD and F LAN, + Significant difference between M LD and M LAN, # Significant difference between F LD and M LD, $ Significant difference between F LAN and M LAN, % Significant difference between F LD and M LAN, ^ Significant difference between F LAN and M LD.
| Gene | ZT2 | ZT8 | ZT14 | ZT 20 | Statistical Significance |
|---|---|---|---|---|---|
| clock | +,^ | No change | No change | No change | p<0.05 Tukey’s multiple comparisons |
| bmal1 | No change | No change | No change | No change | p<0.05 Tukey’s multiple comparisons |
| per1 | No change | No change | No change | No change | p<0.008 t-tests with Bonferroni correction |
| per2 | No change | No change | No change | No change | p<0.008 t-tests with Bonferroni correction |
| rev-erbα | No change | No change | No change | No change | p<0.008 t-tests with Bonferroni correction |
| cry1 | +,^ | No change | No change | No change | p<0.05 Tukey’s multiple comparisons |
| cry2 | ^ | No change | No change | No change | p<0.008 t-tests with Bonferroni correction |
DISCUSSION
The current study sought to examine the rapid effects of LAN (5 lux) on the expression of depressive-like behavior in male and female mice, as well as measures of neuroinflammation and neurotrophin expression within the hippocampus. Taken together, our results establish that acute exposure to low level light at night (LAN) induces depressive-like behavior with concurrent changes in hippocampal expression of neurotrophins, clock genes, and a proinflammatory cytokine. Similar changes in neurotrophins and cytokine expression are associated with alterations in mood. Previous studies examining chronic LAN exposure in several nocturnal and diurnal rodent species demonstrate increased depressive-like behavior following 3–4 weeks of exposure to LAN12–15. However, there are studies demonstrating no association between LAN an mood in C57Bl/6 mice; suggesting a potential strain specific effect of LAN in rodents76–77. The current study establishes that in Swiss Webster mice the depressive-like behavioral effects of LAN emerge within the first three nights of exposure (Figure 1A–E), and when considered in the context of prior studies, suggests that the depressive effects of LAN may persist for weeks. Additionally, these behavioral data are consistent with two recent cross-sectional studies which report an association between increased nighttime light exposure and depressive symptomology in middle-aged and elderly people8,43. The rapid onset of the neurological and behavioral changes associated with LAN may also have implications for critically ill patients because 3.28 days is the average length of stay in intensive care units within the United States44 and maintaining dim lighting throughout the night in patient rooms is standard practice.
As part of this study, we sought to determine a potential mechanism by which acute LAN may be increasing depressive-like behavior in male and female mice. The neurotrophic hypothesis of major depressive disorder (MDD) postulates that a reduction in neurotrophin signaling is a contributing factor to the development of depression. Indeed, multiple studies have linked changes in bdnf, igf1, and VEGF with the development of MDD45–49. In the present study both male and female mice housed in LAN had reduced hippocampal VEGF-A concentrations (Figure 3C); additionally, male LAN mice had reduced hippocampal BDNF mRNA relative to mice housed in dark nights (Figure 3A). However, there were no changes in IGF1 mRNA expression (Figure 3B), suggesting LAN may have targeted effects on neurotrophin expression. Importantly, this reduction of neurotrophic signaling (BDNF and VEGF) within the hippocampus may contribute to the development of the observed depressive-like phenotype. Notably, the BDNF data presented here for males (Figure 2A) are consistent with a previous study demonstrating depressive-like behavior and reduced BDNF following chronic (4 weeks) exposure to LAN in a different mouse strain (C3H/HeNHsd)14. Additionally, converging evidence from post mortem tissue and serum samples suggest a role for reduced BDNF in clinical MDD; specifically, BDNF is reduced in post-mortem brain tissue and serum from individuals with MDD45,50,51. Further, antidepressant treatment increases hippocampal BDNF, and both antidepressant therapy and electroconvulsive shock therapy increase BDNF in blood52. A similar role in MDD has been proposed for VEGF, although the clinical data are not as compelling as for BDNF and a comprehensive analysis of VEGF in post-mortem brain tissue of individuals with MDD has not yet been reported52. However, clinical studies have demonstrated an association between alterations in the concentration of VEGF in the serum and plasma with the development of MDD53–55. Additionally, a recent clinical study has identified VEGF related polymorphisms as a risk factor for the development of MDD49. Rodent studies have confirmed causal relationships between both BDNF and VEGF and the expression of depressive-like behavior under a variety of conditions23,56. Together, these data indicate that LAN results in a rapid decline in hippocampal VEGF in both males and females, and a decline in BDNF mRNA in males that appears to persist across weeks of exposure to LAN. Although, the effect of acute LAN on VEGF-A was comparable in male and female mice, the apparent sex difference in hippocampal BDNF mRNA expression after acute LAN suggests that BDNF downregulation is not essential for the development of the depressive-like phenotype in females. Additional studies are needed to determine whether the reduction in VEGF and BDNF described here contributes to the decreased neurogenesis or alteration in spine density within the hippocampus.
Because of the demonstrated interaction between increased neuroinflammation and reduced neurotrophin signaling48, we next sought to examine the effects of acute LAN on proinflammatory cytokine expression within the hippocampus. In the present study female mice exposed to acute LAN displayed increased hippocampal IL-1β mRNA expression (Figure 3F). This increase in hippocampal IL-1β gene expression in females may also contribute to the development of the depressive-like phenotype. These data are consistent with the cytokine hypothesis of depression, and specifically there are compelling clinical and basic science data to support a causal role for increased IL-1β precipitating depressive symptoms21,22. Whether IL-1β increases at a later time point in males exposed to LAN remains to be determined. In contrast to the effects of chronic LAN, which increases TNF-α mRNA in both male mice57 and female hamsters15, there was no effect of 4 nights of LAN on hippocampal TNF-α mRNA in either sex in the current study (Figure 3G). Notably, IL-1β and TNF-α have interactive effects, and whether they interact over time to promote, and then maintain the depressive-like behavior associated with LAN, remains to be determined.
Additionally, because of the interaction between IL-1β and the expression of VEGF/alterations in VEGF signaling58,59, we examined the expression of vegfr1 and vegfr2 within the hippocampus. Females housed in LAN displayed increased expression of VEGFR1 mRNA in the hippocampus relative to female mice exposed to dark nights (Figure 3D). However, no changes in VEGFR1 mRNA expression were demonstrated in male mice. Additionally, no changes in VEGFR2 mRNA expression were demonstrated in either sex (Figure 3E). Notably, VEGFR1 has additional roles outside of angiogenesis that include macrophage activation and monocyte trafficking60,61. Due to the increased expression of IL-1β and VEGFR1 specifically in females housed in LAN and recent studies demonstrating peripheral monocytes migrating into the brain as the main producers of IL-1β62; future studies should examine the effects of acute LAN on monocyte trafficking to determine whether increased monocyte trafficking may explain the increased IL-1β and VEGFR1 mRNA expression in females reported in the current study. Additionally, studies should examine the effects of LAN on other VEGF family proteins that bind to VEGFR1 such as VEGF-B and PIGF.
Next, we sought to assess the effects of acute LAN exposure on the circadian system by examining body temperature rhythms, activity rhythms, and circadian gene expression throughout the day within the hippocampus. There were no differences within sexes on the period (tau) of activity or body temperature rhythms (Figure 4D). However, there was an effect of LAN on acrophase of activity and body temperature rhythms, specifically female mice housed in LAN delayed their acrophase relative to their sex matched controls (Figure 4E and K). Exposure to LAN (light pulse) can advance or delay the phase depending on the timing and intensity of the light signal63. Studies examining the effects of low level LAN on the phase of activity rhythms have yielded inconsistent results. One such study examining changes in the phase of activity rhythms in mice in response to three weeks of low level light at night (20 lux) concluded that the phase of activity rhythms remained stable64. However, a significant phase change in the activity rhythms of Syrian hamsters has been demonstrated; specifically, hamsters transferred from LD conditions to low level LAN increased the duration of the active phase by ~3hrs relative to complete darkness65; a similar delay in the acrophase of activity rhythms occured in the present study (~4hrs). Notably, no changes were detected in the amplitude of activity rhythms, suggesting that the behavioral effects were not a side effect of masking (Figure 4F). These data are consistent with previous studies in hamsters and rats demonstrating no side effect of masking following low level light at night exposure13,65. Circadian gene expression within the hippocampus remained largely unchanged (Figure 5 and Table 2). No alterations in bmal1, per1, per2, rev-erbα, or cry2 were demonstrated at any time point. Notably, rhythms in per1, per2, and cry2 expression appear less robust and represent a potential limitation in concluding there were no significant changes in per1, per2, and cry2 expression in response to LAN exposure. This likely reflects an region specific effect, as clock gene expression was assessed within the hippocampus and not within the hypothalamus. However, there were significant increases in the expression of clock and cry1 at ZT2 in male mice exposed to LAN relative to sex matched controls. Although not explicitly tested, this may represent a potential compensatory mechanism in male mice, as a previous study examining the effects of anti-depressants on clock gene expression within the hippocampus demonstrate increased expression of clock and cry1 in response to treatment with an antidepressant (fluoxetine)66. Additionally, we sought to assess the effects of acute LAN exposure on the hypothalamic-pituitary-adrenal axis by measuring corticosterone concentrations within the serum, as well as the expression of hippocampal glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). There were no significant changes in serum corticosterone concentrations or hippocampal GR expression (data not shown). However, males mice exposed to LAN increased the expression of hippocampal MR at ZT2 (Figure 5P). This increase in male hippocampal MR expression may explain the male specific reduction in hippocampal bdnf expression, as signaling via MR reduces bdnf expression within the hippocampus67
Whereas anxiety-like responses following acute LAN exposure were not the primary focus of this study, acute exposure to LAN did produce an anxiolytic effect, with increased central tendency during the open field test in females (Figure 2B). However, no changes in anxiety-like behavior were demonstrated in either sex during elevated plus maze testing (Figure 2D–F). Studies examining the effects on anxiety following LAN exposure have produced varying results. For example, male Swiss-Webster mice exposed to LAN for three weeks display increased number of rears during open field testing, increased number of open arm entries on the elevated plus maze, reduction in time to enter the open arm12. However, another study examining the effects of 5 weeks of LAN in male Swiss-Webster mice demonstrated no changes in anxiety-like behavior following LAN exposure68. These inconsistencies in anxiety-like behavior following LAN exposure may explain the differences between the results of open field testing and elevated plus maze in the current study.
In summary, based on this study and the existing literature, we propose that the mechanism through which acute LAN increases depressive-like behavior in mice involves an increase in neuroinflammation (at least in females), which reduces neurotrophin expression, in turn reducing neuronal complexity in the hippocampus and inducing depressive-like behavior. Although the neural circuitry underlying the physiological and behavioral responses to acute, low level light at night has not been specifically examined, it is known that intrinsically photosensitive retinal ganglion cells (ipRGCs) are critical for transducing information about environmental light, and that these cells project not only to the suprachiasmatic nucleus (SCN, the “master clock”), but also to numerous brain regions associated with cognitive and affective behaviors69. Furthermore, we are confident that ipRGCs mediate the effects of chronic LAN on depressive-like behavior because these changes can only be evoked by light within the wavelength range that stimulate these ipRGC cells16. Microglia, the resident macrophages of the central nervous system, are the most likely source of increased CNS proinflammatory cytokines after exposure to LAN70. Increased CNS proinflammatory cytokine expression precipitates depressive-like behavior in a wide range of contexts71. Likewise, our laboratory has previously demonstrated that chronic (4 weeks) LAN exposure in hamsters increases neuroinflammation, reduces neurotrophin (BDNF) concentrations, and reduces neuronal complexity within the hippocampus, concurrent with an increase in depressive-like behavior18. The relationships between LAN-induced neuroinflammation and neurotrophin expression, neuroplasticity, and depressive-like behavior are likely causal; hippocampal neuroplasticity and depressive-like behavior are normalized by ICV administration of a TNFα inhibitor to hamsters maintained under chronic LAN conditions15. Furthermore, studies examining the effects of altered neurotrophin expression and neuroinflammation have demonstrated that changes in neuronal morphology, neuronal excitability, and synaptic transmission begin to occur within minutes72–75. Therefore, three nights of LAN is a sufficient amount of time for changes in neuronal morphology and synaptic transmission to occur in response to alterations in neurotrophin expression and neuroinflammation within the hippocampus.
In conclusion, the current study supports the hypothesis that increased exposure to LAN may be contributing to the rising rates of MDD. Whereas prior research has focused on the neurological and behavioral effects of chronic LAN, the current data demonstrates that proinflammatory cytokine and neurotrophin concentrations in the hippocampus change within days of being exposed to LAN, and there is a concomitant increase in depressive-like behavior. Thus, even acute exposure to LAN may result in adverse health effects.
ACKNOWLEDGEMENTS
We thank the animal care staff for expert care of the animals. This research was supported by NINDS (R01NS092388).
Footnotes
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest to report.
REFERENCES
- 1.Gaston KJ, Bennie J, Davies TW, Hopkins J. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol Rev 2013; 88: 912–927. [DOI] [PubMed] [Google Scholar]
- 2.Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K et al. The new world atlas of artificial night sky brightness. Sci Adv 2016; 2: e1600377–e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry 2017; 7: e1017–e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. JNCI J Natl Cancer Inst 2001; 93: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 5.Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (lan) and cancers of prostate, colon, and lung in men. Chronobiol Int 2009; 26: 108–125. [DOI] [PubMed] [Google Scholar]
- 6.Kloog I, Stevens RG, Haim A, Portnov BA. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 2010; 21: 2059–2068. [DOI] [PubMed] [Google Scholar]
- 7.Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab 2013; 98: 337–344. [DOI] [PubMed] [Google Scholar]
- 8.Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. Exposure to light at night and risk of depression in the elderly. J Affect Disord 2013; 151: 331–6. [DOI] [PubMed] [Google Scholar]
- 9.Obayashi K, Saeki K, Kurumatani N. Light exposure at night is associated with subclinical carotid atherosclerosis in the general elderly population: The HEIJO-KYO cohort. Chronobiol Int 2015; 32: 310–317. [DOI] [PubMed] [Google Scholar]
- 10.Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol Int 2014; 2: 394–400. [DOI] [PubMed] [Google Scholar]
- 11.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 2005; 65: 11174–84. [DOI] [PubMed] [Google Scholar]
- 12.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res 2009; 205: 349–354. [DOI] [PubMed] [Google Scholar]
- 13.Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 2012; 27: 319–327. [DOI] [PubMed] [Google Scholar]
- 14.Fonken LK, Nelson RJ. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res 2013; 243: 74–78. [DOI] [PubMed] [Google Scholar]
- 15.Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry 2013; 18: 930–936. [DOI] [PubMed] [Google Scholar]
- 16.Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. doi: 10.1523/JNEUROSCI.5734-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opperhuizen A-L, Stenvers DJ, Jansen RD, Foppen E, Fliers E, Kalsbeek A. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia 2017; 60: 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 2011; 36: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 19.Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 2012; 27: 319–327. [DOI] [PubMed] [Google Scholar]
- 20.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 2009; 24: 27–53. [DOI] [PubMed] [Google Scholar]
- 21.Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets 2012; 16: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 22.Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain Behav Immun 2013; 31: 31–47. [DOI] [PubMed] [Google Scholar]
- 23.Castrén E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis 2017; 97: 119–126. [DOI] [PubMed] [Google Scholar]
- 24.Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C et al. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 2002; 114: 795–805. [DOI] [PubMed] [Google Scholar]
- 25.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 2005; 192: 348–356. [DOI] [PubMed] [Google Scholar]
- 26.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 2003; 23: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function Lisa. 2004. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khawaja X, Xu J, Liang JJ, Barrett JE. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: implications for depressive disorders and future therapies. J Neurosci Res 2004; 75: 451–460. [DOI] [PubMed] [Google Scholar]
- 29.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E et al. Gene profile of electroconvulsive seizures induction of neurotrophic and angigogenic factors. J Neurosci 2003; 23: 10841–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci 2002; 99: 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 2004; 36: 827–835. [DOI] [PubMed] [Google Scholar]
- 32.Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci 2011; 108: 5081–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson MF, Åberg MAI, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Dev Brain Res 2002; 134: 115–122. [DOI] [PubMed] [Google Scholar]
- 34.Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuro-Psychopharmacology Biol Psychiatry 2016; 64: 277–284. [DOI] [PubMed] [Google Scholar]
- 35.Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun 2013; 27: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res 2013; 23: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker WH II, Borniger JC, Surbhi, Zalenski AA, Muscarella SL, Fitzgerald JA et al. Mammary tumors induce central pro-inflammatory cytokine expression, but not behavioral deficits in balb/c mice. Sci Rep 2017; 7: 8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borniger JC, Walker WH Ii, Surbhi, Emmer KM, Zhang N, Zalenski AA et al. A role for hypocretin/orexin in metabolic and sleep abnormalities in a mouse model of non-metastatic breast cancer. Cell Metab 2018; 0. doi: 10.1016/j.cmet.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: 45e–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borniger JC, Maurya SK, Periasamy M, Nelson RJ. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol Int 2014; 31: 917–925. [DOI] [PubMed] [Google Scholar]
- 41.Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms 2013; 28: 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ. Strengths and limitations of period estimation methods for circadian data. PLoS One 2014; 9. doi: 10.1371/journal.pone.0096462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endo T, Kripke DF, Ancoli-Israel S. Wake up time, light, and mood in a population sample age 40–64 years. Psychiatry Investig 2015; 12: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lilly CM, Zuckerman IH, Badawi O, Riker RR. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest 2011; 140: 1232–1242. [DOI] [PubMed] [Google Scholar]
- 45.Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuro-Psychopharmacology Biol Psychiatry 2006; 30: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 46.Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 2008; 11: 1169–1180. [DOI] [PubMed] [Google Scholar]
- 47.Viikki M, Anttila S, Kampman O, Illi A, Huuhka M, Setälä-Soikkeli E et al. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci Lett 2010; 477: 105–108. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment and pathophysiology. Neuropsychopharmacology 2011; 36: 2375–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie T, Stathopoulou MG, de Andrés F, Siest G, Murray H, Martin M et al. VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl Psychiatry 2017; 7: e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry J-M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002; 109: 143–8. [DOI] [PubMed] [Google Scholar]
- 51.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase b in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003; 60: 804. [DOI] [PubMed] [Google Scholar]
- 52.Sharma AN, da Costa e Silva BFB, Soares JC, Carvalho AF, Quevedo J. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J Affect Disord 2016; 197: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carvalho AF, Köhler CA, McIntyre RS, Knöchel C, Brunoni AR, Thase ME et al. Peripheral vascular endothelial growth factor as a novel depression biomarker: A meta-analysis. Psychoneuroendocrinology 2015; 62: 18–26. [DOI] [PubMed] [Google Scholar]
- 54.Elfving B, Buttenschøn HN, Foldager L, Poulsen PHP, Grynderup MB, Hansen ÅM et al. Depression and BMI influences the serum vascular endothelial growth factor level. Int J Neuropsychopharmacol 2014; 17: 1409–1417. [DOI] [PubMed] [Google Scholar]
- 55.Clark-Raymond A, Meresh E, Hoppensteadt D, Fareed J, Sinacore J, Halaris A. Vascular endothelial growth factor: a potential diagnostic biomarker for major depression. J Psychiatr Res 2014; 59: 22–7. [DOI] [PubMed] [Google Scholar]
- 56.Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 2012; 46: 1–10. [DOI] [PubMed] [Google Scholar]
- 57.Hogan MK, Kovalycsik T, Sun Q, Rajagopalan S, Nelson RJ. Combined effects of exposure to dim light at night and fine particulate matter on C3H/HeNHsd mice. Behav Brain Res 2015; 294: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murata M, Yudoh K, Nakamura H, Kato T, Inoue K, Chiba J et al. Distinct signaling pathways are involved in hypoxia- and IL-1-induced VEGF expression in human articular chondrocytes. J Orthop Res 2006; 24: 1544–1554. [DOI] [PubMed] [Google Scholar]
- 59.Jung YD, Liu W, Reinmuth N, Ahmad SA, Fan F, Gallick GE et al. Vascular endothelial growth factor is upregulated by interleukin-1β in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis 2001; 4: 155–162. [DOI] [PubMed] [Google Scholar]
- 60.Barleon B, Sozzani S, Zhou D, Weich H, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996; 87. [PubMed] [Google Scholar]
- 61.Clauss M, Weich H, Breier G, Knies U, Röckl W, Waltenberger J et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996; 271: 17629–34. [DOI] [PubMed] [Google Scholar]
- 62.McKim DB, Weber MD, Niraula a, Sawicki CM, Liu X, Jarrett BL et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry 2017; 00: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents - IV. Entrainment: Pacemaker as clock. J Comp Physiol 1976; 106: 291–331. [Google Scholar]
- 64.Shuboni D, Yan L. Nighttime dim light exposure alters the responses of the circadian system. Neuroscience 2010; 170: 1172–1178. [DOI] [PubMed] [Google Scholar]
- 65.Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. J Biol Rhythms 2007; 22: 356–367. [DOI] [PubMed] [Google Scholar]
- 66.Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A et al. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience 2005; 134: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 67.Hansson AC, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M et al. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. Eur J Neurosci 2000; 12: 2918–2934. [DOI] [PubMed] [Google Scholar]
- 68.Castro JPMV, Frussa-Filho R, Fukushiro DF, Chinen CC, Abílio VC, Silva RH. Effects of long-term continuous exposure to light on memory and anxiety in mice. Physiol Behav 2005; 86: 218–223. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D et al. Light affects mood and learning through distinct retina-brain pathways. Cell 2018; 175: 71–84.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fonken LK, Weil ZM, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun 2013; 34: 159–163. [DOI] [PubMed] [Google Scholar]
- 71.Liberman AC, Trias E, da Silva Chagas L, Trindade P, dos Santos Pereira M, Refojo D et al. Neuroimmune and inflammatory signals in complex disorders of the central nervous system. Neuroimmunomodulation 2018; : 1–25. [DOI] [PubMed] [Google Scholar]
- 72.Gallo G, Letourneau P. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci 1998; 18: 5403–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lemann V, Gottmann K, Heumann R. BDNF, and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport 1994; 6: 21–25. [DOI] [PubMed] [Google Scholar]
- 74.Mishra A, Kim HJ, Shin AH, Thayer SA. Synapse loss induced by interleukin-1β requires pre-and post-synaptic mechanisms. J Neuroimmune Pharmacol 2012; 7: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoshino K, Hasegawa K, Kamiya H, Morimoto Y. Synapse-specific effects of IL-1β on long-term potentiation in the mouse hippocampus. Biomed Res 2017; 38: 183–188. [DOI] [PubMed] [Google Scholar]
- 76.Martynhak B, Hogben A, Zanos P, Georgiou P, Andreatini R, Kitchen I, et al. “Transient anhedonia phenotype and altered circadian timing of behaviour during night-time dim light exposure in Per3−/− mice, but not wildtype mice.” Scientific Reports 2017; 7: 40399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cleary-Gaffney M, Coogan AN. “Limited evidence for affective and diurnal rhythm responses to dim light-at-night in male and female C57Bl/6 mice.” Physiology & Behavior 2018; 189: 78–85. [DOI] [PubMed] [Google Scholar]