Abstract
Asparaginase (ASNase) causes pancreatitis in approximately 10% of leukemia patients, and the mechanisms underlying this painful complication are not known. ASNase primarily depletes circulating asparagine, and the endogenously expressed enzyme, asparagine synthetase (ASNS), replenishes asparagine. ASNS was suggested previously to be highly expressed in the pancreas. In this study, we determined the expression pattern of ASNS in the pancreas and the mechanism for increased pancreatic ASNS abundance. Compared with other organs, ASNS was highly expressed in both the human and mouse pancreas, and, within the pancreas, ASNS was present primarily in the acinar cells. The high baseline pancreatic ASNS was associated with higher baseline activation of protein kinase R-like endoplasmic reticulum kinase (PERK) signaling in the pancreas, and inhibition of PERK in acinar cells lessened ASNS expression. ASNase exposure, but not the common pancreatitis triggers, uniquely up-regulated ASNS expression, indicating that the increase is mediated by nutrient stress. The up-regulation of acinar ASNS with ASNase exposure was owing to increased transcriptional rather than delayed degradation. Knockdown of ASNS in the 266-6 acinar cells provoked acinar cell injury and worsened ASNase-induced injury, whereas ASNS overexpression protected against ASNase-induced injury. In summary, ASNS is highly expressed in the pancreatic acinar cells through heightened basal activation of PERK, and ASNS appears to be crucial to maintaining acinar cell integrity. The implications are that ASNS is especially hardwired in the pancreas to protect against both baseline perturbations and nutrient deprivation stressors, such as during ASNase exposure.
Keywords: Leukemia, Asparaginase-Associated Pancreatitis, Asparagine Synthetase, PERK Signaling
Abbreviations used in this paper: AAP, asparaginase-associated pancreatitis; ASNase, asparaginase; Asn, asparagine; ASNS, asparagine synthetase; ATF4, activating transcription factor 4; cDNA, complementary DNA; CHX, cycloheximide; eIF2α, eukaryotic initiation factor 2 subunit 1; ER, endoplasmic reticulum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA; PERK, protein kinase R-like endoplasmic reticulum kinase; PI, propidium iodide; qPCR, quantitative reverse-transcription polymerase chain reaction; shRNA, short hairpin RNA
Summary.
Asparagine synthetase, the endogenously expressed counteracting enzyme to asparaginase, is highly expressed in pancreatic acinar cells through heightened basal protein kinase R-like endoplasmic reticulum kinase signaling. The acinar cells have the ability to further up-regulate asparagine synthetase with asparaginase exposure.
Asparaginase (ASNase) is a chemotherapeutic drug that has been in use since the 1970s, primarily for childhood acute lymphoblastic leukemia and some types of non-Hodgkin’s lymphoma.1 Acute lymphoblastic leukemia is the most common childhood cancer in the United States, with an incidence of 3.5 per 100,000 children per year.2, 3 The adverse events for ASNase include allergic reactions (in 20%–40%), thrombotic events (in <5%), hepatotoxicity, and hyperlipidemia. However, the most problematic adverse event is the painful, life-threatening disorder of the pancreas: pancreatitis.4, 5 The frequency of pancreatitis with ASNase treatment is upward of 10%. This ranks ASNase as among one of the most likely drugs to induce pancreatitis.6 Furthermore, patients with asparaginase-associated pancreatitis (AAP) are more likely to die from pancreatic complications of pseudocyst formation, necrosis, and long-term insulin dependence compared with other etiologies of pancreatitis. Discontinuation of ASNase because of pancreatitis leads to a suboptimal duration of ASNase treatment, and this shortfall could jeopardize event-free survival from leukemia.
Although there are several recent reports characterizing genotype and phenotype relationships in AAP5, 7, 8 and some aspects of signaling,9, 10 the mechanisms underlying AAP are not clear. ASNase is effective in killing leukemic cells primarily by deamidating asparagine (Asn) to aspartic acid and thus depleting Asn. Although Asn is a nonessential amino acid, leukemic cells develop Asn addiction for 2 major reasons. first, the cells rapidly consume Asn because of their high rate of protein synthesis. Second, they have low expression of the counteracting enzyme asparagine synthetase (ASNS).
ASNS is a 561 amino acid–long adenosine triphosphate–dependent enzyme that in mammals uses glutamine as a nitrogen donor to amidate aspartic acid to Asn.11 It has been suggested, mostly based on crude tissue enzymatic activity measurements, that ASNS is expressed preferentially in the pancreas.12, 13 Pancreatic sections from the Human Gene Atlas14 roughly point to its expression primarily in the main parenchymal cell of the pancreas: the pancreatic acinar cell. In the current study, we investigated the expression pattern of ASNS in the pancreas, the mechanism for pancreatic ASNS expression at baseline, and whether the pancreatic acinar cell has the capacity to up-regulate ASNS further. We show that ASNS is expressed predominantly in pancreatic acinar cells and the mechanism for this high expression is through the basal activation of the endoplasmic reticulum (ER) stress–associated kinase protein kinase R-like endoplasmic reticulum kinase (PERK). We also show that pancreatic acinar cells have the capacity to up-regulate ASNS further, specifically in the setting of ASNase exposure, through both PERK and the amino acid response, a signaling pathway induced by limitation of any one of the amino acids required for protein synthesis.
Results
ASNS Is Expressed Predominantly in the Pancreas
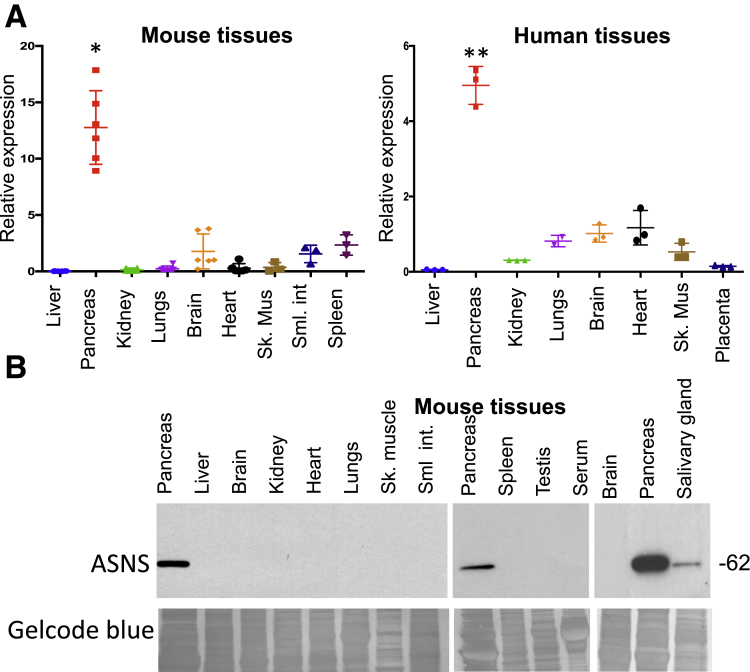
In mammals, ASNS is expressed in a variety of organs, and several early publications had suggested, mostly by crude tissue enzymatic assays, that ASNS activity levels are highest in the pancreas.12, 13 In quantitative reverse-transcription polymerase chain reaction (qPCR) analysis of ASNS messenger RNA (mRNA) content in an array of organs in the mouse, we found that ASNS was most highly expressed in the pancreas. It was expressed at an intermediate level in brain, heart, small intestine, and spleen, whereas liver, kidney, skeletal muscle, and lung had low expression (Figure 1A, left). Similar trends were seen in human organs (Figure 1A, right). Western blot for ASNS protein also showed that, compared with other mouse organs, ASNS was expressed predominantly in the pancreas (Figure 1B). Interestingly, ASNS expression in the salivary gland, which is also an exocrine gland,15 was readily detectable, although it was much less than that of the pancreas. Under the exposure conditions of these Western blots, none of the other organs showed detectable expression. Thus, there is high baseline expression of both ASNS mRNA and protein in the pancreas.
Figure 1.
ASNS is expressed predominantly in the pancreas. (A) qPCR from mouse (n = 3–6; *P < .05) and human tissues (n = 3, normalized to expression from brain; *P < .05; **P < .01). (B) Western blot from mouse tissues with GelCode Blue (Thermo Scientific, Pittsburgh, PA) loading control (n = 5). Sk Mus, skeletal muscle; Sml int, small intestine.
Within the Pancreas, ASNS Is Expressed Primarily in Acinar Cells
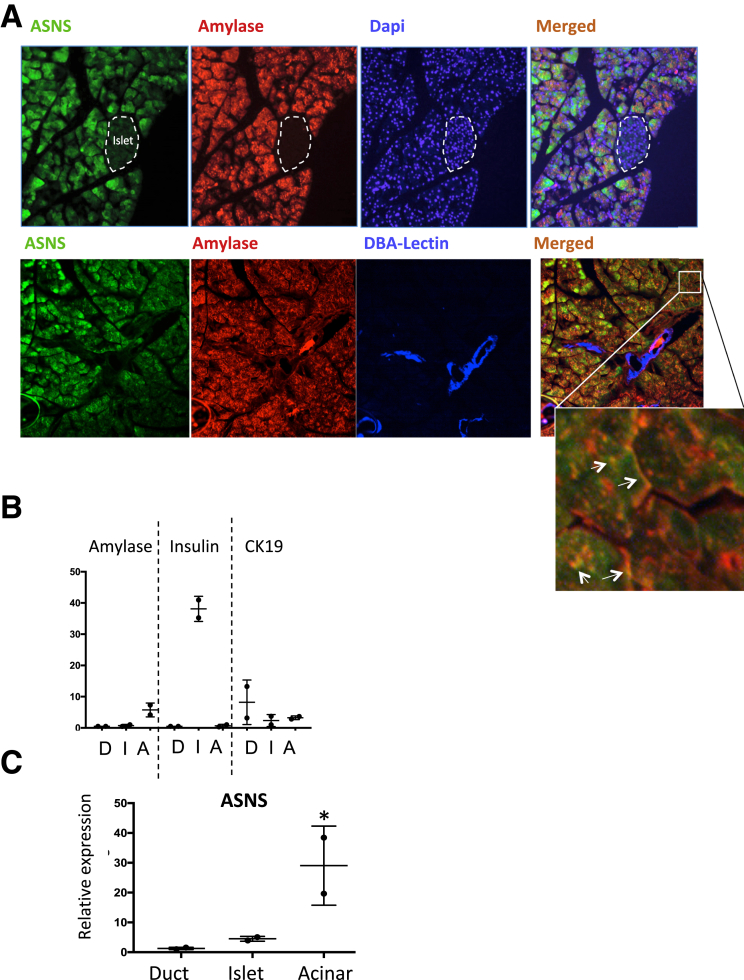
Immunofluorescence staining from mouse pancreas showed that ASNS is expressed primarily in acinar cells because ASNS staining corresponded with the acinar cell marker amylase (Figure 2A). Within the acinar cell, ASNS primarily appeared to be cytosolic, although there was partial co-localization of ASNS with amylase in the apical secretory pole. This is in comparison with immunofluorescence images from the Human Protein Atlas14 in the human cell line U-2 osteosarcoma, which shows mostly diffuse cytosolic ASNS labeling. In U937 cells with forced ASNS expression, however, ASNS was found to be partially localized to the plasma membrane.16 No expression was detected in the islets or duct cells (the latter was counterlabeled with the ductal cell marker Dolichos biflorus agglutinin lectin). To complement the data from the immunofluorescence, we isolated each of the major cells of the pancreas from the mouse. The purity of the islet, ductal, and acinar cells was confirmed by performing qPCR for insulin, Cytokeratin 19, and amylase, respectively (Figure 2B). qPCR for ASNS from these cell populations further showed that ASNS expression was mostly in acinar cells (Figure 2C).
Figure 2.
ASNS is expressed predominantly in pancreatic acinar cells. (A) Immunofluorescence for ASNS (green), amylase (red), and Dolichos biflorus agglutinin-lectin (blue, bottom row) in mouse pancreatic tissue (n = 3). Inset: the white arrows point to amylase within the acinar cells. (B) Relative expression by qPCR of amylase, insulin, and Cytokeratin 19 from isolated ductal (D), islet (I), and acinar (A) cells, to confirm the purity of the isolation method, and (C) qPCR for ASNS in the isolated fractions (n = 2; *P < .05).
Basal PERK Signaling in the Pancreas Mediates the High Expression of ASNS
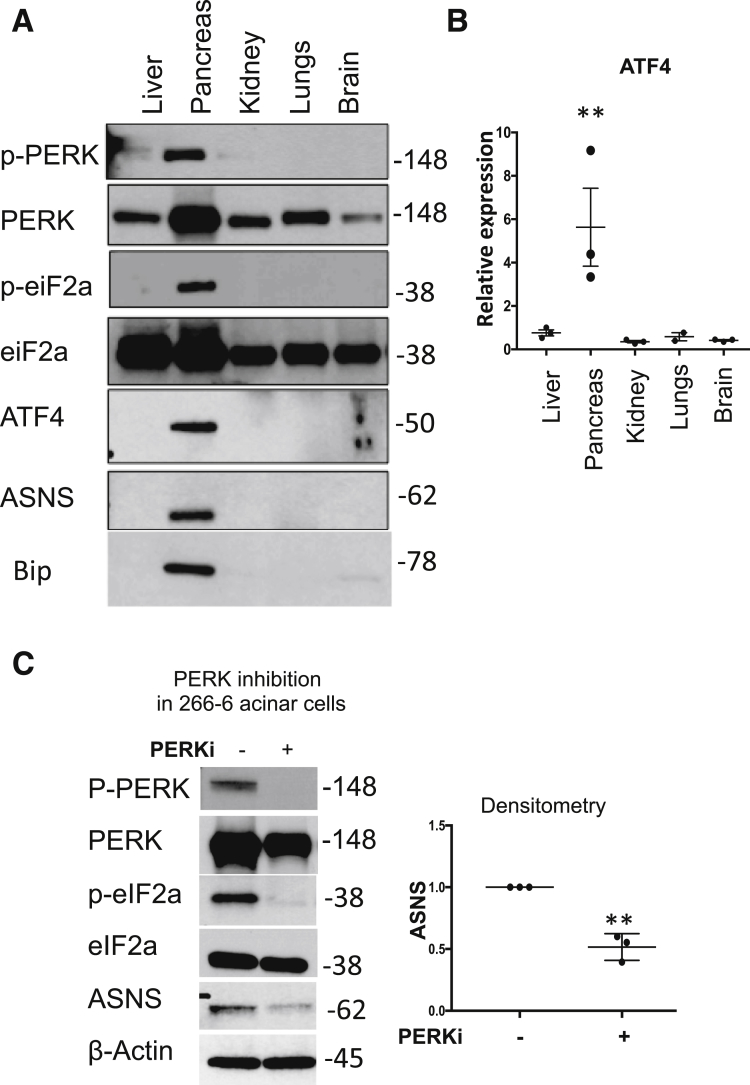
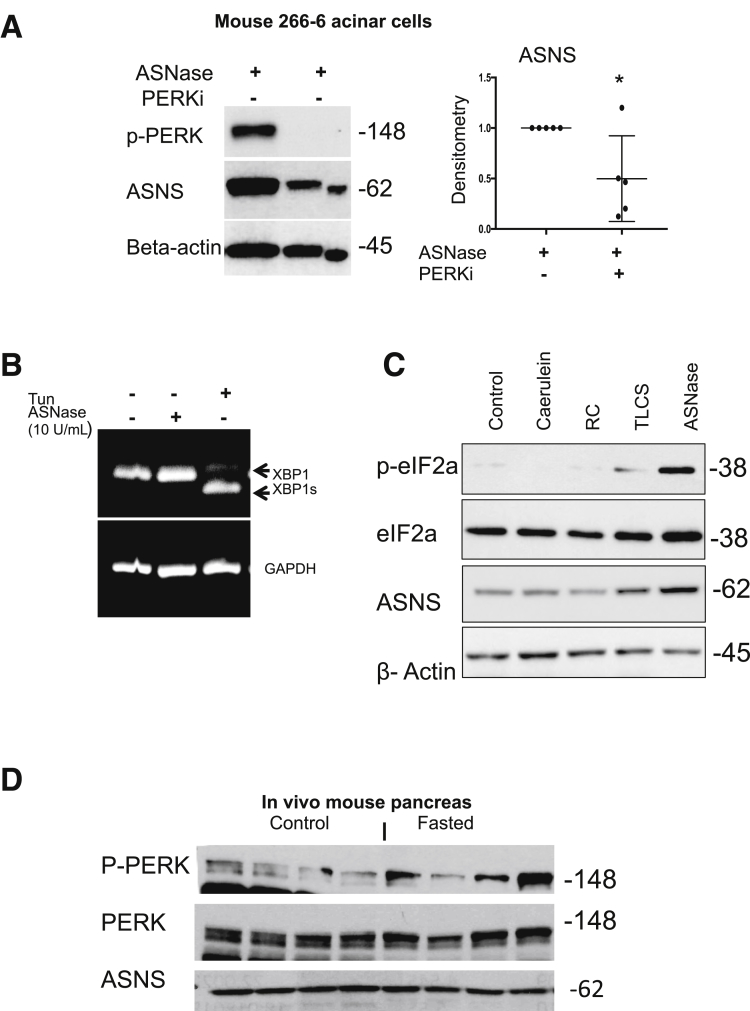
The PERK activation pathway is well characterized in situations of ER stress.17 However, its function at baseline, and specifically in the pancreas, is not clear. Here, we show that compared with other organs in mice, PERK signaling is highly active in the pancreas at baseline, evidenced by phosphorylation of PERK and eukaryotic initiation factor 2 subunit 1 (eIF2α) (Figure 3A). Phosphorylation of eIF2α by PERK globally suppresses protein synthesis, and yet it selectively increases translation of activating transcription factor 4 (ATF4).18 We found that ATF4 was highly expressed in the pancreas compared with several other organs, both at the protein and mRNA levels (Figure 3A and B). ATF4 activates transcription of the ASNS gene in response to a variety of upstream stress signals, including PERK.12 Indeed, increased phosphorylated PERK and ATF4 were accompanied by high expression of ASNS. The induction of Glucose binding protein 78/binding immunoglobulin protein is a marker of ER stress.19 We observed that binding immunoglobulin protein also was more highly expressed in the pancreas in mice than in several other solid organs (Figure 3A). Furthermore, in the 266-6 acinar cells, PERK inhibition with GSK260641420 prevented ASNase-induced eIF2α phosphorylation and led to lower baseline ASNS expression (Figure 3C).
Figure 3.
PERK signaling maintains the high baseline expression of ASNS in the pancreas. (A) Western blot for the components of the PERK signaling pathway in mouse tissues (n = 3). (B) qPCR of ATF4 in mouse tissues (n = 3; **P < .01). (C) Treatment of the mouse pancreatic 266-6 acinar cell with the PERK inhibitor GSK2606414 (PERKi; 10 μmol/L) for 16 hours led to reduced baseline ASNS expression (n = 3; **P < .01). Bip, Binding immunoglobulin protein; p-eIF2a, phosphorylated eIF2a; p-PERK, phosphorylated PERK.
Acinar ASNS Expression Is Induced in Response to ASNase Exposure Through PERK
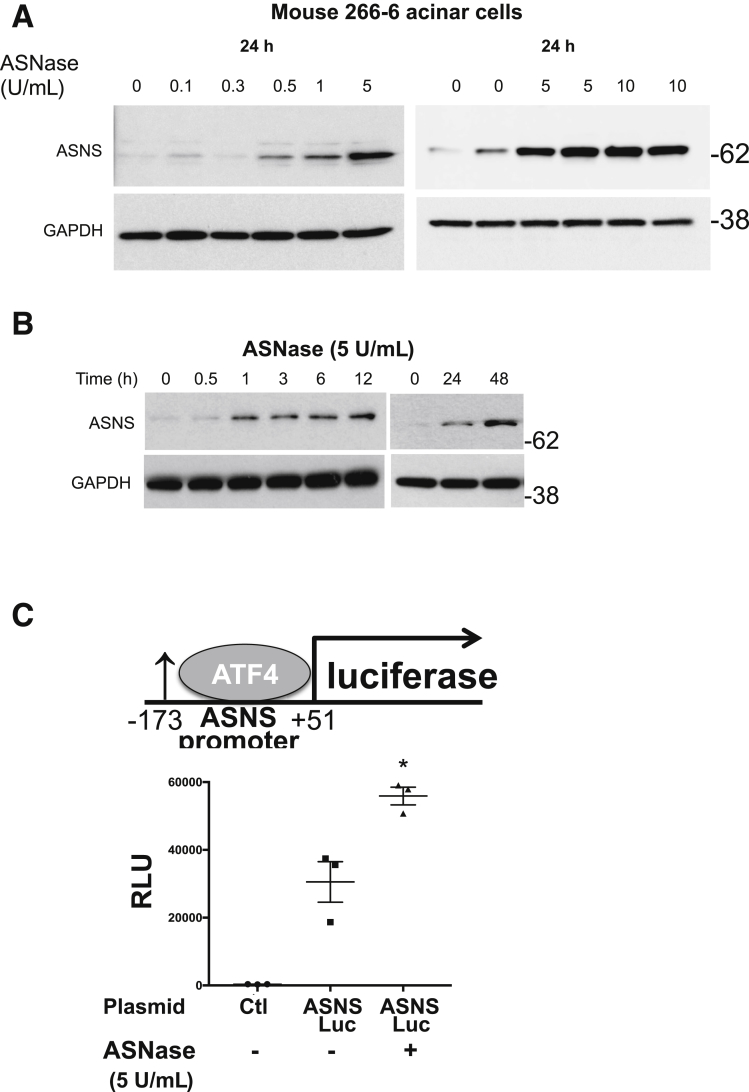
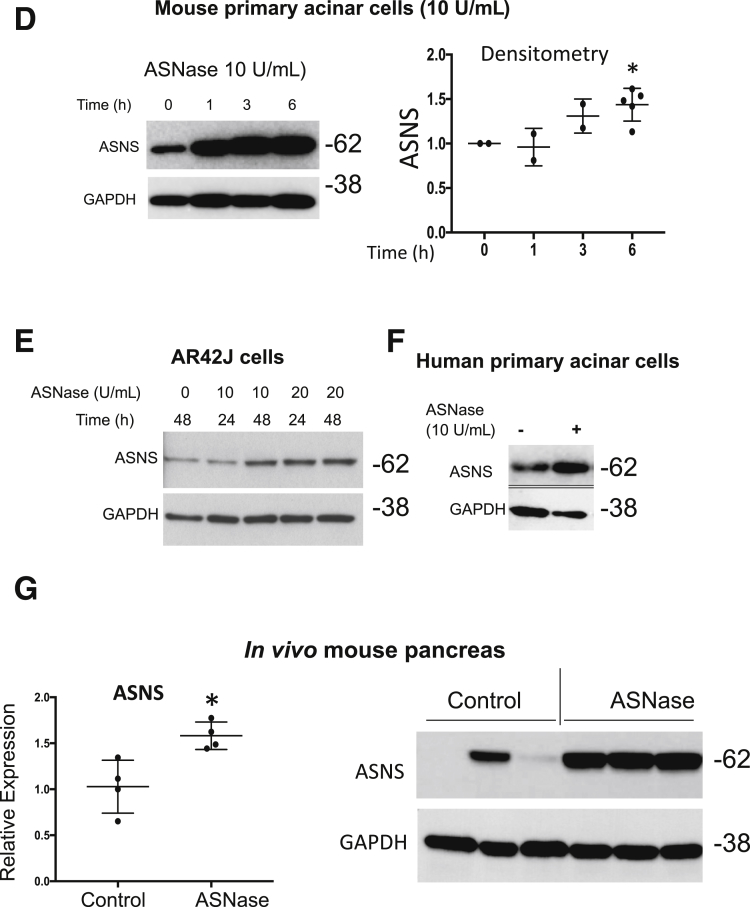
ASNS expression is up-regulated in mammalian cells and tissues in response to amino acid deprivation, including after exposure to ASNase.21, 22, 23 However, the characteristics of ASNS in acinar cells with ASNase exposure is not known. In clinical studies, a minimum target level of 0.1 U/mL plasma ASNase activity was necessary to deplete plasma Asn, and the highest levels of plasma ASNase approached 10 U/mL.24, 25 Using this background knowledge of the pharmacologically relevant concentrations observed in the systemic circulation in vivo, in an ex vivo setting, we incubated mouse 266-6 acinar cells with ASNase concentrations of 0.1 to 10 U/mL. A total of 5 U/mL was optimal for induction of ASNS expression (Figure 4A). Furthermore, we incubated mouse 266-6 acinar cells with ASNase (5 U/mL) for up to 48 hours and observed a steady time-dependent increase in ASNS up-regulation (Figure 4B). After transfection of 266-6 cells with a luciferase reporter containing the ATF4 binding site of the ASNS promoter,26 ASNase exposure led to increased luciferase activity (Figure 4C). Treatment of mouse primary acinar (Figure 4D), rat AR42J acinar (Figure 4E), and human primary pancreatic acinar cells (Figure 4F) with ASNase also resulted in an increase in ASNS protein expression. To investigate whether ASNase exposure also can lead to the up-regulation of ASNS in vivo, we injected 4- to 5-month-old female Swiss-Webster mice with L-ASNase (3000 U/kg each day for 5 days). Both qPCR and Western blot confirmed a significant up-regulation of ASNS expression in the ASNase-treated group, compared with the controls (Figure 4G). Inhibition of PERK, using GSK2606414, in mouse pancreatic acinar 266-6 cells resulted in a reduction in ASNase-induced ASNS expression (Figure 5A). However, there was no X-box binding protein 1 cleavage with ASNase (Figure 5B).
Figure 4.
Acinar ASNS expression is up-regulated in response to ASNase exposure. With ASNase treatment, there was a (A) concentration- and (B) time-dependent increase in ASNS in 266-6 acinar cells. (C) Transfection of a luciferase construct containing the ATF4 binding site of the ASNS promoter (-173 to +51) in 266-6 cells treated with or without ASNase (5 U/mL; n = 3; *P < .05). (D) Time-dependent increase in ASNS expression in mouse primary acinar cells (n = 2–4; *P < .05, relative to 0 h) and (E) in the rat pancreatic acinar AR42J cell. (F) Increase in ASNS expression in primary human pancreatic acinar cells. (G) In the mouse pancreas by qPCR (left; n = 4; *P < .05) and Western blot (right; n = 3; *P < .05), there was also an increase in ASNS expression with in vivo ASNase exposure. Ctl, control.
Figure 5.
ASNase-induced ASNS up-regulation is mediated through PERK, and compared with the other pancreatitis triggers, only ASNase exposure leads to activation of eIF2α and up-regulation of ASNS. (A) In 266-6 acinar cells, the PERK inhibitor GSK2606414 (PERKi; 10 μmol/L) suppressed ASNase-induced up-regulation of ASNS (n = 5; *P < .05). (B) By qPCR, there was no difference in X-box binding protein 1 cleavage with ASNase (n = 3). Tunicamycin (Tun; 5 μg/mL for 6 h) served as a positive control for inducing ER stress. (C) Mouse 266-6 acinar cells were treated for 6 hours with pathologically relevant concentrations of pancreatitis stimuli. Cerulein (100 nmol/L), radiocontrast (RC; 16%), and taurolithocholic acid 3-sulfate (TLCS; 500 umol/L). (D) Pancreatic ASNS and p-PERK/total PERK in mice fasted for 12 hours (n = 4). p-PERK, phosphorylated PERK.
Acinar ASNS Up-Regulation Uniquely Occurs in Response to ASNase, but Not With the Common Pancreatitis Stimuli
To evaluate whether ASNS induction occurs with stimuli that cause pancreatitis in vivo and induce acinar injury ex vivo, 266-6 acinar cells were treated for 6 hours with supraphysiologic concentrations (100 nmol/L) of the cholecystokinin analog cerulein, 16% radiocontrast, or 500 μmol/L of the bile acid taurolithocholic acid 3-sulfate, compared with 5 U/mL ASNase. These agents induce pancreatitis in vivo and acinar cell injury ex vivo.27, 28, 29, 30 We observed strong phosphorylation of eIF2α and up-regulation of ASNS only in the case of ASNase treatment (Figure 5C). Short-term fasting of mice (<48 h) has been shown to induce ASNS expression in a few tissues, but the pancreas was not evaluated.31 To investigate this, we fasted mice for 12 hours, with access only to water, and we did not observe changes in pancreatic ASNS or PERK expression, compared with nonfasted mice (Figure 5D). These results suggest that up-regulation of ASNS in pancreatic acinar cells occurs with ASNase exposure, but not with the other known acinar cell injury stressors.
ASNase Exposure Does Not Alter the Rate of ASNS Protein Degradation
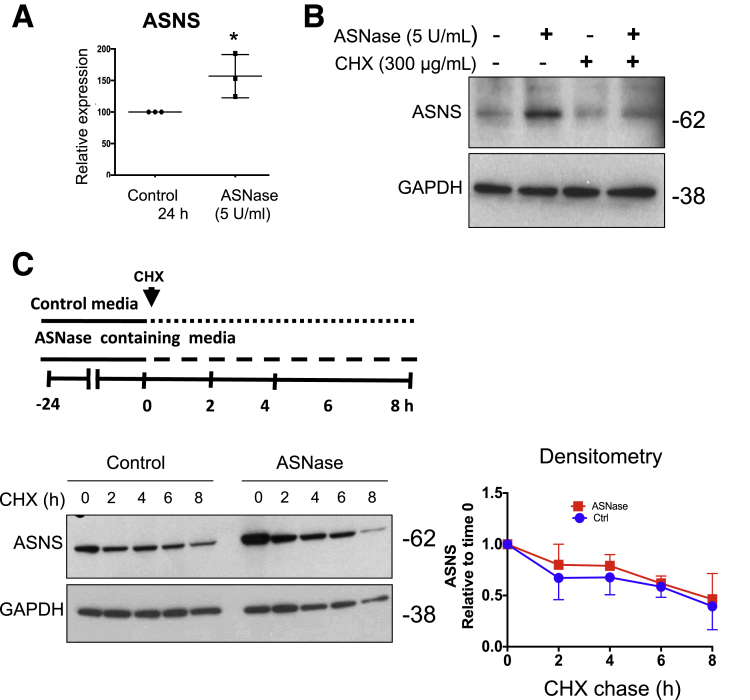
An increase in ASNS protein levels after ASNase treatment can result from either increased ASNS synthesis or decreased ASNS protein turnover. By qPCR, 5 U/mL ASNase exposure for 24 hours led to a significant induction of ASNS mRNA in 266-6 acinar cells (Figure 6A). After 8 hours of ASNase exposure with or without the protein synthesis inhibitor cycloheximide (CHX),32 the increase in ASNS protein content was largely blocked (Figure 6B). To analyze the effect of ASNase on ASNS turnover, a CHX-chase experiment was performed. The cells were first treated with ASNase to induce ASNS expression. They then were incubated with CHX, and ASNS content was assessed every 2 hours for up to 8 hours. After normalizing ASNS content to the value at the beginning of the chase period (t = 0 h), no difference was observed in the rate of ASNS protein degradation (Figure 6C). These results suggest that the net up-regulation of ASNS is owing solely to increased ASNS transcript and has no contribution from reduced protein degradation.
Figure 6.
ASNase-induced up-regulation of acinar ASNS is owing to increased transcription, not reduced protein degradation. (A) qPCR for ASNS from 266-6 acinar cells with ASNase exposure for 24 hours (n = 3; *P < .05). (B) Western blot for ASNS from 266-6 cells incubated with CHX (n = 3). (C) An 8-hour CHX chase with or without ASNase exposure showed no difference in ASNS degradation. Densitometry is normalized to 0 hours for each comparison group (n = 3).
ASNS Knockdown Provokes Pancreatic Acinar Cell Injury and Worsens With ASNase Exposure, Whereas ASNS Overexpression Protects Against ASNase-Induced Injury
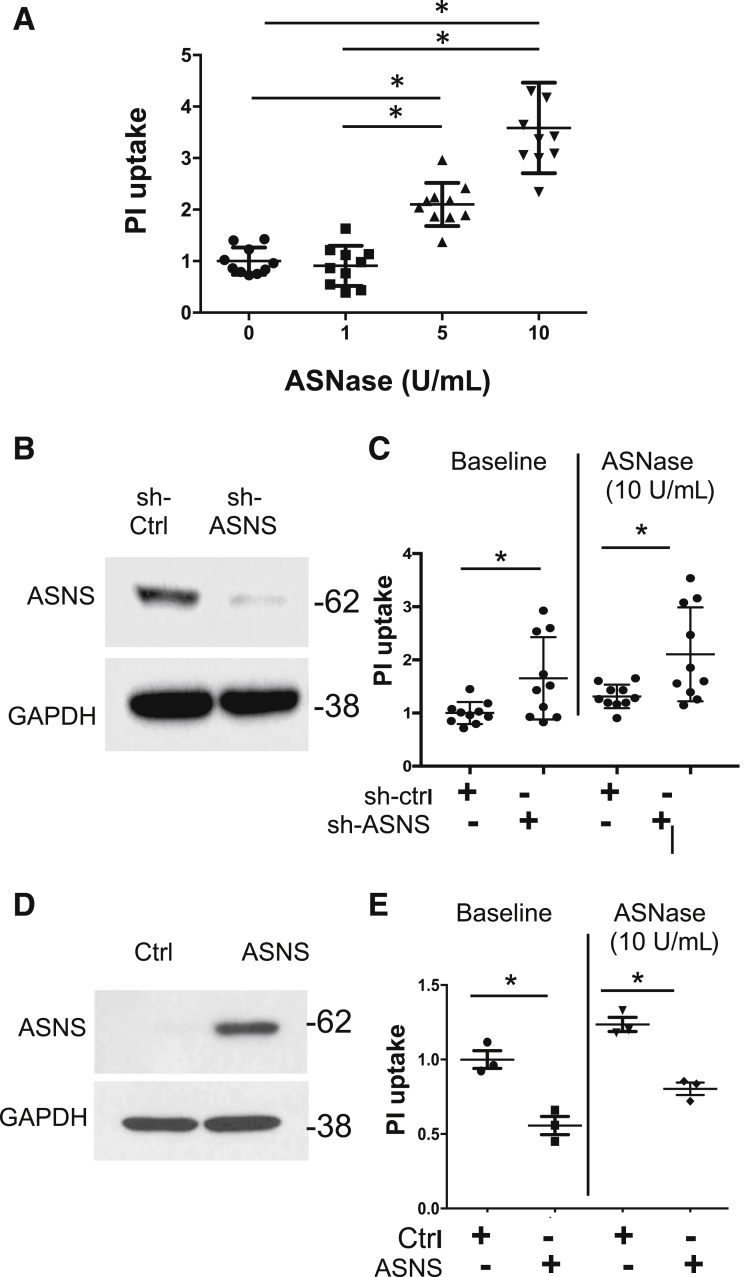
Knockdown of ASNS in melanoma cells and in breast cancer cell lines led to suppression of growth and caused cell-cycle arrest.33, 34 However, there are no reports of the effect of ASNS knockdown on pancreatic acinar cells. To validate whether ASNase exposure can lead to pancreatic acinar cell injury, we exposed 266-6 cells to ASNase and observed a concentration-dependent increase in propidium iodide (PI) uptake (Figure 7A). Transient transfection of ASNS short hairpin RNA (shRNA) in mouse 266-6 acinar cells significantly reduced ASNS protein expression (Figure 7B). At baseline, ASNS knockdown induced cell injury compared with a scrambled control, and exposure to ASNase worsened the degree of injury (Figure 7C). We next examined whether, conversely, ASNS overexpression was protective (Figure 7D). As hypothesized, overexpression of ASNS reduced both baseline cell injury in culture over 24 hours and ASNase-induced cellular injury (Figure 7E). Taken together, the results indicate that ASNS has a cytoprotective effect on acinar cells.
Figure 7.
ASNS knockdown provokes pancreatic acinar cell injury and worsens with ASNase exposure, whereas ASNS overexpression protects against ASNase-induced injury. (A) In 266-6 pancreatic acinar cells, ASNase exposure led to a concentration-dependent increase in PI uptake (n = 9–10; *P < .05). (B) Western blot shows knockdown of ASNS in 266-6 acinar cells by shRNA (n = 4). (C) ASNS knockdown induced cell injury in culture over 24 hours, compared with a scrambled control, and knockdown worsened injury with ASNase exposure (n = 10; *P < .05). (D) Western blot shows ASNS plasmid overexpression. (E) Compared with a scrambled control, ASNS overexpression led to reduced PI uptake both at baseline and with ASNase exposure (n = 3; *P < .05). Ctrl, control.
Discussion
The key findings of the current work are as follows: (1) ASNS is highly expressed in pancreatic acinar cells, (2) the mechanism for this high expression is through PERK signaling, (3) acinar cells have the capacity to up-regulate ASNS further in the setting of ASNase, and (4) ASNS maintains acinar cell integrity, both at baseline and with ASNase exposure.
The high expression of ASNS in the pancreas (Figure 1), and in the pancreatic acinar cells within the pancreas in particular (Figure 2), suggest that acinar cell ASNS might have a special role in maintaining pancreatic acinar cell homeostasis. Seventy percent of the transcripts from the pancreas encode secreted proteins, compared with 10%–20% in most other tissues.14 To supply the demands of large amounts of secreted enzymes for digestion, the pancreatic acinar cells are adapted to possess one of the highest protein biosynthetic rates of any epithelial cell.35, 36 This characteristic prompted us to investigate the mechanism underlying the high basal ASNS expression in normal pancreas. We found that PERK phosphorylation, which occurs during ER stress from the unfolded protein response, was much higher in the pancreas at baseline, compared with several other organs (Figure 3). Consistent with increased PERK activity, eIF2α phosphorylation and ATF4 expression also were higher. Inhibition of PERK signaling led to repression of ASNS expression, indicating that the high baseline ASNS expression is dependent on PERK activation. The heightened ER stress response presumably is protective in the context of the unique translational nature of the pancreatic acinar cells, and it is consistent with reports that these cells possess high basal autophagy.37 Our finding of high ASNS expression in the parotid gland, albeit lower than in the pancreas, is consistent with the histologic similarity of both exocrine glands15 and the clinical observation that ASNase occasionally is associated with the complication of parotitis.38
Besides the high expression of pancreatic ASNS at baseline, ASNS also is up-regulated at both the transcript and protein level with ASNase exposure (Figure 4). ASNS up-regulation, however, was not seen with several of the common pancreatitis stimuli or with short-term food fasting (Figure 5). The up-regulation was mediated exclusively through increased transcription, rather than through reduced ASNS turnover (Figure 6). The results suggest that pancreatic acinar cells have the capacity to induce ASNS expression uniquely in response to selective situations of amino acid deprivation.
Patients with hypomorphic mutations in ASNS develop central nervous system defects.11 Although they do not overtly develop pancreatitis, it is not known what effect ASNS deficiency has on the pancreas. In the current work, ASNS knockdown in the mouse acinar 266-6 cells induced cell injury and worsened the damage with ASNase exposure, whereas ASNS overexpression mitigated the damage (Figure 7). The findings point to a cytoprotective effect of ASNS on the acinar cells, likely through replenishment of Asn.
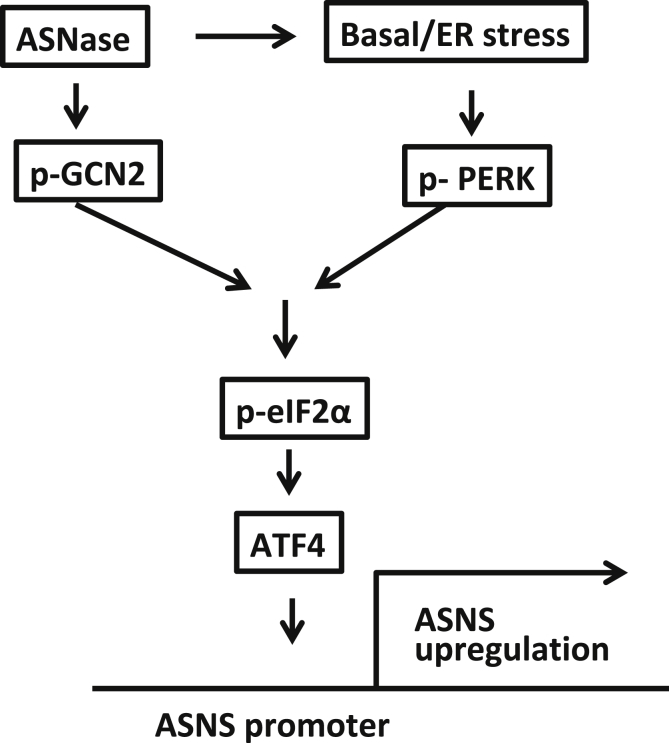
Overall, the findings in this study of high expression patterns of ASNS in the pancreatic acinar cell and the ability to up-regulate ASNS further—both aspects at least partially through the PERK arm of the ER stress response—indicate that ASNS is hardwired in the pancreas to protect against perturbations in amino acid availability both at baseline and with nutrient stress (Figure 8). Further work is needed to probe whether defects in the wider repertoire of pancreatic ASNS signaling predispose patients to AAP.
Figure 8.
Schematic of the mechanism for high ASNS expression in the pancreatic acinar cell and its up-regulation with ASNase. Heightened basal ER stress activates PERK, which in turn phosphorylates eIF2α (p-eIF2α) and activates transcription of ATF4. Binding of ATF4 on the amino acid response element of the ASNS promoter induces ASNS expression. In addition, ASNase exposure can up-regulate ASNS further through a combination of greater PERK signaling and the nutrient deprivation response through the amino acid sensor general control nonderepressible 2 (GCN2).
Materials and Methods
Materials
Two independent anti-ASNS antibodies were used (ab40850, Abcam, San Francisco, CA; 50-560-171, Fisher Scientific, Pittsburgh, PA). Rabbit anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (G8795-100UL), mouse anti–β-actin, and anti–α-amylase antibodies were purchased from Sigma-Aldrich (St. Louis, MO). ASNase (ab73439, Abcam; A3809, Sigma-Aldrich) was prepared as an aqueous stock at 1 U/μL concentration in water. Dolichos biflorus agglutinin-lectin (Vector Labs, Burlingame, CA), rabbit antibodies to phosphorylated PERK, PERK, phosphorylated p-eIF2α, and eIF2α were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-ATF4 antibody and the reporter plasmid ASNS-173/+51/luciferase were kindly provided by Michael S. Kilberg. The latter was made by inserting an ASNS promoter fragment (nucleotides -173/+51) upstream of the Firefly luciferase reporter gene,26 using the restriction site HindIII site of the luciferase reporter vector, pGL3 plasmid (Promega, Madison, WI).
Cell Culture
The 266-6 mouse pancreatic acinar cell line and the AR42J rat acinar cell line were obtained from American Type Culture Collection and cultured according to standard mammalian tissue culture protocols and sterile technique. Media was supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine. Tissue culture media and supplements were obtained from Thermo Fisher Scientific (Pittsburgh, PA).
Mouse Primary Acinar Cell Isolation
All animal experiments were performed using a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Mouse primary acinar cells were isolated from both male and female 3-month-old Swiss Webster mice using a previously described method,39 with minor modifications. The pancreas was removed and then minced for 5 minutes in Dulbecco’s modified Eagle medium/F12 without phenol red (Invitrogen), plus 0.1% bovine serum albumin and 2 mg/mL type 4 collagenase (Worthington, Lakewood, NJ). The suspension was incubated briefly for 5 minutes at 37°C while shaking at 90 rpm. The buffer was removed and replaced with new collagenase buffer and then incubated for 35 minutes. The suspension was filtered through a 300-μm mesh (Sefar American, Buffalo, NY) and then washed 3 times with collagenase-free buffer. Acinar cells were allowed to equilibrate for 5 minutes at 37°C before use.
Western Blot
Cells were lysed in NP40 lysis buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, and 1% NP-40. The lysates were processed for Western blot using 20 μg per sample. Results were normalized to either GAPDH or β-actin.
RNA Isolation and qPCR
Total RNA was isolated from a variety of mouse tissues using TRIzol reagent (Invitrogen), and complementary DNA (cDNA) was made using a High-Capacity RNA-to-cDNA kit (Fisher Scientific) following the manufacturer’s instructions. A tissue panel of normalized first-strand human cDNA preparations was purchased from Clontech (Mountain View, CA) (#636742). qPCR was performed using an ABI 7300HT instrument, with the commercial target probes and Master mix (Applied Biosystems, Foster City, CA). Specific TaqMan Gene Expression Assays (Thermo Fisher Scientific) were used to detect mouse and human ASNS (Mm00803785_m1 and Hs04186194_m1, respectively) along with the mouse and human reference gene TATA-box binding protein (TBP) (Mm01277042_m1 and Hs00427620_m1, respectively). Real-time reactions were run using the following cycle parameters: 95°C for 12 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Differential gene expression was calculated by the 2-ΔΔCT (delta delta cycle threshold) method.
Analysis of Protein Degradation
To analyze ASNS protein synthesis, 266-6 cells were cultured for 24 hours before treating them with or without 5 U/mL ASNase and 300 μg/mL CHX. After 8 hours, the cells were harvested and lysed, and the protein extracts were immunoblotted for ASNS, along with GAPDH as a loading control. For the CHX chase, the cells were cultured with or without ASNase for 24 hours, and then CHX was added to the wells. Cells then were harvested for Western blot at 0, 2, 4, 6, and 8 hours after application of the CHX.
Transfection of Plasmids and shRNA
Cells were seeded at approximately 50% confluency, allowed to adhere for 24 hours, and then transfected using Lipofectamine according to the manufacturer's protocol (Fisher Scientific). ASNS shRNA plasmid was purchased from Sigma-Aldrich (TRCN0000324928), and 1 μg of ASNS shRNA plasmid per well in 6-well plates was transfected in parallel with a scrambled control plasmid. Cells were exposed to ASNase 24 hours after transfection, and then harvested after another 24 hours. The ASNS overexpression plasmid was purchased from Addgene (Watertown, MA).
PI Uptake
The 266-6 cells were seeded and grown in a 48-well plate for 24 hours in Dulbecco’s modified Eagle medium. Cell injury was measured using a PI uptake assay. Cells treated with ASNase or other stimuli were washed twice in phosphate-buffered saline before incubating in 25 μg/mL PI at 37°C, and the percentage of PI-positive cells was measured by spectrophotometry (excitation, 536 nm; emission, 617 nm).
Footnotes
Author contributions Amitava Mukherjee, Nayyar Ahmed, Sohail Z. Husain, and Michael S. Kilberg conceptualized the study; Amitava Mukherjee, Nayyar Ahmed, Fateema Rose, Abraheem N. Ahmad, Tanveer A. Javed, Li Wen, Rita Bottino, Xiangwei Xiao, and Sohail Z. Husain contributed in methodology; Amitava Mukherjee and Nayyar Ahmed performed the investigation; Amitava Mukherjee, Nayyar Ahmed, and Sohail Z. Husain wrote the original draft of the manuscript; Amitava Mukherjee, Nayyar Ahmed, Li Wen, Sohail Z. Husain, and Michael S. Kilberg Writing reviewed and edited the manuscript; Sohail Z. Husain and Michael S. Kilberg acquired funding; Sohail Z. Husain provided resources; and Amitava Mukherjee and Sohail Z. Husain were responsible for study supervision. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institutes of Health grants DK083327, DK093491, and DK03002 (S.Z.H.) and CA203565 (M.S.K.), and a start-up award from the University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh (A.M.).
References
- 1.Batool T., Makky E.A., Jalal M., Yusoff M.M. A comprehensive review on L-asparaginase and its applications. Appl Biochem Biotechnol. 2016;178:900–923. doi: 10.1007/s12010-015-1917-3. [DOI] [PubMed] [Google Scholar]
- 2.McNeil D.E., Cote T.R., Clegg L., Mauer A. SEER update of incidence and trends in pediatric malignancies: acute lymphoblastic leukemia. Med Pediatr Oncol. 2002;39:554–557. doi: 10.1002/mpo.10161. discussion 552–553. [DOI] [PubMed] [Google Scholar]
- 3.Hjalgrim L.L., Rostgaard K., Schmiegelow K., Soderhall S., Kolmannskog S., Vettenranta K., Kristinsson J., Clausen N., Melbye M., Hjalgrim H., Gustafsson G. Age- and sex-specific incidence of childhood leukemia by immunophenotype in the Nordic countries. J Natl Cancer Inst. 2003;95:1539–1544. doi: 10.1093/jnci/djg064. [DOI] [PubMed] [Google Scholar]
- 4.Oparaji J.A., Rose F., Okafor D., Howard A., Turner R.L., Orabi A.I., Byersdorfer C., Mi Q., Ritchey K., Lowe M.E., Husain S.Z. Risk factors for asparaginase-associated pancreatitis: a systematic review. J Clin Gastroenterol. 2017;51:907–913. doi: 10.1097/MCG.0000000000000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolthers B.O., Frandsen T.L., Baruchel A., Attarbaschi A., Barzilai S., Colombini A., Escherich G., Grell K., Inaba H., Kovacs G., Liang D.C., Mateos M., Mondelaers V., Moricke A., Ociepa T., Samarasinghe S., Silverman L.B., van der Sluis I.M., Stanulla M., Vrooman L.M., Yano M., Zapotocka E., Schmiegelow K., Ponte di Legno Toxicity Working Group Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017;18:1238–1248. doi: 10.1016/S1470-2045(17)30424-2. [DOI] [PubMed] [Google Scholar]
- 6.Bai H.X., Ma M.H., Orabi A.I., Park A., Latif S.U., Bhandari V., Husain S.Z. Novel characterization of drug-associated pancreatitis in children. J Pediatr Gastroenterol Nutr. 2011;53:423–428. doi: 10.1097/MPG.0b013e318228574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Tanfous M., Sharif-Askari B., Ceppi F., Laaribi H., Gagne V., Rousseau J., Labuda M., Silverman L.B., Sallan S.E., Neuberg D., Kutok J.L., Sinnett D., Laverdiere C., Krajinovic M. Polymorphisms of asparaginase pathway and asparaginase-related complications in children with acute lymphoblastic leukemia. Clin Cancer Res. 2015;21:329–334. doi: 10.1158/1078-0432.CCR-14-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Yang W., Devidas M., Cheng C., Pei D., Smith C., Carroll W.L., Raetz E.A., Bowman W.P., Larsen E.C., Maloney K.W., Martin P.L., Mattano L.A., Jr., Winick N.J., Mardis E.R., Fulton R.S., Bhojwani D., Howard S.C., Jeha S., Pui C.H., Hunger S.P., Evans W.E., Loh M.L., Relling M.V. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;34:2133–2140. doi: 10.1200/JCO.2015.64.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng S., Gerasimenko J.W., Tsugorka T., Gryshchenko O., Samarasinghe S., Petersen O.H., Gerasimenko O.V. Calcium and adenosine triphosphate control of cellular pathology: asparaginase-induced pancreatitis elicited via protease-activated receptor 2. Philos Trans R Soc Lond B Biol Sci. 2016;371:1700. doi: 10.1098/rstb.2015.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillipson-Weiner L., Mirek E.T., Wang Y., McAuliffe W.G., Wek R.C., Anthony T.G. General control nonderepressible 2 deletion predisposes to asparaginase-associated pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1061–G1070. doi: 10.1152/ajpgi.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomelino C.L., Andring J.T., McKenna R., Kilberg M.S. Asparagine synthetase: function, structure, and role in disease. J Biol Chem. 2017;292:19952–19958. doi: 10.1074/jbc.R117.819060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balasubramanian M.N., Butterworth E.A., Kilberg M.S. Asparagine synthetase: regulation by cell stress and involvement in tumor biology. Am J Physiol Endocrinol Metab. 2013;304:E789–E799. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milman H.A., Cooney D.A. The distribution of L-asparagine synthetase in the principal organs of several mammalian and avian species. Biochem J. 1974;142:27–35. doi: 10.1042/bj1420027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Ponten F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 15.Rakonczay Z., Jr., Vag J., Foldes A., Nagy K., Nagy A., Hegyi P., Varga G. Chronic inflammation in the pancreas and salivary glands--lessons from similarities and differences in pathophysiology and treatment modalities. Curr Pharm Des. 2014;20:1104–1120. doi: 10.2174/13816128113199990415. [DOI] [PubMed] [Google Scholar]
- 16.He Y., Li B., Luo C., Shen S., Chen J., Xue H., Tang J., Gu L. Asparagine synthetase is partially localized to the plasma membrane and upregulated by L-asparaginase in U937 cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31:159–163. doi: 10.1007/s11596-011-0243-4. [DOI] [PubMed] [Google Scholar]
- 17.Hetz C., Papa F.R. The unfolded protein response and cell fate control. Mol Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Rozpedek W., Pytel D., Mucha B., Leszczynska H., Diehl J.A., Majsterek I. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Radford H., Moreno J.A., Verity N., Halliday M., Mallucci G.R. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 2015;130:633–642. doi: 10.1007/s00401-015-1487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikonorova I.A., Al-Baghdadi R.J.T., Mirek E.T., Wang Y., Goudie M.P., Wetstein B.B., Dixon J.L., Hine C., Mitchell J.R., Adams C.M., Wek R.C., Anthony T.G. Obesity challenges the hepatoprotective function of the integrated stress response to asparaginase exposure in mice. J Biol Chem. 2017;292:6786–6798. doi: 10.1074/jbc.M116.768408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Pan Y.X., Dudenhausen E.E., Kilberg M.S. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem. 2004;279:50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- 23.Clausen N., Nielsen J.H. Direct long-term effects of L-asparaginase on rat and human pancreatic islets. Pediatr Res. 1989;26:158–161. doi: 10.1203/00006450-198908000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Riccardi R., Holcenberg J.S., Glaubiger D.L., Wood J.H., Poplack D.G. L-asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res. 1981;41:4554–4558. [PubMed] [Google Scholar]
- 25.Avramis V.I., Sencer S., Periclou A.P., Sather H., Bostrom B.C., Cohen L.J., Ettinger A.G., Ettinger L.J., Franklin J., Gaynon P.S., Hilden J.M., Lange B., Majlessipour F., Mathew P., Needle M., Neglia J., Reaman G., Holcenberg J.S., Stork L. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99:1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 26.Su N., Kilberg M.S. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem. 2008;283:35106–35117. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S., Orabi A.I., Le T., Javed T.A., Sah S., Eisses J.F., Bottino R., Molkentin J.D., Husain S.Z. Exposure to radiocontrast agents induces pancreatic inflammation by activation of nuclear factor-kappaB, calcium signaling, and calcineurin. Gastroenterology. 2015;149:753–764 e11. doi: 10.1053/j.gastro.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muili K.A., Wang D., Orabi A.I., Sarwar S., Luo Y., Javed T.A., Eisses J.F., Mahmood S.M., Jin S., Singh V.P., Ananthanaravanan M., Perides G., Williams J.A., Molkentin J.D., Husain S.Z. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem. 2013;288:570–580. doi: 10.1074/jbc.M112.428896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orabi A.I., Wen L., Javed T.A., Le T., Guo P., Sanker S., Ricks D., Boggs K., Eisses J.F., Castro C., Xiao X., Prasadan K., Esni F., Gittes G.K., Husain S.Z. Targeted inhibition of pancreatic acinar cell calcineurin is a novel strategy to prevent post-ERCP pancreatitis. Cell Mol Gastroenterol Hepatol. 2017;3:119–128. doi: 10.1016/j.jcmgh.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saluja A.K., Dudeja V. Relevance of animal models of pancreatic cancer and pancreatitis to human disease. Gastroenterology. 2013;144:1194–1198. doi: 10.1053/j.gastro.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto J., Kamata S., Miura A., Nagata T., Kainuma R., Ishii I. Differential adaptive responses to 1- or 2-day fasting in various mouse tissues revealed by quantitative PCR analysis. FEBS Open Bio. 2015;5:357–368. doi: 10.1016/j.fob.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao S.H., Wang W.L., Chen C.Y., Chang Y.L., Wu Y.Y., Wang Y.T., Wang S.P., Nesvizhskii A.I., Chen Y.J., Hong T.M., Yang P.C. Analysis of protein stability by the cycloheximide chase assay. Bio Protoc. 2015;5 doi: 10.21769/BioProtoc.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H., Zhou F., Du W., Dou J., Xu Y., Gao W., Chen G., Zuo X., Sun L., Zhang X., Yang S. Knockdown of asparagine synthetase by RNAi suppresses cell growth in human melanoma cells and epidermoid carcinoma cells. Biotechnol Appl Biochem. 2016;63:328–333. doi: 10.1002/bab.1383. [DOI] [PubMed] [Google Scholar]
- 34.Ueno T., Ohtawa K., Mitsui K., Kodera Y., Hiroto M., Matsushima A., Inada Y., Nishimura H. Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia. 1997;11:1858–1861. doi: 10.1038/sj.leu.2400834. [DOI] [PubMed] [Google Scholar]
- 35.Logsdon C.D., Ji B. The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol. 2013;10:362–370. doi: 10.1038/nrgastro.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Case R.M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978;53:211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 37.Antonucci L., Fagman J.B., Kim J.Y., Todoric J., Gukovsky I., Mackey M., Ellisman M.H., Karin M. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci U S A. 2015;112:E6166–E6174. doi: 10.1073/pnas.1519384112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan G.C., Chiang A.K., Ha S.Y., Lau Y.L., Ong J.B., Lee A.C. Asparaginase-induced acute parotitis: an uncommon and self-limiting complication. Med Pediatr Oncol. 2002;39:73–74. doi: 10.1002/mpo.10068. [DOI] [PubMed] [Google Scholar]
- 39.Orabi A.I., Muili K.A., Wang D., Jin S., Perides G., Husain S.Z. Preparation of pancreatic acinar cells for the purpose of calcium imaging, cell injury measurements, and adenoviral infection. J Vis Exp. 2013;77 doi: 10.3791/50391. [DOI] [PMC free article] [PubMed] [Google Scholar]