Graphical abstract
Highlights
-
•
The present work aimed to find new growth inhibitory agents against Spodoptera littoralis (Boisd.).
-
•
New derivatives of 4-aminophenol (as insect growth regulators) were synthesized in the laboratory
-
•
The chemically structure of every one of target compounds was elucidated by spectroscopic techniques.
-
•
The insecticidal activity of the synthesized compounds was evaluated against Spodopetera littoralis (Boisd.).
-
•
The activity was compared with control compound Fenoxycarb.
Keywords: Spodoptera littoralis(Boisd), Fenoxycarb, Toxicity, Insect growth regulators
Abstract
The 13 new compounds were chemically synthesized and their spectroscopic analysis was done to determine their chemical structure. All the compounds were screened for their insecticidal potential against Spodoptera littoralis (Boisd.). Among the tested compounds, the compound 13 was found to be the most potent. It displayed one fold more activity than a reported insect growth regulator, fenoxycarb. The other target compounds demonstrated weak to strong toxicological activities against Spodoptera littoralis (Boisd.).
1. Introduction
Spodoptera littoralis (Boisd.) is considered one of key insect that reason incredible harm to cotton plants and other different plants in Egypt [1,2] the instar larvae of this insect can feed on about ninety economically plant kind belonging to 40 families. To battle the insect growth, producers utilize prepared organic insecticides [3] and some biorational operators, for example, Bacillus thuringiens is Berliner, however the accomplished control isn't successful enough because high ability to create opposition toward of the majority of conventional compounds. Consequently, researchers and producers are looking for elective materials that are viable against this insect, safe to people, natural well disposed, and good inside focused bug the board (IPM) practices [4]. The elective control strategies that is promising as a potential instrument in S. littoralis safe administration projects is the utilization of biorational control specialists, for example, synthetic insect growth regulators (IGRs) and those dependent on normally inferred product [5,6]. IGRs are professed to be more secure for valuable creatures than customary items, and they have been effectively utilized in IPM programs against many tree and little fruit insects [7]. There is a need for different insecticides having different modes of action. We found while searching at the desired and synthesis of juvenile hormone analogs [8,9] of pests to be evaluate against the S. littoralis (Boisd.).The prepared compounds displayed a variable level of action activity against this pests, and a number of them were most dynamic activity than the normally juvenile hormones [[10], [11], [12], [13]]. Considering that the pests, after treatment with JHAs, were less defenseless to characteristic contaminations with the S. littoralis than normal non treated insects [14]. Shockingly, they demonstrated a changeful level of activity, some of them being very active in inhibiting cell expansion of this pests [15]. Toward the start, the well-known insect growth regulator fenoxycarb was utilized as standard control since it carried on as an exceedingly active operator against larvae of S. littoralis [10]. Be that as it may, some adjusted chemical structures have the 4-phenoxyan carbamate were observed to be more active than fenoxycarb in investigations against S. littoralis cells [16]. The mode of action of these compounds have been studied and there is evidence that there is a restrain sterol biosynthesis inside the cells [17].
2. Materials and methods
Estimating of the MP. for all prepared target compounds was completed on a Fisher-John mechanical technique. By utilizing a Vario EL C, H, N, S analyzer, basic examinations (C, H, N, and S) were elucidated. On a Pye-Unicam SP3-100 spectrophotometer IR spectra were gotten by utilizing the KBr disc technique. 1H NMR and 13C NMR spectra were estimated on Bruker 400 MHz spectrometers utilizing tetramethylsilane (TMS) as a source of perspective and concoction movements were accounted for as ppm. By utilizing a Jeol JMS-400 mass spectra were completed. Fenoxycarb juvenile hormone analogues as an insect growth regulators insecticide was buyfrom Sigma-Aldrich. The numbers of S. littoralis insects were gathered from cotton leave worm, fields of Assiut University. Toxic activity of the thirteen compounds comparing with fenoxycarb as reported insecticide was tested against the instar larvae of S. littoralis.
3. Result and Discussion
3.1. Chemistry
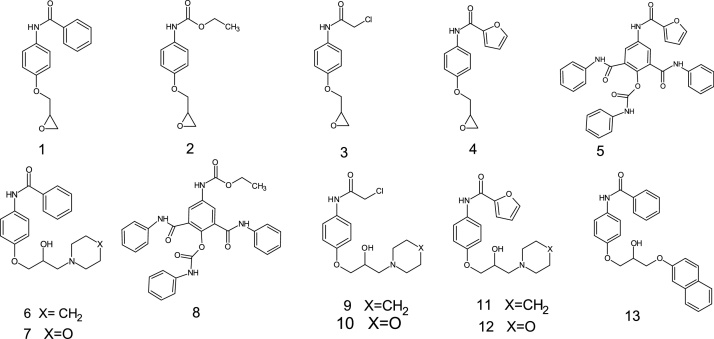
As following our project in prepared and toxicity evaluate the biological activity of juvenile hormones analogues, here in thirteen tested compounds where shown in (Fig. 1) to determined their toxicity as insecticides. The thirteen compounds, namely, N-[4-(oxiran-2-ylmethoxy)phenyl]benzamide 1, ethyl[4-(oxiran-2-ylmeth-oxy)phenyl]carb- amate 2, 2-chloro-N-[4(oxiran-2-ylmethoxy)phenyl]acetamide 3, N-[4-(oxiran-2-ylmethoxy)phenyl]furan-2-carboxamide 4, 4-(furan-2-carboxamido)-2,6-bis(phenylcabamoyl)phenylphenylcarbamate 5, N-{4-[2-hydroxy-3-(piperidine-1-yl)propoxy]phenyl}benzamide 6, N-{4-[2-hydroxy-3-(morpholin-4-yl)propoxy]phenyl}benzamide 7, Ethyl(3,5-bis(phenylcarbmoyl)((phenylcarbamoyl)oxy)pheny) carbamate 8, 2-chloro-N-{4-[2-hydroxy-3-(piperidin-1-yl)propoxy]phenyl}acetamide 9, 2-chloro-N-{4-[2-hydroxy-3-(morpholin-4-yl)propoxy]phenyl}acetamide 10, N-{4-[2-hydroxy-3-(piperidin-1-yl)propoxy]phenyl}furan-2-carboxamide 11, N-{4-[2-hydroxy-3-(morpholin-4-yl)propoxy]phenyl}furan carboxamide, N-{4-[2-hydroxy-3-(morpholin-4-yl)propoxy]phenyl}furan-2-carboxamide 12 and N-[4-(2-hydroxnapthoxypropoxy)phenyl]benzamide 13.
Fig. 1.
Purposed compounds that tested against S. littoralis.
3.2. Experimental
A Fisher-Johns instruments were practiced to register the melting points of every prepared compounds. Infra-red and elemental analyses (C, H, N, and S) were achieved through a PyeUnicam SP3-100 spectro-photometer utilizing the KBr disk strategy and a Vario EL C, H, N, S analyzer, separately. A Bruker 400 MHz spectrometer was utilized to measure DEPT 135 spectra and the 1H and 13C NMR spectra within the TMS as an interior standard. Reaction headway and perfection of the prepared sections were checked by thin layer chromatography.
General procedure of synthetic oxirane ring (1-4) By reaction of 4-hydroxyphenyl acetamide derivatives (0.04 mol) with epichlorohydrin (0.12 mol) in presence of sodium hydroxide 25 % in water was stirred in ice bath for 4 h, compounds (1–4) were prepared, The formed precipitate was filtered off and recrystallized from methanol.
3.3. N-[4-(oxiran-2-ylmethoxy)phenyl]benzamide (1)
Pale red crystals. Yield: 83 %; MP: 102−104 °C. IR (ν) (KBr) cm−1: 3328 (NH), 3050, 3027 (C–H Aromatic), 2922, 2827 (C–H aliphatic).1HNMR (DMSO-d6): δ 10.11 (s, 1H, NH), 6.19 − 7.99 (m, 9H Ar-H), 4.33 (s, 1H, CH), 3.8 (m, 2H, CH2), 2.8 (s, 1H, CH), 2.7 (s, 1H, CH). 13C NMR (DMSO-d6): δ 165.61, 155.02, 135.54, 13307, 132.66, 131.82, 128.80, 128.00, 122.44, 69.60, 50.22, 40.69. Dept 135: δ 131.80, 128.78, 127.99, 122.49, 122.47, 115.01, 114.91, 69.63(CH2, 44.24 (CH2). Elemental analysis calculated for C16H15NO3 (%) Calcd. /found; C: 71.36/71.34, H: 5.61/5.60, N: 5.20/5.21.
3.4. Ethyl [4-(oxiran-2-ylmethoxy)phenyl]carbamate (2)
White crystals. Yield: 90%; MP: 86-89 °C. IR (ν) (KBr) cm−1: 3322 (NH), 3059, 3018 (C-H Aromatic), 2901, 2880 (C-H aliphatic) 1710 (C = O).1HNMR (DMSO-d6): δ 9.35 (s, 1H, NH), 7.36 − 7.38 (s, 2H Ar-H), 6.80 − 6.90 (s, 2H Ar-H), 4.27 (s, 1H, CH), 4.26 (s, 2H, CH2), 3.95 (s, 1H, CH), 3.31 (s, 1H, CH), 2.83 (s, 1H, CH), 2.84 (s, 1H, CH), 1.3 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 157.01 153.31, 149.92, 136.04, 119.12, 89.22, 39.44, 38.14, 28.22, 27.69. Elemental analysis calculated for C12H15NO4 (%) Calcd. /found; C: 60.75/60.74, H: 6.37/6.35, N: 5.90/5.89.
3.5. 2-Chloro-N-[4-(oxiran-2-ylmethoxy)phenyl]acetamide (3)
White crystals. Yield: 72%; MP: 146-148 °C. IR (ν) (KBr) cm−1: 3267 (NH), 3093 (C–HAromatic), 2920 (C–H aliphatic), 1660 (C = O).1HNMR (DMSO-d6): δ 10.10 (s, 1H, NH), 7.50 − 7.52 (s, 2H Ar-H), 6.93 − 6.95 (s, 2H Ar-H), 4.30 (s, 1H, CH), 4.27 (s, 2H, CH2), 3.85 (s, 1H, CH), 3.31 (s, 1H, CH), 2.85 (s, 1H, CH), 2.82 (s, 1H, CH). 13C NMR (DMSO-d6): δ 158.02, 153.41, 143.12, 133.59, 126.12, 117.03, 38.14, 27.22, 28.01. Elemental analysis calculated for C11H12ClNO3 (%) Calcd. /found; C: 54.67/54.55, H: 5.00/4.98, N: 5.80/5.78.
3.6. N-[4-(oxiran-2-ylmethoxy)phenyl]furan-2-carboxamide (4)
Brown powder. Yield: 69 %; MP: 166−168 °C. IR (ν) (KBr) cm−1: 3346 (NH), 3185 (C–HAromatic), 2941 (C–H aliphatic), 1661 (C = O).1HNMR (DMSO-d6): δ 10.03 (s, 1H, NH), 6.69 − 7.90 (m, 7H Ar-H), 4.32 (s, 1H CH), 3.33 (s, 1H, CH), 3.29 (s, 2H, CH2), 2.85 (s, 1H, CH), 2.71 (s, 1H, CH). 13C NMR (DMSO-d6): δ 158.43, 156.31, 143.44, 131.59, 129.63, 127.22, 126.12, 120.17, 117.03, 114.33, 38.13, 28.201. Elemental analysis calculated for C14H13NO4 (%) Calcd. /found; C: 64.86/64.88, H: 5.05/5.505, N: 5.40/5.39.
General procedure of synthetic compounds (5, 8)via reaction of 4-hydroxyphenyl acetamide derivatives (0.09 mmol) with phenyl isocyanate (0.02 mmol) and tow drops of triethylamine as catalyst in 30 ml 1,4-dioxan, the reaction mixture was refluxed for 5 h, The formed precipitate was filtered off and recrystallized from methanol.
3.7. 4-(Furan-2-carboxamido)-2,6-bis(phenylcabamoyl)phenylphenylcarbamate (5)
White powder. Yield: 70%; MP: 143-146 °C. IR (ν) (KBr) cm−1: 3288 (2NH), 3138 (C-H Aromatic), 1716, 1648 (C = O).1HNMR (DMSO-d6): δ 10.22 (s, 1H, NH), 10.14 (s, 1H, NH), 8.61 (s, 2H, 2NH), 6.71 − 7.93 (m, 20H Ar-H). 13C NMR (DMSO-d6): δ 156.43, 153.31, 150.44, 130.01, 127.32, 127.19, 119.63, 118.22, 118.02, 112.92. Elemental analysis calculated for C32H24N4O6 (%) Calcd. /found; C: 68.56/68.49, H: 4.32/4.30, N: 9.99/9.98.
General procedure of synthetic compounds (6-13) A mixture of oxirane ring derivatives (compounds 1-4) (1 mmol), the nucleophilic reagent added (piperidine, morpholine, 2-naphthol) (3 mmol), add drops of triethylamine in ethanol was stirred and refluxed at ambient temperature for 5 h to give a precipitate which filtered off and recrystallized from 1,4-Dioxan.
3.8. N-{4-[2-hydroxy-3-(pipridin-4-yl)propoxy]phenyl}benzamide (6)
White powder. Yield: 62%; MP: 155-158 °C. IR (ν) (KBr) cm−1:3496, 3395 (NH, OH), 3055 (C–HAromatic), 2924(C–H aliphatic), 1627 (C = O). 1HNMR (DMSO-d6): δ 10.11 (s, 1H, NH), 7.50 − 7.97 (m, 9H Ar-H), 5.52 (s, 1H, OH), 4.00 (m, 3H, CH), 3.8 (m, 9H, 4CH2 +CH), 2.2 (s, 1H, CH), 1.4(s, 2CH, CH2). 13C NMR (DMSO-d6): δ 158.03, 155.32, 140.44, 136.01, 128.39, 127.19, 118.22, 118.02, 112.9, 77.20, 56.15, 39.22, 31.13, 29.15 28.32. Elemental analysis calculated for C21H26N2O3 (%) Calcd. /found; C: 71.16/71.09, H: 7.39/7.40, N: 7.90/7.89.
3.9. N-{4-[2-hydroxy-3-(morpholin-4-yl)propoxy]phenyl}benzamide (7)
White powder. Yield: 73 %; MP: 205−208 °C. IR (ν) (KBr) cm−1:3277 (OH), 3115 (C–HAromatic), 2903 (C–H aliphatic), 1689 (C = O). 1HNMR (DMSO-d6): δ 10.11 (s, 1H, NH), 7.50 − 7.97 (m, 9H Ar-H), 5.52 (s, 1H, OH), 4.00 (m, 3H, CH), 3.8 (m, 8H, 4CH2), 1.4(s, 2CH, CH2). 13C NMR (DMSO-d6): δ 157.03, 145.70, 137.15, 127.39, 127.19, 119.55, 118.15, 111.09, 81.20, 75.15, 35.22, 33.13, 27.15 27.32. Elemental analysis calculated for C20H24N2O4 (%) Calcd. /found; C: 67.40/67.38, H: 6.74/6.72, N: 7.86/7.88.
3.10. Ethyl(3,5-bis(phenylcarbmoyl)-4-((phenylcarbamoyl)oxy)pheny)carbamate (8)
White crystals. Yield: 83 %; MP: 186−189 °C. IR (ν) (KBr) cm−1: 3326 (NH), 3194, 3132 (C–H Aromatic), 2978 (C–H aliphatic), 1733, 1694, 1645 (C = O).1HNMR (DMSO-d6): δ 10.13 (s, 1H, NH), 9.63 (s, 1H, NH), 8.64 (s, 1H, NH), 6.96 − 7.56 (s, 17H Ar-H), 4.18 (s, 2H, CH2), 1.3 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 157.32, 157.03, 156.70, 147.15, 140.89, 139.25, 137.25, 130.00, 123.23, 122.23, 121.58, 119.68, 119.05, 117. 23, 116.35, 59.93, 12.3. Elemental analysis calculated for C30H26N4O6 (%) Calcd. /found; C: 66.91/66.90, H: 4.87/4.88, N: 10.40/10.38.
3.11. 2-Chloro-N-{4-[2-hydroxy-3-(piperidin-1-yl)propoxy]phenyl}acetamide (9)
Brown powder. Yield: 44 %; MP: 158−160 °C. IR (ν) (KBr) cm−1: 3267 (NH), 3093 (C–HAromatic), 2920 (C–H aliphatic), 1658 (C = O).1HNMR (DMSO-d6): δ 10.10 (s, 1H, NH), 7.50 − 7.52 (m, 4H Ar-H), 4.30 (s, 1H, OH), 4.27 (s, 1H, CH), 3.85 (m, 4H, 2CH2), 2.85 (8, 8H, 4CH2), 1.82 (s, 4H, 2CH2). 13C NMR (DMSO-d6): δ 157.20, 134.81, 129.69, 127.21, 126.77, 123.92, 119.2, 107.25, 71.78, 55.32, 26.19, 24.64. Elemental analysis calculated for C16H23ClN2O3 (%) Calcd. /found; C: 58.80/58.78, H: 7.09/7.07, N: 8.57/8.55.
3.12. 2-Chloro-N-{4-[2-hydroxy-3-(morpholin-4-yl)propoxy]phenyl}acetamide (10)
White powder. Yield: 72 %; MP: 146−148 °C. IR (ν) (KBr) cm−1: 3267 (NH), 3093 (C–HAromatic), 2920 (C–H aliphatic), 1660 (C = O).1HNMR (DMSO-d6): δ 10.13 (s, 1H, NH), 7.98 (s, 2H Ar-H), 7.82 (s, 2H Ar-H), 4.90 (s, 1H, OH), 3.29 (s, 1H, CH), 3.85 (s, 2H, CH2), 3.12 (s, 2H, CH2), 2.20 (m, 8H, 4CH2), 1.82 (s, 2H, CH2).13C NMR (DMSO-d6): δ 157.40, 139.69, 127.71, 127.17, 119.2, 107.25, 71.78, 67.25, 55.02, 25.29, 23.44. Elemental analysis calculated for C15H21ClN2O4 (%) Calcd. /found; C: 54.79/54.77, H: 6.44/7.41, N: 8.52/8.50.
3.13. N-{4-[2-Hydroxy-3-(piperidin-1-yl)propoxy]phenyl}furan-2-carboxamide (11)
Yellow crystals. Yield: 63 %; MP: 196−198 °C. IR (ν) (KBr) cm−1: 3326 (NH), 3178, (C–H Aromatic), 2925 (C–H aliphatic), 1694, (C = O).1HNMR (DMSO-d6): δ 10.01 (s, 1H, NH), 6.98 − 7.90 (s, 7H Ar-H), 4.72 (s, 1H, OH), 3.9 (m, 3H, CH + CH2), 2.4 (s, 2H, CH2), 2.3 (s, 2H, CH2), 1.4 (m, 8H, 4CH2). 13C NMR (DMSO-d6): δ 157.33, 150.03, 159.70, 149.15, 147.89, 146.25, 140.25, 128.63, 128.23, 127.23, 52.36, 44.99, 28.98, 18. 23, 16.35. Elemental analysis calculated for C19H24N2O4 (%) Calcd. /found; C: 66.26/66.23, H: 7.02/7.00, N: 8.13/8.15.
3.14. N-{4-[2-Hydroxy-3-(morpholin-4-yl)propoxy]phenyl}furan-2-carboxamide (12)
White powder. Yield: 26 %; MP: 218-220 °C. IR (ν) (KBr) cm−1: 3326 (NH), 3302 9OH), 3078, (C–H Aromatic), 2909 (C–H aliphatic), 1653, (C = O).1HNMR (DMSO-d6): δ 10.10 (s, 1H, NH), 6.90 − 7.63 (s, 7H Ar-H), 4.72 (s, 1H, OH), 3.72 (s, 1H, CH), 2.5 (m, 4H, 2CH2), 1.3 (m, 8H, 4CH2). 13C NMR (DMSO-d6): δ 157.83, 159.70, 148.15, 148.89, 128.85, 128.65, 128.01, 172.93, 127.23, 52.36, 48.23, 38.98, 17. 23, 16.33. Elemental analysis calculated for C18H22N2O5 (%) Calcd. /found; C: 62.42/62.40, H: 6.40/6.38, N: 8.09/8.09.
3.15. N-[4-(2-Hydroxy-3-napthoxypropoxy)phenyl]benzamide (13)
White crystals. Yield: 81 %; MP: 220−222 °C. IR (ν) (KBr) cm−1: 3330 (NH), 3051 (C–H Aromatic), 2922 (C–H aliphatic), 1645 (C = O).1HNMR (DMSO-d6): δ 10.13 (s, 1H, NH), 6.96 − 7.98 (s, 16H Ar-H), 4.33 (s, 1H, OH), 4.1 (m, 5H, CH). 13C NMR (DMSO-d6): δ 166.39, 157.16, 154.75, 148.39, 139.68, 134.78, 129.74, 128.95, 127.96, 127.12, 126.82, 123.99, 119.25, 111.15, 111.27, 107.10, 107.59, 67.16, 61.33. Elemental analysis calculated for C30H26N4O6 (%) Calcd. /found; C: 66.91/66.90, H: 4.87/4.88, N: 10.40/10.38 Elemental analysis calculated for C26H23NO4 (%) Calcd. /found; C: 75.53/75.50, H: 6.61/5.63, N: 3.39/3.40.
4. Laboratory bioassay
The method that measure toxicity of the target compounds was tested by leaf dipping bioassay [18]. Results of research facility screening to discover the suitable concentrations of the objective target compounds which are deformation in the insect to kill half 50 % LC50 of instar larvae were proclaimed here. Five concentrations of arrangement of each synthesized compound in addition to 0.1 % Triton X-100 as a surfactant were used. The number of ten 2nd instar larvae and 4th instar larvae of insects, nearly have the same size, plates (9 cm. distance across) of castor bean leaves in which dunked in the objective treatment concentrations for 10 s then left to dry and offered to larvae, which starved for 4−6 reatment was reproduced multiple times (10 larvae for each). Control was dunked in distilled water only. The larvae were permitted to benefit from treated plates for 48 h., then transferred to the untreated ones. Mortality percentages were recorded after 72 h. for all insecticides. Mortality was redressed by Abbott’s formula [19]. The doses mortality relapse lines were statistically investigated by probit analysis [20]. Toxicity Index and Relative Potency determined by Sun equations [21]:
Slope esteems and middle deadly focused concentrations LC50 of the title target compounds were determined through a Probit relapse investigation program and recorded in (ppm) [20]. Were inundated for 10 s in each concentration multiple times (3 times). Pests which treated were leaved to dry at room temperature for about half hour. Control clumps of utilized pests were likewise used. The insecticidal action trial of each compound was rehashed multiple times (2 time) and the gotten data were rectified by Abbott's equation [19]. By utilizing a modernized probit relapse investigation program, middle deadly fixations (LC50) and incline estimations of objective target compounds were figured and revealed as (ppm) [20].
5. Insecticidal activity
The objective tested compounds have been used for insecticidal activity as explained beneath:
5.1. Toxicological activity of compounds against 2nd instar larvae
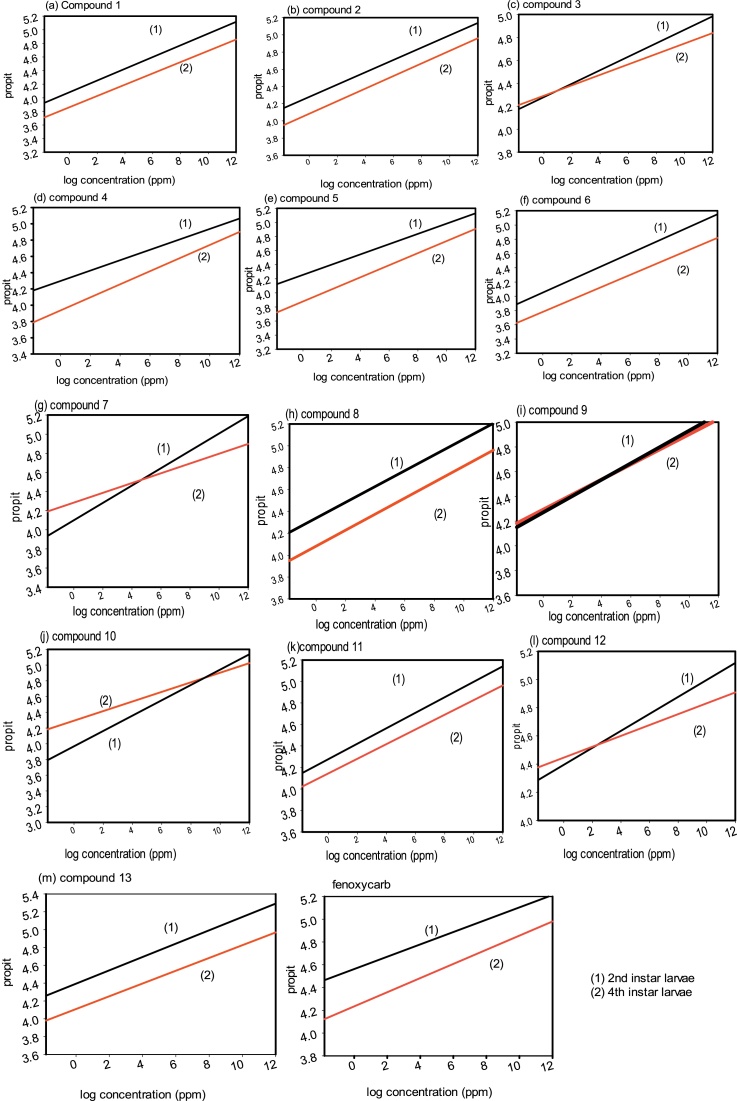
As shown in (Table 1) target compounds were tested of their activity as insecticides in which shown beneath. Thirteen previously mentioned compounds displayed strong to weak toxic action against the 2nd instar larvae in light of the fact that various of them were active than fenoxycarb after 72 h. of the test with LC50 qualities differ from 4.066 ppm for 2nd instar larvae, while fenoxycarb LC50 was 5.943 ppm for 2nd instar larvae. For example, LC50 values of compounds 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12and 13 were 26.546, 12.505, 57.622, 25.414, 15.720, 20.478, 21.424, 8.032, 37.445, 37.495, 13.885, 14.106 and 4.066 ppm, respectively in a specific order, and LC50 of fenoxycarb was 5.943 ppm. From outcomes in over, the toxicity of compound 13 against the S. littoralis larvae 4.066 ppm after 72 h of the test on the grounds that LC50 estimation of reported fenoxycarb was 5.943 ppm.
Table 1.
Insecticidal activity of compounds 1–13and juvenile hormone analogue fenoxycarb against the larvae of S. littoralis (Boisd.), after 72 h of treatments.
| 2nd instar larvae | 4th instar larvae | |||||
|---|---|---|---|---|---|---|
| Comp. | LC50(ppm) | slope | Toxic ratio | LC50 (ppm) | slope | Toxic ratio |
| fenoxycarb | 5.943 | 0.298 ± 0.0808 | 0.684 | 59.914 | 0.225 ± 0.0870 | 0.954 |
| 1 | 26.546 | 0.246 ± 0.0805 | 0.153 | 145.369 | 0.301 ± 0.0991 | 0.393 |
| 2 | 12.505 | 0.246 ± 0.0793 | 0.325 | 67.908 | 0.297 ± 0.0893 | 0.840 |
| 3 | 57.622 | 0.246 ± 0.0791 | 0.070 | 254.471 | 0.225 ± 0.0820 | 0.224 |
| 4 | 25.414 | 0.233 ± 0.0756 | 0.159 | 128.376 | 0.297 ± 0.0978 | 0.445 |
| 5 | 15.720 | 0.196 ± 0.0858 | 0.258 | 81.406 | 0.231 ± 0.0880 | 0.702 |
| 6 | 20.478 | 0.239 ± 0.0796 | 0.198 | 91.360 | 0.341 ± 0.0987 | 0.625 |
| 7 | 21.424 | 0.209 ± 0.0756 | 0.191 | 113.203 | 0.293 ± 0.0966 | 0.505 |
| 8 | 8.032 | 0.262 ± 0.0793 | 0.506 | 67.670 | 0.266 ± 0.0912 | 0.844 |
| 9 | 37.445 | 0.213 ± 0.0794 | 0.108 | 148.565 | 0.302 ± 0.0986 | 0.384 |
| 10 | 37.495 | 0.221 ± 0.0794 | 0.108 | 152.260 | 0.239 ± 0.0985 | 0.375 |
| 11 | 13.885 | 0.261 ± 0.0802 | 0.292 | 68.670 | 0.260 ± 0.0912 | 0.832 |
| 12 | 14.106 | 0.218 ± 0.0776 | 0.288 | 77.624 | 0.202 ± 0.0793 | 0.742 |
| 13 | 4.066 | 0.196 ± 0.0756 | 1 | 57.170 | 0.264 ± 0.0905 | 1 |
5.2. Toxicological activity of compounds against 4th instar larvae
As shown in (Table 1) target compounds were tested for their activity as insecticides and this is shown beneath. Thirteen previously mentioned compounds displayed strong to weak toxic action against the 4th instar larvae in light of the fact that various them were active than fenoxycarb after 72 hs of the treatment in which LC50 values changed from 57.170–254.471 ppm, while fenoxycarb LC50 was 59.914 ppm. Compounds 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13 gave a high toxicity with LC50 values of 145.369, 67.908, 254.471, 128.376, 81.406, 91.360, 113.203, 67.670, 148.565, 152.260, 68.670, 68.670 and 57.170 ppm. Comparing with fenoxycarb compound 13 shown that the highest toxicity while compounds 8, 11, 12 and 5 give a very good activity in which LC50 values are67.670, 68.670, 68.670 and 81.406 ppm, respectively.
6. Structure-activity relationship
As a resumption of our search, the structure-activity relationships were accounted for here as indicated by the poisonous activity peaks in Table 1 underneath and (Fig. 2) too. It is demonstrated that the 4-aminophenol derivatives 13 is progressively active against of S. littoralis than different compounds that prepared. The large activity related with compounds 8 and 5 might be because of the closeness of the carbamate and fuoryl group moiety independently in their chemically structure and the general qualities of the synthesized compounds.
Fig. 2.
Compounds 1–13 and reference juvenile hormone analogue fenoxycarb as insecticidal activities against the 2nd and 4th instar larvae of S. littoralis after 72 h of treatment.
7. Conclusion
A chain of 4-alkyloxyphenyl amide derivatives which are analogues to fenoxycarb juvenile hormone in which contain phenoxy group were chemically synthesized. The toxic activity of the tested target compounds was assessed against 2nd and 4th instar larvae demonstrated that some of the synthesized target compounds have great toxicological activity, though some of them uncovered sensible aphicidal activity. Particularly, compound 13 was the most toxic action since it surpassed the aphicidal activity of a reference juvenile hormone analogue fenoxycarb. The activity concerning compound 13 might be because of the presence of the ethers group joined to the aminophenol in its atomic structure. Our examination showed that the new aminophenol analogues containing ethers group moiety could successfully control of S. littoralis. These results are lively and gainful for additional work on the improvement of new and strong insecticides.
Declaration of Competing Interest
We are declared that there is no conflict of interest including any financial, personal or other relationships with other people or organizations within five years of beginning the submitted our paper that could inappropriately influence, or be perceived to influence, our work
Acknowledgements
This work was financially supported by the plant protection institute, agriculture research center of Egypt, and chemistry department, Sohag University.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00394.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Raslan S.A. Preliminary report on initial and residual mortality of the natural product, spinosad for controlling cotton leaf worm egg masses in 2002 cotton season at sharkia governorate, Egypt. Plant Protection Research Institute, Cairo, Egypt; 2002. pp. 635–637. [Google Scholar]
- 2.Al-Shannaf H.M., Desuky W.M., Abd El-Halim S.M. Effect of some compounds on cotton leafworm, Spodoptera littoralis (Boisd.) and their predators. Egypt. J. Appl. Sci. 2006;21:646–660. [Google Scholar]
- 3.Özmen D., Kilincer N. Research on the effects of diflubenzuron and hexaflumuron on Spodoptera littoralis (Boisduval) (Lepidoptera: noctuidae) larvae. Türkiye Entomoloji Dergisi. 2002;26:21–32. [Google Scholar]
- 4.Nasr H.M., Badawy M.E.I., Rabea E.I. Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera littoralis. Pestic. Biochem. Physiol. 2010;98:198–205. [Google Scholar]
- 5.Wheeler D.E., Nijhout H.F. A perspective for understanding the modes of juvenile hormone action as a lipid signaling system. bioEssays. 2003;25:994–1001. doi: 10.1002/bies.10337. [DOI] [PubMed] [Google Scholar]
- 6.Riddiford L.M. Juvenile hormone action: a 2007 perspective. J. Insect Physiol. 2008;(54):895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 7.El-Sheikh E.A., Kamita S.G., Vu K., Hammock B.D. Improved insecticidal efficacy of a recombinant baculovirus expressing mutated JH esterase from Manduca sexta. Biol. Control. 2011;58:354–361. [Google Scholar]
- 8.Bortolotti L., Porrini C., Sbrenna A.M., Sbrenna G. Ovicidal action of fenoxycarb on a predator, Chrysoperla carnea (Neuroptera: chrysopidae) Appl. Entomol. Zool. (Jpn.) 2000;35:265–270. [Google Scholar]
- 9.Khalil S.M.S., Anspaugh D.D., Roe R.M. Role of juvenile hormone esterase and epoxide hydrolase in reproduction of the cotton bollworm, Helicoverpa zea. J. Insect Physiol. 2006;52:669–678. doi: 10.1016/j.jinsphys.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Wilson T.G. The molecular site of action of juvenile hormone and juvenile hormone insecticides during metamorphosis: how these compounds kill insects. J. Insect Physiol. 2004;50:111–121. doi: 10.1016/j.jinsphys.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran A., Granett J., Ennis T. Insect growth regulators. In: Kerkut G., Gilbert L.I., editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology, 12. Pergamon Press; New York: 1985. pp. 529–601. [Google Scholar]
- 12.Monconduit H., Mauchamp B. Fenoxycarb a potent contaminant of the silkworm, Bombyx mori L., does not influence its juvenile hormone titer. Arch. Insect Biochem. Physiol. 1999;40:141–149. [Google Scholar]
- 13.Staal G. BInsect growth regulators with juvenile hormone activity. Annu. Rev. Entomol. 1975;20:417–460. doi: 10.1146/annurev.en.20.010175.002221. [DOI] [PubMed] [Google Scholar]
- 14.Kramer S.J., Law J.H. Synthesis and transport of juvenile hormones in insects. Acc. Chem. Res. 1980;20:297–303. [Google Scholar]
- 15.Riddiford L.M. Juvenile hormone action: a 2007 perspective. J. Insect Physiol. 2008;(54):895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Hammock B.D. Regulation of juvenile hormone titer: degradation. In: Kerkut G.A., Gilbert L.I., editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Pergamon Press; New York: 1985. pp. 431–472. [Google Scholar]
- 17.Abdel-Aal Y.A.I., Hammock B.D. Transition state analogs as ligands for affinity purification of juvenile hormone esterase. Science. 1986;233:1073–1076. doi: 10.1126/science.3738525. [DOI] [PubMed] [Google Scholar]
- 18.Kamita S.G., Hammock B.D. Juvenile hormone esterase: biochemistry and structure. J. Pestic. Sci. 2010;35:265–274. doi: 10.1584/jpestics.R10-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott W. S. Method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- 20.Finney D.J. Cambridge University Press; Cambridge, U. K: 1952. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve. [Google Scholar]
- 21.Sun Y.P. Toxicity index - an improved method of comparing the relative toxicity of insecticides. J. Econ. Entomol. 1950;1:45–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.