Abstract
Background
Low-dose mercury (Hg) exposure has been associated with cardiovascular diseases, diabetes, and obesity in adults, but it is unknown the metabolic consequence of in utero Hg exposure. This study aimed to investigate the association between in utero Hg exposure and child overweight or obesity (OWO) and to explore if adequate maternal folate can mitigate Hg toxicity.
Methods
This prospective study included 1442 mother-child pairs recruited at birth and followed up to age 15 years. Maternal Hg in red blood cells and plasma folate levels were measured in samples collected 1–3 days after delivery (a proxy for third trimester exposure). Adequate folate was defined as plasma folate ≥ 20.4 nmol/L. Childhood OWO was defined as body mass index ≥ 85% percentile for age and sex.
Results
The median (interquartile range) of maternal Hg levels were 2.11 (1.04–3.70) μg/L. Geometric mean (95% CI) of maternal folate levels were 31.1 (30.1–32.1) nmol/L. Maternal Hg levels were positively associated with child OWO from age 2–15 years, independent of maternal pre-pregnancy OWO, diabetes, and other covariates. The relative risk (RR = 1.24, 95% CI 1.05–1.47) of child OWO associated with the highest quartile of Hg exposure was 24% higher than those with the lowest quartile. Maternal pre-pregnancy OWO and/or diabetes additively enhanced Hg toxicity. The highest risk of child OWO was found among children of OWO and diabetic mothers in the top Hg quartile (RR = 2.06; 95% CI 1.56–2.71) compared to their counterparts. Furthermore, adequate maternal folate status mitigated Hg toxicity. Given top quartile Hg exposure, adequate maternal folate was associated with a 34% reduction in child OWO risk (RR = 0.66, 95% CI 0.51–0.85) as compared with insufficient maternal folate. There was a suggestive interaction between maternal Hg and folate levels on child OWO risk (p for interaction = 0.086).
Conclusions
In this US urban, multi-ethnic population, elevated in utero Hg exposure was associated with a higher risk of OWO in childhood, and such risk was enhanced by maternal OWO and/or diabetes and reduced by adequate maternal folate. These findings underscore the need to screen for Hg and to optimize maternal folate status, especially among mothers with OWO and/or diabetes.
Keywords: Diabetes, Folate, In utero, Mercury, Metal, Nutrient, Overweight, Obesity
Background
Mercury (Hg) is a persistent and widespread environmental pollutant and is highly toxic to human health worldwide. Exposure to toxic Hg is ubiquitous in the general US population [1]. The National Health and Nutrition Examination Survey found that 85.2% of the population had detectable levels of Hg [1]. Methylmercury (MeHg, the major constituent of organic Hg) is of particular concern due to its ability to cross the placenta and blood-brain barrier during pregnancy, as well as bioaccumulation [2]. Neuro-toxic effects of MeHg have been well studied [3], and MeHg’s role in cardiometabolic health is beginning to be recognized [4, 5]. Studies in adults have found that low-dose Hg exposure was associated with obesity [6] and visceral adipose tissue [7], suggesting that Hg is an obesogen.
The developing fetus is particularly vulnerable to nutritional and environmental exposures [8, 9]. Previous studies suggest that obesity epidemic could be, in part, due to in utero exposures to adverse environments [10, 11]. Although Hg has been recognized as an obesogen [6, 12, 13], literature is limited for the link of in utero Hg exposure and obesity in later life. Especially, the role of Hg in the inter-generational risk of obesity is yet to be explored. Given childhood obesity persists into adulthood and is linked to cardiovascular disease in later life, a critical unanswered question is whether in utero low-dose Hg exposure increases a child’s risk for obesity in childhood and beyond.
In light of widespread maternal exposure to Hg in the USA, there is an urgent need to identify potentially important mitigating factors. Because antioxidants are desirable in the process of Hg detoxification [14], folate, an essential B vitamin, may be a candidate due to its antioxidant and anti-inflammatory properties [15, 16]. Studies have demonstrated that folate has protective effects on cardiovascular diseases [17] and might reduce the adverse impacts of environmental chemicals on the fetus [18]. One study has documented an inverse relationship between serum folate levels and blood Hg concentration during pregnancy [19]. Furthermore, our previous studies unveiled a protective effect of folate on inter-generational risks of obesity and elevated blood pressure [11, 20]. However, the role of folate in the setting of in utero Hg exposure and inter-generational link of obesity is yet to be examined.
Using a well-established, population-based birth cohort, this study sought to characterize the potential adverse effect of in utero Hg exposure on childhood overweight or obesity (OWO) up to 15 years of age. We also assessed whether maternal pre-pregnancy OWO and/or diabetes enhances the toxic effect of in utero Hg exposure. In addition, we evaluated to what extent adequate maternal folate status during pregnancy counteracts the toxicity of in utero Hg exposure on childhood OWO.
Study population and methods
Study population
The study protocol was approved by the Institutional Review Boards of Boston Medical Center and Johns Hopkins Bloomberg School of Public Health. Written informed consent was obtained from each study child’s biological mother or the study child. Participants of this study came from the Boston Birth Cohort (BBC), who were enrolled at birth and followed prospectively thereafter at the Boston Medical Center. Detailed information on participant enrollment has been described previously [21]. Briefly, the mother-infant pairs were enrolled 24 to 72 h after delivery. After obtaining written informed consent, trained research staff interviewed mothers using a standardized questionnaire. Maternal blood was drawn for measurement of Hg in red blood cell (RBC) and plasma folate concentrations. Pertinent clinical information was obtained by a review of maternal and infant medical records, including prenatal ultrasonographic reports, laboratory reports, and information on pregnancy complications, and birth outcomes. As of July 2018, 3163 children who enrolled in the BBC and received primary care at the Boston Medical Center were followed. Of these children, 1551 mothers had measurements taken for Hg concentration in the RBCs. There were 109 children excluded due to a lack of body mass index (BMI) data between ages 2–15 years. Finally, this study included 1442 mother-child pairs who had complete data (Additional file 1: Figure S1). The characteristics were similar between the included and excluded samples, except that more Black women and less preterm births were in the included samples (Additional file 1: Table S1).
Ascertainment of maternal Hg levels in RBCs
Maternal RBC-Hg concentrations were measured using inductively coupled plasma mass spectrometry (ICP-MS) on an Agilent 8900 QQQ (Agilent Technologies Inc., Santa Clara, CA) at the New Jersey Department of Health, Environmental and Chemical Laboratory Services, Metals Laboratory, Trenton, New Jersey. This laboratory is certified by the US Centers for Disease Control and Prevention, Clinical Laboratory Improvement Amendment, and Clinical Laboratory Improvement Services for analysis of clinical samples. The analytical procedures followed standard protocols for quality control and assurance. The quality control includes a calibration curve comprised of a minimum of five points, continuous calibration verification, continuous calibration blank verification, duplicate samples, laboratory fortified matrix samples, blind duplicates, and a minimum of four second source quality control samples for every run. Calibration stock solution (catalog# SM-2107-042-50, High-Purity Standards, SC) and second source quality control samples (Blood Metals Quality Control Specimens, Lot 018, Wisconsin State Laboratory of Hygiene, WI) were used as National Institute of Standards and Technology (NIST)-traceable certified reference materials to calibrate and monitor the accuracy and interday and intraday repeatability of the analysis. The second source samples must recover within the established recovery range set by the manufacturer or within a tighter range established by the laboratory. The coefficient of variation (CV) was < 5.0%. The limit of detection (LOD) was 0.28 μg/L. If the concentration was below the LOD, the value was assigned to be the value of the LOD divided by the square root of two.
Ascertainment of maternal plasma folate concentrations
Maternal plasma folate concentrations were measured by a commercial laboratory via chemiluminescent immunoassay using a MAGLUMI 2000 Analyzer (Snibe Co., Ltd., Shenzhen, China). Each run included a calibration curve with five points and three internal quality controls. If the value of folate was above the maximum LOD, plasma samples were diluted at a ratio of 1:2 and then re-run. The inter-assay CV was < 4% [22]. In our previous study [11], we found that maternal plasma folate level ≥ 20.4 nmol/L was associated with a lower risk of child OWO and mitigated the inter-generational link of obesity. As such, we defined adequate maternal folate as maternal plasma folate ≥ 20.4 nmol/L.
Definition of maternal characteristics
Maternal variables, including age at time of delivery, race/ethnicity, parity, education, pre-pregnancy height and weight, cigarette smoking, and fish consumption, were based on maternal questionnaire interview. Maternal pre-pregnancy BMI was calculated as pre-pregnancy weight (kg) divided by squared height (m), and further dichotomized into non-OWO (BMI < 25 kg/m2) and OWO (BMI ≥ 25 kg/m2). Fish consumption was grouped into none, 1–2 servings/week, and ≥ 3 servings/week. Maternal pregnancy complications, including diabetes mellitus (either gestational diabetes or pre-existing diabetes), hypertensive disorders (pre-eclampsia, eclampsia, gestational hypertension, chronic hypertension, and HELLP [hemolysis, elevated liver enzymes, and low platelets syndrome]), were abstracted from medical records. Gestational age was estimated based on the first day of the last menstrual period, as recorded in the maternal medical record, or early (< 20 weeks) prenatal ultrasonographic results, as detailed in a previous publication [21].
Assessment of child’s birth outcomes and breastfeeding status
Child’s birthweight and gender were abstracted from the medical records. Birthweight for gestational age was calculated according to an established local reference population, and controlled for infant gender, gestational age, and ethnicity [21]. Fetal growth pattern was defined by birthweight for gestational age and grouped into small for gestational age (SGA) (gestational age specific birthweight < 10th percentile), large for gestational age (LGA) (birthweight > 90th percentile), and appropriate for gestational age (AGA) (birthweight in the 10th to 90th percentile for gestational age). Information regarding infant breastfeeding history was primarily assessed within the first 2 years of follow-up visits, and grouped into exclusively breastfeeding, exclusively formula feeding, or mixed breast and formula feeding [23].
Child BMI and overweight or obesity in childhood
Child weight and height were extracted from the medical records, which were measured by trained medical staff using the same clinical protocol and equipment during well-child visits. Before data analyses, careful data checking and cleaning of weight and height data was performed. At first, we removed extreme or biologically implausible values. Then, we identified outliners or erroneous height and weight values based on growth curve. When possible, erroneous height and weight values were corrected, otherwise the points were deleted. Age- and sex-specific BMI z-scores and percentiles were calculated using US national reference data [24], derived from a US representative sample. OWO was defined as BMI ≥ 85th percentile for age and sex [25]. The BBC has used rolling enrollment, so the length of postnatal follow-up and number of well-child visits varied greatly across study participants. It is known that OWO at an older age is more likely to persist into adulthood. Thus, we chose the last visit in each time period as the end point for OWO.
Statistical analysis
Demographic and clinical data are presented as either mean ± standard deviation (SD) or n(%) stratified by maternal Hg categories. Unadjusted trend p values across maternal Hg categories were calculated by Mantel-Haenszel χ2 for categorical variables and linear regression for continuous variables. The relationship between maternal Hg and child OWO is displayed using locally weighted regression smoothing plots (implemented using PROC LOESS in SAS, a nonparametric regression method).
Relative risk (RR) was estimated by Poisson regression with robust error variance to determine an association between maternal Hg and child OWO, adjusting for important covariates, including maternal age, race/ethnicity, education, parity, smoking, pre-pregnancy OWO, diabetes, hypertensive disorders, preterm birth, fetal growth pattern, and child’s breastfeeding status. To examine the persistence of the association from preschool age to school age to adolescents, we performed similar analyses stratified by child age at outcome assessment (e.g., at age 2–5 years, 6–9 years, and 10–15 years). In addition, we evaluated the joint risk attributed to maternal Hg categories and either or both pre-pregnancy OWO and diabetes. We tested the interaction of maternal pre-pregnancy OWO (as a binary variable) and Hg levels (as a continuous variable) or the interaction of maternal diabetes status and Hg levels on child’s risk of OWO by adding a multiplicative term in the models. Effect modification was assessed by the likelihood ratio test using an a priori α value of 0.05. We used same methods to investigate whether maternal adequate folate status can ameliorate the adverse effect of maternal Hg on child risk of OWO.
Finally, to examine the robustness of the results and biological plausibility, we conducted a series of sensitivity analyses: subgroup analyses, including among fish consumers, term births, Black children, and breastfed children, and sequential models adding more covariables of interest. We also performed similar analyses stratified by child’s sex. All p values were from two-sided tests, and all statistical analyses were performed using SAS v.9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Our study population consisted of 1442 mother-child pairs of which 722 (50·1%) children were boys and 967(67·1%) were Black. The age range of children at his/her last visit was 2–15 years. Hg was detected in 89% of mothers. The median [interquartile range (IQR)] of maternal RBC-Hg was 2.11 (1.04–3.70) μg/L. The distribution of maternal RBC-Hg is presented according to race/ethnicity and maternal OWO/diabetes status (Additional file 1: Figure S2). Geometric mean [95% confidence interval (CI)] of maternal folate plasma was 31.1 (30.1–32.1) nmol/L. In all, 21.4% of mothers had plasma folate levels < 20.4 nmol/L. Maternal and child characteristics stratified by maternal Hg quartiles are presented in Table 1. Older, non-smoking, Black, and multiparous mothers had higher Hg levels. A higher frequency of fish intake was associated with higher Hg levels. Lower plasma folate levels were associated with increasing Hg levels. Children of mothers with higher Hg levels tended to be female, older, and breastfed.
Table 1.
The characteristics of the study population (n = 1442)
| Maternal RBC mercury level (μg/L) in quartiles | |||||
|---|---|---|---|---|---|
| Q1 0·39–1·04 |
Q2 1·02–2·10 |
Q3 2·12–3·68 |
Q4 3·70–27·8 |
P trend | |
| Maternal characteristics | |||||
| n | 360 | 361 | 359 | 362 | |
| Age, years | 26·8 ± 6·5 | 28·0 ± 6·0 | 29·1 ± 6·7 | 30·3 ± 6·3 | < 0·001 |
| Race | < 0·001 | ||||
| Black | 192 (53·3) | 225 (62·3) | 265 (73·8) | 285 (78·7) | |
| Non-black | 168 (46·7) | 136 (37·7) | 94 (26·2) | 77 (21·3) | |
| Education | 0·367 | ||||
| High school and less | 234 (65·0) | 243 (67·3) | 233 (64·9) | 226 (62·4) | |
| Beyond high school | 126 (35·0) | 118 (32·7) | 126 (35·1) | 136 (37·6) | |
| Smoking | < 0·001 | ||||
| Not smoker | 279 (77·5) | 284 (78·7) | 305 (85·0) | 324 (89·5) | |
| Smoker | 81 (22·5) | 77 (21·3) | 54 (15·0) | 38 (10·5) | |
| Parity | 0·001 | ||||
| Nulliparous | 177 (49·2) | 150 (41·6) | 139 (38·7) | 136 (37·6) | |
| Multiparous | 183 (50·8) | 211 (58·4) | 220 (61·3) | 226 (62·4) | |
| Pre-pregnancy BMI, kg/m2 | 26·4 ± 6·8 | 27·2 ± 7·6 | 27·2 ± 6·7 | 26·5 ± 5·7 | 0·769 |
| Overweight or obesity | 176 (48·9) | 185 (51·2) | 203 (56·6) | 197 (54·4) | 0·063 |
| Diabetes | 44 (12·2) | 45 (12·5) | 45 (12·5) | 50 (13·8) | 0·537 |
| Hypertensive disorder | 45 (12·5) | 50 (13·9) | 61 (17·0) | 60 (16·6) | 0·068 |
| Fish intake (serving/week) | < 0·001 | ||||
| 0 | 163 (45·3) | 80 (22·2) | 55 (15·3) | 35 (9·7) | |
| 1–2 | 183 (50·8) | 253 (70·1) | 246 (68·5) | 239 (66·0) | |
| ≥ 3 | 14 (3·9) | 28 (7·7) | 58 (16·2) | 88 (24·3) | |
| Plasma folate (nmol/L)* | 34·6 (32·5–36·8) | 30·1 (28·3–32·1) | 29·4 (27·5–31·3) | 30·7 (28·8–32·7) | < 0·001 |
| Child’s characteristics | |||||
| Age, years | 7·6 ± 2·9 | 8·0 ± 3·2 | 8·6 ± 3·3 | 8·4 ± 3·0 | 0·001 |
| Gender | 0·002 | ||||
| Boy | 196 (54·4) | 190 (52·6) | 180 (50·1) | 156 (43·1) | |
| Girl | 164 (45·6) | 171 (47·4) | 179 (49·9) | 206 (56·9) | |
| Birthweight, g | 2982 ± 773 | 2955 ± 776 | 2972 ± 790 | 3015 ± 818 | 0·775 |
| Gestational age, weeks | 38·0 ± 3·2 | 37·9 ± 3·3 | 37·8 ± 3·2 | 38·1 ± 3·1 | 0·998 |
| Preterm birth | 88 (24·4) | 90 (24·9) | 85 (23·7) | 85 (23·5) | 0·681 |
| Fetal growth | 0·176 | ||||
| AGA | 290 (80·6) | 286 (79·2) | 279 (77·7) | 281 (77·6) | |
| SGA | 38 (10·5) | 37 (10·3) | 39 (10·9) | 37 (10·2) | |
| LGA | 32 (8·9) | 38 (10·5) | 41 (11·4) | 44 (12·2) | |
| Breastfeeding | 0·013 | ||||
| Formula only | 90 (25·0) | 105 (29·1) | 88 (24·5) | 65 (17·9) | |
| Breastfeed exclusively | 28 (7·8) | 31 (8·6) | 21 (5·9) | 39 (10·8) | |
| Both | 242 (67·2) | 225 (62·3) | 250 (69·6) | 258 (71·3) | |
| Overweight or obesity | 138 (38·3) | 154 (42·7) | 146 (40·7) | 174 (48·1) | 0·019 |
| Number of BMI measures | 3·8 ± 2·3 | 4·0 ± 2·5 | 4·1 ± 2·6 | 4·0 ± 2·4 | < 0·001 |
*Geometric mean (95% CI); Q quartile, RBC red blood cell, AGA appropriate for gestational age, SGA small for gestational age, LGA large for gestational age
Maternal RBC-Hg levels and child OWO
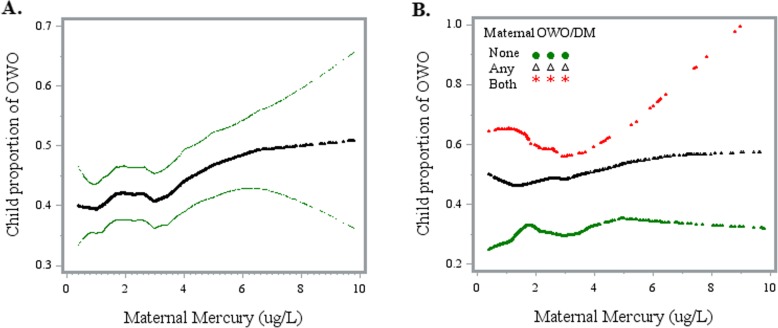
As shown in Fig. 1, maternal Hg levels were positively associated with an increased risk of child OWO (panel a). When stratified by maternal pre-pregnancy OWO and/or diabetes, the association was strongest among OWO and diabetic mothers (panel b). Furthermore, the risk of child OWO among OWO and diabetic mothers was the highest across the whole Hg spectrum. Multivariate regression models (Table 2) showed that the increased risk of child OWO was mainly concentrated in the top Hg quartile (Q4) with an RR of 1.24 (95% CI 1.05–1.47), while second (Q2) and third (Q3) Hg quartiles were not significantly associated with the risk of child OWO, as compared with the lowest quartile (Q1). When we combined Q1–Q3 as the reference, the top Hg quartile was associated with increased risk of child OWO (RR = 1·19; 95% CI 1.05–1.35; p = 0·007). These associations persisted from preschool age (2–5 years), to school age (6–9 years) and into early adolescence (10–15 years).
Fig. 1.
Association between maternal RBC-Hg concentrations and offspring overweight or obesity. Abbreviation: OWO, overweight or obesity; DM, diabetes. Panel a displays the crude association between maternal RBC-Hg concentration and offspring proportion of OWO. Panel b displays the association stratified by maternal OWO/DM condition. None, non-OWO and non-diabetic; Any, either OWO or diabetes; Both, OWO and diabetes
Table 2.
Association of maternal mercury levels with child risk of overweight or obesity
| Age group | Mercury quartile | Crude | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Case, n(%) | RR | 95% CI | p | RR | 95% CI | p | ||
| Total sample 2–15 years (n = 1442) | |||||||||
| Q1 | 360 | 138 (38·3) | 1·00 | 1·00 | |||||
| Q2 | 361 | 154 (42·7) | 1·11 | 0·93–1·33 | 0·238 | 1·09 | 0·92–1·30 | 0·313 | |
| Q3 | 359 | 146 (40·7) | 1·06 | 0·89–1·27 | 0·522 | 1·03 | 0·86–1·23 | 0·748 | |
| Q4 | 362 | 174 (48·1) | 1·25 | 1·06–1·49 | 0·009 | 1·24 | 1·05–1·47 | 0·013 | |
| P trend | 0·047 | 0·098 | |||||||
| Q1–Q3 | 1080 | 438 (40·6) | 1·00 | 1·00 | |||||
| Q4 | 362 | 174 (48·1) | 1·19 | 1·04–1·35 | 0·010 | 1·19 | 1·05–1·35 | 0·007 | |
| 2–5 years (n = 1395) | |||||||||
| Q1 | 346 | 113 (32·7) | 1·00 | 1·00 | |||||
| Q2 | 347 | 139 (40·1) | 1·23 | 1·01–1·50 | 0·044 | 1·20 | 0·99–1·45 | 0·061 | |
| Q3 | 347 | 138 (39·8) | 1·22 | 1·00–1·49 | 0·053 | 1·15 | 0·95–1·41 | 0·160 | |
| Q4 | 355 | 159 (44·8) | 1·37 | 1·13–1·66 | 0·001 | 1·33 | 1·10–1·61 | 0·003 | |
| P trend | 0·015 | 0·067 | |||||||
| Q1–Q3 | 1040 | 390 (37·5) | 1·00 | 1·00 | |||||
| Q4 | 355 | 159 (44·8) | 1·19 | 1·04–1·37 | 0·013 | 1·19 | 1·03–1·36 | 0·016 | |
| 6–9 years (n = 1030) | |||||||||
| Q1 | 250 | 100 (40·0) | 1·00 | 1·00 | |||||
| Q2 | 255 | 118 (46·3) | 1·16 | 0·95–1·41 | 0·156 | 1·15 | 0·94–1·39 | 0·171 | |
| Q3 | 258 | 116 (45·0) | 1·12 | 0·92–1·38 | 0·259 | 1·09 | 0·89–1·34 | 0·399 | |
| Q4 | 267 | 142 (53·2) | 1·33 | 1·10–1·61 | 0·003 | 1·30 | 1·07–1·58 | 0·007 | |
| P trend | 0·028 | 0·100 | |||||||
| Q1–Q3 | 763 | 334 (43·8) | 1·00 | 1·00 | |||||
| Q4 | 267 | 142 (53·2) | 1·21 | 1·06–1·40 | 0·006 | 1·20 | 1·04–1·38 | 0·010 | |
| 10–15 years (n = 449) | |||||||||
| Q1 | 91 | 38 (41·8) | 1·00 | 1·00 | |||||
| Q2 | 106 | 47 (44·3) | 1·06 | 0·77–1·47 | 0·716 | 1·14 | 0·84–1·56 | 0·383 | |
| Q3 | 138 | 67 (48·6) | 1·16 | 0·86–1·57 | 0·320 | 1·22 | 0·91–1·64 | 0·184 | |
| Q4 | 114 | 62 (54·4) | 1·30 | 0·97–1·75 | 0·079 | 1·40 | 1·04–1·88 | 0·028 | |
| P trend | 0·057 | 0·058 | |||||||
| Q1–Q3 | 335 | 152 (45·4) | 1·00 | 1·00 | |||||
| Q4 | 114 | 62 (54·4) | 1·20 | 0·98–1·47 | 0·083 | 1·22 | 0·99–1·50 | 0·061 | |
Q quartile, RR relative risk, CI conference interval. Mercury quartile range: Q1 0.39–1.04 μg/L; Q2 1.04–2.10 μg/L; Q3 2.12–3.68 μg/L; Q4 3.70–27.8 μg/L.
Adjusted for maternal age, race, smoking, education, parity, pre-pregnancy overweight or obesity, diabetes, hypertensive disorder, preterm birth, fetal growth pattern, and breastfeeding status
Combined effects of maternal pre-pregnancy OWO/diabetes and Hg levels on child OWO
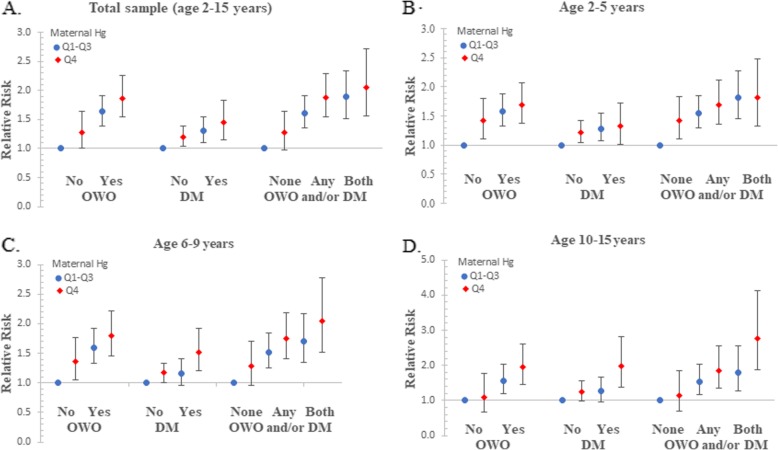
We found a significant combined effect of maternal Hg levels and either or both pre-pregnancy OWO and diabetes on child OWO risk. Children of OWO mothers in the top quartile of Hg had an increased risk of OWO (RR = 1.87; 95% CI 1.55–2.25) compared to those with non-OWO mothers in lower quartiles of Hg (Fig. 2, a). Similarly, the risk of OWO was significantly higher in children of diabetic mothers in the top quartile of Hg than those with non-diabetic mothers in lower quartiles of Hg (RR = 1.45; 95% CI 1·15–1.83). When the presence of maternal OWO and diabetes was categorized into three groups: none (without OWO and diabetes), any (either OWO or diabetes), and both (OWO and diabetes), the risk of OWO (RR = 2.06, 95% CI 1.56–2.71) was highest in children born to mothers with both OWO and diabetes and in the top quartile of Hg, compared with children of mothers without OWO and diabetes and in low quartiles of Hg (Fig. 2a). However, there was no evidence of an interaction between maternal RBC-Hg and pre-pregnancy OWO or between maternal Hg and diabetes with respect to the risk of child OWO (p > 0.05). These association patterns were similar for preschool age children (Fig. 2b), school age children (c), and adolescents (d).
Fig. 2.
Combined effect of maternal OWO and/or DM and RBC-Hg on the risk of child OWO. OWO, overweight or obesity; DM, diabetes; Q, quartile; The presence of maternal OWO and diabetes was categorized into three groups: none (without OWO and diabetes), any (either OWO or diabetes), and both (OWO and diabetes). Panel a displays the associations among total sample at age from 2-15 years; panel b displays the associations among children at age from 2-5 years; panel c displays the associations among children at age from 6-9 years; panel d displays the associations among children at age from 10-15 years
Counteracting effects of maternal folate status against RBC-Hg
Maternal plasma folate was inversely related with Hg concentration (Additional file 1: Figure S3). As shown in Table 3, the rate of child OWO was significantly lower in children whose mothers in the highest Hg quartile and with adequate maternal folate than children whose mothers with a low folate levels (43.6% vs. 60.9%; RR = 0·66; 95% CI 0.51–0·85; p = 0.001). Adequate maternal folate status was not associated with reduced child OWO risk (RR = 0.96; 95% CI 0.80–1.15; p = 0.658) among children of mothers with Hg levels in the lower three quartiles. The interaction between maternal RBC-Hg and plasma folate on child OWO risk was marginal (p = 0.086 for interaction).
Table 3.
Modifying effects of maternal folate levels with mercury levels on child overweight or obesity risk
| Mercury quantile | Maternal folate | Crude | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Case, n(%) | RR | 95% CI | p | RR | 95% CI | p | ||
| Q4 | Low | 64 | 39 (60.9) | 1.00 | 1.00 | ||||
| Adequate | 236 | 103 (43.6) | 0.72 | 0.56–0.91 | 0.007 | 0.66 | 0.51–0.85 | 0.001 | |
| Q1-Q3 | Low | 195 | 85 (43.6) | 1.00 | 1.00 | ||||
| Adequate | 714 | 289 (40.5) | 0.93 | 0.77–1.11 | 0.427 | 0.96 | 0.80–1.15 | 0.658 | |
| P for interaction | 0.095 | 0.086 | |||||||
Q quartile. Mercury quartile range: Q1 0.39–3.68 μg/L; Q4 3.70–27.8 μg/L. Adequate folate was defined as plasma folate ≥ 20.4 nmol/L; low folate was defined as plasma folate < 20.4 nmol/L
Adjusted for maternal race, smoking, education, parity, pre-pregnancy overweight or obesity, diabetes, hypertensive disorder, preterm birth, fetal growth pattern, and breastfeeding status
Subgroup and sensitivity analyses
To control for other potential confounders, we further adjusted for frequency of maternal fish consumption (Additional file 1: Table S2), maternal RBC-selenium levels (Additional file 1: Table S3), and maternal RBC-lead levels (Additional file 1: Table S4). The results were similar to those reported above. A series of subgroup analyses were performed, and the associations were not changed materially among fish consumers (Additional file 1: Table S5), term births (Additional file 1: Table S6), Blacks (Additional file 1: Table S7), boys (Additional file 1: Table S8), girls (Additional file 1: Table S9), and breastfed children (Additional file 1: Table S10). To further evaluate the associations, we analyzed child BMI z-score as a continuous outcome (Additional file 1: Table S11), and again the results were consistent.
Discussion
To the best of our knowledge, this is the first study to investigate in utero Hg exposure and OWO in childhood using a prospective, longitudinal birth cohort. This study provided several new findings. Maternal RBC-Hg levels were positively associated with the risk of child OWO. The highest risk of child OWO was seen among children of mothers with Hg levels in the top quartile. The associations were consistent from preschool age to school age to adolescence. The Hg-related risk of child OWO was further enhanced by maternal pre-pregnancy OWO and/or diabetes but mitigated by adequate maternal folate status. These data suggest that in utero Hg exposure has a long-term consequence on child metabolic health, and optimal folate in utero may act to reduce Hg toxicity. Our findings underscore the need for screening for blood Hg and folate levels in pregnant women, particularly in OWO and/or diabetic women.
Hg is well-known to be a neurotoxicant and is beginning to be recognized as an obesogen [6, 12, 13]. Studies in adults have suggested that blood Hg levels were associated with obesity [6] and visceral adipose tissue (measured by dual-energy X-ray absorptiometry) [7]. Measures of Hg in human hair were also associated with BMI [13] and obesity [12]. Our study extends these findings, suggesting a positive association between maternal Hg levels and child risk of OWO from preschool age to school age to adolescence. These results were consistent across an array of sensitivity analyses. Our findings provide new evidence that in utero Hg exposure may play a role in the development of childhood OWO.
The underlying mechanism for the association between in utero Hg exposure and risk of child OWO is not clear. One potential mechanism is via MeHg-induced oxidative stress and inflammation. Experimental studies have demonstrated that MeHg exposure induces oxidative stress and systemic inflammation [26], which, in turn, can cause disturbances in glucose metabolism and lipid peroxidation [27]. Consistently, a human study showed that MeHg exposure was associated with reduced activity of Paraoxonase 1 (PON1) [28], an enzyme that inhibits systemic oxidative stress and guards against atherosclerosis and obesity [29].
Nutrition has been proposed as a safe, simple, and inexpensive method to mitigate the detrimental effects of exposure to environmental toxicants [30, 31]. One of the nutrients that might reduce the adverse impacts of chemicals on the fetus is folate [18]. However, the literature is limited on the relationship between folate status and Hg toxicity. Previous studies showed that folate and vitamin B12 deficiency magnify the adverse effects of Hg [32] and that folate may play a role in alleviating Hg toxicity [19]. Our findings reiterate the potential importance of optimal maternal folate status in the setting of Hg exposure.
Our study has important public health implications. MeHg is a major contaminant in some fish and seafoods. The overall health impact of fish consumption may reflect both the beneficial effects of nutrients, such as omega-3 long-chain fatty acids, and the detrimental effects of contaminants, such as Hg, found in fish. The results of the current study emphasize the need to carefully weigh the nutritional benefits of fish consumption with the risks of increased exposure to Hg during pregnancy to reduce the risk of childhood OWO. Our study findings raise the prospect to screen for Hg and folate and to optimize maternal folate status during gestation, especially among mothers with OWO and/or diabetes.
Our study has a couple of strengths. We measured child BMI longitudinally, which enabled us to define OWO over 15 years of follow-up and explore temporal characteristics of the association between in utero Hg exposure and OWO risk. In addition, we assessed in utero Hg exposure in maternal RBCs 1–3 days after delivery, reflecting third trimester exposure and free from hemodilution. However, several potential limitations of the study need to be noted. We measured total Hg, not MeHg, though Hg concentrations in RBCs are the best biomarker of MeHg exposure insofar as ∼ 80% of MeHg is stored in red blood cells [2]. Second, we did not measure child Hg exposure during childhood. Third, although we controlled for many potential confounders, such as maternal age, education, race/ethnicity, parity, smoking, and other pertinent covariates in the models, we could not completely eliminate the potential for residual confounding due to unknown or uncontrolled confounders. Finally, the fact that our study population was dominantly urban, low-income Blacks might limit the generalizability of our findings.
Conclusion
In this large, long-term prospective birth cohort study of a US urban low-income population, there was a significant dose-response relationship between in utero Hg exposure and risk of child OWO. Maternal pre-pregnancy OWO and/or diabetes enhanced the Hg-child OWO associations, while adequate maternal folate status mitigated the associations. These findings, if further confirmed, underscore the need to screen for Hg and folate and to optimize maternal folate status during gestation, especially among mothers with OWO and diabetes.
Supplementary information
Additional file 1: Figure S1. Flowchart of study population. Figure S2. The distribution of maternal RBC-mercury stratified by maternal race and prepregnancy overweight or obesity (OWO) and/or diabetes (DM) status. Figure S3. The relationship between maternal plasma folate and RBC-Hg concentrations. Table S1. Comparison of pre- and peri-natal characteristics between total enrolled sample, follow-up sample, included sample of this analysis and subset with folate data. Table S2. Individual and combined effects of maternal OWO and/or DM and mercury and child risk of OWO, with additional adjustment for frequency of fish consumption. Table S3. Individual and combined effects of maternal OWO and/or DM and mercury on child risk of OWO, with additional adjustment for maternal selenium level. Table S4. Individual and combined effects of maternal OWO and/or DM and mercury on child risk of OWO, with additional adjustment for maternal lead level. Table S5. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among fish consumers only (n=1109). Table S6. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among term births only (n=1094). Table S7. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among black children (n=967). Table S8. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among boys (n=722). Table S9. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among girls (n=720). Table S10. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among breastfed children only (n=1094). Table S11. Individual and combined effects of maternal OWO and/or DM and mercury on child BMI z-scores.
Acknowledgements
The authors wish to acknowledge Thomas Kirn, PhD, Tina Fan, PhD, and Doug Haltmeier, MS in the New Jersey Department of Health for their leadership, input, and support throughout this project. The authors also wish to thank the study participants, the nursing staff at Labor and Delivery of the Boston Medical Center and the field team for their contributions to the Boston Birth Cohort. Linda Rosen, MSEE, and the Clinical Data Warehouse assisted in obtaining relevant clinical information. She was compensated for her time. Clinical Data Warehouse service is supported by Boston University’s Clinical and Translational Institute and the National Institutes of Health Clinical and Translational Science Award (grant U54-TR001012).
Role of the funder/sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by the National Institutes of Health, the Health Resources and Services Administration, the US Department of Health and Human Services, or the US government, or the New Jersey Department of Health.
Abbreviations
- AGA
Appropriate for gestational age
- BBC
The Boston Birth Cohort
- BMI
Body mass index
- CI
Confidence interval
- CV
Coefficient variation
- HELLP
Hemolysis, elevated liver enzymes, and low platelets syndrome
- Hg
Mercury
- IQR
Interquartile range
- LGA
Large for gestational age
- LOD
The limit of detection
- MeHg
Methylmercury
- OWO
Overweight or obesity
- RR
Relative risk
- SD
Standard deviation
- SGA
Small for gestational age
Authors’ contributions
GW, JD, EB, and XW were responsible for study concept and design. GW, JD, and TRB were responsible for drafting of the manuscript. EB, AMS, and JM performed laboratory assays and quality control. GW, JD, DCB, MCW, XH, MWK, and TLC assumed major responsibilities for statistical analyses and data presentations. GW and YJ prepared blood samples for lab assay. XW was responsible for acquisition of the epidemiological and clinical data as well as biospecimen. All the authors (GW, JD, EB, AMS, JM, TRB, DCB, XH, YJ, MCW, TLC, MWK, XW) were responsible for critical review and revision of the manuscript and contributed to data interpretations. GW and XW have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
The Boston Birth Cohort (the parent study) is supported in part by the National Institutes of Health (NIH) under grants number R01HD086013 and 2R01HD041702, and by the Health Resources and Services Administration of the U.S. Department of Health and Human Services under UJ2MC31074 Autism Longitudinal Data Project. Dr. Guoying Wang receives grant R03ES029594 from the NIH/National Institute of Environmental Health Science.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study has been approved by the Institutional Review Boards of Boston Medical Center and Johns Hopkins Bloomberg School of Public Health. Written informed consent was obtained from each study child’s biological mother and assent from the study child.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guoying Wang and Jessica DiBari Co-first authors: these authors made equal contributions.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12916-019-1442-2.
References
- 1.Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: the United States NHANES, 2007-2012. J Toxicol Environ Health A. 2017;80(9):502–512. doi: 10.1080/15287394.2017.1330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 3.Grandjean P, Cordier S, Kjellstrom T, Weihe P, Budtz-Jorgensen E. Health effects and risk assessments. In: Pirrone N, Mahaffey KR, editors. Dynamics of mercury pollution on regional and global scales: atmospheric processes and human exposure around the world. Norwell: Springer; 2005. pp. 499–523. [Google Scholar]
- 4.Guallar E, Sanz-Gallardo MI, van't Veer P, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersma RA, Martin-Moreno JM, Kok FJ, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347(22):1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 5.He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA trace element study. Diabetes Care. 2013;36(6):1584–1589. doi: 10.2337/dc12-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho S, Jacobs DR, Jr, Park K. Population correlates of circulating mercury levels in Korean adults: the Korea National Health and Nutrition Examination Survey IV. BMC Public Health. 2014;14:527. doi: 10.1186/1471-2458-14-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JS, Ha KH, He K, Kim DJ. Association between blood mercury level and visceral adiposity in adults. Diabetes Metab J. 2017;41(2):113–120. doi: 10.4093/dmj.2017.41.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mone SM, Gillman MW, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113(4 Suppl):1058–1069. [PubMed] [Google Scholar]
- 9.De Long NE, Holloway AC. Early-life chemical exposures and risk of metabolic syndrome. Diabetes Metab Syndr Obes. 2017;10:101–109. doi: 10.2147/DMSO.S95296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, Paige D, Bartell T, Hong X, Caruso D, et al. Association between maternal prepregnancy body mass index and plasma Folate concentrations with child metabolic health. JAMA Pediatr. 2016;170(8):e160845. doi: 10.1001/jamapediatrics.2016.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skalnaya MG, Demidov VA. Hair trace element contents in women with obesity and type 2 diabetes. J Trace Elem Med Biol. 2007;21(Suppl 1):59–61. doi: 10.1016/j.jtemb.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Skalnaya MG, Tinkov AA, Demidov VA, Serebryansky EP, Nikonorov AA, Skalny AV. Hair toxic element content in adult men and women in relation to body mass index. Biol Trace Elem Res. 2014;161(1):13–19. doi: 10.1007/s12011-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 14.Quig D. Cysteine metabolism and metal toxicity. Altern Med Rev. 1998;3(4):262–270. [PubMed] [Google Scholar]
- 15.Zhao M, Chen YH, Dong XT, Zhou J, Chen X, Wang H, Wu SX, Xia MZ, Zhang C, Xu DX. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS One. 2013;8(12):e82713. doi: 10.1371/journal.pone.0082713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001;30(12):1390–1399. doi: 10.1016/S0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 17.Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22(1):6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang F, Longnecker MP, Venners SA, Johnson S, Korrick S, Zhang J, Xu X, Christian P, Wang MC, Wang X. Preconception serum 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane and B-vitamin status: independent and joint effects on women's reproductive outcomes. Am J Clin Nutr. 2014;100(6):1470–1478. doi: 10.3945/ajcn.114.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H, Kim KN, Hwang JY, Ha EH, Park H, Ha M, Kim Y, Hong YC, Chang N. Relation between serum folate status and blood mercury concentrations in pregnant women. Nutrition. 2013;29(3):514–518. doi: 10.1016/j.nut.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Mueller NT, Li J, Sun N, Huo Y, Ren F, Wang X. Association of maternal plasma folate and cardiometabolic risk factors in pregnancy with elevated blood pressure of offspring in childhood. Am J Hypertens. 2017;30(5):532–540. doi: 10.1093/ajh/hpx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. Jama. 2014;311(6):587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. Jama. 2015;313(13):1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 23.Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, Hao K, Pearson C, Ortiz K, Bonzagni A, et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol. 2011;128(2):374–381. doi: 10.1016/j.jaci.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. CDC growth charts, United States. 2000. http://www.cdc.gov/growthcharts/. Accessed 26 Nov 2013.
- 25.National Center for Health Statistics. Overweight and obesity, defining childhood obesity. Page last reviewed: July 3, 2018. Accessed 30 July 2019.
- 26.Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131(1):1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes. 2006;55(6):1614–1624. doi: 10.2337/db06-0029. [DOI] [PubMed] [Google Scholar]
- 28.Ayotte P, Carrier A, Ouellet N, Boiteau V, Abdous B, Sidi EA, Chateau-Degat ML, Dewailly E. Relation between methylmercury exposure and plasma paraoxonase activity in inuit adults from Nunavik. Environ Health Perspect. 2011;119(8):1077–1083. doi: 10.1289/ehp.1003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. Jama. 2008;299(11):1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furst A. Can nutrition affect chemical toxicity? Int J Toxicol. 2002;21(5):419–424. doi: 10.1080/10915810290096649. [DOI] [PubMed] [Google Scholar]
- 31.Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, Silverstone A, Watkins B, Suk WA. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect. 2007;115(4):493–495. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgson NW, Waly MI, Al-Farsi YM, Al-Sharbati MM, Al-Farsi O, Ali A, Ouhtit A, Zang T, Zhou ZS, Deth RC. Decreased glutathione and elevated hair mercury levels are associated with nutritional deficiency-based autism in Oman. Exp Biol Med (Maywood) 2014;239(6):697–706. doi: 10.1177/1535370214527900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Flowchart of study population. Figure S2. The distribution of maternal RBC-mercury stratified by maternal race and prepregnancy overweight or obesity (OWO) and/or diabetes (DM) status. Figure S3. The relationship between maternal plasma folate and RBC-Hg concentrations. Table S1. Comparison of pre- and peri-natal characteristics between total enrolled sample, follow-up sample, included sample of this analysis and subset with folate data. Table S2. Individual and combined effects of maternal OWO and/or DM and mercury and child risk of OWO, with additional adjustment for frequency of fish consumption. Table S3. Individual and combined effects of maternal OWO and/or DM and mercury on child risk of OWO, with additional adjustment for maternal selenium level. Table S4. Individual and combined effects of maternal OWO and/or DM and mercury on child risk of OWO, with additional adjustment for maternal lead level. Table S5. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among fish consumers only (n=1109). Table S6. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among term births only (n=1094). Table S7. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among black children (n=967). Table S8. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among boys (n=722). Table S9. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among girls (n=720). Table S10. Individual and combined effects of maternal OWO and/or DM and mercury on child OWO among breastfed children only (n=1094). Table S11. Individual and combined effects of maternal OWO and/or DM and mercury on child BMI z-scores.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.