Abstract
Osteosarcoma is predominant in the adolescent and the elderly population, but few studies have described the characteristics and prognostic factors of patients older than 60 years. In this study, the Surveillance, Epidemiology, and End Results registry database was used to identify all patients diagnosed with primary osteosarcoma from 1973 to 2014. We utilized Cox proportional hazards regression analysis to evaluate the association between patient overall survival and relevant characteristics, including gender, race, disease stage, treatment methods, primary tumor site, differentiation grade, and histologic subtype. In the data set, a total of 1139 patients with osteosarcoma older than 60 years old were identified. The overall rate of distant metastatic cases was 28.6%. Osteosarcoma occurred equally in men and women (49.5% vs 50.5%). Of all, 41.3% of tumors were located in axial location (pelvis, spine, and ribs), 34.1% of tumors were located in extremity (long or short bones of the upper or lower extremity), and 24.6% in other location (mandible, skull, and other atypical locations). Male (hazard ratio [HR] = 1.201; 95% confidence interval [CI]: 1.056-1.366), axial location (HR = 1.342; 95% CI: 1.157-1.556), distant metastasis (HR = 2.369; 95% CI: 2.015-2.785), non-surgery perform (HR = 2.108; 95% CI: 1.814-2.451) were independent risk factors for 5-year overall survival. This study revealed distinct clinicopathological features of patients with osteosarcoma older than 60 years. Male gender, tumor in axial site, nonsurgery perform, and distant metastasis indicated worse prognosis survival. Performing surgery is still an effective and reliable treatment method for patients older than 60 years.
Keywords: sarcoma, osteosarcoma, SEER program, survival analysis, risk factors
Introduction
Osteosarcoma was the most common primary malignant bone tumor, with 2 peaks of incidence occurring in adolescence and the individuals aged >60 years,1 but few reports described the characteristics and prognostic factors of elderly patients with osteosarcoma. In 1986, Huvos firstly reported and analyzed the clinical characteristics and outcomes of 117 patients older than 60 years with osteogenic sarcoma under adjuvant and neoadjuvant chemotherapy.2 Longhi et al observed 43 patients with high-grade osteosarcoma who were older than 65 years and found that these patients had a worse prognosis compared to younger patients due to a longer time lapse from the onset of symptoms to diagnosis, more metastatic cases at diagnosis, less use of limb salvage, and fewer patients receiving chemotherapy.3 In 2009, the Japanese Musculoskeletal Oncology Group conducted a retrospective multicenter analysis, which included 95 patients older than 60 years from 27 institutions and revealed that there was a predilection for axial localization in osteosarcoma in an elderly population, and surgical treatment had a significant impact on patient’s prognosis.4 Owing to the small number of patients, the above studies had limited statistical power.2-4 A larger population-based study is needed to estimate the survival rate of elderly patients with osteosarcoma, to identify prognostic factors, and to establish standard treatment strategy.
Materials and Methods
The Surveillance, Epidemiology, and End Results (SEER) registry, founded by the US National Cancer Institute, collects cancer-related survival data on cancer cases from 1973 and represents 28% of America’s population today.5 Patients diagnosed with osteosarcoma from 1973 to 2014 were identified using the Histologic International Classification of Disease for Oncology, 3rd Edition (IDO-O-3) Codes 9180 to 9187 and 9192 to 9195, including osteosarcoma, not otherwise specified (NOS); chondroblastic osteosarcoma; fibroblastic osteosarcoma; telangiectatic osteosarcoma; osteosarcoma in Paget disease; and parosteal osteosarcoma. Patient age is recorded in the SEER database as a categorical variable in 5-year intervals, beginning at 0 year and ending at 85 years or more. Information on patients with osteosarcoma older than 60 years of age at diagnosis was available in the database, including patient demographics, primary tumor site, metastatic status, histologic subtype, differentiation grade, treatment methods, and follow-up information (survival months and cause of death). The primary tumor site was categorized as axial (pelvis, spine, and ribs), extremity (long and short bones of the upper and lower extremity), or other (mandible, skull, and other atypical locations). We classified patients coded with “distant” as metastatic, while patients coded with “localized” or “regional” were classified as nonmetastatic. Differentiation grade is reported on a scale from I to IV, grades I and II were defined to be low-grade tumors, while grades III and IV were defined to be high-grade tumors. Primary outcome was defined as time in months from diagnosis to death from any cause for overall survival, while time from diagnosis to death specific to the cancer-related diagnosis for disease-specific survival. Patients were censored when a patient was alive at the time of last follow-up.
Statistical Analysis
SPSS (version 17.0.1; Chicago, Illinois) was used for statistical analysis. The Kaplan-Meier method was utilized to calculate the median survival time and overall survival rate. The log-rank test was applied to test the differences formally. Statistical significance was taken at probability value <.01. Multivariate analyses were carried out using Cox proportional hazards ratios in order to identify independent predictors of overall survival. Hazard ratio (HR) >1 revealed that prognostic factor was associated with decreasing survival, while HR <1 revealed that prognostic factor was associated with increasing survival rate compared to the reference. Hazard ratio = 1 indicated that there was no significant relationship between them.
Result
In the database from 1973 to 2014, we identified 1139 patients with osteosarcoma older than 60 years totally (Table 1). Demographically, 564 (49.5%) were male and 575 (50.5%) were female. Nine hundred sixty-two whites represent 84.4% of cases followed by blacks, representing 9.7% of 111 cases, and 5.4% were of other races. In 471 (41.3%) cases, the primary tumors were located in axial (mandible, skull, pelvis, spine, and ribs); 389 (34.1%) cases of tumors were located in extremity (long or short bones of the upper or lower extremity), while 24.6% in other atypical locations. Histologically, 45.5% of tumors were of unknown grade, 6.9% were low-grade tumors, 47.6% were high-grade tumors. The most common histological subtype is NOS osteosarcoma (78.6%), followed by chondroblastic osteosarcoma (7.3%), osteosarcoma with Paget disease (6.6%), fibroblastic osteosarcoma (3.3%), parosteal osteosarcoma (1.4%), telangiectatic osteosarcoma (1.1%), central osteosarcoma (0.9%), small cell osteosarcoma (0.3%), and other rare histological subtypes. The overall rate of distant metastatic cases in our cohort was 28.6%, and 58.8% of the cases received a surgical treatment.
Table 1.
Patient Characteristics.
| Category | Age ≥60, n = 1139 (%) |
|---|---|
| Sex | |
| Female | 575 (50.5) |
| Male | 564 (49.5) |
| Race | |
| White | 962 (84.4) |
| Black | 111 (9.7) |
| Other | |
| Therapy | |
| Surgery | 469 (41.2) |
| Nonsurgery | 670 (58.8) |
| Disease stage | |
| Localized/regional | 630 (55.3) |
| Distant | 326 (28.6) |
| Unstage | 183 (16.1) |
| Primary site | |
| Extremity | 389 (34.1) |
| Axial | 471 (41.3) |
| Other | 279 (24.6) |
| Grade | |
| Well differentiated | 32 (2.8) |
| Moderately | 47 (4.1) |
| Poorly | 194 (17.0) |
| Undifferentiated | 349 (30.6) |
| Unknown | 517 (45.5) |
| Histology | |
| Osteosarcoma, NOS | 895 (78.6) |
| Chondroblastic OS | 84 (7.3) |
| Fibroblastic OS | 38 (3.3) |
| OS in Paget | 75 (6.6) |
| Telangiectatic OS | 13 (1.1) |
| Other | 34 (3.1) |
Abbreviations: NOS, not otherwise specified; OS, osteosarcoma.
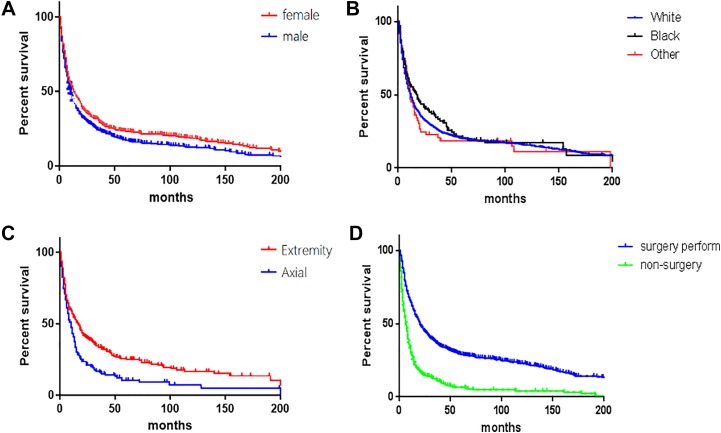
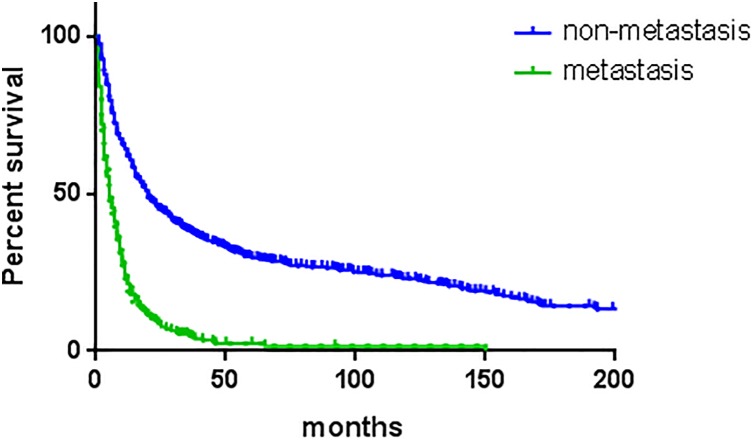
Overall survival and disease-specific survival were 27.6% and 49.6% at 3 years, 20.2% and 43.2% at 5 years, and 13.2% and 39.6% at 10 years. Analysis of Kaplan-Meier survival curves with the log-rank test revealed that gender (P = .002), primary site (P < .001), disease stage (P < .001), differentiation grade (P < .001), therapy method (P < .001), and histologic subtype (P < .001) were associated with the decreased 5-year overall survival. Multivariate analyses were performed using Cox proportional hazards ratios in order to identify independent predictors (Table 2). Male (HR = 1.201; 95% confidence interval [CI]: 1.056-1.366), axial location (HR = 1.342; 95% CI: 1.157-1.556), distant metastasis (HR = 2.369; 95% CI: 2.015-2.785), and nonsurgery perform (HR = 2.108; 95% CI: 1.814-2.451) were independent risk factors for 5-year overall survival.
Table 2.
Univariate Analysis of Factors Affecting 5-Year Overall Survival and 5-Year Disease-Specific Survival of Patients With Osteosarcoma Older Than 60 Years.a
| Factors | Overall Survival | Disease-Specific Survival | ||
|---|---|---|---|---|
| 5-Year Survival (%) | P Value | 5-Year Survival (%) | P Value | |
| Overall | 20.2 | 43.2 | ||
| Sex | .004 | .086 | ||
| Female | 23.2 | 46.9 | ||
| Male | 17.2 | 39.2 | ||
| Race | .397 | .332 | ||
| White | 20.3 | 42.9 | ||
| Black | 21.5 | 45.6 | ||
| Other | 19.4 | 50.4 | ||
| Therapy | .001 | .001 | ||
| Surgery | 28.9 | 52.1 | ||
| Nonsurgery | 6.6 | 24.9 | ||
| Disease stage | .001 | .001 | ||
| Metastasis | 2.5 | 9.0 | ||
| Nonmetastasis | 29.2 | 57.2 | ||
| Primary site | .001 | .001 | ||
| Extremity | 21.6 | 38.8 | ||
| Axial | 9.2 | 28.3 | ||
| Other | 14.7 | 30.2 | ||
| Grade | 0.001 | 0.001 | ||
| Well differentiated | 56.1 | 82.3 | ||
| Moderately | 54.5 | 71.4 | ||
| Poorly | 17.1 | 45.2 | ||
| Undifferentiated | 21.3 | 43.7 | ||
| Histology | .001 | .001 | ||
| Osteosarcoma, NOS | 17.9 | 40.2 | ||
| Chondroblastic OS | 27.1 | 65.9 | ||
| Fibroblastic OS | 38.3 | 56.8 | ||
| OS in Paget | 12.1 | 29.3 | ||
| Telangiectatic OS | 21.9 | 38.5 | ||
Abbreviations: NOS, not otherwise specified; OS, osteosarcoma
a Multivariate Cox regression results have been added as follow.
Discussion
Osteosarcoma is the most common malignant bone tumor with double peak of incidence. The majority arise in adolescence, with a significant second peak in elderly populations.1 As the proportion of older populations in society is significantly increasing, the absolute number of elderly patients with osteosarcoma is expected to increase. Accordingly, a larger population-based study is needed to estimate the survival rate of elderly patients with osteosarcoma, to identify prognostic factors, and to establish standard treatment strategy. By means of the SEER database, we observed the latest updated epidemiologic characteristics and identified the association of survival with some prognosis factors for 1139 patients with osteosarcoma older than 60 years of age at diagnosis. In this study, overall survival and disease-specific survival were 27.6% and 49.6% at 3 years, 20.2% and 43.2% at 5 years, and 13.2% and 39.6% at 10 years.
Few studies6-8 have reported survival differences between males and females with osteosarcoma. Previous study6 from SEER (1991-2010) demonstrated that males with high-grade osteosarcoma were associated with decreased cause-specific survival and assumed that males had poor compliance with recommended treatment protocol. Petrilli et al7 analyzed 92 patients with nonmetastatic osteosarcoma and found that male sex was a predictive factor of tumor recurrence and a poor prognostic indicator of death. Smeland et al8 reported that female gender was an independent favorable prognostic factor in a study of 113 patients with osteosarcoma who received neoadjuvant chemotherapy. Based on 1702 patients with osteosarcoma, Bielack et al9 attributed the differences between males and females to the poorer response to neoadjuvant chemotherapy for male patients with osteosarcoma. Our results involved in elderly patients with osteosarcoma were congruent with above studies. In some basic biological literatures, endogenous sex hormone had been found to play a role in osteosarcoma cell, which might explain the difference in clinical survival rate between females and males.10,11 Wagle et al10 reported that knocking down an androgen receptor coactivator and androgen receptor inhibited proliferation-related signaling in osteosarcoma cell and decreased cellular proliferation. Fang et al11 observed that the proliferation of osteosarcoma cell was remarkably affected by 17β-estradiol (E2). High dose of E2 treatment inhibited proliferation, migration, and invasion processes of osteosarcoma cells. Although gender differences in survival rate may be associated with some society-related factors to some extent,9 it is worth noting that sex hormone may play a role in the progression of osteosarcoma and it was an important area for further study.
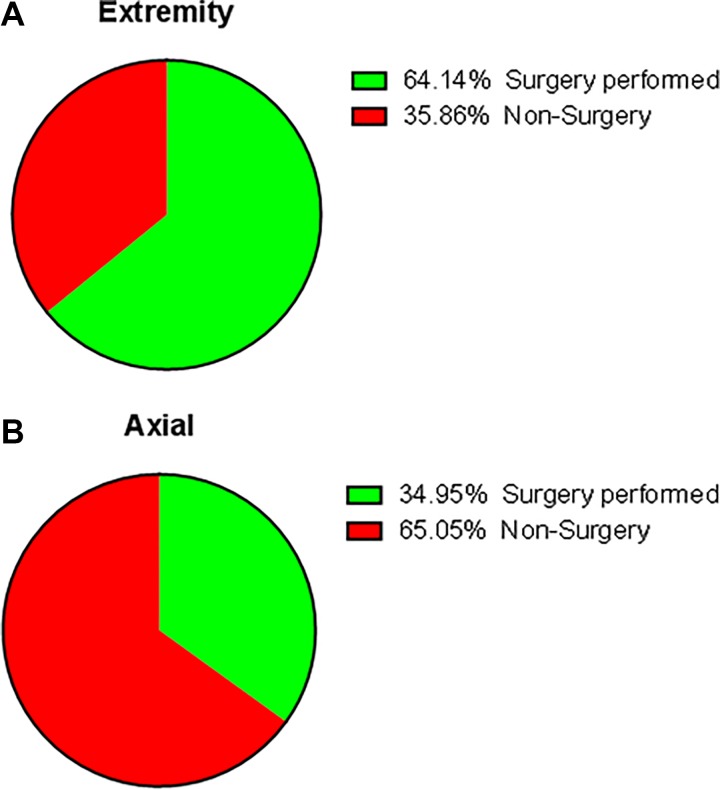
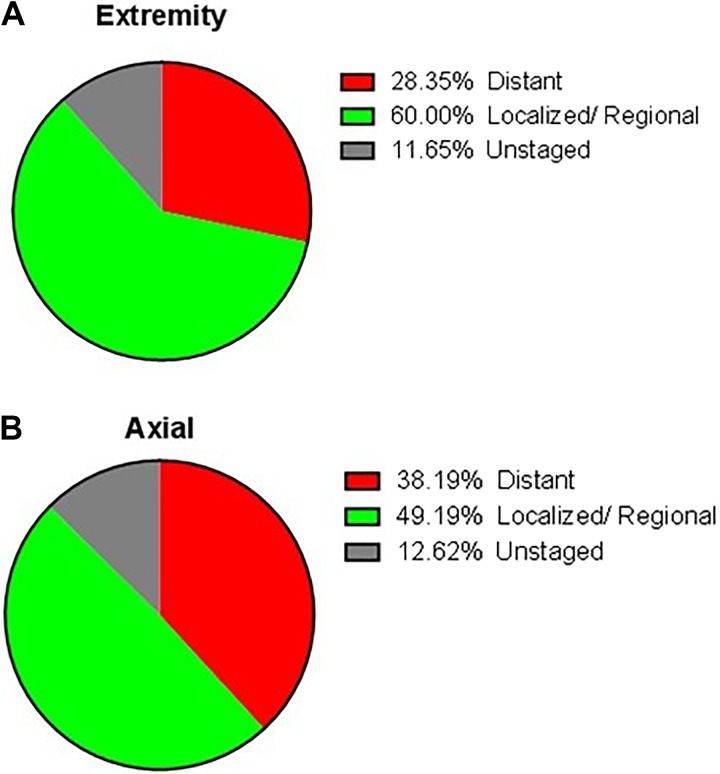
Although extremities were still the most common site of osteosarcoma occurring, there was a prominent tendency that osteosarcoma was common in axial localization in patients older than 40 years compared to the young.2,3,12,13 The incidence of axial osteosarcoma was from 20.5% to 49% based on previous researches.2,4,14 In regard to primary patients with osteosarcoma older than 60 years, Nishida et al4 reported 31% of cases were located in the axial site, while 41.3% in the extremity site. Kumar et al15 showed that the axial skeleton involved in primary osteosarcoma was 42% in patients older than age 50 years as compared to only 18.4% axial osteosarcoma in the younger age groups. Consistence with previous studies, the incidence of axial osteosarcoma was 41.3% in our study. Additionally, our findings indicated that axial location was statistically associated with worsened overall survival, which was consistent with previous studies.6,16,17 Firstly, the poor prognosis may attribute to the higher metastatic rate of osteosarcoma in axial localization. In our cohort, the rate of distant metastasis in extremity localization was 28.4%, while 38.2% in axial localization. Bielack et al18 and Miller et al19 reported that patients with osteosarcoma of the axial skeleton were more likely to show metastases at diagnosis compared to extremity. The latter stated that higher distant metastasis rate in axial osteosarcoma perhaps resulted from delayed diagnosis or age-specific differences in tumor biology. In addition, Fahey20 found that osteosarcoma localized in axial was in closer proximity to large venous sinuses, which may lead to hematogenous metastasis more easily. Secondly, the tumor in extremity was easy to be detected at an early stage because of a lump or some swelling with pain which attracted the attention of patients easily. However, when the tumor located at vertebral column or sacrum, localized pain was the first and even the only symptom,21 which can be caused by some benign spinal disease, such as lumbar muscle strain, degenerative lumbar disc disease, spinal stenosis, and so on. These may confound patients and their physicians and lead to a delayed diagnosis and a high risk of metastases. Thirdly, the anatomical complexities of axial location partly made radical operation difficult and lead to more complications, thus resulted in worse prognosis. For instance, the en bloc resection, the most effective surgical intervention for the spinal tumor, may result in serious complications such as huge blood loss, implant failure, or paralysis. When the tumor invaded the upper cervical region, vertebral artery foramen, or located into the odontoid, inadequacy of the margins of excision was unavoidable.
As is well known, operative resection with wide margins in osteosarcoma could offer the best long-term prognosis, while inadequacy of the margins of excision increased the risk of local recurrence and indicate a dismal prognosis.22,23 Nishida et al4 showed that 5-year overall survival rate for the elderly patients who underwent surgical treatment is 53.2% compared to 8.7% in the nonsurgery cohort. Longhi et al3 showed that 5-year overall survival for patients older than 65 years who underwent surgical treatment is nearly 30%, while 0% in the nonsurgery cohort. In our study, 5-year overall survival of patients with surgical treatment was 28.9%, while those who failed to receive surgery was only 6.6%, which was consistent with previous studies, but we had to admit that we failed to distinguish the patients who had missed surgical indications from the patients who were unwilling to undergo operation because of no records of these data in the SEER database.
The present study has a few limitations. Firstly, this study is limited by the fact that SEER does not contain sufficient information on specific chemotherapy regimens and radiation therapy, and tumor grade and specific histological diagnoses in every patient, so that these were not incorporated in our study. Although the SEER database reports whether surgical intervention was performed, it is restricted in its ability to analyze certain other variables, such as margin status, extent of surgical resection, and postoperative tumor recurrence. Secondly, since our study is retrospective, it is inevitable that certain data of patients are missing. This may have decreased the number of eligible cases. Despite these shortcomings, the SEER Program database serves as an unparalleled resource when investigating rare cancers.
Conclusion
This study revealed distinct clinicopathological features of patients with osteosarcoma older than 60 years. The prognosis of osteosarcoma in the old was worse in comparison to the young. Male gender, tumor in axial site, nonsurgery treatment, and distant metastasis indicated worse prognosis of 5-year overall survival. Performing surgery was effective and reliable treatment method for elderly patients. The delayed diagnosis in axial location contributed to the larger size of neoplasm and formed distant organ metastasis, which led patients miss effective surgery at an early stage and made terminal malignant tumors unresectable.
Figure 1.
The proportion of patients who underwent operation stratified by different tumor location.
Figure 2.
The proportion of patients with distant metastasis stratified by different tumor location.
Figure 3.
Kaplan-Meier estimated overall survival in patients with osteosarcoma older than 60 years, stratified by (A) gender (female vs male; P < .01), (B) race (white vs black vs other; P = .39), (C) primary site (vertebral column vs sacrum/pelvis; P < .01), and (D) therapy method (nonsurgery vs surgery perform; P < .01). The log-rank test results have been added in KM plot; the colors of the figures have been changed instead of red and light green.
Figure 4.
Kaplan-Meier estimated overall survival in osteosarcoma older than 60 years, stratified by metastasis status at presentation.
Table 3.
Multivariate Cox Analysis of Factors Affecting Overall Survival Rate of Patients With Osteosarcoma Older Than 60 Years.a
| Factors | Overall Survival | |
|---|---|---|
| HR (95% CI) | P Value | |
| Sex | .005 | |
| Female | Ref | |
| Male | 1.201 (1.056-1.366) | |
| Race | .637 | |
| White | Ref | |
| Black | 1.240 (0.396-3.880) | |
| Other | 1.039 (0.782-1.379) | |
| Therapy | <.001 | |
| Surgery | Ref | |
| Nonsurgery | 2.108 (1.814-2.451) | |
| Disease stage | <.001 | |
| Nonmetastasis | Ref | |
| Metastasis | 2.369 (2.015-2.785) | |
| Primary site | <.001 | |
| Extremity | Ref | |
| Axial | 1.342 (1.157-1.556) | |
| Other | 0.799 (0.660-0.967) | |
| Grade | .121 | |
| Well differentiated | Ref | |
| Moderately | 1.904 (1.133-3.201) | |
| Poorly | 2.124 (2.065-2.435) | |
| Undifferentiated | 2.257 (2.109-2.668) | |
| Histology | .081 | |
| Osteosarcoma, NOS | Ref | |
| Chondroblastic OS | 0.669 (0.516-0.867) | |
| Fibroblastic OS | 0.547 (0.377-0.794) | |
| OS in Paget | 1.227 (0.965-1.560) | |
| Telangiectatic OS | 0.864 (0.615-1.285) | |
Abbreviations: CI, confidence interval; HR, Hazard ratio; NOS, not otherwise specified; OS, osteosarcoma.
an = 1139
Footnotes
Authors’ Note: All data in this study are extracted from the SEER registry database.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zhehao Dai, MD, PhD  https://orcid.org/0000-0001-9285-2494
https://orcid.org/0000-0001-9285-2494
References
- 1. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer. 1986;57(7):1442–1449. [DOI] [PubMed] [Google Scholar]
- 3. Longhi A, Errani C, Gonzales-Arabio D, Ferrari C, Mercuri M. Osteosarcoma in patients older than 65 years. J Clin Oncol. 2008; 26(33):5368–5373. [DOI] [PubMed] [Google Scholar]
- 4. Nishida Y, Isu K, Ueda T, et al. Osteosarcoma in the elderly over 60 years: a multicenter study by the Japanese Musculoskeletal Oncology Group. J Surg Oncol. 2009;100(1):48–54. [DOI] [PubMed] [Google Scholar]
- 5. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) program. 2014. http://seer.cancer.gov/data. Accessed December 22, 2018.
- 6. Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) program database. Cancer Epidemiol. 2015;39(4):593–599. [DOI] [PubMed] [Google Scholar]
- 7. Petrilli AS, Gentil FC, Epelman S, et al. Increased survival, limb preservation, and prognostic factors for osteosarcoma. Cancer. 1991;68(4):733–737. [DOI] [PubMed] [Google Scholar]
- 8. Smeland S, Müller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488–494. [DOI] [PubMed] [Google Scholar]
- 9. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–790. [DOI] [PubMed] [Google Scholar]
- 10. Wagle S, Park SH, Kim KM, et al. DBC1/CCAR2 is involved in the stabilization of androgen receptor and the progression of osteosarcoma. Sci Rep. 2015;5:13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang D, Yang H, Lin J, et al. 17β-estradiol regulates cell proliferation, colony formation, migration, invasion and promotes apoptosis by upregulating miR-9 and thus degrades MALAT-1 in osteosarcoma cell MG-63 in an estrogen receptor-independent manner. Biochem Biophys Res Commun. 2015;457(4):500–506. [DOI] [PubMed] [Google Scholar]
- 12. Joo MW, Shin SH, Kang YK, et al. Osteosarcoma in Asian populations over the age of 40 years: a multicenter study. Ann Surg Oncol. 2015;22:3557–3564. [DOI] [PubMed] [Google Scholar]
- 13. Iwata S, Ishii T, Kawai A, et al. Prognostic factors in elderly osteosarcoma patients: a multi-institutional retrospective study of 86 cases. Ann Surg Oncol. 2014;21(11):263–268. [DOI] [PubMed] [Google Scholar]
- 14. Jeon DG, Lee SY, Cho WH, Song WS, Park JH. Primary osteosarcoma in patients older than 40 years of age. J Korean Med Sci. 2006;21(4):715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar R, Kumar M, Malhotra K, Patel S. Primary osteosarcoma in the elderly revisited: current concepts in diagnosis and treatment. Curr Oncol Rep. 2018;20(2):13. [DOI] [PubMed] [Google Scholar]
- 16. Pruksakorn D, Phanphaisarn A, Arpornchayanon O, Uttamo N, Leerapun T, Settakorn J. Survival rate and prognostic factors of conventional osteosarcoma in Northern Thailand: a series from Chiang Mai University Hospital. Cancer Epidemiol. 2015;39(6):956–963. [DOI] [PubMed] [Google Scholar]
- 17. Min D, Lin F, Shen Z, et al. Analysis of prognostic factors in 333 Chinese patients with high-grade osteosarcoma treated by multidisciplinary combined therapy. Asia Pac J Clin Oncol. 2013;9(1):71–79. [DOI] [PubMed] [Google Scholar]
- 18. Bielack SS, Wulff B, Delling G, et al. Osteosarcoma of the trunk treated by multimodal therapy: experience of the Cooperative Osteosarcoma Study Group (COSS). Med Pediatr Oncol. 1995;24(1):6–12. [DOI] [PubMed] [Google Scholar]
- 19. Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am. 2013;95(13):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fahey M, Spanier SS, Vander Griend RA. Osteosarcoma of the pelvis. A clinical and histopathological study of twenty-five patients. J Bone Joint Surg Am. 1992;74(3):321–330. [PubMed] [Google Scholar]
- 21. Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am. 2009;40(1):21–36. [DOI] [PubMed] [Google Scholar]
- 22. Grimer RJ, Taminiau AM, Cannon SR, et al. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br. 2002;84(3):395–400. [DOI] [PubMed] [Google Scholar]
- 23. Weeden S, Grimer RJ, Cannon SR, et al. The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer. 2001;37(1):39–46. [DOI] [PubMed] [Google Scholar]