Abstract
Background
To study the effects of different positive end expiratory pressure (PEEP) on blood pressure and heart function in elderly patients with hypertension.
Methods
Forty elderly patients above 65 years of age treated with mechanical ventilation were divided into two groups: a control group of non-hypertensive subjects (n = 18) and a hypertension group (n = 22) patients with essential hypertension. Changes in blood pressure, central venous pressure (CVP), central venous oxygen saturation (ScvO2), heart rate, and airway pressure were determined in response to different selected PEEP levels of 0, 2, 4, 6, 8, 10 and 12 cm H2O under SIMV(PC) + PSV mode throughout the study.
Results
In both groups, the increase in PEEP led to an increase in CVP and airway pressure. When PEEP was above 4 cm H2O in the hypertension group, a decrease in blood pressure and ScvO2, and an increase of heart rate were observed. These results indicated that cardiac output significantly decreased.
Conclusion
High levels of PEEP can significantly influence changes in blood pressure and heart function in elderly patients with hypertension.
Trial registration
This trial was retrospectively registered, The Chinese trial registration number is ChiCTR-ROC-17012873. The date of registration is 10-2-2017.
Keywords: Positive end expiratory pressure, Hemodynamics, Elderly patients with hypertension, Mechanical ventilation
Background
The number of elderly patients with essential hypertension is increasing annually at a rapid rate worldwide [1, 2]. Recent data from the National Health and Nutrition Examination Survey indicate that 70% of older adults have hypertension [3]. Elastic arteries of elderly hypertensives dilate and stiffen, which decreases arterial capacitance and limited recoil and is thus unable to accommodate the changes that occur during the cardiac cycle [4, 5]. Therefore, if cardiac output decreases, the blood pressure drops immediately in elderly hypertensives.
Multiple methods exist for the measurement of cardiac output. However, accurate measurement of cardiac output, such as that obtained using a SwanGanz catheter and cardiac color ultrasound requires advanced instrumentation. Another important factor for hemodynamic stability is the balance between oxygen delivery (DO2) and consumption (VO2). The most often used bedside parameter to assess the relationship between oxygen supply and consumption is central venous oxygen saturation (ScvO2) [6, 7]. ScvO2 decreases under low oxygen supply conditions as in occurs during low cardiac output, decreased hemoglobin, decreased arterial oxygen saturation, or in cases of increased oxygen demand that occurs with shivering, fever, agitation, and hypermetabolic state [8, 9]. Therefore, if hemoglobin, arterial oxygen saturation and oxygen demand are relatively invariant, ScvO2 is associated with cardiac output.
Elderly hypertensive patients have poor cardiorespiratory function and often require ventilator assisted breathing [10]. As far as we know, the elasticity of arterial vessels in elderly patients with hypertension is diminished while the adjustment function of arterial vessels is poor; therefore, hemodynamics is susceptible to external factors such as positive pressure ventilation [11]. If positive end expiratory pressure (PEEP) is improperly used, it may have negative effects on blood dynamics and oxygen metabolism in elderly hypertensive patients, and may even cause multiple organ dysfunction and death [12].
This study aimed to explore the effects of different PEEP levels on blood pressure and heart function in elderly patients with hypertension, and to minimize the adverse effects of PEEP on hemodynamics.
Methods
Study design
For all selected patients, the SIMV (PC) + PSV ventilator mode (Vela ventilator, American) was utilized to assist breathing. The tidal volume was controlled (10 ml/kg) to maintain pulmonary oxygenation and promote carbon dioxide exhalation, as well as the inhalation time (1.2 s), and respiratory frequency (18 times/min). Oxygen concentration maintenance at 40% made the peripheral oxygen saturation more than 95%. All patients underwent radial artery puncture and subclavian venous catheter. Phlegm was removed from the airway, and subjects were placed at a comfortable 30° half supine position with infusion speed regulated at 40–50 drops/min. Midazolam 0.05 mg/kg/h was used to limit spontaneous breathing. Tidal volume was maintained relatively constant, and changes in blood pressure, central venous pressure (CVP), ScvO2, heart rate (HR), and airway pressure were determined in response to different selected PEEP levels of 0, 2, 4, 6, 8, 10 and 12 cm H2O. Each PEEP level exposure lasted 2 h. The researchers were blinded to patient treatment conditions. If a significant decrease in blood pressure and an obvious decrease of peripheral oxygen saturation (SpO2) were noted, the study was terminated to ensure patient safety. No patients withdrew from the study.
Participants
This prospective, observational study was conducted between January 2015 and January 2017 at the Intensive Care Unit in Fujian Medical University Union Hospital. Chinese clinical trial registration number is ChiCTR-ROC-17012873. Forty elderly patients over 65 years of age with respiratory failure undergoing mechanical ventilation treatment were divided into two groups: a control group of non-hypertension subjects (n = 18) and a hypertension group of patients (n = 22) with essential hypertension (Fig. 1). Respiratory failure in these 40 elderly patients resulted from pneumonia. All patients or their legally authorized representatives provided written informed consent. The study was approved by the Ethics Committee of Union Hospital Affiliated to Fujian Medical University. We excluded patients with secondary hypertension, shock, arrhythmia, and severe heart failure (NYHA cardiac function grade III - IV).
Fig. 1.
Consort Flow Diagram
Lung function and hemodynamic measurements
To ensure that the subclavian vein catheter (Arrow, American) was located in the superior vena cava, the position of the catheter was confirmed by bedside X-ray or CT. The subclavian vein catheter was connected to a multi-parameter cardioelectric monitor (GEcicpro/8000i, American) to measure CVP. Extraction of 1.5 ml central venous catheter blood was used by blood gas analysis (i-stat300 blood gas analyzer, Abbott, United States) for ScvO2 monitoring. In addition, a multi-parameter cardioelectric monitor continuously assessed radial blood pressure, HR, and SpO2. A ventilator continuously monitored peak airway pressure (Ppeak), mean airway pressure (Pmean), tidal volume (VT) and minute ventilation volume (MV).
Evaluation criteria
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg under sufficient sedation during mechanical ventilation without man-machine confrontation and/or the self-reported use of antihypertensive medication in the previous 2 weeks [13].
Statistics analysis
We determined the sample size for the study using statistical analysis software, NCSS PASS. All data were presented as mean ± SD or median (interquartile range) as indicated by data distribution tested by the Shapiro-Wilk test. To compare data between groups, a t test, analysis of variance (ANOVA), Mann–Whitney test, or Kruskal–Wallis test was utilized depending on their distribution and number of variables. Correlation was analyzed by Pearson correlation analysis. Confounding factors were controlled by multiple linear regression analysis. Statistical significance was considered at P < 0.05.
Results
Participants characteristics
Descriptive characteristics are summarized in Table 1. SBP, DBP, mean arterial blood pressure (MABP), serum creatinine (Scr) and uric acid (UA) levels of the hypertension group were elevated compared to the control group (P < 0.05). There were no statistically significant differences in age, triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterin (LDL-C), fasting plasma glucose (FPG), hemoglobin (HB), or body temperature (T) between the two groups (P > 0.05). When PEEP was 0 cm H2O, there was also no statistically significant difference in CVP between the groups (P > 0.05).
Table 1.
Baseline characteristics. Data are shown as mean ± SD or median (interquartile)
| Control group (n = 18) | Hypertension group (n = 22) | |
|---|---|---|
| Sex M/F (n) | 12/6 | 14/8 |
| Age (years) | 78.1 ± 9.2 | 74.5 ± 6.3 |
| SBP (mmHg) | 119.1 ± 9.9 | 151.6 ± 13.2* |
| DBP (mmHg) | 67.4 ± 5.3 | 82.0 ± 12.0* |
| MABP(mmHg) | 84.7 ± 3.7 | 105.1 ± 10.1* |
| Scr (umol/L) | 68.0 (48.5,83.3) | 110.4 (57.3202.3)* |
| UA (umol/L) | 163.0 (81.5,311.3) | 434.5 (312.0,509.0)* |
| TG (mmol/L) | 1.1 (0.8,1.7) | 1.7 (1.0,3.0) |
| TC (mmol/L) | 3.6 ± 1.2 | 3.8 ± 0.9 |
| LDL-C (mmol/L) | 2.2 ± 1.0 | 2.2 ± 0.8 |
| FPG (mmol/L) | 7.6 (5.4,8.7) | 6.5 (4.8,9.1) |
| HB (g/L) | 102.1 ± 26.0 | 91.8 ± 21.6 |
| T (°C) | 37.1 ± 0.4 | 36.9 ± 0.5 |
| CVP(mmHg, PEEP 0 cm H2O) | 7.2 ± 1.2 | 8.1 ± 3.3 |
SBP Systolic blood pressure, DBP Diastolic blood pressure, MABP Mean arterial blood pressure, Scr Serum creatinine, UA Uric acid, TG Triglyceride, TC Total cholesterol, LDL-C Low density lipoprotein cholesterin, FPG Fasting plasma glucose, HB Hemoglobin, T Body temperature, CVP Central venous pressure, PEEP Positive end expiratory pressure
*: P<0.05
Results per study group
Effect of different PEEP levels on blood pressure
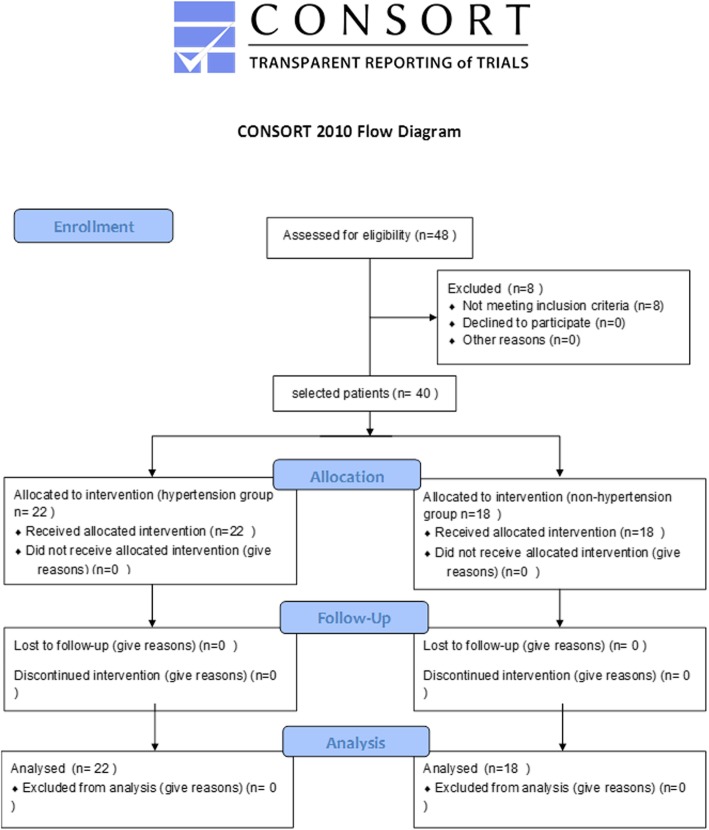
In the control group, SBP, DBP, and MABP were unaffected by PEEP (P > 0.05). In the hypertension group, when PEEP was below 4 cm H2O, blood pressure was also unaffected by PEEP (P > 0.05). However, when PEEP was above 4 cm H2O, this increase in PEEP led to an decrease in blood pressure (P < 0.05) (Table 2, Fig. 2). Moreover, in the hypertension group, when PEEP above 4 cm H2O, PEEP was negatively correlated with SBP (r = − 0.390, P < 0.001), DBP (r = − 0.266, P = 0.005), and MABP (r = − 0.374, P < 0.001). Using multiple linear regression analysis to control confounding factors, the results showed that with the increase of controlling factors, the negative correlation between PEEP and blood pressure still existed (Table 3).
Table 2.
Effect of different PEEP levels on hemodynamics. Data are shown as mean ± SD
| PEEP(cmH2O) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| SBP (mmHg) | 0 | 119.1 ± 9.9 | 120.2 ± 8.4 | 119.9 ± 9.2 | 121.3 ± 6.6 | 121.9 ± 9.4 | 120.8 ± 9.2 | 122.6 ± 9.1 |
| 1 | 151.6 ± 13.2 | 152.6 ± 9.4 | 154.2 ± 10.0 | 146.1 ± 14.2 | 142.8 ± 14.4 | 139.8 ± 15.3bc | 137.1 ± 14.1abc | |
| DBP (mmHg) | 0 | 67.4 ± 5.3 | 68.6 ± 6.7 | 67.9 ± 5.7 | 69.6 ± 6.2 | 70.5 ± 6.9 | 69.7 ± 7.5 | 69.6 ± 8.7 |
| 1 | 82.0 ± 12.0 | 84.1 ± 11.4 | 82.5 ± 12.9 | 77.4 ± 13.1 | 76.0 ± 12.4 | 73.3 ± 13.8 | 72.2 ± 11.7b | |
| MABP (mmHg) | 0 | 84.7 ± 3.7 | 85.7 ± 4.3 | 85.6 ± 4.3 | 86.8 ± 4.9 | 87.6 ± 4.9 | 86.7 ± 4.6 | 87.1 ± 5.4 |
| 1 | 105.1 ± 10.1 | 107.6 ± 7.9 | 106.0 ± 9.7 | 100.2 ± 10.9 | 98.1 ± 10.4b | 95.3 ± 11.5bc | 93.8 ± 10.0abc | |
| CVP (mmHg) | 0 | 7.2 ± 1.2 | 7.4 ± 1.2 | 7.6 ± 1.2 | 7.8 ± 1.3 | 8.0 ± 1.2 | 8.2 ± 1.3 | 8.4 ± 1.3 |
| 1 | 8.1 ± 3.3 | 8.4 ± 3.1 | 9.0 ± 4.2 | 8.7 ± 2.6 | 9.0 ± 2.9 | 9.1 ± 2.8 | 9.3 ± 2.9 | |
| ScvO2 (%) | 0 | 68.4 ± 7.3 | 68.6 ± 5.8 | 69.3 ± 6.0 | 69.9 ± 7.4 | 71.3 ± 5.7 | 70.4 ± 8.0 | 72.0 ± 6.6 |
| 1 | 67.3 ± 8.5 | 69.3 ± 8.9 | 70.8 ± 7.6 | 67.8 ± 5.7 | 65.3 ± 6.4 | 64.0 ± 5.7c | 61.9 ± 6.2cd | |
| HR (bpm) | 0 | 82.6 ± 12.5 | 82.9 ± 12.7 | 83.3 ± 12.9 | 83.8 ± 13.5 | 82.1 ± 13.2 | 82.6 ± 13.3 | 82.7 ± 12.8 |
| 1 | 80.2 ± 12.5 | 78.5 ± 12.2 | 76.9 ± 10.4 | 80.9 ± 12.7 | 82.7 ± 10.7 | 85.1 ± 9.8 | 88.2 ± 11.5c | |
| Ppeak (cmH2O) | 0 | 20.4 ± 4.9 | 21.5 ± 4.6 | 22.3 ± 4.4 | 23.6 ± 4.5 | 24.6 ± 4.4 | 25.7 ± 4.5a | 27.3 ± 4.8ab |
| 1 | 20.8 ± 4.1 | 21.8 ± 4.3 | 23.3 ± 4.6 | 24.6 ± 5.1 | 26.1 ± 4.9a | 27.4 ± 4.8ab | 29.3 ± 4.7abc | |
| Pmean (cmH2O) | 0 | 10.1 ± 2.1 | 11.1 ± 2.2 | 11.9 ± 2.3 | 13.1 ± 2.5a | 13.9 ± 2.2ab | 15.1 ± 2.4abc | 16.2 ± 2.7abcd |
| 1 | 10.3 ± 2.3 | 11.2 ± 2.0 | 12.3 ± 2.1 | 13.7 ± 2.2ab | 14.7 ± 2.1abc | 16.0 ± 2.2abcd | 17.5 ± 2.2abcde | |
| VT (ml) | 0 | 644.6 ± 95.3 | 649.7 ± 96.8 | 654.0 ± 96.1 | 651.9 ± 94.3 | 642.6 ± 106.2 | 646.3 ± 106.4 | 645.3 ± 107.2 |
| 1 | 584.0 ± 119.1 | 587.0 ± 118.3 | 591.1 ± 114.1 | 589.6 ± 115.4 | 586.9 ± 117.7 | 587.4 ± 116.3 | 585.3 ± 117.3 | |
| MV (L/min) | 0 | 11.7 ± 1.6 | 11.7 ± 1.6 | 11.8 ± 1.6 | 11.8 ± 1.5 | 11.6 ± 1.8 | 11.7 ± 1.8 | 11.7 ± 1.9 |
| 1 | 10.5 ± 2.1 | 10.6 ± 2.1 | 10.8 ± 2.0 | 10.7 ± 2.0 | 10.8 ± 2.1 | 10.9 ± 2.0 | 10.7 ± 2.0 | |
| SpO2 | 0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| (%) | 1 | 99.9 ± 0.5 | 99.9 ± 0.3 | 100.0 ± 0.2 | 100.0 ± 0.2 | 100.0 ± 0.2 | 100.0 ± 0.2 | 99.9 ± 0.5 |
SBP Systolic blood pressure, DBP Diastolic blood pressure, MABP Mean arterial blood pressure, CVP Central venous pressure, ScvO2 Central venous oxygen saturation, HR Heart rate, Ppeak Peak airway pressure, Pmean Mean airway pressure, VT Tidal volume, MV Minute ventilation volume, SpO2 Peripheral oxygen saturation; 0: Control group; 1: Hypertension group;
aP < 0.05, compared with PEEP 2 cm H2O: bP < 0.05, compared with PEEP 4 cm H2O: cP < 0.05, compared with PEEP 6 cm H2O: dP < 0.05, compared with PEEP 8 cm H2O: eP < 0.05. In the same group of blood pressure, compared with PEEP 0 cm H2O: aP < 0.05, compared with PEEP 2 cm H2O: bP < 0.05, compared with PEEP 4 cm H2O: cP < 0.05, compared with PEEP 6 cm H2O: dP < 0.05, compared with PEEP 8 cm H2O: eP < 0.05
Fig. 2.
Effect of different PEEP levels on systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial blood pressure (MABP). a: Effect of different PEEP levels on SBP, b: Effect of different PEEP levels on DBP, c: Effect of different PEEP levels on MABP. Data are shown as mean and standard deviation. In the same group of blood pressure, aindicated significant difference (p < 0.05) compared with PEEP 0 cmH2O. bindicated significant difference compared with PEEP 2 cmH2O. cindicated significant difference compared with PEEP 4 cmH2O
Table 3.
Multiple linear regression analysis of blood pressure and PEEP
| Hypertension group (PEEP≥4cmH2O) | Model1 | Model 2 | Model 3 |
|---|---|---|---|
| SBP | |||
| Beta | −0.417 | − 0.428 | −0.473 |
| P | < 0.001 | < 0.001 | < 0.001 |
| DBP | |||
| Beta | −0.277 | −0.307 | − 0.341 |
| P | 0.004 | 0.002 | 0.001 |
| MABP | |||
| Beta | −0.394 | − 0.423 | −0.467 |
| P | < 0.001 | < 0.001 | < 0.001 |
Model 1: Adjustment for age and gender; Model 2: Adjustment for age, gender, triglyceride, total cholesterol, low density lipoprotein cholesterin, fasting plasma glucose, serum creatinine and uric acid; Model 3: Adjustment for hemoglobin, body temperature, minute ventilation volume and peripheral oxygen saturation on the basis of Model 2
SBP Systolic blood pressure, DBP Diastolic blood pressure, MABP Mean arterial blood pressure
Effect of different PEEP levels on cardiac function
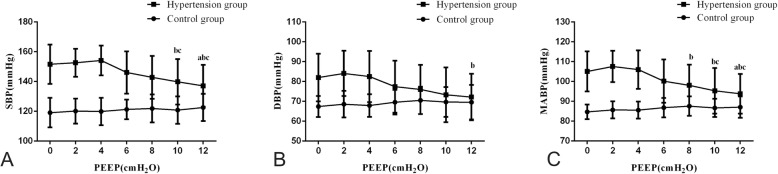
In both groups, an increase in PEEP led to an increase of CVP (Table 2, Fig. 3). Moreover, PEEP was positively correlated with the CVP (r = 0.149, P = 0.013). With an increase in controlling factors, a positive correlation between PEEP and CVP was still observed (Table 4). In the control group, HR and ScvO2 were unaffected by PEEP (P > 0.05). In the hypertension group, when PEEP was less than 4 cm H2O, HR and ScvO2 were also unaffected (P > 0.05). However, when PEEP was above 4 cm H2O, an increase of HR and a decrease of ScvO2 were observed (Table 2, Fig. 4). Furthermore, in the hypertension group, when PEEP was above 4 cm H2O, PEEP was positively correlated with HR (r = 0.333, P < 0.001) and negatively correlated with ScvO2 (r = − 0.441, P < 0.001). Using multiple linear regression analysis to control confounding factors, the correlation between PEEP and HR, ScvO2 still existed (Table 5).
Fig. 3.
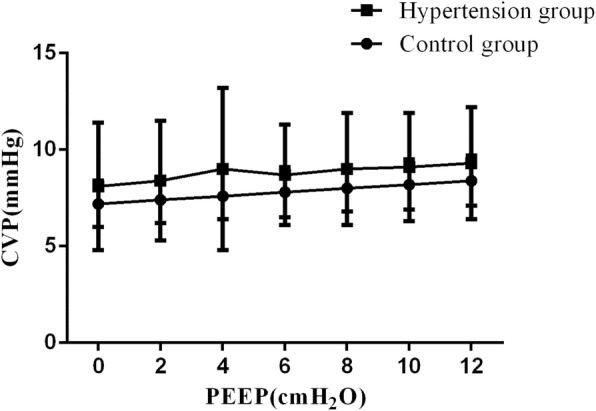
Effect of different PEEP levels on central venous pressure (CVP). Data are shown as mean and standard deviation
Table 4.
Multiple linear regression analysis of CVP and PEEP
| All subjects | Model1 | Model2 | Model3 |
|---|---|---|---|
| CVP | |||
| Beta | 0.150 | 0.152 | 0.174 |
| P | 0.012 | 0.012 | 0.004 |
Model 1: Adjustment for age and gender; Model 2: Adjustment for age, gender, triglyceride, total cholesterol, low density lipoprotein cholesterin, fasting plasma glucose, serum creatinine and uric acid; Model 3: Adjustment for hemoglobin, body temperature, minute ventilation volume and peripheral oxygen saturation on the basis of Model 2
CVP Central venous pressure
Fig. 4.
4 Effect of different PEEP levels on central venous oxygen saturation (ScvO2) and heart rate (HR). a: Effect of different PEEP levels on ScvO2, b: Effect of different PEEP levels on HR. Data are shown as mean and standard deviation. In the same group of blood pressure, cindicated significant difference (p < 0.05) compared with PEEP 4 cmH2O. dindicated significant difference compared with PEEP 6 cmH2O
Table 5.
Multiple linear regression analysis of cardiac function and PEEP
| Hypertension group (PEEP ≥4 cm H2O) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| ScvO2 | |||
| Beta | −0.455 | − 0.552 | −0.592 |
| P | < 0.001 | < 0.001 | < 0.001 |
| HR | |||
| Beta | 0.333 | 0.345 | 0.428 |
| P | < 0.001 | < 0.001 | < 0.001 |
Model 1: Adjustment for age and gender; Model 2: Adjustment for age, gender, triglyceride, total cholesterol, low density lipoprotein cholesterin, fasting plasma glucose, serum creatinine and uric acid; Model 3: Adjustment for hemoglobin, body temperature, minute ventilation volume and peripheral oxygen saturation on the basis of Model 2
ScvO2 Central venous oxygen saturation, HR Heart rate
Effects of different PEEP levels on airway pressure
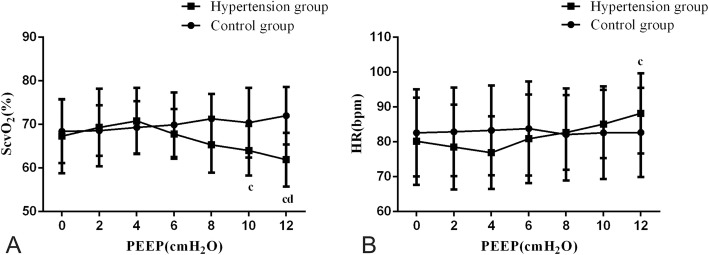
In both groups, VT, MV, and SpO2 were unaffected by PEEP, but the increase in PEEP led to an increase of Ppeak and Pmean (Table 2, Fig. 5). In addition, PEEP was positively correlated with the Ppeak (r = 0.489, P < 0.001) and Pmean (r = 0.708, P < 0.001). Using multiple linear regression analysis to control confounding factors, a correlation between PEEP and airway pressure was still observed (Table 6).
Fig. 5.
Effect of different PEEP levels on peak airway pressure (Ppeak) and mean airway pressure (Pmean). a: Effect of different PEEP levels on Ppeak, b: Effect of different PEEP levels on Pmean. Data are shown as mean and standard deviation. In the same group of blood pressure, aindicated significant difference (p < 0.05) compared with PEEP 0 cmH2O. bindicated significant difference compared with PEEP 2 cmH2O. cindicated significant difference compared with PEEP 4 cmH2O. dindicated significant difference compared with PEEP 6 cmH2O. eindicated significant difference compared with PEEP 8 cmH2O
Table 6.
Multiple linear regression analysis of airway pressure and PEEP
| All subjects | Model1 | Model2 | Model3 |
|---|---|---|---|
| Ppeak | |||
| Beta | 0.489 | 0.514 | 0.515 |
| P | < 0.001 | < 0.001 | < 0.001 |
| Pmean | |||
| Beta | 0.713 | 0.740 | 0.744 |
| P | < 0.001 | < 0.001 | < 0.001 |
Model 1: Adjustment for age and gender; Model 2: Adjustment for age, gender, triglyceride, total cholesterol, low density lipoprotein cholesterin, fasting plasma glucose, serum creatinine and uric acid; Model 3: Adjustment for hemoglobin, body temperature, minute ventilation volume and peripheral oxygen saturation on the basis of Model 2
Ppeak Peak airway pressure, Pmean Mean airway pressure
Discussion
PEEP is applied during the end of expiration to maintain alveolar pressure above atmospheric pressure. The benefit of PEEP has been demonstrated in terms of preventing collapsed alveoli and improving oxygenation [14, 15]. However, applying PEEP may affect cardiac function and vital organ perfusion via complex mechanisms, especially for elderly hypertensives [16]. Our study found that when PEEP was below 4 cm H2O in the both control and hypertension groups, blood pressure was unaffected by PEEP. However, when PEEP was above 4 cm H2O, the increase in PEEP led to decreased blood pressure and PEEP was negatively correlated with blood pressure in the hypertension group. Upon control confounding factors, this negative correlation still existed. This observation may be due to diminished elasticity in elderly hypertensives’ blood vessels and subsequent functional decline, suggesting a high level of PEEP would be a negative influence on hemodynamics [17].
To further study why elevated PEEP induced a drop in blood pressure, we explored the relationship between PEEP and cardiac function. As volume status was of high importance for hemodynamic instability in patients ventilated with high tidal volume and different PEEP levels, a sedative drug called midazolam was used to minimize spontaneous breathing, and tidal volume was maintained relatively constant in our study. Moreover, prior to the study, when PEEP was 0 cm H2O, there was no statistically significant difference in CVP between the hypertension and control groups. This result indicated that initial fluid status was similar between the two groups. During our study we controlled patients’ body position and infusion speed to ensure that the two groups’ fluid intake was as similar as possible. We found that in both groups, VT, MV, and SpO2 were unaffected by PEEP, but increased PEEP led to an increase of CVP and airway pressure. When PEEP was raised above 4 cm H2O, in the hypertension group, the increase in PEEP led to a reduction in ScvO2 and an increase in HR. Furthermore, PEEP was positively correlated with HR and negatively correlated with the ScvO2. To control for confounding factors such as hemoglobin, arterial oxygen saturation and oxygen demand, we found that the correlation between PEEP and HR and ScvO2 still existed. This indicated that elevated levels of PEEP caused decreased cardiac output in elderly hypertensive patients [18].
In general, the increase in PEEP caused higher airway pressure which resulted in increased intrathoracic pressure (ITP) [19]. Elevation of CVP by increasing ITP resulted in a reduction in venous return [20]. However, for non-hypertensive subjects, the effects on increasing ITP on venous return, especially by PEEP, did not always lead to decreased cardiac output because of their strong cardiovascular regulation [21]. In elderly hypertensive patients, the thickened and stiffened vein wall caused slower venous return than that of non-hypertension subjects, which resulted in reduced right ventricular (RV) preload [22]. PEEP not only decreased RV preload by impeding systemic venous return, but also increased RV afterload in combination with high tidal volume. In our study, we used high tidal volume of 10 ml/kg body weight to maintain pulmonary oxygenation and promote carbon dioxide exhalation. High PEEP with high tidal volume led to increased end inspiratory lung volume, which was responsible for the RV afterload. Vieilaard-Baron [23] and Protti [24] demonstrated that afterload for RV did not influence capillary compression, but rather the end inspiratory lung volume that caused this compression and reduced the flow through the lung. As a result, the reduction in RV ejection caused by PEEP and high tidal volume decreased left ventricular filling in both groups [25]. However, the hemodynamics of elderly hypertensive patients was more affected by blood volume deficiency than non- hypertensive patients. Therefore, the lower the stroke volume, the faster HR that occurs for elderly hypertensive individuals [26]. The high tidal volume was able to reduce flow through the lung and decrease left ventricular filling, so it was also used to highlight the difference in the response to hypovolemia between the two groups in the present study.
In our study, we found that the increase in PEEP led to an increase in CVP but a decrease in cardiac output. Studies assessing fluid status have shown that CVP monitoring is as effective, and safer, than more invasive means such as pulmonary artery occlusion pressure [27]. It is clear, however, that CVP does not tell the entire story, as patients with positive pressure ventilation may have high CVP, but reduced circulating volume [28]. Currently, many scholars have not advocated the use of CVP to predict changes in pre-cardiac load in mechanical ventilation [29, 30]. However, accurate measurement of cardiac output, such as by SwanGanz catheter and cardiac color ultrasound requires advanced instrumentation and incurs greater cost [31]. Recent studies have found that ScvO2 can be used to evaluate the condition of left cardiac ejection, which is simple and reliable and can be used in clinical application [18]. A study by Lanspa et al. reported that assessment of cardiac function often relied on ScvO2, with higher ScvO2 corresponding with increased cardiac output [32]. As such, our study used ScvO2 to reflect left cardiac ejection.
For elderly hypertensive patients, this study showed that PEEP of approximately 4 cm H2O could have maximal impact on improving oxygenation, and minimize its impact on hemodynamics. However, due to the limited patient source, the appropriate PEEP value setting should be confirmed by the large sample multicenter clinical study.
Conclusions
Traditional static index CVP cannot accurately reflect changes in cardiac preload with positive pressure ventilation, though ScvO2 shows obvious advantages, which can be used to predict cardiac preload under mechanical ventilation. For elderly patients with hypertension, low levels of PEEP have less effect on blood pressure and cardiac output, while higher levels of PEEP can significantly affect blood pressure and cardiac output. Therefore, the effects of PEEP on the hemodynamics of elderly patients with hypertension should be taken into consideration.
Acknowledgements
We would like to thank all of our colleagues who recruited and treated the patients.
Abbreviations
- ANOVA
Analysis of variance
- CVP
Central venous pressure
- DBP
Diastolic blood pressure
- DO2
Oxygen delivery
- FPG
Fasting plasma glucose
- HB
Hemoglobin
- HR
Heart rate
- ITP
Increased intrathoracic pressure
- LDL-C
low density lipoprotein cholesterin
- MABP
Mean arterial blood pressure
- MV
Minute ventilation volume
- PEEP
Positive end expiratory pressure
- Pmean
Mean airway pressure
- Ppeak
Peak airway pressure
- PVR
Pulmonary vascular resistance
- RV
Right ventricular
- SBP
Systolic blood pressure
- Scr
Serum creatinine
- ScvO2
Central venous oxygen saturation
- SpO2
Peripheral oxygen saturation
- T
Body temperature
- TC
Total cholesterol
- TG
Triglyceride
- UA
Uric acid
- VO2
Consumption
Authors’ contributions
LZ designed the experiment, conducted the experiment, analyzed the data and wrote the manuscript. GC designed the experiment, conducted the experiment and wrote the manuscript. ZX and QW investigated the patients and collected the data. QY investigated the patients and analyzed the data. CC designed the experiment, funded the study and wrote the manuscript. LZ, GC, ZX, QW, QY and CC contributed to drafting and revision of the manuscript and approval of the final version to be submitted for consideration for publication.
Funding
This study was supported by Nation and Fujian Province’s Key Clinical Specialty Discipline Construction Program, Fujian Medical University Union Hospital Project(2015-GJLN-3-08) and the Fujian Provincial Science and Technology Guiding Project (No.2017Y0041,2018Y0033).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Union Hospital Affiliated to Fujian Medical University. Chinese clinical trial registration number is ChiCTR-ROC-17012873. All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
All subjects signed informed consent to participate in the study.
Consent for publication
Consent for publication was not required because all patient data were anonymised.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lili Zhou and Guoen Cai contributed equally to this work and should be considered co-first authors.
Contributor Information
Lili Zhou, Email: 41120096@qq.com.
Guoen Cai, Email: cgessmu@126.com.
Zhihui Xu, Email: 37277936@qq.com.
Qinyong Weng, Email: wengqinyon@sina.com.
Qinyong Ye, Email: unionqyye@163.com.
Cunrong Chen, Email: ccrcgezll@sina.com.
References
- 1.Wu L, He Y, Jiang B, Sun D, Wang J, Liu M, Yang S, Wang Y. Trends in prevalence, awareness, treatment and control of hypertension during 2001-2010 in an urban elderly population of China. PLoS One. 2015;10(8):e0132814. doi: 10.1371/journal.pone.0132814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser EA, Lotze U, Schäfer HH. Increasing complexity: which drug class to choose for treatment of hypertension in the elderly? Clin Interv Aging. 2014;9:459–475. doi: 10.2147/CIA.S40154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis N, Mendrinos D, Sanidas E, Favatas G, Georgopoulou M. Hypertension in the elderly. World J Cardiol. 2012;4(5):135–147. doi: 10.4330/wjc.v4.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buford TW. Hypertension and Aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikor A, Trásy D, Németh MF, Osztroluczki A, Kocsi S, Kovács I, Demeter G, Molnár Z. Continuous central venous oxygen saturation assisted intraoperative hemodynamic management during major abdominal surgery: a randomized, controlled trial. BMC Anesthesiol. 2015;15:82. doi: 10.1186/s12871-015-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H, Long Y, Liu D, Wang X, Zhou X. Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care. 2015;19(1):330. doi: 10.1186/s13054-015-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laine GA, Hu BY, Wang S, Thomas Solis R, Reul GJ., Jr Isolated high lactate or low central venous oxygen saturation after cardiac surgery and association with outcome. J Cardiothorac Vasc Anesth. 2013;27(6):1271–1276. doi: 10.1053/j.jvca.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Burša F, Pleva L. Anaerobic metabolism associated with traumatic hemorrhagic shock monitored by microdialysis of muscle tissue is dependent on the levels of hemoglobin and central venous oxygen saturation: a prospective, observational study. Scand J Trauma Resusc Emerg Med. 2014;22:11. doi: 10.1186/1757-7241-22-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandy D, Aronow WS, Banach M. Current perspectives on treatment of hypertensive patients with chronic obstructive pulmonary disease. Integr Blood Press Control. 2013;6:101–109. doi: 10.2147/IBPC.S33982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palatini P, Casiglia E, Gąsowski J, Głuszek J, Jankowski P, Narkiewicz K, Saladini F, Stolarz-Skrzypek K, Tikhonoff V, Van Bortel L, Wojciechowska W, Kawecka-Jaszcz K. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag. 2011;7:725–739. doi: 10.2147/VHRM.S25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas M, Sutherasan Y, Gregoretti C, Pelosi P. PEEP role in ICU and operating room: from pathophysiology to clinical practice. ScientificWorldJournal. 2014;2014:852356. doi: 10.1155/2014/852356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hypertension Study Group Prevalence, awareness, treatment and control of hypertension among the elderly in Bangladesh and India: a multicentre study. Bull World Health Organ. 2001;79(6):490–500. [PMC free article] [PubMed] [Google Scholar]
- 14.Garnero A, Tuxen D, Corno G, Durand-Gasselin J, Hodgson C, Arnal J-M. Dynamics of end expiratory lung volume after changing positive end-expiratory pressure in acute respiratory distress syndrome patients. Crit Care. 2015;19(1):340. doi: 10.1186/s13054-015-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nespoulet H, Rupp T, Bachasson D, Tamisier R, Wuyam B, Lévy P, Verges S. Positive expiratory pressure improves oxygenation in healthy subjects exposed to hypoxia. PLoS One. 2013;8(12):e85219. doi: 10.1371/journal.pone.0085219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Collino F, Maiolo G, Rapetti F, Romitti F, Tonetti T, Vasques F, Quintel M. Positive end-expiratory pressure: how to set it at the individual level. Ann Transl Med. 2017;5(14):288. doi: 10.21037/atm.2017.06.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denker MG, Cohen DL. What is an appropriate blood pressure goal for the elderly: review of recent studies and practical recommendations. Clin Interv Aging. 2013;8:1505–1517. doi: 10.2147/CIA.S33087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor PM, Dhawan I, Jain P, Chowdhury U. Lactate, endothelin, and central venous oxygen saturation as predictors of mortality in patients with tetralogy of Fallot. Ann Card Anaesth. 2016;19(2):269–276. doi: 10.4103/0971-9784.179619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luecke T, Pelosi P. Clinical review: positive end-expiratory pressure and cardiac output. Crit Care. 2005;9(6):607–621. doi: 10.1186/cc3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyton AC, Lindsey AW, Abernathy B, et al. Venous return at various right atrial pressures and the normal venous return curve. Am J Phys. 1957;189:609–615. doi: 10.1152/ajplegacy.1957.189.3.609. [DOI] [PubMed] [Google Scholar]
- 21.Fares WH, Carson SS. The relationship between positive end expiratory pressure and cardiac index in patients with acute respiratory distress syndrome. J Crit Care. 2013;28(6):10. doi: 10.1016/j.jcrc.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orde SR, Behfar A, Stalboerger PG, Barros-Gomes S, Kane GC, Oh JK. Effect of positive end-expiratory pressure on porcine right ventricle function assessed by speckle tracking echocardiography. BMC Anesthesiol. 2015;15:49. doi: 10.1186/s12871-015-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (1985) 1999;87(5):1644–1650. doi: 10.1152/jappl.1999.87.5.1644. [DOI] [PubMed] [Google Scholar]
- 24.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, Masson S, Cressoni M, Gattinoni L. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013;41(4):1046–1055. doi: 10.1097/CCM.0b013e31827417a6. [DOI] [PubMed] [Google Scholar]
- 25.Rankin JS, Olsen CO, Arentzen CE, et al. The effects of airway pressure on cardiac function in intact dogs and man. Circulation. 1982;66:108–120. doi: 10.1161/01.CIR.66.1.108. [DOI] [PubMed] [Google Scholar]
- 26.Yu K, Nakashima T, Iseri T, Yonetani C, Ueda K, Fujimoto Y, Kato S. The impact of inspiratory pressure on stroke volume variation and the evaluation of indexing stroke volume variation to inspiratory pressure under various preload conditions in experimental animals. J Anesth. 2015;29(4):515–521. doi: 10.1007/s00540-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D-k, Wang X-t, Liu D-w. Association between elevated central venous pressure and outcomes in critically ill patients. Ann Intensive Care. 2017;7:83. doi: 10.1186/s13613-017-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.House AA, Haapio M, Lentini P, Bobek I, de Cal M, Cruz DN, Virzì GM, Carraro R, Gallo G, Piccinni P, Ronco C. Volume Assessment in Mechanically Ventilated Critical Care Patients Using Bioimpedance Vectorial Analysis, Brain Natriuretic Peptide, and Central Venous Pressure. Int J Nephrol. 2011;2011:413760. doi: 10.4061/2011/413760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D, Ahmed SM, Ali S, Ray U, Varshney A, Doley K. Correlation between central venous pressure and peripheral venous pressure with passive leg raise in patients on mechanical ventilation. Indian J Crit Care Med. 2015;19(11):648–654. doi: 10.4103/0972-5229.169338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundaram SC, Salins SR, Kumar AN, Korula G. Intra-Operative Fluid Management in Adult Neurosurgical Patients Undergoing Intracranial Tumour Surgery: Randomised control trial comparing pulse pressure variance (PPV) and central venous pressure (CVP) J Clin Diagn Res. 2016;10(5):UC01–UC05. doi: 10.7860/JCDR/2016/18377.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinsky MR, editor. Functional Hemodynamic Monitoring. Crit Care Clin. 2015;31(1):89–111. [DOI] [PMC free article] [PubMed]
- 32.Lanspa MJ, Pittman JE, Hirshberg EL, Wilson EL, Olsen T, Brown SM, Grissom CK. Association of left ventricular longitudinal strain with central venous oxygen saturation and serum lactate in patients with early severe sepsis and septic shock. Crit Care. 2015;19(1):304. doi: 10.1186/s13054-015-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.