Abstract
Metabolic endotoxemia initiates low-grade chronic inflammation in metabolic syndrome (MetS) and provokes the progression towards more advanced cardiometabolic disorders. Our recent works in obese rodent models demonstrate that catechin-rich green tea extract (GTE) improves gut barrier integrity to alleviate the translocation of gut-derived endotoxin and its consequent pro-inflammatory responses mediated through Toll-like receptor-4/nuclear factor κB (TLR4/NFκB) signaling. The objective of this clinical trial is to establish the efficacy of GTE to alleviate metabolic endotoxemia-associated inflammation in persons with MetS by improving gut barrier function. We plan a double-blind, placebo-controlled cross-over trial in persons with MetS and age- and gender-matched healthy persons (18–65 y; n = 20/group) who will receive a low-energy GTE-rich (1 g/day; 890 mg total catechins) confection snack food while following a low-polyphenol diet for 28 days. Assessments will include measures of circulating endotoxin (primary outcome) and secondary outcomes including biomarkers of endotoxin exposure, region-specific measures of intestinal permeability, gut microbiota composition, diversity, and functions, intestinal and systemic inflammatory responses, and catechins and microbiota-derived catechin metabolites. Study outcomes will provide the first report of the GTE-mediated benefits that alleviate gut barrier dysfunction in relation to endotoxemia-associated inflammation in MetS persons. This is expected to help establish an effective dietary strategy to mitigate the growing burden of MetS that currently affects ~35% of Americans.
Keywords: Catechin, Endotoxemia, Gut barrier function, Gut dysbiosis, Gut microbiota, Inflammation, Metabolic syndrome, Tea
Abbreviations: BMI, body mass index; GTE, green tea extract; LPS, lipopolysaccharides; MetS, metabolic syndrome; NFκB, nuclear factor κB; TLR4, Toll-like receptor-4; TNF- α, tumor necrosis factor-α; SCFA, short chain fatty acid; LBP, LPS binding protein; PCoA, principal coordinates analysis
1. Introduction
The prevalence of metabolic syndrome (MetS) is at an alarming rate with ~35% of Americans afflicted [1]. MetS is diagnosed based on the presence of ≥3 of 5 cardiometabolic criteria (hypertension, central obesity, hyperglycemia, hypertriglyceridemia, or low HDL-C) [2]. Because MetS symptoms are often subclinical, most individuals are left unmanaged other than to encourage lifestyle modification. Without intervention, MetS is likely to progress to more serious disorders (e.g. type II diabetes, fatty liver and cardiovascular diseases) [3]. Thus, identifying dietary strategies that alleviate MetS and its pathological progression is critical to public health.
Progression of MetS is provoked by a low-grade inflammation that is mediated, in part, by metabolic endotoxemia [4]. Metabolic endotoxemia is characterized by 2–3 times higher circulating levels of the Gram-negative bacteria component endotoxin [5]. The intestinal barrier normally serves as the first-line of defense against the translocation of gut-derived bacterial endotoxins (e.g. lipopolysaccharides; LPS). However, poor lifestyle and excess visceral adiposity encourage bacterial overgrowth, impair gut barrier integrity, and increase endotoxin translocation [[6], [7], [8], [9]]. Consequently, endotoxin binds to Toll-like receptor-4 (TLR4) [10], which signals the activation of nuclear factor κB (NFκB) to upregulate pro-inflammatory mediators causing low-grade inflammation [11]. Hence, lifestyle strategies that enhance gut barrier function would be expected to limit the translocation of gut-derived bacterial toxins and thwart MetS progression.
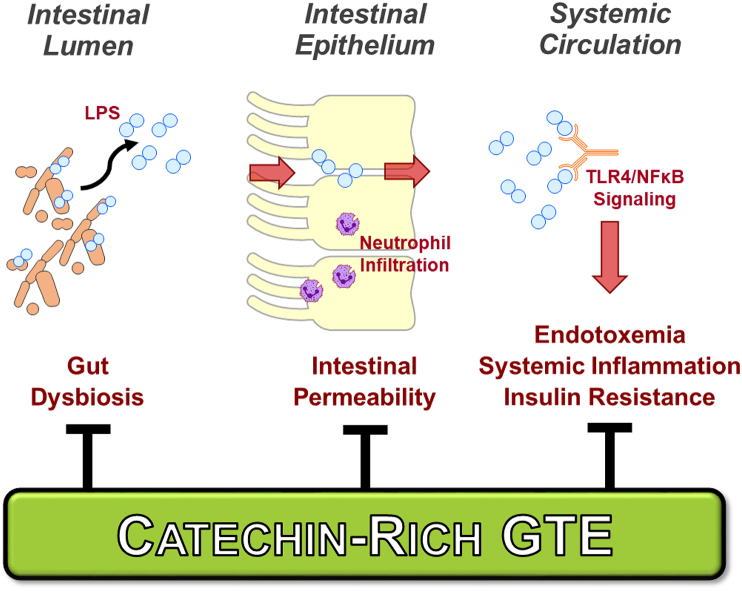
Catechin-rich green tea (Fig. 1) has received significant interest due to epidemiological evidence suggesting that its consumption lowers cardiometabolic risk [12]. Evidence from studies in rodents indicate that green tea extract (GTE) alleviates MetS complications including insulin resistance, liver steatosis, and hepatocellular injury [[13], [14], [15], [16]]. These benefits are likely attributed to several mechanisms that limit gut-derived LPS translocation (Fig. 2). Specifically, GTE in mice fed a high-fat diet favorably altered gut microbiota composition (e.g. decreased Firmicutes/Bacteroidetes) and functions [e.g. decreased LPS biosynthesis, increased short chain fatty acid (SCFA) metabolism] [17]. It also decreased LPS translocation in association with improved intestinal tight junction protein expression and downregulated adipose and intestinal TLR4/NFκB inflammation [17]. GTE in wild-type mice fed a high-fat diet also alleviated liver steatosis, hepatocellular ballooning, lipid peroxidation, NFκB activation, and mRNA expression of pro-inflammatory mediators (i.e. TNFα, iNOS, myeloperoxidase); each of these were lowered to the extent attributed to the loss of TLR4 signaling in TLR4 mutant mice [18]. These findings suggest that the hepatic benefits of GTE occur, at least in part, in a TLR4-dependent manner and likely involve a separate gut-level mechanism that limits metabolic endotoxemia.
Fig. 1.
Chemical structures of major catechins in green tea. Adapted from Masterjohn and Bruno [16].
Fig. 2.
GTE potentially alleviates systemic metabolic syndrome complications attributed to metabolic endotoxemia-associated inflammation. GTE is hypothesized to favorably alter gut microbiota composition to increase commensal populations that biosynthesize short chain fatty acids while decreasing pathogenic bacteria populations that provoke intestinal inflammation. GTE is also likely to limit the absorption of Gram-negative bacteria-derived endotoxins that otherwise activate TLR4/NFκB signaling. Abbreviations: GTE, green tea extract; LPS, lipopolysaccharide (i.e. endotoxin); NFκB, nuclear factor κB; TLR4, Toll-like receptor-4. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Despite compelling evidence from preclinical studies that GTE decreases hepatic and adipose TLR4/NFκB inflammation and improves gut barrier function [[17], [18], [19], [20]], evidence-based dietary recommendations for GTE are limited by the absence of studies in humans examining its effects on metabolic endotoxemia. Our planned double-blind, randomized, placebo-controlled cross-over intervention is the first to examine GTE in MetS persons compared with age- and gender-matched healthy persons on gut dysbiosis, intestinal permeability, metabolic endotoxemia, intestinal and systemic responses implicated TLR4/NFκB inflammation-driven cardiometabolic disorders.
2. Objective, aims, and hypotheses
The objective of this trial is to establish evidence-based recommendations for green tea catechins based on improvements in metabolic endotoxemia and restored gut barrier function. The following specific aims and hypotheses will be addressed:
Aim 1. Assess GTE on intestinal permeability and gut-derived endotoxin translocation. We hypothesize that GTE will decrease metabolic endotoxemia that is otherwise elevated in persons with MetS compared with healthy persons by improving intestinal barrier integrity.
Aim 2. Evaluate GTE on microbiota composition and functions. We hypothesize that GTE consumption in MetS persons will alleviate gut dysbiosis, decrease populations of pathogenic LPS-containing Gram-negative bacteria, and increase SCFAs that improve gut barrier function.
Aim 3. Establish intestinal and systemic anti-inflammatory activities of GTE. We hypothesize that GTE in MetS persons will decrease intestinal inflammation in association with reduced circulating NFκB-dependent inflammatory proteins and expression levels of genes involved in TLR4/NFκB signaling.
The primary outcome of this controlled trial is serum endotoxin following GTE intervention in MetS and healthy persons. Secondary outcomes include measures of endotoxin exposure in the circulation (LPS binding protein, soluble CD14), gut microbiota composition (diversity, populations of commensal and pathogenic bacteria), fecal SCFAs, region-specific intestinal permeability, intestinal inflammatory protein biomarkers measured in fecal samples (calprotectin, myeloperoxidase), circulating pro-inflammatory proteins (TNFα, IL-6 and -8, C-reactive protein), expression levels of TLR4/NFκB signaling genes from peripheral blood mononuclear cells (TLR4, MyD88, p65, IL-6 and -8, TNFα, myeloperoxidase, MCP-1), and measures of circulating and fecal GTE catechins and their microbial-derived metabolites (e.g. valerolactones).
3. Study design and methods
3.1. Clinical trial design
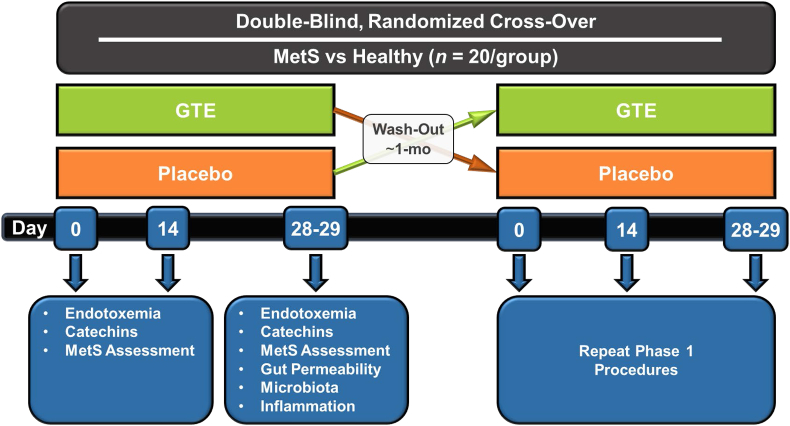
This study protocol has been approved by the Institutional Review Board at The Ohio State University and registered at ClinicalTrials.gov (NCT03973996). The randomized controlled trial will be conducted in MetS (n = 20) and age- and gender-matched healthy persons (n = 20) who will be recruited from the Columbus, Ohio area. In a double-blind, placebo-controlled crossover design (Fig. 3), participants fulfilling study entry criteria (Table 1) will be randomized to receive placebo or GTE (1 g containing 890 mg of total catechins) confections daily for 4-wk. During each study arm, they will be instructed to abstain from all tea varieties and follow a low-polyphenol diet [21]. Blood will be collected at day 0, 14, and 28 for metabolic assessments and safety monitoring by assessing a serum chemistry panel [e.g. liver function (aminotransferases), glucose, insulin]; blood collected on day 28 will be also used to isolate RNA from peripheral blood mononuclear cells for gene expression studies. On day 0, 14, and 28, blood pressure and anthropometrics will be also assessed, and dietary nutrient and flavonoid intakes will be evaluated from 3-day food records as we described [22,23]. On day 28, participants will provide three fecal samples from any three of the four immediately preceding days. Following blood collection, they will complete a gut permeability test and collect 24-h urine through day 29. Upon completing these procedures, participants will undergo ~1-month wash-out before repeating the study identically, but with crossover to the alternate treatment (Fig. 3).
Fig. 3.
Study design of the planned double-blind, placebo-controlled, randomized cross-over trial. Persons with metabolic syndrome (MetS) and age- and gender-matched healthy adults will be recruited from the Columbus, Ohio area. Fasted blood samples will be collected on days 0, 14, and 28 for metabolic assessments (e.g. endotoxemia, catechins, systemic inflammatory responses, clinical chemistries). Gut microbiota composition and functions will be assessed from pooled fecal samples collected on any three of four terminal days of the intervention. On day 28, participants will ingest a 5-sugar probe solution and collect urine for 24-h for the assessment of region-specific gut permeability.
Table 1.
Participant eligibility criteria.
| Inclusion Criteria |
|---|
| Population: men and women 18–65 y of age |
| Persons with MetS (≥3 of 5 of established MetS criteria) |
| Fasting glucose: >100 mg/dL |
| Waist circumference: >89 cm in women or >102 cm in men |
| HDL-C: <50 mg/dL in women or <40 mg/dL in men |
| Triglyceride: >150 mg/dL |
| Blood pressure: >130/85 mmHg |
| Healthy persons |
| BMI: 19–25 kg/m2 |
| Fasting glucose: <100 mg/dL |
| HDL-C: >50 mg/dL in women or >40 mg/dL in men |
| Triglyceride: <150 mg/dL |
| Blood pressure: <120/80 mmHg |
| Exclusion Criteria |
| Concurrent tea consumption (Camellia sinensis or herbal varieties) |
| History of liver disease, cardiovascular disease, hypertension (blood pressure >140/90 mmHg), cancer, gastrointestinal disorders or surgeries, chronic diarrhea, hemochromatosis, anemia, Parkinson's disease |
| Use of medications to manage diabetes, hypertension, or hyperlipidemia |
| Use of dietary supplements, prebiotics, probiotics, antibiotics, or anti-inflammatory agents within the past 1 mo |
| Use of medications known to be contraindicated for use with green tea (e.g. blood thinning medications, antipsychotic medications, monoamine oxidase inhibitors) |
| Smoker |
| >2 alcoholic drinks/day |
| Food allergies, intolerances, or dietary restrictions to any of the confection ingredients |
| Women who are pregnant, intending to become pregnant, lactating, or initiating or changing birth control within the past 3-mo |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein-cholesterol; MetS, metabolic syndrome.
3.2. Test confections: dose and formulation
The confection provides GTE at 1 g/day (890 mg total catechins). Based on brewed green tea containing 150–180 mg catechins [16], GTE at 1 g corresponds to ~5 servings/day of green tea. This intake level is in agreement with epidemiological observations suggesting that green tea lowers cardiometabolic risk [[24], [25], [26]]. When extrapolated on the basis of energy intake as detailed [17], it also reflects the lowest known dose that attenuates hepatic NFκB activation in obese rodents [20] and is similar to the dose that lowers TLR4/NFκB inflammation and endotoxemia by improving gut barrier integrity [18]. A 4-wk study period has been selected to translate evidence from preclinical studies examining GTE on endotoxemia [19] and is consistent with studies examining microbiota composition, and green tea (10–30 days), at similar doses to our approach, increasing Bifidobacterium in the human microbiota [27,28].
We established a novel starch-based GTE-rich confection for human trials [22], which has been modified into a gelatin-based vehicle (i.e. “gummy”) with desirable sensory characteristics to achieve objectives of the present trial. The 100 g daily dose is low-energy (51 kcal/day) while delivering 1 g of decaffeinated GTE. The catechin profile was verified by HPLC [22] to contain 89% total catechins (62% epigallocatechin gallate, 14% epigallocatechin, 10% epicatechin gallate, 13% epicatechin, and 2% catechin), which is similar to that of freshly brewed green tea [16]. The confection consists of 84.5% water, 1 g decaffeinated GTE powder (Taiyo International, Inc.), 2% sucrose, 6% gelatin, 0.5% citric acid, and lime-flavored gelatin (6%) for treatment blinding and to mask the bitter notes of green tea. Sucrose was selected as a sweetener since sugar alcohols and fructose may alter microbiota composition [29], which could confound interpretation by masking the benefits of GTE. Confections will be prepared as described [[30], [31], [32]]. To deliver 1 g GTE, 6 confections (~4 cm3 and ~16 g each) will be consumed; 2 with each daily meal. Placebo confections will be prepared identically, but without GTE. Participants will be provided dietary instruction to substitute confections for a snack of equivalent energy (~50 kcal). Confections will be packaged into oxygen-impermeable wrappers and coded for treatment blinding. In-house analysis indicates that the recovery of each catechin from freshly prepared confections is 84–105% and stability has been verified to be at least 1-mo at ambient temperature. However, to best preserve sensory and textural characteristics, participants will refrigerate confections. We will also verify catechin composition in GTE at 3-mo intervals or upon receipt of a new lot of GTE.
3.3. Dietary control
To improve diet homogeneity and limit potential confounding effects of polyphenol intakes on study outcomes, participants will follow a low-polyphenol diet during each study arm, as we described [21]. In brief, participants will receive weekly dietary education under the auspice of a registered dietitian to omit or limit polyphenol-rich foods. Specifically, participants will be provided oral instruction and written educational materials to abstain from foods containing >50 mg/serving of total polyphenols and to limit those having 10–50 mg/serving of polyphenols to a maximum of 2 servings/day. Foods containing <10 mg/serving will be unrestricted. With this approach, dietary polyphenols of adults decreased by 45% compared with usual intakes (1568 mg/day), and study subjects achieved 95% adherence to the dietary modifications [21]. Fiber intakes with this dietary approach are also similar to the low intakes of the typical American diet (~15 g/day) [33].
3.4. Participant recruitment and eligibility
Study recruitment is expected to last 12–18 months in order to achieve participant enrollment goals. Participants will be recruited through posted flyers, e-mail, electronic and newsprint advertisements, word of mouth, and social media. The advertisements will instruct interested participants to call the study center. The research coordinator will provide an overview of study procedures and perform a short questionnaire to determine preliminary qualification to the study (e.g. age, non-smoking status, non-use of dietary supplements, absence of overt disease). Persons meeting preliminary eligibility will be scheduled for an in-person visit to review all study procedures, obtain written informed consent, and to collect a fasting blood sample to determine whether biochemical parameters meet eligibility requirements for MetS or healthy status (Table 1). If participants meet all eligibility requirements, subsequent visits for baseline, mid-study, and terminal study procedures (Fig. 3) will be scheduled.
3.5. Experimental procedures
During the study, participants will be instructed to arrive at the clinic after a 10–12 h overnight fast and to avoid any vigorous physical activity during the preceding 48 h. At each appointment, participants will be queried on the use of any dietary supplements, antibiotics, anti-inflammatory or other medications, and any adverse effects since the last visit. Anthropometrics, blood pressure, dietary intake data, and adherence to the intervention will be evaluated at baseline, mid-study, and terminal study visits. Blood will be collected at the same intervals to assess serum endotoxin, plasma catechins, and a panel of serum chemistries. Gut permeability, microbiota composition, and intestinal and systemic inflammation will be measured in blood, urine, or fecal samples provided at the terminal study visit.
3.5.1. Anthropometrics, blood pressure, and dietary intake
Participants will rest for 15 min prior to determining blood pressure using an automated cuff (Omron BP760). Measures will be performed twice separated by 1 min. If values differ significantly, a third measure will be performed and all values will be averaged. Body mass index (BMI) will be calculated from height determined from a wall-mounted stadiometer (Model 216; Seca) and weight from a calibrated scale (Model 869; Seca); participants will wear minimal clothing during the latter. Waist circumference will be assessed at the level of the umbilicus using a nonflexible measuring tape. Dietary intakes will be assessed using 3-day food records prior to baseline to obtain usual intakes and then again at mid-study and study termination to verify dietary adherence. Food records (3-day) will be evaluated for energy and nutrients using Nutrition Data System for Research (NDSR 2018; University of Minnesota) and the Nutrition Coordinating Center Flavonoid and Proanthocyanin database will be used to assess flavonoid intakes as we described [22,23].
3.5.2. Endotoxemia
Serum endotoxin will be measured using a high-sensitivity fluorometric kit (PyroGene™ recombinant Factor C Assay; Lonza International) and endotoxin-free consumables on a fluorescence-capable microplate reader, as we described [18,19], from fasting blood samples obtained at day 0, 14 and 28 of each cross-over period. In brief, recombinant factor C is activated by its binding to endotoxin, and the bound complex then induces cleavage of a fluorogenic substrate that is detectable at 380/440 nm (excitation/emission). Endotoxin quantification is performed by linear regression against an endotoxin standard prepared in parallel that has a concentration traceable to the manufacturer. In our hands, inter- and intra-assay CV is 4.2–6.1%.
To further evaluate endotoxin exposure, plasma LPS binding protein (LBP; Hycult Biotech) and soluble CD14 (sCD14; R&D System) will be measured (days 0, 14, 28) using separate ELISA kits as we described [34]. From the measured proteins, the ratio of LBP:sCD14 will be calculated as an index of endotoxin exposure consistent with pro-inflammatory signaling by LPS that is mediated by its binding to LBP and sCD14 prior to activating the TLR4 signaling complex.
3.5.3. Gut permeability
At wk 4 (d 28), fasted participants will ingest a sugar probe solution [sucrose (40 g; Spectrum), lactulose (5 g; Akorn Pharmaceuticals), mannitol (1 g; Spectrum), sucralose (1 g; Spectrum), and erythritol (1 g; Spectrum)] as described [35]. Meals devoid of sucrose and the above artificial sweeteners will be provided for 24 h (through day 29). Urinary sugars will be assessed by LC-MS as described [35], but with minor modification to use a Prominence LCMS-2020 instrument (Shimadzu). In brief, urine samples will be diluted in acetonitrile:water (85:15), spiked with 13C-glucose (internal standard; Sigma), centrifuged at 10,000 g (15 min, 4 °C), and the collected supernatant injected. Isocratic separation will be performed using an Acquity UPLC BEH Amide column (100 × 2.1 mm; 1.7 μm; Waters Corp) and acetonitrile:water (65:35) containing 0.1% (v/v) ammonium hydroxide as the mobile phase. Single-ion monitoring will be performed to detect each compound at their mass/charge ratios and corresponding retention times confirmed by authentic standards [sucrose (Tokyo Chemical Industry); sucralose, lactulose, mannitol, erythritol (Sigma)]. Intestinal permeability is then defined by the urinary excretion (% of dose) and excretion ratios of lactulose:mannitol and sucralose:erythritol at 0–5 h (proximal intestinal permeability) and 6–24 h to reflect upper and lower intestinal permeability, respectively [[36], [37], [38]]. This approach is based on lactulose being absorbed in the small intestine, but not the colon due to bacterial degradation, whereas sucralose is unaffected by gut microbiota and is absorbed in the colon; sucrose is used to reflect gastric permeability [39]. Thus, these studies will define the interactive effects of MetS and GTE on site-specific gut permeability.
3.5.4. Microbiota composition and genomic function
Microbiota will be characterized phylogenetically from pooled fecal samples collected during three of any four terminal days of each study arm in accordance with our established procedures [40,41]. In brief, total DNA will be extracted as we described [42]. Amplicon libraries each with a unique barcode will be prepared from individual samples, pooled, and subjected to MiSeq sequencing (Illumina, 2 × 300 paired-end protocol) [40]. Sequence data will be analyzed with QIIME2 [43] by filtering out low-quality reads (Q < 25), joining the two sequence reads, trimming primers and barcodes, picking operational taxonomic units (OTUs, 97% similarity against Silva reference database) [44,45], and identifying and removing chimera sequences using ChimeraSlayer against Greengenes database. α-Diversity measures (e.g. richness, evenness, Chao1 richness estimate, Shannon-Wiener diversity index, and Faith's phylogenetic diversity) are then calculated. Principal coordinates analysis (PCoA) is performed at the OTU level to compare the overall microbiota. Bacteria that differ in relative abundance by health status and GTE treatment will be determined as described elsewhere [17]. The PCoA results will be assessed using permutational multivariate analysis of variance (PERMANOVA) to determine if the GTE treatment significantly alter the overall microbiota [46]. Microbial functions will be predicted using PICRUSt2 [47] and subsequent comparison with the KEGG Orthology classification scheme and MetaCyc pathway database prior to statistical analysis of the functions with STAMP V2.1.3 [48]. Welch's t-test with Benjamini-Hochberg false discovery rate correction is used to define pair-wise differences using a q-quality filter of P ≤ 0.05. Bacterial genera and functional feature biomarkers indicative of the GTE treatment will be identified using linear discriminant analysis effect size [49]. We will also use correlation analysis to identify the genera and functional features that correspond to specific phenotypic measurements.
3.5.5. Short chain fatty acids
SCFAs will be analyzed from pooled fecal samples collected on days 24–28 using LC-MS/MS as described [50]. We will evaluate ten C2–C6 straight chain SCFAs (e.g. butyrate, acetate, propionate) and branched chain SCFAs (e.g. isobutyric acid, isovaleric acid). Straight chain SCFAs are predominantly from bacterial fermentation of fibers whereas branched chain SCFAs are derived mainly from bacteria metabolism of branched chain amino acids. The former are often depleted with gut dysbiosis whereas the latter are often increased. In brief, fecal samples will be homogenized 1:2 in propanol and centrifuged (4000g, 4 °C, 10 min). The collected supernatant containing 13C-butyrate (internal standard) is incubated for 30 min at 40 °C with 200 mM 3-nitrophenylhydrazine (3NPH) and 120 mM N-(3-dimethylaminopropyl)-Nʹ-ethylcarbodiimide to generate 3NPH-SCFA derivatives. The sample is then diluted in 10% acetonitrile prior to injection on a Q-Exactive Hybrid Mass Spectrometer connected to a Vanquish HPLC system operated with electrospray ionization in negative mode (ThermoFisher Scientific). Quantification of derivatized SCFAs is performed against area ratios of derivatized authentic standards prepared in parallel relative to 3NPH-13C-butyrate.
3.5.6. Intestinal and systemic inflammation
The interactive effects of GTE and MetS status on intestinal and systemic inflammation will be evaluated at day 28. From the collected fecal samples, protein concentrations of calprotectin (Hycult Biotech) and myeloperoxidase (Eagle Biosciences) will be determined using commercially available ELISA kits as described [51]. Systemic inflammatory responses will be evaluated from plasma by determining protein concentrations of high-sensitivity C-reactive protein, IL-6 and -8, TNFα, and myeloperoxidase using separate commercially available ELISA kits (obtained from Biocheck, Inc or R&D Systems). In addition, expression levels of genes involved in TLR4/NFκB signaling (TLR4, MyD88, p65, IL-6 and -8, TNFα, MPO, MCP-1) will be assessed from RNA isolated from peripheral blood mononuclear cells using qRT-PCR as we described [18].
3.5.7. Plasma and fecal catechins and metabolites
An Ultimate 3000 HPLC system and TSQ Quantiva triple quadruple mass spectrometer (ThermoFisher Scientific) equipped with an electrospray ionization source operated in both positive and negative mode will be used assess catechins and their related metabolites (Table 2). GTE catechins and metabolites in pooled fecal samples (days 24–28) and plasma (days 0, 14, 28) will be subjected to enzymatic hydrolysis with β-glucuronidase/sulfatase and the ethyl acetate extract will be dried under nitrogen gas. Reconstituted samples will then be injected on an Xterra RP-C18 column (Waters Corporation, 3.9 × 100 mm, 3.5 μm) for binary gradient separation at 0.9 mL/min using mobile phase A (89.9:10.0:0.1 water:acetonitrile:formic acid) and mobile phase B (69.9:30.0:0.1 water:acetonitrile:formic acid). Separation time is 11 min. The retention time and selected reaction monitoring (SRM transitions of target compounds) are established from purified standards (Cayman Chemical). Therefore, orthogonal information on the retention time and two pairs of SRM transitions can be used to detect and identify phenolic compounds (Table 2). When analyzing biological samples, a mixture of polyphenol standards will be evaluated every 10 injections to monitor instrument stability.
Table 2.
Mass spectrometry detection parameters for select phenolic compounds.1
| Compound | RT (min) | RT Window (min) | Polarity | Precursor (m/z) | Product (m/z) | Collision Energy (V) | RF Lens (V) |
|---|---|---|---|---|---|---|---|
| Epigallocatechin | 2.59 | 1 | Negative | 305.1 | 125.1 | 22.2 | 82 |
| 179.1 | 15.9 | ||||||
| 3,4-Dihydroxybenzoic acid | 2.63 | 1 | Negative | 153.3 | 91.0 | 25.6 | 61 |
| 109.1 | 15.6 | ||||||
| m-Coumaric acid | 2.72 | 1 | Negative | 163.2 | 91.2 | 25.5 | 57 |
| 119.0 | 16.5 | ||||||
| Caffeic acid | 3.30 | 1 | Negative | 179.2 | 107.1 | 22.0 | 72 |
| 135.1 | 15.3 | ||||||
| 5-(3′,4′-Dihydroxyphenyl)-valerolactone | 3.52 | 1 | Negative | 207.2 | 122.1 | 19.6 | 50 |
| 161.0 | 23.6 | ||||||
| 5-(3′,5′)-Dihydroxyphenyl-valerolactone | 3.55 | 1 | Negative | 209.1 | 163.1 | 20.0 | 34 |
| 191.1 | 20.0 | ||||||
| Chlorogenic acid | 3.58 | 1 | Negative | 353.1 | 171.1 | 32.1 | 65 |
| 191.1 | 19.1 | ||||||
| Phlorizin | 3.81 | 1 | Negative | 435.2 | 273.0 | 15.4 | 100 |
| 297.0 | 17.3 | ||||||
| Catechin | 4.11 | 1 | Negative | 289.1 | 187.1 | 22.7 | 65 |
| 203.1 | 21.0 | ||||||
| Epicatechin | 4.11 | 1 | Negative | 289.1 | 175.0 | 20.9 | 82 |
| 205.0 | 17.3 | ||||||
| Procyanidin B2 | 4.31 | 1 | Negative | 577.2 | 407.1 | 25.0 | 106 |
| 425.1 | 15.5 | ||||||
| Epigallocatechin gallate | 5.28 | 1 | Negative | 457.2 | 125.0 | 39.4 | 65 |
| 168.9 | 17.2 | ||||||
| Ferulic acid | 6.43 | 1 | Negative | 193.2 | 134.0 | 14.6 | 62 |
| 178.2 | 10.3 | ||||||
| Epicatechin gallate | 6.63 | 1 | Negative | 441.1 | 245.0 | 26.5 | 65 |
| 259.0 | 20.8 | ||||||
| Quercetin | 6.76 | 1 | Negative | 301.0 | 151.1 | 24.0 | 117 |
| 179.1 | 18.0 | ||||||
| Quercetin 3-D-galacoside | 6.81 | 1 | Negative | 463.1 | 271.0 | 43.0 | 113 |
| 299.9 | 26.6 | ||||||
| Naringenin | 6.82 | 1 | Negative | 271.2 | 119.1 | 27.6 | 75 |
| 151.0 | 20.0 |
1Abbreviations: m/z, mass-to-charge ratio; RF, radio frequency; RT, retention time.
4. Data analysis and management
4.1. Power analysis
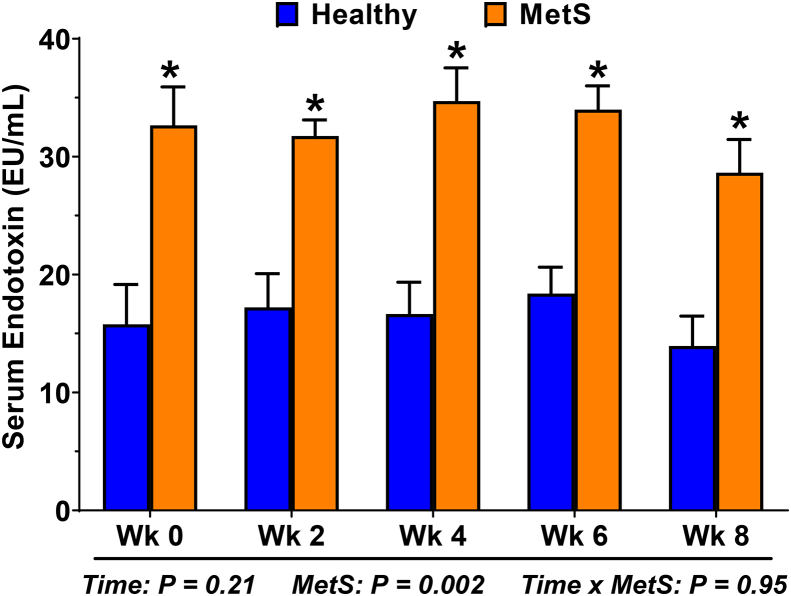
A power analysis was performed using serum endotoxin (day 28) as the primary outcome of the clinical trial. Mean serum endotoxin of MetS and healthy adults (32.4 ± 4.4 vs 16.4 ± 7.8; Fig. 4), which was determined from five measures performed at 2-wk intervals, was used to predict a conservative 30% improvement by GTE in MetS persons; this corresponds to a 10 EU/mL decrease in endotoxin in MetS. Simulations were used to estimate power based on 20 healthy and 20 MetS adults randomized to treatment vs. control in a 50/50 ratio, respectively (Fig. 3). Based on a linear mixed model with fixed treatment, health status, and time period effects and a random effect for subject to account for repeated measures on each subject, we expect >90% power (α = 0.05) to detect the primary contrast of interest (GTE vs. placebo in MetS adults) and >85% power (α = 0.05) to detect an interaction between treatment and health status.
Fig. 4.
Persons with metabolic syndrome (MetS) have metabolic endotoxemia. Serum endotoxin was evaluated at 2-wk intervals in persons with MetS and age-/gender-matched healthy adults who completed an earlier study [51]. Measures were performed using a high-sensitivity fluorescence-based endotoxin kit according to the manufacturer's instructions (PyroGene recombinant Factor C Assay; Lonza Int). Data (means ± SE; n = 10/group) were analyzed by 2-way RM ANOVA to assess effects due to time and MetS status. Endotoxin was unaffected by time, but was >2-times higher among MetS persons at each time point (*, P < 0.05).
4.2. Statistical analysis
Data (means ± SD) will be reported by cohort, and by gender within cohort. Most data will be analyzed by linear mixed effects models (LMMs) with random effect for subjects (to account for repeated measures) and fixed effects for health status, gender, GTE treatment, time period, and their interactions. Treatment by time period interaction will test for whether carry-over effects are significant, and data visualization will assess whether baseline levels are consistent between the two time periods. Multivariable regression analysis will define correlations between study variables with consideration of potential covariates. In all cases, modeling assumptions (e.g. normality) will be evaluated, and if substantially violated, accounted for by performing suitable transformations and/or alternative modeling approaches (e.g. generalized estimating equations). Our group will follow the CONSORT 2010 standards for reporting results from randomized trials [52] and the TiDieR standards for description of interventions [53]. Use of R Markdown to encapsulate code and generate reports in a single document will be followed to ensure reproducibility of all reported results. Statistical significance for all analyses will be set at P ≤ 0.05.
4.3. Data management
Various assays will be performed upon collection to preserve analyte stability and some when all samples are collected to decrease assay variability. The principal investigator (RSB) will be ultimately responsible for data quality and control and will oversee: the clinical trial, protection of subject and data confidentiality and assignment of subject numbers, and most biomolecular studies. A collaborating biostatistician (GNB) will be responsible for the randomization scheme and executing the statistical analysis plan. A collaborating microbiologist (ZY) and MS-metabolomics expert (JZ) will also assist with genomic and mass spectrometry experiments, respectively, and a food scientist (YV) will oversee the manufacture of test confections. To coordinate data archival and analysis, a data management plan (DMP) will be housed on an encrypted cloud-based service that ensures accessibility to team members. The DMP details standard operating procedures for biospecimen collection and procedures to report each clinical and/or experimental endpoint (e.g. file naming, data format) and integrating those data into a database that will be housed on the cloud-based service. Each investigator will be responsible for data quality control, maintaining original data sheets, and uploading raw and final data to the cloud-based service in a format readable to all team members. Annual training of the DMP will be performed and individual sessions as needed for new personnel. In the event that any personnel leave the project, contingencies are in place to restrict their access to the cloud-based service.
5. Discussion
This planned investigation is the first clinical intervention trial to examine GTE on metabolic endotoxemia relative to the interactive effects of gut barrier dysfunction, dysbiosis, and chronic inflammatory responses implicated in MetS progression. Based on our preclinical evidence in murine models [18,19], GTE is expected to decrease serum endotoxin in a time-dependent manner and to a greater extent in MetS persons due to their underlying metabolic endotoxemia (Fig. 4). Essentially, endotoxemia can be alleviated by decreasing gut-derived endotoxin translocation by (1) improving gut barrier integrity and/or (2) decreasing pathogenic LPS-containing Gram-negative bacteria populations (e.g. Proteobacteria) of the gut microbiota; this study considers both aspects. Indeed, region-specific measures of gut permeability are expected to demonstrate that GTE restores intestinal integrity to limit endotoxin absorption, especially in the distal gut (i.e. based on urinary sucralose/erythritol6–24 h) where GTE has been reported to improve tight junction protein expression in obese mice [17]. Further, antimicrobial and prebiotic activities of GTE catechins [27,[54], [55], [56]] are expected to alleviate gut dysbiosis in association with increasing commensal, SCFA-producing bacteria (e.g. Clostridia). Not only do SCFA (e.g. butyrate) regulate intestinal tight junction expression [57,58], their anti-inflammatory activities help to mitigate oxidative tissue injury [16]. Regardless of the mechanism by which GTE decreases endotoxemia, lowered serum endotoxin is expected to correlate with decreased plasma LBP:sCD14 and mRNA expression levels of TLR4/NFκB-dependent inflammatory genes in PBMCs. Thus, the gut-level health benefits of GTE catechins that reduce host inflammatory responses could help block and/or reverse the pathogenic progression of MetS, which would be evaluated in a longer-term intervention.
5.1. Strengths and limitations
Strengths of this study include its innovative focus, rigorous cross-over experimental design, and significance for public health. The planned research is a substantial departure from the status quo that has historically evaluated dietary interventions on cardiometabolic endpoints (e.g. insulin resistance, dyslipidemia). Rather, our innovative approach targets an early, intestinal-level pathological insult that drives MetS complications. Indeed, no controlled trials have examined GTE on metabolic endotoxemia in relation to health-promoting gut barrier functions that alleviate host inflammatory responses that initiate the sequela of MetS [4]. Further, our approach with a GTE-rich confection and matched placebo confection ensures treatment blinding, promotes adherence, and potential translation of product to commercial market. This approach also recognizes consumers’ increased preference for healthy snacks [59]; it overcomes the inconvenience of brewing tea or taking supplements; and it circumvents a barrier in that no foods in the American diet are catechin-rich other than green tea, which is consumed to a lesser extent that catechin-deplete black tea [60,61].
Our cross-over experimental design in both healthy persons and those with MetS is an additional strength of the planned study focusing on gut health. Despite evidence that diet and microbiota interact towards the development of MetS, few studies in humans have examined GTE on gut microbiota; the findings have been equivocal with some showing favorable alterations in bacteria populations [62,63] whereas others reported no detectable effect on microbiota composition [64]. These discrepancies may be explained by the non-randomized [62,63] or parallel design of the trials [64] or the enrollment of only healthy persons who are unlikely to have gut dysbiosis that is frequently observed in obese individuals [65,66]. Further, these studies had limited dietary control, especially with regard to polyphenol intakes. Thus, our cross-over design with GTE and placebo confections, enrollment of MetS and healthy persons, and dietary control to limit polyphenols and dietary fiber will enable effective hypothesis testing of the interaction between MetS status and GTE on microbiota composition and functions.
Lastly, our planned studies are anticipated to have public health and scientific significance because they represent the first translational study in MetS persons examining GTE to manage disease-promoting, intestinal-level responses leading to endotoxemia and TLR4/NFκB inflammation. Indeed, outcomes of this intervention that integrate clinical, biochemical, metagenomic, and metabolomics aspects could advance a timely evidence-based dietary recommendation with an easily implementable GTE confection to help mitigate the growing burden of MetS by ameliorating endotoxin-TLR4 signaling.
The primary challenge of this study is that the contribution of each of the proposed mechanisms (altered microbiota community vs. improved gut barrier function vs. reduced TLR4/NFκB signaling; Fig. 2) cannot be assessed independently. Rather, we will measure the cumulative impact of GTE on all three outcomes. However, regression analysis will be used to assess the strength of these relationships for future hypothesis testing in preclinical models. Because our investigative approach utilizes a “whole food” GTE intervention, we will also not be able to assess the individual benefits of specific catechins (Fig. 1). While the latter may be perceived as a limitation, it actually addresses consumers’ growing interest in health-promoting dietary patterns and foods rather than isolated food constituents or dietary supplements. Nonetheless, provided our hypothesis is confirmed, future studies examining the reciprocal benefits of catechins and their microbial metabolites (e.g. valerolactones) with the gut microbiota will improve an understanding of the GTE mechanism of action. Another limitation of this study is its short duration. For this first of its kind trial, we are targeting metabolic endotoxemia in relation to gut barrier function rather than efficacy to alleviate MetS symptoms. Provided that our hypothesis is correct in that GTE resolves metabolic endotoxemia, studies of extended duration will be needed to evaluate the efficacy of GTE on the development and progression of MetS. Future studies will also be needed to examine the dose-response effects of GTE catechins and the benefits of GTE on MetS-related complications (e.g. type 2 diabetes, nonalcoholic fatty liver).
5.2. Conclusions
Growing evidence supports the premise that increased intestinal permeability and consequent absorption of gut-derived endotoxin and its activation of TLR4/NFκB signaling is an underlying risk factor for MetS and related cardiometabolic diseases. However, no controlled studies in humans have evaluated the potential gut-level benefits of GTE to alleviate endotoxemia-associated inflammation. The protocol described herein will address this critical knowledge gap concerning the benefits of GTE catechins acting at the gut to attenuate endotoxin absorption. Thus, outcomes will not only provide foundation for mechanistic studies in vitro examining prebiotic and antimicrobial activities of GTE catechins relative to gut barrier function, but also help to establish evidence-based dietary recommendations for a health-promoting, commercially translatable, catechin-rich food that can potentially reduce cardiometabolic morbidity.
Acknowledgements
This work is primarily supported by USDA-NIFA (USDA-NIFA 2019-67017-29259), and ancillary support has been provided by USDA-HATCH (OHO01452-MRF) and The Ohio State University (OSU) Ohio Agricultural Research and Development Center as well as the Center for Applied Plant Sciences.
References
- 1.Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Prevalence of the metabolic syndrome in the United States, 2003-2012. J. Am. Med. Assoc. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Vol. 120. National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity, Circulation; 2009. pp. 1640–1645. (International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity, Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention). [DOI] [PubMed] [Google Scholar]
- 3.Cornier M.A., Dabelea D., Hernandez T.L., Lindstrom R.C., Steig A.J., Stob N.R., Van Pelt R.E., Wang H., Eckel R.H. The metabolic syndrome. Endocr. Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 5.Neves A.L., Coelho J., Couto L., Leite-Moreira A., Roncon-Albuquerque R., Jr. Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 2013;51:R51–R64. doi: 10.1530/JME-13-0079. [DOI] [PubMed] [Google Scholar]
- 6.Fialho A., Fialho A., Thota P., McCullough A., Shen B. Higher visceral to subcutaneous fat ratio is associated with small intestinal bacterial overgrowth. Nutr. Metab. Cardiovasc. Dis. 2016;26:773–777. doi: 10.1016/j.numecd.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Carnevale R., Pastori D., Nocella C., Cammisotto V., Baratta F., Del Ben M., Angelico F., Sciarretta S., Bartimoccia S., Novo M., Targher G., Violi F. Low-grade endotoxemia, gut permeability and platelet activation in patients with impaired fasting glucose. Nutr. Metab. Cardiovasc. Dis. 2017;27:890–895. doi: 10.1016/j.numecd.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Mokkala K., Pellonpera O., Roytio H., Pussinen P., Ronnemaa T., Laitinen K. Increased intestinal permeability, measured by serum zonulin, is associated with metabolic risk markers in overweight pregnant women. Metabolism. 2017;69:43–50. doi: 10.1016/j.metabol.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Navarrete J.M., Sabater M., Ortega F., Ricart W., Fernandez-Real J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 11.Traber M.G., Buettner G.R., Bruno R.S. The relationship between vitamin C status, the gut-liver axis, and metabolic syndrome. Redox Biol. 2019;21 doi: 10.1016/j.redox.2018.101091. 101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai K., Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ. 1995;310:693–696. doi: 10.1136/bmj.310.6981.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park H.J., DiNatale D.A., Chung M.Y., Park Y.K., Lee J.Y., Koo S.I., O'Connor M., Manautou J.E., Bruno R.S. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J. Nutr. Biochem. 2011;22:393–400. doi: 10.1016/j.jnutbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Chung M.Y., Park H.J., Manautou J.E., Koo S.I., Bruno R.S. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J. Nutr. Biochem. 2012;23:361–367. doi: 10.1016/j.jnutbio.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung M.Y., Mah E., Masterjohn C., Noh S.K., Park H.J., Clark R.M., Park Y.K., Lee J.Y., Bruno R.S. Green tea lowers hepatic COX-2 and prostaglandin E2 in rats with dietary fat-induced nonalcoholic steatohepatitis. J. Med. Food. 2015;18:648–655. doi: 10.1089/jmf.2014.0048. [DOI] [PubMed] [Google Scholar]
- 16.Masterjohn C., Bruno R.S. Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutr. Rev. 2012;70:41–56. doi: 10.1111/j.1753-4887.2011.00440.x. [DOI] [PubMed] [Google Scholar]
- 17.Dey P., Sasaki G.Y., Wei P., Li J., Wang L., Zhu J., McTigue D., Yu Z., Bruno R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019;67:78–89. doi: 10.1016/j.jnutbio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Sasaki G.Y., Dey P., Chitchumroonchokchai C., Labyk A.N., McDonald J.D., Kim J.B., Bruno R.S. Green tea extract protects against hepatic NFkappaB activation along the gut-liver axis in diet-induced obese mice with nonalcoholic steatohepatitis by reducing endotoxin and TLR4/MyD88 signaling. J. Nutr. Biochem. 2018;53:58–65. doi: 10.1016/j.jnutbio.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Sapper T.N., Mah E., Moller M.V., Kim J.B., Chitchumroonchokchai C., McDonald J.D., Bruno R.S. Green tea extract treatment reduces NFkappaB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 2017;41:34–41. doi: 10.1016/j.jnutbio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Park H.J., Lee J.Y., Chung M.Y., Park Y.K., Bower A.M., Koo S.I., Giardina C., Bruno R.S. Green tea extract suppresses NFkappaB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. J. Nutr. 2012;142:57–63. doi: 10.3945/jn.111.148544. [DOI] [PubMed] [Google Scholar]
- 21.Roberts K.M., Grainger E.M., Thomas-Ahner J.M., Hinton A., Gu J., Riedl K.M., Vodovotz Y., Abaza R., Schwartz S.J., Clinton S.K. Application of a low polyphenol or low ellagitannin dietary intervention and its impact on ellagitannin metabolism in men. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapper T.N., Mah E., Ahn-Jarvis J., McDonald J.D., Chitchumroonchokchai C., Reverri E.J., Vodovotz Y., Bruno R.S. A green tea-containing starch confection increases plasma catechins without protecting against postprandial impairments in vascular function in normoglycemic adults. Food Funct. 2016;7:3843–3853. doi: 10.1039/c6fo00639f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y., Mah E., Davis C.G., Jalili T., Ferruzzi M.G., Chun O.K., Bruno R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013;57:896–905. doi: 10.1002/mnfr.201200619. [DOI] [PubMed] [Google Scholar]
- 24.Kuriyama S. The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J. Nutr. 2008;138 doi: 10.1093/jn/138.8.1548S. 1548S-53S. [DOI] [PubMed] [Google Scholar]
- 25.Yang J., Mao Q.X., Xu H.X., Ma X., Zeng C.Y. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang J., Zhang Z., Zheng T.Z., Bassig B.A., Mao C., Liu X., Zhu Y., Shi K., Ge J., Yang Y.J., Dejia H., Bai M., Peng Y. Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta-analysis. Int. J. Cardiol. 2016;202:967–974. doi: 10.1016/j.ijcard.2014.12.176. [DOI] [PubMed] [Google Scholar]
- 27.Okubo T., Ishihara N., Oura A., Serit M., Kim M., Yamamoto T., Mitsuoka T. In vivo effects of tea polyphenol intake on human intestinal microflora and metabolism. Biosci. Biotechnol. Biochem. 1992;56:588–591. doi: 10.1271/bbb.56.588. [DOI] [PubMed] [Google Scholar]
- 28.Jin J.S., Touyama M., Hisada T., Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012;56:729–739. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 29.Suez J., Korem T., Zilberman-Schapira G., Segal E., Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microb. 2015;6:149–155. doi: 10.1080/19490976.2015.1017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J., Ahn-Jarvis J.H., Vodovotz Y. Development and characterization of different black raspberry confection matrices designed for delivery of phytochemicals. J. Food Sci. 2015;80:E610–E618. doi: 10.1111/1750-3841.12808. [DOI] [PubMed] [Google Scholar]
- 31.Fisher E.L., Ahn-Jarvis J., Gu J., Weghorst C.M., Vodovotz Y. Assessment of physicochemical properties, dissolution kinetics and storage stability of a novel strawberry confection designed for delivery of chemopreventive agents. Food Struct. 2014;1:171–181. [Google Scholar]
- 32.Sessler T., Weiss J., Vodovotz Y. Influence of pH and soy protein isolate addition on the physicochemical properties of functional grape pectin confections. Food Hydrocolloids. 2013;32:294–302. [Google Scholar]
- 33.King D.E., Mainous A.G., 3rd, Lambourne C.A. Trends in dietary fiber intake in the United States, 1999-2008. J. Acad. Nutr. Diet. 2012;112:642–648. doi: 10.1016/j.jand.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Pei R., DiMarco D.M., Putt K.K., Martin D.A., Gu Q., Chitchumroonchokchai C., White H.M., Scarlett C.O., Bruno R.S., Bolling B.W. Low-fat yogurt consumption reduces biomarkers of chronic inflammation and inhibits markers of endotoxin exposure in healthy premenopausal women: a randomised controlled trial. Br. J. Nutr. 2017;118:1043–1051. doi: 10.1017/S0007114517003038. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell C.M., Davy B.M., Halliday T.M., Hulver M.W., Neilson A.P., Ponder M.A., Davy K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials. 2015;45:328–337. doi: 10.1016/j.cct.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dastych M., Dastych M., Jr., Novotna H., Cihalova J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn's disease. Dig. Dis. Sci. 2008;53:2789–2792. doi: 10.1007/s10620-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 37.Camilleri M., Nadeau A., Lamsam J., Nord S.L., Ryks M., Burton D., Sweetser S., Zinsmeister A.R., Singh R. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neuro Gastroenterol. Motil. 2010;22:e15–26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Wijck K., van Eijk H.M., Buurman W.A., Dejong C.H., Lenaerts K. Novel analytical approach to a multi-sugar whole gut permeability assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2794–2801. doi: 10.1016/j.jchromb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Del Valle-Pinero A.Y., Van Deventer H.E., Fourie N.H., Martino A.C., Patel N.S., Remaley A.T., Henderson W.A. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin. Chim. Acta. 2013;418:97–101. doi: 10.1016/j.cca.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kigerl K.A., Hall J.C., Wang L., Mo X., Yu Z., Popovich P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 2016;213:2603–2620. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park T., Yu Z. Do ruminal ciliates select their preys and prokaryotic symbionts? Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01710. 1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z., Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36:808–812. doi: 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]
- 43.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodriguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., 2nd, Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft JJJ, Vargas F., Vazquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019 doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole J.R., Wang Q., Fish J.A., Chai B., McGarrell D.M., Sun Y., Brown C.T., Porras-Alfaro A., Kuske C.R., Tiedje J.M. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson M.J., Walsh D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol. Monogr. 2013;83:557–574. [Google Scholar]
- 47.Douglas G.M., Maffei V.J., Zaneveld J., Yurgel S.N., Brown J.R., Taylor C.M., Huttenhower C., Langille M.G.I. 2019. PICRUSt2: an Improved and Extensible Approach for Metagenome Inference. bioRxiv (Preprint) 672295. [Google Scholar]
- 48.Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-6-r60. R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J., Lin K., Sequeira C., Borchers C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta. 2015;854:86–94. doi: 10.1016/j.aca.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Mah E., Sapper T.N., Chitchumroonchokchai C., Failla M.L., Schill K.E., Clinton S.K., Bobe G., Traber M.G., Bruno R.S. alpha-Tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: a randomized, double-blind, crossover trial. Am. J. Clin. Nutr. 2015;102:1070–1080. doi: 10.3945/ajcn.115.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz K.F., Altman D.G., Moher D., Group C. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann T.C., Glasziou P.P., Boutron I., Milne R., Perera R., Moher D., Altman D.G., Barbour V., Macdonald H., Johnston M., Lamb S.E., Dixon-Woods M., McCulloch P., Wyatt J.C., Chan A.W., Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348 doi: 10.1136/bmj.g1687. g1687. [DOI] [PubMed] [Google Scholar]
- 54.Lee H.C., Jenner A.M., Low C.S., Lee Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Tzounis X., Vulevic J., Kuhnle G.G., George T., Leonczak J., Gibson G.R., Kwik-Uribe C., Spencer J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 56.Fan F.Y., Sang L.X., Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22 doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A., Weir T.L., Ehrentraut S.F., Pickel C., Kuhn K.A., Lanis J.M., Nguyen V., Taylor C.T., Colgan S.P. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H.B., Wang P.Y., Wang X., Wan Y.L., Liu Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 59.Piernas C., Popkin B.M. Snacking increased among U.S. adults between 1977 and 2006. J. Nutr. 2010;140:325–332. doi: 10.3945/jn.109.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim K., Vance T.M., Chun O.K. Estimated intake and major food sources of flavonoids among US adults: changes between 1999-2002 and 2007-2010 in NHANES. Eur. J. Nutr. 2016;55:833–843. doi: 10.1007/s00394-015-0942-x. [DOI] [PubMed] [Google Scholar]
- 61.Song W.O., Chun O.K. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J. Nutr. 2008;138 doi: 10.1093/jn/138.8.1543S. 1543S-7S. [DOI] [PubMed] [Google Scholar]
- 62.Yuan X., Long Y., Ji Z., Gao J., Fu T., Yan M., Zhang L., Su H., Zhang W., Wen X., Pu Z., Chen H., Wang Y., Gu X., Yan B., Kaliannan K., Shao Z. Green tea liquid consumption alters the human intestinal and oral microbiome. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin J.S., Touyama M., Hisada T., Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012;56:729–739. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 64.Janssens P.L., Penders J., Hursel R., Budding A.E., Savelkoul P.H., Westerterp-Plantenga M.S. Long-term green tea supplementation does not change the human gut microbiota. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 66.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]