Abstract
Background
The pathogenesis of chemotherapy-induced neuropathy, a dose-dependent adverse effect of cisplatin, involves mitochondrial dysfunction. PTEN-induced putative kinase 1 (PINK1)/Parkin-mediated mitophagy removes damaged mitochondria under various pathological conditions. The objective of this study was to determine mitophagy status and its effects on mitochondrial function and neuronal cell damage after cisplatin treatment using an in vitro model of cisplatin-induced neurotoxicity.
Material/Methods
PC12 cells were transfected with Parkin or Parkin siRNA using lentiviral particles and Lipofectamine 3000™, respectively, and then were exposed to 10 μM cisplatin. The expression of autophagic proteins was measured by Western blot analysis. Mitophagy in PC12 cells was detected by confocal microscopy analysis of mitochondria-lysosomes colocalization and autophagic flux. The effects of PINK1/Parkin-mediated mitophagy on cisplatin-induced neurotoxicity were assessed via mitochondrial function, neuritic length, nuclear diameter, and apoptosis.
Results
Cisplatin activated PINK1/Parkin-mediated mitophagy in PC12 cells. Autophagic flux analysis revealed that cisplatin inhibits the late stage of the autophagic process. The knockdown of Parkin suppressed cisplatin-induced mitophagy, aggravating cisplatin-induced depolarization of mitochondria, cellular ATP deficits, reactive oxygen species outburst, neuritic shortening, nuclear diameter reduction, and apoptosis, while Parkin overexpression enhanced mitophagy and reversed these effects.
Conclusions
PINK1/Parkin-regulated mitophagy can protect against cisplatin-related neurotoxicity, suggesting therapeutic enhancement of mitophagy as a potential intervention for cisplatin-induced peripheral neuropathies. The interference of cisplatin with autophagosome-lysosome fusion may be partly responsible for cisplatin-induced neurotoxicity.
MeSH Keywords: Antineoplastic Agents, Apoptosis, Cisplatin, Mitochondria, Mitochondrial Degradation, Peripheral Nervous System Diseases
Background
Cisplatin is a platinum-based chemotherapeutic agent for solid cancers (e.g., lung, bladder, and ovarian cancers). Oncological treatment with cisplatin can lead to chemotherapy-induced peripheral neuropathy (CIPN), a dose-limiting adverse effect. As a result, 20% of patients must discontinue anticancer therapy [1,2]. Furthermore, patients often have incomplete recovery from CIPN, and neurotoxic symptoms may persist for several months or even years after treatment cessation, and progressively worsen over time [3,4]. Consequently, patients may be cancer-free but suffer from functional disruptions and chronic pain evoked by cisplatin treatment.
The mechanism of cisplatin-induced CIPN remains elusive, although it likely involves direct effects on sensory neuron viability as well as mitochondria-specific consequences. Mitochondria provide energy to neurons and preserve several inter-related pathways for cellular processes, including the apoptotic signaling pathways [5] and reactive oxygen species (ROS) generation [6]. Mitochondria are the primary and early targets in cisplatin-induced neurotoxicity [7]. Morphological changes [8] and dysfunction of mitochondria, including insufficient ATP production, increased ROS production [9], and potential membrane collapse [7], have been observed after cisplatin treatment both in vivo and in vitro. Since damaged or dysfunctional mitochondria contribute to CIPN, elimination of these impaired organelles may protect against CIPN.
Mitophagy is selective removal of defective mitochondria through autophagy and plays a crucial role in the preservation of a healthy pool of mitochondria for cellular homeostasis. Autophagy and mitophagy use similar molecular pathways to form autophagosomes and autolysosomes [10,11]. The PINK1/Parkin pathway, the most well-documented mitophagy-specific signaling cascade, can initiate recruitment of autophagy machinery and facilitate engulfment of damaged mitochondria into autophagosomes [12,13]. Mitophagy impairment is linked to aging and pathological conditions, such as neurodegenerative diseases, inflammation, and cancer [14]. Recent studies show that PINK1/Parkin-mediated mitophagy may protect against cisplatin-induced kidney injury [15,16]. Whether mitophagy can be activated to prevent CIPN is unknown. It is clear that the effect of mitophagy on disease progress is complex and depends on both the cell and disease. The purpose of this study was to determine mitophagy status, and its effects on mitochondrial function and cell apoptosis, after cisplatin treatment using an in vitro model of cisplatin-induced neurotoxicity [17]. These data provide an increased understanding of PINK1/Parkin-mediated mitophagy related to the neurotoxicity of cisplatin. We hope this study can provide a potential new avenue for treatment of CIPN.
Material and Methods
Cell culture
Rat pheochromocytoma PC12 cells (Shanghai Institute of Biochemistry and Cell Biology, CAS) were maintained following standard protocol [18] in RPMI 1640 liquid medium containing 10% heat-inactivated horse serum, 5% heat-inactivated fetal bovine serum, and a 1% penicillin-streptomycin mixture and kept in a humidified incubator with 95% air and 5% CO2 at 37°C (Thermo Electron Co.). PC12 cells were subcultured at a confluence of 70–80% and used at the 3rd to 10th passage, with the medium refreshed every 3–4 days.
Transfections
PC12 cells were transfected with Parkin overexpression plasmids or an empty expression vector (Hanbio Biotechnology, China) using lentiviral particles [19]. We transfected Parkin siRNA (Target sequence: 5′ AGTCGGAACATCACTTGCA 3′) and scrambled control siRNA (Ribobio, China) into cells using Lipofectamine 3000™ (Life Technologies, USA) [19] according to the manufacturer’s instructions, and confirmed transfection by qPCR within 48 h following cell harvest. Cells transfected with the empty vector and control siRNA were used as negative controls, along with untransformed cells. PC12 cells were cultured for at least 24 h after transfections and then exposed to 10 μM cisplatin (KeyGEN, KGR0036, China) for 24 h; these prepared cells were then used in subsequent experiments.
Western blotting
Western blotting was performed as previously described [20]. Prepared cells were rinsed twice with cold PBS and lysed in lysis buffer. Equivalent protein was resolved on 10–12% polyacrylamide gels by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) blotting membranes by an electrophoretic transfer system with cold-block (Bio-Rad Laboratories, CA). Membranes were blocked using nonfat milk in TBST at room temperature for 1.5 h and sequentially incubated overnight at 4°C with the appropriate primary antibodies according to experimental requirements. The primary antibodies were diluted as follows: Parkin (Abcam, ab77924, 1: 200); PINK1 (Abcam, ab23707, 1: 1000); Beclin-1 (Boster Biological, PB0014, 1: 500); LC3 (Abcam, ab192890, 1: 800); and β-actin (Abcam, ab207612, 1: 2000). Diluted antibodies were then incubated with secondary antibodies of goat anti-rabbit IgG-HRP (Abcam, ab6721, 1: 3500) or goat anti-mouse IgG-HRP (Abbkine, A21010-1, 1: 2000) at room temperature for 1 h. Immuno-reactive bands were visualized using chemiluminescence and analyzed by Gel-Pro 32 (Li-Cor Bioscience, USA).
Mitophagy detection
Prepared PC12 cells were seeded at a density of 1×105 in 35-mm cell culture dishes coated with poly-L-lysine (Corning, USA). After 24 h incubation for adhesion, cells were treated with 10 μM cisplatin diluted in PC12 cell medium or vehicle control for 24 h. Lyso-Tracker Green and Mito-Tracker Red staining were used for determining colocalization of mitochondria and lysosomes, as previously described [21]. Cells were harvested and washed twice, co-incubated in the dark at 37°C for 30 min with 50 nM Lyso-Tracker Green (LTG; KeyGEN Biotech, China) and Mito-Tracker Red (MTR; KeyGEN Biotech, China), and then washed again before mounting in fluorescence mounting medium for rapid detection using a confocal microscope (Carl Zeiss, Germany). Five fields of cells (each containing at least 2 transfected cells) were randomly selected and Pearson’s correlation coefficients for numbers of mitochondria (Red fluorescence) and lysosomes (Green fluorescence) were generated using Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Autophagic flux monitoring
As mitophagy is a dynamic process, we evaluated the autophagic flux of cisplatin-induced mitophagy using an mRFP-GFP-LC3 tandem construct [22]. Early in the autophagic process, yellow fluorescent dots (GFP+RFP+) in the cytoplasm indicate recruitment of LC3 proteins to autophagosomes. At the late phase (fusion of autophagosomes with lysosomes), the low pH of the lysosome can reduce the GFP fluorescent signal, while the mRFP (GFP−RFP+) signal remains strong [23]. PC12 cells were transfected by mRFP-GFP-LC3B adenovirus with polybrene at a multiplicity of infection (MOI)=30 and incubated for 36 h. Cells treated with 10 μM cisplatin for 24 h were washed twice with 10 mM of PBS, then fixed with 4% paraformaldehyde for 30 min at room temperature and sealed with 50% glycerin/PBS. Imaging of LC3B expression was done with a confocal microscope (Carl Zeiss, Germany). The number of yellow (GFP+RFP+) and red (GFP−RFP+) mRFP-GFP-LC3 puncta were quantified using Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) to assess autophagic flux.
Mitochondrial membrane potential change assay
Mitochondrial membrane potential was tested using the JC-1 mitochondrial membrane potential detection kit (KeyGEN, KGA602, China). Aggregated JC-1 emits red fluorescence, whereas the monomorphic form emits green fluorescence. In impaired mitochondria, with a depolarized mitochondria membrane potential, most JC-1 is in monomeric form, giving a low red-to-green fluorescence ratio [24]. Pretreated PC12 cells were washed and incubated with JC-1 (2 μg/ml) for 15 min at 37°C following the manufacturer’s instructions. Immediately after washing cells twice with incubation buffer, cell fluorescence emission was measured using a flow cytometer (Merck, Amnis Flowsight, USA).
ATP assay
Intracellular ATP levels were measured in prepared cells using a luciferase-based assay [25] with a commercial ATP detection kit (Beyotime, China) following the manufacturer’s protocol. ATP values were calculated based on a standard curve of known ATP concentrations prepared daily. ATP content is given as nmol/105 cells.
Detection of mitochondrial ROS
Mitochondrial ROS level was measured by the fluorometric assay, as previously described [26]. Prepared PC12 cells seeded in 96-well microplates (106 cells/well) were incubated with MitoSOX red reagent (Invitrogen, M36008, USA) at a concentration of 4 μM in the dark at 37°C for 10 min. Cells were then washed twice with warm PBS. The ROS level was identified using a SpectraMax M5e Multi-Mode microplate reader (Molecular Devices, USA).
Annexin V-APC/7-AAD apoptosis assay
Apoptosis of PC12 cells was determined with an apoptosis detection kit (KeyGEN, KGA104, China) [27]. Prepared cells (6×106) were seeded in 6-cm dishes coated with poly-L-lysine (Corning, USA) and treated with 10 μM cisplatin for 24 h. After 24 h incubation, cells were harvested for apoptosis detection. Cells were washed twice with PBS and then re-suspended in binding buffer. Following standard protocol, cells were subsequently stained with 5 μl annexin V-APC and 5 μl 7-AAD at room temperature in the dark for 20 min. The apoptosis ratio was evaluated via flow cytometry within 1 h (Merck, Amnis Flowsight, USA). Early apoptotic (Annexin V+/7-AAD−) and late apoptotic cells (Annexin V+/7-AAD+) were considered as apoptotic cells.
Determination of the neuritic length and nuclear diameter in PC12 cells
The detection of average neuritic length and nuclear diameter was carried out as previously described elsewhere [28]. PC12 cells after cisplatin treatment were seeded in 12-well plates coated with poly-L-lysine (Corning, USA). After cisplatin treatment, cells were fixed in 4% paraformaldehyde and assessed by phase-contrast microscope (Olympus Model IX51, Japan, 200×magnification). For nuclear diameter evaluation, cells were stained with Hoechst 33342 dye. The neuritic length and nuclear diameter were identified using Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Three non-overlapped micrographs were randomly captured from each group, and the average neuritic length (μm) and nuclear diameter (μm) were calculated from 30 neurites and 30 nuclei, respectively.
Statistical analyses
Data are given as the mean±standard deviation (SD). A t test or one-way ANOVA followed by Tukey’s multiple comparison tests were performed to compare the differences between groups. All analyses were carried out using SPSS v.19 (SPSS, Inc., Chicago, IL, USA). P values of less than 0.05 were considered statistically significant.
Results
Cisplatin induced PINK1/Parkin-mediated mitophagy
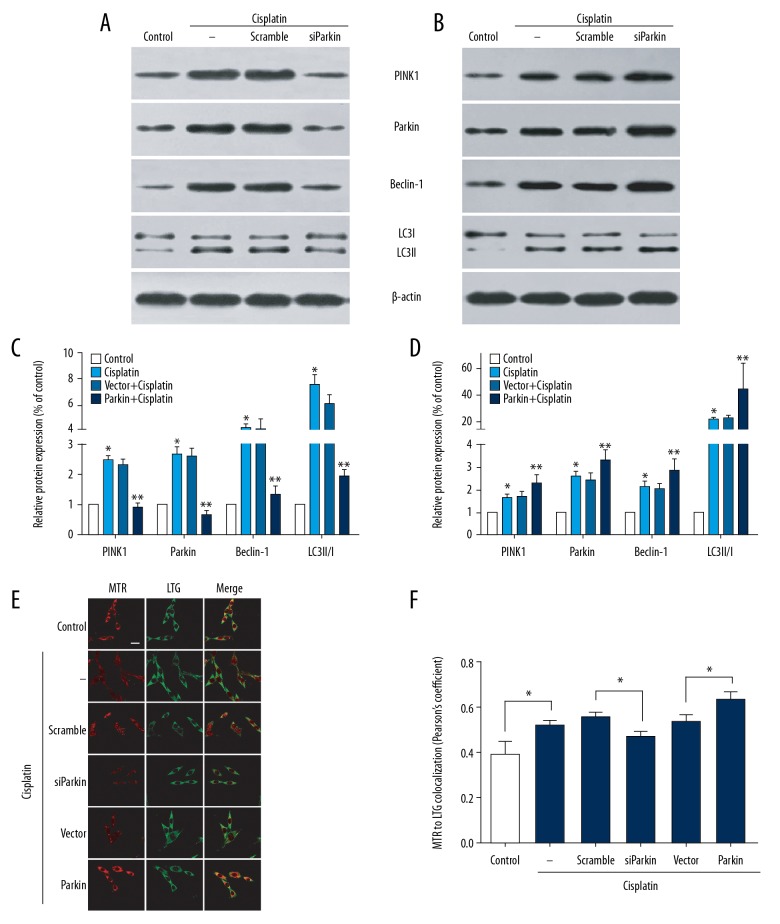
Mitophagy shares many characteristic pathways with autophagy. Beclin-1 and microtubule-associated protein 1 light chain 3 (LC3) as critical autophagy proteins have been reported to be involved in the initiation of mitophagy [29] and to promote the expansion of isolation membranes around the mitochondrion [12]. Thus, we used their relative levels to assess whether cisplatin activates mitophagy in PC12 cells. Western blot analysis indicated that the LC3-II to LC3-I ratio and Beclin-1 expression were increased in cisplatin-treated PC12 cells compared to the control group (Figure 1A–1D). Next, we used MTR and LTG to co-stain PC12 cells and evaluated the colocalization of mitochondria and lysosomes by confocal microscopy. The correlation coefficient of red dots (mitochondria) to green dots (lysosomes) in cisplatin-treated cells was 0.52±0.02 compared with 0.39±0.06 in control cells (Figure 1E, 1F). These observations indicate that cisplatin induces mitophagy.
Figure 1.
Cisplatin induced PINK1/Parkin-mediated mitophagy. Naive PC12 cells and transfected-PC12 cells were treated with vehicle (as control) or cisplatin (10 μM) for 24 h. (A, C) The expressions of PINK1, Parkin, Beclin-1, LC3-I, and LC3-II were detected by immunoblotting. Representative blots are presented. (B, D) Densitometric analysis of blots. Data are expressed as mean±SD from 3 independent experiments. * P<0.05 versus control group. ** P<0.05 versus scramble or vector group. (E) Mitochondria and lysosomes colocalization in cisplatin-treated PC12 cells. Representative images (scale bars, 20 μm) from confocal microscopy analysis of LTG and MTR colocalization. (F) Pearson’s correlation coefficient for colocalization of LTG and MTR. Data presented as mean±SD of at least 5 fields of view from 3 independent experiments. * P<0.05. LTG – Lyso-Tracker Green; MTR – Mito-Tracker Red.
Mitophagy regulated by the PINK1/Parkin pathway is involved in multiple pathological contexts. We next investigated whether cisplatin-induced mitophagy in PC12 is mediated through the PINK1/Parkin pathway. PINK1 and Parkin expression was significantly increased in cisplatin-treated cells (Figure 1A–1D). To further confirm this result, we silenced and overexpressed Parkin in PC12 cells before treatment with cisplatin. As shown in Figure 1C and 1D, Parkin siRNA reduced the levels of PINK1, Parkin, LC3-II/LC3-I, and Beclin-1 in cisplatin-exposed cells. Moreover, the colocalization of LTG (lysosomes) and MTR (mitochondria) decreased in those cells, with a colocalization coefficient of 0.47±0.02 compared to 0.56±0.01 in the scramble group (Figure 2A, 2B). Conversely, overexpression of Parkin led to increased expressions of PINK1, Parkin, LC3-II/LC3-I, and Beclin-1 compared to non-Parkin-transfected cells (Figure 1C, 1D). Overexpressed Parkin in the cisplatin-treated cells also increased the colocalization of LTG (lysosomes) and MTR (mitochondria), with a coefficient of 0.64±0.03 (Figure 1E, F). These observations suggest that knockdown of Parkin suppresses cisplatin-induced mitophagy, whereas overexpression of Parkin promotes this process.
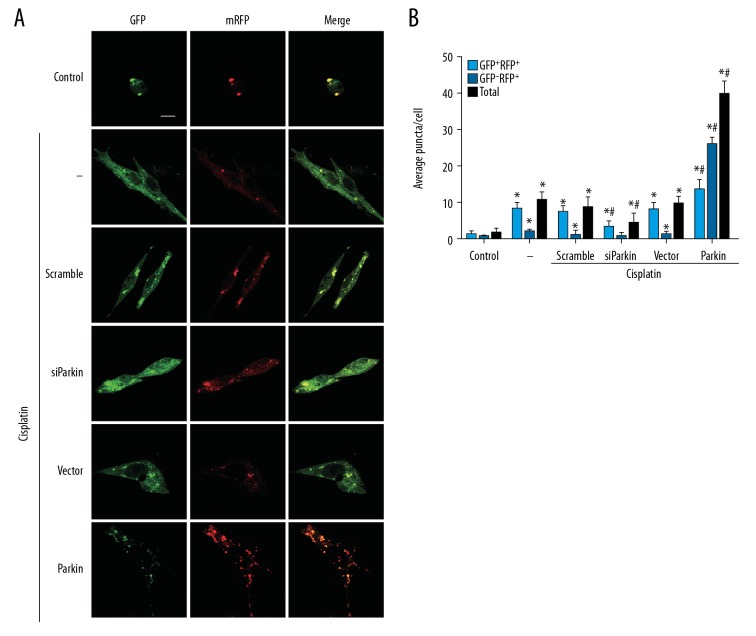
Figure 2.
Autophagic flux in cisplatin-treated PC12 cells. PC12 cells were transfected with mRFP-GFP-LC3 for 36 h and then transfected with Parkin siRNA or Parkin plasmid. After exposure to 10 μM cisplatin for 24 h, yellow and red fluorescence in cells was identified by confocal microscopy. (A) Representative images (scale bars, 20 μm) from each group. (B) Quantification of LC3 puncta observed in A. Light blue columns show amounts of yellow puncta (GFP+RFP+), blue columns show red puncta (GFP−RFP+), black columns show total LC3 puncta. Data are expressed as mean±SD from 3 independent experiments. * P<0.05 versus control group. # P<0.05 versus scramble or vector group.
Cisplatin inhibited autophagy flux in PC12 cells
Stress conditions that activate autophagy sometimes result in defects of the autophagy process [30]. To elucidate whether the neurotoxic role of cisplatin in PC12 cells depends on the interference of the autophagy flux, we assessed the autophagic flux in cisplatin-treated cells. In the autophagic flux assay, cells treated with cisplatin had fewer red puncta than yellow puncta (Figure 2A, 2B), indicating that most LC3 puncta were autophagosomes. In siParkin-transfected cells, yellow (GFP+RFP+) puncta accumulation was lower compared to vehicle-transfected cells (Figure 2A, 2B). Consistent with the Western blot and colocalization results, Parkin overexpression led to an increased number of red (GFP−RFP+) and yellow (GFP+RFP+) puncta, with more red (GFP−RFP+) puncta present in cells (Figure 2A, 2B). Collectively, these results imply that cisplatin initiates mitophagy but suppresses the terminal stage of the autophagic process, and Parkin overexpression partly restores autophagy flux.
The effects of PINK1/Parkin-mediated mitophagy on cisplatin-induced mitochondrial dysfunction
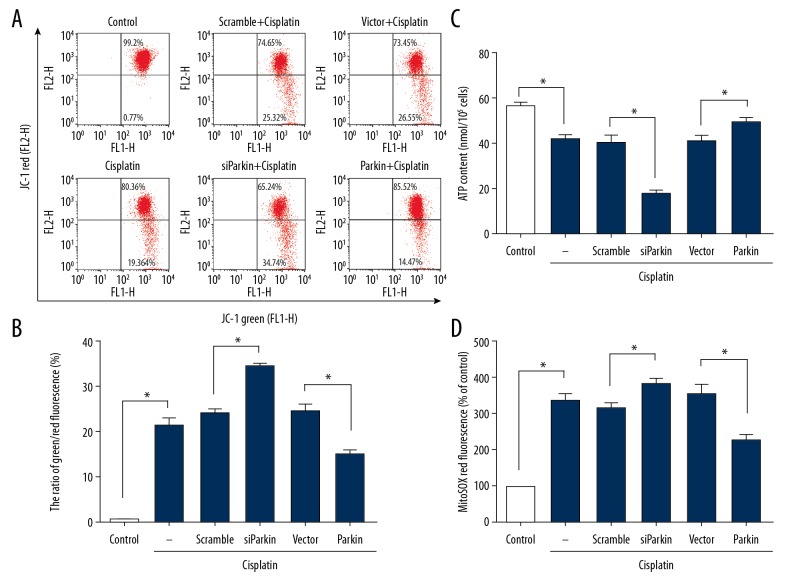
To evaluate the role of mitophagy in cisplatin-induced mitochondrial dysfunction, we measured mitochondrial membrane potential, cellular ATP level, and mitochondrial ROS generation. In the presence of 10 μM cisplatin, cells had decreased mitochondrial membrane potential (Figure 3A, 3B) and deficits of cellular ATP (Figure 3C). Knockdown of Parkin in PC12 cells further aggravated the cisplatin-induced depolarization of mitochondria (Figure 3A, 3B) and decreased cellular ATP level (Figure 3C). In contrast, we found significant increases in mitochondrial membrane potential (Figure 3A, 3B) and cellular ATP concentration in Parkin-transfected cisplatin cells (Figure 3C).
Figure 3.
Effects of cisplatin on mitochondrial potential, ATP contents, and ROS generation. Naive PC12 cells and cells transfected with Parkin siRNA or Parkin plasmid followed by treatment with vehicle (as control) or cisplatin (10 μM) for 24 h. (A) Mitochondrial membrane potential was measured by flow cytometry. Representative data from each group. (B) Mitochondrial membrane potential expressed as the ratio of the green/red fluorescence. (C) ATP contents. (D) Mitochondrial ROS level as MitoSOX fluorescence intensity standardized to the percent of control. Data are expressed as mean±SD from 3 independent experiments. * p<0.05.
Exposure to cisplatin significantly increased ROS formation by 3.3-fold compared with the control (Figure 3D). Following Parkin knockdown, the level of mitochondrial ROS increased by approximately 22% compared with vehicle-transfected cells (Figure 3D), while Parkin-overexpressed cells had a lower ROS generation compared with the vector group (Figure 3D). These results suggest that PINK1/Parkin-mediated mitophagy exerts a protective effect on cisplatin-induced mitochondrial dysfunction in PC12 cells, and Parkin overexpression can further enhance this effect.
The effects of PINK1/Parkin-mediated mitophagy on cisplatin-induced PC12 cell damage
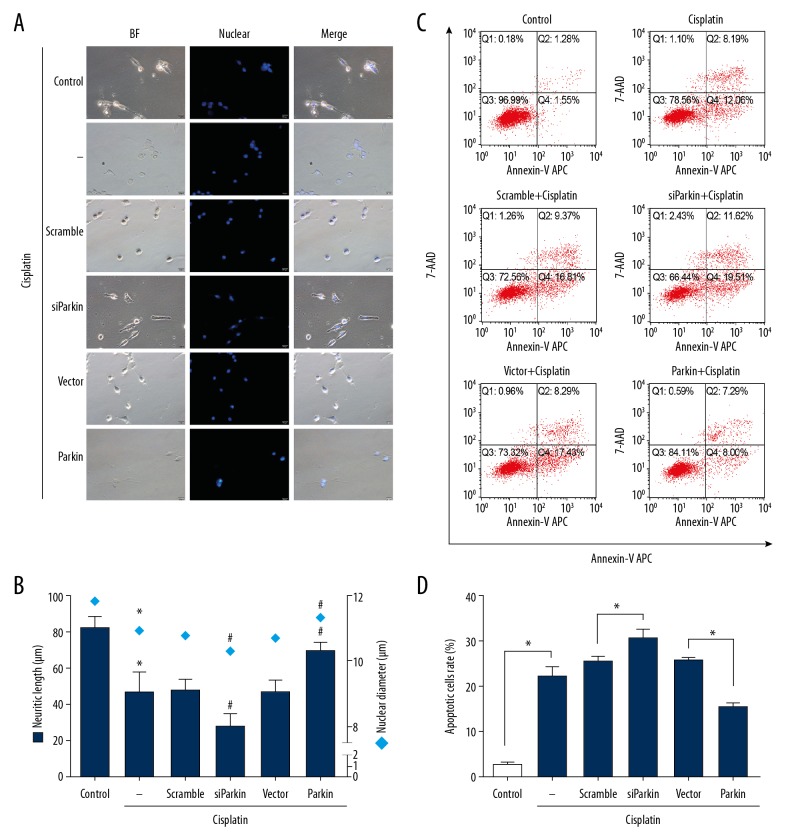
PINK1/Parkin-mediated mitophagy plays a protective role against cisplatin-induced mitochondria damage in PC12 cells; therefore, we investigated whether PINK1/Parkin-mediated mitophagy can alleviate cisplatin neurotoxicity. We measured neuritic length, nuclear diameter, and apoptosis rate in cisplatin-treated PC12 cells. Compared with the control cells, treatment with cisplatin significantly decreased the neuritic length and nuclear diameter (Figure 4A, 4B). Parkin knockdown resulted in further neuritic shortening and nuclear diameter size reduction in cisplatin-exposed cells, while Parkin overexpression markedly increased the average neuritic length and nuclear diameter compared with control vector cells (Figure 4A, 4B).
Figure 4.
The effects of PINK1/Parkin-mediated mitophagy on cisplatin-induced PC12 cell damage. Naive PC12 cells and transfected-PC12 cells were treated with vehicle (as control) or cisplatin (10 μM) for 24 h. (A) Morphological alterations of PC12 cells under bright-field illumination after Hoechst 33342 staining (scale bars, 20 μm). (B) The mean neuritic length (deep blue bar) and nuclear diameter (blue square) were calculated. * P<0.05 versus control group. # P<0.05 versus scramble or vector group. (C) Annexin V-APC/7-AAD was used to measure apoptosis by flow cytometry. Early apoptotic (Annexin V+/7-AAD−) and late apoptotic cells (Annexin V+/7-AAD+) were considered as apoptotic cells. (D) Apoptosis rate for all cells. Data are expressed as mean±SD from 3 independent experiments. * p<0.05.
We also examined whether mitophagy could rescue cell death associated with cisplatin treatment. Cell death was measured by Annexin V-APC/7-AAD apoptosis assay with flow cytometric analysis. As revealed in Figure 4C and 4D, 10-μM cisplatin exposure significantly increased the apoptotic rate by 7.7-fold compared with the control (Cisplatin, 22.3±2.0% versus Control, 2.89±0.2%). The level of apoptosis increased by approximately 17% in Parkin knockdown cells compared with vehicle-transfected cells (siParkin, 30.6±1.85% versus Scramble 25.4±1.0%) (Figure 4C, 4D). On the contrary, Parkin-overexpressing cells exhibited a lower apoptotic rate (Parkin, 15.4±0.7% versus Vector 25.66±0.6%) (Figure 4C, 4D). Similar to what we saw in mitochondrial dysfunction, PINK1/Parkin-mediated mitophagy exerted protection in PC12 cells against cisplatin-induced neurotoxicity and apoptosis.
Discussion
Mitophagy is a selective form of autophagy that clears unwanted or damaged mitochondria. PINK1/Parkin-mediated mitophagy induction and its protective effect are well-established in cisplatin nephrotoxicity [15,16]. However, little is known about the effect of mitophagy in cisplatin neurotoxicity. In this study, we presented findings that provide new insights into the underlying mechanism of CIPN and possible prevention strategies. We showed that cisplatin activates PINK1/Parkin-mediated mitophagy in PC12 cells. Moreover, cisplatin impedes autophagosome maturation into autolysosomes, and Parkin overexpression partially restores autophagic flux. Finally, we found that mitophagy inhibition aggravates cisplatin effects, while mitophagy enhancement improves mitochondrial and neuronal cell health, as well as apoptosis of cisplatin-damaged cells. From these results, we can conclude that PINK1/Parkin-mediated mitophagy prevents neurotoxicity in PC12 cells treated with cisplatin. Notably, an impaired autophagy process might be responsible for cisplatin-induced neurotoxicity.
In mammalian cells, the depolarization of mitochondria activates PINK1/Parkin-mediated mitophagy [31]. The collapsed mitochondrial transmembrane potential promotes PINK1 stabilization on the outer mitochondrial membrane, where PINK1 recruits and activates Parkin by phosphorylating its ubiquitin-like domain. Once Parkin is at the mitochondria, it ubiquitinates several different mitochondrial proteins that then serve as signals, facilitating the recruitment of autophagy receptors for informing autolysosomes [12]. In the current study, we found depolarization of mitochondria occurs in cisplatin-treated PC12 cells. Furthermore, detection of autophagic markers, mitochondria-lysosomes colocalization, and expressions of PINK1 and Parkin increased in cisplatin-treated cells, but decreased in Parkin-knockdown cisplatin cells, suggesting cisplatin activates PINK1/Parkin-mediated mitophagy in PC12 cells.
The PINK1/Parkin-mediated mitophagy pathway has been identified as a paradigm for clearing dysfunctional mitochondria in mammalian cells [32]. Our work shows that PINK1/Parkin-mediated mitophagy can protect against cisplatin-induced neurotoxicity, indicating that the PINK1/Parkin pathway is a common pathway of mitophagy, protecting against cisplatin-induced neurotoxicity and nephrotoxicity. Not all mitophagy processes work through the PINK1/Parkin pathway, however, as PINK1/Parkin-independent mitophagy is also involved in the elimination of dysfunctional mitochondria in neurons. For example, Nip3-like protein X can restore mitophagy and mitochondrial function in neurons and fibroblasts with deficient PINK1/Parkin [33,34]. Whether PINK1/Parkin-independent pathways participate in cisplatin-induced mitophagy in neurons, and their effects on cisplatin neurotoxicity, requires further investigation.
As a form of selective autophagy, mitophagy utilizes machinery extensively similar to that used in autophagy to form autophagosomes and autolysosomes [10,11]. Thus, autophagic flux can accurately represent the mitophagy process. In cisplatin-treated PC12 cells, we observed increased autophagosome synthesis and a higher accumulation of autophagosomes than autolysosomes, indicating cisplatin activates autophagy/mitophagy but blocks its late stage. The formation of autophagosomes is an energy-consuming process. When autophagosome-lysosome fusion is defective, excessive autophagosomes synthesis is ineffective in mitophagy and neurons, resulting in further decreased energy and nutrition. Additionally, non-fused autophagosomes ultimately result in the accumulation of cisplatin-damaged mitochondria. These defective mitochondria cannot produce sufficient energy, which in turn becomes a source of cellular ROS. These excessive ROS cause the activation of apoptotic pathways [5] and are related to direct damage to neuronal components, thereby exerting neurotoxicity. Our results using Parkin-overexpressed cells support this idea, as overexpression of Parkin restored autophagy flux and consequently stabilized mitochondrial networks and decreased apoptosis, which further alleviated neuronal damage. However, at present, we do not know how Parkin is involved in autophagosome-lysosome fusion, as neither of the identified Parkin substrates is known to affect this process [12]. On the other hand, a previous report suggests that mitochondrial impairments elicit disruption of lysosomal activity in neurons, and this results in inhibition of autophagy flux [35]. Parkin directly improves mitochondrial function by keeping mtDNA from oxidative damage and stimulating mtDNA repair [36]. We hypothesize that Parkin restores the autophagy flux by improving mitochondrial function and thus promoting lysosomal activity. Taken together, these results suggest that cisplatin impairment of the autophagy/mitophagy process may be a potential mechanism underlying cisplatin-induced neurotoxicity and the therapeutic benefit of enhancing mitophagy to treat CIPN.
One possible mechanism to account for the effects of cisplatin on autophagic flux stems from the ability of cisplatin to deplete the activity of various enzymes and receptors. Previous studies showed that cisplatin leads to conformational changes of ubiquitin [37], which may suppress the ubiquitin-proteasome pathway. Furthermore, cisplatin can damage neuronal DNA and inhibit DNA replication and transcription through the formation of platinum adducts with both nuclear and mitochondrial DNA [1,8]. Therefore, we hypothesize that triggered by the cisplatin toxicity, proteins from different protein complexes that participate in autophagosome-lysosome fusion are susceptible to cisplatin toxicity, thus blocking autophagic flux. Malfunctional lysosomes may be another candidate contributor, as the lysosomal membrane is severely damaged after cisplatin treatment [38]. The mechanism of cisplatin-inhibited autophagic flux is clearly a multifactorial phenomenon that needs further research.
Our study has certain limitations. First, we explored a single concentration of cisplatin and a single time-point after cisplatin treatment, and whether the observed effects of Parkin on mitophagy and apoptosis are temporary or permanent is not known. In addition, we used a pheochromocytoma-derived cell line that may not adequately represent the mitophagy and neuronal death related to cisplatin effects when used in chemotherapy. Further studies in primary neurons or in vivo are required to explore this.
Mitochondrial dysfunction is involved in the pathogenesis of CIPN. PINK1/Parkin-mediated mitophagy may be an initial compensatory mechanism to eliminate damaged mitochondria. However, incomplete mitophagy is detrimental because neurons depend on mitochondrial respiration [39], and the effects of impaired mitochondria are not diluted through cell division. Instead, further damage will occur to mitochondria and other cellular components, resulting in ever-increasing impairment of neuronal functions and, ultimately, peripheral neuropathy.
Conclusions
Our work reveals that PINK1/Parkin-mediated mitophagy can be induced by cisplatin exposure in PC12 cells and plays a protective role in limiting mitochondrial dysfunction and cell apoptosis. These findings also indicate that the accumulation of dysfunctional mitochondria caused by cisplatin-impeded mitophagy may be responsible for cisplatin-induced neurotoxicity. Moreover, our results demonstrate that therapeutic enhancement of mitophagy is a potential target of peripheral neuropathies associated with cisplatin.
Acknowledgments
The authors would like to thank Linlin Zhao for assistance with the artwork.
Footnotes
Source of support: This work was supported by Nanjing Medical University Science and Technology Development Grant [grant number 2017NJMU081]. The research was also supported in part by the National Natural Science Foundation of China [grant numbers 81500944 and 81271242]
Conflicts of interest
None.
References
- 1.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: A potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–13. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br J Anaesth. 2017;119:737–49. doi: 10.1093/bja/aex229. [DOI] [PubMed] [Google Scholar]
- 3.Siegal T, Haim N. Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer. 1990;66:1117–23. doi: 10.1002/1097-0142(19900915)66:6<1117::aid-cncr2820660607>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem. 2010;47:99–114. doi: 10.1042/bse0470099. [DOI] [PubMed] [Google Scholar]
- 6.Yowtak J, Lee KY, Kim HY, et al. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–52. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podratz JL, Lee H, Knorr P, et al. Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve. Neurobiol Dis. 2017;97:60–69. doi: 10.1016/j.nbd.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41:661–68. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Guo C, Vasko MR, Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008;68:6425–34. doi: 10.1158/0008-5472.CAN-08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci. 2012;125:1488–99. doi: 10.1242/jcs.094110. [DOI] [PubMed] [Google Scholar]
- 11.Kanki T, Wang K, Baba M, et al. A genomic screen for yeast mutants defective in selective mitochondria autophagy. Mol Biol Cell. 2009;20:4730–38. doi: 10.1091/mbc.E09-03-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 2016;26:733–44. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Pickles S, Vigie P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–85. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palikaras K, Daskalaki I, Markaki M, Tavernarakis N. Mitophagy and age-related pathologies: Development of new therapeutics by targeting mitochondrial turnover. Pharmacol Ther. 2017;178:157–74. doi: 10.1016/j.pharmthera.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Tang C, Cai J, et al. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018;9:1113. doi: 10.1038/s41419-018-1152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C, Chen Z, Xu X, et al. Pink1/Parkin-mediated mitophagy play a protective role in cisplatin induced renal tubular epithelial cells injury. Exp Cell Res. 2017;350:390–97. doi: 10.1016/j.yexcr.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Li DW, Sun JY, Wang K, et al. Attenuation of cisplatin-induced neurotoxicity by cyanidin, a natural inhibitor of ROS-mediated apoptosis in PC12 Cells. Cell Mol Neurobiol. 2015;35:995–1001. doi: 10.1007/s10571-015-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng KK, Angelastro JM, Cunningham ME, Greene LA. Chapter 21 – Cultured PC12 cells: A model for neuronal function, differentiation, and survival. In: Celis JE, editor. Cell Biology. Third Edition. Burlington: Academic Press; 2006. pp. 171–76. [Google Scholar]
- 19.Hawley-Nelson P, Ciccarone V, Moore ML. Transfection of cultured eukaryotic cells using cationic lipid reagents. Curr Protoc Mol Biol. 2008;Chapter 9(Unit 9.4) doi: 10.1002/0471142727.mb0904s81. [DOI] [PubMed] [Google Scholar]
- 20.Zuo W, Zhang S, Xia CY, et al. Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: The role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology. 2014;86:103–15. doi: 10.1016/j.neuropharm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Dolman NJ, Chambers KM, Mandavilli B, et al. Tools and techniques to measure mitophagy using fluorescence microscopy. Autophagy. 2013;9:1653–62. doi: 10.4161/auto.24001. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–60. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 24.Castedo M, Ferri K, Roumier T, et al. Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods. 2002;265:39–47. doi: 10.1016/s0022-1759(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 25.Morciano G, Sarti AC, Marchi S, et al. Use of luciferase probes to measure ATP in living cells and animals. Nat Protoc. 2017;12:1542–62. doi: 10.1038/nprot.2017.052. [DOI] [PubMed] [Google Scholar]
- 26.Kauffman ME, Kauffman MK, Traore K, et al. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React Oxyg Species (Apex) 2016;2:361–70. doi: 10.20455/ros.2016.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z-R, Yang L, Zhen J, et al. Nobiletin protects PC12 cells from ERS-induced apoptosis in OGD/R injury via activation of the PI3K/AKT pathway. Exp Ther Med. 2018;16:1470–76. doi: 10.3892/etm.2018.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Chen S, Zhang Y, et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017;8:e2738. doi: 10.1038/cddis.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choubey V, Cagalinec M, Liiv J, et al. BECN1 is involved in the initiation of mitophagy: it facilitates PARK2 translocation to mitochondria. Autophagy. 2014;10:1105–19. doi: 10.4161/auto.28615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid HO, Yadav RK, Kim HR, Chae HJ. ER stress: Autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–77. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin SM, Lazarou M, Wang C, et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–42. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Koentjoro B, Park JS, Sue CM. Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Sci Rep. 2017;7:44373. doi: 10.1038/srep44373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Y, Zheng Y, Zhang X, et al. BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy. 2017;13:1754–66. doi: 10.1080/15548627.2017.1357792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demers-Lamarche J, Guillebaud G, Tlili M, et al. Loss of mitochondrial function impairs lysosomes. J Biol Chem. 2016;291:10263–76. doi: 10.1074/jbc.M115.695825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothfuss O, Fischer H, Hasegawa T, et al. Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum Mol Genet. 2009;18:3832–50. doi: 10.1093/hmg/ddp327. [DOI] [PubMed] [Google Scholar]
- 37.Williams JP, Phillips HI, Campuzano I, Sadler PJ. Shape changes induced by N-terminal platination of ubiquitin by cisplatin. J Am Soc Mass Spectrom. 2010;21:1097–106. doi: 10.1016/j.jasms.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Pourahmad J, Hosseini MJ, Eskandari MR, et al. Mitochondrial/lysosomal toxic cross-talk plays a key role in cisplatin nephrotoxicity. Xenobiotica. 2010;40:763–71. doi: 10.3109/00498254.2010.512093. [DOI] [PubMed] [Google Scholar]
- 39.Bolanos JP, Almeida A, Moncada S. Glycolysis: A bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–49. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]