Highlights
-
•
A novel taxol-producing A. fumigatus strain explored from the Northern Himalayan region.
-
•
dbat and ITS genes were used as molecular markers for screening and identification.
-
•
Hyper-production (1.6 g/L) of taxol in S7 medium, highest from an endophytic fungus.
Abbreviations: bapt, baccatin III-aminophenylpropanoyl-13-O-transferase; dbat, 10-deacetylbaccatin III-10-O-acetyl transferase; ITS, Internal Transcribed Spacer; ts, taxadiene synthase; AIDS, Acquired Immuno-Deficiency Syndrome; BLAST, Basic Local Alignment Search Tool; DNA, Deoxyribose Nucleic Acid; HPLC, High Performance Liquid Chromatography; MMA, Modified Mycological Agar; MEGA, Molecular Evolutionary Genetics Analysis 7; PCR, Polymerase Chain Reaction; FTIR, Fourier Transform Infrared Spectroscopy; TLC, Thin Layer Chromatography; UV, Ultra-Violet; MS, Mass Spectroscopy; NMR, Nuclear Magnetic Resonance
Keywords: Taxol, Endophytes, Aspergillus fumigatus, Cancer, Taxus sp.
Abstract
Taxol® (generic name Paclitaxel) is a chemotherapeutic drug, effective against head, neck, breast, lung, bladder, ovary, and cervix cancers. Rising demands in chemotherapy and limited supply of natural taxol have ultimately increased the cost of the drug. Semi synthesis using taxol precursors is not able to meet the global supply and has intensified the need to find alternative ways of taxol production. In the present study, 34 different endophytes were isolated from Taxus sp. collected from Shimla, Himachal Pradesh (India). Primary screening of taxol-producing fungi was carried out based on the presence of dbat gene, essential for the taxol biosynthetic pathway. A fungal isolate TPF-06 was screened to be a taxol-producing strain based on the PCR amplification results. It was characterized and identified as Aspergillus fumigatus by 18S rRNA (Accession No. KU-837249). Multiple sequence alignment (MSA) of nuclear ribosomal internal transcribed spacer (ITS) region and phylogenetic analysis confirmed that strain belonged to A. fumigatus clade (Accession No. MF-374798) and is endophytic in nature. Presence of taxol was detected and quantified by High-Performance Liquid Chromatography (HPLC) and characterized by using Thin Layer Chromatography (TLC), Ultraviolet (UV) spectroscopy, Mass spectrometry (MS), Fourier-Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR) spectroscopy. Microbial fermentation in the S7 medium yielded 1.60 g/L of taxol, which to the best of our knowledge is the highest taxol production from an endophytic fungus. Findings of the present study suggest that the A. fumigatus is an excellent alternate source for taxol supply, and it may become a highly potent strain on a commercial scale. The involvement of dbat gene in A. fumigatus KU-837249 strain further suggested a way of increasing taxol yield in fungi by medium engineering and recombinant DNA technology in the future.
1. Introduction
Taxol® (generic name Paclitaxel) is a poly-oxygenated cyclic di-terpenoid with a characteristic taxane ring system [1]. It is the most effective and widely used chemotherapeutic drug for the treatment of cancers and virus-related sarcoma [[2], [3], [4]]. Each year, approximately 8.1 million new cancer cases are diagnosed worldwide, including India. Around five hundred patients are treated by 1 kg of taxol, which requires 10 tons of bark equivalent to 300 trees [5]. First time, taxol was isolated from the Taxus brevifolia (bark, roots, and branches) [1]. So far, the source of taxol is either semi-synthetic precursors like baccatin III and 10-deacetylbaccatin III or natural yew tree. Although the concentration of taxol is very low (0.01 %–0.05 %) from the natural sources, still, the bark of yew (Taxus) is the principal source. The high cost of the drug is attributed to the inadequate supply of natural taxol and increasing application in chemotherapy.
Recently, microbial fermentation technology emerged as an alternative approach for cheaper and higher yield of taxol. Particularly, isolation and identification of taxol-producing endophytic fungus is a very prospective and feasible approach for the production of a large amount of taxol [6,7]. Endophytes are designated as a promising source of novel natural metabolites exhibiting a variety of biological activities, including anti-cancer properties [[8], [9], [10], [11]]. Recently, several endophytes from different genera such as T. Anderanae, Alternaria alternate, Fusarium sp. are reported to produce taxol [12]. Production of secondary metabolites is significantly affected by genetic, developmental, and environmental factors. Recombination DNA technologies like gene manipulation and metabolic pathway alterations may improve the endophytic strains and enhance taxol production [13].
In the past decades, numerous taxol-producing endophytic fungi have been isolated. However, none of them achieved an industrial production platform because of the low amount of taxol production. Therefore, researchers are looking for newer approaches using recombinant technology to improve the yield from isolated taxol-producing fungi, in addition to search novel high taxol-producing stable microbial isolates from nature. However, due to the rapidly growing market, low availability, and the fact that Taxus spp. are rare, endangered [14,15] and grow very slowly, an alternative source is needed to produce taxol at large commercial scale. Considering these factors, the current study was carried out on isolation, identification and extracellular production of taxol from Aspergillus fumigatus isolated from Taxus sp. collected from the Northern Himalayan region of India.
2. Materials and methods
2.1. Chemicals and molecular reagents
All the reagents and chemicals were of high purity and analytical grade. Standard paclitaxel was procured from MP Biomedicals (USA). Media components used for the growth and maintenance of taxol-producing endophytes were purchased from Hi-Media (Mumbai). The electrophoresis reagents, molecular grade chemicals used for DNA isolation were purchased from SDFCL (India) and Sigma-Aldrich (USA), respectively. Pre-coated Silica gel 60, F254 TLC plates, and HPLC solvents were of HPLC grade and procured from Merck (Germany). Universal primers for ITS and dbat genes were procured from Sigma (USA) and Bioservice, respectively.
2.2. Collection of plant samples and isolation of taxol-producing endophytes
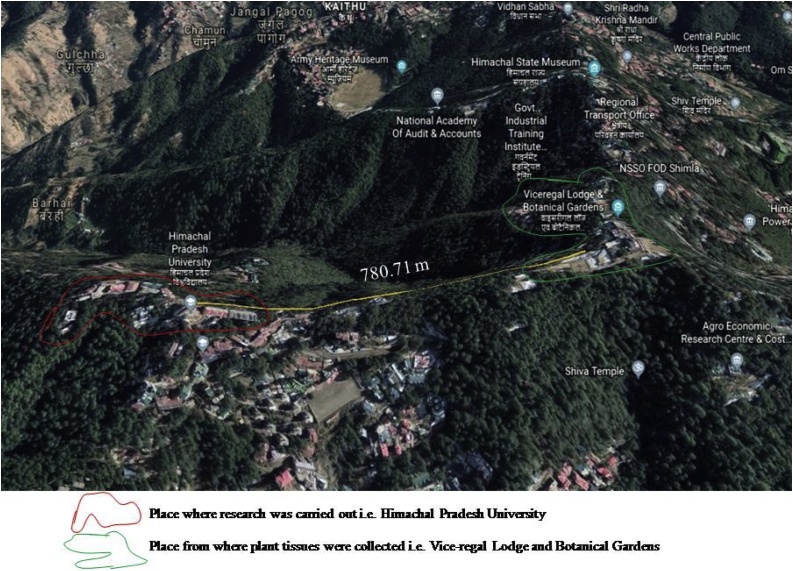
Taxol-producing endophytes were isolated from different plant tissues (bark, stem, and needle) of Taxus sp. collected from Shimla, Himachal Pradesh (India). The location map of the plant tissue collection sites is shown in Fig. 1. The bark, stem, and needle samples were surface sterilized under a laminar airflow chamber with ethanol (70 %; v/v) for 30 s and sodium hypochlorite (3.5 %; v/v) for 2 min, followed by washing with sterilized water. Subsequently, the outer surface was peeled off using a sterilized surgical blade. The bark, stem, and needles were chopped into small pieces of ∼ 0.5 × 0.5 × 0.5 cm and were aseptically placed on the surface of modified mycological agar (MMA) medium [16], composed of glucose 40 g/L, bacto-soytone 10 g/L, sodium acetate 1 g/L, sodium benzoate 50 mg/L, bacto-agar 15 g/L, pH 6.0–6.5 and were incubated at 25 ± 1 °C for 72 h. Morphologically different colonies were picked up depending upon shape, size, and color. Pure line cultures were established by repeatedly streaking single colonies on the MMA medium, and pure cultures were maintained on slants at 4 °C. It was decided to further restrict the studies to endophytic fungi in a quest for taxol-producing microorganisms.
Fig. 1.
Location map of the plant tissues collection sites.
2.3. Molecular screening of taxol-producing endophytic Fungi
All the isolated fungal cultures were grown individually in Erlenmeyer flasks containing 25 mL modified S7 liquid broth [16] consisted of glucose 3 g/L, sodium acetate 1 g/L, sucrose 18 g/L, beef-extract 5 g/L, fructose 9 g/L, soytone 1 g/L, thiamine 1 mg/L, biotin 1 mg/L, pyridoxal 1 mg/L, calcium pantothenate 1 mg/L, magnesium sulphate 3.6 mg/L, calcium nitrate 6.5 mg/L, copper nitrate 1 mg/L, zinc sulphate 2.5 mg/L, manganese chloride 5 mg/L, iron (III) chloride 2 mg/L, phenylalanine 5 mg/L, sodium benzoate 100 mg/L, pH 6.0–6.5 and 1 mL of 1 M sodium phosphate buffer, pH 6.8 and incubated at 25 ± 1 °C for 5–7 days at 150 rpm in an incubator shaker. Genomic DNA extraction was carried out from the harvested mycelia.
2.3.1. Fungal DNA extraction
Genomic DNA was extracted using the CTAB method [17] with necessary modifications. Briefly, the mycelia collected from the cultures were ground using mortar-pestle in liquid nitrogen into a fine powder. 200 mg of mycelium powder was suspended in 1000 μL of DNA extraction buffer. To the above suspension, 200 μL of 5 M NaCl and 100 μL of 10 % cetyltrimethylammonium bromide (CTAB) were added. The resulting mixture was incubated in a water bath for 3 min at 45 °C with occasional inversion. An equal volume of Chloroform: Isoamyl alcohol (24:1; v/v) was added to the lysed mixture and centrifuged at 12,000×g for 15 min. The upper aqueous layer was collected gently and incubated with an equal amount of isopropanol overnight at −20 °C for precipitation. Post incubation, tubes were centrifuged at 10,000×g for 10 min (4 °C), followed by washing of upper aqueous phase with 70 % ethanol. Finally, the pellet was dissolved in nuclease-free water (30 μL) and stored at −20 °C till further use.
2.3.2. PCR based molecular screening using dbat and ITS genes
The internal transcribed spacer (ITS) fragments and 10-deacetylbaccatin III-10-O-acetly transferase (dbat) gene were amplified by using universal primers [18] ITS1 (5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ); ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ); dbat F (5ʹ-GGGAGGGTGCTCTGTTTG-3ʹ) and dbat R (5ʹ-GTTACCTGAACCACCAGAGG-3ʹ) were purchased from Sigma Aldrich (USA) and Bioservice respectively. The standard PCR reaction of 25 μL consisted of 3 μL genomic DNA (∼100 ng), 1.5 μL forward and reverse primers each (10 μM), 0.4 μL DNA Taq polymerase (2 U), 2.5 μL 10X Taq buffer (Thermo), 2.5 μL MgCl2 (25 mM), 2.5 μL dNTP mix (2 mM), and 11.1 μL nuclease-free water (Thermo). The PCR reaction was performed by initial denaturation at 94 °C (3 min), followed by 30 cycles at 94 °C (30 s), 55 °C (30 s), 72 °C (1 min) and final extension at 72 °C (5 min) using thermocycler (Eppendorf Mastercycler™, Germany). Besides this, taxadiene synthase (ts) and C-13 phenylpropanoid side chain-CoA acyltransferase (bapt) genes involved in the taxol synthesis pathway were also screened for PCR amplification using primer sets for ts and bapt [19]. The reaction and temperature profile for ts and bapt gene were similar for ITS gene, as mentioned above. Finally, the PCR products of ITS and dbat genes were analyzed in 2 % agarose gel and visualized using Gel Doc system (CI50 Azure Biosystem, USA).
2.3.3. Nucleotide sequencing and phylogenetic analysis
Specific bands of ITS and dbat genes were sliced from the agarose gel and purified by QIAquick Gel Extraction Kit (Qiagen, Germany). Sequencing PCR of the purified product was done by Big-Dye Terminator v 3.1 Cycle Sequencing Kit (Applied Biosystems USA) using appropriate sense and antisense primers for ITS and dbat gene with standard reaction and temperature profile. Specific amplified products were precipitated, and finally, the samples were loaded into AB 3500 XL Dx Genetic Analyzer (Applied Biosystem, USA). Sequence analysis and comparison using the Basic Local Alignment Search Tool (BLAST) was done in ABI 3500 automated DNA sequencer platform. Multiple sequence alignment and phylogenetic tree analysis were done using Clustal X 2.0.11 and Molecular Evolutionary Genetics Analysis (MEGA) version 7.0 (www.megasoftware.net) software respectively, based on the internal transcribed spacer sequences of similar fungal species.
2.4. Identification and characterization of taxol-producing endophytic fungus
Fungal isolate TPF-06 found positive in molecular screening based on dbat gene expression, was further characterized using microscopic and molecular tools. The endophytic fungal strain TPF-06 was grown on 90 mm Petri plates containing MMA medium and characterized based on colony morphology, spores, reproductive structures, and 18S rRNA sequences.
2.4.1. Morphological characterization using microscopy
Microscopic studies were carried out using fungal mycelia on a glass slide stained with Lactophenol Cotton Blue (LPCB) dye. Fungal mycelia were carefully teased using a needle, and a coverslip was placed onto the thin preparation. Morphology was observed under an upright biological microscope at 40X (Olympus, Japan).
2.4.2. Molecular identification using 18S rRNA
A fresh plate of the fungal isolate TPF-06 was prepared on MMA medium and outsourced to the Xcelris Genomics, Ahmedabad, Gujarat (India) for 18S rRNA sequencing. Sequencing data was analyzed and compared with similar sequences from NCBI (USA) using BLAST [20]. Clustal W version 2.0 and MEGA version 7.0 [21] were used to align the partial 18S rRNA sequences and to build a phylogenetic tree for a selected fungal isolate.
2.5. Hyper-production and extraction of taxol from endophytic fungus
Erlenmeyer flask containing 100 mL modified S7 liquid medium [16] was seeded with 4.79 × 104 spores per mL of the fungal isolate TPF-06 and incubated at 25 ± 1 °C for 21 days with agitation speed at 150 rpm in an incubator shaker. Post 21 days of incubation, microbial biomass was removed from fungal isolates by passing the cultures through four layers of cheesecloth. The fatty acid concentration was minimized by the addition of 0.25 g sodium carbonate to culture filtrate and later extracted with two equal volumes of ethyl acetate. Under reduced pressure at 40 °C (vacuum evaporator), the solvent was removed, leaving behind dry solid residues which were re-dissolved in methanol. The crude extract containing taxol was subjected to TLC, HPLC, UV-spectroscopy, FTIR spectroscopy, MS, and NMR analysis for the presence, quantity, and purity of taxol by comparing with standard Paclitaxel procured from M.P Biomedicals (USA).
2.6. Characterization and analysis of extracted taxol
2.6.1. Thin Layer Chromatography (TLC)
For the detection of taxol, the crude sample was spotted on 0.25 mm (10 × 20 cm) aluminum pre-coated silica gel plates along with standard paclitaxel as internal standard, and the plate was developed in chloroform:methanol at 7:1 (v/v) successively. Taxol was detected by spraying 1 % vanillin (w/v) in sulfuric acid after gentle heating [22] or by using spray reagent consisting of 20 g of antimony trichloride in a mixture of 20 mL glacial acetic acid and 60 mL chloroform [23]. The Retention factor (Rf) value of sample was calculated according to the following equation from the chromatogram and compared with standard taxol.
2.6.2. Ultra violet (UV) spectroscopic analysis
The UV spectroscopy analysis of the crude extracted sample was performed by scraping off the area of silica TLC plate containing putative taxol at the appropriate Rf. After dissolution in methanol, the spectrum of crude taxol samples was plotted in Beckman DU-40 spectrophotometer (USA) and quantified by comparing with that of the standard taxol.
2.6.3. Fourier-Transform Infrared (FTIR) spectroscopic analysis
The extracted sample (crude taxol) was mixed and grounded with potassium bromide (KBr, IR grade) in a 1:10 ratio and pressed under vacuum to form pellet disc using spectra pelletizer. FTIR of the crude taxol was recorded and compared to standard paclitaxel with Nicolet 5700 in transmittance mode with a higher solution (1 cm–1) and a wide scan range of 4000 cm–1 to 500 cm–1 at Department of Chemistry, HP University, Shimla (India).
2.6.4. Mass spectrometry (MS) analysis
The crude taxol was dissolved in methanol: water: acetic acid (50:50:1; v/v), and at 50 V, 2 μL sample was injected by the loop injection method [1]. Mass spectroscopy (MS) analysis of extracted taxol and standard taxol was performed using Waters Micromass Q-Tof Micro with electrospray ionization (ESI) and atmospheric pressure chemical ionization (APcI) sources having mass range of 4000 amu in quadruple and 20,000 amu in ToF at SAIF/CIL, Punjab University, Chandigarh (India) and NIPER, Mohali (India) to confirm the presence of taxol.
2.6.5. Nuclear Magnetic Resonance (NMR) analysis
1H NMR of fungal taxol was recorded at 23 °C in CDCl3 using Bruker Advance-II 400 NMR spectrometer (Germany) with a cryomagnet of field strength 9.4 T, to confirm the structure. 1H NMR spectra were obtained at 400 Mhz following standard pulse sequences and phase programs supplied with NMR spectrometer [24].
2.6.6. High-Performance Liquid Chromatography (HPLC) analysis
HPLC was performed to estimate taxol production in the sample extracts. For HPLC analysis, the sample extracts were diluted in the mobile phase and subjected to HPLC (Perkin Elmer, USA), performed using 200 Ic pump (Perkin Elmer) equipped with reverse phase C18 5 μm column (Merck, LiChrosolv) and 785A Absorbance Detector (Applied Biosystem) [16]. Briefly, extracted test samples (crude taxol) were filtered through a 0.2 μm filter. The mobile phase consisted of methanol: water, 80:20 (v/v). 10 μL of the crude sample was injected each time with 1 mL per min flow rate and was detected at 227 nm [25]. NetWin Software (Netel Chromatographs, India) was used to monitor absorbance at 227 nm. Taxol presence was verified by comparing the retention time of the test samples with that of the standard taxol (Paclitaxel, M.P Biomedicals).
2.7. Quantification of fungal taxol
The calibration curve was constructed using HPLC by injecting the different known concentrations of standard taxol. The area under the peak of known concentrations was used for quantification. The average of four independent experiments was used to estimate the concentration of taxol production per liter from the extracted samples.
3. Results and discussion
3.1. Isolation of taxol-producing endophytes from Taxus sp
Endophytes are well recognized as a novel resource of bioactive compounds. Isolation of endophytic microorganisms producing paclitaxel is extensively studied worldwide, and more than 100 taxol-producing strains have been isolated till now [11,22]. However, yield in the reported isolates was extensively low to be explored further for the commercial applications [26,27].
In the present study, an attempt has been made to isolate a hyper taxol-producing endophyte having industrial applications. In total, 34 different endophytes were isolated from the bark, stem, and needle tissue samples of Taxus sp. collected from Shimla, Himachal Pradesh (India) (Supplementary information Table 1S). The isolated endophytes include bacterial, fungal, and actinomycetes, which were sub-cultured on MMA medium to eliminate taxol or other taxane traces carried over from the plant tissue. Only the fungal cultures were further screened, identified, and characterized for taxol production.
3.2. Molecular screening of Taxol-producing Fungi based on dbat and ITS genes
Detection of taxol using biochemical and spectrometric techniques is a time-intensive procedure. A couple of studies have used dbat, ts, and bapt genes encoding for the taxol biosynthetic pathway as molecular markers for screening taxol-producing fungi [9,[28], [29], [30]]. ITS gene coding for the nuclear ribosomal internal transcribed spacer region is a universal DNA barcode marker for fungal identification.
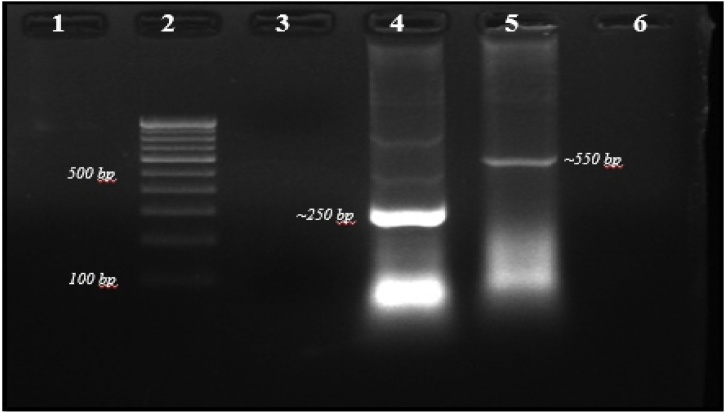
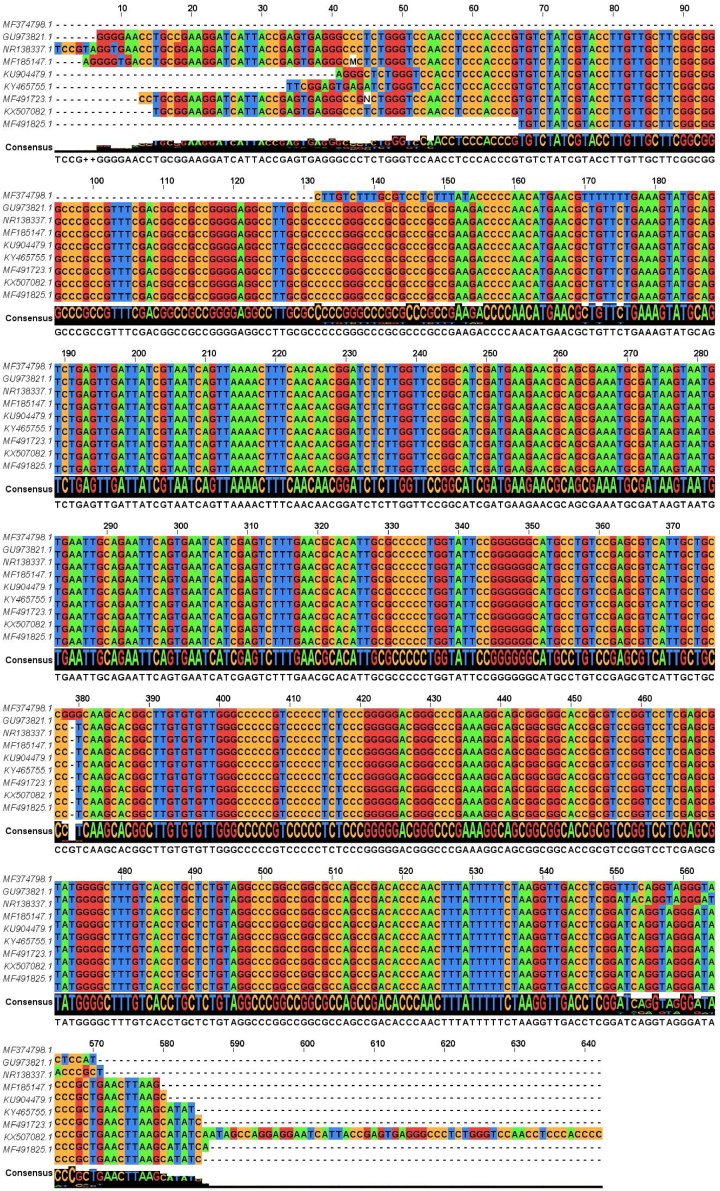
Therefore, dbat and ITS genes were used as molecular markers for screening and identification of taxol-producing endophytic fungi in the present study. CTAB method used for genomic DNA isolation was found to be efficient. The concentration and purity of the DNA were confirmed by nanodrop. PCR amplification confirms that the fungal isolate, TPF-06, was found positive for dbat and ITS gene. Agarose gel electrophoresis showed a band at approximately ∼250 bp and ∼530 bp, respectively (Fig. 2). The sequencing of the amplicons was carried out (sequence file provided as Supplementary information in Table 2S). BLAST analysis of the ITS sequence of fungal isolate TPF-06 revealed 93 % similarity with Aspergillus fumigatus (query coverage 97 %). Results of phylogenetic analysis clustered TPF-06 with A. fumigatus species based on the evolutionary distance (Fig. 3). The ITS gene sequence is submitted to NCBI with accession number MF-374798.
Fig. 2.
PCR analysis for the presence of dbat gene in A. fumigatus; Lane 2: Molecular Marker (100 bp); Lane 4: dbat gene (∼250 bp); Lane 5: ITS gene (∼550 bp); Lane 1, 3, 6: Empty.
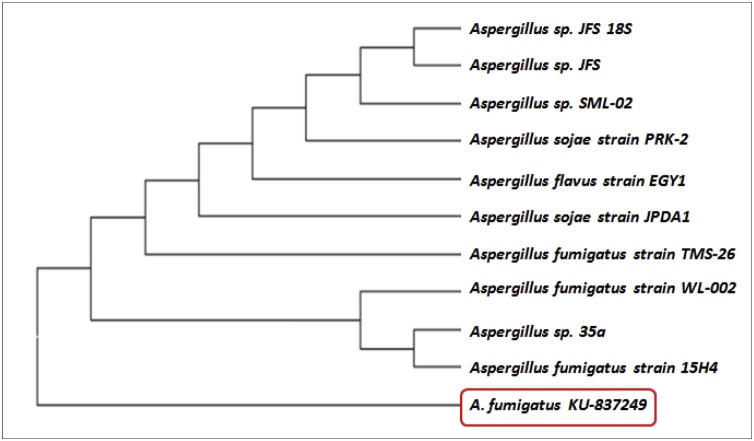
Fig. 3.
Phylogenetic dendrogram of selected isolate 18S rRNA sequence based on neighbor joining method.
Similar results were found during the primary screening of taxol-producing endophytic fungi by Zhou et al. [31] and Zhang et al. [32], reinforced the utility of ITS, ts, dbat and bapt genes as molecular markers [29]. Zhou et al. [33], used a gene coding for taxadiene synthase (TS), which is a rate-limiting enzyme in the taxol biosynthetic pathway as a molecular marker to screen for taxol-producing fungi. Jennewein et al. [34], on the contrary, suggested that dbat and bapt genes are more diagnostic than the ts gene because more than ten enzymatic steps after TS, are required to reach Baccatin III and taxol itself. Roopa et al. [29] showed the presence of dbat and bapt gene implicated in taxol biosynthesis and ITS gene (∼540 bp) in Alternaria, Fusarium and A. niger isolated from Salacia oblonga. Xiong et al. [35] isolated and identified Guignardia, Nigrospora, Phomopsis, and Phoma from T. media based on ITS rDNA sequences.
3.3. Morphological Identification and Phylogenetic Analysis based on 18 s RNA
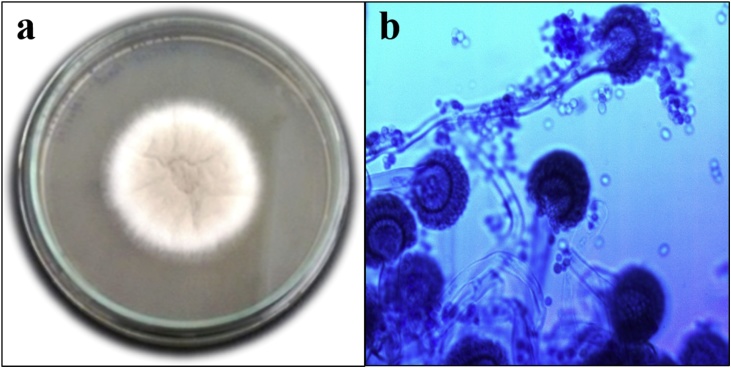
The pure culture of fungal isolate TPF-06, found positive based on dbat gene expression, was prepared and maintained at 4 °C. TPF-06 was aerobic, spore former with septate mycelium. The colonies were white-creamish in appearance from the front side and yellowish-white from reverse side (Fig. 4; a) on the MMA plate. Morphological observations of TPF-06 under microscope indicated stipe and conidial head consisted of single series of phialides, and rounded conidia were dispersed in long and parallel dry chains upon staining with LPCB dye at 40X magnification (Fig. 4; b). Based on the morphological features observed, the endophytic fungus was further confirmed to belong to the genus Aspergillus.
Fig. 4.
Morphological characterization of A. fumigatus (a) on Modified Mycological Agar Medium (b) under Upright Biological Microscope at 40X.
Sequence analysis of 18S rRNA elucidated the taxonomic position of taxol-producing fungus. A strong relationship was revealed between selected isolate and members of genus Aspergillus using the BLAST comparison of 767 bp sequence with other similar sequences available in the GenBank database [20]. The maximum similarity resulted in a cluster that included many different species of Aspergillus was showing a close relationship with the fungus isolated in the present study. Homology comparison conferred 94 % homology with A. fumigatus strain-A (001) and A. fumigatus 15H4-PO-P1-1strain (Fig. 5). Based on the morphological features, 18S rRNA gene sequence homology, and phylogenetic tree analysis, the isolate was identified and designated as A. fumigatus KU-837249. Also, the close association of fungal isolate TPF-06 with the endophytic A. flavus and A. neoellipticus proved the endophytic nature of A. fumigatus KU-837249. To the best of author’s knowledge and as per the information available in the literature, it is revealed that A. fumigatus from Taxus sp. of the Northern Himalayan region was not reported for taxol production.
Fig. 5.
Conservation of taxol producing ITS region in other selected Aspergillus spp.
Zhou et al. [33] identified the taxol-producing endophytic fungus as Mucor sp. based on 18S rRNA sequence. Similarly, the HD86-9 strain revealed morphological and molecular similarity (98 %) to A. niger using 18S rRNA and internal transcribed spacer (ITS) region analysis [33].
3.4. Hyper-production and extraction of taxol from endophytic fungus A. fumigatus
Current sources of Taxol production include extraction from cultivated Taxus spp., chemical semi-synthesis, in vitro plant tissue and cell culture, metabolic engineering in bacterial and fungal endophytes. The strategy to extract taxol from natural bark source is limited at the global scenario because of the slow growth of Taxus spp. and low-yield [36]. Thus, chemical semi-synthesis [[37], [38], [39], [40], [41]] and in vitro plant tissue culture [[42], [43], [44], [45]] techniques prevail as the primary source for taxol supply at clinical level. Chemical semi-synthesis of paclitaxel from 10-deacetylbaccatin III (10-DAB) was reported for the first time in 1988 [46]. Plant tissue culture/cell culture-based strategies used for the production of taxol from Taxus spp. has been summarized in Table 1. However, low and unstable product yield, high production costs [55] and dependence on the yew tree material are burning problems in these widely used methods [56].
Table 1.
Plant tissue culture/cell culture based production of taxolfrom Taxus species.
| Plant species | Culture type | Taxol yeild | References |
|---|---|---|---|
| Taxus cuspidata | Callus culture in Shake flask | 0.020 % DW | [47] |
| Taxus cuspidata | Callus culture in Shake flask | 431 mg/L | [48] |
| Taxus brevifolia | Callus culture | 0.01 % of the dry weight of the bark | [49] |
| Taxus media | Cell suspension | 115.2 mg/L | [50] |
| Taxus x media | Hairy root culture | 221.8 μg/L | [51] |
| Taxus chinensis | Cell culture in a bioreactor | 612 mg/L | [52] |
| Taxus chinensis | Cell suspension culture in Fed batch conditions | 900 mg/L | [53] |
| Taxus baccata | Cell culture with methyl jasmonate induction | 295 mg/L | [43] |
| Taxus baccata | Cell suspension cultures immobilized within Ca2+ alginate beads in Stirred bioreactor | 43.43 mg/L | [54] |
Since the discovery of first taxol-producing fungi in 1993 [16], continuous interest from researchers all over the world to explore a different approach to produce the drug from fungal endophytes. More than 50 taxol-producing fungal endophytes have been isolated over the past decades. A comprehensive list of fungal endophytes isolated from different plant species with the taxol yield has been highlighted in Table 2. Numerous issues had delayed the fungal production of the drug at the commercial scale. One of the most highlighted challenges is the low yield in fungal strains. Besides this, the stains will loose their taxol-producing capabilities after long-term culturing [74]. To overcome this issue, the heterologous expression of genes from taxol biosynthetic pathway has been attempted to produce the compound using genetic engineering techniques [13,75]. An E. coli and yeast strain were engineered to produce taxadiene in high titers of 1 g/L and 8.7 ± 0.85 mg/L respectively [76,77]. However, the lack of availability of a complete set of genes involved in paclitaxel biosynthesis is at present a limiting factor, especially in case of endophytic microorganisms [26].
Table 2.
Taxol producing endophytic fungi isolated from different plant hosts.
| Endophytic fungi | Plant Host | Concentration (μg/L) | References |
|---|---|---|---|
| Taxomyces andeanae | Taxus brevifolia | 0.024–0.05 | [16] |
| Pestalotiopsis microspora | Taxus walachiana | 60–70 | [57] |
| Pestalotiopsis guepinii | Wollemia nobilis | 0.49 | [58] |
| Periconia sp. | Torreyagra ndifolia | 0.03–0.83 | [59] |
| Pestalotiopsis microspora | Maguireothamnusspeciosus | 0.11 | [60] |
| Tubercularia sp. | Taxus mairei | 185.40 | [6] |
| Trichothecium sp. | Taxus wallichiana | 0.17 | [61] |
| Phoma sp. | Taxus yunnanensis | 32.93 | [62] |
| Aspergillus niger | Taxus yunnanensis | 1000.00 | [63] |
| Nodulisporum sylviforme | Taxus cuspidata | 392 | [64] |
| Ectosroma sp. | T. chinensis varmairei | 276.75 | [65] |
| Bionectria sp. | T. chinensis varmairei | 33.90–430.46 | [66] |
| Aspergillus fumigatus | Podocarpus sp. | 560.0 | [7] |
| Metarhizlum anisopliae | Taxus chinensis | 846.1 | [67] |
| Nodulisporum sylviforme | Taxus cuspidata | 468.6 | [68] |
| Phomabetae | Gingko biloba | 795.00 | [69] |
| Fusariumredolens | Taxus baccata subsp. wallichiana | 66 | [30] |
| Penicillium aurantiogriseum | Corylusa vellana | 70–350 | [70] |
| Cladosporium oxysporum | Moringa oleifera | 550 | [71] |
| Phoma Medicaginis | Taxus wallichianavar, mairei | 1215 | [72] |
| Aspergillus aculeatinus | Taxus chinensis var. mairei | 334.92–1137.56 | [73] |
| Aspergillus fumigatus | Taxus sp. | 1590.00 | Present Study |
In the present study, taxol from A. fumigatus (TPF-06) was produced by growing fungal isolate in modified S7 medium incubated at 25 ± 1 °C for 21 days with sucrose as carbon and beef extract as a nitrogen source. S7 medium was chosen for fungal fermentation because the sugar ratio in the S7 medium was identical to the inner bark of Taxus sp. [16]. Since the benzoyl ring in plant-derived taxol is from phenylalanine [78,79], modified S7 medium was supplemented with phenylalanine, and sodium acetate to act as precursors during the metabolism of these endophytic fungi.
Similarly, in earlier investigations [80,81], S7 medium have been used for taxol production. Xu et al. [82], reported taxol yield of 20 μg/L with Fusarium maire in the basal medium consisted of glucose (80 g); NH4NO3 (5 g); MgSO4 (0.5 g); KH2PO4 (0.5 g); ZnSO4 (1 mg); Cu(NO3)2 (1 mg); FeCl3 (2 mg); NaOAc (1 g), vitamin B1 (50 mg) and l-tyrosine (5 mg). Chakravarthi et al. [83] used potato dextrose liquid medium for Cladosporium cladosporioides MD2 (T. media) and Fusarium solani (T. celebica) for better taxol yield.
After 21 days of fermentation, culture filtrates were extracted with equal volumes of ethyl acetate, and the organic phase was collected. The solvent was evaporated under vacuum to get dry solid residues of fungal extracts. These extracts were dissolved in methanol and examined for taxol production by TLC, UV-Spectroscopy, FTIR, MS, and HPLC techniques. The results were compared with the standard taxol from MP Biomedical (USA) to confirm the presence of taxol.
3.5. Characterization and analysis of extracted taxol
3.5.1. Thin-layer Chromatography (TLC) analysis
TLC analysis detected crude taxol on 0.25 mm silica gel plates developed in chloroform:methanol (7:1, v/v) with 1 % (w/v) vanillin in sulphuric acid reagent after gentle heating. The spot appeared blue and faded to dark grey after 24 h (Fig. 6), indicated the presence of taxol in the sample mixture when compared with standard. Rf value was found to be 0.90, which was similar to standard taxol [84]. A similar chromatographic result was also reported for taxol production from Pestalotiopsis malicola and P. pauciseta VM1 [85,86]. Gangadevi and Muthumary [87], using TLC confirmed taxol production by Colletotrichum gloeosporioides JGC-9 isolated from medicinal plant Justicia gendarussa.
Fig. 6.
Detection of taxol using TLC;ST: Standard Taxol; and TS: Test Samples.
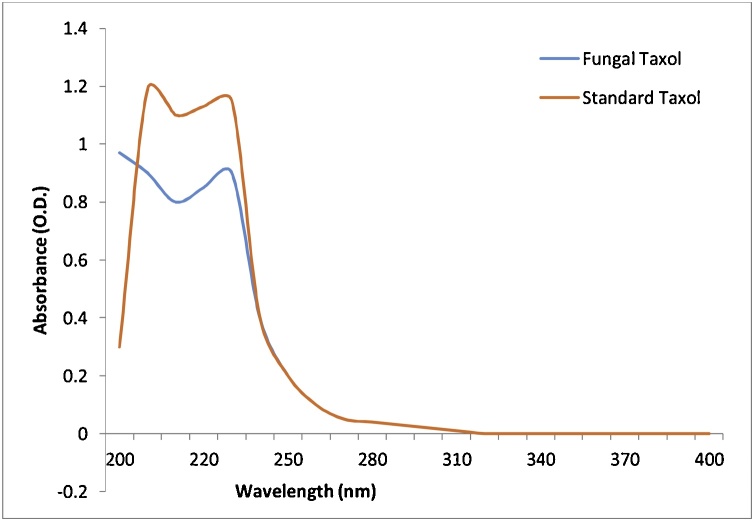
3.5.2. Ultra violet (UV) spectroscopic analysis
UV spectroscopic analysis showed λmax for sample taxol at 227 nm ranges as comparable to λmax for standard paclitaxel is observed at 227 nm range with minor variations in absorbance [88]. This confirmed the existence of taxol molecule in the sample mixture (Fig. 7). The λmax corresponds to the existence of the benzoyl group. The fungal compound isolated from Tubercularia sp. TF5 showed a similar UV absorption spectrum to that of standard taxol at 228 nm [6,82]. The UV absorption spectrum of fungal taxol isolated from Pestalotiopsis pauciseta VM1 was similar to that of standard taxol with maximum absorption at 235 nm, and 232 nm [86] whereas Gangadevi et al. [89], reported similar UV spectrum with maximum absorption at 273 nm.
Fig. 7.
UV spectrum of standard and fungal taxol produced from A. fumigatus.
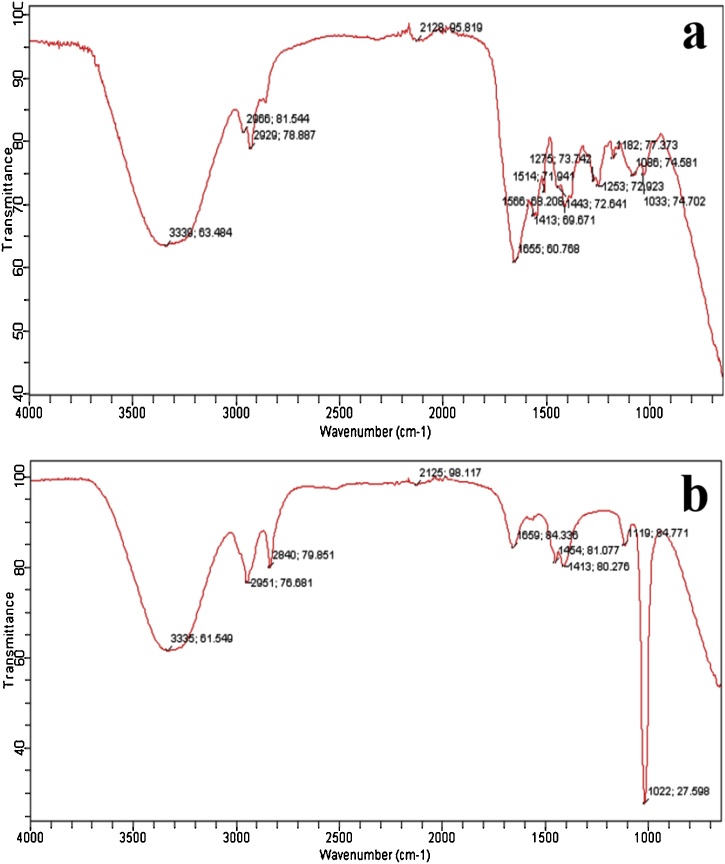
3.5.3. Fourier Transfer Infrared (FTIR) spectroscopic analysis
The IR spectra of taxol produced by A. fumigatus was almost superimposed on the spectrum of standard taxol with small variations in peaks (Fig. 8; a,b). A broad peak in the range of 3336 to 3436 cm–1 was observed due to hydroxyl (−OH) and amide (–NH) groups stretch in addition to aliphatic CH stretch in the range of 2920 to 2939 cm–1. The registration peak was observed in the range of 2356–2364 cm–1 and 1045 to 1068 cm–1, due to amine (NH) group and aromatic C and H bands stretching frequency. The aromatic ring (C C) stretching frequency and the esters and ketone (C O) groups stretching was observed in the range of 1590 to 1735 cm–1. Based on the IR analysis, this fungus showed a positive sign for the production of taxol in the culture medium in comparison to the standard taxol, which was also reported in the earlier work of Kumaran et al. [90] and Gangadevi and Muthumary [91]. A similar IR spectrum with identical group stretching was obtained for taxol obtained from an endophytic fungus P. pauciseta VM1 [86].
Fig. 8.
IR spectrum of (a) standard taxol; and (b) sample taxol from A. fumigatus.
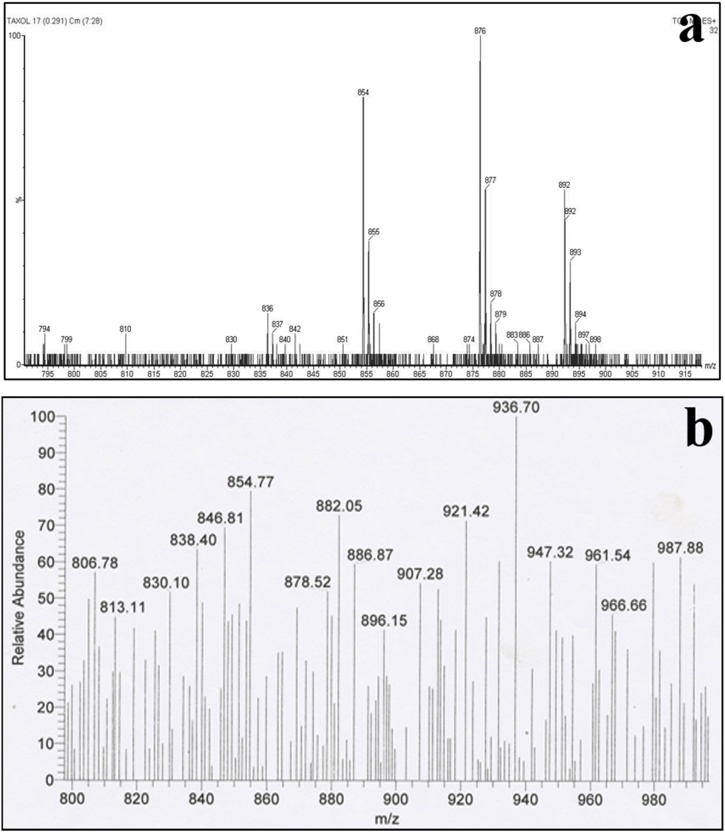
3.5.4. Mass spectrometry (MS) analysis
High-resolution MS revealed the complex structure of crude taxol produced from A. fumigatus with empirical formula C47H51NO14 and molecular weight of 853.9. Chromatogram analysis of standard taxol yields both (M+H)+ and (M + Na)+ peak at an 854 m/z and 876 m/z, respectively (Fig. 9; a). Crude taxol sample also produced both peaks (M+H)+ and (M + Na)+ at 854.77 m/z and 878.52 m/z, respectively (Fig. 9; b), with small variations and confirmed the presence of taxol in test samples. These results showed that A. fumigatus produced taxol in the appreciable amount [57]. Electrospray mass spectra of fungal taxol isolated from A. niger from Taxus cuspidate and A. candidus MD3 showed (M+H)+ and (M + Na)+ peak at 855 m/z and 876 m/z respectively and was similar to the standard taxol [33,92].
Fig. 9.
MS chromatogram of (a) standard taxol; and (b) sample taxol from A. fumigatus.
3.5.5. Nuclear magnetic resonance (NMR) analysis
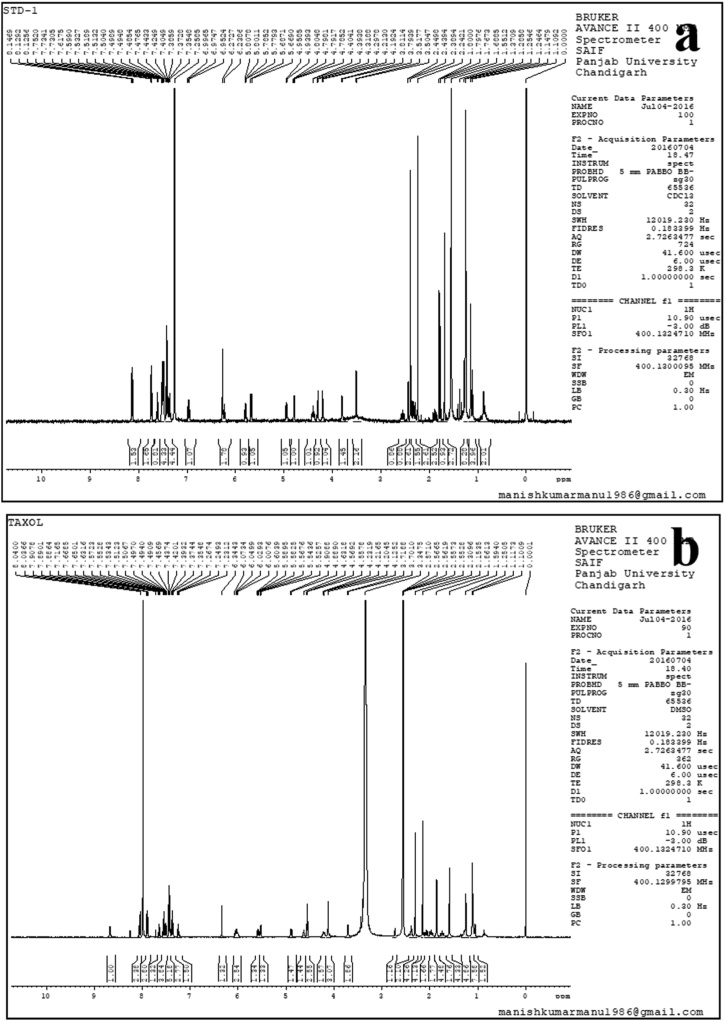
1H NMR spectra of taxol showed good distribution and resolution of all the signals in the 1.0 ppm–8.5 ppm range. The strong three-proton signals caused by the methyl and acetate groups contributed to the strong three-proton signals and lie in the range of 1.0 ppm–2.5 ppm along with multiplets caused by few methylene moieties. The taxane skeleton and the side chain are distributed by majority of the protons and observed in the region between 2.5 ppm and 7.0 ppm, whereas C-2 benzoate, C-30 phenyl, and C-30 benzamide groups contributed the aromatic proton signals between 7.0 ppm and 8.3 ppm. The characteristic chemical shifts of taxol are shown in Fig. 10 (a, b). Similar, 1H NMR characteristic chemical shifts of taxol were obtained from the previous findings of Zhang et al. [92] and Gangadevi and Muthumary [91] from the A. candidus MD3 (T. media) and fungus Bartaliniaro billardoides (Aegle marmelos) respectively. Pandi et al. [3] have also noticed an identical NMR spectrum of taxol produced from an endophytic fungus of Lasiodiplodiatheo bromae isolated from Morinda citrifolia medicinal plant.
Fig. 10.
1H NMR spectrum of (a) standard paclitaxel; and (b) crude paclitaxel from A. fumigatus.
3.5.6. High-Performance Liquid Chromatography (HPLC) analysis
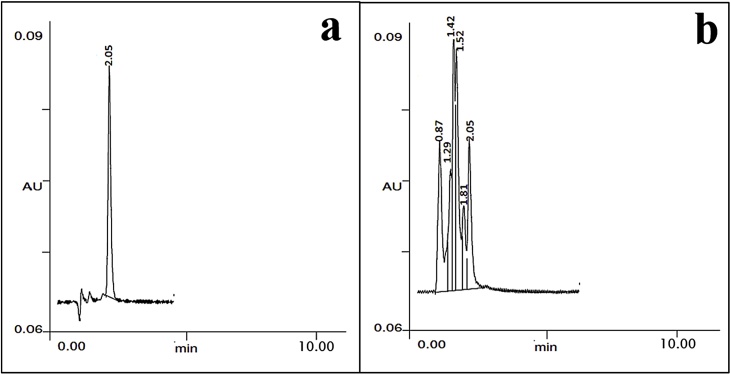
First taxol-producing fungus Taxomyces andreanae with a very low yield of 24−70 ng/L was reported by Sterile et al. [16]. Since then, few reports on the isolation of taxol-producing endophytic fungi [57,81,82,85,93] have been documented. However, the unstable production and lower yield is the major problem for taxol production using fungal fermentation. HPLC analysis recorded a peak with a specific retention time of 2.05, which is identical to standard paclitaxel and confirmed the presence of taxol in test samples (Fig. 11; a, b).
Fig. 11.
HPLC Chromatogram of (a) standard paclitaxel (retention time = 2.05); and (b) sample taxol (retention time = 2.05).
Srinivasan and Kathiravan [84,94] also reported taxol yield of 92 μg/L and 0.064 mg/L from P. funereal and P. breviseta fungus and quantified with HPLC with a similar retention time of 2.822 and 2.210, respectively as standard taxol. Even Metarhizium anisopliae and Cladosporium cladosporioides MD2 fungal strains are very promising taxol producers with up to 800 mg/L yield quantified by HPLC [95].
3.6. Quantification of fungal taxol
The taxol content from the stain TPF-06 identified as A. fumigatus after fungal fermentation in S7 media for 21 days was quantified using HPLC. The area under the peak of different known concentrations of standard taxol served as the standard curve for taxol quantification (Supplementary information Fig. 1S). The total amount of taxol produced was recorded after an average of multiple measurements from samples of single cultivation and found to be 1.60 g/L, which proposed the utility of this fungus for the production of taxol in the culture medium. Although taxol production from Aspergillus sp. has been reported previously. However, this is the first report for the isolation, identification, and characterization of Aspergillus fumigatus from Taxus spp. from the Northern Himalayan region, India which has ability to produce taxol at a high amount of 1.6 g/L. To boost the supply and to bring down the price of cancer treatment, different research strategies including plant cell culture, strain improvement in endophytes using metabolic engineering should be employed in future to satisfy the demand.
4. Conclusion
Endophytes associated with the tissues of higher plants are emerging as a promising alternative and a novel source for microbial taxol production. However, botanical resources and chemical semi-synthesis are not able to satisfy the huge demand for the anti-cancerous drug taxol. In the present work, a new source for microbial taxol production has been explored from the Northern Himalayan region, India, based on the genes involved in taxol biosynthesis and nuclear ribosomal internal transcribed spacer (ITS) region. From the study, it was revealed that identical results exist between the fungal taxol and standard taxol in all the respective spectroscopic and chromatographic techniques. Taxol produced by fungal endophyte Aspergillus fumigatus was found 1.60 g/L, which is so far the highest yield of microbial taxol production recorded till date according to the literature cited. Thus, fermentation processes using taxol-producing endophytes may be an alternative yet promising way to boost the supply of this multibillion-dollar drug (taxol), thereby reduce the increasing cost of drug therapy.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
There is no conflict of interest among the authors.
Acknowledgments
The authors are highly grateful to the Department of Biotechnology, Himachal Pradesh University, Shimla, India, for providing the laboratory and chemical facilities during the study. DNA sequencing facility at the Centre of Advanced Research in Medical Mycology, PGIMER, Chandigarh for helping in gene sequencing and the Computational Facility of Bioinformatics Centre, Himachal Pradesh University Shimla is also duly acknowledged.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00395.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Wani M.C., Taylor H.L., Wall M.E., Coggon P., Mcphail A.T. Plant antitumor agents.VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia2. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Ojima I. In: Taxol®: Science and Applications. Suffness Matthew., editor. CRC Press; Boca Raton, FL: 1995. 426 pp. 18.5 × 26 cm. ISBN 0-8493-8382-X. $129.95., J. Med. Chem. 39 (1996) 807. [DOI] [Google Scholar]
- 3.Pandi M., Senthilkumaran R., Rajapriya P., Yogeswari S., Muthumary J. Taxol, A potential drug for the treatment of cancer. Biores. Bull. 2011;2:1–12. [Google Scholar]
- 4.Skeel R.T., Ganz P.A. Handb. Cancer Chemother. 5th ed. Lippincott Williams Wilkins; Philadelphia: 1999. Systematic assessment of the patient with cancer and long-term medical complications of treatment. 42. [Google Scholar]
- 5.Wheeler N.C., Jech K., Masters S., Brobst S.W., Alvarado A.B., Hoover A.J., Snader K.M. Effects of genetic, epigenetic, and environmental factors on taxol content in Taxus brevifolia and related species. J. Nat. Prod. 1992;55:432–440. doi: 10.1021/np50082a005. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Li G., Lu H., Zheng Z., Huang Y., Su W. Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 2000;193:249–253. doi: 10.1111/j.1574-6968.2000.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun D., Ran X., Wang J. Isolation and identification of a taxol-producing endophytic fungus from Podocarpus. Wei Sheng Wu Xue Bao. 2008;48:589–595. [PubMed] [Google Scholar]
- 8.Zhou D.P., Ping W.X., Sun J.Q., Zhou X.H., Liu X.L., Yang D.Z. Study on isolation of taxol-producing fungus. J. Microbiol. 2001;21:18–20. [Google Scholar]
- 9.Malik S., Cusidó R.M., Mirjalili M.H., Moyano E., Palazón J., Bonfill M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem. 2011;46:23–34. doi: 10.1016/j.procbio.2010.09.004. [DOI] [Google Scholar]
- 10.Elavarasi A., Rathna G.S., Kalaiselvam M. Taxol producing mangrove endophytic fungi Fusarium oxysporum from Rhizophora annamalayana. Asian Pac. J. Trop. Biomed. 2012;2:S1081–S1085. doi: 10.1016/S2221-1691(12)60365-7. [DOI] [Google Scholar]
- 11.Kumaran R.S., Kim H.J., Hur B.-K. Taxol promising fungal endophyte, Pestalotiopsis species isolated from Taxus cuspidata. J. Biosci. Bioeng. 2010;110:541–546. doi: 10.1016/j.jbiosc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X., Zhu H., Liu L., Lin J., Tang K. A review: recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010;86:1707–1717. doi: 10.1007/s00253-010-2546-y. [DOI] [PubMed] [Google Scholar]
- 13.Dejong J.M., Liu Y., Bollon A.P., Long R.M., Jennewein S., Williams D., Croteau R.B. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2006;93:212–224. doi: 10.1002/bit.20694. [DOI] [PubMed] [Google Scholar]
- 14.Ved D.K., Kinhal G.A., Ravikumar K., Prabhakaran V., Ghate U., Sankar R.V., Indresha J.H. Revitalisation Local Heal. Tradit.; 2003. Conservation Assessment & Management Prioritisation for the Medicinal Plants of Jammu & Kashmir, Himachal Pradesh Found. [Google Scholar]
- 15.Sajwan B.S., Prakash K.C. Conservation of medicinal plants: conventional and contemporary strategies, regulations and executions. Indian For. 2007;133:484–495. [Google Scholar]
- 16.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science (80-.) 1993;260 doi: 10.1126/science.8097061. 214 LP – 216. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D., Yang Y., Castlebury L.A., Cerniglia C.E. A method for the large scale isolation of high transformation efficiency fungal genomic DNA. FEMS Microbiol. Lett. 1996;145:261–265. doi: 10.1111/j.1574-6968.1996.tb08587.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y.C., Eisner J.D., Kattar M.M., Rassoulian-Barrett S.L., LaFe K., Yarfitz S.L., Limaye A.P., Cookson B.T. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 2000;38:2302–2310. doi: 10.1128/jcm.38.6.2302-2310.2000. https://www.ncbi.nlm.nih.gov/pubmed/10834993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H., Luo L., Zhou S., Bi P., Liu K., Zhao J. Separation and purification of taxol and cephalomannine from Taxus cuspidada by normal phase chromatography and twice-reversed-phase chromatography. Chem. Nat. Compd. 2007;43:478–480. doi: 10.1007/s10600-007-0169-z. [DOI] [Google Scholar]
- 20.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J., Zhou L., Wang J., Shan T., Lingyun Z., Liu X., Gao L. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010;1 [Google Scholar]
- 23.Kaufmann H.P., Gupta A.K. Terpenes as constituents of the unsaponifiables of fats. Chem. Ber. 1964;97:2652. [Google Scholar]
- 24.Chmurny G.N., Hilton B.D., Brobst S., Look S.A., Witherup K.M., Beutler J.A. 1H- and 13C-NMR assignments for taxol, 7-epi-Taxol, and Cephalomannine. J. Nat. Prod. 1992;55:414–423. doi: 10.1021/np50082a002. [DOI] [PubMed] [Google Scholar]
- 25.Cardellina J.H. HPLC separation of taxol and cephalomannine. J. Liq. Chromatogr. 1991;14:659–665. doi: 10.1080/01483919108049278. [DOI] [Google Scholar]
- 26.Flores-Bustamante Z.R., Rivera-Ordũa F.N., Martínez-Cárdenas A., Flores-Cotera L.B. Microbial paclitaxel: advances and perspectives. J. Antibiot. (Tokyo) 2010;63:460–467. doi: 10.1038/ja.2010.83. [DOI] [PubMed] [Google Scholar]
- 27.Visalakchi S., Johnpaul M. Taxol (anticancer drug) producing endophytic fungi. Int. J. Pharma Bio Sci. 2010;1:1–9. [Google Scholar]
- 28.Das A., Rahman M.I., Ferdous A.S., Amin A.-, Rahman M.M., Nahar N., Uddin M.A., Islam M.R., Khan H. An endophytic Basidiomycete, Grammothele lineata, isolated from Corchorus olitorius, produces paclitaxel that shows cytotoxicity. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roopa G., Madhusudhan M.C., Sunil K.C.R., Lisa N., Calvin R., Poornima R., Zeinab N., Kini K.R., Prakash H.S., Geetha N. Identification of Taxol-producing endophytic fungi isolated from Salacia oblonga through genomic mining approach. J. Genet. Eng. Biotechnol. 2015;13:119–127. doi: 10.1016/j.jgeb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garyali S., Kumar A., Reddy M.S. Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew. J. Microbiol. Biotechnol. 2013;23:1372–1380. doi: 10.4014/jmb.1305.05070. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X., Wang Z., Jiang K., Wei Y., Lin J., Sun X., Tang K. Screening of taxol-producing endophytic fungi from Taxus chinensis var. mairei. Appl. Biochem. Microbiol. 2007;43:439–443. doi: 10.1134/S000368380704014X. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P., Zhou P.P., Jiang C., Yu H., Yu L.J. Screening of Taxol-producing fungi based on PCR amplification from Taxus. Biotechnol. Lett. 2008;30:2119–2123. doi: 10.1007/s10529-008-9801-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhao K., Ping W., Li Q., Hao S., Zhao L., Gao T., Zhou D. Aspergillus niger var. taxi, a new species variant of taxol-producing fungus isolated from Taxus cuspidata in China. J. Appl. Microbiol. 2009;107:1202–1207. doi: 10.1111/j.1365-2672.2009.04305.x. [DOI] [PubMed] [Google Scholar]
- 34.Jennewein S., Long R.M., Williams R.M., Croteau R. Cytochrome P450 taxadiene 5α-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chem. Biol. 2004;11:379–387. doi: 10.1016/j.chembiol.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Xiong Z.Q., Yang Y.Y., Zhao N., Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. 2013;13:71. doi: 10.1186/1471-2180-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann B., Christen P. Recent extraction techniques for natural products: microwave‐assisted extraction and pressurised solvent extraction. Phytochem. Anal. An Int. J. Plant Chem. Biochem. Tech. 2002;13:105–113. doi: 10.1002/pca.631. [DOI] [PubMed] [Google Scholar]
- 37.Holton R.A., Somoza C., Kim H.B., Liang F., Biediger R.J., Boatman P.D., Shindo M., Smith C.C., Kim S. First total synthesis of taxol. 1. Functionalization of the B ring. J. Am. Chem. Soc. 1994;116:1597–1598. [Google Scholar]
- 38.Holton R.A., Kim H.B., Somoza C., Liang F., Biediger R.J., Boatman P.D., Shindo M., Smith C.C., Kim S. First total synthesis of taxol. 2. Completion of the C and D rings. J. Am. Chem. Soc. 1994;116:1599–1600. [Google Scholar]
- 39.Mukaiyama T., Shiina I., Iwadare H., Saitoh M., Nishimura T., Ohkawa N., Sakoh H., Nishimura K., Tani Y., Hasegawa M. Asymmetric total synthesis of taxol\R. Chem. Eur. J. 1999;5:121–161. [Google Scholar]
- 40.Holton R.A., Biediger R.J., Boatman P.D. CRC Press; Boca Raton, FL: 1995. Semisynthesis of Taxol and Taxotere. [Google Scholar]
- 41.Patel R.N., Banerjee A., Nanduri V. Enzymatic acetylation of 10-deacetylbaccatin III to baccatin III by C-10 deacetylase from Nocardioides luteus SC 13913. Enzyme Microb. Technol. 2000;27:371–375. doi: 10.1016/s0141-0229(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 42.Jaziri M., Zhiri A., Guo Y.-W., Dupont J.-P., Shimomura K., Hamada H., Vanhaelen M., Homès J. Taxus sp. cell, tissue and organ cultures as alternative sources for taxoids production: a literature survey. Plant Cell Tissue Organ Cult. 1996;46:59–75. [Google Scholar]
- 43.Tabata H. Biomanufacturing. Springer; 2004. Paclitaxel production by plant-cell-culture technology; pp. 1–23. [DOI] [PubMed] [Google Scholar]
- 44.Wu J., Lin L. Enhancement of taxol production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl. Microbiol. Biotechnol. 2003;62:151–155. doi: 10.1007/s00253-003-1275-x. [DOI] [PubMed] [Google Scholar]
- 45.Khosroushahi A.Y., Valizadeh M., Ghasempour A., Khosrowshahli M., Naghdibadi H., Dadpour M.R., Omidi Y. Improved Taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biol. Int. 2006;30:262–269. doi: 10.1016/j.cellbi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Denis J.N., Greene A.E., Guenard D., Gueritte-Voegelein F., Mangatal L., Potier P. Highly efficient, practical approach to natural taxol. J. Am. Chem. Soc. 1988;110:5917–5919. [Google Scholar]
- 47.Fett-Neto A.G., DiCosmo F., Reynolds W.F., Sakata K. Cell culture of Taxus as a source of the antineoplastic drug taxol and related taxanes. Bio/Technology. 1992;10:1572. doi: 10.1038/nbt1292-1572. [DOI] [PubMed] [Google Scholar]
- 48.Fett-Neto A.G., Melanson S.J., Sakata K., DiCosmo F. Improved growth and taxol yield in developing calli of Taxus cuspidata by medium composition modification. Bio/Technology. 1993;11:731. doi: 10.1038/nbt0693-731. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee S., Upadhyay N., Kukreja A.K., Ahuja P.S., Kumar S., Saha G.C., Sharma R.P., Chattopadhyay S.K. Taxanes from in vitro cultures of the Himalayan yew Taxus wallichiana. Planta Med. 1996;62:329–331. doi: 10.1055/s-2006-957895. [DOI] [PubMed] [Google Scholar]
- 50.Yukimune Y., Hara Y., Nomura E., Seto H., Yoshida S. The configuration of methyl jasmonate affects paclitaxel and baccatin III production in Taxus cells. Phytochemistry. 2000;54:13–17. doi: 10.1016/S0031-9422(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 51.Syklowska-Baranek K., Pietrosiuk A., Kokoszka A., Furmanowa M. Enhancement of taxane production in hairy root culture of Taxus x media var. Hicksii. J. Plant Physiol. 2009;166:1950–1954. doi: 10.1016/j.jplph.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z.-Y., Zhong J.-J. Combination of conditioned medium and elicitation enhances taxoid production in bioreactor cultures of Taxus chinensis cells. Biochem. Eng. J. 2002;12:93–97. doi: 10.1016/S1369-703X(02)00044-X. [DOI] [Google Scholar]
- 53.Bringi V., Kadkade P.G., Prince C.L., Schubmehl B.F., Kane E.J., Roach B. 1995. Enhanced Production of Taxol and Taxanes by Cell Cultures of Taxus species. [Google Scholar]
- 54.Bentebibel S., Moyano E., Palazón J., Cusidó R.M., Bonfill M., Eibl R., Pinol M.T. Effects of immobilization by entrapment in alginate and scale‐up on paclitaxel and baccatin III production in cell suspension cultures of Taxus baccata. Biotechnol. Bioeng. 2005;89:647–655. doi: 10.1002/bit.20321. [DOI] [PubMed] [Google Scholar]
- 55.Frense D. Taxanes: perspectives for biotechnological production. Appl. Microbiol. Biotechnol. 2007;73:1233–1240. doi: 10.1007/s00253-006-0711-0. [DOI] [PubMed] [Google Scholar]
- 56.Hao X., Pan J., Zhu X. Taxol producing fungi. Nat. Prod. Phytochem. Bot. Metab. Alkaloids, Phenolics Terpenes. 2013:2797–2812. [Google Scholar]
- 57.Strobel G., Yang X., Sears J., Kramer R., Sidhu R.S., Hess W.M. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology. 1996;142:435–440. doi: 10.1099/13500872-142-2-435. [DOI] [PubMed] [Google Scholar]
- 58.Strobel G.A., Hess W.M., Li J.-Y., Ford E., Sears J., Sidhu R.S., Summerell B. Pestalotiopsis guepinii, a taxol-producing endophyte of the Wollemi pine, Wollemia nobilis. Aust. J. Bot. 1997;45:1073–1082. [Google Scholar]
- 59.Li J.Y., Sidhu R.S., Ford E.J., Long D.M., Hess W.M., Strobel G.A. The induction of taxol production in the endophytic fungus—Periconia sp from Torreya grandifolia. J. Ind. Microbiol. Biotechnol. 1998;20:259–264. [Google Scholar]
- 60.Strobel G.A., Ford E., Li J.Y., Sears J., Sidhu R.S., Hess W.M. Seimatoantlerium tepuiense gen. nov., a unique epiphytic fungus producing taxol from the Venezuelan Guyana. Syst. Appl. Microbiol. 1999;22:426–433. doi: 10.1016/S0723-2020(99)80052-6. [DOI] [PubMed] [Google Scholar]
- 61.Shrestha K., Strobel G.A., Shrivastava S.P., Gewali M.B. Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med. 2001;67:374–376. doi: 10.1055/s-2001-14307. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y.J., Zhang Z., Wang Y., Su Y., Zhang R. Screening endophytic fungus to produce taxol from Taxus yunnanensis. Biotechnology. 2003;13:10–11. [Google Scholar]
- 63.Chen J.H., Liu J.J., Zang G.G., Li Y.J., Zhao L.N. Screening of taxol-producing endophytic fungi and regulation of fermentation conditions. J Cent. South Univ. 2004;35:65–69. [Google Scholar]
- 64.Zhou D., Zhao K., Ping W., Ge J., Ma X., Jun L. Study on the mutagensis of protoplasts from taxol-producing fungus Nodulisporium sylviforme. J. Am. Sci. 2005;1:62. [Google Scholar]
- 65.Hu K., Tan F., Tang K., Zhu S., Wang W. Isolation and screening of endophytic fungi synthesizing taxol from Taxus chinensis var. mairei. J. Southwest China Norm. Univ. (Nat. Sci. Ed. 2006;31:134–137. [Google Scholar]
- 66.Yu Y., Hu C. Separation and identification of a new Taxus chinensis var. mairei endophytic fungus (Bionectria sp.) and the activity of its metabolites. J. Southwest Univ. (Nat. Sci. Ed.) 2007;29:131–135. [Google Scholar]
- 67.Liu W.H., Yao B., Zhu S.Q., LIAO Z. Advances in studies on biosynthetic pathway of taxol precursor and its correlative biotechnology. Chin. Tradit. Herb. Drugs. 2009;40:1327–1331. [Google Scholar]
- 68.Zhao K., Sun L., Wang X., Li X., Zhou D. Screening of high taxol producing fungi by mutagenesis and construction of subtracted cDNA library by suppression subtracted hybridization for differentially expressed genes. Wei Sheng Wu Xue Bao= Acta Microbiol. Sin. 2011;51:923–933. [PubMed] [Google Scholar]
- 69.Kumaran R.S., Choi Y.-K., Lee S., Jeon H.J., Jung H., Kim H.J. Isolation of taxol, an anticancer drug produced by the endophytic fungus, Phoma betae. Afr. J. Biotechnol. 2012;11:950–960. [Google Scholar]
- 70.Yang Y., Zhao H., Barrero R.A., Zhang B., Sun G., Wilson I.W., Xie F., Walker K.D., Parks J.W., Bruce R. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genom. 2014;15:69. doi: 10.1186/1471-2164-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raj K.G., Manikandan R., Arulvasu C., Pandi M. Anti-proliferative effect of fungal taxol extracted from Cladosporium oxysporum against human pathogenic bacteria and human colon cancer cell line HCT 15. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;138:667–674. doi: 10.1016/j.saa.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 72.Zaiyou J., Li M., Xiqiao H. An endophytic fungus efficiently producing paclitaxel isolated from Taxus wallichiana var. mairei. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiao W., Ling F., Yu L., Huang Y., Wang T. Enhancing taxol production in a novel endophytic fungus, Aspergillus aculeatinus Tax-6, isolated from Taxus chinensis var. mairei. Fungal Biol. 2017;121:1037–1044. doi: 10.1016/j.funbio.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Staniek A., Woerdenbag H.J., Kayser O. Taxomyces andreanae: a presumed paclitaxel producer demystified? Planta Med. 2009;75:1561–1566. doi: 10.1055/s-0029-1186181. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S., Yeo Y., Greenhagen B.T., McMullin T., Song L., Maurina‐Brunker J., Rosson R., Noel J.P., Chappell J. Metabolic engineering of sesquiterpene metabolism in yeast. Biotechnol. Bioeng. 2007;97:170–181. doi: 10.1002/bit.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engels B., Dahm P., Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 2008;10:201–206. doi: 10.1016/j.ymben.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Ajikumar P.K., Xiao W.-H., Tyo K.E.J., Wang Y., Simeon F., Leonard E., Mucha O., Phon T.H., Pfeifer B., Stephanopoulos G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science (80-.) 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strobel G.A., Stierle A., van Kuijk F.J.G.M. Factors influencing the in vitro production of radiolabeled taxol by Pacific yew, Taxus brevifolia. Plant Sci. 1992;84:65–74. [Google Scholar]
- 79.Fleming P.E., Mocek U., Floss H.G. Biosynthesis of taxoids. Mode of formation of the taxol side chain. J. Am. Chem. Soc. 1993;115:805–807. [Google Scholar]
- 80.Sreekanth D., Syed A., Sarkar S., Sarkar D., Santhakumari B., Ahmad A., Khan M.I. Production, purification, and characterization of taxol and 10-DABIII from a new endophytic fungus Gliocladium sp. isolated from the Indian yew tree, Taxus baccata. J. Microbiol. Biotechnol. 2009;19:1342–1347. doi: 10.4014/jmb.0904.04041. [DOI] [PubMed] [Google Scholar]
- 81.Zhou L., Zhao J., Shan T., Cai X., Peng Y. Spirobisnaphthalenes from Fungi and their biological activities. Mini-Rev. Med. Chem. 2010;10:977–989. doi: 10.2174/138955710792007178. [DOI] [PubMed] [Google Scholar]
- 82.Li C.T., Li Y., Wang Q.J., Sung C.K. Taxol production by Fusarium arthrosporioides isolated from yew, Taxus cuspidata. J. Med. Biochem. 2008;27:454–458. doi: 10.2478/v10011-008-0022-3. [DOI] [Google Scholar]
- 83.Chakravarthi B.V.S.K., Das P., Surendranath K., Karande A.A., Jayabaskaran C. Production of paclitaxel by Fusarium solani isolated from Taxus celebica. J. Biosci. 2008;33:259–267. doi: 10.1007/s12038-008-0043-6. [DOI] [PubMed] [Google Scholar]
- 84.Kathiravan G., Sri Raman V. In vitro TAXOL production, by Pestalotiopsis breviseta—a first report. Fitoterapia. 2010;81:557–564. doi: 10.1016/j.fitote.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 85.Bi J., Ji Y., Pan J., Yu Y., Chen H., Zhu X. A new taxol-producing fungus (Pestalotiopsis malicola) and evidence for taxol as a transient product in the culture. Afr. J. Biotechnol. 2011;10:6647–6654. [Google Scholar]
- 86.Vennila R., Johnpaul M. Taxol from Pestalotiopsis pauciseta VM1, an endophytic fungus of Tabebuia pentaphylla. Biomed. Prev. Nutr. 2011;1 doi: 10.1016/j.bionut.2010.12.005. [DOI] [Google Scholar]
- 87.Gangadevi V., Johnpaul M. Isolation of Colletotrichum gloeosporioides, a novel endophytic taxol-producing fungus from the leaves of a medicinal plant. Justicia gendarussa. 2008;5 [Google Scholar]
- 88.Metz A., Haddad A., Worapong J., Long D.M., Ford E.J., Hess W.M., Strobel G.A. Induction of the sexual stage of Pestalotiopsis microspora, a taxol-producing fungus. Microbiology. 2000;146(Pt 8):2079–2089. doi: 10.1099/00221287-146-8-2079. [DOI] [PubMed] [Google Scholar]
- 89.Gangadevi V., Murugan M., Johnpaul M. Taxol Determination from Pestalotiopsis pauciseta, a Fungal Endophyte of a Medicinal Plant. Sheng Wu Gong Cheng Xue Bao. 2008;24:1433–1438. doi: 10.1016/S1872-2075(08)60065-5. [DOI] [PubMed] [Google Scholar]
- 90.Kumaran R.S., Muthumary J., Hur B.-K. Isolation and identification of an anticancer drug, taxol from Phyllosticta tabernaemontanae, a leaf spot fungus of an angiosperm, Wrightia tinctoria. J. Microbiol. 2009;47:40. doi: 10.1007/s12275-008-0127-x. [DOI] [PubMed] [Google Scholar]
- 91.Gangadevi V., Muthumary J. A Novel Endophytic Taxol-Producing Fungus Chaetomella raphigera Isolated From a Medicinal Plant, Terminalia arjuna. Appl. Biochem. Biotechnol. 2009;158:675–684. doi: 10.1007/s12010-009-8532-0. [DOI] [PubMed] [Google Scholar]
- 92.Zhang P., Zhou P.P., Yu L.J. An endophytic taxol-producing fungus from Taxus x media, Aspergillus candidus MD3. FEMS Microbiol. Lett. 2009;293:155–159. doi: 10.1111/j.1574-6968.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 93.Li J.Y., Strobel G., Sidhu R., Hess W.M., Ford E.J. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiology. 1996;142(Pt 8):2223–2226. doi: 10.1099/13500872-142-8-2223. [DOI] [PubMed] [Google Scholar]
- 94.Srinivasan J.M.K. Taxol production from Pestalotiopsis sp an endophytic fungus Isolated from Catharanthus roseus. J. Ecobiotechnology. 2009;1 https://updatepublishing.com/journal/index.php/jebt/article/view/7 [Google Scholar]
- 95.Gond S.K., Kharwar R.N., White J.F. Will fungi be the new source of the blockbuster drug taxol? Fungal Biol. Rev. 2014;28:77–84. doi: 10.1016/j.fbr.2014.10.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.