Abstract
Robustness of superhydrophobic materials has been gradually taken into consideration for practical applications; however, little attention has been paid to the impact resistance of the superhydrophobicity of the materials. The present study demonstrated a new route for improving the mechanical durability, especially the impact resistance, of the superhydrophobic materials. First, poly(styrene-co-butadiene)/poly(ethylene-vinyl acetate) (SBR/EVA) composite monoliths with microscale cellular structures were manufactured by vulcanization-foaming processes. Then the composite monoliths were treated with sandpaper to create nanostructures above the revealed micropores after removing the uppermost skin, forming micro/nanotextured surfaces and giving improvements in superhydrophobicity. Due to the elastomeric nature of SBR and EVA, the superhydrophobicity of the monoliths can be maintained even while the material is mechanically impacted or compressed, and wearing helps improvement or recovery of the superhydrophobicity because of the self-similarity of the cellular structure inside the monoliths. Additionally, the obtained superhydrophobic materials are resistant to acidic, alkali, and salt liquors as well as organic solvents and have easy healing capacity of superhydrophobicity with a simple sanding treatment when destroyed by exposure to oxygen plasma.
Introduction
Improvement in durability of superhydrophobic materials has received much attention due to their wide range of possible applications including self-cleaning,1 oil–water separation,2−5 anti-icing,6,7 drag reduction,8 anticorrosion,9−11 and other areas. The mechanical stability of the topographic structure significantly affects the durability12 of superhydrophobicity. One strategy to improve the structural stability is enhancing the interfacial interactions between the micro/nanotextured structures and the materials by electrostatic assembly,13,14 hydrogen bonds,15,16 chemical bonds,17,18 and polymer or inorganic binding19−22. For example, durable superhydrophobic coating was fabricated through spraying the mixture of inorganic binder aluminum phosphate and nanoparticle on the glass substrate.16 The coating could remain superhydrophobic after sanding 500 times on account of the hydroxyl cross-linking reaction between the inorganic binder and glass substrate. Another is to create roughening structures directly from the bulk matrix of materials at the surface. The roughening structure is part of the substrate matrix as a whole, which avoids the interfacial problems in the previous method that introduces extra materials onto substrates. Many techniques have been introduced into the construction of robust superhydrophobic structures including chemical etching,23−25 plasma processing,26−28 electrochemical reaction,29,30 and laser ablating31,32. Natural33,34 or artificial templates35,36 were also adopted in a few studies that make for the forming of micro-nanostructures, which have the same composition as the substrate.37
Recently, bulk superhydrophobic materials have been getting much attention, which not only have hierarchical monolithic structures with the bulk substrate at the surface but also have self-similar structures inside. These materials have micro/nanotextured structures abound in the entire system.38−41 Therefore, even if the upper portion of the material is worn away, the exposed portion at the bottom still has a similar structure. In addition, wear-resistant elastomer materials (such as silicone composite42,43 and EVA44) can be introduced during the preparation of the superhydrophobic surface to enhance its durable properties. Davis et al.45 reported that superhydrophobic PDMS monoliths with porous micro/nanotextured structures were produced through the emulsion technique. The monoliths retained their antiwetting properties even after being subjected to rigorous surface wear treatment because of their self-similarity. In addition, due to the elastomeric nature of PDMS, superhydrophobicity can be maintained even while the material is mechanically strained or compressed. This method took into consideration not only the antiabrasion property but also the strain and compress properties, which is very useful for design of mechanically durable superhydrophobic materials.
In this work, taking into consideration the mechanical impact resistant property, durable elastomer materials (SBR and EVA) were introduced to fabricate superhydrophobic surfaces utilizing their unique elasticity, durability, and wear resistance. Additionally, micropores were formed inside the composite monoliths during the fabrication process without using any solvent through vulcanization-foaming and with the synergy effect of the cross-linking agent dicumyl peroxide (DCP) as well as sulfur (S) and blowing agent azodicarbonamide (AC), making the composite materials superhydrophobic after sanding treatment, as shown in Figure 1. The superhydrophobicity of the obtained SBR/EVA surfaces can be maintained even while the material is mechanically impacted or compressed, and wearing of the materials surfaces could result in improvement rather than loss of the superhydrophobicity.
Figure 1.

Illustration of the fabrication of superhydrophobic composite surfaces. (a) Preparation of hydrophobic SBR/EVA composite monoliths. (b) Sanding treatment of hydrophobic composite.
Results and Discussion
Morphology and Hydrophobicity of SBR/EVA Composite
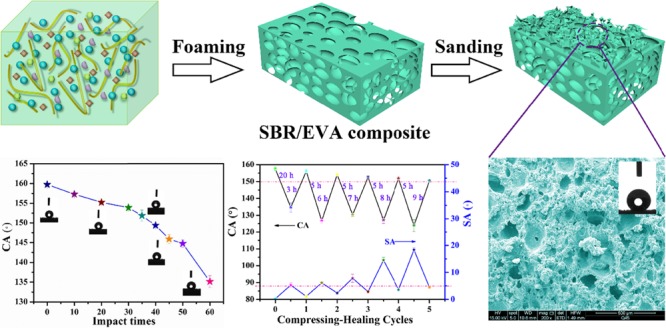
The SBR/EVA composite was fabricated by mixed SBR (60 wt %), EVA (40 wt %), WCB, cross-linking agent (DCP, S), and blowing agent (AC) together evenly in the internal mixer, as shown in Figure 1a. Compared to the small density of pure SBR and poor elasticity of pure EVA, 60 wt % SBR and 40 wt % EVA were chosen to fabricate hydrophobic composite monoliths because of their good dimensional stability and suitable cell distribution.46 During the process of composite vulcanization-foaming, cells were generated and grew on account of swelling of the system with the blowing agent, which decomposed into nitrogen and affected the number and distribution of nucleated cells. The SBR/EVA composite could maintain a stable state when taken out from the flat-panel curing to room temperature, which made the bubble cells to gradually stop growing. SEM images and CAs of SBR/EVA composites with different contents of the blowing agent are shown in Figure 2. Different sizes of cells formed during the process of composite vulcanization-foaming due to the poor compatibility between SBR and EVA. It was found in Figure 2a–g that, as the content of the blowing agent increased, the cellular diameter increased. Different sizes of cells gave rise to microscale structures. The relationship between the contact angle and blowing agent is shown in Figure 2h. The contact angle could reach 145° when the blowing agent of AC was 2 wt %.
Figure 2.
SEM images and CA values of SBR/EVA composite with different contents of blowing agent: (a) 0, (b) 0.5, (c) 1, (d) 1.5, (e) 2, (f) 2.5, and (g) 3 wt %. (h) The relationship between contact angle (CA) and blowing agent.
To explore the influence of the content of the blowing agent on the porous structure and the hydrophobicity of the composite materials, cell distribution was measured by Software Nano Measurer 1.2. It was demonstrated in Figure S1 that different contents of the blowing agent resulted in various cell distributions. With the increase of the blowing agent content, large cells appeared gradually through the vulcanization-foaming process. Nonlinear fit was used to measure the messy degree of cellular, which was better when the R2 value is close to 1 commonly. Conversely, that of departure means more messy microscale rough structures in this paper; that is to say, wider cell distribution was obtained. As shown in Figure S1d, the R2 value was 0.88182 when the blowing agent was 2 wt %, which demonstrated that the widest distribution with different sizes of cells appeared. It was obvious that a messy bubble phase produced a rougher structure, which contributed to achieving superhydrophobicity. The 2 wt % foaming agent was chosen as the optimal microstructure monoliths because different sizes of cells were distributed widely. Therefore, the SBR/EVA composite with the blowing agent of 2 wt % was chosen as the primary sample for investigation in this paper.
Superhydrophobic Property of the Sanded SBR/EVA Composite
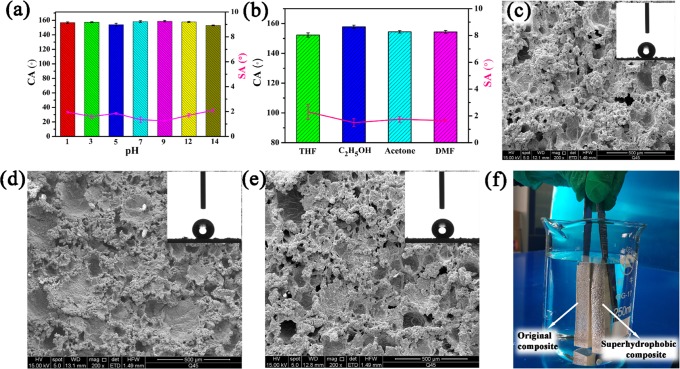
As shown in Figure 1b, the SBR/EVA composite was fixed on the friction instrument, and sandpaper (400 mesh) was pasted on the top of the stainless-steel column as the external force and moved repeatedly at a pressure of 45 KPa for 50 cycles. SEM images of superhydrophobic SBR/EVA composites with different contents of the blowing agent are shown in Figure 3a and Figure S2. It was found that sanding treatment produced micro-nanostructures on the SBR/EVA composite surfaces in which the cellular structure generated in the process of composite vulcanization-foaming provides the microscale structure, while nanoscale roughness was obtained on the basis of micropores after sanding, and the fabrication was conducted without using any solvents. Figure 3c shows the relationship between the CA, SA of the superhydrophobic composite, and the amount of blowing agent used. It was found that CAs of all samples could reach 150° with SAs being less than 5°. Importantly, the superhydrophobicity could be maintained even when layers of composites were gradually sanded away, as shown in Figure S3. When the upper layer was worn away, the exposed new composite layer still had a similar contact angle. That is to say, the microporous structure abounded in the entire material. To further verify that the as-generated microscale structure helped to achieve superhydrophobicity, the SBR/EVA composite without adding a blowing agent was also investigated. Figure 2a shows that the original unfoamed SBR/EVA sample was plain with a contact angle of 118°. With the sanding treatment, there was an increase of contact angle with cycles of sanding. As shown in Figure S4, the unfoamed SBR/EVA sample required 250 abrasion cycles to reach a contact angle of 152°. However, the foaming composite could reach the state of superhydrophobicity with only 50 cycles of abrasion. This demonstrated that the microscale roughness from foaming of the SBR/EVA composite played an important role in superhydrophobicity. Furthermore, different wearing approaches using various mesh sandpaper, cloth, and stainless-steel column were conducted to illustrate whether the material in our work has wear-independent similarity performance.47 It was found in Figure S5 that CAs of all sample could reach (or close to) 150° and SAs could reach less than (or close to) 5°, which demonstrated superhydrophobicity. The CAs first decreased then increased. It might be because that some of the roughness was sanded away and finally exposed again on the composite surface. Therefore, the superhydrophobic SBR/EVA composite has wear-independent superhydrophobicity without losing its nonwetting performance after various wear conditions.
Figure 3.
(a) SEM of superhydrophobic SBR/EVA composite with 2 wt % blowing agent after sanding. (b) Higher magnification of panel (a). (c) The relationship between the CA/SA value and blowing agent.
Impact Resistance of Superhydrophobic SBR/EVA Composite
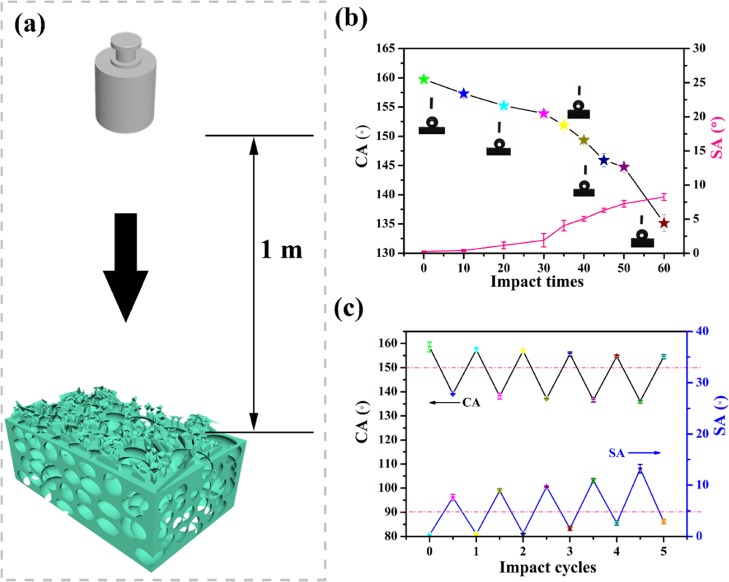
The impact resistance of the superhydrophobic SBR/EVA material is very important for outdoor uses. The sample was evaluated by dropping a given weight to impact the surface from 1 m height (Figure 4a) followed by CA and SA value measuring. It was found that the superhydrophobic monoliths could withstand 40 times impact without deformation and maintain the CA greater than 150° and SA less than 5°, which is the state of superhydrophobicity. Further impacting caused loss of the superhydrophobicity. However, the superhydrophobicity could recover through placement of the sample at room temperature for 4 h, showing the excellent healing ability of the superhydrophobicity after intense impacting, as shown in Figure S6. Importantly, although the recovering time increased with increasing impact cycles, the sample could retain the superhydrophobicity with a CA value greater than 150° and SA value less than 5° after 5 cycles of impacting and healing, as shown in Figure 4c. The morphology changes of the SBR/EVA composite displayed in Figure 5 demonstrated that the roughness decreased after dynamic impacts and could recover when the sample was placed at room temperature. 3D images of the color difference of the sample show the roughness changes in accordance with the SEM images of the sample treated. In the impacting process, the samples were only affected by the dynamic mechanical force; therefore, the decrease or loss of superhydrophobicity might be mainly caused by the decrease of the surface roughness after the intense dynamic force. After placement at room temperature, the roughening structure could recover due to the elastic property of SBR and EVA, making the material surface superhydrophobic. To further test the impact resistance of the SBR/EVA composite, 1 kg mass of weight was also used to hit the sample by the same method. The results shown in Figure S7 demonstrated that the SBR/EVA composite also retained superhydrophobicity after 18 times of impacting, and the sample which lost superhydrophobicity could recover its wetting state after 4 h of room-temperature placement.
Figure 4.
(a) Illustration of impact of superhydrophobic composite with 0.5 kg weight. (b) CA/SA value change with impact times. (c) Impact-healing cycles of the sample after 60 times of impact.
Figure 5.
SEM and 3D images of original (a,d), impacted 60 times (b,e), and recovered in 4 h (c,f) SBR/EVA composite. In panels (d–f), the color difference means height change from a minimum position.
In order to check if the material has the ability to heal the lost superhydrophobicity caused by long-time static force, the superhydrophobic SBR/EVA composite was compressed with a pressure of 0.5 MPa for a given time, and the CA and SA values were measured. It was found that the SBR/EVA composite could remain superhydrophobic for 20 h at a pressure of 0.5 MPa with the CA value decreasing and SA value increasing gradually. However, the superhydrophobicity of the compressed sample restored automatically after 3 h of placement at room temperature, showing healing capability. The sample could retain superhydrophobicity after 5 cycles of compressing-healing. The composite molecular chain could rebound rapidly after force release for SEM testing of the compressed sample, which would cause errors between SEM result and real morphology. Therefore, we used the hot press method to compress and cure the sample before SEM testing. In Figure 6b, it could be seen that the surface of the composite was made flat after hot compressing. However, after 3 h of placement at room temperature, the rough structure appeared obviously as shown in Figure 6c. Optical profilometer images of the sample also show that the roughness changes in Figure S8 are in accordance with the SEM images. The surface roughness value of the sample changed from 2.598 (Figure S8a) to 1.697 μm (Figure S8b) in the compressing process followed by the loss of superhydrophobicity. The surface roughness could recover to 2.272 μm (Figure S8c) after placement at room temperature. The reborn rough morphology might come from the recovery of the structure due to the elasticity of the material, which helped the healing of the structure for superhydrophobicity.
Figure 6.
SEM images of (a) superhydrophobic SBR/EVA composite, (b) hot-compressed composite, and (c) after 3 h of placement at room temperature. (d) CA/SA value change with compressing-healing cycles.
Chemical Stability of the Superhydrophobic SBR/EVA Composite
In practical applications, superhydrophobic materials are commonly exposed to the external environment and are susceptible to be corroded by chemicals. It is meaningful to consider the chemical stability of the superhydrophobic SBR/EVA composite. The tests were conducted by dipping samples into different pH solutions and various organic solvents. Figure 7a shows that the CA of the superhydrophobic SBR/EVA composite remained above 150° and the SA was less than 5° that changed little even after soaking in different pH solutions for 3 days. This indicated that SBR/EVA composite surfaces were resistant to acidic, alkali, and salt liquors. SEM images of the composite are displayed in Figure 7c–e, which were dipped in hydrochloric acid (pH = 1, Figure 7c), sodium chloride solution (pH = 7, Figure 7d), and sodium hydroxide solution (pH = 14, Figure 7e). The morphology of the superhydrophobic SBR/EVA composite surfaces changed little showing no obvious damage. For comparison, the original and superhydrophobic SBR/EVA composites were dipped in water. The superhydrophobic SBR/EVA composite showed a layer of bright plastron on its surface in water as demonstrated in Figure 7f. This phenomenon might be caused by the air layer trapped at the interface that separated the water and the composite. This air layer could avoid direct contact between the solution and the composite, making the superhydrophobic SBR/EVA composite durable to different chemicals. Superhydrophobic SBR/EVA composites were also soaked in various organic solvents for 3 days, as shown in Figure 7b. CA/SA values demonstrated that the as-obtained composites were stable enough to remain superhydrophobic with little change of the structure as shown in the SEM images (Figure S9).
Figure 7.
CA/SA value of superhydrophobic SBR/EVA composite treated by (a) immersion in different pH solutions for 3 days and (b) immersion in various organic solvents for 3 days. SEM images of superhydrophobic composite after dipping in (c) hydrochloric acid, pH = 1; (d) sodium chloride solution, pH = 7; and (e) sodium hydroxide solution, pH = 14. (f) Immersion of superhydrophobic SBR/EVA composite and original composite in water.
Healing Capability of Superhydrophobic SBR/EVA Composite through Wearing
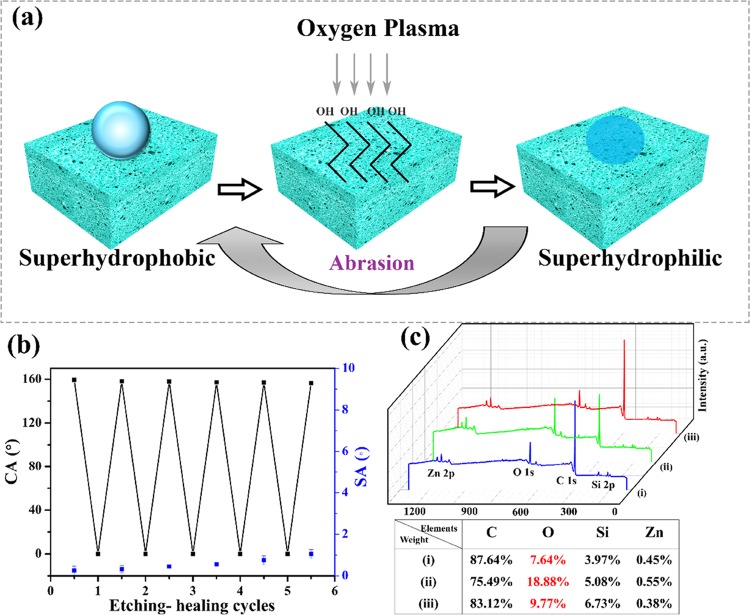
In order to show the easy-healing ability of the superhydrophobic composite, samples were etched by oxygen plasma for 2 min and worn by sandpaper. It should be noted that oxygen plasma treatment usually makes materials hydrophilic due to the breakdown of the carbon chain of polymers, such as SBR and EVA, and introduction of hydroxyl groups onto the surface. Therefore, the superhydrophobic composite turned superhydrophilic with contact angle decreasing from 160 to 0°. However, the composite could easily regenerate its superhydrophobicity through abrasion, as shown in Figure 8a. Abrasion made the composite lose the plasma caused hydrophilic layer and exposed a new hydrophobic roughening layer, obtaining superhydrophobicity. Figure 8b indicates that the etching-healing cycles could be repeated to get a superhydrophobic surface unless the monoliths were worn out. The element composition change of the SBR/EVA composite was measured by XPS. It was found that the content of oxygen in the sample treated by oxygen plasma was 2 times higher than that of the original composite. Sanding treatment reduced the content of oxygen obviously, showing recovery of the low-surface-energy property in addition to roughening the surface by abrasion.
Figure 8.
(a) Schematic illustration of wettability control process of the SBR/EVA composite. (b) Healing cycles of composite healed by abrasion. (c) XPS spectra of superhydrophobic SBR/EVA composite (i) before and (ii) after oxygen plasma treatment and (iii) after sanding treatment.
Conclusions
Durable superhydrophobic SBR/EVA composite surfaces were fabricated through vulcanization-foaming and sanding without using any solvents. The cellular structure provided microscale roughness in the process of composite foaming, and sanding gave rise to nanoscale roughness on the micropores of the cell, favoring superhydrophobicity with the contact angle above 150° and sliding angle less than 5°. The superhydrophobicity of the monoliths can be maintained even while the material is mechanically impacted or compressed. Wearing helps improvement or recovery of the superhydrophobicity. Additionally, the obtained superhydrophobic materials are resistant to acidic, alkali, and salt liquors as well as organic solvents and have easy healing capacity of superhydrophobicity with a simple sanding treatment.
Experimental Section
Materials
Poly(styrene-co-butadiene) (SBR-1502 M) was purchased from Petro China Co., Ltd. Poly(ethylene-vinyl acetate) (EVA) was purchased from Jinju International Trade Co., Ltd. (Shanghai, China). White carbon black (WCB) was supplied by Jitong Chemical & Technology Co., Ltd. (Shi Jiazhuang, China). Sulfur (S) and dicumyl peroxide (DCP) were purchased from Tailong Plastic Technology Co., Ltd. (Jinan, China). Azodicarbonamide (AC) was provided by Huanzong International Trade Co., Ltd. (Dongwan, China). Solid paraffin was supplied by Fuluo Biotechnology Co., Ltd. (Hangzhou, China). Zinc oxide (ZnO) and stearic acid (St) were purchased from Guangdong South BASF Wax Co., Ltd. Sandpaper was purchased from Tongcheng Tianli Emery Cloth Co., Ltd. (Hubei, China).
Preparation of the SBR/EVA Porous Monoliths
Three main steps were adopted to prepare SBR/EVA composite monoliths. First, 60 wt % SBR, 40 wt % EVA, 0.5 wt % sulfur, and 0.6 wt % DCP as the cross-linking agent, 25 wt % WCB, and 5 wt % paraffin as the reinforce reagent, 9 wt % ZnO, and 3 wt % St as the blowing promoter, and 2 wt % AC as the blowing agent were mixed into an internal mixer (SM-0.5 L-K, Suyan, Jiangsu, China) for 20 min at 120 °C. Then the mixed compounds were transferred to a two-roll mill (XH-401C, Xihua, Guangdong, China) at 80 °C for 10 min to form composite sheets. Finally, the SBR/EVA composite sheets were treated by flat-panel curing press (XH-406, XiHua, Guangdong, China) at a temperature of 180 °C and a pressure of 10 MPa for 450 s to obtain SBR/EVA composite monoliths.
In order to evaluate the influence of the content of the blowing agent on the inside of the porous structure and the property of the composite materials, 0, 0.5, 1, 1.5, 2.5, and 3 wt % AC were also used with other conditions unchanged.
Sanding Treatment of SBR/EVA Composite
The SBR/EVA composite was fixed on the friction instrument (Figure 1b). Sandpaper (400 mesh) was pasted on the top of the stainless-steel column. When the machine started to work, the friction instrument bearing, which connected to the stainless-steel column, moved back and forth on the surface of the composite. Then the superhydrophobic SBR/EVA composite was obtained after the sanding process.
Characterization
An FEI Q45 Environmental scanning electron microscope (SEM) was used to analyze the surface morphology of the material, which was sputter-coated with gold beforehand on its surface. Water contact angles (CA) and sliding angles (SA) were measured by a video optical contact angle system (OCA 20, DataPhysics, Germany) with deionized water of 5 μL.
In order to evaluate the mechanical impact resistant ability of the SBR/EVA composite, the sample was impacted by 0.5 and 1.0 kg weights from 1 m height for given times followed by CA and SA value measuring. Meanwhile, the SBR/EVA composite was compressed under a pressure of 0.5 MPa, and then the SEM and CA values of deformed and recovered composites were measured at room temperature. 3D images of composite roughness changes were also recorded on an HK8700 Ultra-Depth Microscope (Kyocera, Japan) and optical profilometer (Bruker Countor GT K, Beijing).
Chemical stability testing was conducted by dipping the SBR/EVA composite samples into solutions with different pH and organic solvents (ethanol, THF, acetone, and DMF) for 72 h followed by SEM investigation and CA/SA measurement of the dried samples.
In order to evaluate the healing ability of the superhydrophobicity, the superhydrophobic SBR/EVA sample was exposed to oxygen plasma (Tangshan Yanzhao Science and Technology Institute, China) etching for 2 min. Then the plasma-etched sample was abraded using sandpaper. CA and SA values were measured, and the element content change was analyzed by a K-alpha Thermo Fisher Scientific X-ray photoelectron spectroscope (XPS).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51572161).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02535.
Cell distribution of SBR/EVA composite; SEM images and CA values of the superhydrophobic composite in different conditions (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Xue C.-H.; Li Y.-R.; Zhang P.; Ma J.-Z.; Jia S.-T. Washable and Wear-Resistant Superhydrophobic Surfaces with Self-Cleaning Property by Chemical Etching of Fibers and Hydrophobization. ACS Appl. Mater. Interfaces 2014, 6, 10153–10161. 10.1021/am501371b. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Huang S.; Li F.; Zhao X.; Wang W. Coexistence of Superhydrophilicity and Superoleophobicity: Theory, Experiments and Applications in Oil/Water Separation. J. Mater. Chem. A 2018, 6, 15057–15063. 10.1039/C8TA04725A. [DOI] [Google Scholar]
- Li J.; Kang R.; Tang X.; She H.; Yang Y.; Zha F. Superhydrophobic Meshes That Can Repel Hot Water and Strong Corrosive Liquids Used for Efficient Gravity-Driven Oil/Water Separation. Nanoscale 2016, 8, 7638–7645. 10.1039/C6NR01298A. [DOI] [PubMed] [Google Scholar]
- Li F.; Wang Z.; Huang S.; Pan Y.; Zhao X. Flexible, Durable, and Unconditioned Superoleophobic/Superhydrophilic Surfaces for Controllable Transport and Oil-Water Separation. Adv. Funct. Mater. 2018, 28, 1706867. 10.1002/adfm.201706867. [DOI] [Google Scholar]
- Yang Y.; Li X.; Zheng X.; Chen Z.; Zhou Q.; Chen Y. 3D-Printed Biomimetic Super-Hydrophobic Structure for Microdroplet Manipulation and Oil/Water Separation. Adv. Mater. 2018, 30, 1704912. 10.1002/adma.201704912. [DOI] [PubMed] [Google Scholar]
- Wang L.; Gong Q.; Zhan S.; Jiang L.; Zheng Y. Robust Anti-Icing Performance of a Flexible Superhydrophobic Surface. Adv. Mater. 2016, 28, 7729–7735. 10.1002/adma.201602480. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Huang J.; Cheng Y.; Yang H.; Chen Z.; Lai Y. Bioinspired Surfaces with Superwettability for Anti-Icing and Ice-Phobic Application: Concept, Mechanism, and Design. Small 2017, 13, 1701867. 10.1002/smll.201701867. [DOI] [PubMed] [Google Scholar]
- Hwang G. B.; Patir A.; Page K.; Lu Y.; Allan E.; Parkin I. P. Buoyancy Increase and Drag-Reduction through a Simple Superhydrophobic Coating. Nanoscale 2017, 9, 7588–7594. 10.1039/C7NR00950J. [DOI] [PubMed] [Google Scholar]
- Wang N.; Xiong D.; Deng Y.; Shi Y.; Wang K. Mechanically Robust Superhydrophobic Steel Surface with Anti-Icing, UV-Durability, and Corrosion Resistance Properties. ACS Appl. Mater. Interfaces 2015, 7, 6260–6272. 10.1021/acsami.5b00558. [DOI] [PubMed] [Google Scholar]
- Wei H.; Wang Y.; Guo J.; Shen N. Z.; Jiang D.; Zhang X.; Yan X.; Zhu J.; Wang Q.; Shao L.; Lin H.; Wei S.; Guo Z. Advanced Micro/Nanocapsules for Self-Healing Smart Anticorrosion Coatings. J. Mater. Chem. A 2015, 3, 469–480. 10.1039/C4TA04791E. [DOI] [Google Scholar]
- Valdez B.; Kiyota S.; Stoytcheva M.; Zlatev R.; Bastidas J. M. Cerium-Based Conversion Coatings to Improve the Corrosion Resistance of Aluminium Alloy 6061-T6. Corros. Sci. 2014, 87, 141–149. 10.1016/j.corsci.2014.06.023. [DOI] [Google Scholar]
- Milionis A.; Loth E.; Bayer I. S. Recent Advances in the Mechanical Durability of Superhydrophobic Materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. 10.1016/j.cis.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Chen S.; Li X.; Li Y.; Sun J. Intumescent Flame-Retardant and Self-Healing Superhydrophobic Coatings on Cotton Fabric. ACS Nano 2015, 9, 4070–4076. 10.1021/acsnano.5b00121. [DOI] [PubMed] [Google Scholar]
- Guo X.-J.; Xue C.-H.; Li M.; Li X.; Ma J.-Z. Fabrication of Robust, Superhydrophobic, Electrically Conductive and UV-Blocking Fabrics via Layer-by-Layer Assembly of Carbon Nanotubes. RSC Adv. 2017, 7, 25560–25565. 10.1039/C7RA02111A. [DOI] [Google Scholar]
- Wang H.; Yao Q.; Wang C.; Fan B.; Sun Q.; Jin C.; Xiong Y.; Chen Y. A Simple, One-Step Hydrothermal Approach to Durable and Robust Superparamagnetic, Superhydrophobic and Electromagnetic Wave-Absorbing Wood. Sci. Rep. 2016, 6, 35549. 10.1038/srep35549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Li J.; Hou Y.; Guo Z. Inorganic Adhesives for Robust Superwetting Surfaces. ACS Nano 2017, 11, 1113–1119. 10.1021/acsnano.6b08348. [DOI] [PubMed] [Google Scholar]
- Si Y.; Zhu H.; Chen L.; Jiang T.; Guo Z. A Multifunctional Transparent Superhydrophobic Gel Nanocoating with Self-Healing Properties. Chem. Commun. 2015, 51, 16794–16797. 10.1039/C5CC06977G. [DOI] [PubMed] [Google Scholar]
- Wang H.; Liu Z.; Wang E.; Yuan R.; Gao D.; Zhang X.; Zhu Y. A Robust Superhydrophobic PVDF Composite Coating with Wear/Corrosion-Resistance Properties. Appl. Surf. Sci. 2015, 332, 518–524. 10.1016/j.apsusc.2015.01.213. [DOI] [Google Scholar]
- Wong W. S. Y.; Stachurski Z. H.; Nisbet D. R.; Tricoli A. Ultra-Durable and Transparent Self-Cleaning Surfaces by Large-Scale Self-Assembly of Hierarchical Interpenetrated Polymer Networks. ACS Appl. Mater. Interfaces 2016, 8, 13615–13623. 10.1021/acsami.6b03414. [DOI] [PubMed] [Google Scholar]
- Feng L.; Song Y.; Zhai J.; Liu B.; Xu J.; Jiang L.; Zhu D. Creation of a Superhydrophobic Surface from an Amphiphilic Polymer. Angew. Chem., Int. Ed. 2003, 42, 800–802. 10.1002/anie.200390212. [DOI] [PubMed] [Google Scholar]
- Pan S.; Guo R.; Björnmalm M.; Richardson J. J.; Li L.; Peng C.; Bertleff-Zieschang N.; Xu W.; Jiang J.; Caruso F. Coatings Super-Repellent to Ultralow Surface Tension Liquids. Nat. Mater. 2018, 17, 1040–1047. 10.1038/s41563-018-0178-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang L.; Xiao Z.; Wang S.; Yu X. Fabrication of Robust and Repairable Superhydrophobic Coatings by an Immersion Method. Chem. Eng. J. 2019, 369, 1–7. 10.1016/j.cej.2019.03.021. [DOI] [Google Scholar]
- Yoon D.; Chae S.; Kim W.; Lee D.; Choi D. Superhydrophobic Plasmonic Nanoarchitectures Based on Aluminum Hydroxide Nanotemplates. Nanoscale 2018, 10, 17125–17130. 10.1039/C8NR04873H. [DOI] [PubMed] [Google Scholar]
- Dou W.; Wu J.; Gu T.; Wang P.; Zhang D. Preparation of Super-Hydrophobic Micro-Needle CuO Surface as a Barrier against Marine Atmospheric Corrosion. Corros. Sci. 2018, 131, 156–163. 10.1016/j.corsci.2017.11.012. [DOI] [Google Scholar]
- Li L.; Breedveld V.; Hess D. W. Creation of Superhydrophobic Stainless Steel Surfaces by Acid Treatments and Hydrophobic Film Deposition. ACS Appl. Mater. Interfaces 2012, 4, 4549–4556. 10.1021/am301666c. [DOI] [PubMed] [Google Scholar]
- Cortese B.; Morgan H. Controlling the Wettability of Hierarchically Structured Thermoplastics. Langmuir 2011, 28, 896–904. 10.1021/la203741b. [DOI] [PubMed] [Google Scholar]
- Xie L.; Tang Z.; Jiang L.; Breedveld V.; Hess D. W. Creation of Superhydrophobic Wood Surfaces by Plasma Etching and Thin-Film Deposition. Surf. Coat. Technol. 2015, 281, 125–132. 10.1016/j.surfcoat.2015.09.052. [DOI] [Google Scholar]
- Ellinas K.; Tsougeni K.; Petrou P. S.; Boulousis G.; Tsoukleris D.; Pavlatou E.; Tserepi A.; Kakabakos S. E.; Gogolides E. Three-Dimensional Plasma Micro-Nanotextured Cyclo-Olefin-Polymer Surfaces for Biomolecule Immobilization and Environmentally Stable Superhydrophobic and Superoleophobic Behavior. Chem. Eng. J. 2016, 300, 394–403. 10.1016/j.cej.2016.04.137. [DOI] [Google Scholar]
- Liu Q.; Chen D.; Kang Z. One-Step Electrodeposition Process to Fabricate Corrosion-Resistant Superhydrophobic Surface on Magnesium Alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. 10.1021/am507586u. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Sheng X.; Cheng X.; Chen L.; Jin J.; Feng X. Robust Electrochemical Metal Oxide Deposition Using an Electrode with a Superhydrophobic Surface. Nanoscale 2017, 9, 87–90. 10.1039/C6NR07421A. [DOI] [PubMed] [Google Scholar]
- Boinovich L. B.; Domantovskiy A. G.; Emelyanenko A. M.; Pashinin A. S.; Ionin A. A.; Kudryashov S. I.; Saltuganov P. N. Femtosecond Laser Treatment for the Design of Electro-Insulating Superhydrophobic Coatings with Enhanced Wear Resistance on Glass. ACS Appl. Mater. Interfaces 2014, 6, 2080–2085. 10.1021/am4051603. [DOI] [PubMed] [Google Scholar]
- Boinovich L. B.; Modin E. B.; Sayfutdinova A. R.; Emelyanenko K. A.; Vasiliev A. L.; Emelyanenko A. M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. 10.1021/acsnano.7b04634. [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Xiao J.; Zeng J.; Wang C.; Liu J.; Xing S.; Jiang D.; Du G.; Yang F.; Peng C.; Chen H.; Ye Q.; Tang J. Facile Method to Prepare a Novel Honeycomb-Like Superhydrophobic Polydimethylsiloxan Surface. Surf. Coat. Technol. 2010, 205, 1947–1952. 10.1016/j.surfcoat.2010.08.085. [DOI] [Google Scholar]
- Mele E.; Girardo S.; Pisignano D. Strelitzia Reginae Leaf as a Natural Template for Anisotropic Wetting and Superhydrophobicity. Langmuir 2012, 28, 5312–5317. 10.1021/la300243x. [DOI] [PubMed] [Google Scholar]
- Xu L.; Zhu D.; Lu X.; Lu Q. Transparent, Thermally and Mechanically Stable Superhydrophobic Coating Prepared by an Electrochemical Template Strategy. J. Mater. Chem. A 2015, 3, 3801–3807. 10.1039/C4TA06944G. [DOI] [Google Scholar]
- Xu Q. F.; Mondal B.; Lyons A. M. Fabricating Superhydrophobic Polymer Surfaces with Excellent Abrasion Resistance by a Simple Lamination Templating Method. ACS Appl. Mater. Interfaces 2011, 3, 3508–3514. 10.1021/am200741f. [DOI] [PubMed] [Google Scholar]
- Xue C.-H.; Ma J.-Z. Long-lived Superhydrophobic Surfaces. J. Mater. Chem. A 2013, 1, 4146. 10.1039/c2ta01073a. [DOI] [PubMed] [Google Scholar]
- Deng X.; Mammen L.; Butt H. J.; Vollmer D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. 10.1126/science.1207115. [DOI] [PubMed] [Google Scholar]
- Jin H.; Tian X.; Ikkala O.; Ras R. H. A. Preservation of Superhydrophobic and Superoleophobic Properties upon Wear Damage. ACS Appl. Mater. Interfaces 2013, 5, 485–488. 10.1021/am302541f. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Guo Y.; Chen H.; Zhu W.; Zhang P. A Novel Damage-Tolerant Superhydrophobic and Superoleophilic Material. J. Mater. Chem. A 2014, 2, 9002–9006. 10.1039/C4TA00869C. [DOI] [Google Scholar]
- Zhang W.; Xiang T.; Liu F.; Zhang M.; Gan W.; Zhai X.; Di X.; Wang Y.; Liu G.; Wang C. Facile Design and Fabrication of Superwetting Surfaces with Excellent Wear-Resistance. ACS Appl. Mater. Interfaces 2017, 9, 15776–15784. 10.1021/acsami.7b02158. [DOI] [PubMed] [Google Scholar]
- Lv C.; Wang H.; Liu Z.; Wang C.; Li H.; Zhao Y.; Zhu Y. A Fluorine-Free Superhydrophobic PPS Composite Coating with High Thermal Stability, Wear Resistance, Corrosion Resistance. Prog. Org. Coat. 2017, 110, 47–54. 10.1016/j.porgcoat.2017.04.049. [DOI] [Google Scholar]
- Ju J.; Yao X.; Hou X.; Liu Q.; Zhang Y. S.; Khademhosseini A. A Highly Stretchable and Robust Non-Fluorinated Superhydrophobic Surface. J. Mater. Chem. A 2017, 5, 16273–16280. 10.1039/C6TA11133E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Yu X.; Zhang Y. Large-Scale Fabrication of Translucent, Stretchable and Durable Superhydrophobic Composite Films. J. Mater. Chem. A 2017, 5, 23489–23496. 10.1039/C7TA08203G. [DOI] [Google Scholar]
- Davis A.; Surdo S.; Caputo G.; Bayer I. S.; Athanassiou A. Environmentally Benign Production of Stretchable and Robust Superhydrophobic Silicone Monoliths. ACS Appl. Mater. Interfaces 2018, 10, 2907–2917. 10.1021/acsami.7b15088. [DOI] [PubMed] [Google Scholar]
- Ji Z.; Ma J.; Qin X.; Wu Y.; Xu R.; Ma Z.; Xue C.; Qin J.; Shao L. Improved Dimensional Stability of Styrene Butadiene Rubber/Ethylene Vinyl Acetate Composite Foams with Skeleton Support Structure Based on Alternately Cross-Linking Process. Polymer 2018, 157, 103–110. 10.1016/j.polymer.2018.10.028. [DOI] [Google Scholar]
- Steele A.; Davis A.; Kim J.; Loth E.; Bayer I. S. Wear Independent Similarity. ACS Appl. Mater. Interfaces 2015, 7, 12695–12701. 10.1021/acsami.5b00725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.