Clavibacter michiganensis subsp. michiganensis, the causal agent of tomato bacterial canker disease, is one of the economically important pathogens of solanaceous crops (e.g., eggplant, pepper, and tomato) around the world. The disease occurs in many countries, with a particular importance in regions characterized by high precipitation and humid environmental conditions. As a seed-borne pathogen, C. michiganensis subsp. michiganensis is included in the A2 (high risk) list of quarantine pathogens by the European and Mediterranean Plant Protection Organization (EPPO). Bacterial canker disease was reported for the first time in 1993 in Iran, while the geographic distribution, genetic diversity, and phylogenetic position of the causal agent remain undetermined. In this study, using the multilocus sequence analysis and typing (MLSA/MLST) approach, we provided a phylogeographic scheme for the C. michiganensis subsp. michiganensis strains isolated in Iran. Furthermore, global-scale phylogenetic analyses led to determination of phylogenetic position of Iranian C. michiganensis subsp. michiganensis strains among worldwide population of the pathogen. Based on diversity parameters and population structure, we suggest relatively higher genetic diversity of the bacterial canker pathogen in Iran than has so far been observed in the other areas of the world. Results obtained in this study provide a novel insight into the genetic diversity and population structure of the bacterial canker pathogen on a global scale.
KEYWORDS: tomato bacterial canker, MLSA/MLST, Solanum lycopersicum, solanaceous crops
ABSTRACT
Tomato bacterial canker caused by Clavibacter michiganensis subsp. michiganensis is one of the most important seed-borne tomato diseases around the globe. The disease was initially reported in 1993 in Iran, and it became a rising threat for the multibillion dollar tomato industry of the country during the last decade. In this study, using phylogeographic analyses, we determined genetic diversity and geographic distribution of C. michiganensis subsp. michiganensis in Iran. Our field surveys showed that the pathogen is expanding into the southern and eastern areas of the country. Furthermore, multilocus sequence analysis and typing (MLSA/MLST) using the sequences of five housekeeping genes (atpD, gyrB, ppk, recA, and rpoB) revealed that 37 C. michiganensis subsp. michiganensis strains isolated in Iran had high genetic diversity and placed in 15 sequence types (STs), while all the available 184 worldwide C. michiganensis subsp. michiganensis sequences were placed in 43 STs. MLSA divided the worldwide C. michiganensis subsp. michiganensis strains into two phylogroups (I and II). Among the 37 strains isolated in Iran, 30 strains clustered in phylogroup I, while 7 strains clustered in phylogroup II. Phylogeographic data inferred from the allelic profile of the five housekeeping genes suggested multiple introductions of C. michiganensis subsp. michiganensis inoculum into Iran, while the geographic origin of the Iranian C. michiganensis subsp. michiganensis strains remains undetermined. Further analyses using higher numbers of strains are warranted to decipher the evolutionary history of C. michiganensis subsp. michiganensis in Iran. Additionally, stricter seed/transplant inspections are recommended to reduce the risk of pathogen expansion to areas with no history of the disease.
IMPORTANCE Clavibacter michiganensis subsp. michiganensis, the causal agent of tomato bacterial canker disease, is one of the economically important pathogens of solanaceous crops (e.g., eggplant, pepper, and tomato) around the world. The disease occurs in many countries, with a particular importance in regions characterized by high precipitation and humid environmental conditions. As a seed-borne pathogen, C. michiganensis subsp. michiganensis is included in the A2 (high risk) list of quarantine pathogens by the European and Mediterranean Plant Protection Organization (EPPO). Bacterial canker disease was reported for the first time in 1993 in Iran, while the geographic distribution, genetic diversity, and phylogenetic position of the causal agent remain undetermined. In this study, using the multilocus sequence analysis and typing (MLSA/MLST) approach, we provided a phylogeographic scheme for the C. michiganensis subsp. michiganensis strains isolated in Iran. Furthermore, global-scale phylogenetic analyses led to determination of phylogenetic position of Iranian C. michiganensis subsp. michiganensis strains among worldwide population of the pathogen. Based on diversity parameters and population structure, we suggest relatively higher genetic diversity of the bacterial canker pathogen in Iran than has so far been observed in the other areas of the world. Results obtained in this study provide a novel insight into the genetic diversity and population structure of the bacterial canker pathogen on a global scale.
INTRODUCTION
Tomato (Solanum lycopersicum L.) is the most widely cultivated vegetable around the globe, reaching worldwide production of 182 million tonnes in 2017 (1). In the early 16th century, tomato was introduced from western South America to Europe by Spanish conquistadors, and the crop has been distributed around the globe in a short period of time during the 19th and 20th centuries (2). In parallel to the global trend in tomato cultivation, risks of crop losses due to biotic stresses have also increased over the years (3). Emerging bacterial tomato diseases—caused by seed-borne agents—are the main consequence of the increased global trade in tomato seed industry (4–6). Seed-borne bacterial pathogens are of great economic importance, since they are capable of distributing over distant geographic areas via infected seed lots, leading to substantial crop losses. Furthermore, even a low rate of pathogen transmission (e.g., 0.01%) from seed to seedling can initiate a serious epidemic in commercial tomato fields (4).
Bacterial canker of tomato caused by the Gram-positive bacterium Clavibacter michiganensis subsp. michiganensis affects tomato production under different environmental conditions around the globe (7). The disease was first identified on tomato in Michigan (USA) in 1909 (8) and currently is widespread through all the six continents (9). The causal agent is included in the A2 list of quarantine pathogens by the European and Mediterranean Plant Protection Organization (9). The pathogen is seed borne; hence, infected seeds are the main source of inoculum for long-distance dissemination (10). Furthermore, warm and humid growing environments (for both greenhouse- and field-grown tomatoes) coupled with frequent overhead irrigation maximize bacterial growth and spread among the plants, leading to the yield losses of 20 to 85% due to the canker and wilting symptoms (11, 12).
Bacterial canker is of high economic importance in countries with fast-growing tomato industries, e.g., Iran and Turkey (13, 14). Following China, India, the United States, Turkey, and Egypt, Iran is the sixth largest tomato producer in the world, possessing one of the fast-growing tomato industries in the past decades. Indeed, while worldwide tomato production has increased only 5-fold over the past 50 years, the Iranian tomato industry has grown 46-fold in the same time frame (1). Among the top 10 tomato-producing countries, yield losses due to severe outbreaks of the bacterial canker disease were recorded in the United States (15), Turkey (13), and Iran (16). Widespread occurrence of the disease was also reported in the European countries, e.g., Italy (17) and Belgium (18), as well as in South America, e.g., Chile (19) and Uruguay (20). Regarding the seed-borne nature of the pathogen, since the mid-20th century, international tomato seed trades have spread the causal agent on intra- and intercontinental scales (9). In Iran, bacterial canker was first reported in northwestern areas of the country in 1993 (21). Severe outbreaks of the bacterial canker were rarely reported in Iran until the recent expansion of the disease into the northern areas (16). However, the genetic diversity of the pathogen in Iran, phylogenetic position of the Iranian C. michiganensis subsp. michiganensis strains among the worldwide population of the pathogen, and geographic origin of C. michiganensis subsp. michiganensis inoculum in the country remain uninvestigated.
Phylogenetic and phylogeographic studies using genomic DNA-based approaches (e.g., multilocus sequence analysis and typing [MLSA/MLST]) are capable of elucidating the population structure, evolutionary descent, and transmission routes of the causal agents of epidemic diseases (22). During the past decade, a number of molecular phylogenetic studies were performed to clarify the phylogenetic position and taxonomy of C. michiganensis strains (23), which led to the reclassification of tomato-associated pathogenic and nonpathogenic strains into different taxa (24). However, so far phylogenetic investigations on C. michiganensis subsp. michiganensis strains have been mostly country specific, including only a portion of the worldwide diversity of the pathogen in the American and Western European countries (15, 19, 20). Furthermore, despite the widespread distribution of the bacterial canker pathogen in the Middle Eastern countries (e.g., Iran), phylogenetic relationships of C. michiganensis subsp. michiganensis strains isolated in these areas with European and American strains have not yet been determined. Hence, a global-scale phylogeographic analysis using the information of all the available C. michiganensis subsp. michiganensis strains is warranted to infer the patterns of evolutionary descent among the worldwide population of the pathogen.
The purposes of the present study were to (i) determine the geographic distribution of the bacterial canker pathogen in tomato-growing areas in Iran and (ii) elucidate the phylogenetic position of 37 Iranian C. michiganensis subsp. michiganensis strains among the worldwide population of the pathogen (147 strains, retrieved from the NCBI GenBank database) using MLSA/MLST of five housekeeping genes (i.e., atpD, gyrB, ppk, recA, and rpoB). Phylogeographic analyses revealed that the worldwide C. michiganensis subsp. michiganensis population could be divided into two lineages (phylogroups) based on the date of isolation, while the Iranian strains have high genetic diversity and are distributed in both phylogroups of the subspecies.
RESULTS
Distribution of bacterial canker disease in Iran.
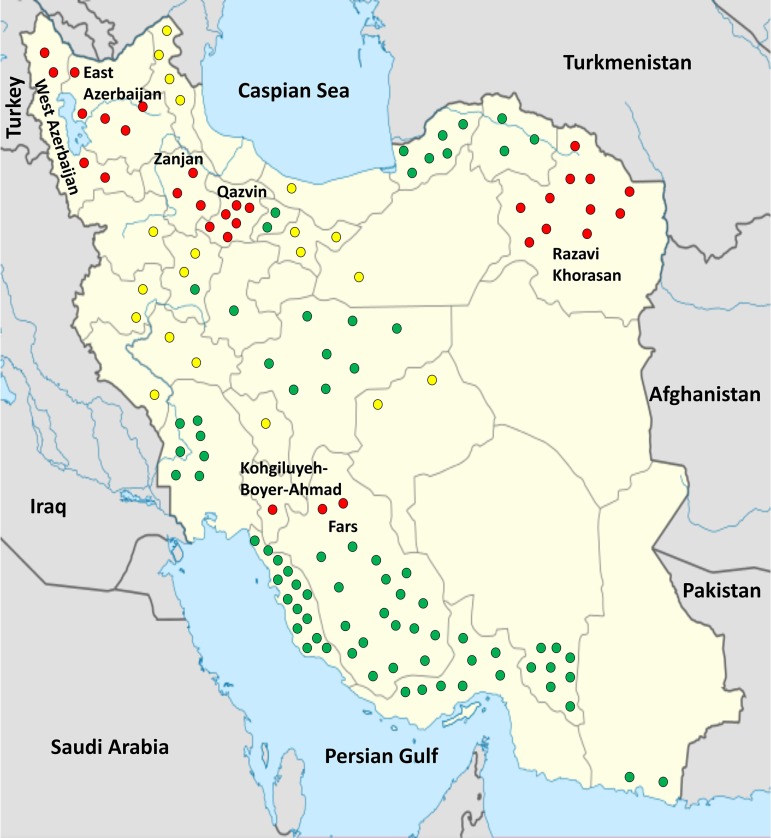
Field surveys across tomato-growing areas in Iran during 2015 to 2017 revealed the incidence of bacterial canker in regions with no history of the disease (Fig. 1). Until recently, bacterial canker was limited to the northwestern and northern provinces of Iran, while our surveys indicated the incidence of the disease in seven geographically distant provinces, i.e., East Azerbaijan, Fars, Kohgiluyeh-Boyer-Ahmad, Qazvin, Razavi Khorasan, West Azerbaijan, and Zanjan (Table 1). Bacterial canker symptoms were rarely observed in southern provinces, where only two strains and one suspected strain were isolated in Kohgiluyeh-Boyer-Ahmad and northern areas of Fars province, respectively (Fig. 1). A total of 37 yellow-pigmented Gram-positive bacterial strains were identified as C. michiganensis subsp. michiganensis according to their mucoidal colony characteristics on yeast extract-dextrose-calcium carbonate (YDC) medium and the results of subspecific PCRs using the primer pair PSA-4/PSA-R, which directed the amplification of the expected 271-bp DNA fragment in all the strains. All the strains were isolated from tomato plants, and no bacterial canker symptom was observed in the surveyed eggplant and pepper fields in Iran. All 37 strains were pathogenic on tomato plants under greenhouse conditions, while none of them were pathogenic on chili pepper, bell pepper, or black nightshade (Solanum nigrum).
FIG 1.
Distribution of tomato bacterial canker disease caused by Clavibacter michiganensis subsp. michiganensis in tomato-producing areas of Iran. Each circle represents the annual production of 50,000 tonnes of tomatoes in each area according to the Iran Ministry of Agriculture (25). Red circles indicate the occurrence of bacterial canker, while green circles indicate the absence of the disease. Yellow circles represent the areas not surveyed in this study. The source map is from https://commons.wikimedia.org/wiki/File:Iran_location_map.svg.
TABLE 1.
Clavibacter michiganensis subsp. michiganensis strains isolated in Iran, their area/date of isolation, and their sequence types and allelic profile based on the sequences of five housekeeping genes (i.e., atpD, gyrB, ppk, recA, and rpoB)
| Strain | Region |
Yr | Allelic profilea |
Sequence typeb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Province | County | atpD | gyrB | ppk | recA | rpoB | |||
| gh1 | Qazvin | Takestan | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| gh12 | Qazvin | Takestan | 2017 | 5 | 2 | 2 | 1 | 1 | 39 |
| gh17 | Qazvin | Takestan | 2017 | 6 | 6 | 4 | 1 | 1 | 12 |
| gh19 | Qazvin | Qazvin | 2017 | 3 | 5 | 6 | 1 | 1 | 3 |
| gh22 | Qazvin | Qazvin | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| M13 | Razavi Khorasan | Chenaran | 2017 | 3 | 3 | 6 | 1 | 1 | 4 |
| M3 | Razavi Khorasan | Chenaran | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| O1 | West Azerbaijan | Urmia | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| O11 | West Azerbaijan | Urmia | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| O15 | West Azerbaijan | Urmia | 2017 | 2 | 6 | 1 | 3 | 1 | 33 |
| O16 | West Azerbaijan | Urmia | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| O27 | West Azerbaijan | Urmia | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| O36 | West Azerbaijan | Urmia | 2017 | 1 | 6 | 6 | 1 | 1 | 8 |
| O6 | West Azerbaijan | Urmia | 2017 | 1 | 6 | 6 | 1 | 1 | 8 |
| Sh4 | Fars | Eghlid | 2017 | 6 | 5 | 6 | 1 | 1 | 10 |
| T210 | Kohgiluyeh-Boyer-Ahmad | Yasuj | 2016 | 6 | 6 | 6 | 1 | 1 | 13 |
| Ta10 | East Azerbaijan | Marand | 2017 | 5 | 6 | 6 | 1 | 1 | 9 |
| Ta13 | East Azerbaijan | Marand | 2017 | 5 | 1 | 2 | 1 | 1 | 37 |
| Ta15 | East Azerbaijan | Marand | 2017 | 4 | 1 | 3 | 1 | 1 | 23 |
| Ta17 | East Azerbaijan | Marand | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| Ta25 | East Azerbaijan | Marand | 2017 | 5 | 1 | 2 | 1 | 1 | 37 |
| Ta3 | East Azerbaijan | Marand | 2017 | 5 | 1 | 2 | 2 | 1 | 38 |
| Ta4 | East Azerbaijan | Marand | 2017 | 5 | 1 | 2 | 1 | 1 | 37 |
| Ta59 | East Azerbaijan | Tabriz | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| Ta80 | East Azerbaijan | Osku | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| Tom808 | Zanjan | Zanjan | 2015 | 5 | 6 | 6 | 1 | 1 | 9 |
| Tom826 | West Azerbaijan | Mahabad | 2015 | 3 | 6 | 6 | 1 | 1 | 1 |
| Tom835 | Qazvin | Takestan | 2015 | 5 | 6 | 6 | 1 | 1 | 9 |
| Y5 | Kohgiluyeh-Boyer-Ahmad | Yasuj | 2017 | 6 | 6 | 6 | 1 | 1 | 13 |
| Z12 | Zanjan | Zanjan | 2017 | 3 | 6 | 5 | 1 | 1 | 2 |
| Z13 | Zanjan | Zanjan | 2017 | 1 | 6 | 6 | 1 | 1 | 8 |
| Z20 | Zanjan | Zanjan | 2017 | 5 | 6 | 6 | 1 | 1 | 9 |
| Z21 | Zanjan | Zanjan | 2017 | 3 | 6 | 6 | 1 | 1 | 1 |
| Z32 | Zanjan | Abhar | 2017 | 6 | 6 | 6 | 1 | 1 | 13 |
| Z5 | Zanjan | Soltaniyeh | 2017 | 6 | 4 | 6 | 1 | 1 | 11 |
| Z7 | Zanjan | Soltaniyeh | 2017 | 6 | 6 | 6 | 1 | 1 | 13 |
| Zol2 | East Azerbaijan | Marand | 2015 | 5 | 1 | 2 | 1 | 1 | 37 |
Phylogenetic analyses.
Phylogenetic tree constructed using the concatenated sequences of housekeeping genes in 37 C. michiganensis subsp. michiganensis strains isolated in Iran along with the sequences of 147 worldwide strains retrieved from the NCBI GenBank database revealed that all 184 C. michiganensis subsp. michiganensis strains clustered in a monophyletic clade and were differentiated from their closest nonpathogenic neighbor group, which was recently nominated as Clavibacter michiganensis subsp. californiensis (see Fig. S1 in the supplemental material). Since the rpoB gene sequences were not available in the strains isolated in Chile and Uruguay, we have constructed a phylogenetic tree consisting of 184 worldwide strains using the concatenated sequences of atpD, gyrB, ppk, and recA. Results obtained from the four-gene MLSA were in congruence with those obtained from the five-gene phylogeny (Fig. 2 and Fig. S2). However, variable results were obtained when the sequences of individual housekeeping genes were subjected to the phylogenetic analysis (Fig. S3).
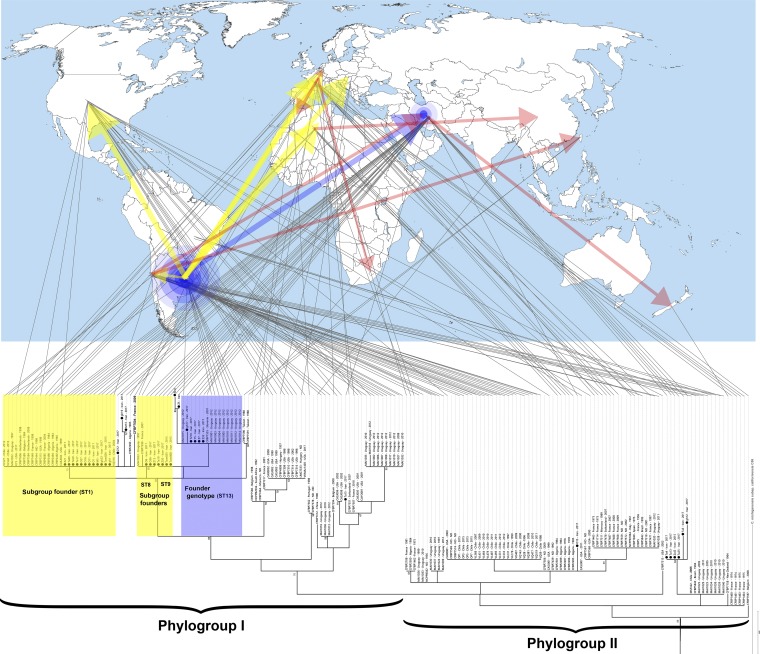
FIG 2.
Phylogeny of Clavibacter michiganensis subsp. michiganensis strains isolated in this study among the worldwide population of the pathogen based on the concatenated sequences of four housekeeping genes (i.e., atpD, gyrB, ppk, and recA). Clavibacter michiganensis subsp. californiensis was used as an outgroup to root the tree. The bar presents numbers of substitutions per site. Gray lines linking the phylogenetic tree to the world map indicate geographic origin of a given strain. Strains isolated in Iran are indicated by black circles. Worldwide C. michiganensis subsp. michiganensis strains were divided into two phylogroups (I and II) based on the date of isolation. Blue highlighting on the tree indicates the founder sequence type (ST13), while the yellow highlighting shows the subgroup founders. Blue concentric circles on the map indicate the predicted center of diversity of the pathogen. Yellow arrows show the hypothetical transmission route of subgroup founders, while red arrows represent the subsequent transmission routes of the pathogen. The source map is from https://commons.wikimedia.org/wiki/File:A_large_blank_world_map_with_oceans_marked_in_blue.PNG.
MLSA of the worldwide C. michiganensis subsp. michiganensis population revealed that the strains used in this study were divided into two phylogroups (I and II) based on their dates of isolation (Fig. 2). Phylogroup I consisted of post-1984 strains with the exception of CFBP 4999, which was isolated in Hungary in 1957, while phylogroup II includes all the pre-1980 strains, although a number of post-1980 strains also clustered in this clade. Among the 37 strains isolated in Iran, 30 strains clustered with the post-1984 strains (phylogroup I), while 7 strains (i.e., gh12, O15, Ta3, Ta4, Ta13, Ta25, and Zol2) clustered in phylogroup II (Fig. 2). Iranian strains within phylogroup I were scattered through several subclusters and were phylogenetically close to the strains isolated in South America (i.e., Chile and Uruguay), Western Europe (i.e., Belgium, France and Netherlands), and northern Africa (i.e., Algeria). Iranian strains in phylogroup II were unique in the concatenated sequences of the evaluated genes, sharing no sequence identity with the strains isolated elsewhere (Fig. 2). Moreover, country-specific patterns were observed among both phylogroups I and II. For instance, all the strains isolated in eastern Asia (i.e., Taiwan and China), Eastern Europe (i.e., Hungary and Slovenia), South Africa, and Portugal clustered within phylogroup I, while the strains isolated in Brazil, Italy, and Spain clustered in phylogroup II. Among 19 strains isolated in France, six strains clustered in phylogroup I, while the remaining 13 strains clustered in phylogroup II. As for the strains isolated in the American continent, the proportions of the strains in phylogroups I/II were 3/22, 24/15, and 15/5 in the strains isolated in Chile, Uruguay, and the United States, respectively (Fig. 2).
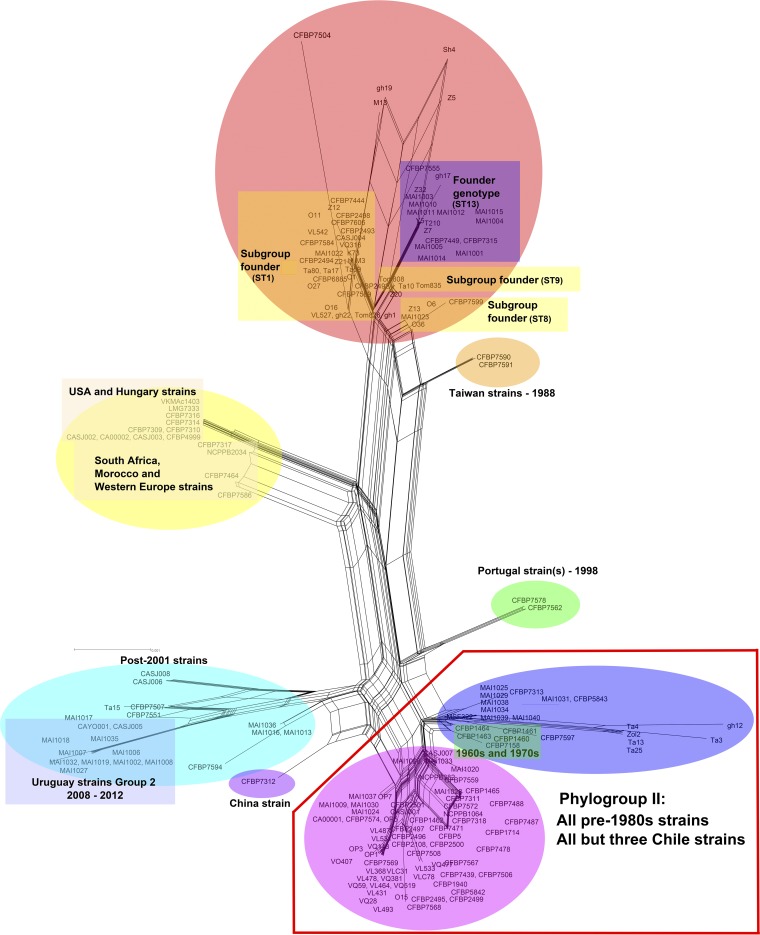
Because the maximum likelihood phylogenies showed conflicting topologies, phylogenetic networks were generated using the NeighborNet method implemented in SplitsTree version 4.14.4 for all the individual genes, as well as the concatenated data set of sequences (Fig. 3). The NeighborNet network constructed using the concatenated sequences of four housekeeping genes was in congruence with the maximum likelihood phylogenetic tree, where all the pre-1980 strains clustered in a monophyletic group, outlined in red in Fig. 3. Interestingly, 22 out of 25 Chilean strains, all of which were isolated in the period from 1996 to 2015, clustered among the pre-1980 strains (phylogroup II). Strain CFBP 7312, isolated in China, strains CFBP 7590 and CFBP 7591, isolated in Taiwan, and strain CFBP 7562, isolated in Portugal, clustered in separate clades in the NeighborNet network, indicating their distinct phylogenetic positions. Although a chronological relationship could be inferred among various clusters in the NeighborNet network, no country-specific clustering was observed, indicating the impact of international tomato seed transportation on the global distribution of the pathogen.
FIG 3.
NeighborNet network of Clavibacter michiganensis subsp. michiganensis strains constructed using SplitsTree version 4.14.4. The network confirms division of the worldwide C. michiganensis subsp. michiganensis strains into two phylogroups (I and II) based on their dates of isolation. All the pre-1980 strains along with a number of post-1984 strains clustered in a monophyletic clade, outlined in red.
Genetic diversity.
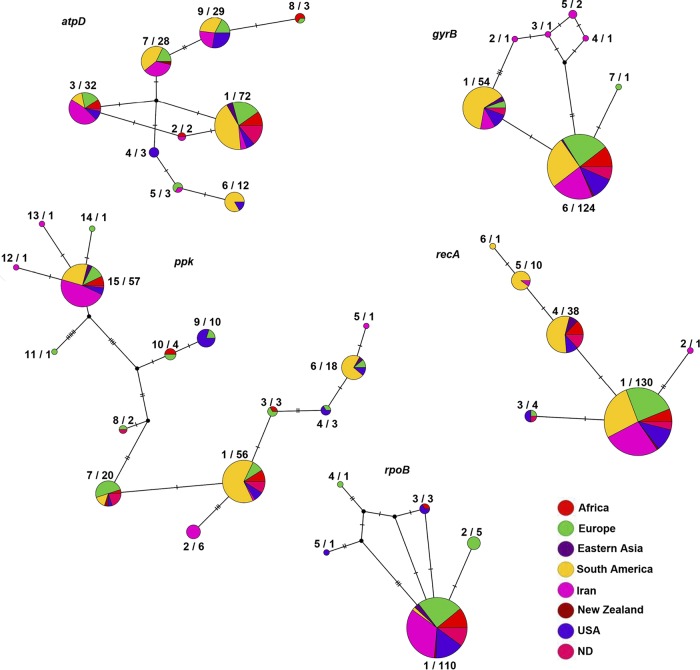
Thirty-seven C. michiganensis subsp. michiganensis strains isolated in Iran carried various allelic forms and were clustered in different allele groups based on the sequences of individual housekeeping genes. Accordingly, six, six, six, three, and one allele were detected in the partial sequences of the atpD, gyrB, ppk, recA, and rpoB genes, respectively (Table 1). On the other hand, among 184 worldwide strains, 9, 7, 15, and 6 alleles were detected in the sequences of the atpD, gyrB, ppk, and recA genes, while five alleles were detected in the rpoB gene sequences of 120 strains (excluding the Chile and Uruguay strains) (Fig. 4, Fig. S3, and Table S1). Since rpoB gene sequences were lacking in the Chile and Uruguay strains, we performed diversity analyses on the worldwide C. michiganensis subsp. michiganensis strains using the sequences of four genes (i.e., atpD, gyrB, ppk, and recA), which demonstrated that the strains isolated in Iran were placed in 15 sequence types (STs) (Fig. S4), while a total of 43 STs were detected when all 184 worldwide C. michiganensis subsp. michiganensis strains were considered (Table S1 and Fig. S5). Sequence variation statistics and diversity parameters of the strains isolated in Iran were calculated using DnaSP 5.10 software and compared with those of 147 strains isolated around the globe (Tables 2 and 3). According to the haplotype frequency parameter (number of haplotypes/number of strains) in the concatenated sequences of five genes, the strains isolated in Qazvin and Zanjan provinces in Iran had the highest allelic richness, showing 0.833 and 0.750 haplotype frequency, respectively (Table 2). The strains isolated in Zanjan province did not show any nucleotide diversity in the gyrB, recA, or rpoB gene sequences. As for the neutrality tests, significant departure from the mutation/drift equilibrium was observed in ppk gene sequences among the strains isolated in West Azerbaijan. However, due to the insignificant neutrality values in the remaining four genes, further evidence is needed to draw a conclusion on the impact of departure from the mutation/drift equilibrium in the strains isolated in different geographic areas in Iran. On the other hand, given the low number of C. michiganensis subsp. michiganensis strains isolated in Fars, Kohgiluyeh-Boyer-Ahmad, and Razavi Khorasan provinces, we were unable to perform diversity analyses on these strains.
FIG 4.
TCS haplotype (allelic) networks generated using PopART program from the individual sequences of five housekeeping genes (i.e., atpD, gyrB, ppk, recA, and rpoB) in 184 worldwide Clavibacter michiganensis subsp. michiganensis strains. Each circle represents a unique allele within the sequences of a given gene. The size of the circles indicates the relative frequency of strains belonging to a particular allele. Hatch marks along the branches indicate the number of mutations. Numbers on the left side of the radix character represent the corresponding allele as shown in Table S1, while the numbers on the right side of the radix character represent the number of strains in each allele. Each color indicates a different geographic location, i.e., Africa (strains from Algeria, Morocco, and South Africa), Eastern Asia (strains from China and Taiwan), Europe (strains from Belgium, France, Hungary, Italy, the Netherlands, Portugal, Slovenia, Spain, and Switzerland), Iran, South America (strains from Brazil, Chile, and Uruguay), New Zealand, and the United States.
TABLE 2.
Sequence variation statistics and diversity parameters among 37 Clavibacter michiganensis subsp. michiganensis strains isolated in Iran calculated using DnaSP 5.10
| Province | Gene | No. of: |
Haplotype frequencya | Total no. of segregating sites | % polymorphic sites | Nucleotide diversity (π) | No. of mutations (η) | Haplotype (gene) diversity | Value for indicated neutrality testb |
Minimum no. of recombination events | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Nucleotides | Haplotypes | Tajima’s D | Fu and Li’s D* | Fu and Li’s F* | |||||||||
| East Azerbaijan | atpD | 10 | 455 | 3 | 0.300 | 4 | 0.879 | 0.308 × 10−2 | 4 | 0.600 | −0.037 NS | −0.338 NS | −0.296 NS | 0 |
| gyrB | 10 | 468 | 2 | 0.200 | 1 | 0.213 | 0.114 × 10−2 | 1 | 0.533 | 1.302 NS | 0.804 NS | 1.026 NS | 0 | |
| ppk | 10 | 495 | 3 | 0.300 | 16 | 3.232 | 0.154 × 10−1 | 16 | 0.644 | 1.627 NS | 0.799 NS | 1.132 NS | 0 | |
| recA | 10 | 415 | 3 | 0.300 | 3 | 0.722 | 0.225 × 10−2 | 3 | 0.644 | −0.431 NS | −0.804 NS | −0.798 NS | 0 | |
| rpoB | 10 | 277 | 1 | 0.100 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| Concatenated | 10 | 2,110 | 5 | 0.500 | 24 | 1.137 | 0.498 × 10−2 | 24 | 0.800 | 1.135 NS | 0.405 NS | 0.662 NS | 1 | |
| Qazvin | atpD | 6 | 455 | 3 | 0.500 | 4 | 0.879 | 0.410 × 10−2 | 4 | 0.733 | 0.355 NS | 0.071 NS | 0.139 NS | 0 |
| gyrB | 6 | 468 | 3 | 0.500 | 5 | 1.068 | 0.484 × 10−2 | 5 | 0.600 | 0.196 NS | 0.362 NS | 0.348 NS | 0 | |
| ppk | 6 | 495 | 3 | 0.500 | 14 | 2.828 | 0.943 × 10−2 | 14 | 0.600 | −1.466 NS | −1.504*, P < 0.05 | −1.623 NS | 0 | |
| recA | 6 | 415 | 1 | 0.166 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| rpoB | 6 | 277 | 1 | 0.166 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| Concatenated | 6 | 2,110 | 5 | 0.833 | 23 | 1.090 | 0.417 × 10−2 | 23 | 0.933 | −0.793 NS | −0.834 NS | −0.900 NS | 1 | |
| West Azerbaijan | atpD | 8 | 455 | 3 | 0.375 | 2 | 0.439 | 0.212 × 10−2 | 2 | 0.607 | 0.931 NS | 1.111 NS | 1.167 NS | 0 |
| gyrB | 8 | 468 | 1 | 0.125 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| ppk | 8 | 495 | 2 | 0.250 | 10 | 2.020 | 0.505 × 10−2 | 10 | 0.250 | −1.741*, P < 0.05 | −1.906*, P < 0.05 | 2.069*, P < 0.05 | 0 | |
| recA | 8 | 415 | 2 | 0.250 | 3 | 0.722 | 0.181 × 10−2 | 3 | 0.250 | −1.447 NS | −1.565 NS | −1.685 NS | 0 | |
| rpoB | 8 | 277 | 1 | 0.125 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| Concatenated | 8 | 2,110 | 3 | 0.375 | 15 | 0.710 | 0.200 × 10−2 | 15 | 0.607 | −1.389 NS | −1.504 NS | −1.641 NS | 0 | |
| Zanjan | atpD | 8 | 455 | 4 | 0.500 | 5 | 0.098 | 0.502 × 10−2 | 5 | 0.821 | 0.840 NS | 0.747 NS | 0.846 NS | 0 |
| gyrB | 8 | 468 | 1 | 0.125 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| ppk | 8 | 495 | 2 | 0.250 | 1 | 0.202 | 0.051 × 10−2 | 1 | 0.250 | −1.054 NS | −1.126 NS | −1.203 NS | 0 | |
| recA | 8 | 415 | 1 | 0.125 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | |
| rpoB | 8 | 277 | 1 | 0 | 0.000 | 0.000 | 0 | 0.000 | ND | ND | ND | ND | ||
| Concatenated | 8 | 2,110 | 6 | 0.750 | 9 | 0.426 | 0.156 × 10−2 | 9 | 0.929 | −0.261 NS | −0.386 NS | −0.396 NS | 0 | |
Number of haplotypes/number of strains.
ND, not determined; NS, not significant. Asterisks with data, P ≤ 0.05.
TABLE 3.
Sequence variation statistics and diversity parameters among the worldwide strains of Clavibacter michiganensis subsp. michiganensis calculated using DnaSP 5.10
| Country | Gene | No. of: |
Haplotype frequencya |
Total no. of segregating sites |
% of polymorphic sites |
Nucleotide diversity (π) |
No. of mutations (η) |
Haplotype (gene) diversity |
Value for indicated neutrality testb |
Minimum no. of recombination events | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Nucleotides | Haplotypes | Tajima’s D | Fu and Li’s D* | Fu and Li’s F* | |||||||||
| Worldwide | atpD | 184 | 455 | 9 | 0.049 | 8 | 1.758 | 0.493 × 10−2 | 8 | 0.768 | 1.391 NS | 1.215 NS | 1.523 NS | 1 |
| gyrB | 184 | 468 | 7 | 0.038 | 6 | 1.282 | 0.131 × 10−2 | 6 | 0.462 | −0.837 NS | 0.043 NS | −0.307 NS | 2 | |
| ppk | 184 | 495 | 15 | 0.081 | 28 | 5.656 | 0.190 × 10−2 | 28 | 0.789 | 0.617 NS | −2.069 NS | −1.166 NS | 2 | |
| recA | 184 | 415 | 6 | 0.032 | 6 | 1.445 | 0.140 × 10−2 | 6 | 0.457 | −0.901 NS | −2.012 NS | −1.940 NS | 0 | |
| rpoB | 120 | 277 | 5 | 0.041 | 8 | 2.888 | 0.101 × 10−2 | 9 | 0.159 | −2.027* | −2.553* | −2.815* | 0 | |
| Concatenated | 184 | 1,833 | 43 | 0.233 | 48 | 2.618 | 0.509 × 10−2 | 48 | 0.936 | 0.374 NS | −1.553 NS | −0.862 NS | 7 | |
| Iran | atpD | 37 | 455 | 6 | 0.162 | 7 | 1.538 | 0.429 × 10−2 | 7 | 0.739 | 0.462 NS | −0.202 NS | 0.003 NS | 1 |
| gyrB | 37 | 468 | 6 | 0.162 | 5 | 1.068 | 0.245 × 10−2 | 5 | 0.488 | −0.109 NS | 1.124 NS | 0.875 NS | 2 | |
| ppk | 37 | 495 | 6 | 0.162 | 18 | 3.363 | 0.914 × 10−2 | 18 | 0.450 | 0.164 NS | −0.207 NS | −0.100 NS | 0 | |
| recA | 37 | 415 | 3 | 0.081 | 5 | 1.204 | 0.139 × 10−2 | 6 | 0.368 | −1.628 NS | −2.856* | −2.898* | 0 | |
| rpoB | 37 | 277 | 1 | 0.027 | 0 | 0.000 | 0.000 × 10−2 | 0 | 0.000 | NA | NA | NA | NA | |
| Concatenated | 37 | 1,833 | 15 | 0.405 | 35 | 1.909 | 0.447 × 10−2 | 36 | 0.869 | −0.173 NS | −0.643 NS | −0.573 NS | 4 | |
| Europec | atpD | 32 | 455 | 6 | 0.187 | 7 | 1.538 | 0.456 × 10−2 | 7 | 0.734 | 0.563 NS | 1.269 NS | 1.231 NS | 1 |
| gyrB | 32 | 468 | 2 | 0.062 | 1 | 0.213 | 0.037 × 10−2 | 1 | 0.175 | −0.448 NS | 0.587 NS | 0.344 NS | 1 | |
| ppk | 32 | 495 | 9 | 0.281 | 16 | 3.232 | 0.104 × 10−2 | 16 | 0.847 | 1.258 NS | 1.185 NS | 1.420 NS | 1 | |
| recA | 32 | 415 | 4 | 0.125 | 2 | 0.481 | 0.116 × 10−2 | 3 | 0.425 | −0.816 NS | −0.282 NS | −0.506 NS | 0 | |
| rpoB | 32 | 277 | 3 | 0.093 | 5 | 1.805 | 0.189 × 10−2 | 5 | 0.325 | 1.555 NS | −2.413 NS | −2.511 NS | 0 | |
| Concatenated | 32 | 1,833 | 13 | 0.406 | 26 | 1.418 | 0.447 × 10−2 | 27 | 0.909 | 0.787 NS | 1.221 NS | 1.273 NS | 3 | |
| Chile | atpD | 25 | 455 | 3 | 0.120 | 3 | 0.659 | 0.127 × 10−2 | 3 | 0.290 | −0.670 NS | −0.202 NS | −0.386 NS | 0 |
| gyrB | 25 | 468 | 2 | 0.080 | 1 | 0.213 | 0.047 × 10−2 | 1 | 0.220 | −0.280 NS | 0.617 NS | 0.429 NS | 0 | |
| ppk | 25 | 495 | 2 | 0.080 | 10 | 2.020 | 0.444 × 10−2 | 10 | 0.220 | −0.559 NS | 1.407* | 0.955 NS | 0 | |
| recA | 25 | 415 | 2 | 0.080 | 2 | 0.481 | 0.135 × 10−2 | 2 | 0.280 | 0.124 NS | 0.831 NS | 0.731 NS | 0 | |
| rpoB | NA | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Concatenated | 25 | 1,833 | 3 | 0.120 | 16 | 0.872 | 0.194 × 10−2 | 16 | 0.290 | −0.564 NS | 1.198 NS | 0.772 NS | 0 | |
| Uruguay | atpD | 39 | 455 | 5 | 0.128 | 8 | 1.758 | 0.622 × 10−2 | 8 | 0.781 | 1.423 NS | 0.636 NS | 1.035 NS | 0 |
| gyrB | 39 | 468 | 2 | 0.510 | 1 | 0.213 | 0.089 × 10−2 | 1 | 0.416 | 1.089 NS | 0.566 NS | 0.823 NS | 0 | |
| ppk | 39 | 495 | 4 | 0.102 | 13 | 0.026 | 0.073 × 10−2 | 13 | 0.714 | 2.277* | 1.505* | 2.067** | 0 | |
| recA | 39 | 415 | 4 | 0.102 | 4 | 0.963 | 0.315 × 10−2 | 4 | 0.538 | 0.914 NS | −0.030 NS | 0.294 NS | 1 | |
| rpoB | NA | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Concatenated | 39 | 1,833 | 10 | 0.256 | 26 | 1.418 | 0.538 × 10−2 | 26 | 0.860 | 2.060* | 1.189 NS | 1.756* | 2 | |
| USA | atpD | 20 | 455 | 5 | 0.250 | 8 | 1.758 | 0.636 × 10−2 | 8 | 0.784 | 0.956 NS | 1.342 NS | 1.425 NS | 0 |
| gyrB | 20 | 468 | 2 | 0.100 | 1 | 0.213 | 0.094 × 10−2 | 1 | 0.442 | 1.025 NS | 0.649 NS | 0.856 NS | 0 | |
| ppk | 20 | 495 | 6 | 0.300 | 15 | 3.030 | 0.124 × 10−2 | 15 | 0.795 | 1.165 NS | 1.177 NS | 1.362 NS | 1 | |
| recA | 20 | 415 | 3 | 0.150 | 2 | 0.481 | 0.127 × 10−2 | 2 | 0.484 | −0.155 NS | 0.866 NS | 0.677 NS | 0 | |
| rpoB | 20 | 277 | 3 | 0.150 | 6 | 2.166 | 0.249 × 10−2 | 6 | 0.279 | −1.887* | −2.258 NS | −2.487 NS | 0 | |
| Concatenated | 20 | 1,833 | 8 | 0.400 | 26 | 1.418 | 0.515 × 10−2 | 26 | 0.821 | 1.111 NS | 1.420* | 1.546 NS | 3 | |
Number of haplotypes/number of strains.
ND, not determined; NA, not applicable; NS, not significant. *, P ≤ 0.05; **, P ≤ 0.01.
Includes strains isolated in Belgium, France, Spain, Portugal, and the Netherlands.
To provide precise insight into the genetic diversity of C. michiganensis subsp. michiganensis populations in different corners of the globe, we have separately calculated diversity parameters for the strains isolated in Iran, Western Europe, Chile, Uruguay, and the United States (Table 3). Country/region-specific analyses revealed that the strains isolated in Western Europe showed six, two, nine, four, and three alleles in the sequences of the atpD, gyrB, ppk, recA, and rpoB genes, respectively, while the strains isolated in the United States showed five, two, six, three, and three alleles in the same order of the genes. Three, two, two, and two alleles were observed in the Chilean strains, while five, two, four, and four alleles were detected in the Uruguayan strains in the sequences of atpD, gyrB, ppk, and recA, respectively (Table 3). On the other hand, evidence for geographic structure in allelic profile was detected in TCS networks generated using PopART version 1.7 software (Fig. 4). For instance, in the atpD gene sequences, allele 4 (A4) was represented by only U.S. strains. In gyrB gene sequences, alleles A2, A3, A4, and A5 were represented by only Iranian strains, while A7 was represented by a European strain. Furthermore, in ppk gene sequences, A2, A5, A12, and A13 were represented by the strains isolated in Iran, while A11 and A14 were represented by the strains isolated in Europe. As for recA gene sequences, A2 and A6 were represented by the strains isolated in Iran and South America, respectively. In the rpoB gene, A2 and A4 were represented by the strains isolated in Europe, while A5 was represented by a U.S. strain (Fig. 4).
Considering the haplotype/allele frequency (HF) index among the strains isolated in different geographic areas, the HF indices in atpD gene sequences were 0.162, 0.187, 0.120, 0.128, and 0.250 for the strains isolated in Iran, Western Europe, Chile, Uruguay, and the United States, respectively. The HF indices were 0.162, 0.062, 0.080, 0.510, and 0.100 in gyrB gene sequences and were 0.162, 0.281, 0.080, 0.102, and 0.300 in ppk gene sequences and 0.081, 0.125, 0.080, 0.102, and 0.150 in recA gene sequences among the strains isolated in Iran, Western Europe, Chile, Uruguay, and the United States, respectively (Table 3). As for the rpoB gene sequences, the HF indices were 0.027, 0.093, and 0.150 for the strains isolated in Iran, Europe, and the United States, respectively. Analysis of the concatenated sequences of four genes (excluding rpoB) showed that the HF indices were 0.405, 0.406, 0.120, 0.256, and 0.400 for the strains isolated in Iran, Western Europe, Chile, Uruguay, and the United States, respectively, indicating higher genetic diversity of the pathogen in Iran and Western Europe than those observed in the American continent. As far as the data set of concatenated sequences was concerned, the HF indices of the strains isolated in Iran and Western Europe—0.405 and 0.406, respectively—were far above the HF index observed in the worldwide collection of the strains (HF index = 0.233). On the other hand, the HF index of the strains isolated in Chile was almost half the worldwide HF value (Table 3).
None of the population neutrality indices (i.e., Tajima’s D, Fu and Li’s D*, and Fu and Li’s F*) was significant among the strains isolated in Western Europe, indicating that the population is evolving as per mutation/drift equilibrium, and this is an indication of no evidence of selection among the population (Table 3). However, 37 strains isolated across seven provinces in Iran showed significant departure from the mutation/drift equilibrium in the recA gene sequences, where both the Fu and Li’s D* (−2.856) and Fu and Li’s F* (−2.898) indices were statistically significant (Table 3). Although the negative values in recA gene sequences indicate selective sweeps and an excess of singletons among the population, and suggest demographic expansion after a recent bottleneck, insignificant values for atpD, gyrB, ppk, and rpoB gene sequences prevent us from making a precise statement on the neutrality status of the C. michiganensis subsp. michiganensis population in Iran. Interestingly, all the neutrality indices were statistically significant in ppk gene sequences of the strains isolated in Uruguay (Tajima’s D = 2.277, Fu and Li’s D* = 1.505, and Fu and Li’s F* = 2.067). Similar results were obtained for the concatenated sequences of the strains isolated in Uruguay, as well as the Fu and Li’s D* index in the ppk gene sequences of the strains isolated in Chile (Table 3). A positive value for Tajima’s D index signifies low levels of both low- and high-frequency polymorphisms (lack of rare alleles), indicating a balancing selection among the South American population of the pathogen. Furthermore, positive values for Fu and Li’s D* as well as Fu and Li’s F* parameters indicate a lack of singletons in the population. Significant deviation from the standard neutral model in C. michiganensis subsp. michiganensis strains isolated in South America was in contrast with the hypothesis that plant pathogens evolve in a mutation/drift equilibrium in the center of origin of their host plants. However, since the significant neutrality indices were found for a limited number of evaluated genes, further investigations using the sequences of additional housekeeping genes are needed to prove these observations.
The pairwise homoplasy index (PHI test) calculated using the SplitsTree software rejected the hypothesis of no recombination and detected statistically significant evidence for recombination in atpD (P = 0.049) and ppk (P = 0.012), as well as the concatenated data set of sequences (P = 5.829 × 10−7) in the worldwide population of the pathogen. Despite the results of the PHI test, Recombination Detection Program (RDP) did not find statistically significant evidence for recombination in any of the evaluated genes. We also estimated recombination events thorough all the available Clavibacter species sequences as listed in Fig. S1. No evidence for recombination was detected between the C. michiganensis subsp. michiganensis population and those of remaining species/subspecies of the genus in the RDP analyses (data not shown). On the other hand, DnaSP did find recombination among the strains isolated in Iran (in atpD and gyrB), Western Europe (in atpD, gyrB, and ppk), Uruguay (in recA), and the United States (in ppk) (Table 3). Taken together, due to the variable results obtained using different programs/algorithms, further analyses using the sequences of additional housekeeping genes would shed light on the genetic exchange history and evolutionary patterns of the pathogen on a global scale. Furthermore, to elucidate if recombination in the atpD and ppk genes had any distorting effect on the phylogenetic inference of the strains, a maximum likelihood phylogenetic tree was constructed using the sequences of three recombination-free genes, i.e., gyrB, recA, and rpoB. Results obtained from the concatenated sequences of gyrB, recA, and rpoB were in congruence with those obtained from the five-gene phylogeny, indicating that the worldwide C. michiganensis subsp. michiganensis strains clustered in a monophyletic clade apart from the remaining subspecies/species of Clavibacter (Fig. S2 and S6).
Phylogeography of C. michiganensis subsp. michiganensis strains.
Province-specific STs were detected among the strains isolated in Iran. For instance, ST23, ST37, and ST38 were isolated in East Azerbaijan, ST3, ST12, and ST39 were isolated in Qazvin, and ST2 and ST11 were isolated in Zanjan province (Fig. S4). Furthermore, ST4, ST10, and ST33 were isolated in Razavi Khorasan, Fars, and West Azerbaijan provinces, respectively (Fig. S4). On the other hand, several strains isolated in different provinces of Iran shared the same ST with strains isolated in other countries. For instance, 12 strains isolated in Iran clustered along with the strains isolated in South America, Europe, and northern Africa, all belong to the ST1 (Fig. 5 and Table S1). Furthermore, strains O6, O36, and Z13 shared the same ST (ST8) with the strain MAI1023 isolated in Uruguay in 1997. Strains T210, Y5, Z7, and Z32 also shared the same ST (ST13) with nine Uruguayan strains (Fig. 1 and 5). However, strains Z12 (ST2), gh19 (ST3), M13 (ST4), Ta10, Z20, Tom835, and Tom808 (ST9), Sh4 (ST10), Z5 (ST11), gh17 (ST12), Ta15 (ST23), O15 (ST33), Ta4, Ta13, Zol2, and Ta25 (ST37), Ta3 (ST38), and gh12 (ST39) belonged to unique STs which were Iran specific, with no exact similarity to the strains isolated in other geographic areas (Fig. 5 and Table S1). Further country/continent-specific STs were also detected among the worldwide C. michiganensis subsp. michiganensis population (Fig. 5). For instance, ST5 was isolated in Algeria and ST6, ST7, and ST16 were isolated in France, while ST21 and ST43 were isolated in Belgium. ST14, ST19, and ST41 were isolated in Taiwan, China, and New Zealand, respectively. ST22 and ST25 were isolated in the United States in 2002 and 2001, respectively. ST20, ST26, ST27, ST29, and ST30 were isolated in Uruguay, while ST31 was isolated in Chile and California. The ST40 strains were also isolated in southern America. Four strains assigned as ST42 (Fig. 5) were isolated in France in 1974 and 1975. Interestingly, the type strain of C. michiganensis subsp. michiganensis (CFBP 4999; isolated in Hungary in 1957), which was the only pre-1984 strain in phylogroup I, shared the same ST (ST17) as the strains isolated in the United States in the period from 1998 to 2017 (Fig. 2 and 5).
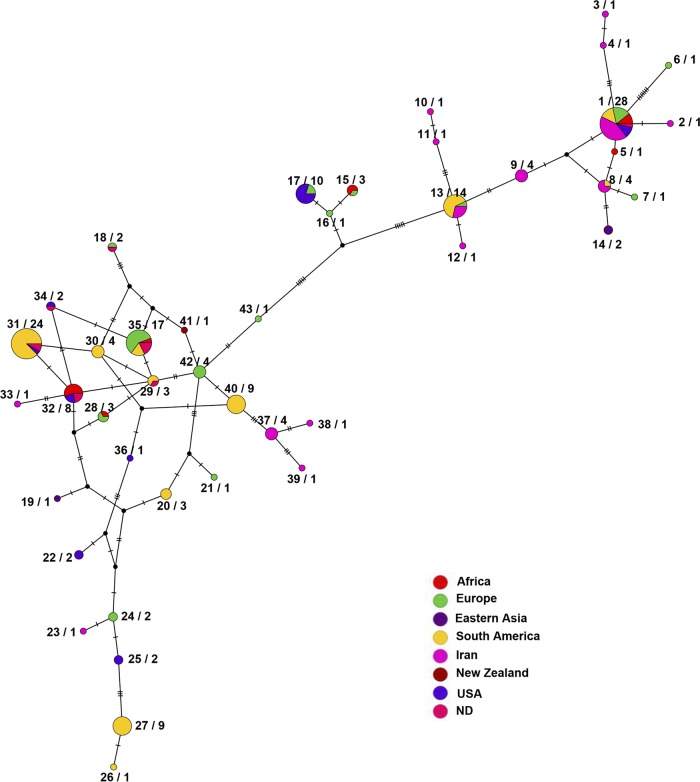
FIG 5.
TCS sequence type network generated using the PopART program from the concatenated sequences of four housekeeping genes (i.e., atpD, gyrB, ppk, and recA) in 184 worldwide Clavibacter michiganensis subsp. michiganensis strains. Each circle represents an ST, while sizes of the circles indicate the relative frequency of strains belonging to a particular ST (43 STs). Hatch marks along the branches indicate the number of mutations. Numbers on the left side of the radix character represent the corresponding ST as shown in Table S1, while the numbers on the right side of the radix character represent the number of strains in each ST. Each color indicates a different geographic location, i.e., Africa (strains from Algeria, Morocco, and South Africa), Eastern Asia (strains from China and Taiwan), Europe (strains from Belgium, France, Hungary, Italy, the Netherlands, Portugal, Slovenia, Spain, and Switzerland), Iran, South America (strains from Brazil, Chile, and Uruguay), New Zealand, and the United States.
On the other hand, eBURST analyses assigned the worldwide C. michiganensis subsp. michiganensis strains into 43 STs consisting of three groups (i.e., I, II, and III) and four singleton sequences. Group I included 163 strains and 34 STs as shown in Fig. S7, while groups II and III included 5 and 10 strains and 3 and 2 STs, respectively (data not shown). Although the founder ST (ST13) and the corresponding subgroups in group I were supported with high bootstrap values, assignment of founder genotype in groups II and III was not supported with high bootstrap values (>40); hence, they were not considered reliable founders. In the group I, ST13 was identified as the founder ST of the population (Fig. S7). The founder ST consisted of 10, 12, and 9 single-, double-, and triple-locus variants, respectively. Three single-locus variants of the ST13 (i.e., ST1, ST8, and ST9) were identified as the subgroup founders, each with its own cluster of linked single-locus variants (Fig. S7). Four out of 14 strains in ST13 were isolated in Iran, while 9 strains were isolated in Uruguay and strain CFBP 7555 was isolated in Slovenia. Results obtained from the eBURST analyses were in congruence with those of maximum likelihood, NeighborNet, and POPArt analyses (Fig. 2, 3, and 5). However, since representative strains of the pathogen from the center of origin of the host plant (i.e., Ecuador, Peru, and Mexico) were lacking in this study, exact estimation of the founder population needs analysis of a larger collection of C. michiganensis subsp. michiganensis strains from South American countries.
DISCUSSION
In this study, we created a distribution map for tomato bacterial canker disease in Iran. Until recently, C. michiganensis subsp. michiganensis was restricted to the northwestern and northern provinces of Iran (16), while our field surveys showed that the pathogen is expanding southward into Fars and Kohgiluyeh-Boyer-Ahmad provinces and eastward into the Razavi Khorasan province in the country (Fig. 1). Annual tomato production in these areas with new bacterial canker incidence reaches more than three million tons, highlighting the potential upcoming negative consequences of disease incidence on the tomato industry in Iran. Furthermore, MLSA-based phylogenetic analyses revealed that C. michiganensis subsp. michiganensis strains isolated in Iran shared the same STs as a set of geographically diverse strains expanding from Western Europe to Northern Africa, as well as the American continent. These data suggest multiple introductions of C. michiganensis subsp. michiganensis into Iran, highlighting the impact of seed-borne primary inoculum in the global distribution of the pathogen. On the other hand, the worldwide population of C. michiganensis subsp. michiganensis could be delineated into two distinct phylogroups based on the date of isolation. All the post-1984 strains were clustered in a monophyletic clade, while the other phylogroup consisted of both pre-1980 and post-1984 strains (Fig. 2). The strains isolated in Iran were scattered through both phylogroups, confirming the variability in C. michiganensis subsp. michiganensis population in terms of sequential introduction to the country.
The global tomato seed market is projected to reach $1.5 billion (U.S. dollars) by 2024. China and India are considered the largest producers of tomato seeds, following the United States. As for Iran, although the proportional data from each of seed provider are not available, most of the cultivated tomato seeds in Iran are imported from China, India, and Western European countries, e.g., the Netherlands (25). International tomato seed trade is thought to be the leading force for the global distribution of the bacterial canker pathogen (4, 9). Tomato species originated from the Peru-Ecuador and Chile-Andean areas (26, 27), and after spreading northward, possibly as a weed, in pre-Columbian times, the tomato was not extensively domesticated until it reached Mexico, and from there the cultivated forms were dispersed throughout the world (28). There is limited information regarding C. michiganensis subsp. michiganensis diversity in the South and Central American countries. The pathogen is present in Argentina, Belize, Brazil, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Grenada, Guadeloupe, Martinique, Mexico, Panama, Peru, and Uruguay (9). However, in most of these countries there is no official report or detailed information regarding the distribution and diversity of the pathogen. Phylogenetic analysis performed in this study included available sequence data from Chile and Uruguay (19, 20). Further field surveys and bacterial isolation from cultivated and wild tomato species in American countries would shed light on the center of diversity and origin of this pathogen.
In the absence of representative C. michiganensis subsp. michiganensis strains from the other American countries (e.g., Peru, Ecuador, and Mexico), the South American origin of the pathogen comes from multiple observations, including the eBURST analyses which suggested ST13 (including several strains isolated in Uruguay) as the founder ST. Uruguayan strains of the pathogen remain the most diverse population in the region. High genetic diversity of the pathogen in Uruguay is due more likely to seed lot transmission from different origins into the country. The use of tomato cultivars in Uruguay has been influenced by regional and global trends, while introduction of C. michiganensis subsp. michiganensis-infected tomato seeds could have happened from the Columbian era during the successive waves of immigration from European countries. In the 1990s, seed trade intensified due to the incorporation of hybrid cultivars that replaced the production and maintenance of seed at the local level. Indeed, Uruguay does not have its own seed production system, and the country possesses a long history of using imported seeds with no phytosanitary control. This reinforces the idea that C. michiganensis subsp. michiganensis strains have been introduced to the country through seed lots imported from different locations every year. Geographics origins of the imported seeds are diverse and have been changing over the years from different countries in Asia, Europe and more recently in South America (i.e., Chile and Peru). The same situation could be observed in Iran, where tomato seed lots are imported to the country from different countries with no sanitary checks for C. michiganensis subsp. michiganensis infection. Further evidence for the potential risk of uninspected tomato seeds comes from the fact that tomato bacterial spot pathogens, i.e., Xanthomonas euvesicatoria pv. euvesicatoria and Xanthomonas euvesicatoria pv. perforans, were reported for the first time in the country in 2013 and 2015, respectively (29, 30). Uninspected trades of crop seeds in Iran have also led to the occurrence of other economically important quarantine diseases, including bacterial wilt of dry beans caused by Curtobacterium flaccumfaciens pv. flaccumfaciens (31), common bacterial blight of beans caused by Xanthomonas axonopodis pv. phaseoli (32, 33), and bacterial leaf spot of alfalfa caused by Xanthomonas euvesicatoria pv. alfalfae (34).
Recombination is recognized as one of the main evolutionary forces driving bacterial diversity and adaptation to a given environment and to hosts they may colonize (35). Congruent results were inferred from the RDP and SplitsTree analyses as well as the NeighborNet network, suggesting that intersubspecies recombination has rarely occurred in the C. michiganensis species complex. Furthermore, recombination does not play a substantial role in the intrasubspecific diversity of C. michiganensis subsp. michiganensis strains. Indeed, multiple introductions of seed-borne inoculum to the areas with bacterial canker incidence raises the genetic diversity of the pathogen in a given area and plays at the same time a pivotal role in the global distribution of the pathogen. Several pieces of evidence for the multiple introductions of C. michiganensis subsp. michiganensis from the New World into the Old World can be inferred from the haplotype arrangements among the global population of the pathogen. For instance, ST1 consisted of strains isolated in different countries, i.e., Algeria, Belgium, Chile, France, Iran, the Netherlands, Uruguay, and the United States. ST1 was identified as a subgroup founder which was descended from the founder ST (ST13) by one single-locus variant, while ST2, ST3, ST4, and ST6 were derived from ST1 each by one single-locus variant (Fig. S7). Nevertheless, more strict evidence using comparative genomics data is needed to determine the exact founder population of the pathogen on the global scale.
Interestingly, ST17 consisted of eight U.S. strains and the type strain of C. michiganensis subsp. michiganensis (CFBP 4999) isolated in Hungary in 1957, which is the only pre-1980 strain in phylogroup I (Fig. 2). Two different interpretations could be inferred in this regard: (i) the type strain of the pathogen (CFBP 4999) was transferred from the United States into Hungary in the mid-20th century, while the strains possessing the same ST are still established in the United States and were recovered from tomato plants from 1998 to 2017; (ii) C. michiganensis subsp. michiganensis strains which were identical to the type strain of the pathogen were transferred from Hungary to the United States and established in the States. However, regarding the fact that ST17 was descended from ST13 by a single-locus variant, the U.S.-Europe movement hypothesis is more likely to have happened in the mid-20th century.
In conclusion, this study provides novel insight into the geographic distribution of bacterial canker disease in Iran, while elucidating the phylogenetic relationships of C. michiganensis subsp. michiganensis strains isolated in the country with the worldwide population of the pathogen. Phylogeographic analyses revealed that multiple founding C. michiganensis subsp. michiganensis inocula have been introduced to Iran, more likely via infected tomato seed lots. Distribution of plant pathogens is one of the consequences of expanded international transportation networks causing plant sanitary issues to become a global challenge. Hence, as far as the quarantine of seed-borne pathogens is concerned, it is important to take a global approach to combat phytosanitary issues. In this study, although a number of country-specific patterns were traced within the worldwide C. michiganensis subsp. michiganensis population based on the ST scheme of the strains, several STs were shown to be shared among the strains isolated from different corners of the globe. As for the Iranian C. michiganensis subsp. michiganensis population, 12 unique STs were found, most of which were single-locus variants of the other STs. This highlights the higher genetic diversity of the bacterial canker pathogen than has so far been described and underlines at the same time the potential threats due to the increasing global distribution of the pathogen. On the other hand, it emphasizes the need to develop new and state-of-the-art detection techniques to prevent the spread of C. michiganensis subsp. michiganensis via infected tomato seeds and plantlets. Concerning the Iranian tomato industry, since the pathogen has not yet been detected in the most important tomato-growing areas on the southern coasts of the country (Fig. 1), stricter field surveys and plant material inspections are recommended to reduce the risk of pathogen entry into the region.
MATERIALS AND METHODS
Bacterial strains.
Comprehensive field surveys were conducted across tomato-, pepper-, and eggplant-growing areas in Iran from 2015 to 2017 through northern, northeastern, northwestern, central, and southern provinces of the country (Fig. 1). This study follows a countrywide quarantine inspection program for the monitoring of seed-borne bacterial diseases of solanaceous crops in Iran (30). Surveying strategies, sample collection, and bacterial isolation were the same as described previously (36). Bacterial strains were resuspended in sterile distilled water (SDW) and stored at 4°C for further use. For long-term storage, the strains were maintained in 15% glycerol at –70°C. All the Gram-positive yellow-pigmented bacterial strains possessing mucoidal colonies on yeast extract-dextrose-calcium carbonate (YDC) agar medium were subjected to a C. michiganensis subsp. michiganensis-specific PCR test using the primer pair PSA-4/PSA-R (37). DNA extraction was carried out using an Expin Combo GP (GeneAll, Tic Tech Centre, Singapore) DNA extraction kit as recommended by the manufacturer. The quality and quantity of DNAs were spectrophotometrically evaluated and adjusted to 50 ng/μl using NanoDrop ND-100 (NanoDrop Technologies, Waltham, MA) for further use. For PCRs, a universal PCR kit—Ampliqon Taq DNA polymerase Master Mix Red (Ampliqon A/S, Odense, Denmark)—was applied according to the manufacturer’s recommendations. For each strain, a 50-μl PCR mixture, including 100 ng total DNA and 2 μl of each primer (10 pmol/μl), was used. The type strain of C. michiganensis subsp. michiganensis (ICMP 2550 = CFBP 4999) was used as a positive control, while a peach-colored tomato-associated nonpathogenic Clavibacter species strain (ICMP 22100 [38]) was used as a negative control.
Pathogenicity tests and host range.
All the bacterial strains (Table 1) were evaluated for their pathogenicities on the host of isolation (tomato; cv. Sunseed 6189) as well as bell pepper (cv. Sereno), chili pepper (cv. Aziz), and black nightshade (Solanum nigrum) plants under greenhouse conditions using the method as described previously (14). Positive- and negative-control plants were inoculated in the same manner using the type strain of C. michiganensis subsp. michiganensis (ICMP 2550) and sterile distilled water, respectively. Koch’s postulates were accomplished by reisolating the inoculated strains on yeast extract-peptone-glucose agar (YPGA) medium from all inoculated plants. Confirmation of the reisolated bacteria was made by determining Gram reaction and colony characteristics on YDC medium, as well as by using the subspecific primer pair PSA-4/PSA-R (37) (Table 4). The pathogenicity tests were conducted twice.
TABLE 4.
Primer pairs used in this study
| Primer name | Sequence (5′–3′) | Size of amplicon (bp) | Annealing temp (°C) | Target | Reference |
|---|---|---|---|---|---|
| PSA-4 | TCATTGGTCAATTCTGTCTCCC | 271 | 58 | Clavibacter michiganensis subsp. michiganensis | 37 |
| PSA-R | TACTGAGATGTTTCACTTCCCC | ||||
| atpD2F | GACATCGAGTTCCCGCAC | 1,104 | 55 | atpD | 23 |
| atpD2R | CGATGATCTCCTGGAGCTCCTTGT | ||||
| 2F | ACCGTCGAGTTCGACTACGA | 977 | 57 | gyrB | 47 |
| 6R | AGSACGATCTTGTGGTA | ||||
| ppkF | GAGAACCTCATCCAGGCCCT | 604 | 60 | ppk | 23 |
| ppkR | CGAGCTTGCAGTGGGTCTTGAG | ||||
| recaF | GACCGCGCTCGCACAGATCGACCG | 724 | 63 | recA | 23 |
| recaR | GCCATCTTGTTCTTGGACGACCTTG | ||||
| 3Fs | GACAACTTCTACTTCAAC | 447 | 57 | rpoB | 48 |
| 4Rs | GTTGTTCTGGTCCATGAAC |
Phylogenetic analyses.
Based on the results of C. michiganensis subsp. michiganensis-specific PCRs and pathogenicity tests, 37 strains were identified as C. michiganensis subsp. michiganensis. To obtain precise and reliable data on the phylogenetic position of the strains, they were subjected to the MLSA/MLST analyses using the sequences of five housekeeping genes (i.e., atpD, gyrB, ppk, recA, and rpoB) as recommended previously (23). These loci were shown to provide robust phylogeny and are sufficient to reliably resolve evolutionary relationships of C. michiganensis strains at an intrasubspecies level. PCR parameters were the same as described above, while the sequences and annealing temperature of primer pairs are shown in Table 4. Purified PCR products were sent to Bioneer Corporation (Daejeon, South Korea) to be sequenced via Sanger sequencing technology.
To determine the phylogenetic position of the strains isolated in this study among the worldwide population of the pathogen, corresponding sequences of five housekeeping genes in a collection of 147 C. michiganensis subsp. michiganensis strains were retrieved from the NCBI GenBank database and included in the phylogenetic analysis (Table S1). Sequences were concatenated following the alphabetic order of the genes, ending in a sequence of 2,110 bp: nucleotides 1 to 455 for atpD, 456 to 923 for gyrB (468 bp), 924 to 1418 for ppk (495 bp), 1419 to 1833 for recA (415 bp), and 1834 to 2110 for rpoB (277 bp). The phylogenetic tree was constructed using the maximum likelihood method with MEGA 6.06 software (39). The model of evolution for maximum likelihood analysis was determined using Modeltest tab in MEGA 6.06. Clavibacter michiganensis subsp. californiensis C55T was used to root the phylogenetic tree, and it was constructed with bootstrapping (1,000 replications). The maximum likelihood method was used to generate phylogenetic trees from the sequences of individual housekeeping genes using the procedure as described above. Furthermore, to evaluate the homogeneity of the C. michiganensis subsp. michiganensis strains included in this study, a phylogenetic tree was constructed using the sequences of five housekeeping genes in all the available Clavibacter species strains.
Genetic diversity.
Nucleotide diversity, haplotype (allele) frequency, haplotype diversity, percentage of polymorphic sites, number of alleles and STs, and the minimum number of fixed recombination events were estimated using DnaSP 5.10 software (40). DnaSP is a multipropose program that allows exhaustive DNA polymorphism analysis. The program implements statistical methods to infer haplotype (allelic) phase and prepares the data for subsequent analyses (41). The class I neutrality indices (Tajima’s D, Fu and Li’s D*, and Fu and Li’s F* statistics) were also calculated for detecting departure from the mutation/drift equilibrium (40). Multiple methods were used to detect recombination events among the strains. Detection of potential recombinant sequences and identification of likely parental sequences were carried out using a set of seven nonparametric detection methods (i.e., RDP, Geneconv, MaxChi, Chimera, BootScan, SiScan, and 3Seq) implemented in Recombination Detection Program (RDP) version 4.80 (42). RDP4 is a program for detecting and analyzing recombination or genomic reassortment signals and for stripping evidence of recombination in a set of aligned DNA sequences. The analyses were performed with default settings for the different detection methods, and the Bonferroni-corrected P value cutoff was set at 0.05. Recombination events were accepted when they were identified by at least four out of seven detection methods (42). Furthermore, a NeighborNet network was constructed to detect and visualize conflicting phylogenetic signals in the data set, and the pairwise homoplasy index (PHI) was calculated using SplitsTree version 4.14.4 (43). SplitsTree4 aims to provide a framework for evolutionary analysis and computes unrooted phylogenetic networks from aligned DNA sequence data (43). These calculations were performed once for 37 Iranian strains and again for the entire data set of 184 worldwide strains using all the individual genes, as well as the concatenated sequences (43). Furthermore, to have a precise country/continent-specific overview on the population structure of the pathogen, the strains isolated in Western Europe (i.e., France, Belgium, Spain, Portugal, and the Netherlands), Chile, Uruguay, and the United States were considered separate populations and subjected to the above-mentioned statistical analyses.
Phylogeographic analyses.
To visualize the relationships between DNA sequences of 37 C. michiganensis subsp. michiganensis strains isolated in Iran with those of 147 strains isolated in different corners of the globe, haplotype networks (allelic networks) were generated for individual housekeeping genes using the TCS algorithm (44) implemented in PopART version 1.7 software (45). The primary function of PopART is the inference and visualization of genetic relationships among intraspecific sequences. In addition, the software allowed us to display the number of mutations among neighbor haplotypes. The geographic origin of 184 C. michiganensis subsp. michiganensis strains (Table S1) was delineated into the haplotype network of each of the housekeeping genes, as well as the concatenated data set as described by Leigh and Bryant (45). The haplotype information was displayed as pie charts at the nodes of the networks showing relative frequency of the strains isolated in a given geographic area. To facilitate the illustration of a differentiable colorful diagram for the haplotype networks, the strains were grouped into seven assemblages based on their area of isolation as follows: Africa (i.e., strains from Algeria, Morocco, and South Africa), Eastern Asia (i.e., strains from China and Taiwan), Europe (i.e., strains from Belgium, France, Hungary, Italy, the Netherlands, Portugal, Slovenia, Spain, and Switzerland), Iran, South America (i.e., strains from Brazil, Chile, and Uruguay), New Zealand, and the United States. The country-specific alleles and shared STs between the countries were inferred from the resulting networks. Furthermore, a hypothetical phylogeographic structure was visualized using the eBURST algorithm to explore the patterns of evolutionary descent according to the ST distribution (46). The eBURST algorithm divides the MLST data set into groups of related strains and clonal complexes, predicts the founding (ancestral) genotype of each clonal complex, and computes the bootstrap support for the founding genotype assignment. Then it displays the most parsimonious patterns of descent of all the strains in each clonal complex from the predicted founder(s) (46). The analyses were performed using the stringent (default) group definition, in which sequence types are included within the same clonal complex only if they share identical alleles at three or four out of the four MLST loci with at least one other allele in the population. As a default setting, 1,000 resamplings were performed for bootstrapping the resulted evolutionary network.
Accession number(s).
The sequenced nucleotides in the 37 C. michiganensis subsp. michiganensis strains isolated in Iran were deposited into the NCBI GenBank database under the following accession numbers: MK568135 to MK568166 for atpD, MK568167 to MK568198 for gyrB, MK568199 to MK568230 for ppk, MK568231 to MK568262 for recA, and MK568263 to MK568294 for rpoB.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Shiraz University (PhD grant to M.A.).
E.O. and M.A. conceived and designed the study, with assistance from S.M.T. M.A. carried out the experiments. E.O. analyzed and interpreted the data with assistance from M.A., H.H., and M.I.S. E.O. prepared the paper, with assistance from M.A., M.V., and M.I.S. All the coauthors revised the final manuscript, and E.O. acted as the corresponding author.
We declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02098-19.
REFERENCES
- 1.FAOSTAT. 2018. The agriculture production domain. http://www.fao.org/faostat/en/?#compare.
- 2.Smith AF. 2001. The tomato in America: early history, culture, and cookery. University of Illinois Press, Champaign, IL. [Google Scholar]
- 3.Jones JB, Zitter TA, Momol TM, Miller SA. 2016. Compendium of tomato diseases and pests, 2nd ed APS Press, St. Paul, MN. [Google Scholar]
- 4.Gitaitis R, Walcott R. 2007. The epidemiology and management of seed borne bacterial diseases. Annu Rev Phytopathol 45:371–397. doi: 10.1146/annurev.phyto.45.062806.094321. [DOI] [PubMed] [Google Scholar]
- 5.Munkvold G. 2009. Seed pathology progress in academia and industry. Annu Rev Phytopathol 47:285–311. doi: 10.1146/annurev-phyto-080508-081916. [DOI] [PubMed] [Google Scholar]
- 6.Schaad NW, Abrams J, Madden LV, Frederick RD, Luster DG, Damsteegt VD, Vidaver AK. 2006. An assessment model for rating high-threat crop pathogens. Phytopathology 96:616–621. doi: 10.1094/PHYTO-96-0616. [DOI] [PubMed] [Google Scholar]
- 7.Eichenlaub R, Gartemann KH. 2011. The Clavibacter michiganensis subspecies: molecular investigation of Gram-positive bacterial plant pathogens. Annu Rev Phytopathol 49:445–464. doi: 10.1146/annurev-phyto-072910-095258. [DOI] [PubMed] [Google Scholar]
- 8.Smith EF. 1910. A new tomato disease of economic importance. Science 31:794–796. [Google Scholar]
- 9.EPPO. 2016. PM 7/42 (3) Clavibacter michiganensis subsp. michiganensis. Bull OEPP/EPPO Bull 46:202–225. doi: 10.1111/epp.12302. [DOI] [Google Scholar]
- 10.Gitaitis RD, Beaver RW, Voloudakis AE. 1991. Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Dis 75:834–838. doi: 10.1094/PD-75-0834. [DOI] [Google Scholar]
- 11.Hausbeck MK, Bell J, Medina-Mora C, Podolsky R, Fulbright DW. 2000. Effect of bactericides on population sizes and spread of Clavibacter michiganensis subsp. michiganensis on tomatoes in the greenhouse and on disease development and crop yield in the field. Phytopathology 90:38–44. doi: 10.1094/PHYTO.2000.90.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Nandi M, Macdonald J, Liu P, Weselowski B, Yuan ZC. 2018. Clavibacter michiganensis ssp. michiganensis: bacterial canker of tomato, molecular interactions and disease management. Mol Plant Pathol 19:2036–2050. doi: 10.1111/mpp.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen Y, Aysan Y, Mirik M, Ozdemir D, Meijer-Dekens F, van der Wolf JM, Visser RGF, van Heusden S. 2018. Genetic characterization of Clavibacter michiganensis subsp. michiganensis population in Turkey. Plant Dis 102:300–308. doi: 10.1094/PDIS-02-17-0276-RE. [DOI] [PubMed] [Google Scholar]
- 14.Osdaghi E, Ansari M, Taghavi SM, Zarei S, Koebnik R, Lamichhane JR. 2018. Pathogenicity and phylogenetic analysis of Clavibacter michiganensis strains associated with tomato plants in Iran. Plant Pathol 674:957–970. doi: 10.1111/ppa.12801. [DOI] [Google Scholar]
- 15.Thapa SP, Pattathil S, Hahn MG, Jacques MA, Gilbertson RL, Coaker G. 2017. Genomic analysis of Clavibacter michiganensis reveals insight into virulence strategies and genetic diversity of a Gram-positive bacterial pathogen. Mol Plant Microbe Interact 30:786–802. doi: 10.1094/MPMI-06-17-0146-R. [DOI] [PubMed] [Google Scholar]
- 16.Nazari F, Niknam GR, Ghasemi A, Taghavi SM, Momeni H, Torabi S. 2007. An investigation on strains of Clavibacter michiganensis subsp. michignensis in north and north west of Iran. J Phytopathol 155:563–569. doi: 10.1111/j.1439-0434.2007.01304.x. [DOI] [Google Scholar]
- 17.Ialacci GM, Bella P, Licciardello G, Strano CP, Eichenlaub R, Gartemann KH, La Rosa R, Catara V. 2016. Clonal populations of Clavibacter michiganensis subsp. michiganensis are responsible for the outbreaks of bacterial canker in greenhouse tomatoes in Italy. Plant Pathol 65:484–495. doi: 10.1111/ppa.12424. [DOI] [Google Scholar]
- 18.Zaluga J, Stragier P, Van Vaerenbergh J, Maes M, De Vos P. 2013. Multilocus variable-number-tandem-repeats analysis (MLVA) distinguishes a clonal complex of Clavibacter michiganensis subsp. michiganensis strains isolated from recent outbreaks of bacterial wilt and canker in Belgium. BMC Microbiol 13:126. doi: 10.1186/1471-2180-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenzuela M, Besoain X, Durand K, Cesbron S, Fuentes S, Claverías F, Jacques MA, Seeger M. 2018. Clavibacter michiganensis subsp. michiganensis strains from central Chile exhibit low genetic diversity and sequence types match strains in other parts of the world. Plant Pathol 67:1944–1954. doi: 10.1111/ppa.12911. [DOI] [Google Scholar]
- 20.Croce V, Pianzzola MJ, Durand K, Gonz Alez-Arcos M, Jacques MA, Siri MI. 2016. Multilocus sequence typing reveals high variability among Clavibacter michiganensis subsp. michiganensis strains affecting tomato crops in Uruguay. Eur J Plant Pathol 144:1–13. doi: 10.1007/s10658-015-0738-0. [DOI] [Google Scholar]
- 21.Mazarei M, Orumchi S, Lora C. 1993. Investigation of bacterial canker of tomato in West Azarbaijan, Iran, p 160 Proceedings of the 11th Iranian Plant Protection Congress. Secretariat of the Congress, University of Guilan, Guilan, Iran. [Google Scholar]
- 22.Gray RR, Salemi M. 2012. Integrative molecular phylogeography in the context of infectious diseases on the human-animal interface. Parasitology 139:1939–1951. doi: 10.1017/S0031182012001102. [DOI] [PubMed] [Google Scholar]
- 23.Jacques MA, Durand K, Orgeur G, Balidas S, Fricot C, Bonneau S, Quillévéré A, Audusseau C, Olivier V, Grimault V, Mathis R. 2012. Phylogenetic analysis and polyphasic characterization of Clavibacter michiganensis strains isolated from tomato seeds reveal that nonpathogenic strains are distinct from C. michiganensis subsp. michiganensis. Appl Environ Microbiol 78:8388–8402. doi: 10.1128/AEM.02158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasuhara-Bell J, Alvarez AM. 2015. Seed-associated subspecies of the genus Clavibacter are clearly distinguishable from Clavibacter michiganensis subsp. michiganensis. Int J Syst Evol Microbiol 65:811–826. doi: 10.1099/ijs.0.000022. [DOI] [PubMed] [Google Scholar]
- 25.Radmehr A. 2017. Agricultural statistics, vol 1 2015–2016 cropping seasons, p 156 Ministry of Jihad-e-Agriculture, Tehran, Iran. (In Persian.) [Google Scholar]
- 26.Peralta IE, Spooner DM, Knapp S. 2008. Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst Bot Monogr 84:1–186. [Google Scholar]
- 27.Chetelat RT, Pertuzé RA, Faúndez L, Graham EB, Jones CM. 2009. Distribution, ecology and reproductive biology of wild tomatoes and related nightshades from the Atacama Desert region of northern Chile. Euphytica 167:77–93. doi: 10.1007/s10681-008-9863-6. [DOI] [Google Scholar]
- 28.Jenkins JA. 1948. The origin of the cultivated tomato (1948). Econ Bot 2:379. doi: 10.1007/BF02859492. [DOI] [Google Scholar]
- 29.Osdaghi E, Taghavi SM, Hamzehzarghani H, Lamichhane JR. 2016. Occurrence and characterization of the bacterial spot pathogen Xanthomonas euvesicatoria on pepper in Iran. J Phytopathol 64:722–734. doi: 10.1111/jph.12493. [DOI] [Google Scholar]
- 30.Osdaghi E, Taghavi SM, Hamzehzarghani H, Fazliarab A, Lamichhane JR. 2017. Monitoring the occurrence of tomato bacterial spot and range of the causal agent Xanthomonas perforans in Iran. Plant Pathol 66:990–1002. doi: 10.1111/ppa.12642. [DOI] [Google Scholar]
- 31.Osdaghi E, Taghavi SM, Hamzehzarghani H, Fazliarab A, Harveson RM, Lamichhane JR. 2016. Occurrence and characterization of a new red-pigmented variant of Curtobacterium flaccumfaciens, the causal agent of bacterial wilt of edible dry beans in Iran. Eur J Plant Pathol 146:129–145. doi: 10.1007/s10658-016-0900-3. [DOI] [Google Scholar]
- 32.Osdaghi E. 2014. Occurrence of common bacterial blight on mungbean (Vigna radiata) in Iran caused by Xanthomonas axonopodis pv. phaseoli. New Dis Rep 30:9. doi: 10.5197/j.2044-0588.2014.030.009. [DOI] [Google Scholar]
- 33.Osdaghi E, Shams-Bakhsh M, Alizadeh A, Lak MR. 2010. Study on common bean seed lots for contamination with Xanthomonas axonopodis pv. phaseoli by BIO-PCR technique. Int J Agric Technol 6:503–513. [Google Scholar]
- 34.Yaripour Z, Taghavi SM, Osdaghi E, Lamichhane JR. 2018. Host range and phylogenetic analysis of Xanthomonas alfalfae causing bacterial leaf spot of alfalfa in Iran. Eur J Plant Pathol 150:267–274. doi: 10.1007/s10658-017-1271-0. [DOI] [Google Scholar]
- 35.Didelot X, Maiden MC. 2010. Impact of recombination on bacterial evolution. Trends Microbiol 18:315–322. doi: 10.1016/j.tim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osdaghi E, Taghavi SM, Hamzehzarghani H, Fazliarab A, Harveson RM, Tegli S, Lamichhane JR. 2018. Epiphytic Curtobacterium flaccumfaciens strains isolated from symptomless solanaceous vegetables are pathogenic on leguminous but not on solanaceous plants. Plant Pathol 67:388–398. doi: 10.1111/ppa.12730. [DOI] [Google Scholar]
- 37.Pastrik KH, Rainey FA. 1999. Identification and differentiation of Clavibacter michiganensis subspecies by polymerase chain reaction-based techniques. J Phytopathol 147:687–693. doi: 10.1046/j.1439-0434.1999.00442.x. [DOI] [Google Scholar]
- 38.Osdaghi E, Portier P, Briand M, Taghouti G, Jacques M-A. 2018. Draft genome sequences of the type strains of three Clavibacter subspecies, and atypical peach-colored strains isolated from tomato. Microbiol Resour Announc 7:e01357-18. doi: 10.1128/MRA.01357-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 41.Rozas J. 2009. DNA sequence polymorphism analysis using DnaSP. Methods Mol Biol 537:337–350. doi: 10.1007/978-1-59745-251-9_17. [DOI] [PubMed] [Google Scholar]
- 42.Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. 2015. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol 1:vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 44.Clement M, Posada D, Crandall KA. 2000. TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 45.Leigh JW, Bryant D. 2015. Data from: popART: full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 46.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. doi: 10.1128/jb.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richert K, Brambilla E, Stackebrandt E. 2005. Development of PCR primers specific for the amplification and direct sequencing of gyrB genes from microbacteria, order Actinomycetales. J Microbiol Methods 60:115–123. doi: 10.1016/j.mimet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Richert K, Brambilla E, Stackebrandt E. 2007. The phylogenetic significance of peptidoglycan types: molecular analysis of the genera Microbacterium and Aureobacterium based upon sequence comparison of gyrB, rpoB, recA and ppk and 16SrRNA genes. Syst Appl Microbiol 30:102–108. doi: 10.1016/j.syapm.2006.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.