Abstract

Practical methods for the preparation of selectively fluorinated compounds are in extremely high demand in nearly every sector of the pharmaceutical and agrochemical industries. Here we provided an account of the recent methodological breakthrough dealing with detrifluoroacetylative in situ generation of cyclic fluoro-enolates and their application for the preparation of various polyfunctional compounds featuring quaternary C–F stereogenic carbon. The reactions include aldol, Mannich, Michael addition reactions, SN2/SN2′ alkylations, and the additions to azo compounds. The detrifluoroacetylative protocol for in situ generation of cyclic fluoro-enolates is operationally simple and scalable and proceeds at ambient temperature. Generally, the stereochemical outcome, controlled by the stoichiometric chiral auxiliary of the chiral catalyst, is synthetically useful, allowing preparation of enantiomerically pure compounds of high pharmaceutical potential.
1. Introduction
Due to the remarkable performance of fluorine-containing drugs and agrochemicals, synthesis of fluoro-organic compounds is currently one of the most rapidly developing areas of organic chemistry.1 The properties of fluorine confer unmatched flexibility among the elements commonly used in drug design, with the majority of the effects falling into three general categories: First, the modified reactivity and metabolism, as in the increased stability toward oxidative degradation and design of mechanism-based enzyme inhibitors. Second, the modified physicochemical properties, as in the control of acidity/basicity, lipophilicity, and membrane permeability. Finally, the fine-tuning of three-dimensional structure and conformations using electrostatic and hydrogen-bonding properties of fluorine atoms.2 Considering the ever-increasing number of fluorinated marketed drugs, it would be reasonable to assume that fluorine editing/fluorine scanning will become a mainstream paradigm in modern drug design.3
However, the progress in the development of fluoro-organic methodology has been rather uneven. For example, enantio-controlled synthesis of compounds possessing carbon–fluorine quaternary stereogenic carbons is still a challenging endeavor. As a reflection of this methodological predicament, the pharmaceuticals featuring quaternary C–F stereogenic centers are exceptionally rare. Motivated by this synthetic deficiency, we joined the efforts of many other research groups4 focusing on the development of modern, advanced procedures for practical preparation of quaternary C–F compounds in enantiomerically pure form. In this mini-review we provide a brief summary of the discovery and development of detrifluoroacetylative in situ generation of cyclic fluoro-enolates and their synthetic applications.
2. Synthesis and Detrifluoroacetylative in Situ Generation of Cyclic Fluoro-enolates
The cyclic ketone-based 1 and cyclic amide-based hydrates 2 (Figure 1) were developed by Han, Soloshonok, and co-workers, and these two trifluorinated ketone hydrates contain a fluoro atom at the α-position, which were used as the precursors for the detrifluoroacetylative in situ generation of cyclic fluoro-enolates.5,6
Figure 1.

Hydrates 1 and 2.
The cyclic-ketone-based hydrate 1 was synthesized via two steps with cyclic ketones 3 as the starting materials (Scheme 1).5 First, the cyclic ketone 3 reacted with ethyl trifluoroacetate in the presence of sodium methoxide in ether at room temperature for 24 h, affording the intermediate 4. Then, intermediate 4 was treated by Selectfluor in acetonitrile at room temperature for 24 h to give the desired hydrates 1 in good chemical yields. The cyclic-amide-based hydrates 2 were prepared via the similar two-step method with oxindoles 6 as the starting materials. Only one modification was needed, and it involved substitution of sodium hydride for sodium methoxide in the first step as the oxindoles containing a less acidic C–H bond.
Scheme 1. Synthesis of Enolate Precursors 1 and 2 and Detrifluoroacetylative in Situ Generation of Enolates 5 and 8.
These hydrates (1 and 2) underwent the C–C bond cleavage in the presence of organic base to give the fluoro-enolates (5 and 8) via the release of trifluoroacetic acid.
3. Aldol Addition Reactions
3.1. Keto-Type Enolates
As shown in Scheme 1, the β-keto-α-fluorohydrates 1 could easily undergo the C–C bond cleavage with the release of trifluoroacetic acid in the presence of organic base, resulting in the fluoro-enolates, potentially versatile nucleophiles in asymmetric organic transformations. The first synthetic application of hydrates 1 was the Cu-catalyzed aldol reaction with aromatic aldehydes, which was reported by the Han group in 2015 (Scheme 2).5 These detrifluoroacetylative aldol reactions were carried out with the combination of copper triflate and bidentate bis(oxazoline) 9 as chiral catalyst and DIPEA as base in THF at 0–20 °C. The reactions showed wide substrate scope and tolerated various substation patterns (34 substrates), providing the corresponding products, bearing C–F quaternary stereogenic centers, in 75–96% yield, 40–98% de, and 67–97% ee. It should be mentioned that the cyclic keto hydrates 1 bearing five-membered, six-membered, seven-membered, and even heterocyclic rings were all well tolerated in this asymmetric transformation with excellent outcomes.
Scheme 2. Asymmetric Aldol Reactions of Hydrates 1 with Aromatic Aldehydes.
Here we would like to focus the readers’ attention on the self-disproportionation of enantiomer (SDE) phenomenon and problems associated with an accurate determination of enantiomeric purity of products obtained in catalytic asymmetric transformations in general7 and fluorine-containing chiral compounds in particular.8 The SDE phenomenon is ubiquitous in nature,9 always taking place when an enantiomerically enriched compound is subjected to any type of physicochemical phase transition.10 Of particular relevance to organic synthesis are the SDE cases via sublimation and achiral chromatography.11 In these asymmetric aldol reactions, a relatively high magnitude of the SDE phenomenon was detected.5 As reported, one aldol product with an initial 84% ee was subjected to the achiral chromatography, which provided an enantiomerically enriched first fractions (88% ee) and correspondingly enantiomerically depleated last fractions (77% ee).
After the synthetically useful results were obtained for the detrifluoroacetylative aldol reactions with aromatic aldehydes, Han, Soloshonok, and co-workers tried to extend this reaction by using usually less reactive aliphatic aldehydes as the starting materials (Scheme 3).12 The reaction conditions were carefully optimized, and the use of Cu(OTf)2/ligand 9 was demonstrated to be the best one. A series of aliphatic aldehydes worked well in this asymmetric aldol reaction and provided the corresponding aldol adducts 11 in good yields and high stereoselectivities. It should be mentioned that the byproduct 12 was observed in almost all the cases with the yields from 5% to >80%. For the selected examples, the aldehydes with low steric hindrance, such as n-butylaldehyde and n-octylaldehyde, good yields of products 11, and less than 10% yields of byproducts 12 were obtained. However, in the case of bulky aldehydes, almost no alodol adducts (11c or 11d) were observed, and >80% yield of byproducts was isolated.
Scheme 3. Asymmetric Aldol Reactions of Hydrates 1 with Aliphatic Aldehydes.
In 2017, the Han group developed a cascade detrifluoroacetylative aldol/intramolecular cyclization/oxidation reaction between hydrates 1 and ortho-phthalaldehyde (Scheme 4).13 Initially, compound 13 was designed as the corresponding product via the reaction sequence of detrifluoroacetylation, aldol, and cyclization in the presence of triethylamine and lithium bromide. However, reversibility of the cyclization of intermediate 13 resulted in poor diastereoselectivity. Thus, direct in situ oxidation with the addition of pyrindium chlorochromate (PCC) was conducted, which successfully provided the desired lactone derivatives in 46–84% yields and 56:44–89:11 diastereoselectivities. In this work, the authors also tried the asymmetric catalytic reactions of hydrates 1 and ortho-phthalaldehyde with Cu(OTf)2 as catalyst and bis(oxazoline) 9 as chiral ligand. However, very poor enantioselectivities were detected.
Scheme 4. Asymmetric Aldol/Cyclization/Oxidation Reactions of Hydrates 1 with ortho-Phthalaldehyde.
As the fluorine-containing β-keto-ester 14 backbone shows the high pharmaceutical potential, the Han group continued their interests to develop the detrifluoroacetylative methodology to assemble these compounds. They found that using 2-formylbenzoate, instead of ortho-phthalaldehyde, could solve the reversibility problem, thus providing an excellent outcome (Scheme 5).14 After careful optimization of reaction conditions, the combination of Cu(OTf)2/ligand 9 was chosen as the best one, and the reaction proceeded smoothly to give the corresponding product 14 in good yields and high diastereo- and enantioselectivities. Furthermore, this reaction showed wide substrate scope, and a total of 25 examples were scrutinized in this system. Several kinds of γ-lactones and δ-lactones containing C–F quaternary stereogenic centers were synthesized under mild reaction conditions.
Scheme 5. Asymmetric Aldol/Cyclization Reactions of Hydrates 1 with 2-Formylbenzoate.
3.2. Indole-Type Enolates
As a continuation of work on the asymmetric aldol reactions of the β-keto-α-fluorohydrates 1, the cyclic-amide-based hydrates 2 were then used as the precursors of the corresponding fluoro amide-enolates 8 in the aldol reaction in 2017.15 Compared with the aldol reaction of hydrates 1,12−14 this reaction used CuI as the metal catalyst and phenyl-substituted bis(oxazoline) 15 as the chiral ligand. Based on the studies of optimization of reaction conditions, the solvent played a key role in the reaction outcome, and the use of a cosolvent THF/i-PrOH (1:1) provided the best results. Under the optimized conditions, a wide range of aldehydes, including aromatic and aliphatic aldehydes, worked very well, affording the desired products 16 in 29–93% yields, 57:43–91:9 diastereoselectivities, and 18–92% enantioselectivities. In particular, one hydrate-containing unprotected N-H moiety also was well tolerated in the system and reacted with benzaldehyde smoothly to give the product in 74% yield (Scheme 6).
Scheme 6. Asymmetric Aldol Reactions of Hydrates 2.
4. Mannich Addition Reactions
4.1. Keto-Type Enolates
Our previous experience with chiral N-sulfinylimines16 was exceptionally positive in terms of the synthetic versatility and generally observed stereochemical outcome in addition reactions with various C-nucleophiles,17 including acyclic fluoro-enolates.18
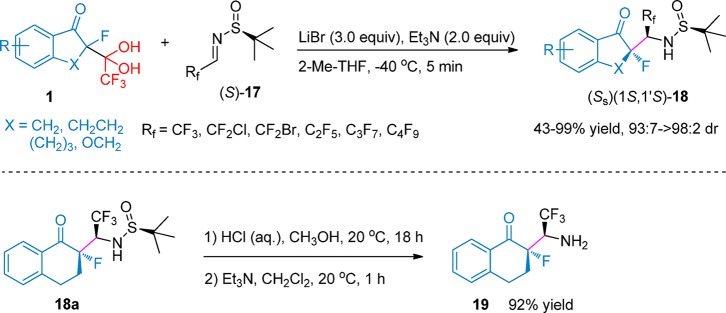
Thus, these fluorohydrates 1 were studied as nucleophiles for the Mannich reactions with chiral N-sulfinylimines. It was found that the asymmetric Mannich additions did happen and provided the targeted C–F quaternary α-fluoroalkyl-β-keto-amines (Scheme 7).19 The optimization studies of the asymmetric Mannich reaction showed that it could be conducted under very operationally simple conditions with the addition of triethylamine and lithium bromide at low temperature (−40 °C). It should be mentioned that these reactions were completed within 5 min and afforded the corresponding products (S)(1S,1’S)-18 in excellent yields and high diastereoselectivities (43–99% yield, 93:7–>98:2 dr). For the reactions of chiral CF3-sulfinylimine, several hydrates 1 bearing electron-withdrawing or electron-donating group substituted aromatic rings worked very well under optimized conditions. Besides CF3-sulfinylimine, other sulfinylimines 17 containing CF2Cl, CF2Br, C2F5, C3F7, and C4F9 have also been tried in this system. These reactions also could be completed within 5 min, affording the desired products with a similarly high stereochemical outcome.
Scheme 7. Asymmetric Mannich Reactions of Hydrates 1 and Fluoroalkyl Imines.
The N-protecting group was easily removed in the presence of hydrochloric acid and then was treated by triethylamine in dichloromethane, which provided the free chiral α-trifluoromethyl-α-fluoro-β-keto-amine in 92% yield.
4.2. Indole-Type Enolates
After successful results obtained from the asymmetric detrifluoroacetylative Mannich reactions of hydrates 1 and fluoroalkyl imines, hydrates 2 were used as nucleophiles for this Mannich reaction (Scheme 8).6 It was found that the reactions between hydrates 2 and imine 17 could be carried out under similar conditions,19 being completed within 5 min or less. The reactions showed wide substrate scope, and all of the 32 examples bearing various types of substituents were well tolerated, affording the corresponding products in excellent and high diastereoselectivities (79–97% yield, 92:8–>98:2 dr). In particular, only one diastereomer was observed for the cases of CF2Cl-, CF2Br-, C2F5-, C3F7-, and C4F9-containing imines. The sulfinyl group in the products can be conveniently removed under the acidic conditions. When the (R)-imine was used as the starting material, the product with opposite absolute configuration (3R,2′R)-20a was obtained with the same level of chemical and stereochemical outcome.
Scheme 8. Asymmetric Mannich Reactions of Hydrates 2 and Fluoroalkyl Imines.
The less electrophilic, nonfluorinated imines 21 can also be used as Mannich acceptors in the addition reaction with hydrates 2 (Scheme 9).20 These transformations took a bit longer time for completion (10 min), being conducted in the presence of triethylamine and lithium bromide, resulting in the corresponding products 22 in good to excellent yields (64–96%). Several types of imines, bearing aromatic, alkyl, alkenyl, and alkynyl groups, worked very well in this detrifluoroacetylative Mannich reaction and afforded only one diastereomer for all the cases (all >98:2 dr). It should be mentioned that hydrates 2 bearing N-H, N-Me, N-allyl, and even N-Ph moieties were all well tolerated in this reaction. These reactions were shown to proceed via the chelated transition states involving the Li coordination to the S–O oxygen.
Scheme 9. Asymmetric Mannich Reactions of Hydrates 2 and Nonfluorinated Imines.
5. Michael Additions
After the development of aldol and Mannich reactions for these two types of enolate precursors, the Han and Soloshonok group turned their attention to detrifluoroacetylative Michael addition reactions. Initially, they used various types of α,β-unsaturated carbonyl derivatives, including quite reactive N-(enoyl)oxazolidinones, as Michael acceptors for the in situ generated fluoro-enolates. However, no positive results were obtained in these reactions.21 Then, (ethene-1,1-diyldisulfonyl)dibenzene (23) was used as a Michael acceptor to react with hydrates 1 under the Cu-catalyzed conditions.5 After careful scan of the chiral ligands, (1S,2S)-1,2-diphenylethane-1,2-diamine 24, bearing a sterically bulky anthracenyl group, was demonstrated to be the best in catalyzing the reaction with 2-fluoro-2-(2,2,2-trifluoro-1,1-dihydroxyethyl)-3,4-dihydronaphthalen-1(2H)-one (1a). The addition proceeded smoothly to give the desired product in 99% yield and 93% ee. The substrate structural generality of hydrates 1 was examined, showing that different substituents on the aromatic ring had almost no effect on the reaction outcome, resulting in 86–99% yields and 60–96% enantioselectivities. This method provides an easy access to γ-sulfonyl-α-fluoroketones containing quaternary C–F stereogenic carbon centers 25 (Scheme 10).
Scheme 10. Asymmetric Michael Reactions of Hydrates 1.
In 2019, Han and coauthors extended this detrifluoroacetylative Michael reaction and used the indole-based hydrates 2 as the nucleophiles instead of ketone-based hydrates 1.22 The reaction was conducted under similar conditions, using the combination of Cu(OTf)2/ligand 24 as a catalyst and DIPEA as a base in THF at −20 °C. These reactions afforded the corresponding products in excellent yields and high enantioselectivities.
6. Alkylation Reactions
In 2017, Han, Soloshonok, and coauthors further explored the chemistry of detrifluoroacetylative-generated fluoro-enolates to the alkylation reactions. They found that hydrates 2 could react with Morita–Baylis–Hillman carbonates 26 in the presence of organic base and lithium bromide at room temperature (Scheme 11).23 Different from the previous reports,5,6 1,1,3,3-tetramethyl guanidine (TMG) was found to be the best base in catalyzing the reaction which afforded the corresponding products 27 in excellent yields and stereoselectivities (80–95% yield, 85:15 → 99:1 E/Z). The reaction also showed a wide substrate scope, and a variety of substitution patterns on hydrates 2 and Morita–Baylis–Hillman carbonates 26 were all well tolerated in this detrifluoroacetylative protocol.
Scheme 11. SN2′ Alkylation Reactions of Hydrates 2.
As one can expect, the nucleophilic substitution reaction could take place at two positions (Figure 2). Notably, this reaction proceeded in a highly chemoselective manner resulting in the SN2′ products 27, and almost no SN2 products were detected.
Figure 2.

Possible reaction pathways in the alkylation reaction.
Then, the authors continued their studies to find an appropriate condition to obtain the corresponding SN2 products 29. It was found that the reaction selectively gave the SN2 products with the Pd2(dba)3/SKP-type ligand 28 as a catalyst, TMG as a base, and 1-propanol as a solvent at room temperature for 12 h (Scheme 12).24 The reaction proceeded smoothly, and the examined 24 variously substituted substrates all showed excellent chemoselectivity with more than 10:1 ratio of 29:27.
Scheme 12. SN2 Alkylation Reactions of Hydrates 2.
Importantly, the hydrates 2 were shown to have clear synthetic advantage over 3-fluoro-1-methylindolin-2-one (30) as the fluoro-enolate precursor in the synthesis of fluorine-containing compounds bearing a C–F quaternary stereogenic center. Thus, under the same reaction conditions, substrate 30 gave lower yield and enantioselectivity of the corresponding alkylation product 29a (60% yield and 63% ee) (Scheme 13).
Scheme 13. SN2 Alkylation Reactions of 30.
7. Reactions with Azo-Compounds
An unusual detrifluoroacetylative reaction between hydrates 2 and diethyl azodicarboxylate was reported in 2017. Under the typical detrifluoroacetylative reaction conditions, the in situ generated 3-fluoroindolin-2-one enolates derived from hydrates 2 reacted with diethyl azodicarboxylates to afford isatin hydrazone derivatives (Scheme 14).25 Several hydrates, bearing various substituents on the aromatic ring, were tried in this reaction, resulting in 58–81% yield of products 31.
Scheme 14. Reactions of Hydrates 2 and 30.
It is interesting that the loss of the C–F bond in products 31 was obtained instead of the regular addition process. A plausible mechanistic rationale is provided in Scheme 15. It is assumed that the Michael addition of enolate to azodicarboxylates gives intermediate 32, which exists in equilibrium with more stable intermediate 33. The latter undergoes intramolecular substitution with the C–F bond cleavage affording intermediate 34. Finally, the nucleophilic attack on the carbonyl group of the ester and ring opening give the final product 31.
Scheme 15. Proposed Mechanism.
8. Conclusions
The data discussed in this mini-review on the discovery and development of detrifluoroacetylative generation of cyclic fluoro-enolates underscore the methodological potential of these intermediates for the preparation of structurally complex compounds bearing quaternary stereogenic C–F centers. The reaction-type chemistry for these in situ generated enolates is shown on the examples of asymmetric aldol, Mannich, Michael additions, and alkylation reactions. It is particularly noteworthy that the detrifluoroacetylative conditions are compatible with catalytic enantioselective transformations, requiring formation, also in situ, of the corresponding catalytic species. The most attractive features of this approach, such as the operational convenience, substrate generality, and, in most cases, excellent stereochemical outcome bode well for its widespread application for the preparation of C–F containing biologically relevant compounds.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21761132021) and IKERBASQUE, Basque Foundation for Science.
Biographies
Jiang Liu obtained his B.Sc. from Nanjing Forestry University in 2019. He is currently a graduate student in the research group of Professor Han at Nanjing Forestry University.
Ziyi Li received his B.Sc. from Nanjing Forestry University in 2019. He is currently a graduate student in the research group of Professor Han at Nanjing Forestry University.
Haibo Mei obtained his B.Sc. in 2009 and Ph.D. in 2014 in organic chemistry from Nanjing University. Then he joined Nanjing University as a research fellow in the area of asymmetric synthesis. In 2018, he moved to Nanjing Forestry University and became an associate professor there. His research focuses on fluorine chemistry and asymmetric synthesis.
Vadim A. Soloshonok graduated from Kiev State University in 1983 and received his Ph.D. in 1987 from the Ukrainian Academy of Sciences. He is the Ikerbasque Research Professor at the University of Basque Country, San Sebastian, Spain. He is currently serving as a member of the advisory editorial board of the Journal of Fluorine Chemistry, Synthesis Editor of Amino Acids, and Editor-in-Chief of Organic Section of the Molecules MDPI, is Past-Chair of the ACS Fluorine Division, and is the author of 350+ research papers. His current research interests are fluorine chemistry, asymmetric synthesis, and self-disproportionation of enantiomers.
Jianlin Han received his Ph.D. in organic Chemistry in 2007 from Nanjing University. He then carried out postdoctoral studies for one year at Texas Tech University. In 2008, he moved to the University of Oklahoma to continue postdoctoral research for nearly one year. In 2009, he took the position of Associate Professor at the Nanjing University. In 2019, he moved to Nanjing Forestry University and became a professor there. His current research interests are organic fluorine chemistry, asymmetric synthesis, and radical methodologies.
The authors declare no competing financial interest.
References
- Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Wang J.; Gu Z.; Wang S.; Zhu W.; Aceña J. L.; Soloshonok V. A.; Izawa K.; Liu H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- Mei H.; Han J. L.; Fustero S.; Medio-Simon M.; Sedgwick D. M.; Santi C.; Ruzziconi R.; Soloshonok V. A. Fluorine-Containing Drugs Approved by the FDA in 2018. Chem. - Eur. J. 2019, 25, 11797–11819. 10.1002/chem.201901840. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Han J. L.; Wang J.; Shibata N.; Sodeoka M.; Soloshonok V. A.; Coelho J. A. S.; Toste F. D. Modern Approaches for Asymmetric Construction of Carbon-Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev. 2018, 118, 3887–3964. 10.1021/acs.chemrev.7b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C.; Wu L.; Han J. L.; Soloshonok V. A.; Pan Y. Assembly of Fluorinated Quaternary Stereogenic Centers through Catalytic Enantioselective Detrifluoroacetylative Aldol Reactions. Angew. Chem., Int. Ed. 2015, 54, 6019–6023. 10.1002/anie.201500908. [DOI] [PubMed] [Google Scholar]
- Xie C.; Zhang L.; Sha W.; Soloshonok V. A.; Han J. L.; Pan Y. Detrifluoroacetylative in Situ Generation of Free 3-Fluoroindolin-2-one-Derived Tertiary Enolates: Design, Synthesis, and Assessment of Reactivity toward Asymmetric Mannich Reactions. Org. Lett. 2016, 18, 3270–3273. 10.1021/acs.orglett.6b01516. [DOI] [PubMed] [Google Scholar]
- Han J.; Kitagawa O.; Wzorek A.; Klika K. D.; Soloshonok V. A. The self-disproportionation of enantiomers (SDE): a menace or an opportunity?. Chem. Sci. 2018, 9, 1718–1739. 10.1039/C7SC05138G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorochinsky A. E.; Aceña J. L.; Soloshonok V. A. Self-Disproportionation of Enantiomers of Chiral, Non-Racemic Fluoroorganic Compounds: Role of Fluorine as Enabling Element. Synthesis 2013, 45, 141–152. 10.1055/s-0032-1316812. [DOI] [Google Scholar]
- Han J.; Soloshonok V. A.; Klika K. D.; Drabowicz J.; Wzorek A. Chiral sulfoxides: advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 2018, 47, 1307–1350. 10.1039/C6CS00703A. [DOI] [PubMed] [Google Scholar]
- Han J.; Nelson D. J.; Sorochinsky A. E.; Soloshonok V. A. Self-Disproportionation of Enantiomers via Sublimation; New and Truly Green Dimension in Optical Purification. Curr. Org. Synth. 2011, 8, 310–317. 10.2174/157017911794697303. [DOI] [Google Scholar]
- Zhang L.; Zhang W.; Sha W.; Mei H.; Han J. L.; Soloshonok V. A. Detrifluoroacetylative generation and chemistry of fluorine containing tertiary enolates. J. Fluorine Chem. 2017, 198, 2–9. 10.1016/j.jfluchem.2016.12.007. [DOI] [Google Scholar]
- Zhang L.; Xie C.; Dai Y.; Mei H.; Han J. L.; Soloshonok V. A.; Pan Y. Catalytic asymmetric detrifluoroacetylative aldol reactions of aliphatic aldehydes for construction of C-F quaternary stereogenic centers. J. Fluorine Chem. 2016, 184, 28–35. 10.1016/j.jfluchem.2016.01.008. [DOI] [Google Scholar]
- Sha W.; Zhang L.; Wu X.; Mei H.; Han J. L.; Soloshonok V. A.; Pan Y. Detrifluoroacetylative cascade reactions of bicyclic fluoro-enolates with ortho-phthalaldehyde: Aspects of reactivity, diastereo- and enantioselectivity. J. Fluorine Chem. 2017, 196, 14–23. 10.1016/j.jfluchem.2016.06.008. [DOI] [Google Scholar]
- Sha W.; Zhang L.; Zhang W.; Mei H.; Soloshonok V. A.; Han J. L.; Pan Y. Catalytic cascade aldol-cyclization of tertiary ketone enolates for enantioselective synthesis of keto-esters with a C-F quaternary stereogenic center. Org. Biomol. Chem. 2016, 14, 7295–7303. 10.1039/C6OB01152G. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang W.; Mei H.; Han J.; Soloshonok V. A.; Pan Y. Catalytic asymmetric aldol addition reactions of 3-fluoro-indolinone derived enolates. Org. Biomol. Chem. 2017, 15, 311–315. 10.1039/C6OB02454H. [DOI] [PubMed] [Google Scholar]
- Mei H.; Xie C.; Wu L.; Soloshonok V. A.; Han J.; Pan Y. Asymmetric Mannich reactions of imidazo[2,1-b]-thiazole-derived nucleophiles with (SS)-N-tertbutanesulfinyl-(3,3,3)-trifluoroacetaldimine. Org. Biomol. Chem. 2013, 11, 8018–8021. 10.1039/c3ob41785a. [DOI] [PubMed] [Google Scholar]
- Xie C.; Mei H.; Wu L.; Soloshonok V. A.; Han J.; Pan Y. LDA-promoted asymmetric synthesis of ß-trifluoromethyl-ß-amino indanone derivatives with virtually complete stereochemical outcome. RSC Adv. 2014, 4, 4763–4768. 10.1039/C3RA45773G. [DOI] [Google Scholar]
- Xie C.; Wu L.; Mei H.; Soloshonok V. A.; Han J.; Pan Y. Generalized access to fluorinated β-keto amino compounds through asymmetric additions of α,α-difluoroenolates to CF3-sulfinylimine. Org. Biomol. Chem. 2014, 12, 7836–7843. 10.1039/C4OB01575D. [DOI] [PubMed] [Google Scholar]
- Xie C.; Dai Y.; Mei H.; Han J.; Soloshonok V. A.; Pan Y. Asymmetric synthesis of quaternary α-fluoro-β-keto-amines via detrifluoroacetylative Mannich reactions. Chem. Commun. 2015, 51, 9149–9152. 10.1039/C5CC02256H. [DOI] [PubMed] [Google Scholar]
- Xie C.; Sha W.; Zhu Y.; Han J. L.; Soloshonok V. A.; Pan Y. Asymmetric synthesis of C-F quaternary α-fluoro-β-amino-indolin-2-ones via Mannich addition reactions; facets of reactivity, structural generality and stereochemical outcome. RSC Adv. 2017, 7, 5679–5683. 10.1039/C6RA27710A. [DOI] [Google Scholar]
- Zhu Y.; Zhang W.; Mei H.; Han J. L.; Soloshonok V. A.; Pan Y. Catalytic Enantioselective Michael Addition Reactions of Tertiary Enolates Generated by Detrifluoroacetylation. Chem. - Eur. J. 2017, 23, 11221–11225. 10.1002/chem.201702091. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Ni Y.; Soloshonok V. A.; Han J. L.; Pan Y. Catalytic enantioselective Michael addition reactions between in situ detrifluoroacetylatively generated 3-fluorooxindole-derived enolates and 1-(1-(phenylsulfonyl)vinylsulfonyl)benzene. J. Fluorine Chem. 2019, 219, 32–38. 10.1016/j.jfluchem.2018.12.009. [DOI] [Google Scholar]
- Zhu Y.; Mei H.; Han J. L.; Soloshonok V. A.; Zhou J.; Pan Y. Chemoselective SN2’ Allylations of Detrifluoroacetylatively In Situ Generated 3-Fluoroindolin-2-one-Derived Tertiary Enolates with Morita-Baylis-Hillman Carbonates. J. Org. Chem. 2017, 82, 13663–13670. 10.1021/acs.joc.7b02409. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Mao Y.; Mei H.; Pan Y.; Han J. L.; Soloshonok V. A.; Hayashi T. Palladium-Catalyzed Asymmetric Allylic Alkylations of Colby Pro-Enolates with MBH Carbonates: Enantioselective Access to Quaternary C-F Oxindoles. Chem. - Eur. J. 2018, 24, 8994–8998. 10.1002/chem.201801670. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Zhang W.; Mei H.; Han J. L.; Soloshonok V. A.; Pan Y. Unusual reactivity of fluoro-enolates with dialkyl azodicarboxylates: Synthesis of isatin-hydrazones. J. Fluorine Chem. 2017, 203, 99–103. 10.1016/j.jfluchem.2017.06.010. [DOI] [Google Scholar]