Abstract
We report two malignant sacrococcygeal tumors in infants that were associated with pathogenic DICER1 variation. These tumors were composed of primitive neuroepithelium, embryonal rhabdomyosarcoma and cartilage and initially diagnosed as immature teratomas. One child developed intracranial metastasis and died. The second child underwent surgery and chemotherapy and achieved complete remission. This child subsequently developed five additional DICER1-associated neoplasms by age nine. Genetic analysis revealed that both tumors harbored biallelic pathogenic DICER1 variation. We believe these cases represent another novel subtype of DICER1-associated tumor. This new entity, which we propose to call DICER1-associated presacral malignant teratoid neoplasm, may be difficult initially to distinguish from immature teratoma, but recognizing it as an entity can facilitate appropriate classification as an aggressive malignancy and facilitate appropriate genetic counseling, DICER1 germline variant testing, screening and education.
Keywords: Immature teratoma, sacrococcygeal teratoma, yolk sac tumor, neural plate, DICER1, DICER1 syndrome, intracranial metastasis
Introduction
DICER1 encodes an RNase III endonuclease and plays a pivotal role in miRNA processing [1]. Since it was first reported as the causative gene for familial pleuropulmonary blastoma, pathogenic DICER1 germline variation has been identified in a number of tumor types of seemingly diverse histogenesis including pleuropulmonary blastoma, ovarian Sertoli-Leydig cell tumor, cystic nephroma and pituitary blastoma and some non-cancerous conditions, including multinodular goiter and macrocephaly [2–6]. DICER1-associated tumors typically harbor biallelic DICER1 variation: a loss-of-function variant (usually germline) and a somatic “hotspot” variant within the RNase IIIb domain (E1705, D1709, G1809, D1810, or E1813). Here, we report two infants who had pathologically unique presacral tumors that harbored biallelic DICER1 variants. We would propose that these neoplasms with their collage of dysembryonic tissue patterns, initially interpreted as immature teratomas, represent another unique DICER1-related tumor. However, the various pathologic representatives in these neoplasms overlapped to a considerable degree with the other DICER1-related dysontogenetic neoplasms with their own teratoid morphologic attributes.
Material and Methods
Human subjects review.
The study was approved by the Institutional Review Boards at all participating institutions. Patient 1 was enrolled in the Japanese Children’s Cancer Group observational study. Patient 2 was enrolled in the National Cancer Institute Natural History of DICER1 Syndrome study (NCT-01257597). Eligibility criteria included a history of a DICER1-associated neoplasm or documentation of a pathogenic germline DICER1 variant. Informed, written consent was obtained from the patients’ parents.
DICER1 genetic analyses.
For the tumor from patient 1, targeted sequencing for all coding exons from 93 genes (Supplemental Table 1) was performed on an Ion Proton Sequencer (Life Technologies). Reads were mapped onto the hg19 human reference genome sequence and common variation from dbSNP build (http://www.ncbi.nlm.nih.gov/projects/SNP/), the 1000 Genomes database (http://www.1000genomes.org), and Human Genetic Variation Database (http://www.hgvd.genome.med.kyoto-u.ac.jp/) was excluded. In Patient 2, germline DICER1 testing was performed by Invitae on DNA extracted from blood. Somatic DICER1 sequencing of the tumor performed on DNA extracted from the paraffin block was performed as previously described [7].
Results
Patient 1
A one-week-old boy was admitted because of loss of lower extremity movement. His prenatal period and family history were unremarkable. Magnetic resonance imaging showed an extradural tumor within the spinal canal extending from L2 to the sacral region (Fig. 1A, B). The levels of serum alpha-fetoprotein and β-human chorionic gonadotropin were within the normal ranges for his age. Following the partial resection of a hemorrhagic mass, the patient received one course of chemotherapy with etoposide and carboplatin and subsequently underwent gross total resection. Pathologic examination of the resected masses revealed a multi-patterned neoplasm composed of primitive neuroepithelial and mesenchymal elements. The neuroepithelial components consisted of primitive neuronal cells forming occasional true rosettes with a resemblance to medulloepithelioma (Fig. 2A, B). There were scattered nodules of primitive cartilage rimmed by spindle cells (Fig. 2C). The remainder of the tumor was composed of embryonal rhabdomyosarcoma showing a full range of differentiation from blastema to maturing skeletal muscle (Fig. 2D). Neither epithelial lined cysts, cutaneous structures, or fetal-appearing tissues like liver or pancreas nor malignant germ cell elements like endodermal sinus tumor (yolk sac tumor) were identified.
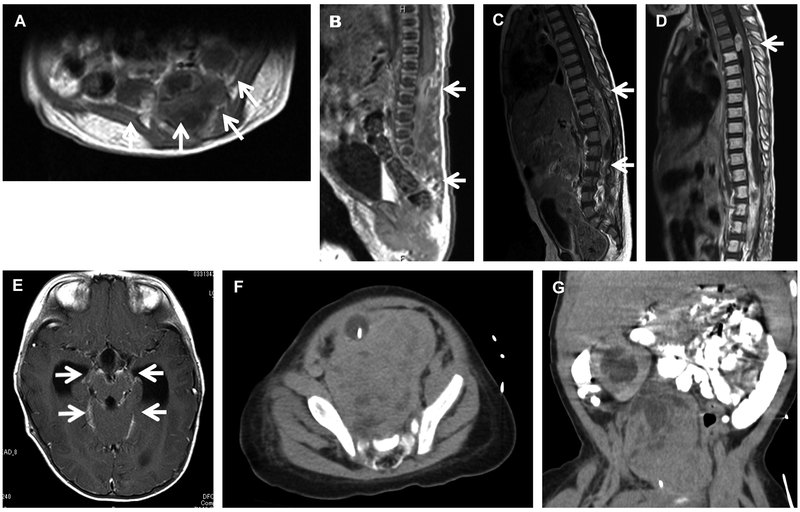
Figure 1.
Radiological findings, with arrow indicating tumor. Gadolinium-enhanced magnetic resonance imaging at first diagnosis (A, B), the first relapse (C), and the second relapse after radiotherapy (D, E) in Patient 1. Patient 2 axial (F) and coronal (G) views from pre-operative computed axial tomography imaging with oral and intravenous contrast showing a heterogenous, paramedian pelvic mass and asymmetric kidneys. There is delayed urinary excretion on the right due to obstruction from the mass. In the axial image (F), the balloon of the urinary catheter is apparent and displaced ventrally and significantly away from the midline.
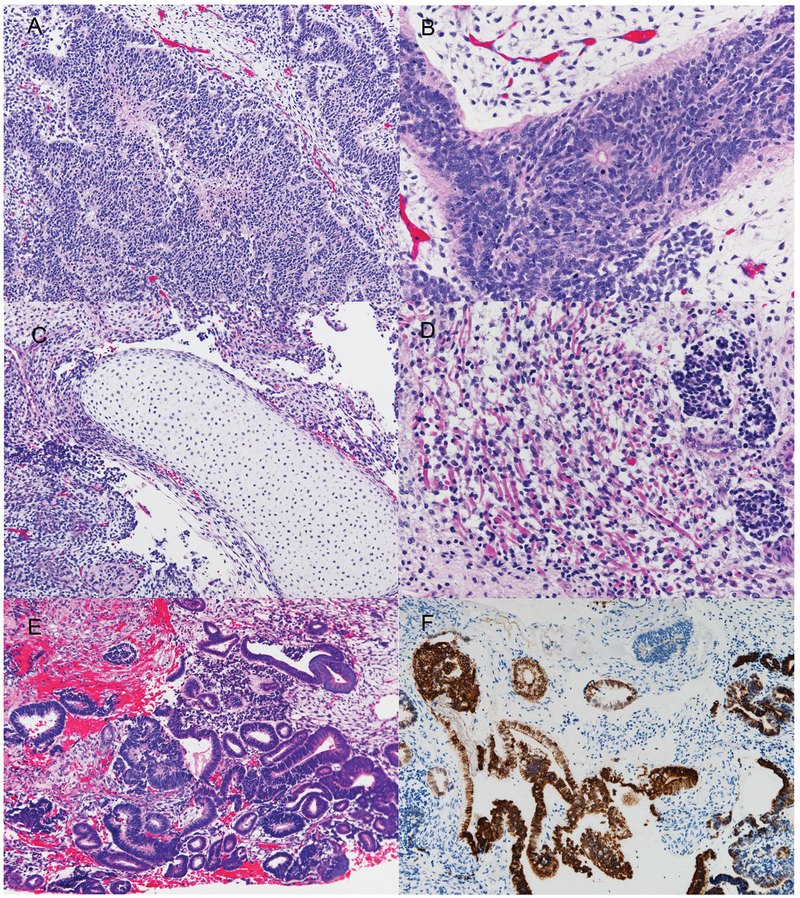
Figure 2.
Microscopic appearance of the multipatterned tumor from Patient 1. The primary tumor from Patient 1 showed immature neuroepithelial components including multilayered rosettes and medulloepithelioma-like component (A, B), and immature mesenchymal tissues, including mature cartilage and skeletal muscle cells (C, D). The recurrent tumor was composed predominantly of neuroepithelial tubular structures in loose mesenchymal tissue (E). These tubules were positive for cytokeratin CAM 5.2 (F). Hematoxylin and eosin (A-E); Anti-CAM 5.2 (F): Original magnification x 100 (A); x 200 (C, E, F), x 400 (B, D).
The patient received no post-operative chemotherapy, but eight months later, the tumor recurred and had extended to thoracic level 9 (Fig.1C). The patient underwent partial resection followed by local radiation (45 Gy) and chemotherapy. Although the tumor responded transiently to the salvage therapy, it recurred outside the irradiation field (Th3/Th4) and metastasized to the midbrain (Fig.D, E). The patient died of the disease 25 months after the initial diagnosis.
The recurrent tumor consisted of neuroepithelial tubular structures and loose mesenchymal tissue (Fig. 2E). Cytokeratin CAM5.2 was positive in the neuroepithelial tubules (Fig. 2F).
Patient 2
A four-month-old girl with an unremarkable prenatal and a limited family history presented with abdominal distention, poor feeding and blood in her diaper. Abdominal and pelvic computed tomography scans showed a heterogeneous 6.5 × 6.5 ×5.0 cm mass within the pelvis and associated high-grade obstruction of the right kidney with bilateral hydronephrosis, right greater than left (Fig. 1F, G). Spine magnetic resonance imaging showed a large presacral mass with heterogenous contrast enhancement. She underwent resection of the pelvic/presacral mass.
Pathologically, the tumor was composed predominantly of immature neuroepithelium in epithelial cords (Fig.3A), clusters of neuroblasts with rare true rosettes (Fig. 3B) and differentiating neurocytic areas in a neuropil background (Fig. 3C). Rare immature cartilage nodules were seen (Fig. 3D). Embryonal rhabdomyo- sarcomatous areas with skeletal muscle differentiation were also present (Fig. 3E,3F). Neither endodermal sinus tumor nor other germ cell patterns were identified.
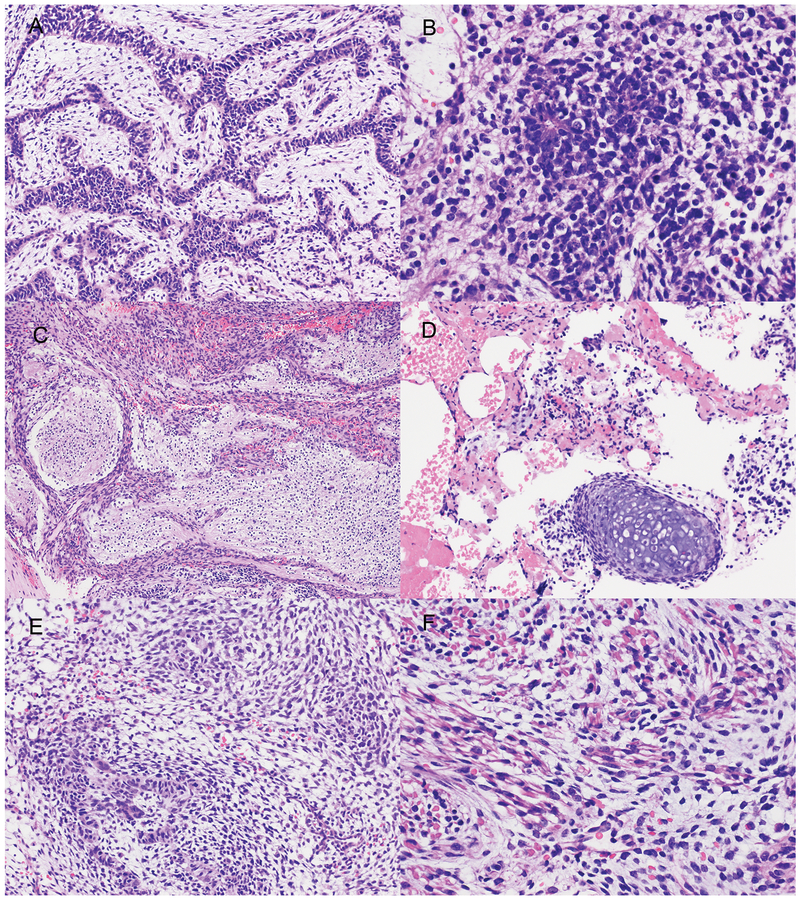
Figure 3.
Microscopic appearance of the multipatterned tumor from Patient 2.The tumor from Patient 2 was composed predominantly of immature neuroepithelium in epithelial cords (A), clusters with neuroblasts with rare true rosettes (B) and differentiating neurocytic areas in a neuropil background (C). Rare immature cartilage nodules were seen (D). Embryonal rhabdomyosarcomatous areas with skeletal muscle differentiation were also present (E, F). Hematoxylin and eosin: Original magnification x 200 (A, D, E); x 100 (C), x 400 (B, F).
She was treated with chemotherapy and two tandem auto stem cell transplantations. Subsequently she developed a vaginal embryonal rhabdomyosarcoma (age five years), sarcoma of the right kidney arising in a cystic nephroma (age eight years), papillary thyroid microcarcinoma and nasal chondromesenchymal hamartoma (age eight years) and cystic nephroma of the left kidney (age nine years).
DICER1 sequencing
In Patient 1, two nonsynonymous variants in DICER1 (c.2233C>T, p.Arg745* and c.5437G>A, p.Glu1813Lys) were detected, with a variant allelic frequency (VAF) of 52.3% and 34.2%, respectively. No other nonsynonymous variation in other genes on the panel was detected. These results were established after the patient’s death and germline or family testing was not performed.
Patient 2 underwent germline DICER1 testing at age eight years. Sequence results showed a pathogenic frameshift variant (c.4407_4410delTTCT, p.Ser 1470 Leufs*19); this variant was also harbored by the patient’s father, who has an unremarkable medical history. Somatic DICER1 sequencing of the tumor performed on tissue from the paraffin block showed the germline DICER1 variant (VAF: 51%) and a DICER1 “hotspot” variant (c.5113G>A; p.Glu1705Lys; VAF: 41.4%).
Discussion
Given the age at presentation and the sacrococcygeal location, it is not surprising that these two neoplasms were initially diagnosed as immature teratomas. However, the detection of tumor-associated pathogenic DICER1 variation in Patient 1 and the occurrence of multiple DICER1-related tumors in Patient 2 (and a germline DICER1 pathogenic variant) promoted re-consideration of the pathology in these two cases. Though primitive neuroepithelial rosettes and tubules were present in both tumors, the predominant pattern was embryonal rhabdomyosarcoma in the absence of the typical spectrum of teratomatous elements or endodermal sinus tumor which are frequent findings in immature teratoma of the sacrococcygeum [8]. Sarcomas including rhabdomyosarcoma are known to occur in germ cell neoplasms, principally in the mediastinum and gonads, but are unknown in infantile sacrococcygeal teratomas to the best of our knowledge [9, 10]. The combination of primitive neuroepithelial tubular profiles, rhabdomyoblastic and cartilaginous nodules in these two cases are similar to those observed in several of the other DICER1-related tumors including ciliary body medulloepithelioma and cervical embryonal rhabdomyosarcoma [3].
The immature or sarcomatous cartilage and embryonal rhabdomyosarcomatous elements are features of pleuropulmonary blastoma types I through III. Primitive neuroepithelial structures and rhabdomyosarcoma are found in pituitary blastoma and heterologous Sertoli-Leydig cell tumor of the ovary, respectively. Neither of these two neoplasms had foci of yolk sac tumor which are often identified as microscopic foci in immature teratomas of the sacrococcygeum. Theses suggest the present cases are previously unidentified pathological subtype of presacral tumor and a novel DICER1-associated tumor.
The International Pleuropulmonary Blastoma /DICER1 and Ovarian and Testicular Stromal Tumor Registries performed a search of all treating institution and central review diagnoses (search terms: germ cell, teratoma, yolk sac tumor, sacrococcygeal, tumor, seminoma, germinoma) for enrolled individuals with germline DICER1 pathogenic variation. There were no centrally-reviewed cases of germ cell tumors in either Registry (706 patients from more than 500 families). As the pathological findings of present cases resembles immature teratoma, we then reviewed the published accounts of DICER1 variation in germ cell tumors. As shown in Table 1, there were numerous tumors with somatic truncating or hotspot variation in DICER1, but there were no reports of pathogenic germline DICER1 variation [4, 11–16]. In addition, the tumors with DICER1 variation typically do not include detailed pathologic descriptions, and thus we cannot verify with certainty that they are not phenotypic mimics of either known or yet uncharacterized malignant neoplasms. Taken together, we could not find evidence that germ cell tumors are associated with pathogenic germline DICER1 variation.
Table 1.
Published reports of germ cell tumors with DICER1 variation
| Diagnosis | Demographics (Sex/Age) | DICER1 Somatic/Germline | Comment on Histology | Follow-up | Reference |
|---|---|---|---|---|---|
| Sacrococcygeal teratoma with nephroblastoma | Female/3 years | N/A | Composed predominantly of nephroblastomatous tissue; ultrastructural features like WIlms | Alive and well after 12 months | Ward Cancer 1974 |
| Seminoma (1 of 72 in series) | Male/32 years | No data/c.G4740G: p.Q1580H | No data | Alive at 46 years | Slade JMG 2011 |
| Primitive germ-cell tumor: Yolk sac (PGCTYS-01)* | No data | c.G5125A: p.D1709N/ No data | No data | No data | Heravi-Moussavi NEJM 2012 |
| Primitive germ-cell tumor: Yolk sac (PGCTYS-02)* | No data | c.T5127A: p.D1709E and trans c.G5492A: p.Y1831Ter/ No data | No data | No data | Heravi-Moussavi NEJM 2012 |
| Teratoma: Mature$ | No data | c.G5428T: p.D1810Y/ No data | No data | No data | Heravi-Moussavi NEJM 2012 |
| Testicular germ cell tumor (seminoma)# | No data | p.R1725Q/ No data | No data | No data | De Boer BMC Research Notes 2012 |
| Mixed germ cell tumor (yolk sac tumor/immature teratoma)^ | Female/27 years | c.G5428T: p.D1810Y/ No data | No data | No data | Witkowski BJC 2013 |
| Mixed gonadoblastoma/dysgerminoma^ | Female/15 years | c.A5429G: p.E1788fs*41/ No data | No data | No data | Witkowski BJC 2013 |
| Mixed germ cell tumor (dysgerminoma/yolk sac tumor)^ | Female/9 years | c.A5438G: p.E1788fx*41/ No data | No data | No data | Witkowski BJC 2013 |
| Mixed germ cell tumor (embryonal carcinoma/ immature teratoma/ choriocarcinoma)^ | Male/ 12 years | c.A5438G: p.E1788fx*41/ No data | No data | No data | Witkowski BJC 2013 |
| Yolk sac tumor^ | Male/ 1 year | c.A5438G: p.E1788fx*41/ No data | No data | No data | Witkowski BJC 2013 |
| Familial testicular germ cell tumors | 43 probands | Numerous likely non-pathogenic germline variants | No data | No data | Sabbaghian BMC Research Notes 2013 |
| Malignant thyroid teratoma | Female/59 years | c.A5438G: p.E1813G%/ No data | Post-chemotherapy pathology showed residual teratoma with extensive hyalinization, necrosis, dystrophic calcification, chronic inflammation and pigment-laden histiocytes consistent with treatment effects | Alive at 4-year follow-up | Rabinowits Thyroid 2017 |
Of 15 yolk-sac tumors sequenced, two harbored somatic DICER1 variation
Of 5 immature and 9 mature teratomas sequenced, one mature teratoma harbored somatic DICER1 variation
A somatic DICER1 variant found in 96 TGCT tumors.
This somatic DICER1 variant was observed in 56% of reads; no copy-number changes were observed. Two TP53 variants were also observed (c.G743A: p.R248Q and c.376_splice:p.Y126_splice) and an NF1 variant (c.A3160C: p.N1054H) in 6–8% of reads, suggesting a subclonal tumor population.
Data on five tumors reported in Table 3 of Witkowski 2013. A total of 99 ovarian germ cell tumors and 19 testicular germ cell tumors were sequenced resulting in 5/118 (4.2%) with a somatic DICER1 variant. Germline DNA was available for only 5 tumors (not specified) and did not show DICER1 variation.
Importantly, to date, no reported case has suggested an association between DICER1 variation and immature teratomas or infantile presacral tumor. Also of note, Patient 2 developed subsequently four DICER1-associated neoplasms; this number of the tumors was relatively high as Brenneman et al. reported the mean and median number of lesions in children with pathogenic germline DICER1 variation as 1.8 and 2.0, respectively [7].
Furthermore, immature teratomas typically are not associated with normal AFP levels and rarely disseminate intracranially, unlike the clinical presentation of Patient 1. [17, 18]. However, metastasis to the central nervous system is the most common site of distant spread by pleuropulmonary blastoma. Patient 1 did not receive chemotherapy after total resection of tumor due to the limited efficacy of chemotherapy against extracranial immature teratoma. In contrast, Patient 2 received intensive chemotherapy and remains in complete remission although the initial tumor was incompletely resected. Therefore, this tumor was aggressive, and the patient may have benefited from adjuvant chemotherapy.
In summary, we present two infantile sacrococcygeal tumors mimicking immature teratoma in two unrelated children (one with germline pathogenic DICER1 variation) that may be a new subtype of DICER1-related infantile tumors. We had not previously seen tumors in this location in DICER1 carriers in the International Pleuropulmonary Blastoma Registry suggesting they may be one of the rarer tumors associated with DICER1 pathogenic variation. We anticipate that an increased use of multi-gene next generation sequencing panels may lead to further expansion of the spectrum of disease associated with DICER1. Recognition of a pathogenic germline DICER1 variant permits cascade genetic testing and potential identification of other family members at increased risk of malignancy. Surveillance guidelines and quantification of neoplasm risk and for DICER1-carriers are available [19, 20].
This new entity, which we propose to call DICER1-associated presacral malignant teratoid neoplasm, may be difficult to distinguish from immature teratoma, however recognition can facilitate appropriate classification as an aggressive malignancy and will facilitate appropriate genetic counseling, DICER1 germline variant testing, screening and education.
Supplementary Material
Acknowledgments
The authors thank the patients and family members of the case for participating in this research. We thank M. Honda-Kitahara and R. Nagano for their technical support. This work is supported in part by the NCI Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Institutes of Health grant NCI R01CA143167 (Hill), the Japan Children’s Cancer Group (JCCG) and the Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and Development, AMED [No. 17ck0106330h0001].
Footnotes
Conflict of Interest
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. [DOI] [PubMed] [Google Scholar]
- 2.Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer. 2014;14:662–72. [DOI] [PubMed] [Google Scholar]
- 4.Slade I, Bacchelli C, Davies H, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet 2011;48:273–8. [DOI] [PubMed] [Google Scholar]
- 5.Khan NE, Bauer AJ, Doros L, et al. Macrocephaly associated with the DICER1 syndrome. Genet Med. 2017;19:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan NE, Bauer AJ, Schultz KAP, et al. Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J Clin Endocrinol Metab. 2017;102:1614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenneman M, Field A, Yang J, et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in pleuropulmonary blastoma / DICER1 syndrome: a unique variant of the two-hit tumor suppression model. F1000Res. 2015;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenney JK, Heerema-McKenney A, Rouse RV. Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv Anat Pathol. 2007;14:69–92. [DOI] [PubMed] [Google Scholar]
- 9.Malagon HD, Valdez AM, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol. 2007;31:1356–62. [DOI] [PubMed] [Google Scholar]
- 10.Sebire NJ, Fowler D, Ramsay AD. Sacrococcygeal tumors in infancy and childhood; a retrospective histopathological review of 85 cases. Fetal Pediatr Pathol. 2004;23:295–303. [DOI] [PubMed] [Google Scholar]
- 11.Heravi-Moussavi A, Anglesio MS, Cheng SW, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med 2012;366:234–42. [DOI] [PubMed] [Google Scholar]
- 12.de Boer CM, Eini R, Gillis AM, et al. DICER1 RNase IIIb domain mutations are infrequent in testicular germ cell tumours. BMC Res Notes. 2012;5:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witkowski L, Mattina J, Schonberger S, et al. DICER1 hotspot mutations in non-epithelial gonadal tumours. Br J Cancer 2013;109:2744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabbaghian N, Bahubeshi A, Shuen AY, et al. Germ-line DICER1 mutations do not make a major contribution to the etiology of familial testicular germ cell tumours. BMC Res Notes. 2013;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinowits G, Barletta J, Sholl LM, et al. Successful Management of a Patient with Malignant Thyroid Teratoma. Thyroid. 2017;27:125–8. [DOI] [PubMed] [Google Scholar]
- 16.Ward SP, Dehner LP. Sacrococcygeal teratoma with nephroblastoma (Wilm’s tumor): a variant of extragonadal teratoma in childhood. A histologic and ultrastructural study. Cancer. 1974;33:1355–63. [DOI] [PubMed] [Google Scholar]
- 17.Wessell A, Hersh DS, Ho CY, et al. Surgical treatment of a type IV cystic sacrococcygeal teratoma with intraspinal extension utilizing a posterior-anterior-posterior approach: a case report. Childs Nerv Syst. 2018;34:977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider DT, Wessalowski R, Calaminus G, et al. Treatment of recurrent malignant sacrococcygeal germ cell tumors: analysis of 22 patients registered in the German protocols MAKEI 83/86, 89, and 96. J Clin Oncol 2001;19:1951–60. [DOI] [PubMed] [Google Scholar]
- 19.Schultz KAP, Williams GM, Kamihara J, et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res. 2018;24:2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart DR, Best AF, Williams GM, et al. Neoplasm Risk Among Individuals With a Pathogenic Germline Variant in DICER1. J Clin Oncol 2019;37:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.