Abstract
Subarachnoid hemorrhage (SAH) is a devastating disease. Neuronal death is an important pathophysiology in the acute phase of SAH, but the histopathological features of dying neurons have been poorly studied. Using several staining methods including terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and microtubule-associated protein 2 (MAP-2) double immunolabeling, we investigated the morphological changes of nucleus and cytoskeleton in neurons and sought susceptible areas to neuronal death in filament perforation SAH mice under light microscope. TUNEL and MAP-2 double immunolabeling clearly showed morphological features of shrunken cytoplasm and sometimes curl-like fibers in dying neurons, besides nuclear abnormalities. More dying neurons were detected in the moderate SAH group than in the mild SAH group, and the temporal base cortex was the most susceptible area to neuronal death with deoxyribonucleic acid (DNA) damage among the cerebral cortices and hippocampus at 24 hr after SAH (p<0.01, ANOVA). Lesser hippocampal neuronal death was observed at 24 hr, but neuronal death was significantly increased in the CA1 region at 7 days after SAH (p<0.05, unpaired t-test). Using TUNEL and MAP-2 double immunolabeling, morphological features of not only the nucleus but also the cytoplasm in post-SAH neuronal death with DNA damage can be observed in detail under light microscope:
Keywords: double immunostaining, in situ cell death assay, neuronal cell death, neuron-specific marker, stroke
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a devastating disease which has about 50% mortality.1,2 After clipping or coiling a ruptured cerebral aneurysm, survivors sometimes show delayed neurological deterioration.2,3 Vasospasm of major cerebral arteries has been regarded as a main cause of deterioration. Recently, early brain injury (EBI) consisting of intracranial events that occur before the onset of vasospasm is also suggested to contribute to poor outcome in SAH patients.1,2,4–6 EBI may be triggered by blood-derived substances in the subarachnoid space and an acute increase in intracranial pressure (ICP) due to a ruptured cerebral aneurysm, followed by a decrease in cerebral perfusion pressure and cerebral blood flow.4,7 Neuronal apoptosis is supposed to be a constituent of EBI.2,5,6
Because brain consists of several types of cells such as neurons and glia, neuron-specific staining is preferable when observing neuronal apoptosis. Both neurons and astrocytes were demonstrated to become terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL)-positive in a dog SAH model.5 In experimental SAH models, double immunofluorescence technique combining TUNEL and a neuronal marker labeling, which is simple and quick, is popular in detecting neuronal apoptosis.5 Another method, double staining using immunoenzymatic techniques, holds superiority in observing histological details over immunofluorescent methods,8,9 and nowadays multiple immunoenzymatic techniques are widely used in the clinical pathology department.9 However, the usage of double immunoenzymatic staining including TUNEL is relatively minor in SAH research works, and the detailed description of the morphology in dying neurons is hardly found in SAH brain.10–12 In addition, susceptible areas to neuronal apoptosis after SAH have not been investigated so far, especially in mice.
In this study, we investigated the precise morphological features of degenerating neurons in a mice SAH model under light microscope using double immunoenzymatic staining with TUNEL and an antibody for microtubule-associated protein 2 (MAP-2), which is a neuronal cytoplasm-specific marker,13,14 and sought the optimal observation area of post-SAH presumed neuronal apoptosis for future research works. In double immunolabeling, as combining two antigens of different cellular localization is useful to obtain clear color contrast, MAP-2 existing in the cytoplasm and dendrites was selected as a neuronal marker in contradistinction to TUNEL in the nucleus in this study.
Materials and Methods
Study Protocol
All procedures were approved by the Animal Ethics Review Committee of Mie University. The study complies with the institution’s Guidelines for Animal Experiments and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting in vivo experiments. C57BL/6 mice (male; age, 10–12 weeks; body weight, 25–30 g; SLC, Hamamatsu, Japan) were used for SAH or sham modeling. First, at 24 hr after modeling, neurological score was tested, mice were sacrificed, and SAH grades were evaluated. Then, animal’s brain was used for making paraffin-embedded sections. Left (puncture-side) cerebral cortex at 1.5 mm posterior to the bregma was studied. Mice randomly underwent sham or SAH operation until the number of animals reached n=5 in the sham, mild, and moderate SAH groups, respectively. Second, 7-day observation experiment was performed. Sham or SAH modeling was continued until the number of surviving animals at 7 days after modeling reached n=5 per group. Neurological score was tested every day. Animals were sacrificed after assessing neurological score at 7 days after modeling, and animal’s brain was studied as described above.
SAH Modeling and Grading
Endovascular perforation SAH modeling in mice and the assessment of the severity of SAH were conducted as previously described.6 Briefly, mice were anesthetized with an intraperitoneal injection of Avertin® (2,2,2-tribromoethanol; 250 μg/g body weight) solution, positioned supinely, and their skins were cut in the midline. Then, the left carotid artery was exposed. The left external carotid artery (ECA) was cut, and the left internal carotid artery (ICA) and the left common carotid artery (CCA) were clipped. A 4-0 monofilament with a sharpened tip was inserted from the ECA into the ICA. Clip of the ICA was taken off and the filament was pushed further to perforate the bifurcation of the left anterior cerebral artery and the left middle cerebral artery. The filament was withdrawn and the stump of ECA was coagulated. Clip of CCA was also taken off: At that time, a transient decrease in the rate of respiration and carotid artery pulsation was observed, probably reflexing a Cushing reflex,15 which suggested that animals suffered from transient global cerebral ischemia. Sham operation was performed in the same way but did not perforate the artery. Blood pressure and heart rate were monitored via the tail (BP-98A; Softron, Tokyo, Japan) during surgery. Rectal temperature was kept at 37C, in Celsius units, during operation.
SAH grade was evaluated in a blind fashion using the picture of mouse’s brain basal cistern as previously described.6 Briefly, the basal cistern was divided into six segments, and each segment was allotted a grade from 0 to 3 depending on the amount of SAH. A total score ranging from 0 to 18 was determined by summing the scores. In the SAH mice at 24 hr, surviving mice were divided into mild (SAH grades 5–7) and moderate (SAH grades 8–12) SAH groups.
Neurological Score
Neurological evaluation was blindly made as previously described.6 Briefly, neurological scores (3–18) were assessed by summing up six test scores (spontaneous activity, spontaneous movement of four limbs, forepaw outstretching, climbing, body proprioception, and response to whisker stimulation). Higher scores indicate better neurological status. Neurological scores were analyzed by Mann–Whitney U-tests or Kruskal–Wallis tests, followed by Steel–Dwass multiple comparisons.
Hematoxylin and Eosin (H&E) and Cresyl Violet Stainings
Formalin-perfused and paraffin-embedded coronal sections of mouse’s brain were stained with H&E or cresyl violet as previously described.6,16,17 In brief, mice were deeply anesthetized and perfused with cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde for brain fixation. The brains were removed, embedded in paraffin, and coronally cut into 4 μm sections. For H&E staining, sections were dewaxed, rehydrated, and stained with hematoxylin followed by eosin. Then, sections were dehydrated with graded alcohols, placed in xylenes, and mounted. For cresyl violet staining, sections were stained with 0.1% cresyl violet solution (pH 3.7; cat. no. 41021; Muto Pure Chemicals, Tokyo, Japan).
TUNEL Staining
TUNEL staining was performed according to the manufacturer’s instruction (in situ cell death detection kit ver. 15; Roche Inc., Mannheim, Germany). In brief, after being dewaxed and rehydrated, sections were incubated with proteinase K (Roche) solution for 15 min at room temperature, incubated with TUNEL solution at 37C, in Celsius units, for 1 hr, washed with PBS, and incubated with 3% hydrogen peroxide (H2O2) for 10 min. Then, the sections were incubated with peroxidase solution at 37C, in Celsius units, for 30 min and washed with PBS. Diaminobenzidine (DAB) was used as the chromogen. After TUNEL staining, sections were stained lightly with cresyl violet to stain neurons as previously described.16 Incubation with labeling solution without the enzyme (TdT reagent) served as a negative control.
MAP-2 Single Staining
Staining procedures were performed using a commercially available kit (Vectastain Elite ABC Rabbit AP kit; Vector, Burlingame, CA). After being dewaxed and rehydrated, sections underwent heat-induced antigen retrieval (HIAR) in 10 mM citrate buffer (pH 6.0) at 90C, in Celsius units, for 20 min. Then, sections were brought back to room temperature naturally, treated with normal goat serum for 30 min to block nonspecific binding, and incubated with rabbit polyclonal anti-MAP-2 antibody (1:500, cat. no. ab32454; Abcam, Cambridge, MA) at 4C, in Celsius units, overnight. Next, sections were incubated with anti-rabbit secondary antibody for 30 min at room temperature, washed with tris-buffered saline with tween 20 (TBS-T), incubated with avidin–biotin complex conjugated with alkaline phosphatase for 30 min at room temperature, and again washed with TBS-T. Sections were dehydrated, cleared (Lemosol A®, cat, no. 120-04411; Wako, Tokyo, Japan), and mounted with xylene-free medium (Softmount®, cat. no. 192-16301; Wako, Tokyo, Japan). Negative control was incubated with buffer alone instead of anti-MAP-2 antibody.
Double Immunolabeling for TUNEL and MAP-2
As to double immunolabeling, sections were first stained with TUNEL reaction, and peroxidase-mediated color reaction was developed with DAB as described above. Then, HIAR (at 90C, in Celsius units, for 20 min in 10 mM citrate butter, pH 6.0) and peroxidase inactivation with 3% H2O2 were performed as previously described.10 This step was essential to block crossreaction of enzymes or antibodies between the first and the second staining methods. Then, sections were used for immunostaining with anti-MAP-2 antibody, secondary antibody, and avidin–biotin complex and colored by alkaline phosphatase–mediated reaction with vector blue (cat. no. SK-5300; Vector). Color development with vector blue was made more lightly than that with MAP-2 single staining to be able to distinguish the brown color of double-labeled neurons. Two negative controls of double staining were generated by omission of the TdT reagent in the TUNEL assay or anti-MAP-2 antibody in the second staining.
Cell Counting
In the 24-hr experiment, the number of TUNEL-positive neurons in five sequential fields in the left parietal, lateral, and temporal base cortices or in the CA1 and CA3 sectors in the left hippocampus was counted at 400× magnification in each sample in a blinded manner. In the 7-day experiment, the number of both TUNEL-positive and TUNEL-negative neurons in the CA1 and CA3 sectors and in the dentate gyrus (DG) in the left hippocampus was counted in the same way. TUNEL-positive included type I and II TUNEL-positive nuclear staining irrespective of cytoplasmic TUNEL labeling, which is sometimes caused by the leakage of deoxyribonucleic acid (DNA) from the nucleus into the cytoplasm.12 However, TUNEL-positive but MAP-2-negative cells were not counted to exclude non-neuronal cells. Also, cells with TUNEL-negative and normally appeared nuclei were not counted although their cytoplasm appeared to be labeled with TUNEL, because the cytoplasmic TUNEL labeling was considered nonspecific. Thus, the number of double-labeled cells, that is, TUNEL-positive neurons per square millimeter, was measured in each sample. The number of normally appearing TUNEL-negative neurons per square millimeter was also measured in each sample in the 7-day experiment. Data were expressed as mean ± SD and compared among the sham, mild SAH, and moderate SAH groups using one-way ANOVA followed by Student–Neuman–Keuls post hoc tests in the 24-hr experiment and between the sham and SAH groups using unpaired t-tests in the 7-day experiment. Value of p<0.05 was considered significant.
Results
In the 24-hr experiment, one of 11 mice undergoing SAH operation died within 24 hr of surgery. The remaining mice were sacrificed at 24 hr after surgery: Five mice had mild SAH and the other five mice had moderate-grade SAH (Fig. 1A). No mice in the sham group died before euthanasia. Mice in the sham and mild SAH groups showed good neurological performance, whereas neurological deterioration tended to be severe in the moderate SAH group (Fig. 1B).
Figure 1.

Subarachnoid hemorrhage (SAH) grade (A) and neurological score (B). Individual SAH grades are expressed as squares and triangles in the mild and moderate SAH groups, respectively (A). Moderate SAH mice tended to have worse neurological scores compared with the other groups (B; p>0.05, Kruskal–Wallis test).
In the 7-day experiment, because of a high mortality (Supplemental Table S1) and the diverse natural clearance of subarachnoid clot (SAH grade at 7 days—0, 3, 3, 3, and 9, respectively), all surviving SAH mice were compared as a SAH group with the sham group. Neurological scores were significantly worse in the SAH group compared with the sham group at 24 hr after surgery (Supplemental Fig. S1).
H&E, Cresyl Violet, TUNEL Stainings, and Double Immunolabeling for TUNEL and MAP-2 at 24 Hr
In layer II of the left temporal base cortex in a moderate-grade SAH mouse, H&E and cresyl violet stainings revealed pyknotic nuclei, small aggregates of chromatin, and shrunken cell body of degenerating neurons (Fig. 2A and B). Cell body of morphologically abnormal neurons was also detectable in MAP-2 single staining (Fig. 2C). However, condensed nuclei were colored brown, and sometimes it is difficult to distinguish small dying neurons from glia in TUNEL staining with counterstaining of cresyl violet (Fig. 2D). Cytoplasm was also stained lightly in TUNEL staining (Fig. 2D), but negative controls denied nonspecific staining (Supplemental Fig. S2). In TUNEL and MAP-2 double immunolabeling, cells with brown-colored nuclei and blue-colored cytoplasm were detected and were supposed to be neurons with DNA damage (Fig. 2E–G). Intact neurons were morphologically defined as cells with bright oval nuclei and prominent nucleoli (Fig. 2).
Figure 2.
Neuronal deoxyribonucleic acid (DNA) damage in the left temporal base cortex at 24 hr after subarachnoid hemorrhage (SAH) in mice. Serial sections of the same sample from a moderate-grade SAH mouse are used: (A) hematoxylin-eosin staining; (B) cresyl violet staining; (C) microtubule-associated protein 2 (MAP-2) staining; (D) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining counterstained with cresyl violet; (E) double immunolabeling with TUNEL and anti-MAP-2 antibody; (F and G) negative controls of double immunolabeling in which terminal deoxynucleotidyl transferase enzyme (F) or anti-MAP-2 antibody (G) is omitted, showing no crossreaction. Framed areas on panels A to E are magnified in the lower left insets to show morphologically apoptosis-appearing neurons in each staining: shrunken cytoplasm and dense nuclei profiled by hematoxylin and eosin (A), densely stained cells with cresyl violet (B), shrunken neuronal cell body revealed by MAP-2 (C), TUNEL-positive neuron having nucleoli clearly stained with cresyl violet (D), and TUNEL-positive neuron with MAP-2-positive cytoplasm (E). Arrows, morphologically apoptosis-appearing neurons containing small aggregates of chromatin; double arrows, corkscrew-like fibers of morphologically abnormal neurons; single arrowheads, small cells which may be glia; double arrowheads, small DNA-damaged cells densely stained with cresyl violet that may be glia or neuron; white arrows, morphologically intact neurons; white arrowheads, MAP-2-positive (F) or TUNEL-positive (G) neurons. Scale bar, 50 μm in panels and 10 μm in insets.
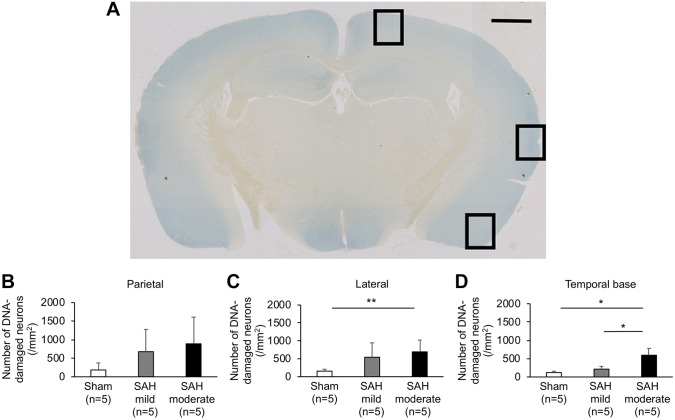
Higher Grade SAH Induces More Neuronal Death With DNA Damage in the Cerebral Cortex at 24 Hr
Double immunolabeling for TUNEL and MAP-2 showed that the majority of neurons in the sham group were morphologically normal in the left parietal, lateral, and temporal base cortices (Figs. 3 and 4). Mild-grade SAH mice had some neurons with DNA damage, and moderate-grade SAH mice had more neurons with DNA damage in all three cortices. However, the number of neurons with DNA damage was significantly different among sham, mild-, and moderate-grade SAH mice only in the left temporal base cortex (p<0.01, one-way ANOVA; Fig. 3). Moderate-grade SAH mice also have significantly more DNA-damaged neurons compared with sham, but not mild-grade SAH mice in the lateral cortex (p<0.05, one-way ANOVA).
Figure 3.
Representative brain slice showing the left parietal (upper rectangle), lateral (middle rectangle), and temporal base (lower rectangle) cortices at 1.5 mm posterior to the bregma (A) and the number of apoptotic neurons in the left parietal, lateral, and temporal base cortex (B–D) at 24 hr after subarachnoid hemorrhage (SAH) in mice evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling and microtubule-associated protein 2 double immunolabeling. In the temporal base cortex, the moderate SAH group has significantly more deoxyribonucleic acid (DNA)-damaged neurons compared with the other groups (one-way ANOVA, *p<0.01). The moderate SAH group also has significantly more DNA-damaged neurons compared with the sham group in the lateral cortex (one-way ANOVA, **p<0.05). Scale bar, 1 mm.
Figure 4.
Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and microtubule-associated protein 2 (MAP-2) double immunolabeling showing neuronal deoxyribonucleic acid (DNA) damage in the left parietal (A–C), lateral (D–F), and temporal base (G–I) cortices at 1.5 mm posterior to the bregma at 24 hr in sham (A, D and G), mild subarachnoid hemorrhage (SAH; B, E, and H), and moderate SAH (C, F, and I) mice. Note that little neuronal DNA damage is shown in the sham group, whereas the moderate SAH group extensively has DNA-damaged neurons. Framed areas on panels A to I are magnified in the lower left insets to show a representative neuron in each group: morphologically intact neurons with bright oval nuclei (A, D, and G), and DNA-damaged neurons with TUNEL-positive nuclei and shrunken MAP-2-positive cell body (B, C, E, F, H, and I). Arrows, DNA-damaged neurons; double arrows, corkscrew-like fibers of DNA-damaged neurons; white arrows, morphologically intact neurons. Scale bar, 50 μm in panels and 10 μm in insets.
As to the left hippocampus, the CA1 and CA3 sectors were evaluated regarding neuronal death with DNA damage using TUNEL and MAP-2 double immunolabeling (Fig. 5). There were only a few neurons with DNA damage found in both sectors even in moderate-grade SAH mice. Neurons in the CA1 and CA3 sectors in the hippocampus seemed less susceptible to DNA damage compared with the cerebral cortex in a setting of experimental SAH by endovascular perforation.
Figure 5.
Representative brain slice showing the CA1 (upper rectangle) and CA3 (right rectangle) sectors of the left hippocampus at 1.5 mm posterior to the bregma (A), and representative terminal deoxynucleotidyl transferase dUTP nick end labeling and microtubule-associated protein 2 double immunolabeling showing neuronal deoxyribonucleic acid (DNA) damage in the CA1 (B–D) and CA3 (E–G) sectors of the left hippocampus at 24 hr in sham (B and E), mild subarachnoid hemorrhage (SAH; C and F), and moderate SAH (D and G) mice. A few DNA-damaged neurons (arrows and framed areas on panels C, D and G) are shown in both sectors in mild and moderate SAH mice. Framed areas on panels B to G are magnified in the lower left insets to show a representative neuron in each group: morphologically intact neurons with bright oval nuclei (B, E, and F), and DNA-damaged neurons (C, D, and G). Scale bar, 500 μm in panel A, 50 μm in panels B to G, and 10 μm in insets. Contrast and brightness enhancement on entire images are performed (A–G).
Morphological Features of Different Stages of Neuronal Death With DNA Damage at 24 Hr After SAH
In TUNEL and MAP-2 double immunolabeling, neurons with different degrees of shrunken nuclei and cytoplasm were found in the left cerebral cortex in moderate-grade SAH mice (Fig. 6). TUNEL-positive shrunken nuclei and MAP-2-positive morphologically abnormal cell bodies indicated relatively early stages of neuronal death with DNA damage, which had MAP-2-positive curl-like structures running vertically in the left lateral and temporal base cortices (Figs. 2, 4 and 6). In late stages of neuronal death with DNA damage, MAP-2 immunoreactivity was found only in shrunken cytoplasm. MAP-2-negative but TUNEL-positive cells were also shown in empty holes and considered to be an end stage of dying neurons with DNA damage or non-neuronal cells (Fig. 6). Empty holes were probably produced through cutting sections or resulted from cell shrinkage (Fig. 6).
Figure 6.
Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and microtubule-associated protein 2 (MAP-2) double immunolabeling showing neurons with different stages of deoxyribonucleic acid (DNA) damage in the left temporal base (A) and lateral (B) cortices at 24 hr in moderate-grade subarachnoid hemorrhage (SAH) mice. Arrows, relatively early stages of DNA-damaged neurons with TUNEL-positive shrunken nuclei and MAP-2-positive morphologically abnormal cell body; dotted arrows, late stages of DNA-damaged neurons in which MAP-2 immunoreactivity is shown only in shrunken cytoplasm; double arrows, vertically running curl-like structures of early stages of DNA-damaged neurons; double dotted arrows, TUNEL-positive but MAP-2-negative cells in empty holes, suggesting end stages of DNA-damaged neurons, glia, or endothelial cells; white arrows, morphologically intact neurons. Scale bar, 50 μm.
Neuronal Death With DNA Damage in the Hippocampus at 7 Days After SAH
In the 7-day experiment, more neurons with DNA damage were observed in all three regions in the hippocampus in the SAH group compared with the sham group, and the difference reached significance only in the CA1 sector (Supplemental Figs. S3 and S4).
Discussion
In this study, we used several different staining methods, including TUNEL-combined double immunolabeling, and visualized both nuclear and cytoplasmic changes in neurons with DNA damage after SAH in mice. This study also showed that the temporal base cortex may be the most suitable area for the future studies of neurons with DNA damage at 24 hr and that hippocampal neuronal death with DNA damage may occur later at 7 days after SAH in mice.
Histological detail is the most important in detecting apoptosis. Classical features of apoptotic cells are chromatin-condensed and shrunken nuclei, shrunken cytoplasm, membrane blebbing, and accompanied apoptotic bodies.11,12,16,18–21 Morphologically apoptotic cells with eosinophilic inclusions are observed in H&E staining.20 Cytoplasmic TUNEL staining is sometimes observed probably due to leakage of DNA from the nucleus.12,22 Cerebral cortex is composed of several cell types such as neurons and glia, and therefore neuron-specific staining is preferable when observing neuronal apoptosis in this area. Cresyl violet (Nissl) staining is usually regarded as a neuron-specific staining method,16,21 but glial nuclei are also stained with this dye,17 indicating that it is sometimes difficult to distinguish small neurons from glia with this staining method. For this reason, we attempted to combine TUNEL staining with MAP-2 immunolabeling to identify dying neurons. Generally, double staining methods are performed with immunofluorescent techniques.5 TUNEL-combined double immunolabeling is relatively uncommonly used,10–12 but the immunolabeling enables observation of precise structures under light microscope.8,9 Using the double immunolabeling method, we could obtain more information about dying neurons after SAH in mice in this study. However, the double immunoenzymatic staining method has some drawbacks, including the problems of nonspecific staining, occasionally poor contrast, and the difficulty in setting the optimal staining conditions according to the localization of target proteins and the characteristics of used antibodies compared with immunofluorescent techniques: Thus, we should use more appropriate method depending on the aim of studies.
Interestingly, our results revealed that dying neurons had both nuclear and cytoskeletal changes after SAH, the latter of which was revealed by MAP-2 staining. Cytoskeletal protein degradation has been suggested to be a key factor in investigating neuronal damage after ischemia: MAP-2 is used as a marker of cytoskeletal breakdown in ischemia models,13,14,23 whereas there seems to be less attention paid to cytoskeletal changes in the field of SAH. The differences in cell death processes such as DNA degradation and cytoskeletal breakdown have been suggested to reflect different degrees of ischemic insults: Mild ischemia such as 5-min bilateral CCA occlusion caused DNA cleavage followed by loss of MAP-2 immunoreactivity, whereas severe ischemia such as 30-min unilateral CCA occlusion or complete ischemia with decapitation resulted in loss of MAP-2 immunoreactivity faster than nuclear morphological changes in the hippocampus of gerbils.13 The former might express apoptotic neuronal death, whereas the latter might show necrotic neuronal death, resulting from differences in the activation of responsible proteases and endonucleases in neurons damaged by diverse severities of ischemic insults.13 Our results showed that nuclear and cytoskeletal changes revealed by TUNEL and MAP-2 double staining occurred roughly parallel in the majority of neurons, suggesting that the type of cell death occurring in the neurons is apoptotic. In addition, our results showed that dying neurons with MAP-2 immunoreactivities had curl-like changes in fibers. Similar findings were reported in animal models of Alzheimer’s disease,24 ibotenic acid neurotoxicity,25 and ischemic stroke.14 Ishida et al.25 observed dying neurons with corkscrew-like dendrites at an early stage of ibotenic acid injection, possibly reflecting diffuse damage on microtubules/neurofilaments.25 Härtig et al.14 reported that corkscrew-like changes in fibers were observed in cerebral ischemia animal models as well as autopsy samples from an ischemic stroke patient: Ischemic insults caused the microtubule-associated protein tau to be more affected, whereas neuronal nuclei to be less diminished, possibly providing cytoskeletal proteins including MAP-2 as key players during the transition processes toward prolonged neuronal injury. Taken together, because DNA-damaged neurons after experimental SAH in mice had cytoskeletal changes like in human ischemic stroke, future experimental and translational works may prove these proteins as new therapeutic targets for neuronal injury after SAH.
The development of neuronal apoptosis was reported in the DG of hippocampus in autopsy cases of clinical SAH.26 Severe global cerebral ischemia results in necrosis, which is irreversible immediately after ictus, and most of the patients cannot survive.2 However, apoptotic neuronal death is treatable, may have important implications for the long-term sequelae of SAH, and therefore can be a therapeutic target to improve outcomes in SAH patients.2 In this study, we used an endovascular perforation SAH model in C57BL/6 mice, which mimics the pathophysiology of human SAH: The model is accompanied with diffuse basal subarachnoid clot and acute ICP elevation followed by global cerebral ischemia.4,7 Besides cytoskeletal changes as described above, our study showed that moderate SAH that was produced by endovascular perforation caused EBI in terms of neuronal death with DNA damage in cerebral cortices at 24 hr1,5–7: In contrast, neuronal death with DNA damage in the hippocampus was uncommon at 24 hr and developed especially in the CA1 region at 7 days after SAH. As far as we know, only one study demonstrated neuronal apoptosis in the entire puncture-side hippocampus at 24 hr after SAH by endovascular perforation in mice,27 although many studies have reported neuronal apoptosis in cerebral cortices in endovascular perforation SAH mice and hippocampal neuronal apoptosis in other animal models of SAH.28 Muroi et al.15 showed Fluoro-Jade C–positive degenerative neurons in the CA1, CA3 and DG regions at days 2 and 3, but revealed TUNEL-positive neurons in the ipsilateral cerebral cortex, not in the hippocampus in endovascular perforation SAH mice. Feiler et al.7 demonstrated neuronal cell loss in the bilateral CA1, CA2, and CA3 regions at 7 days after SAH by endovascular perforation in mice, suggesting the development of neuronal apoptosis in the hippocampus, whereas Milner et al.28 failed to show neuronal cell loss in the CA1 region at 3 weeks after SAH in the same model. The discrepancy could be explained by the difference in SAH severity among studies and arterial anatomy among species and strains: For example, the circle of Willis in ddY and C57BL/6 mice frequently lacks patency of posterior communicating artery compared with other murine strains and rats, and therefore brain tissues in the posterior cerebral artery territory such as the hippocampus are likely to succumb to ischemic insults in these strains.29,30 On the contrary, 5-min cardiac arrest–induced cerebral ischemia in C57BL/6 and SV129 mice was unexpectedly not enough to cause hippocampal neuronal injury.31 Schauwecker and Steward32 reported that hippocampal neurons in C57BL/6 and BALB/c mice had resistance to excitotoxicity, whereas those in SV129 and FVB/N mice showed vulnerability to excitotoxicity as reported in rats. From these findings, there seems a possibility that hippocampal neurons in certain strains of mice including C57BL/6 used in this study have tolerance to ischemia or excitotoxicity possibly at least through different protein synthesis.31,32 More studies are needed as to the vulnerability of the hippocampus when investigating EBI using endovascular perforation SAH models in mice.
This study has some limitations. First, this is a descriptive study, and we observed only TUNEL staining reactivity in degenerating neurons, although many signaling molecules such as caspase-dependent or caspase-independent mechanisms are considered to work in neuronal death with DNA damage after SAH.2,33 In addition, the TUNEL method possibly recognizes different kinds of cell death, that is, not only apoptosis but also necrosis.12 Second, although both negative controls of double staining (TdT or anti-MAP-2 antibody omission) detected no crossreactions, intensive MAP-2 immunoreactivity in dying neurons in this study is somehow beyond explanation. Third, observation in this study was up to 7 days after SAH, and therefore longer observation would be needed to fully evaluate delayed neuronal death.
In conclusion, using the double immunoenzymatic technique, we showed that the temporal base cortex was one of reproducible areas of neuronal death with DNA damage after SAH in C57BL/6 mice and could observe detailed morphological changes of dying neurons after SAH in both the nucleus and the cytoskeleton under light microscope, the latter of which is possibly a new therapeutic target for neuronal injury after SAH.
Supplemental Material
Supplemental material, DS_10.1369_0022155419878181 for Morphological Characteristics of Neuronal Death After Experimental Subarachnoid Hemorrhage in Mice Using Double Immunoenzymatic Technique by Fumi Nakano, Lei Liu, Fumihiro Kawakita, Hideki Kanamaru, Yoshinari Nakatsuka, Hirofumi Nishikawa, Takeshi Okada, Masato Shiba and Hidenori Suzuki in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Chiduru Yamamoto-Nakamura (Department of Neurosurgery, Mie University Graduate School of Medicine) for her assistance with administrative support.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: FN and HS designed the study. FN, FK, HK, HN, and TO performed experiments and acquisition of data. LL, YN, MS, and HS performed data analyses. All authors contributed to interpretation of data. FN and HS wrote and edited the manuscript. LL, FK, HK, YN, HN, TO, and MS critically read and revised the manuscript before submission.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a grant-in-aid for Scientific Research from Japan Society for the Promotion of Science to Drs. Shiba (17K16640) and Suzuki (17K10825).
Contributor Information
Fumi Nakano, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Lei Liu, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Fumihiro Kawakita, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Hideki Kanamaru, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Yoshinari Nakatsuka, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Hirofumi Nishikawa, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Takeshi Okada, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Masato Shiba, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Hidenori Suzuki, Department of Neurosurgery, Graduate School of Medicine, Mie University, Tsu, Japan.
Literature Cited
- 1. Suzuki H. What is early brain injury? Transl Stroke Res. 2015;6:1–3. [DOI] [PubMed] [Google Scholar]
- 2. Cahill WJ, Calvert JH, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki H, Shiba M, Nakatsuka Y, Nakano F, Nishikawa H. Higher cerebrospinal fluid pH may contribute to the development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2017;8:165–73. [DOI] [PubMed] [Google Scholar]
- 4. Friedrich B, Müller F, Feiler S, Schöller K, Plesnila N. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab. 2012;32:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabri M, Kawashima A, Ai J, Macdonald RL. Neuronal and astrocytic apoptosis after subarachnoid hemorrhage: a possible cause for poor prognosis. Brain Res. 2008;1238:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W, Suzuki H. Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl Stroke Res. 2014;5:238–47. [DOI] [PubMed] [Google Scholar]
- 7. Feiler S, Friedrich B, Schöller K, Thal SC, Plesnila N. Standardized induction of subarachnoid hemorrhage in mice by intracranial pressure monitoring. J Neurosci Methods. 2010;190:164–70. [DOI] [PubMed] [Google Scholar]
- 8. Mason DY, Sammons R. Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol. 1978;31:454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanagita E, Kamoshida S, Imagawa N, Itoh T. Immunohistochemistry-based cell cycle detection (iCCD): a novel system to visualize cell kinetics on formalin-fixed paraffin-embedded tissues. Am J Surg Pathol. 2012;36:769–73. [DOI] [PubMed] [Google Scholar]
- 10. Short BG, Zimmerman DM, Schwartz LW. Automated double labeling of proliferation and apoptosis in glutathione S-transferase-positive hepatocytes in rats. J Histochem Cytochem. 1997;45:1299–305. [DOI] [PubMed] [Google Scholar]
- 11. Kokkonen TS, Karttunen TJ. Fas/Fas ligand-mediated apoptosis in different cell lineages and functional compartments of human lymph nodes. J Histochem Cytochem. 2010;58:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lima FB, Bethea CL. Ovarian steroids decrease DNA fragmentation in the serotonin neurons of non-injured rhesus macaques. Mol Psychiatry. 2010;15:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yagita Y, Matsumoto M, Kitagawa K, Mabuchi T, Ohtsuki T, Hori M, Yanagihara T. DNA cleavage and proteolysis of microtubule-associated protein 2 after cerebral ischemia of different severity. Neuroscience. 1999;92:1417–24. [DOI] [PubMed] [Google Scholar]
- 14. Härtig W, Krueger M, Hofmann S, Preißler H, Märkel M, Frydrychowicz C, Mueller WC, Bechmann I, Michalski D. Up-regulation of neurofilament light chains is associated with diminished immunoreactivities for MAP2 and tau after ischemic stroke in rodents and in a human case. J Chem Neuroanat. 2016;78:140–8. [DOI] [PubMed] [Google Scholar]
- 15. Muroi C, Fujioka M, Okuchi K, Fandino J, Keller E, Sakamoto Y, Mishima K, Iwasaki K, Fujiwara M. Filament perforation model for mouse subarachnoid hemorrhage: surgical-technical considerations. Br J Neurosurg. 2014;28:722–32. [DOI] [PubMed] [Google Scholar]
- 16. Capurso SA, Calhoun ME, Sukhov RR, Mouton PR, Price DL, Koliatsos VE. Deafferentation causes apoptosis in cortical sensory neurons in the adult rat. J Neurosci. 1997;17:7372–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gittins R, Harrison PJ. Neuronal density, size and shape in the human anterior cingulate cortex: a comparison of Nissl and NeuN staining. Brain Res Bull. 2004;63:155–60. [DOI] [PubMed] [Google Scholar]
- 18. Al-Abdulla NA, Martin LJ. Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus. Am J Pathol. 1998;153:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Abdulla NA, Portera-Cailliau C, Martin LJ. Occipital cortex ablation in adult rat causes retrograde neuronal death in the lateral geniculate nucleus that resembles apoptosis. Neuroscience. 1998;86:191–209. [DOI] [PubMed] [Google Scholar]
- 20. Bennett SA, Tenniswood M, Chen JH, Davidson CM, Keyes MT, Fortin T, Pappas BA. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 1998;9:161–6. [DOI] [PubMed] [Google Scholar]
- 21. Wang B, Armstrong JS, Lee JH, Bhalala U, Kulikowicz E, Zhang H, Reyes M, Moy N, Spicer D, Zhu J, Yang ZJ, Koehler RC, Martin LJ, Lee JK. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2015;35:781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, Mclntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–83. [PMC free article] [PubMed] [Google Scholar]
- 23. Kitagawa K, Matsumoto M, Saido TC, Ohtsuki T, Kuwabara K, Yagita Y, Mabuchi T, Yanagihara T, Hori M. Species differences in fodrin proteolysis in the ischemic brain. J Neurosci Res. 1999;55:643–9. [DOI] [PubMed] [Google Scholar]
- 24. Szabados T, Dul C, Majtényi K, Hargitai J, Pénzes Z, Urbanics R. A chronic Alzheimer’s model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav Brain Res. 2004;154:31–40. [DOI] [PubMed] [Google Scholar]
- 25. Ishida K, Shimizu H, Hida H, Urakawa S, Ida K, Nishino H. Argyrophilic dark neurons represent various states of neuronal damage in brain insults: some come to die and others survive. Neuroscience. 2004;125:633–44. [DOI] [PubMed] [Google Scholar]
- 26. Nau R, Haase S, Bunkowski S, Brück W. Neuronal apoptosis in the dentate gyrus in humans with subarachnoid hemorrhage and cerebral hypoxia. Brain Pathol. 2002;12:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li M, Wang W, Mai H, Zhang X, Wang J, Gao Y, Wang Y, Deng G, Gao L, Zhou S, Chen Q, Wang X. Methazolamide improves neurological behavior by inhibition of neuron apoptosis in subarachnoid hemorrhage mice. Sci Rep. 2016;6:35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milner E, Holtzman JC, Friess S, Hartman RE, Brody DL, Han BH, Zipfel GJ. Endovascular perforation subarachnoid hemorrhage fails to cause Morris water maze deficits in the mouse. J Cereb Blood Flow Metab. 2014;34:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–9. [DOI] [PubMed] [Google Scholar]
- 30. Okuyama S, Okuyama J, Okuyama J, Tamatsu Y, Shimada K, Hoshi H, Iwai J. The arterial circle of Willis of the mouse helps to decipher secrets of cerebral vascular accidents in the human. Med Hypotheses. 2004;63:997–1009. [DOI] [PubMed] [Google Scholar]
- 31. Kawahara N, Kawai K, Toyoda T, Nakatomi H, Furuya K, Kirino T. Cardiac arrest cerebral ischemia model in mice failed to cause delayed neuronal death in the hippocampus. Neurosci Lett. 2002;322:91–4. [DOI] [PubMed] [Google Scholar]
- 32. Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu L, Fujimoto M, Nakano F, Nishikawa H, Okada T, Kawakita F, Imanaka-Yoshida K, Yoshida T, Suzuki H. Deficiency of tenascin-C alleviates neuronal apoptosis and neuroinflammation after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2018;55:8346–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155419878181 for Morphological Characteristics of Neuronal Death After Experimental Subarachnoid Hemorrhage in Mice Using Double Immunoenzymatic Technique by Fumi Nakano, Lei Liu, Fumihiro Kawakita, Hideki Kanamaru, Yoshinari Nakatsuka, Hirofumi Nishikawa, Takeshi Okada, Masato Shiba and Hidenori Suzuki in Journal of Histochemistry & Cytochemistry