Abstract
The expression of animal digestive enzymes reflects important dietary adaptations. The pangolin, also known as scaly anteater, is a specialized myrmecophage that consumes mainly ants and termites, but its digestive enzymes have not been fully investigated. Therefore, in this study, we used shotgun proteomic analysis to examine the protein components of the saliva and intestinal juice of a Sunda pangolin (Manis javanica) that died shortly after being rescued. The intestinal juice contained greater variety of digestive enzymes, including α-amylase, maltase-glucoamylase, α,α-trehalase, sucrase-isomaltase, pepsin A, trypsin, pancreatic endopeptidase E, carboxypeptidase A1, carboxypeptidase B, dipeptidyl-peptidase 4, and pancreatic triacylglycerol lipase. The digestive enzymes identified in the saliva were maltase-glucoamylase and trypsin, and chitinase which was also found in the intestinal juice. Compared with other animals, the Sunda pangolin has less intestinal protease diversity and lacks key digestive enzymes, such as chymotrypsin and pancreatic elastase. The expression profile of the digestive enzymes of the Sunda pangolin reveals animal’s adaptation to a diet consisting mainly of ants and termites. Our results will facilitate the preparation of artificial food for rescued pangolins and for those in captivity for conservation breeding efforts.
Introduction
The extant mammals, under the powerful selective pressure exerted by the need for successful acquisition of food resources, have evolved a wide variety of dietary niches, ranging from broadly generalized to highly specialized.1 However, food choices are constrained by the capability of an animal’s digestive system to extract nutrients effectively.2 The mammalian digestive system has evolved to include several anatomical structures, a complex microbial flora,3 and digestive enzymes adapted to feeding habits.4,5
Pangolins are specialized myrmecophagous mammals that feed mainly on ants and termites6−12 and have a unique external armor of overlapping scales; hence, they are often called scaly anteaters. Like some other myrmecophagous mammals, such as anteaters, pangolins have powerful front claws that allow them to dig into ant and termite nests. They also have a pointed snout, toothless mouth, and long, flexible, slimy tongue adapted to preying on ants and termites.13,14 In addition to adaptation for predation, the efficient extraction of nutrients from food is another important challenge for pangolins. The polysaccharide chitin, a major exoskeleton component, makes up 5–20% of the dry weight of ants and termites;15 thus, most of the protein value of ants and termites is locked in the exoskeleton,16 and chitin is recalcitrant to digestion. How pangolins get energy and nutrients efficiently from ants and termites is not well understood. There is little research on the pangolin digestive system; published studies have focused on its morphology and structure. The inner gastric surface of the Sunda pangolin (Manis javanica) has a thick layer of keratinized epithelial cells.17 Like Chinese pangolin (Manis pentadactyla), the gastric fundus of Sunda pangolins has many mucosal folds to expand the gastric surface area,17−19 and the pylorus has hard spines called “pyloric teeth”,17,19 which are similar to the stomachs of other ant-eating animals, such as Indian pangolins (Manis crassicaudata),20 grasshopper mouse (Onychomys torridus),21 armadillo, and echidna22 and is thought to be suitable for its ant-eating habit and toothless characteristics.17,22,23 However, no horny ridges are found in the stomachs of the giant pangolin (Smutsia gigantea), long-tailed pangolin (Phataginus tetradactyla), or tree pangolin (Phataginus tricuspis), and this is thought to be a possible difference between Asian and African pangolins.19 Neither the Chinese pangolin nor the Sunda pangolin has a caecum, and there is no obvious boundary between the large and small intestines.24 The small intestine of Chinese pangolin is about nine times its body length, longer than that of a carnivore, and it is thought to take longer time to digest ants and termites.24
Although digestive enzymes and gut microbes play an important role in animal digestion, there is only one report on the digestive enzymes and gut microbes of pangolins. The intestinal microbial community of the Sunda pangolin is similar to that of herbivores but different from that of anteaters and other ant-eating animals.25 It does not conform to the theory that gut microbiomes evolved convergently in the mammals sharing the same feeding habits.26 Another paper verified the presence of chitinase in the stomach of the Sunda pangolin.27 However, there are no reports about the characteristics of digestive enzyme expression in saliva and small intestine. The small intestine, in particular, is the most important site for food digestion. What is more, the pangolins have a higher incidence of gastrointestinal diseases in captivity, and it is speculated to be related to the lack of understanding of pangolins’ digestive physiology, and the artificial food provided is not well digested and absorbed. Hence, we hypothesize that pangolin has formed specialized digestive physiology, which lacks the expression of some proteases, but expresses more certain digestive enzymes, such as chitinase, to adapt to the specialized ant-eating habits.
In recent years, the development of proteomics has allowed investigation of the expression profiles of proteins in specific tissues or cells, and thus determination of the cellular metabolic potential.28,29 The use of proteomics for the large-scale detection of proteins in animal digestive juices can provide insight into animal digestive physiology in an evolutionary environment.30 Digestive enzymes and variation in the digestive tract play key roles in the decomposition of large food molecules into small absorbable compounds and are extremely important in dietary adaptation. Therefore, to understand the digestive function of the Sunda pangolin, this study used shotgun liquid chromatography-tandem mass spectrometry (LC–MS/MS) for the first time to detect the variety and relative abundance of digestive enzymes in saliva and intestinal juice of the Sunda pangolin and to construct an expression profile. Our results provide insight into the mechanisms underlying the digestive adaptation of pangolins and a basis for the selection of artificial food ingredients in the ex situ conservation of these animals.
Results
Identification of Proteins in the Saliva and Intestinal Juice of the Sunda Pangolin
In the tested Sunda pangolin, shotgun LC–MS/MS and MaxQuant software analysis revealed significant differences in the variety of proteins in the two digestive fluids, with 727 distinct proteins in saliva and 2968 in the intestinal juice (Table 1). There were 296 and 1030 proteins with ≥2 unique peptides, respectively (Table 1). Of these identified proteins, 91 and 25 proteins could not be quantified. Myoglobin, keratin, and actin were the most abundant proteins in saliva and intestinal juice, with intensity-based absolute protein quantification (iBAQ) values of 7 351 000 000 and 9 135 400 000, respectively.
Table 1. Proteins Identified in Two Digestive Juices from Sunda Pangolin HS-04.
| unique
peptide |
|||||
|---|---|---|---|---|---|
| sample | protein group | =0 | =1 | ≥2 | iBAQ = 0 |
| saliva | 727 | 45 | 386 | 296 | 91 |
| intestinal juice | 2968 | 224 | 1714 | 1030 | 25 |
Fewer specific proteins were found in saliva (n = 478) than in intestinal juice (n = 2719), whereas the two digestive juices had 249 proteins in common (Figure 1).
Figure 1.
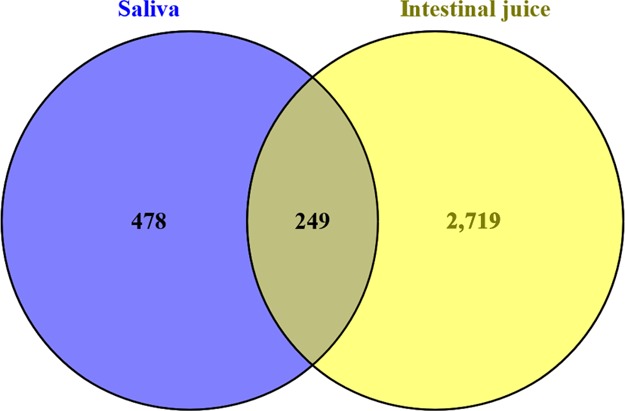
Venn diagram of the common and unique proteins among the total proteins in the saliva and intestinal juice of the Sunda pangolin HS-04.
Functional Predictions for the Proteins Identified in Saliva and Intestinal Juice
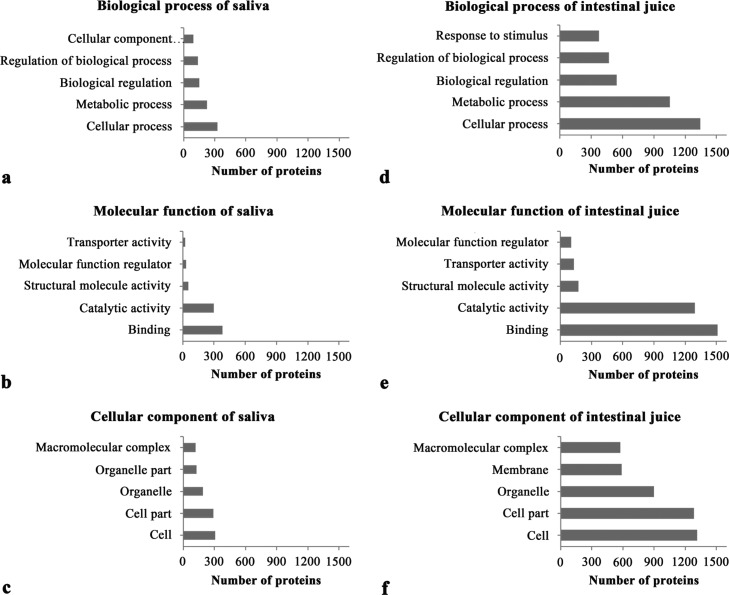
Protein functional annotation provides insight into the physiological function of a sample. Gene ontology (GO) annotation was performed for 719 and 2957 proteins in saliva and intestinal juice of Sunda pangolin HS-04, respectively, of which 8 and 11, respectively, could not be matched because of a lack of annotation. According to prediction analyses, most of the proteins in the two digestive juices were involved in binding and catalytic activity in the molecular function: 383 and 300 proteins in saliva (Figure 2b) and 1513 and 1295 proteins in intestinal juice (Figure 2e). Further analysis assigned most of the proteins from the two digestive fluids to cellular and metabolic processes in the biological process and to cell and cell parts in cellular components (Figure 2).
Figure 2.
Functional classification of the biological processes, molecular functions, and cellular components of saliva (a–c) and intestinal juice (d–f) for Sunda pangolin HS-04 based on GO annotation.
Digestive Enzyme Expression Analysis
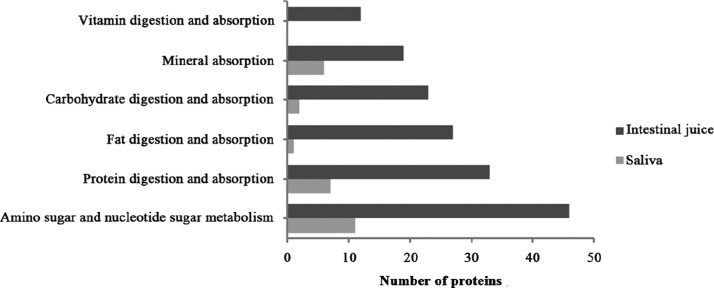
In the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, 316 and 356 pathways were enriched in saliva and intestinal juice of Sunda pangolin HS-04, respectively (Tables S1 and S2). In accordance with the purpose of this study, we focused only on the key pathways associated with digestion and absorption. These five pathways were related to carbohydrate digestion and absorption, protein digestion and absorption, fat digestion and absorption, vitamin digestion and absorption, and mineral absorption. All of these pathways were annotated in the intestinal juices, whereas the vitamin digestion and absorption pathway was not annotated in saliva.
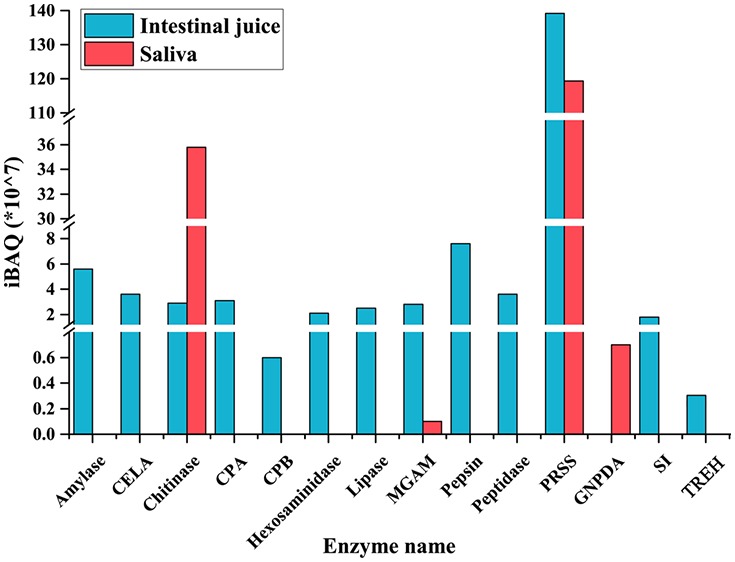
The number of varieties of proteins related to digestion and absorption pathways enriched in saliva and intestinal juice is shown in Figure 3. Among the two digestive fluids of this pangolin, the larger variety of proteins involved in the five digestion and absorption pathways was annotated in intestinal juice, in which the number of types of annotated digestive enzymes was also greater and included α-amylase, maltase-glucoamylase, α,α-trehalase, sucrase-isomaltase, pepsin A, trypsin, pancreatic endopeptidase E, carboxypeptidase A1, carboxypeptidase B, dipeptidyl-peptidase 4, angiotensin-converting enzyme 2, neprilysin, pancreatic triacylglycerol lipase, secretory phospholipase A2, and pancreatic lipase-related protein 1 (Table 2). Only two digestive enzymes, maltase-glucoamylase and trypsin, were annotated in the saliva of the pangolin.
Figure 3.
Five digestion and absorption pathways and the amino sugar and nucleotide sugar metabolism pathways in saliva and intestinal juice of Sunda pangolin HS-04 according to KEGG annotation.
Table 2. Variety of Digestive Enzymes Identified in the Intestinal Juice of Sunda Pangolin HS-04.
| no. | protein ID | name | definition |
|---|---|---|---|
| 1 | G9L1C1 | amylase | α-amylase [EC 3.2.1.1] |
| 2 | A0A287A042 | MGAM | maltase-glucoamylase [EC 3.2.1.20, 3.2.1.3] |
| 3 | M3WU26 | MGAM | maltase-glucoamylase [EC 3.2.1.20, 3.2.1.3] |
| 4 | D2HHN0 | MGAM | maltase-glucoamylase [EC 3.2.1.20, 3.2.1.3] |
| 5 | F7DGG1 | MGAM | maltase-glucoamylase [EC 3.2.1.20, 3.2.1.3] |
| 6 | G1PWG9 | MGAM | maltase-glucoamylase [EC 3.2.1.20, 3.2.1.3] |
| 7 | U6D7J0 | TREH | α,α-trehalase [EC 3.2.1.28] |
| 8 | A0A172ZB06 | SI | sucrase-isomaltase [EC 3.2.1.48, 3.2.1.10] |
| 9 | S7PEU0 | SI | sucrase-isomaltase [EC 3.2.1.48, 3.2.1.10] |
| 10 | E1BGH5 | SI | sucrase-isomaltase [EC 3.2.1.48, 3.2.1.10] |
| 11 | M3YE08 | SI | sucrase-isomaltase [EC 3.2.1.48, 3.2.1.10] |
| 12 | W5NU90 | SI | sucrase-isomaltase [EC 3.2.1.48, 3.2.1.10] |
| 13 | A0A1S2ZY73 | pepsin | pepsin A [EC 3.4.23.1] |
| 14 | P00761 | PRSS | trypsin [EC 3.4.21.4] |
| 15 | L5LNG8 | PRSS | trypsin [EC 3.4.21.4] |
| 16 | M3WP64 | PRSS | trypsin [EC 3.4.21.4] |
| 17 | F1PI75 | CELA | pancreatic endopeptidase E [EC 3.4.21.70] |
| 18 | M3WKB7 | CPA | carboxypeptidase A1 [EC 3.4.17.1] |
| 19 | L5KB43 | CPB | carboxypeptidase B [EC 3.4.17.2] |
| 20 | A0A212C1I6 | peptidase | dipeptidyl-peptidase 4 [EC 3.4.14.5] |
| 21 | L5JZX8 | peptidase | dipeptidyl-peptidase 4 [EC 3.4.14.5] |
| 22 | E2DHI6 | peptidase | angiotensin-converting enzyme 2 [EC 3.4.17.23] |
| 23 | L8IHS5 | peptidase | neprilysin [EC 3.4.24.11] |
| 24 | F5C3N2 | peptidase | neprilysin [EC 3.4.24.11] |
| 25 | P00591 | lipase | pancreatic triacylglycerol lipase [EC 3.1.1.3] |
| 26 | U6DM40 | lipase | secretory phospholipase A2 [EC 3.1.1.4] |
| 27 | D2H719 | lipase | pancreatic lipase-related protein 1 [EC 3.1.1.3] |
| 28 | A0A1S2ZFC8 | lipase | pancreatic lipase-related protein 1 [EC 3.1.1.3] |
| 29 | A0A212CG51 | 3.2.1.14 | chitinase [EC 3.2.1.14] |
| 30 | G1LGG0 | 3.2.1.14 | chitinase [EC 3.2.1.14] |
| 31 | G9K475 | 3.2.1.52 | hexosaminidase [EC 3.2.1.52] |
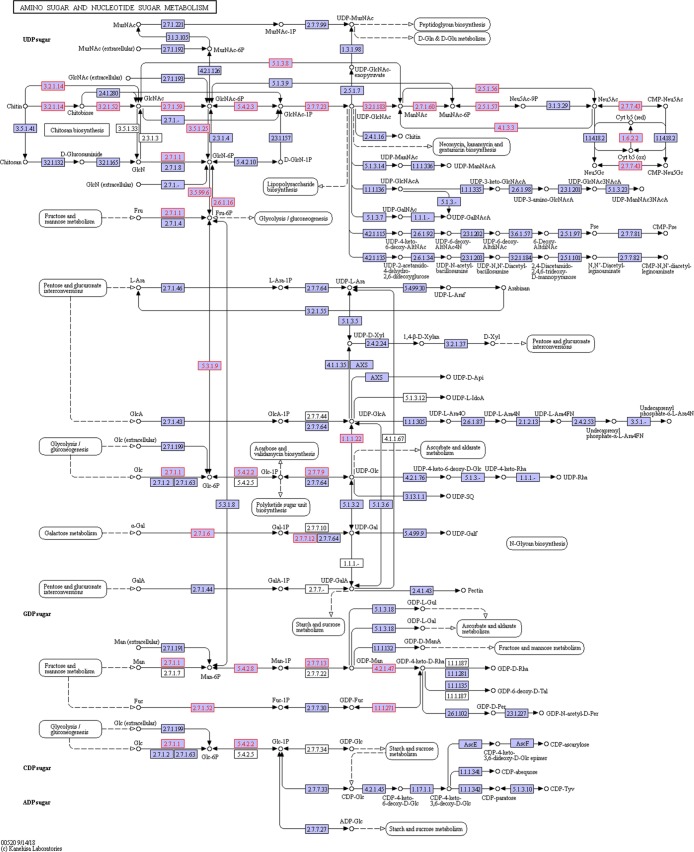
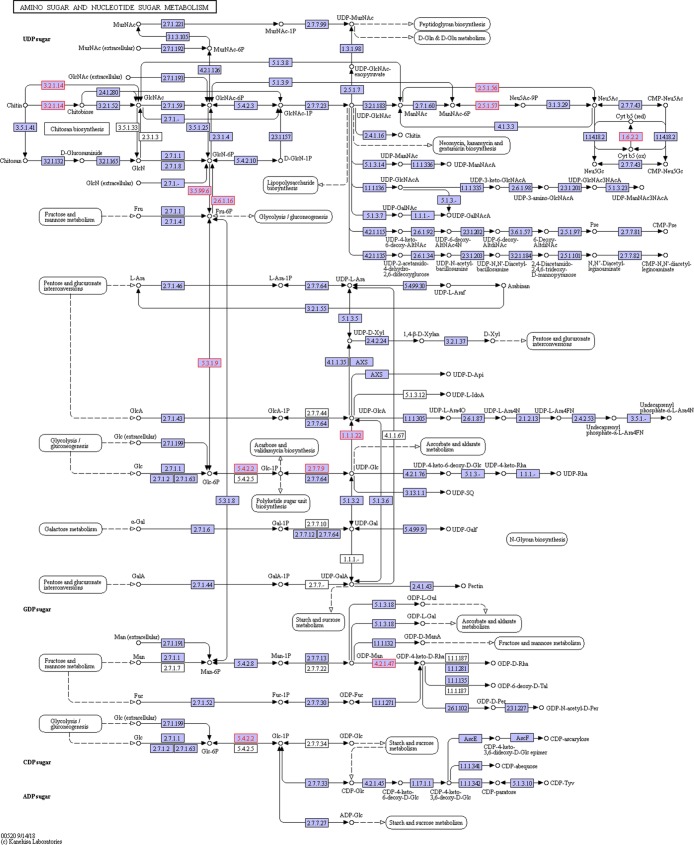
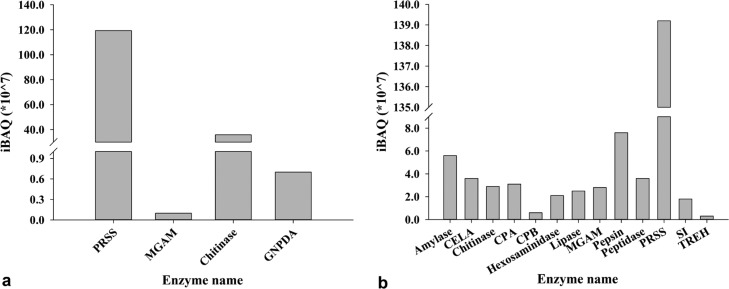
We also considered the amino sugar and nucleotide sugar metabolism pathway as it is related to chitin degradation and the pangolin diet contains a considerable amount of chitin. This pathway was enriched in two digestive juices of this tested pangolin, with the larger variety of related proteins present in the intestinal juice (Figure 3, Table S2). In addition to the chitinolytic enzyme chitinase and hexosaminidase (Table 2), these proteins included multiple enzymes associated with amino sugar metabolism (Figure 4). In the saliva, chitinase and several other enzymes associated with amino sugar metabolism, such as glucosamine-6-phosphate deaminase, were detected (Table 3 and Figure 5). The iBAQ values for these important digestive enzymes identified in the saliva and intestinal juice ranged from 106 to 109 (Figure 6).
Figure 4.
Enzymes (shown in red) enriched in the amino sugar and nucleotide sugar metabolism pathway from the intestinal juice of Sunda pangolin HS-04.
Table 3. Variety of Digestive Enzymes Identified in the Saliva of Sunda Pangolin HS-04.
| no. | protein ID | name | definition |
|---|---|---|---|
| 1 | M3X654 | MGAM | maltase-glucoamylase [EC 3.2.1.20, 3.2.1.3] |
| 2 | P00761 | PRSS1_2_3 | trypsin [EC 3.4.21.4] |
| 3 | G1LGG0 | E3.2.1.14 | chitinase [EC 3.2.1.14] |
| 4 | T0NRJ1 | E3.2.1.14 | chitinase [EC 3.2.1.14] |
Figure 5.
Enzymes (shown in red) enriched in the amino sugar and nucleotide sugar metabolism pathway from the saliva of Sunda pangolin HS-04.
Figure 6.
iBAQ intensities of the digestive enzymes detected in saliva (a) and intestinal juice (b) of Sunda pangolin HS-04.
Discussion
The enzymes in the digestive system of animals are one of the key factors related to their digestive function. Pangolin is a mammal with specialized food, that is, ants and termites. In this study, we speculated that the expression of digestive enzymes in pangolin’s digestive system had formed an adaptation to its specialized food and analyzed the types of digestive enzymes in saliva and intestinal fluid of a Sunda pangolin. Unfortunately, we were unable to detect the types of digestive enzymes in gastric fluid because of problems in sample preservation. The higher diversity of proteins and digestive enzymes in the Sunda pangolin was found in intestinal juice than saliva. Trypsin was detected in two digestive fluids and was the most abundant digestive enzyme (Figure 6). In addition to protein digestion, trypsin plays a central role in regulating the activities of other digestive enzymes.31 This may explain why trypsin was present in the saliva of the pangolin and in its small intestine.
A diet consisting of insects, especially ants and termites, contains considerable amounts of the protein value and other nutrients locked in chitin.16 Although chitin is as an excellent source of carbon, energy, and nitrogen, it must first be broken down by chitinase into n-acetylglucosamine residues that can be transported into the cell.32 In this study, it was found that chitinase was expressed in the saliva and intestinal juice of Sunda pangolin HS-04, and hexosaminidase was also expressed in the intestinal juice. A diet rich in certain nutrients exerts selective pressure on the evolution of genes encoding more specific digestive enzymes in different parts of the digestive system.4,5 Chitinase is commonly found in the stomachs of mammals as acidic mammalian chitinase.15,33−35 For better digestion of chitin in food, in addition to the stomach, chitinase is found in the intestines and saliva of some other insect-eating animals, such as bats and rodents.32,34 It was also detected in the saliva and intestinal juice of the Sunda pangolin, in amounts similar to those of most other digestive enzymes (Figure 6). This implies the existence of strong evolutionary pressure for Sunda pangolins to digest chitin effectively. The expression of chitinase in the mouth, stomach, and small intestine allows efficient nutrient extraction. Moreover, compared to those of carnivores, pangolins have longer intestinal tracts,24 which may allow chitinase more time for chitin digestion in the intestine. Together with the chitinase expressed in its saliva and stomach,27 these constitute an adaptation that allows the utilization of ants and termites as primary food sources.
The wide variety of proteins annotated in the amino sugar and nucleotide sugar metabolism pathway in the tested pangolin intestinal juice indicates not only an evolutionary adaptation for chitin digestion but also the importance of chitin in the pangolin diet. The role of chitin in the dietary nutrition of pangolins and giant anteaters (Myrmecophaga tridactyla) was initially thought to be as a source of dietary fiber.24,36 However, the annotation of other enzymes involved in amino sugar and nucleotide sugar metabolism in the pangolin intestinal tract (besides chitinase and hexosaminidase; Figure 4) suggests that chitin is broken down into n-acetylglucosamine monomers, which are then converted to glucose and nucleotide sugars. Accordingly, chitin likely provides substantial nutrients for pangolins.
There is low protease diversity in the Sunda pangolin intestine, which lacks some protein digestive enzymes. Proteins are eventually digested by proteases into amino acids, which provide key nutrients for growth, development, repair, energy, and many other bodily functions. The significant overlap in functional protease subtypes (i.e., the multiple parallel and redundant systems) in the animal digestive tract ensures an adequate supply of all the key amino acids despite a diverse and inconsistent diet.31 However, we found that several common intestinal proteases, such as chymotrypsin B1, chymotrypsin, chymotrypsin-like protease, pancreatic elastase 2 (EC 3.4.21.71), carboxypeptidase A2 (EC 3.4.17.15), and carboxypeptidase B2 (EC 3.4.17.20), were missing from the protein digestive system of Sunda pangolin HS-04. This is also the case in several primates, such as platyrrhine monkeys, which rely primarily on insect protein and thus have less pepsin diversity than do primates that depend on foliage protein.37,38 Thus, insectivorous species may not need multiple pepsins to digest insect proteins, whereas chitinase is essential for the digestion of insect exoskeletons.2 As the diversity and complexity of proteins in the diet of Sunda pangolins, which feed on ants and termites, are stable, a large number of parallel and redundant systems for dietary protein digestion would not be needed. The digestive enzymes in the animal gut represent the response to dietary diversity, and changes in their expression or type are essential to allow dietary adaptations, including those enabling the use of complex or otherwise indigestible foods.2 Sunda pangolins can digest artificial food made from invertebrates well but not feed containing fish meal, eggs, or dairy products.32 Because different proteases have different specific pockets where single peptide bonds are hydrolyzed,31,39,40 the inability to digest some products may be related to the absence in the pangolin digestive system of chymotrypsin, elastase, and other proteases needed for the breakdown of these proteins. The digestive system of Sunda pangolins also lacks the enzymes needed to break down lactose, which explains why these animals are unable to digest dairy products.2,41 Therefore, artificial food for pangolins should be designed to be compatible with the physiological characteristics of pangolin digestion, including the spectrum of enzymes in its digestive system.
In the study of endangered animals, one of the greatest challenges is obtaining suitable samples.41,42 Some studies can be invasive and difficult to perform with living animals.2 Moreover, even when samples are available, the sample size often fails to meet the general requirements of experimental research. The intensive illegal hunting and trade of pangolins have led to a rapid global decline in the populations of these animals.43,44 They are difficult to study because of their nocturnal habits, rarity, secretiveness, and solitary nature. They cannot be captured for research needs because they are critically endangered on the IUCN Red List of Threatened Species. Although the samples examined in this study were collected from a single dead Sunda pangolin, they provided basic data and an opportunity to elucidate more about the digestive physiology of this species. However, the results of this study only comes from one pangolin, we need to obtain more samples for in-depth studies to validate the results. Then, we will be able to better understand the digestive physiology of this species.
Conclusions
The proteases expressed are simplified in the tested Sunda pangolin intestinal tract. This might indicate that species’ adaptation to its diet is stable with respect to protein diversity and complexity because the pangolin feeds exclusively on ants and termites. Chitinase has been detected in its saliva and intestinal juice and allows the efficient digestion and utilization of ants and termites. This may reflect its adaptation to a chitin-rich diet. KEGG annotation of the proteins in the pangolin’s intestinal juice also implied that chitin provides carbon, nitrogen, and energy, and is therefore a substantial nutrient for the Sunda pangolin. Although only one Sunda pangolin was available for our study, it provided us with a valuable opportunity to understand the digestive physiology and eating habits of this critically endangered species.
Materials and Methods
Subject
Samples were taken from a female Sunda pangolin (HS-04) at the Pangolin Research Base for Artificial Rescue and Conservation Breeding of South China Normal University (PRB-SCNU). The pangolin weighed 1.8 kg when it arrived at PRB-SCNU and died 1 day later because of unknown causes.
Collection of Saliva and Intestinal Juice
Immediately after its death, pangolin HS-04 was dissected. No obvious lesion was found in its oral cavity or intestines. The back of a clean scalpel was used to gently swab the saliva on the tongue surface and the inside of the pangolin’s cheek. Intestinal contents were collected 3–5 cm past the opening of the duodenal bile duct. All collected fluids were placed in a cryopreservation tube and stored immediately in liquid nitrogen, and then shipped to the laboratory on dry ice. Although gastric juice of this pangolin was also collected during sampling, the sample was exposed to room temperature for a long time because of the damage of the packaging box of gastric juice during transportation, which seriously affected the quality of the sample, so it was not tested and analyzed.
The pangolin was housed and post-mortem sampling was conducted in accordance with procedures approved by the Ethics Committee for Animal Research at SCNU and the Guidelines for Animal Care established by the National Institute of Health.
Sample Preparation and Protein Separation by SDS-PAGE
The samples were prepared following the methods of Wiśniewski et al45 After the addition of SDT buffer, the samples were boiled for 15 min and centrifuged at 14 000g for 40 min; the protein in the supernatant was then quantified using a BCA protein assay kit (Bio-Rad, Hercules, CA, USA). The samples were then stored at −80 °C.
For sodium dodecyl sulfate-polyacrylamide gel electrophoresis, 20 μg of protein per sample was mixed with 5× loading buffer and boiled for 5 min. The proteins were separated on a 12.5% polyacrylamide gel (constant 14 mA current, 90 min), and the resulting bands were visualized by Coomassie Blue R-250 staining.
Filter-Aided Sample Preparation
The proteins in the samples were digested using a filter-aided sample preparation method.45 For each sample, 200 μg of protein was mixed with 30 μL of SDT buffer (4% SDS, 100 mM dithiothreitol (DTT), and 150 mM Tris-HCl; pH 8.0). The detergent, DTT, and other low-molecular-weight components were removed using UA buffer (8 M urea and 150 mM Tris-HCl; pH 8.0) by repeated ultrafiltration (Microcon units, 10 kDa). Reduced cysteine residues were blocked by the addition of 100 μL of iodoacetamide (100 mM indole-3-acetic acid in UA buffer). The samples were then incubated for 30 min in the dark, washed three times with 100 μL of UA buffer and twice with 100 μL of 25 mM NH4HCO3 buffer, and then incubated overnight at 37 °C with 4 μg of trypsin (Promega, Madison, WI, USA) in 40 μL of 25 mM NH4HCO3 buffer. The resulting peptides were collected as a filtrate, desalted on C18 cartridges [Empore SPE C18 cartridges (standard density), bed I.D. 7 mm, volume 3 mL; Sigma, St. Louis, MO, USA], concentrated by vacuum centrifugation, and reconstituted in 40 μL of 0.1% (v/v) formic acid. The peptide content was estimated based on the absorption at 280 nm using an extinction coefficient of 1.1 for a 0.1% (g/L) solution, calculated based on the frequencies of tryptophan and tyrosine residues in vertebrate proteins.
Shotgun LC–MS/MS and Data Analysis
Nano LC–MS/MS was performed on the products obtained from the enzymatic hydrolysis of 3 g of samples according to the quantitative results, and each sample was injected once. The peptide mixture was loaded onto a reverse-phase trap column (Thermo Scientific Acclaim PepMap100, 100 μm × 2 cm, nanoViper C18; Thermo Fisher Scientific, Waltham, MA, USA) connected to a C18 reversed-phase analytical column (Thermo Scientific EASY-Column, 10 cm long, 75 μm inner diameter, 3 μm resin; Thermo Fisher Scientific) in buffer A (0.1% formic acid) and separated with a linear gradient of buffer B (84% acetonitrile and 0.1% formic acid) at a flow rate of 300 nL/min, controlled by IntelliFlow technology. The linear gradient was as follows: 0–35% buffer B for 50 min, 35–1100% buffer B for 5 min, and holding in 100% buffer B for 5 min.
LC–MS/MS was performed on a Q Exactive mass spectrometer (Thermo Fisher Scientific) coupled to an EASY-nLC device [Proxeon Biosystems (Thermo Fisher Scientific)] for 60 min. The mass spectrometer was operated in the positive ion mode. MS data were acquired using a data-dependent top 10 method, with dynamic selection of the most abundant precursor ions from the survey scan (300–1800 m/z) for HCD fragmentation. The automatic gain control target was set to 3 × 106, and the maximum injection time to 50 ms. The dynamic exclusion duration was 60.0 s. Survey scans were acquired at a resolution of 70 000 at m/z 200; the resolution for the HCD spectra was set to 17 500 at m/z 200 and the isolation width to 2 m/z. The normalized collision energy was 27 eV, and the underfill ratio, which specifies the minimum percentage of the target value likely to be reached at maximum fill time, was defined as 0.1%. The instrument was run with the peptide recognition mode enabled.
The MS data were analyzed using MaxQuant ver. 1.5.3.17 (Max Planck Institute of Biochemistry, Martinsried, Germany)46 and searched against uniprot_Laurasiatheria_553856_20180403.fasta (553 856 total entries, downloaded 03/04/2018, http://www.uniprot.org). An initial search was set at a precursor mass window of 6 ppm. The search followed an enzymatic cleavage rule of trypsin/P and allowed a maximum of two missed cleavage sites and a mass tolerance of 20 ppm for fragment ions. It was designed as follows: enzyme = trypsin, missed cleavage = 2, fixed modification: carbamidomethyl (C), variable modification: oxidation (M), and decoy database pattern = reverse. The cutoff of the global false discovery rate for peptide and protein identification was set to 0.01.
No unexpected or unusually high safety hazards were encountered.
Acknowledgments
We thank Dr. Guizhen Lv (School of Life Science, South China Normal University), Dr. Chanjuan Ning, Zexin Chen, and Jie He (Shanghai Applied Protein Technology Co. Ltd) for their help in data analysis. We acknowledge and thank the Wildlife Rescue Center of Guangdong Province and the Public Forestry Security of Maoming City (Guangdong Province) for providing the animal used in this study. This research was supported by the National Natural Science Foundation of China (31572286, 31702029), Science and Technology Program of Guangzhou, China (201804010475), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2012).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02845.
Pathways and proteins enriched in the saliva of Sunda pangolin HS-04 and pathways and proteins enriched in the intestinal juice of Sunda pangolin HS-04 (PDF)
Author Contributions
† S.W. and S.L. have contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Feldhamer G. A.Mammalogy: Adaptation, Diversity, Ecology; John Hopkins University Press: Baltimore, Maryland, 2007. [Google Scholar]
- Janiak M. C. Digestive enzymes of human and nonhuman primates. Evol. Anthropol. 2016, 25, 253–266. 10.1002/evan.21498. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Wu Q.; Dai J.; Zhang S.; Wei F. Evidence of cellulose metabolism by the giant panda gut microbiome. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 17714–17719. 10.1073/pnas.1017956108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson E.; Ratnakumar A.; Arendt M.-L.; Maqbool K.; Webster M. T.; Perloski M.; Liberg O.; Arnemo J. M.; Hedhammar Å.; Lindblad-Toh K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 2013, 495, 360–364. 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- Perry G. H.; Dominy N. J.; Claw K. G.; Lee A. S.; Fiegler H.; Redon R.; Werner J.; Villanea F. A.; Mountain J. L.; Misra R.; Carter N. P.; Lee C.; Stone A. C. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 2007, 39, 1256–1260. 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsuc F.; Metcalf J. L.; Wegener Parfrey L.; Song S. J.; González A.; Knight R. Convergence of gut microbiomes in myrmecophagous mammals. Mol. Ecol. 2014, 23, 1301–1317. 10.1111/mec.12501. [DOI] [PubMed] [Google Scholar]
- Heath M. E.; Vanderlip S. L. Biology, husbandry, and veterinary care of captive Chinese pangolins (Manis pentadactyla). Zoo Biol. 1988, 7, 293–312. 10.1002/zoo.1430070402. [DOI] [Google Scholar]
- Lee R. H.; Cheung K.; Fellowes J. R.; Guénard B. Insights into the Chinese pangolin’s (Manis pentadactyla) diet in a Peri-Urban habitat: a case study from Hong Kong. Trop. Conserv. Sci. 2017, 10, 194008291770964. 10.1177/1940082917709648. [DOI] [Google Scholar]
- Lim N. T. L.Autecology of the Sunda pangolin (Manis javanica) in Singapore. M.Sc. Thesis, National University of Singapore: Singapore, 2007. Available from: https://scholarbank.nus.edu.sg/handle/10635/28320. [Google Scholar]
- Redford K. H.Ants and termites as food: patterns of mammalian myrmecophagy. In Current Mammalogy; Genoways H. H., Ed.; Plenum Press: New York, 1987; Vol 1, pp 349–399. [Google Scholar]
- Reiss K. Z. Using phylogenies to study convergence: the case of the ant-eating mammals. Am. Zool. 2001, 41, 507–525. 10.1668/0003-1569(2001)041[0507:uptsct]2.0.co;2. [DOI] [Google Scholar]
- Wu S.; Liu N.; Li Y.; Sun R. Observation on food habits and foraging behavior of Chinese pangolin (Manis pentadactyla). Chin. J. Appl. Environ. Biol. 2005, 11, 337–341. [Google Scholar]
- Nisa C.; Sari R. M.; Agungpriyono S.; Nurhidayat; Novelina S.; Supratikno. Tongue of the Malayan pangolin (Manis javanica). Proceeding of AZWMC, Bogor, Indonesia, Aug 19–22, 2008.
- Wilson A. E. Husbandry of pangolins Manis spp. Int. Zoo Yearb. 1994, 33, 248–251. 10.1111/j.1748-1090.1994.tb03578.x. [DOI] [Google Scholar]
- Paoletti M. G.; Norberto L.; Damini R.; Musumeci S. Human gastric juice contains chitinase that can degrade chitin. Ann. Nutr. Metab. 2007, 51, 244–251. 10.1159/000104144. [DOI] [PubMed] [Google Scholar]
- Redford K. H.; Dorea J. G. The nutritional value of invertebrates with emphasis on ants and termites as food for mammals. J. Zool. 1984, 203, 385–395. 10.1111/j.1469-7998.1984.tb02339.x. [DOI] [Google Scholar]
- Nisa C.; Agungpriyono S.; Kitamura N.; Sasaki M.; Yamada J.; Sigit K. Morphological features of the stomach of Malayan pangolin, Manis javanica. Anat., Histol., Embryol. 2010, 39, 432–439. 10.1111/j.1439-0264.2010.01015.x. [DOI] [PubMed] [Google Scholar]
- Imai M.; Shibata T.; Mineda T.; Suga Y.; Onouchi T. Histological and histochemical investigation on the stomach in man, Janpanese mokey (Macaca fuscata yakui) and some other kinds of animals. Report V. On the stomach of the pangolin (Manis pentadactyla Linne). Okajimas Folia Anat. Jpn. 1973, 49, 433–453. 10.2535/ofaj1936.49.6_433. [DOI] [PubMed] [Google Scholar]
- Krause W. J.; Leeson C. R. The stomach gland patch of the koala (Phascolarctos clnereus). Anat. Rec. 1974, 176, 475–487. 10.1002/ar.1091760410. [DOI] [Google Scholar]
- Pernkopf V. E.; Lehner J.. Vergleichende beschreibung des vorderdarmes bei den einzelnen klassen der kranioten. In Handbuch der Vergleichenden Anatomie der Wirbeltiere; Bolk L., Goppert E., Lubosch W., Ed.; Urban & Schwarzenberg: Berlin und Wien: Band III, 1937; pp 349–476. [Google Scholar]
- Horner B. E.; Taylor J. M.; Padykula H. A. Food habits and gastric morphology of the grasshopper mouse. J. Mammal. 1965, 45, 513–535. 10.2307/1377324. [DOI] [Google Scholar]
- Stevens C. E.; Hume I. D.. Comparative Physiology of the Vertebrate Digestive System, 2nd ed.; Cambridge University Press: Cambridge, 1995. [Google Scholar]
- Walker W. F.Functional Anatomy of the Vertebrates, an Evolutionary Perspective; Saunders College Publishing: Philadelphia, 1987. [Google Scholar]
- Lin M. F.; Chang C.-Y.; Yang C. W.; Dierenfeld E. S. Aspects of digestive anatomy, feed intake and digestion in the Chinese pangolin (Manis pentadactyla) at Taipei Zoo. Zoo Biol. 2015, 34, 262–270. 10.1002/zoo.21212. [DOI] [PubMed] [Google Scholar]
- Ma J.-E.; Jiang H.-Y.; Li L.-M.; Zhang X.-J.; Li G.-Y.; Li H.-M.; Jin X.-J.; Chen J.-P. The fecal metagenomics of Malayan pangolins identifies an extensive adaptation to myrmecophagy. Front. Microbiol. 2018a, 9, 2793. 10.3389/fmicb.2018.02793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge B. D.; Kuczynski J.; Knights D.; Clemente J. C.; Gonzalez A.; Fontana L.; Henrissat B.; Knight R.; Gordon J. I. Diets drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011, 332, 970–974. 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.-E.; Li L.-M.; Jiang H.-Y.; Zhang X.-J.; Li J.; Li G.-Y.; Chen J.-P. Acidic mammalian chitinase gene is highly expressed in the special oxyntic glands of Manis javanica. FEBS Open Bio 2018b, 8, 1247–1255. 10.1002/2211-5463.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Canio M.; Soggiu A.; Piras C.; Bonizzi L.; Galli A.; Urbani A.; Roncada P. Differential protein profile in sexed bovine semen: shotgun proteomics investigation. Mol. BioSyst. 2014, 10, 1264–1271. 10.1039/c3mb70306a. [DOI] [PubMed] [Google Scholar]
- Théron L.; Gueugneau M.; Coudy C.; Viala D.; Bijlsma A.; Butler-Browne G.; Maier A.; Béchet D.; Chambon C. Label-free quantitative protein profiling of vastus lateralis muscle during human aging. Mol. Cell. Proteomics 2014, 13, 283–294. 10.1074/mcp.m113.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-J.; Liu C.-W.; Huang X.-H.; Zhou X.; Zhuo J.-C.; Zhang C.-X.; Bao Y.-Y. Screening and functional analyses of Nilaparvata lugens salivay proteome. J. Proteome Res. 2016, 15, 1883–1896. 10.1021/acs.jproteome.6b00086. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C.; Lowe M. E. Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007, 52, 1–17. 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- Whitaker J. O. Jr; Dannelly H. K.; Prentice D. A. Chitinase in insectivorous bats. J. Mammal. 2004, 85, 15–18. . [DOI] [Google Scholar]
- Boot R. G.; Bussink A. P.; Verhoek M.; de Boer P. A. J.; Moorman A. F. M.; Aerts J. M. F. G. Marked differences in tissue-specific expression of chitinase in mouse and man. J. Histochem. Cytochem. 2005, 53, 1283–1292. 10.1369/jhc.4a6547.2005. [DOI] [PubMed] [Google Scholar]
- Goto M.; Fujimoto W.; Nio J.; Iwanaga T.; Kawasaki T. Immunohistochemical demonstration of acidic mammalian chitinase in the mouse salivary gland and gastric mucosa. Arch. Oral Biol. 2003, 48, 701–707. 10.1016/s0003-9969(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Strobel S.; Roswag A.; Becker N. I.; Trenczek T. E.; Encarnação J. A. Insectivorous bats digest chitin in the stomach using acidic mammalian chitinase. PLoS One 2013, 8, e72770 10.1371/journal.pone.0072770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchner L.; Nofs S. A.; Dierenfeld E. S.; Horvath P. Chitin supplementation in the diets of captive giant anteaters (Myrmecophaga tridactyla) for improved gastrointestinal function. J. Zoo Aquarium Res. 2017, 5, 92–96. 10.19227/jzar.v5i2.170. [DOI] [Google Scholar]
- Kageyama T. New World monkey pepsinogens A and C, and prochymosins. Purification, characterization of enzymatic properties, cDNA cloning, and molecular evolution. J. Biochem. 2000, 127, 761–770. 10.1093/oxfordjournals.jbchem.a022668. [DOI] [PubMed] [Google Scholar]
- Narita Y.; Oda S.-i.; Takenaka O.; Kageyama T. Multiplicities and some enzymatic characteristics of ape pepsinogens and pepsins. J. Med. Primatol. 2000, 29, 402–410. 10.1111/j.1600-0684.2000.290604.x. [DOI] [PubMed] [Google Scholar]
- Reseland J. E.; Larsen F.; Solheim J.; Eriksen J. A.; Hanssen L. E.; Prydz H. A novel human chymotrypsin-like digestive enzyme. J. Biol. Chem. 1997, 272, 8099–8104. 10.1074/jbc.272.12.8099. [DOI] [PubMed] [Google Scholar]
- Schmitz J.Maldigestion and malabsorption. In Pediatric Gastrointestinal Disease: Pathophysiology, Diagnosis, Management; Walker W. A., Goulet O., Kleinman R. E., Sherman P. M., Shneider B. L., Sanderson I. R., Eds.; Decker: Hamilton, Canada, 2004; pp 8–20. [Google Scholar]
- Ingram C. J. E.; Mulcare C. A.; Itan Y.; Thomas M. G.; Swallow D. M. Lactose digestion and the evolutionary genetics of lactase persistence. Hum. Genet. 2009, 124, 579–591. 10.1007/s00439-008-0593-6. [DOI] [PubMed] [Google Scholar]
- Yin H.; Yu G.; Wang G.; Zhou Y.; Wu M. Studying methods of carnivore food habit. J. Anhui Univ. Nat. Sci. Ed. 2008, 32, 90–94. [Google Scholar]
- Challender D.; Nguyen Van T.; Shepherd C.; Krishnasamy K.; Wang A.; Lee B.; Panjang E.; Fletcher L.; Heng S.; Seah Han Ming J.; Olsson A.; Nguyen The Truong A.; Nguyen Van Q.; Chung Y.. Manis javanica. The IUCN Red List of Threatened Species 2014a: e. T12763A45222303. http://dx.doi.org/10.2305/IUCN.UK.2014-2.RLTS.T12763A45222303.en. Download on 16 March 2019.
- Challender D.; Baillie J.; Ades G.; Kaspal P.; Chan B.; Khatiwada A.; Xu L.; Chin S.; KC R.; Nash H.; Hsieh H.. Manis pentadactyla. The IUCN Red List of Threatened Species 2014b: e. T12764A45222544. http://dx.doi.org/10.2305/IUCN.UK.2014-2.RLTS.T12764A45222544.en. Downloaded on 16 March 2019.
- Wiśniewski J. R.; Zougman A.; Nagaraj N.; Mann M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- Cox J.; Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.