Abstract
Reactive oxygen formation plays a mechanistic role in the cardiotoxicity of doxorubicin, a chemotherapeutic agent that remains an important component of treatment programs for breast cancer and hematopoietic malignancies. To examine the role of doxorubicin-induced reactive oxygen species (ROS) in drug-related cardiac apoptosis, murine embryonic fibroblast cell lines were derived from the hearts of glutathione peroxidase 1 (Gpx-1) knockout mice. Cells from homozygous Gpx-1 knockout mice and parental animals were propagated with (Se+) and without (Se-) 100 nM sodium selenite. Activity levels of the peroxide detoxifying selenoprotein glutathione peroxidase (GSHPx) were marginally detectable (<1.6 nmol/min/mg) in fibroblasts from homozygous knockout animals whether or not cells were supplemented with selenium. GSHPx activity in Se- cells from parental murine fibroblasts was also <1.6 nmol/min/mg, whereas GSHPx levels in Se+ parental murine fibroblasts were 12.9 ± 2.7 nmol/min/mg (mean ± SE; P < 0.05). Catalase, superoxide dismutase, glutathione reductase, glutathione S-transferase, glucose 6-phosphate dehydrogenase, and reduced glutathione activities did not differ amongst the four cell lines. Reactive oxygen production increased from 908 ± 122 (arbitrary units) for untreated control cells to 1668 ± 54 following exposure to 1 μM doxorubicin for 24 h in parental fibroblasts not supplemented with selenium (P < 0.03); reactive oxygen formation in doxorubicin-treated parental fibroblasts propagated in selenium was 996 ± 69 (P = not significant compared to untreated control cells). Reactive oxygen levels in homozygous Gpx-1 knockout fibroblasts, irrespective of selenium supplementation status, were increased and equivalent to that in selenium deficient wild type fibroblasts. When cardiac fibroblasts were exposed to doxorubicin (0.05 μM) for 96 h and examined for cell cycle alterations by flow cytometry, and apoptosis by TUNEL assay, marked G2 arrest and TUNEL positivity were observed in knockout fibroblasts in the presence or absence of supplemental selenium, and in parental fibroblasts propagated without selenium. Parental fibroblasts propagated with selenium and exposed to the same concentration of doxorubicin demonstrated modest TUNEL positivity and substantially diminished amounts of low molecular weight DNA. These results were replicated in cardiac fibroblasts exposed to doxorubicin (1–2 μM) for 2 h (to mimic clinical drug dosing schedules) and examined 96 h following initiation of drug exposure. Doxorubicin uptake in cardiac fibroblasts was similar irrespective of the mRNA expression level or activity of GSHPx. These experiments suggest that the intracellular levels of doxorubicin-induced reactive oxygen species (ROS) are modulated by GSHPx and play an important role in doxorubicin-related apoptosis and altered cell cycle progression in murine cardiac fibroblasts.
Keywords: doxorubicin, anthracycline, glutathione peroxidase, reactive oxygen species, apoptosis, heart
Abbreviations: glutathione peroxidase 1 activity, GSHPx-1; reactive oxygen species, ROS; GSH, reduced glutathione; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling
1. Introduction
The anthracycline antibiotic doxorubicin remains a critical component of curative therapy for both breast cancer and hematopoietic malignancies [1]. Unfortunately, a unique form of cardiac toxicity that manifests itself as a congestive cardiomyopathy capable of producing potentially fatal heart failure is associated with the cumulative drug dose administered [2]. A variety of hypotheses have been suggested to explain the damaging effects of doxorubicin on the heart. These include alterations in mitochondrial gene expression [3] and function [4,5], as well as mitochondrial DNA double strand break formation due to topoisomerase IIβ [6,7]. The effectiveness of the iron chelating agent dexrazoxane in preventing clinical cardiac toxicity by limiting the generation of strong oxidant species with the chemical characteristics of the hydroxyl radical in vivo, on the other hand, supports the concept that doxorubicin-related production of cardiac ROS plays an important role in the etiology of this critical drug-related toxicity [8,9].
The mechanism of doxorubicin-related ROS production in the heart involves futile redox cycling of the anthracycline's quinone moiety catalyzed by flavin dehydrogenases and other proteins in essentially every subcellular compartment of cardiac myocytes, including complex I of the mitochondrial electron transport chain, cardiac cytosol, cardiac myoglobin, the sarcoplasmic reticulum, and NADPH oxidase 2 found in cardiac membranes [1,10]. Drug-induced ROS are then capable of interfering with cardiac mitochondrial energy production, the function of the ryanodine-sensitive calcium channels necessary for normal contractility, as well as signal transduction in the heart [2,[11], [12], [13]].
At a morphological level, doxorubicin exposure produces mitochondrial swelling, loss of sarcoplasmic reticular membranes, and the hallmarks of cardiac apoptosis in vivo [14] and in vitro [1,15]. Furthermore, it has been suggested that, as is the case for several other cell types [[16], [17], [18]], doxorubicin-induced apoptosis in the heart is related directly to its ability to generate ROS, in particular H2O2 [10,19].
Alterations in the level of one of the major hydrogen peroxide detoxifying enzymes in mammalian cells, cytoplasmic glutathione peroxidase (GSHPx-1), can modulate peroxide-mediated apoptosis [20,21]; overexpression of Gpx-1 in bovine aortic endothelial cells as well as T47D human breast cancer cells significantly decreases doxorubicin-induced apoptosis [15,20]. It has also been demonstrated that doxorubicin-induced ROS significantly diminish intracellular stores of reduced glutathione (GSH) in mammalian cells [22], supporting the hypothesis that the GSH-GSHPx cycle, which plays a critical role in removing intracellular hydrogen peroxide, is essential for the maintenance of a stable, non-toxic level of peroxidative tone both in vitro and in vivo following doxorubicin exposure [23].
Although a significant body of literature has examined the effects of doxorubicin on cardiac myocytes, substantially less is known about the pharmacological effects of the anthracycline antibiotics on other critical cell types that constitute a major fraction of the murine heart [[24], [25], [26]]. For that reason, we examined the role of GSHPx-1 in protecting cardiac embryonic fibroblasts (that constitute approximately 15% of the mass of the heart in the mouse [24]) from the adverse effects of doxorubicin using cardiac fibroblast cell lines produced from parental and Gpx-1 knockout mice. Using these new cell lines, we found that GSHPx-1 plays an essential role in controlling the extent of doxorubicin-induced ROS production, drug-related apoptosis, and anthracycline-related inhibition of cell cycle progression at the G2/M interface.
2. Materials and methods
2.1. Cell culture and establishment of murine embryonic cardiac fibroblast lines
Fibroblast cell lines were derived from the hearts of parental female C57BL/6 mice (+/+) and from female Gpx-1 knockout mice (−/−). The generation of the knockout animals has been described previously [27]. To produce these cell lines, hearts were removed from (+/+) as well as (−/−) fetuses at day 17 of gestation, minced in iced phosphate-buffered saline (PBS), and then centrifuged at 300×g for 10 min. After removal of the supernatant, collagenase (1 mg/ml) from Boehringer-Mannheim, Corp. (Indianapolis, IN) was added to the cardiac mince which was incubated in a water bath with mild shaking for 1 h at 37 °C. The collagenase-treated cardiac tissue was then washed once with ice-cold PBS and then resuspended in DMEM-F12 medium (Gibco/BRL) containing 10% fetal calf serum with penicillin, streptomycin, and fungizone. Cardiac fibroblasts were then propagated in 15 ml tissue culture dishes at 37 °C in a humidified atmosphere of 5% CO2 in air. Floating cellular debris was aspirated after the initial three days of culture and replaced with fresh media and serum. This procedure was repeated every three days until the cells attached to plastic were of such density that they could be harvested with trypsin/EDTA and re-plated in fresh media with serum. The doubling time for both the knockout and wild-type fibroblasts was ≈14 h.
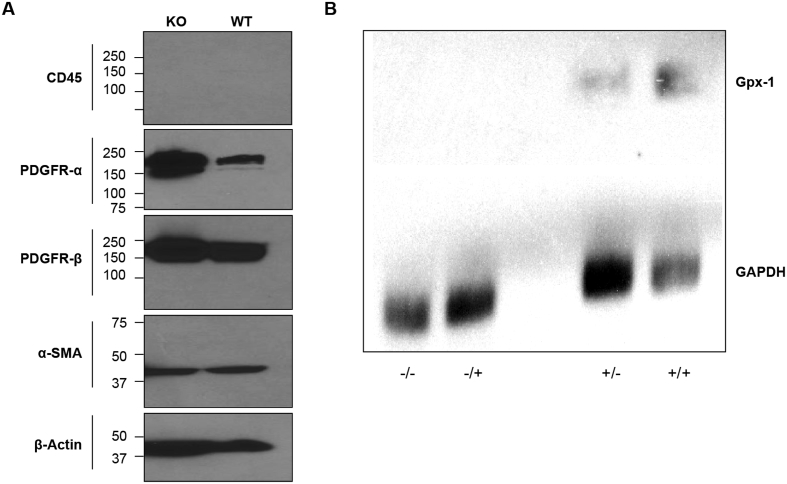
To confirm that the cells used for study were, in fact, murine cardiac fibroblasts, cells were harvested by scraping during their logarithmic growth phase and lysed with RIPA lysis buffer (Millipore cat # 20–188) containing protease and phosphatase inhibitors. Protein lysate (40 μg) was loaded on an Invitrogen 4–20% Tris-glycine gel and transferred to Nylon membrane using an Invitrogen iBlot2 device. The primary antibody was used at 1 to 1000 dilution in 2.5% milk-TBST at 4 °C overnight after 1 h blocking in 5% milk-TBST buffer. The following day, the membrane was washed in TBST buffer for 5 min x 3, and then incubated with a 1 to 3000 dilution of the secondary antibody in 5% milk-TBST at room temperature for 1 h. The membrane was developed after washing three times in TBST with an exposure for 5 min in a 1:1 mixture of exposure solutions (SuperSignal West Pico stable peroxide solution, Thermo Fisher cat #1863097; and SuperSignal West Pico luminol/enhancer solution, Thermo Fisher cat #1863096). The primary antibodies obtained from Cell Signaling were: α-smooth muscle actin (α-SMA) cat #19245s; platelet derived growth factor receptor α (PDGFR-α) cat #3174s; platelet derived growth factor receptor β (PDGFR-β) cat #4564s; CD45 cat #72787s; and β-actin cat # 3700s. Goat anti-rabbit and anti-mouse IgG antibodies were purchased from Santa Cruz. As shown in Fig. 1A, the heart cells from both the wild-type (WT) and Gpx-1 knock out (KO) mice expressed markers of developmental cardiac fibroblasts (PDGFR-α, PDGFR-β, and α-SMA) and not CD45, a marker of cardiac endothelial and mesenchymal cells [24,28].
Fig. 1.
(A) Western blot analysis of the protein expression of the fibroblast markers PDGFR-α, PDGFR-β, and α-SMA, and the mesenchymal marker CD45 in heart cells obtained from parental wild-type (WT) and Gpx-1 knockout (KO) animals. (B) Northern analysis of Gpx-1 expression in cardiac fibroblasts from parental (+) and Gpx-1 knockout (−) C57/Bl6 mice. Cells were passaged without (−/−; +/−) or with (−/+; +/+) supplemental sodium selenite (100 nM). The presence of exogenous selenium increased Gpx-1 mRNA expression by ≈ 3-fold.
Once cardiac fibroblasts with typical morphology had been established in culture, 100 nM sodium selenite was added to both (−/−) and (+/+) lines. Thus, for these studies, four distinct fibroblast cell lines were examined: Gpx-1 (−/−) with or without exogenous selenium; and Gpx-1 (+/+) with or without sodium selenite. For the experiments outlined here, all cells had been passaged no more than four times before use.
2.2. Antioxidant enzyme assays
The specific activities of glutathione peroxidase, catalase, glutathione reductase, glutathione S-transferase, glucose-6-phosphate dehydrogenase, superoxide dismutase, and the level of reduced glutathione in the cardiac fibroblasts were measured in cytosolic preparations of the cells as previously described [29].
2.3. Northern analysis
Analysis of Gpx-1 expression in cardiac fibroblasts was performed using a human Gpx-1 probe as described previously [30].
2.4. Reactive oxygen production and doxorubicin uptake
Exponentially growing fibroblasts either untreated or exposed to doxorubicin (clinical grade) were harvested with trypsin/EDTA and resuspended in iced PBS. After counting with a hemocytometer, the cell suspension was centrifuged for 10 min at 500×g; the cell pellet was resuspended in medium #199 (Gibco/BRL) with the volume adjusted to 106 cells/ml and kept on ice until the flow cytometric analysis was performed. For analysis of reactive oxygen production, 10 μl of a 1 mM solution of DCFH-DA (Molecular Probes #C-6827) in 100% ethanol was added to a 1 ml fibroblast suspension; the cells were then vortexed at room temperature, and fluorescence intensity measured 5 min later using a MoFlo flow cytometer (Cytomation, Fort Collins, CO) using an excitation wavelength of 504 nm and emission of 529 nm. For estimation of doxorubicin content, an identical procedure was used except that no DCFH-DA was added. For estimation of doxorubicin-induced reactive oxygen production, cells were treated for 24 h with the drug (1–2 μM) prior to the addition of DCFH.DA. Untreated control cells were always processed at the same time as doxorubicin-exposed fibroblasts to determine relative fluorescence intensity.
2.5. Doxorubicin-induced apoptosis and effects on cell cycle progression
Apoptosis in Gpx-1 knockout and parental cell lines in the presence or absence of exogenous selenium was determined by TUNEL assay [31]. Briefly, fibroblasts in log-phase growth were treated with doxorubicin either for 2 h (1 μM) or continuously for 96 h (0.05 μM) in their tissue culture flasks. Cells were harvested 96 h after initiation of drug treatment, washed in PBS, resuspended, and fixed with 4% paraformaldehyde in PBS, pH 7.2, for 30 min on ice. After re-centrifugation, cells were resuspended in ice-cold PBS, centrifuged again, and the pellet resuspended in 70% ethanol. After centrifugation, the cells were washed in 1 ml of 0.1% BSA-HBSS, centrifuged, and resuspended using an apoptosis reagent (TdT) kit from Boehringer. The cells were subsequently prepared for flow cytometry exactly as described by the manufacturer. Cells were stained and analyzed with avidin-FITC to examine strand break frequency and with propidium iodide to determine cell cycle progression. Three dimensional plots are displayed showing both parameters. Examples are shown for experiments performed at least in triplicate.
2.6. Statistics
Data have been presented as the mean ± SE for experiments performed in triplicate; means were compared with Student's t-test.
3. Results
3.1. Effect of selenium on glutathione peroxidase expression and antioxidant enzyme levels in cardiac fibroblasts from parental and Gpx-1 knockout mice
As demonstrated in Fig. 1B, cardiac fibroblasts from knockout mice passaged in the presence or absence of 100 nM sodium selenite failed to express Gpx-1 mRNA. Furthermore, cardiac fibroblasts adapted to growth in the absence of selenium supplementation of the media exhibited diminished Gpx-1 mRNA, consistent with previous investigations in human breast cancer cells [30]. In concert with these observations, we found minimal levels of glutathione peroxidase (GSHPx) enzymatic activity in the knockout cells independent of selenium supplementation and in parental cardiac fibroblasts not propagated in supplemental sodium selenite (Table 1). On the other hand, GSHPx activity was substantive in the parental cell line in the presence of selenium. As shown in Table 1, major differences in the enzymatic activity of GSHPx in these four cell lines were not accompanied by significant changes in other antioxidant defenses. In subsequent experiments, these results permitted specific interrogation of the role of GSHPx in protecting cardiac cells from the deleterious effects of doxorubicin-induced oxidative stress.
Table 1.
Effect of glutathione peroxidase expression and selenium on antioxidant levels in cardiac fibroblasts from Gpx-1 (+/+) parental and Gpx-1 (−/−) knockout C57/Bl6 mice.
| Enzyme activity | Gpx-1 (−/−)(-Se)a | Gpx-1 (−/−)(+Se) | Gpx-1 (+/+)(-Se) | Gpx-1 (+/+)(+Se) |
|---|---|---|---|---|
| GSH peroxidase(nmol/min/mg) | 1.61 ± 0.76b | 1.13 ± 0.38 | 1.47 ± 0.21 | 12.9 ± 2.7c |
| GSH reductase(nmol/min/mg) | 7.9 ± 1.4 | 9.1 ± 0.4 | 12.4 ± 1.8 | 11.0 ± 2.0 |
| Catalase(μmol/min/mg) | 1000 ± 410 | 860 ± 200 | 2080 ± 330 | 1870 ± 780 |
| Superoxide dismutase(μg SOD/mg) | 2.33 ± 0.30 | 2.80 ± 0.02 | 2.65 ± 0.10 | 3.02 ± 0.14 |
| G-6-PD(nmol/min/mg) | 30.2 ± 4.3 | 26.7 ± 5.4 | 14.5 ± 4.7 | 12.5 ± 1.7 |
| Glutathione(nmol/106 cells) | 5.85 ± 0.12 | 6.09 ± 0.24 | 7.74 ± 1.03 | 5.84 ± 0.10 |
Cardiac fibroblasts were passaged with (+Se) or without (-Se) 100 nM sodium selenite added to the tissue culture medium.
Mean ± SE; GSHPx activity in the range of 1–2 nmol/mg/min is at the lower limit of detection for this assay.
P < 0.05 versus cells passaged in the absence of sodium selenite or versus Gpx-1 knockout cells.
3.2. GSHPx activity restrains the level of doxorubicin-enhanced reactive oxygen metabolism in cardiac fibroblasts
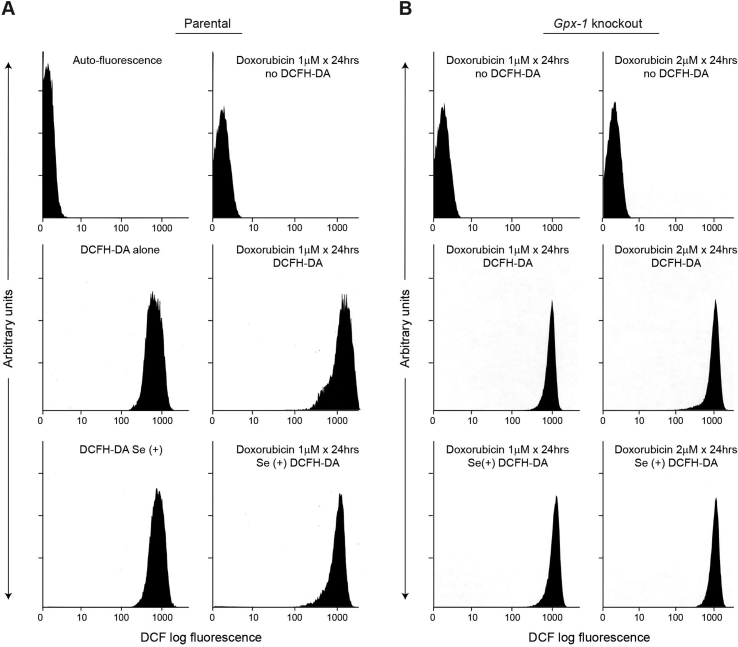
Baseline levels of reactive oxygen species (ROS) in cardiac fibroblasts from parental C57/Bl6 mice, detected as DCF fluorescence, were not significantly altered by passage of these cells in supplemental selenium (Fig. 2A, left middle and bottom panels; 908 ± 122 arbitrary units for Se(+) control cells). However, the significant increase in DCF log fluorescence produced by doxorubicin exposure for 24 h (1668 ± 54 arbitrary units; P < 0.03 versus untreated controls) was decreased to baseline values found in the absence of drug (996 ± 69 arbitrary units) for fibroblasts expressing Gpx-1 that had been propagated in sodium selenite (Fig. 2A, compare right middle and bottom panels). On the other hand, consistent with the absence of measurable GSHPx activity in Gpx-1 knockout heart cells, doxorubicin increased ROS production above baseline levels for two different drug concentrations irrespective of selenium supplementation status (Fig. 2B).
Fig. 2.
Effect of selenium on doxorubicin-enhanced reactive oxygen production. (A) Parental cardiac fibroblasts with or without selenium supplementation. Only the lower two panels represent experiments in which selenium has been added to the cell cultures. High glutathione peroxidase activity levels in selenium supplemented parental fibroblasts almost completely block doxorubicin-enhanced reactive oxygen formation (right middle versus right lower panel). (B) Gpx-1 knockout cardiac fibroblasts demonstrate no effect of selenium supplementation on doxorubicin-enhanced reactive oxygen formation for two different drug concentrations (middle versus lower panels). As is the case in (A), the top and middle panels represent experiments performed for fibroblasts without added selenium (Se-cells). These data are representative of at least three separate experiments.
3.3. GSHPx modulates doxorubicin-induced apoptosis and alterations in murine cardiac fibroblast cell cycle progression
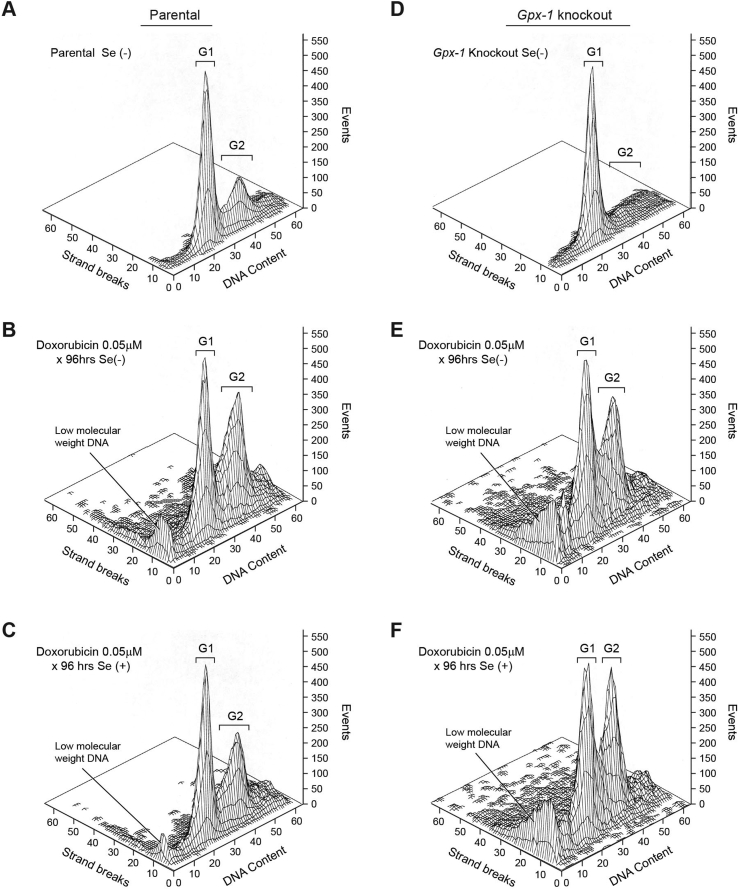
We next evaluated the role of cardiac GSHPx in the control of doxorubicin-induced apoptosis using the TUNEL assay. Because doxorubicin can be administered as both a continuous infusion as well an intravenous bolus [1], we evaluated the effect of doxorubicin on heart fibroblast apoptosis following a 96-h period of drug exposure, as well as 96 h following initiation of a 2-h drug treatment. In the absence of doxorubicin exposure or selenium supplementation, no baseline level of either apoptosis or alteration in cell cycle progression was demonstrable in either parental or Gpx-1 knockout fibroblasts (Fig. 3A and D). On the other hand, a considerable degree of DNA strand scission and accumulation of low molecular weight DNA species was observed at the conclusion of doxorubicin exposure for both parental and knockout cardiac fibroblasts passaged in the absence of selenium (Fig. 3B and E). Apoptotic cells were demonstrable across the cell cycle, including cells stalled in G2/M phase, a hallmark of the cell cycle effect of doxorubicin treatment [32]. In the presence of measurable levels of GSHPx activity, the extent of low molecular weight DNA and DNA strand scission across the cell cycle was markedly decreased, as was the degree of the G2/M block (Fig. 3C). However, as expected, addition of selenium to Gpx-1 knockout fibroblasts during passage neither prevented doxorubicin-induced apoptosis nor altered the blockade of cell cycle progression at the G2/M interface (Fig. 3F).
Fig. 3.
Effect of glutathione peroxidase and selenium on apoptosis and cell cycle arrest produced by a 96-h continuous exposure to doxorubicin in parental and Gpx-1 knockout cardiac fibroblasts. Apoptosis was evaluated by TUNEL assay; cell cycle progression of cells initially in log-phase growth was determined by flow cytometry. TUNEL positive cells were assessed across the cell cycle and are shown as strand breaks measured on the y-axis quantitated as events on the z axis. In these studies, continuous exposure to nanomolar concentrations of doxorubicin leads to accumulation of cells at the G2/M interface. These data are representative of at least three separate experiments.
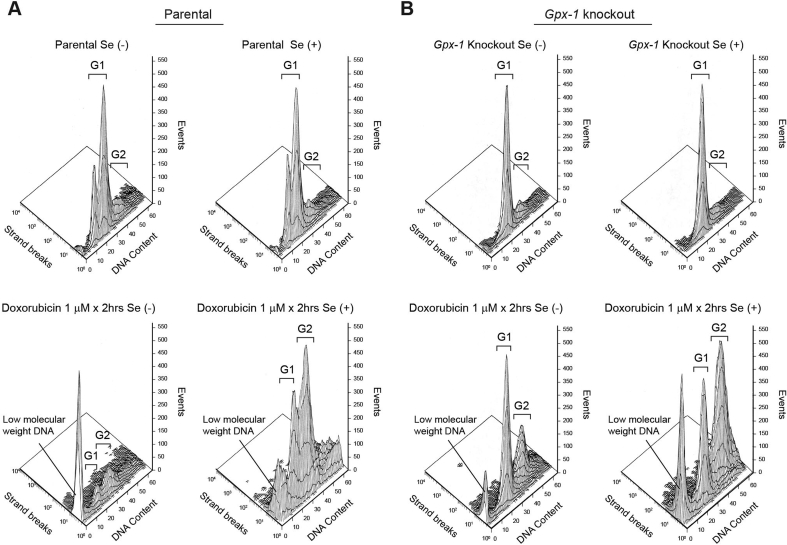
When cardiac fibroblasts were exposed to a higher concentration of doxorubicin (1 μM) for 2 h followed by assessment of apoptosis 96 h after the initiation of treatment (Fig. 4), an extensive degree of apoptosis, reflected by a remarkable amount of low molecular weight DNA and TUNEL positivity, was observed (Fig. 4A, lower left panel). Much lower levels of apoptosis and partial retention of baseline cell cycle progression were observed in parental cardiac fibroblasts passaged in selenium-supplemented media (Fig. 4A, lower right panel). We also found that addition of 100 nM sodium selenite to parental cardiac fibroblasts only during the 2 h of doxorubicin exposure had no effect on the degree of apoptosis that we observed (data not shown). For Gpx-1 knockout fibroblasts, however, passage in selenium-supplemented medium did not prevent extensive drug-induced apoptosis and arrest of cells in G2/M phase (Fig. 4B, lower panels).
Fig. 4.
Effect of glutathione peroxidase and selenium on apoptosis and cell cycle arrest produced by a 2-h exposure to doxorubicin in parental and Gpx-1 knockout cardiac fibroblasts examined 96 h following the initiation of drug treatment. Apoptosis was evaluated by TUNEL assay; cell cycle progression of cells initially in log-phase growth was evaluated by flow cytometry. TUNEL positive cells were assessed across the cell cycle and are shown as strand breaks measured on the y-axis quantitated as events on the z axis. These data are representative of at least three separate experiments.
3.4. GSHPx activity does not alter the uptake of doxorubicin by cardiac fibroblasts
We next evaluated doxorubicin accumulation by analytical cytometry in cardiac fibroblasts with different levels of GSHPx activity (Table 2). Under conditions identical to those used to examine doxorubicin-related ROS formation (Fig. 2), we found no difference in the uptake of doxorubicin in parental or Gpx-1 knockout cardiac fibroblasts whether or not the cells had been passaged in supplemental selenium.
Table 2.
Effect of glutathione peroxidase expression and selenium on doxorubicin accumulation in cardiac fibroblasts from Gpx-1 (+/+) parental and Gpx-1 (−/−) knockout C57/Bl6 mice.
| Treatment | Gpx-1 (−/−)(-Se)a | Gpx-1 (−/−)(+Se) | Gpx-1 (+/+)(-Se) | Gpx-1 (+/+)(+Se) |
|---|---|---|---|---|
| None | 3.4b | 4.1 | ||
| Doxorubicin (1 μM for 24 h) | 6.8 | 6.8 | 8.7 | 7.5 |
Cardiac fibroblasts were passaged with (+Se) or without (-Se) 100 nM sodium selenite added to the tissue culture medium.
These results represent the mean auto-fluorescence of untreated cardiac fibroblasts or the mean fluorescent intensity of doxorubicin assessed by flow cytometry at an excitation wavelength of 504 nm and an emission wavelength of 529 nm for 106 cells treated either with medium or 1 μM doxorubicin for 24 h at 37 °C. For triplicate experiments, fluorescent intensity varied by ≤ 15%.
4. Discussion
The goal of these experiments was to evaluate whether GSHPx could prevent doxorubicin-induced cardiac apoptosis in the absence of other changes in cellular antioxidant defense systems. Using clinically-relevant doxorubicin concentrations and drug exposures that are known to produce oxidative DNA damage in the circulating mononuclear cells of patients undergoing treatment with a 96 h continuous infusion of the drug [33], we found that murine embryonic fibroblasts derived from the hearts of Gpx-1 knockout animals produced significantly more ROS and were substantially more sensitive to the apoptotic effects of doxorubicin than wild type fibroblasts passaged in supplemental selenium to maximize GSHPx activity. ROS-related apoptosis occurred in the absence of GSHPx-related alterations in doxorubicin uptake or changes in the specific activities of a range of antioxidant enzymes, other than GSHPx.
These studies are consistent with our original observation that doxorubicin is significantly more toxic in vivo to the hearts of mice raised on selenium deficient chow (that decreases cardiac GSHPx activity) [34] and, as demonstrated more recently, significantly more damaging to the cardiac function of Gpx-1 knockout mice treated with the anthracycline antibiotic [35]. Conversely, overexpression of Gpx-1 in the murine heart leads to a significant decrease in doxorubicin-related mitochondrial dysfunction as well as drug-induced alterations in myocardial contractility [36]. In both cases, doxorubicin-induced ROS production is likely to set off an apoptotic cascade in heart cells [19]. In this fashion, as is the case for mammalian endothelial cells [15], as well as a variety of human tumor cell lines [18,37], it is the production of H2O2 by doxorubicin (a process modulated by the GSHPx that is present in both cytosol and mitochondria) that plays a critical role in the initiation of cardiac apoptosis. It has been demonstrated previously, furthermore, that a doxorubicin-induced peroxide flux in mammalian cells precedes the loss of mitochondrial membrane permeability that is an initiating event in anthracycline-related activation of the apoptotic cascade [18]. Although the activation of poly-ADP-ribose polymerase that occurs as a consequence of DNA damage from the anthracyclines could contribute to drug-related apoptosis, it has been suggested that this is a secondary event in the temporal scheme of drug-related apoptotic signaling [18].
In this regard, it is appropriate to point out that our laboratory has recently reported that the GSHPx activity of human breast cancer cells can be modified by the addition of exogenous selenium by over ten-fold [38]. It has been reported previously that the selenium content of serum used for tissue culture can vary widely depending on the selenium content of the soil (and hence the grain) on which the species used to supply the serum was raised; in fact, the selenium content of most serum used for research is deficient, leading to substantial geographic variations in the activity of GSHPx in cell lines across laboratories [39,40].
We also found that the presence of substantive GSHPx activity levels in parental cardiac fibroblasts passaged in supplemental selenium also decreased alterations in both G1 and G2/M cell cycle checkpoints produced by doxorubicin (Figs. 3 and 4). Although the arrest of cells in G2/M by doxorubicin has been well characterized [41], and the effects of H2O2 by itself on multiple cell cycle checkpoints has also been described [42], our results demonstrate that the cell cycle effects of doxorubicin, at least in cardiac fibroblasts, may be modulated by drug-enhanced oxidant formation. These results have relevance for both the anti-proliferative and cardiotoxic mechanisms of action of doxorubicin.
In summary, we have found, using murine Gpx-1 knockout fibroblasts, that GSHPx plays an important role in modulating the cardiac toxicity of doxorubicin-induced reactive oxygen formation, supporting the hypothesis that drug-related ROS play a mechanistic role in this therapeutically important side effect of an essential class of anti-cancer agents.
Declaration of competing interest
None.
Acknowledgement
This project has been funded in whole or in part with federal funds from the Center for Cancer Research and the Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health; the work was also conducted, in part, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Doroshow J.H. Topoisomerase II inhibitors: anthracyclines. In: Chabner B.A., Longo D.L., editors. Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott, Williams, and Wilkins Publishers; Philadelphia: 2011. pp. 356–391. [Google Scholar]
- 2.Doroshow J.H. Dexrazoxane for the prevention of cardiac toxicity and treatment of extravasation injury from the anthracycline antibiotics. Curr. Pharm. Biotechnol. 2012;13:1949–1956. doi: 10.2174/138920112802273245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson K.L., Rosenzweig B.A., Zhang J., Knapton A.D., Honchel R., Lipshultz S.E., Retief J., Sistare F.D., Herman E.H. Early alterations in heart gene expression profiles associated with doxorubicin cardiotoxicity in rats. Cancer Chemother. Pharmacol. 2010;66:303–314. doi: 10.1007/s00280-009-1164-9. [DOI] [PubMed] [Google Scholar]
- 4.Diotte N.M., Xiong Y., Gao J., Chua B.H., Ho Y.S. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim. Biophys. Acta. 2009;1793:427–438. doi: 10.1016/j.bbamcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Sardao V.A., Oliveira P.J., Holy J., Oliveira C.R., Wallace K.B. Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in H9c2 cardiomyoblasts. Cancer Chemother. Pharmacol. 2009;64:811–827. doi: 10.1007/s00280-009-0932-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S., Liu X., Bawa-Khalfe T., Lu L.S., Lyu Y.L., Liu L.F., Yeh E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 7.Vejpongsa P., Yeh E.T. Topoisomerase 2beta: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin. Pharmacol. Ther. 2014;95:45–52. doi: 10.1038/clpt.2013.201. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopalan S., Politi P.M., Sinha B.K. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res. 1988;48:4766–4769. [PubMed] [Google Scholar]
- 9.Hasinoff B.B., Herman E.H. Dexrazoxane: how it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc. Toxicol. 2007;7:140–144. doi: 10.1007/s12012-007-0023-3. [DOI] [PubMed] [Google Scholar]
- 10.Gilleron M., Marechal X., Montaigne D., Franczak J., Neviere R., Lancel S. NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem. Biophys. Res. Commun. 2009;388:727–731. doi: 10.1016/j.bbrc.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Jungsuwadee P., Vore M., Butterfield D.A., St Clair D.K. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol. Interv. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 12.Xiang P., Deng H.Y., Li K., Huang G.Y., Chen Y., Tu L., Ng P.C., Pong N.H., Zhao H., Zhang L., Sung R.Y. Dexrazoxane protects against doxorubicin-induced cardiomyopathy: upregulation of Akt and Erk phosphorylation in a rat model. Cancer Chemother. Pharmacol. 2009;63:343–349. doi: 10.1007/s00280-008-0744-4. [DOI] [PubMed] [Google Scholar]
- 13.Simunek T., Sterba M., Popelova O., Adamcova M., Hrdina R., Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 14.Arola O.J., Saraste A., Pulkki K., Kallajoki M., Parvinen M., Voipio-Pulkki L.M. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- 15.Wang S., Konorev E.A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H2O2- and p53-dependent pathways. J. Biol. Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- 16.Pilco-Ferreto N., Calaf G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016;49:753–762. doi: 10.3892/ijo.2016.3558. [DOI] [PubMed] [Google Scholar]
- 17.White S.J., Kasman L.M., Kelly M.M., Lu P., Spruill L., McDermott P.J., Voelkel-Johnson C. Doxorubicin generates a proapoptotic phenotype by phosphorylation of elongation factor 2. Free Radic. Biol. Med. 2007;43:1313–1321. doi: 10.1016/j.freeradbiomed.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizutani H., Tada-Oikawa S., Hiraku Y., Kojima M., Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–1453. doi: 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Spallarossa P., Garibaldi S., Altieri P., Fabbi P., Manca V., Nasti S., Rossettin P., Ghigliotti G., Ballestrero A., Patrone F., Barsotti A., Brunelli C. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J. Mol. Cell. Cardiol. 2004;37:837–846. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Gouaze V., Mirault M.E., Carpentier S., Salvayre R., Levade T., Andrieu-Abadie N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol. Pharmacol. 2001;60:488–496. [PubMed] [Google Scholar]
- 21.Kayanoki Y., Fujii J., Islam K.N., Suzuki K., Kawata S., Matsuzawa Y., Taniguchi N. The protective role of glutathione peroxidase in apoptosis induced by reactive oxygen species. J. Biochem. (Tokyo) 1996;119:817–822. doi: 10.1093/oxfordjournals.jbchem.a021313. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz C., Caja L., Sancho P., Bertran E., Fabregat I. Inhibition of the EGF receptor blocks autocrine growth and increases the cytotoxic effects of doxorubicin in rat hepatoma cells: role of reactive oxygen species production and glutathione depletion. Biochem. Pharmacol. 2008;75:1935–1945. doi: 10.1016/j.bcp.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Doroshow J.H., Akman S., Chu F.-F., Esworthy S. Role of the glutathione-glutathione peroxidase cycle in the cytotoxicity of the anticancer quinones. Pharma Times. 1990;47:359–370. doi: 10.1016/0163-7258(90)90062-7. [DOI] [PubMed] [Google Scholar]
- 24.Pinto A.R., Ilinykh A., Ivey M.J., Kuwabara J.T., D'Antoni M.L., Debuque R., Chandran A., Wang L., Arora K., Rosenthal N.A., Tallquist M.D. Revisiting cardiac cellular composition. Circ. Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., St C.D., Butterfield A., Vore M. Loss of Mrp1 potentiates doxorubicin-induced cytotoxicity in neonatal mouse cardiomyocytes and cardiac fibroblasts. Toxicol. Sci. 2016;151:44–56. doi: 10.1093/toxsci/kfw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levick S.P., Soto-Pantoja D.R., Bi J., Hundley W.G., Widiapradja A., Manteufel E.J., Bradshaw T.W., Melendez G.C. Doxorubicin-induced myocardial fibrosis involves the neurokinin-1 receptor and direct effects on cardiac fibroblasts. Heart Lung Circ. 2019;28:1598–1605. doi: 10.1016/j.hlc.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho Y.S., Magnenat J.L., Bronson R.T., Cao J., Gargano M., Sugawara M., Funk C.D. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 28.Ivey M.J., Tallquist M.D. Defining the cardiac fibroblast. Circ. J. 2016;80:2269–2276. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwelling L.A., Slovak M.L., Doroshow J.H., Hinds M., Chan D., Parker E., Mayes J., Sie K.L., Meltzer P.S., Trent J.M. HT1080/DR4: a P-glycoprotein-negative human fibrosarcoma cell line exhibiting resistance to topoisomerase II-reactive drugs despite the presence of a drug-sensitive topoisomerase II. J. Natl. Cancer Inst. 1990;82:1553–1561. doi: 10.1093/jnci/82.19.1553. [DOI] [PubMed] [Google Scholar]
- 30.Chu F.-F., Esworthy R.S., Akman S., Doroshow J.H. Modulation of glutathione peroxidase expression by selenium: effect on human MCF-7 breast cancer cell transfectants expressing a cellular glutathione peroxidase cDNA and doxorubicin-resistant MCF-7 cells. Nucl. Acids Res. 1990;18:1531–1539. doi: 10.1093/nar/18.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gajewski E., Gaur S., Akman S.A., Matsumoto L., van Balgooy J.N., Doroshow J.H. Oxidative DNA base damage in MCF-10A breast epithelial cells at clinically achievable concentrations of doxorubicin. Biochem. Pharmacol. 2007;73:1947–1956. doi: 10.1016/j.bcp.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu A., Nishida J., Ueoka Y., Kato K., Hachiya T., Kuriaki Y., Wake N. CyclinG contributes to G2/M arrest of cells in response to DNA damage. Biochem. Biophys. Res. Commun. 1998;242:529–533. doi: 10.1006/bbrc.1997.8004. [DOI] [PubMed] [Google Scholar]
- 33.Doroshow J.H., Synold T.W., Somlo G., Akman S.A., Gajewski E. Oxidative DNA base modifications in peripheral blood mononuclear cells of patients treated with high-dose infusional doxorubicin. Blood. 2001;97:2839–2845. doi: 10.1182/blood.v97.9.2839. [DOI] [PubMed] [Google Scholar]
- 34.Doroshow J.H., Locker G.Y., Myers C.E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J. Clin. Invest. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J., Xiong Y., Ho Y.S., Liu X., Chua C.C., Xu X., Wang H., Hamdy R., Chua B.H. Glutathione peroxidase 1-deficient mice are more susceptible to doxorubicin-induced cardiotoxicity. Biochim. Biophys. Acta. 2008;1783:2020–2029. doi: 10.1016/j.bbamcr.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y., Liu X., Lee C.P., Chua B.H., Ho Y.S. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic. Biol. Med. 2006;41:46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Mizutani H., Hotta S., Nishimoto A., Ikemura K., Miyazawa D., Ikeda Y., Maeda T., Yoshikawa M., Hiraku Y., Kawanishi S. Pirarubicin, an anthracycline anticancer agent, induces apoptosis through generation of hydrogen peroxide. Anticancer Res. 2017;37:6063–6069. doi: 10.21873/anticanres.12054. [DOI] [PubMed] [Google Scholar]
- 38.Doroshow J.H., Juhasz A. Modulation of selenium-dependent glutathione peroxidase activity enhances doxorubicin-induced apoptosis, tumour cell killing and hydroxyl radical production in human NCI/ADR-RES cancer cells despite high-level P-glycoprotein expression. Free Radic. Res. 2019;53:882–891. doi: 10.1080/10715762.2019.1641602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helmy M.H., Ismail S.S., Fayed H., El Bassiouni E.A. Effect of selenium supplementation on the activities of glutathione metabolizing enzymes in human hepatoma Hep G2 cell line. Toxicology. 2000;144:57–61. doi: 10.1016/s0300-483x(99)00190-0. [DOI] [PubMed] [Google Scholar]
- 40.Baker R.D., Jr., Baker S.S., Rao R. Selenium deficiency in tissue culture: implications for oxidative metabolism. J. Pediatr. Gastroenterol. Nutr. 1998;27:387–392. doi: 10.1097/00005176-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Potter A.J., Gollahon K.A., Palanca B.J., Harbert M.J., Choi Y.M., Moskovitz A.H., Potter J.D., Rabinovitch P.S. Flow cytometric analysis of the cell cycle phase specificity of DNA damage induced by radiation, hydrogen peroxide and doxorubicin. Carcinogenesis. 2002;23:389–401. doi: 10.1093/carcin/23.3.389. [DOI] [PubMed] [Google Scholar]
- 42.Barnouin K., Dubuisson M.L., Child E.S., Fernandez d.M., Glassford J., Medema R.H., Mann D.J., Lam E.W. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J. Biol. Chem. 2002;277:13761–13770. doi: 10.1074/jbc.M111123200. [DOI] [PubMed] [Google Scholar]