Abstract

ZIF-8 is a flexible zeolitic imidazole-based metal–organic framework and has been extensively studied because of its high structural stability. However, ZIF-8 is hydrolyzed in water at higher temperature, resulting in degradation of its crystalline and porous structure. In order to prevent ZIF-8 from structural collapse due to the hydrolysis reaction of the metal–ligand bond and/or ligand substitution reaction, it is effective to shield the metal–ligand bond from the attack of water molecules. This work reports on the thermal and hydrothermal stability of mechanochemically synthesized ZIF-8 and presents an incredibly simple step to modify the outermost surface of ZIF-8, improving the hydrothermal stability. The partial carbonization resulting in the formation of a carbon-rich outermost layer endowed ZIF-8 with not only high hydrothermal stability but also a high adsorption rate on liquid phase adsorption.
Introduction
Metal–organic frameworks (MOFs) are a unique type of crystalline microporous and inorganic–organic hybrid materials, which can be simply self-assembled from metal ions/clusters and organic ligands.1−6 MOFs offer many interesting opportunities in adsorption and separation technology because of their chemical and structural tunabilities. Recently, the potential application of MOFs has been studied expanding to liquid-phase separation in addition to gas-phase separation. There is growing interest in extending the application of MOFs to liquid-phase separations including bioalcohol dehydration/recovery,7,8 desalination,9−12 removal of trace organic contaminants,13−18 and so on. However, in such separations, especially in aqueous systems, the use of many MOFs is ultimately limited by their hydrothermal stability.
Among the many MOFs, there are two major classes of stable MOFs: the high-valent metal/cluster–carboxylate framework and low-valent metal–imidazolate framework. A major factor for the greater stability of high-valent metal/cluster–carboxylate MOFs, represented by MIL-19 and UiO-type20 structures, is that their high metal coordination numbers create a crowding effect, enhancing the strength of the metal–ligand bond and preventing the formation of water clusters near the metal. A series of ZIFs with the low-valent metal–imidazolate framework have relatively high thermal and chemical stability compared to other common MOFs.21 The relatively high water stability of ZIF is believed to be due to the bonding strength between Zn ions and imidazoles with a higher pKa and formation of the hydrophobic pore and surface. ZIF-8 (Zn(2-methylimidazole)2) is undoubtedly the most extensively studied because of its stability coupled with a facile and diverse synthesis including vapor-,22−24 liquid-,25−29 and solid-phase30−34 processes. However, concerning the stability of ZIF-8, contradicting results have been reported. Yaghi et al.25 and Low et al.21 demonstrated at the outset that the crystalline structure and surface area of ZIF-8 remain unchanged after immersing in boiling water or steaming at 300 °C. On the other hand, Leus et al. reported that the structure and surface area of ZIF-8 partially degrade in water.35 Zhang et al.36 and Duke et al.11 observed dissolution of ZIF-8 and release of Zn2+ into water. Yang et al. showed evidence that ZIF-8 undergoes hydrolysis under hydrothermal conditions where transformation to zinc oxide is thermodynamically preferred.37 Sheng et al.38 and Wang et al.39 also confirmed that ZIF-8 undergoes a phase change in water and transforms into an amorphous or dense crystalline product. More recently, Bhattacharyya et al. investigated the acid gas stability of ZIF-8 and proposed its degradation mechanism, supported by computational study, under CO2, NO2, or SO2 exposure.40−42 These findings strongly suggest that breakthroughs to improve the stability of MOFs are required.
ZIF-8 is now recognized as not having much high hydrothermal stability. The enhancement of stability is one of the most important challenges in the practical application of ZIF-8 for liquid-phase separation. In order to prevent the crystalline and porous structure from structural collapse due to a hydrolysis reaction of the metal–ligand bond and/or ligand substitution reaction, it should be effective to shield the metal–ligand bond from the attack of water molecules. Actually, Walton et al. demonstrated that the water stability of Zn-based MOFs is decreased or improved by incorporating hydrophilic/polar or hydrophobic/nonpolar functional groups in the ligand, respectively.43 Yang et al. presented a strategy to improve the hydrothermal stability of ZIF-8 through the so-called shell–ligand exchange reaction, taking advantage of steric hindrance and water-repellent effects.37 Inspired by these findings, we present here an incredibly simple step to modify the outermost surface of ZIF-8, improving the hydrothermal stability. The strategy in this study is to use thermal treatment for ZIF-8 in an inert atmosphere, partially carbonizing its outermost surface. ZIF-8 mass-produced by mechanochemical synthesis enabled quantitative evaluation of structural stability. In this contribution, we examine the thermal stability of mechanochemically synthesized ZIF-8 and hydrothermal stability of such a thermally treated ZIF-8.
Results and Discussion
Thermal Stability
PXRD patterns of ZIF-8 in the first efforts to evaluate its thermal stability are shown in Figure 1. According to our previous report,32−34 the pristine ZIF-8 was prepared by mechanochemical synthesis using zinc oxide, zinc acetate dihydrate, and 2-methylimidazole. The PXRD pattern of mechanochemically synthesized ZIF-8 corresponds to a family of lattice planes of a sodalite structure. As shown in Figure 1, the pristine ZIF-8 is a white powder, and its color changes from white to brown and finally to black with increasing the thermal treatment temperature. There is no significant difference in the macroscopic morphology observed with FESEM between the pristine ZIF-8 and ZIF/450 (Figure S1). Here, the products thermally treated in a nitrogen flow at x °C are designated as ZIF/x. The sodalite structure remained even after thermal treatment at 550 °C. However, in the PXRD pattern for ZIF/550, the intensity of reflection peaks decreased significantly, and their peak positions shifted slightly toward higher angles, suggesting loss of long-range crystallinity with structural shrinkage. On the other hand, ZIF/650 shows no reflection peak attributed to a sodalite structure and has an amorphous structure. In addition, new reflection peaks with extremely low intensity appeared after thermal treatment above 550 °C, which correspond to a family of lattice planes of zinc oxide.
Figure 1.

Photographs and PXRD patterns of pristine ZIF-8 and ZIF/x. Photograph courtesy of Yasuhito Tanaka. Copyright 2019.
The porous structure of thermally treated ZIF-8 was investigated by nitrogen adsorption/desorption measurements shown in Figure 2. All the products show typical type-I curves with a sharp increase in the adsorbed nitrogen amount near P/P0 = 1, suggesting the hierarchical micro- and mesoporous structure.33 The interparticle porosity between microporous particles forms the mesoporous structure. There is no large difference in the surface area (>1200 m2/g) among the products thermally treated below 550 °C. Given the semilogarithmic scale, there are differences in the type-I isotherms. The pristine ZIF-8 exhibits a unique stepwise isotherm, as reported before by several groups.44−46 ZIF-8 crystallizes into the sodalite topology, generating a resistant structure with large cages (1.16 nm) interconnected via narrow six-ring windows (0.34 nm). The stepwise adsorption isotherm has been explained based on adsorption-induced swinging of the linker and rearrangement of the adsorbed molecules.47 Such adsorption behavior gives ZIF-8 an interesting “gate-opening” functionality; it is interesting to note that ZIF-8 can adsorb molecules with kinetic diameters larger than its window size, such as propane, propylene, and so on.48,49 ZIF/250, ZIF/350, and ZIF/450, which were confirmed to have no structural change from PXRD results, show stepwise isotherms as with the pristine ZIF-8. The first and second branch of the isotherm almost overlaps for the pristine ZIF-8, ZIF/250, ZIF/350, and ZIF/450 as well as for the micropore capacity, indicating no large difference in the pore size distribution. Although the appearance of ZIF/450 visually turned brownish, as a conclusion of the characterization by the combination of PXRD and nitrogen adsorption/desorption results, the pristine ZIF-8 and ZIF/450 are unlikely to make much of a structural difference.
Figure 2.

N2 adsorption isotherms of pristine ZIF-8 and ZIF/x in linear (top) and semilogarithmic (bottom) scales.
Hydrothermal Stability
Next, hydrothermal stability tests were conducted on the pristine ZIF-8 and ZIF/x. Most recently, Zhang et al. pointed out that inconsistent results on water stability reported in the literature were caused by the relative amount of the sample to water, time in contact with water, and sample collection procedure.36 Following this point, in this study, relatively long-term stability tests (for one week) were conducted using a very small amount of samples (50 mg of the sample immersed in 100 mL of water, 0.05 wt %). Since the particle size of mechanochemically synthesized ZIF-8 was relatively large, the solid samples could be easily collected by suction filtration using filter paper with a nominal pore size of 1 μm. As shown in Figure 3, the pristine ZIF-8 preserved its crystalline structure after immersing in water at room temperature for one week, which is in good agreement with an earlier report by Yaghi et al.25 In addition, no changes were observed in nitrogen adsorption isotherms and surface areas, as shown in Figures 4 and 5. However, after immersing in water at 90 °C for one week, the pristine ZIF-8 showed structural collapse of the sodalite as well as transformation to different crystalline structures, as evidenced by the loss of low-angle reflections and appearance of new unknown reflections in the PXRD pattern. The decrease of the nitrogen adsorption amount and surface area was also significant. TEM observation supplemented the transformation of the pristine ZIF-8 into dense crystalline products under hydrothermal conditions (Figure S2).
Figure 3.
PXRD patterns of pristine ZIF-8 and ZIF/x before (bottom) and after immersing water at room temperature (middle) and 90 °C (top).
Figure 4.

N2 adsorption isotherms of pristine ZIF-8 and ZIF/x before and after immersing water at room temperature and 90 °C.
Figure 5.

BET surface areas of pristine ZIF-8 and ZIF/x before and after immersing water at room temperature and 90 °C.
ZIF/250 and ZIF/350 were slightly more stable to hydrolysis than the pristine ZIF-8. Surface area loss for ZIF/250 and ZIF/350 was also suppressed compared to the pristine ZIF-8. On the other hand, ZIF/450 was significantly more stable to hydrolysis than the pristine ZIF-8. The PXRD pattern for ZIF/450 immersed in water at both room temperature and 90 °C shows no structural degradation, as shown in Figure 3. The nitrogen adsorption isotherms and surface areas also hardly changed. In addition, no dense products other than porous ZIF/450 were confirmed by TEM observation after immersing ZIF/450 in water at 90 °C (Figure S2). ZIF-8, which underwent hydrolysis under hydrothermal conditions, could be endowed with high hydrothermal stability after thermal treatment at 450 °C in an inert atmosphere. As for ZIF/550 or ZIF/650 whose structure was partially or totally collapsed by previous thermal treatment, the surface areas significantly decreased after immersing in water even at room temperature. It is suggested that the mechanism of structural collapse between the pristine ZIF-8 and ZIF/550 is different.
Mechanism for Enhancing Hydrothermal Stability
As discussed previously, the structural difference between the pristine ZIF-8 and ZIF/450 could not be found from PXRD and nitrogen adsorption results. Therefore, the difference in chemical structure was examined by FTIR measurement. Figure 6 shows the FTIR spectra of the pristine ZIF-8, which are dominated by intense bands corresponding to methyl group and imidazole ring vibrations. The spectra bands of the mechanochemically synthesized ZIF-8 are in good agreement with those presented by Ordoñez et al.50 and Lin et al.51 The band observed at 420 cm–1 was assigned to Zn–N stretching, while the overall weakening/broadening of the convoluted bands observed at around 750, 1000, and 1400 cm–1 were attributed to entire-ring stretching, in-plane bending, and out-of-plane bending of the imidazolium ring. Even after thermal treatment at 450 °C, the overall chemical structure of ZIF-8 seems to remain unchanged. The band attributed to the physical adsorbed water decreased in intensity, indicating the surface change to more hydrophobicity. On the other hand, for ZIF/650, the breaking of Zn–N, the imidazolium ring, and the methyl group is evident by the loss and broadening of corresponding spectral intensity. In addition, the appearance of a broad band between 3000 and 3500 cm–1 indicates the hydrophilic nature of ZIF/650. In fact, ZIF/650 can be easily dispersed in water, while the pristine ZIF-8 and ZIF/450 repel water and are hard to disperse in water. It is speculated that the low water stability of ZIF/650 is due to the breaking of Zn–N and the resulting elution of Zn-related species into water.
Figure 6.

FTIR of pristine ZIF-8 and ZIF/x.
FTIR results showed little difference in the overall chemical structure between the pristine ZIF-8 and ZIF/450. Next, the surface composition was characterized by XPS to monitor individual regions for Zn 2p, C 1s, and N 1s at higher pass energy and resolution. The usual XPS probing depth range is up to the outermost 10 nm of the sample depending on the takeoff angle.52 The photoelectron escape depth of pelletized samples in this study is estimated to be approximately 4 nm. Figure 7 shows the outermost surface composition of the pristine ZIF-8 and ZIF/x. The outermost surface of the pristine ZIF-8 is found to be Zn-rich relative to 2-methylimidazole in comparison to the expected stoichiometric composition of C8H10N4Zn and terminated by hydroxyl and carbonate groups, as already reported by Benz et al.53 On the other hand, the outermost surface composition ratio of C/Zn increased with increasing the thermal treatment temperature up to 450 °C and reached a maximum of approximately 11 for ZIF/450. This result indicates that a carbon-rich layer without Zn species was formed on the outermost surface of ZIF/450. Although the thermal treatment temperature of 450 °C is much lower than the boiling point of Zn (907 °C), it is believed that the disappearance of Zn in the outermost surface of ZIF-8 can be easily caused by the breaking of Zn–N. Several of the literature has provided evidence of the vapor pressure that solid metals including Zn evaporate under low pressures even at low temperatures.54 With a further increase in the thermal treatment temperature, the C/Zn ratio decreased due to the dominant ligand decomposition. The outermost surface composition ratio of N/C increased after a minimum decline for ZIF/450. It is speculated that the hydrophobic nature of ZIF/450, which repels water, is due to the formation of the carbon-rich layer with the lowest N content. The amorphous layer was slightly observed on the surface of ZIF/450 that was not observed in the pristine ZIF-8 (Figure S3). Although more detailed high-resolution observations are required, it is speculated that the observed layer corresponds to the carbon-rich layer. The thickness of the layer was confirmed to be uniform within a range of approximately 2 to 5 nm. Indeed, the weight loss of the pristine ZIF-8 by thermal treatment at 450 °C was approximately 10% (Figure S4), which corresponds approximately to the weight ratio of the outermost thickness of 5 nm for the pristine ZIF-8. A schematic illustration for the structure of the outermost surface is proposed in Scheme 1. The thermal treatment at 450 °C in an inert atmosphere to form such a carbon layer on the outermost surface endows ZIF-8 with high hydrothermal stability. The extremely simple step for formation of the water-repellent carbon ultrathin layer is effective to shield the metal–ligand bond from the attack of water molecules. On the other hand, the fact that ZIF/550 and ZIF/650 are easily dispersed in water is attributed to the high relative content of N on the surface (Figure S5). This result is in reasonable agreement with the chemical structure including physically adsorbed water estimated by FTIR.
Figure 7.

Outermost surface composition of pristine ZIF-8 and ZIF/x.
Scheme 1. Partial Carbonization and Formation of the Carbon-Rich Layer on the Outermost Surface of ZIF-8.

Liquid-Phase Adsorption Property
ZIF-8 has been recognized as a unique adsorbent with a flexible framework. It is interesting to note that ZIF-8 can adsorb molecules with a kinetic diameter larger than its window size. Herein, we discuss the adsorption performance of organic pollutants on the pristine ZIF-8 and ZIF/x to demonstrate its potential as a liquid-phase adsorbent. Hydroquinone was chosen as a model molecule to test the liquid-phase adsorption property. Figure 8 shows the uptake curves of hydroquinone by the pristine ZIF-8 and ZIF/x. There is no significant difference in the adsorbed amount at equilibrium between the pristine ZIF-8 and ZIF/x thermally treated below 450 °C. The absence of a large difference in the adsorbed amount of hydroquinone results from the lack of a large difference in the overall microstructure including the surface area. Thus, ZIF/550 or ZIF/650 whose structure was partially or totally collapsed by thermal treatment shows lower adsorbed amounts of hydroquinone compared to the pristine ZIF-8.
Figure 8.
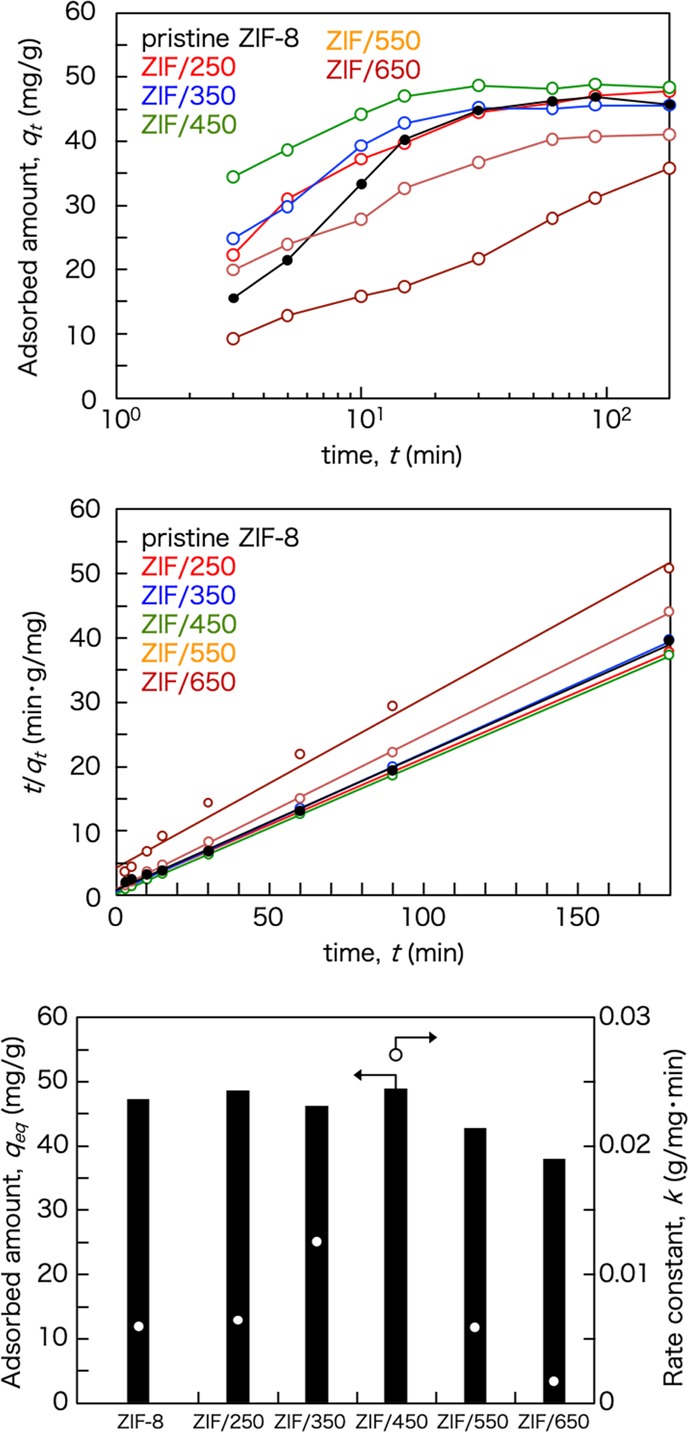
Uptake curves (top) and pseudo-second-order plots (middle) of hydroquinone by pristine ZIF-8 and ZIF/x and their adsorption characteristics (bottom).
Although there is no significant difference in the adsorption capacities, large differences in the adsorption kinetics are confirmed. It was found that the kinetic adsorption performance of hydroquinone on the pristine ZIF-8 and ZIF/x can be well-described by a pseudo-second-order kinetic model55−57 where qt and qeq are the transient and equilibrium adsorption amounts, respectively, and k is the pseudo-second-order rate constant. The constant k can be obtained from the intercept of the plot t/qt against t. Figure 8 shows a good linear relationship between t/qt and t, indicating that the kinetic adsorption behavior is well correlated with the pseudo-second-order kinetic model.
The adsorption rate constant k of hydroquinone on ZIF/450 is much higher than that of hydroquinone on the pristine ZIF-8 and another ZIF/x, as shown in Figure 8. The higher adsorption rate of ZIF/450 with high hydrothermal stability demonstrated it as a better adsorbent for liquid-phase adsorption than the pristine ZIF-8. The enhancement in the adsorption rate suggests that the surface resistance on diffusion of hydroquinone is reduced by pore size enlargement of the outermost surface due to the disappearance of Zn in the carbon-rich layer.
Conclusions
ZIF-8 is recognized as one of the most stable MOFs. Nevertheless, several of the literature shows that ZIF-8 undergoes hydrolysis under hydrothermal conditions. Our experimental results also provided the evidence that the structure and surface area of ZIF-8 degrade in water at higher temperatures regardless of how ZIF-8 is synthesized. In this contribution, we demonstrated an incredibly simple step to improve the hydrothermal stability of ZIF-8. The partial carbonization resulting in the formation of a carbon-rich outermost layer endowed ZIF-8 with not only high hydrothermal stability but also a high adsorption rate on liquid-phase adsorption. This simple strategy can be applied for other MOFs and opens new opportunities for numerous liquid-phase adsorption processes under severe conditions.
Experimental Section
Chemicals
All the commercially available chemicals were used without any further purification. Zinc oxide powders (nominal particle size of 0.02 μm) were purchased from Wako Pure Chemical Industries. Zinc acetate dihydrate, 2-methylimidazole, and hydroquinone were purchased from Sigma-Aldrich, Japan.
Mechanochemical Synthesis of ZIF-8
According to our previous report,32−34 the pristine ZIF-8 was prepared by mechanochemical synthesis using zinc oxide, zinc acetate dihydrate, and 2-methylimidazole. Zinc oxide (1.8 g), 0.55 g of zinc acetate dihydrate, and 4.1 g of 2-methylimidazole were placed in a 250 mL ceramic pot containing 50 YTZ balls and then milled at a rotation rate of 150 rpm for 1 h by using planetary mill Pulverisette 6 (Fritsch Japan). The products were rinsed with methanol and then dried under reduced pressure at 40 °C.
Thermal Treatment for ZIF-8
The pristine ZIF-8 was heated in a tubular furnace with a nitrogen flow at 100 mL/min. The furnace was heated at a ramping rate of 5 °C/min and then kept at 250–650 °C for 3 h. The products treated at x °C were designated as ZIF/x.
Hydrothermal Stability Test
Fifty milligrams of the dried samples, pristine ZIF-8 and ZIF/x, was immersed in 100 mL of water at room temperature or 90 °C for one week with stirring. ZIF/550 and ZIF/650 could be easily dispersed in water, resulting in a suspension. On the other hand, the pristine ZIF-8, ZIF/250, ZIF/350, and ZIF/450 were floated on the water surface at first, and then a suspension could be obtained with vigorous stirring in a few minutes. The solid samples were collected by suction filtration using filter paper with a nominal pore size of 1 μm.
Characterization
Powder X-ray diffraction (PXRD) patterns were recorded on a MiniFlex 600 (Rigaku) by using Cu Kα radiation with λ = 1.5418 Å; the copper anode was operated at 30 kV and 15 mA. N2 adsorption isotherms were measured at 77 K using a BELSORP-max (MicrotracBel Japan). Before adsorption measurement, the samples were degassed at 200 °C under vacuum. The Brunauer–Emmette–Teller (BET) model surface area was calculated from the nitrogen adsorption branch. Field emission scanning electron microscope (FESEM) images were recorded on S-4800 (Hitachi High-Tech). Transmission electron microscope (TEM) images were recorded on JEM-2010 (JEOL). Thermogravimetric analysis (TGA) was carried out with a DTG-60H (Shimadzu) under a nitrogen flow at 100 mL/min. FTIR spectra were recorded on an IRAffinity-1 spectrometer (Shimadzu, Japan) using a KBr pellet method. XPS spectra were recorded on a JPS-9000MX spectrometer (JEOL) using Mg Kα radiation as the energy source.
Liquid-Phase Adsorption
Liquid-phase adsorption experiments were carried out at room temperature by agitating 10 mg of the sample in 10 mL of 50 ppm hydroquinone solution. After adsorption, the hydroquinone concentration was determined using a UV–visible spectrophotometer UV-2450 (Shimadzu).
Acknowledgments
This work was financially supported by the Kurita Water and Environment Foundation (no. KWEF #17A019) and Sasakura Enviro-Science Foundation. This work was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI, Challenging Research (Exploratory) grant no. JP18K18983.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02812.
FESEM images (Figure S1), TEM images (Figure S2 and S3), TGA curve (Figure S4), and additional photographs (Figure S5) (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. 10.1038/46248. [DOI] [Google Scholar]
- Kitagawa S.; Kitaura R.; Noro S. Functional Porous Coordination Polymers. Angew. Chem., Int. Ed. 2004, 43, 2334–2375. 10.1002/anie.200300610. [DOI] [PubMed] [Google Scholar]
- Fujita M.; Tominaga M.; Hori A.; Therrien B. Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res. 2005, 38, 369–378. 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- Yaghi O. M.; O’Keeffe M.; Ockwig N. W.; Chae H. K.; Eddaoudi M.; Kim J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- Serre C.; Mellot-Draznieks C.; Surble S.; Audebrand N.; Filinchuk Y.; Ferey G. Role of Solvent-Host Interactions that Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315, 1828–1831. 10.1126/science.1137975. [DOI] [PubMed] [Google Scholar]
- Férey G. Hybrid Porous Solids: Past, Present, Future. Chem. Soc. Rev. 2008, 37, 191–214. 10.1039/B618320B. [DOI] [PubMed] [Google Scholar]
- Cousin Saint Remi J.; Reḿy T.; Van Hunskerken V.; van de Perre S.; Duerinck T.; Maes M.; De Vos D.; Gobechiya E.; Kirschhock C. E. A.; Baron G. V.; Denayer J. F. M. Biobutanol Separation with the Metal-Organic Framework ZIF-8. ChemSusChem 2011, 4, 1074–1077. 10.1002/cssc.201100261. [DOI] [PubMed] [Google Scholar]
- Liu X. L.; Li Y. S.; Zhu G. Q.; Ban Y. J.; Xu L. Y.; Yang W. S. An Organophilic Pervaporation Membrane Derived from Metal-Organic Framework Nanoparticles for Efficient Recovery of Bio-Alcohols. Angew. Chem., Int. Ed. 2011, 50, 10636–10639. 10.1002/anie.201104383. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Chen Y.; Jiang J. Zeolitic Imidazolate Framework-8 as a Reverse Osmosis Membrane for Water Desalination: Insight from Molecular Simulation. J. Chem. Phys. 2011, 134, 134705. 10.1063/1.3573902. [DOI] [PubMed] [Google Scholar]
- Liu X.; Demir N. K.; Wu Z.; Li K. Highly Water-Stable Zirconium Metal Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. 10.1021/jacs.5b02276. [DOI] [PubMed] [Google Scholar]
- Duke M. C.; Zhu B.; Doherty C. M.; Hill M. R.; Hill A. J.; Carreon M. A. Structural Effects on SAPO-34 and ZIF-8 Materials Exposed to Seawater Solutions, and Their Potential as Desalination Membranes. Desalination 2016, 377, 128–137. 10.1016/j.desal.2015.09.004. [DOI] [Google Scholar]
- Zhu Y.; Gupta K. M.; Liu Q.; Jiang J.; Caro J.; Huang A. Synthesis and Seawater Desalination of Molecular Sieving Zeolitic Imidazolate Framework Membranes. Desalination 2016, 385, 75–82. 10.1016/j.desal.2016.02.005. [DOI] [Google Scholar]
- Jiang J. Q.; Yang C. X.; Yan X. P. Zeolitic Imidazolate Framework-8 for Fast Adsorption and Removal of Benzotriazoles from Aqueous Solution. ACS Appl. Mater. Interfaces 2013, 5, 9837–9842. 10.1021/am403079n. [DOI] [PubMed] [Google Scholar]
- Liu X.; Jin H.; Li Y.; Bux H.; Hu Z.; Ban Y.; Yang W. Metal-Organic Framework ZIF-8 Nanocomposite Membrane for Efficient Recovery of Furfural via Pervaporation and Vapor Permeation. J. Membr. Sci. 2013, 428, 498–506. 10.1016/j.memsci.2012.10.028. [DOI] [Google Scholar]
- Jin H.; Li Y.; Liu X.; Ban Y.; Peng Y.; Jiao W.; Yang W. Recovery of HMF from Aqueous Solution by Zeolitic Imidazolate Frameworks. Chem. Eng. Sci. 2015, 124, 170–178. 10.1016/j.ces.2014.07.017. [DOI] [Google Scholar]
- Jiang X.; Chen H. Y.; Liu L. L.; Qiu L. G.; Jiang X. Fe3O4 Embedded ZIF-8 Nanocrystals with Ultra-High Adsorption Capacity towards Hydroquinone. J. Alloys Compd. 2015, 646, 1075–1082. 10.1016/j.jallcom.2015.06.021. [DOI] [Google Scholar]
- Khan N. A.; Jung B. K.; Hasan Z.; Jhung S. H. Adsorption and Removal of Phthalic Acid and Diethyl Phthalate from Water with Zeolitic Imidazolate and Metal-Organic Frameworks. J. Hazard. Mater. 2015, 282, 194–200. 10.1016/j.jhazmat.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Lan H.; Rönkkö T.; Parshintsev J.; Hartonen K.; Gan N.; Sakeye M.; Sarfraz J.; Riekkola M.-L. Modified Zeolitic Imidazolate Framework-8 as Solid-Phase Microextraction Arrow Coating for Sampling of Amines in Wastewater and Food Samples Followed by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2017, 1486, 76–85. 10.1016/j.chroma.2016.10.081. [DOI] [PubMed] [Google Scholar]
- Férey G.; Mellot-Draznieks C.; Serre C.; Millange F.; Dutour J.; Surblé S.; Margiolaki I. A Chromium Terephthalate-Based Solid With Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. 10.1126/science.1116275. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Low J. J.; Benin A. I.; Jakubczak P.; Abrahamian J. F.; Faheem S. A.; Willis R. R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. 10.1021/ja9061344. [DOI] [PubMed] [Google Scholar]
- Stassen I.; Styles M.; Grenci G.; Van Gorp H.; Vanderlinden W.; De Feyter S.; Falcaro P.; De Vos D.; Vereecken P.; Ameloot R. Chemical Vapour Deposition of Zeolitic Imidazolate Framework Thin Films. Nature Mater. 2016, 15, 304–310. 10.1038/nmat4509. [DOI] [PubMed] [Google Scholar]
- Tanaka S.; Sakamoto K.; Inada H.; Kawata M.; Takasaki G.; Imawaka K. Vapor-Phase Synthesis of ZIF-8 MOF Thick Film by Conversion of ZnO Nanorod Array. Langmuir 2018, 34, 7028–7033. 10.1021/acs.langmuir.8b00948. [DOI] [PubMed] [Google Scholar]
- Ma X.; Kumar P.; Mittal N.; Khlyustova A.; Daoutidis P.; Mkhoyan K. A.; Tsapatsis M. Zeolitic Imidazolate Framework Membranes Made by Ligand-Induced Permselectivation. Science 2018, 361, 1008–1011. 10.1126/science.aat4123. [DOI] [PubMed] [Google Scholar]
- Park K. S.; Ni Z.; Cote A. P.; Choi J. Y.; Huang R.; Uribe-Romo F. J.; Chae H. K.; O’Keeffe M.; Yaghi O. M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10186–10191. 10.1073/pnas.0602439103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z.; Masel R. I. Rapid Production of Metal-Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. 10.1021/ja0635231. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Liu Y.; Zeng G.; Zhao L.; Lai Z. Rapid Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals in an Aqueous System. Chem. Commun. 2011, 47, 2071–2073. 10.1039/c0cc05002d. [DOI] [PubMed] [Google Scholar]
- Tanaka S.; Kida K.; Okita M.; Ito Y.; Miyake Y. Size-Controlled Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Crystals in an Aqueous System at Room Temperature. Chem. Lett. 2012, 41, 1337–1339. 10.1246/cl.2012.1337. [DOI] [Google Scholar]
- Kida K.; Okita M.; Fujita K.; Tanaka S.; Miyake Y. Formation of High Crystalline ZIF-8 in an Aqueous Solution. CrystEngComm 2013, 15, 1794–1801. 10.1039/c2ce26847g. [DOI] [Google Scholar]
- Friščić T.; Fábián L. Mechanochemical Conversion of a Metal Oxide into Coordination Polymers and Porous Frameworks Using Liquid-Assisted Grinding (LAG). CrystEngComm 2009, 11, 743–745. 10.1039/b822934c. [DOI] [Google Scholar]
- Friščić T.; Reid D. G.; Halasz I.; Stein R. S.; Dinnebier R. E.; Duer M. J. Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal-Organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem., Int. Ed. 2010, 49, 712–715. 10.1002/anie.200906583. [DOI] [PubMed] [Google Scholar]
- Tanaka S.; Kida K.; Nagaoka T.; Ota T.; Miyake Y. Mechanochemical Dry Conversion of Zinc Oxide to Zeolitic Imidazolate Framework. Chem. Commun. 2013, 49, 7884–7886. 10.1039/c3cc43028f. [DOI] [PubMed] [Google Scholar]
- Tanaka S.; Nagaoka T.; Yasuyoshi A.; Hasegawa Y.; Denayer J. F. M. Hierarchical Pore Development of ZIF-8 MOF by Simple Salt-Assisted Mechanosynthesis. Cryst. Growth Des. 2018, 18, 274–279. 10.1021/acs.cgd.7b01211. [DOI] [Google Scholar]
- Imawaka K.; Sugita M.; Takewaki T.; Tanaka S. Mechanochemical Synthesis of Bimetallic CoZn-ZIFs with Sodalite Structure. Polyhedron 2019, 158, 290–295. [Google Scholar]
- Leus K.; Bogaerts T.; De Decker J.; Depauw H.; Hendrickx K.; Vrielinck H.; Van Speybroeck V.; Van Der Voort P. Systematic Study of the Chemical and Hydrothermal Stability of Selected “Stable” Metal Organic Frameworks. Microporous Mesoporous Mater. 2016, 226, 110–116. 10.1016/j.micromeso.2015.11.055. [DOI] [Google Scholar]
- Zhang H.; Zhao M.; Lin Y. S. Stability of ZIF-8 in Water under Ambient Conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. 10.1016/j.micromeso.2018.12.035. [DOI] [Google Scholar]
- Liu X.; Li Y.; Ban Y.; Peng Y.; Jin H.; Bux H.; Xu L.; Caro J.; Yang W. Improvement of Hydrothermal Stability of Zeolitic Imidazolate Frameworks. Chem. Commun. 2013, 49, 9140–9142. 10.1039/c3cc45308a. [DOI] [PubMed] [Google Scholar]
- Sheng L.; Yang F.; Wang C.; Yu J.; Zhang L.; Pan Y. Comparison of the Hydrothermal Stability of ZIF-8 Nanocrystals and Polycrystalline Membranes Derived from Zinc Salt Variations. Mater. Lett. 2017, 197, 184–187. 10.1016/j.matlet.2017.03.077. [DOI] [Google Scholar]
- Wang H.; Jian M.; Qi Z.; Li Y.; Liu R.; Qu J.; Zhang X. Specific Anion Effects on the Stability of Zeolitic Imidazolate Framework-8 in Aqueous Solution. Microporous Mesoporous Mater. 2018, 259, 171–177. 10.1016/j.micromeso.2017.10.011. [DOI] [Google Scholar]
- Bhattacharyya S.; Pang S. H.; Dutzer M. R.; Lively R. P.; Walton K. S.; Sholl D. S.; Nair S. Interactions of SO2-Containing Acid Gases with ZIF-8: Structural Changes and Mechanistic Investigations. J. Phys. Chem. C 2016, 120, 27221–27229. 10.1021/acs.jpcc.6b09197. [DOI] [Google Scholar]
- Bhattacharyya S.; Han R.; Kim W.-G.; Chiang Y.; Jayachandrababu K. C.; Hungerford J. T.; Dutzer M. R.; Ma C.; Walton K. S.; Sholl D. S.; Nair S. Acid Gas Stability of Zeolitic Imidazolate Frameworks: Generalized Kinetic and Thermodynamic Characteristics. Chem. Mater. 2018, 30, 4089–4101. 10.1021/acs.chemmater.8b01394. [DOI] [Google Scholar]
- Bhattacharyya S.; Han R.; Joshi J. N.; Zhu G.; Lively R. P.; Walton K. S.; Sholl D. S.; Nair S. Stability of Zeolitic Imidazolate Frameworks in NO2. J. Phys. Chem. C 2019, 123, 2336–2346. 10.1021/acs.jpcc.8b11377. [DOI] [Google Scholar]
- Jasuja H.; Huang Y.-g.; Walton K. S. Adjusting the Stability of Metal–Organic Frameworks under Humid Conditions by Ligand Functionalization. Langmuir 2012, 28, 16874–16880. 10.1021/la304151r. [DOI] [PubMed] [Google Scholar]
- Ania C. O.; García-Pérez E.; Haro M.; Gutiérrez-Sevillano J. J.; Valdés-Solís T.; Parra J. B.; Calero S. Understanding Gas-Induced Structural Deformation of ZIF-8. J. Phys. Chem. Lett. 2012, 3, 1159–1164. 10.1021/jz300292y. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Hu Z.; Jiang J. Sorption-Induced Structural Transition of Zeolitic Imidazolate Framework-8: A Hybrid Molecular Simulation Study. J. Am. Chem. Soc. 2013, 135, 3722–3728. 10.1021/ja401129h. [DOI] [PubMed] [Google Scholar]
- Tanaka S.; Fujita K.; Miyake Y.; Miyamoto M.; Hasegawa Y.; Makino T.; Van der Perre S.; Cousin Saint Remi J.; Van Assche T.; Baron G. V.; Denayer J. F. M. Adsorption and Diffusion Phenomena in Crystal Size Engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. 10.1021/acs.jpcc.5b09520. [DOI] [Google Scholar]
- Tanaka H.; Ohsaki S.; Hiraide S.; Yamamoto D.; Watanabe S.; Miyahara M. T. Adsorption-Induced Structural Transition of ZIF-8: A Combined Experimental and Simulation Study. J. Phys. Chem. C 2014, 118, 8445–8454. 10.1021/jp500931g. [DOI] [Google Scholar]
- Zhang C.; Lively R. P.; Zhang K.; Johnson J. R.; Karvan O.; Koros W. J. Unexpected Molecular Sieving Properties of Zeolitic Imidazolate Framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. 10.1021/jz300855a. [DOI] [PubMed] [Google Scholar]
- Tanaka S.; Okubo K.; Kida K.; Sugita M.; Takewaki T. Grain Size Control of ZIF-8 Membranes by Seeding-Free Aqueous Synthesis and Their Performances in Propylene/Propane Separation. J. Membr. Sci. 2017, 544, 306–311. 10.1016/j.memsci.2017.09.037. [DOI] [Google Scholar]
- Ordoñez M. J. C.; Balkus K. J. Jr.; Ferraris J. P.; Musselman I. H. Molecular Sieving Realized with ZIF-8/Matrimid® Mixed-Matrix Membranes. J. Membr. Sci. 2010, 361, 28–37. 10.1016/j.memsci.2010.06.017. [DOI] [Google Scholar]
- James J. B.; Lin Y. S. Kinetics of ZIF-8 Thermal Decomposition in Inert, Oxidizing, and Reducing Environments. J. Phys. Chem. C 2016, 120, 14015–14026. 10.1021/acs.jpcc.6b01208. [DOI] [Google Scholar]
- Seah M. P.; Dench W. A. Quantitative Electron Spectroscopy of Surfaces: A Standard Data Base for Electron Inelastic Mean Free Paths in Solids. Surf. Interface Anal. 1979, 1, 2–11. 10.1002/sia.740010103. [DOI] [Google Scholar]
- Tian F.; Cerro A. M.; Mosier A. M.; Wayment-Steele H. K.; Shine R. S.; Park A.; Webster E. R.; Johnson L. E.; Johal M. S.; Benz L. Surface and Stability Characterization of a Nanoporous ZIF-8 Thin Film. J. Phys. Chem. C 2014, 118, 14449–14456. 10.1021/jp5041053. [DOI] [Google Scholar]
- Kaye G. W. C.; Ewen D. The Sublimation of Metals at Low Pressures. Proc. R. Soc. A 1913, 89, 58–67. 10.1098/rspa.1913.0063. [DOI] [Google Scholar]
- Tsai W.-T.; Lai C.-W.; Su T.-Y. Adsorption of Bisphenol-A from Aqueous Solution onto Minerals and Carbon Adsorbents. J. Hazard. Mater. 2006, 134, 169–175. 10.1016/j.jhazmat.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Miyake Y.; Ishida H.; Tanaka S.; Kolev S. D. Theoretical Analysis of the Pseudo-Second Order Kinetic Model of Adsorption. Application to the Adsorption of Ag(I) to Mesoporous Silica Microspheres Functionalized with Thiol Groups. Chem. Eng. J. 2013, 218, 350–357. 10.1016/j.cej.2012.11.089. [DOI] [Google Scholar]
- Tanaka S.; Miyashita R. Aqueous-System-Enabled Spray-Drying Technique for the Synthesis of Hollow Polycrystalline ZIF-8 MOF Particles. ACS Omega 2017, 2, 6437–6445. 10.1021/acsomega.7b01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



