Abstract
Objective
Endoplasmic reticulum (ER) stress and mitochondrial function affected intramuscular fat accumulation. However, there is no clear evident on the effect of the regulation of ER stress and mitochondrial function by Angiotensin-converting enzyme 2 (ACE2) on the prevention of intramuscular fat metabolism. We investigated the effects of ACE2 on ER stress and mitochondrial function in skeletal muscle lipid metabolism.
Methods
The triglyceride (TG) content in skeletal muscle of ACE2 knockout mice and Ad-ACE2-treated db/db mice were detected by assay kits. Meanwhile, the expression of lipogenic genes (ACCα, SREBP-1c, LXRα, CPT-1α, PGC-1α and PPARα), ER stress and mitochondrial function related genes (GRP78, eIF2α, ATF4, BCL-2, and SDH6) were analyzed by RT-PCR. Lipid metabolism, ER stress and mitochondrial function related genes were analyzed by RT-PCR in ACE2-overexpression C2C12 cell. Moreover, the IKKβ/NFκB/IRS-1 pathway was determined using lysate sample from skeletal muscle of ACE2 knockout mice.
Results
ACE2 deficiency in vivo is associated with increased lipid accumulation in skeletal muscle. The ACE2 knockout mice displayed an elevated level of ER stress and mitochondrial dysfunctions in skeletal muscle. In contrast, activation of ACE2 can ameliorate ER stress and mitochondrial function, which slightly accompanied by reduced TG content and down-regulated the expression of skeletal muscle lipogenic proteins in the db/db mice. Additionally, ACE2 improved skeletal muscle lipid metabolism and ER stress genes in the C2C12 cells. Mechanistically, endogenous ACE2 improved lipid metabolism through the IKKβ/NFκB/IRS-1 pathway in skeletal muscle.
Conclusions
ACE2 was first reported to play a notable role on intramuscular fat regulation by improving endoplasmic reticulum and mitochondrial function. This study may provide a strategy for treating insulin resistance in skeletal muscle.
Keywords: ACE2, Intramuscular fat, Endoplasmic reticulum, Mitochondrial function
Introduction
Intramuscular fat is an indispensable energy source for skeletal muscle. These lipids play pivotal roles in metabolism not only for skeletal muscle but also for the entire body. The status of the endoplasmic reticulum (ER) is a significant determinant of protein homeostasis in muscle cells. Accumulation of unfolded proteins and other physiological stresses produces ER stress, which initiates the unfolded protein response (UPR). More importantly, the signaling pathway activated by the ER stress has emerged as a critical regulator of lipid biosynthesis, insulin resistance, inflammation, and apoptosis [1, 2].
Excess lipid accumulation and impairment in mitochondrial function have been considered as putative mechanisms for the pathogenesis of skeletal muscle insulin resistance. Mitochondria modulate the balance between lipid metabolism and storage in the skeletal muscle. β-oxidation of fatty acids (FAs) is linked to ATP production, mitochondrial respiration [3], and redox balance [4]. Muscle mitochondria regulate the transport and β-oxidation flux of long-chain fatty acids [5]. Mitochondrial deficiency as the cause of impaired fatty acid oxidation capacity and skeletal muscle fat accumulation, ultimately leading to the mitochondrial-driven ‘lipotoxicity’ hypothesis of insulin resistance [6].
Renin-angiotensin system (RAS) plays an important role in the pathogenesis of IR [7, 8]. Angiotensin-converting enzyme 2 (ACE2) decreases the generation of Ang II by catalyzing the conversion of Ang II to angiotensin-(1–7) (Ang-(1–7)). Ang-(1–7) elicits the opposite effect of Ang II [9]. Recently, we reported that ACE2 regulates mitochondrial function in pancreatic β-cells, and ACE2 inhibits endoplasmic reticulum stress-associated pathway to preserve hepatic insulin resistance and hepatic steatosis [10, 11]. Moreover, the beneficial protection against ER stress in heart and lung by ACE2 is reported [12, 13]. However, there is no direct evidence that ACE2 regulates ERS and mitochondrial function in the skeletal muscle.
Though ACE2 influences the lipid metabolism of adipose tissue and liver, its effect on intramuscular fat has never been reported. A previous study reported that Ang-(1–7) improved the sensitivity of insulin by skeletal muscle glucose uptake increasement in vivo [14]. However, the underlying mechanism is still unclear. In the present study, we investigated the role of ACE2 in regulating intramuscular fat and its possible behavior on endoplasmic reticulum and mitochondrial function to clarify the function of ACE2 in metabolism and provided a potential target for insulin resistance prevention in skeletal muscle.
Materials and methods
Animal
ACE2 KO mice were a gift from Prof. Josef Penninger from the Institute of Molecular Biotechnology, Austria. In all experiments, the ACE2 KO mice were identified by PCR genotyping assays from tail biopsies. Only male ACE2 KO mice (ACE2−/y mice) and their age- and sex-matched wild-type (WT) littermates were performed. The male db/db mice at the age of 6 weeks were purchased from Nanjing Biological Medicine Research Institute, Nanjing University, China. The db/db mice were maintained on the BKS background. All mice were maintained on a 12 h light/dark cycle. All animals were handled in accordance with the protocol approved by the Ethics Committee of Animal Research at Beijing Tongren Hospital, Capital Medical University, Beijing, China.
Biochemical assays
Intramyocellular triglycerides were extracted and determined using a triglyceride assay kit (ThermoFisher Scientific) and normalized to tissue weights.
Histochemistry
Basic muscle morphology was assessed with haematoxylin and eosin staining, according to a standard protocol. All stained, sectioned images were captured under a microscope with a minimum of four fields of view per muscle section at × 100 magnification.
Cell culture
Undifferentiated mouse C2C12 myoblasts were maintained in growth medium (GM) (DMEM, Gibco, 11,965–092, supplemented with 10% fetal bovine serum, heat inactivated, 1% penicillin/streptomycin, 1% L-glutamine and 1-mM sodium pyruvate). To prepare for differentiation, the cells were seeded at a density of 1.6 × 105 cells/well in GM in 6-well plates. Differentiation was initiated 2 days later when the cells became confluent by replacing GM with differentiation medium (DM) containing 2% horse serum in place of 10% FBS. The medium was changed every other day until transfection, which was performed on day 4–5 after initiation of differentiation. The cells were maintained at 37 °C with humidified air at 5% CO2 and passaged by trypsinization.
Overexpression of ACE2 in C2C12 cells and mouse liver
To overexpress ACE2 in the db/db mice, an adenovirus coding for rat ACE2 (rACE2) upstream of an enhanced green fluorescent protein (eGFP) reporter gene (Ad-rACE2-eGFP) and the control eGFP virus (Ad-eGFP) was respectively injected into the db/db mice (male, 5 to 7-week-old) by tail vein (5 × 108 particle forming units (pfu) in 100 μL saline). On the 6th day post-virus injection, glucose tolerance tests (GTT) were performed. On the 7th day, the animals were sacrificed for experimental analysis. The C2C12 cells were infected with 1.0 × 107 pfu Ad-ACE2 or Ad-GFP for 24 h to overexpress the ACE2 protein.
Western blot
Total protein was extracted from muscle tissue and C2C12 cells with RadioImmunoprecipitation Assay (RIPA) lysis buffer, and assessed by the BCA protein assay kit for the amount (Beyotime, China). 30–60 μg protein samples were separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk and incubated with antibodies (Abs) at 4 °C overnight. The blots were probed with HRP-conjugated anti-IgG followed by detection with enhanced chemiluminescence (ECL, Millipore). C/EBP homologous protein (CHOP) Ab, activating transcription factor-4 (ATF4) Ab, Bcl-2 Ab, Bax Ab, NDUFB8 Ab, NFκB Ab, phospho-NFκB Ab, IKKβ Ab, phospho-IKKβ Ab, IRS-1 Ab, phospho-IRS-1 (Ser307) Ab, and tubulin Ab were purchased from Cell Signaling technology. Glucose regulated protein 78 (GRP78) Ab and eIF2ɑ Ab were obtained from Abcam. acetyl-CoA carboxylase α (ACCα) Ab, liver X receptor-α (LXRα) Ab, sterol regulatory element-binding protein-1c (SREBP-1c) Ab, and (carnitine palmitoyltransferase 1 α) CPT-1α Ab were purchased from Santa Cruz. The 68 kDa band of SREBP-1c was used to calculate the gray value.
Total RNA extraction and real-time PCR
Total RNA was extracted by Trizol Reagent (Invitrogen). A total of 500 ng of RNA was applied as the template for the first-strand cDNA synthesis by ReverTraAceqPCR RT Kit (TOYOBO, Osaka, Japan). The transcripts were quantified using Light Cycler 480 Real-Time PCR system (Roche, Basel, Switzerland). Primers were designed by Primer Quest (Integrated DNA Technologies, Inc).
Statistical analysis
All data were presented as the mean ± SD and analyzed by Student’s t-test or one-way ANOVA (with Bonferroni post-hoc tests to compare replicate means) when appropriate. Statistical comparisons were performed by Prism5 (GraphPad Software, San Diego, CA). P value less than 0.05 were identified to be statistically significant. Representative results from at least three independent experiments were shown unless otherwise stated.
Results
ACE2 deficiency in vivo displayed lipid accumulation, ER stress and mitochondrial dysfunction in skeletal muscle
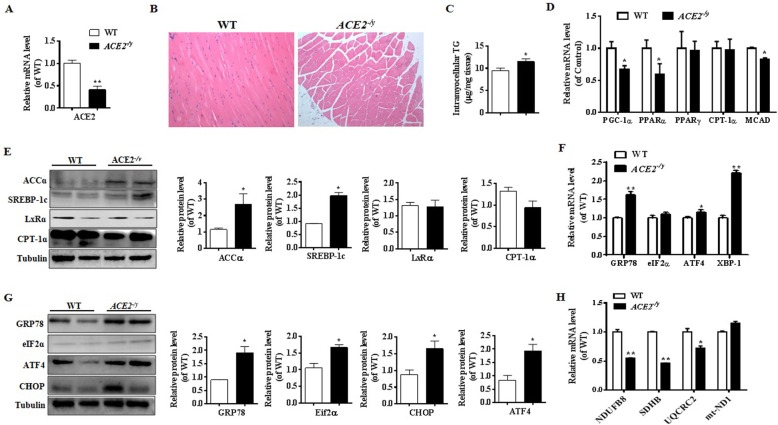
ACE2 knockout mice (ACE2−/y) were used to evaluate its role in skeletal muscle in vivo. The ACE2 mRNA levels were indeed reduced in the skeletal muscle of the ACE2−/y mice (Fig. 1a). Pathological changes were observed in H&E stained ACE2−/y mice skeletal muscle sections. The ACE2−/y mice exhibited breakage of fibers and disorder of morphology (Fig. 1b).
Fig. 1.
Analysis of lipid metabolism, ER stress and mitochondrial function in skeletal muscle of ACE2−/y mice. a: Relative ACE2 expression level in skeletal muscle of ACE2−/y mice. b: H&E-stained histology of gastrocnemius muscle in the ACE2−/y and WT mice (100× H&E). c: Analysis of intramuscular triglyceride concentration in skeletal muscle of ACE2−/y mice. d: Relative gene expression levels of fatty acid oxidation-related genes (PGC-1α, PPARα, PPARγ, CPT-1α, and MCAD). e: Relative protein levels of lipid-metabolizing (ACCα, SREBP-1c, LXRα and CPT-1α). f: Relative gene expression levels of ER stress-related genes (GRP78, Eif2α, ATF4 and XBP-1). g: Relative protein levels of GRP78, Eif2α, ATF4 and CHOP in the skeletal muscle of ACE2−/y mice. h: Relative gene expression levels of NDUFB8, SDHB, UQCRC2 and mt-ND1 in the skeletal muscle of ACE2−/y mice. The data are presented as the mean ± SD of n = 4 independent experiments in ACE2−/y mice. *P < 0.05 and **P < 0.01 versus WT mice by Student’s t test
The skeletal muscle TG content was significantly higher in ACE2−/y mice than the WT mice (Fig. 1c). To further explore these findings, we investigated the expression levels of proteins involved in lipid metabolism using real-time PCR and Western blot. Consistently, the mRNA levels of fatty acid oxidation-related genes, including PPARγ coactivator 1α (PGC-1α), peroxisome proliferator-activated receptor alpha (PPARα), and medium chain acy -CoA dehydrogenase (MCAD) were down-regulated, and little change was observed in PPAR gamma (PPARγ) and CPT-1α in the skeletal muscle of the ACE2−/y mice (Fig. 1d). Coinstantaneous, the protein levels of lipid-metabolizing genes, including ACCα and SREBP-1c were up-regulated, whereas the expression of LXRα and CPT-1α did not exhibit an obvious difference between the ACE2−/y and the WT groups (Fig. 1e). These results suggested that deletion of ACE2 may aggravate intramuscular fat accumulate in the ACE2−/y mice.
Next, we examined the ER stress in the skeletal muscle. During the ER stress, several specific proteins were highly expressed, including GRP78, pancreatic endoplasmic reticulum kinase-eukaryotic translation initiation factor 2α (eIF2α), binding immunoglobulin protein (BiP), also known as inositolrequiring enzyme1α (IRE 1α)-X-box-binding protein-1 (XBP-1), activating transcription factor 4 (ATF4), and CHOP. In this study, the mRNA levels of GRP78, ATF4 and XBP-1 were increased in the ACE2 knockout mice (Fig. 1f). Consistently, the protein levels of GRP78, eIF2α, ATF4, and CHOP were all significantly up-regulated in the skeletal muscle of the ACE2−/y mice (Fig. 1g). These results suggested a progressive accumulation of unresolved ER stress in the skeletal muscle of the ACE2−/y mice.
To study whether the mitochondrial function affect the skeletal muscle lipid metabolism, the gene levels of mitochondrial complexes I- III were measured. As expected, the mRNA levels of NDUFB8 (Complex I), succinate dehydrogenase subunit B (SDHB) (Complex II), and ubiquinol-cytochrome c reductase complex core protein 2 (UQCRC2) (Complex III) were down-regulated significantly (Fig. 1h), whereas no observable difference was detected in mitochondrial encoded NADH dehydrogenase 1 (mt-ND1) group. These results indicated that the endogenous ACE2 may regulate the expressions of proteins related to ER stress, mitochondrial function and cell apoptotic in mice skeletal muscle.
ACE2 improves skeletal muscle lipid metabolism in vitro and in vivo
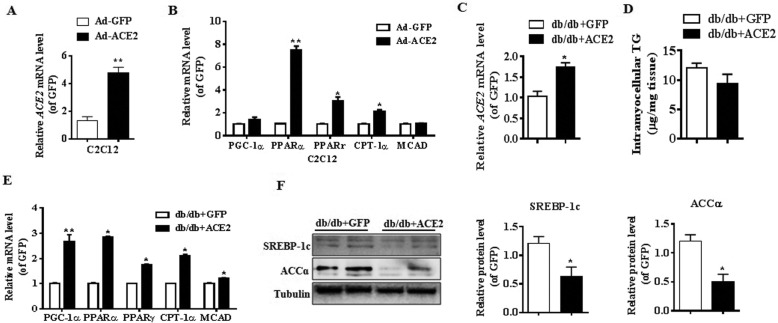
ACE2 was overexpressed in the C2C12 cells to evaluate its role in lipid metabolism and ER stress by RT-PCR analysis in vitro. Firstly, ACE2 has been proved to be indeed overexpressed after the adenoviruses infect (Fig. 2a). Secondly, the mRNA levels of fatty acid oxidation-related genes, PPARα, PPARγ, and CPT-1α were increased, and little change was observed in PGC-1α and MCAD in ACE2-overexpressing C2C12 cells (Fig. 2b). The results showed that the overexpression of ACE2 significantly improved fatty acid oxidation and ER stress.
Fig. 2.
Analysis of lipid metabolism in ACE2-overexpressing C2C12 cells and Ad-ACE2-treated db/db mice. a: Relative ACE2 expression level in ACE2-overexpressing C2C12 cells. b: Relative gene expression levels of fatty acid oxidation-related genes (PGC-1α, PPARα, PPARγ, CPT-1α, and MCAD). c: Relative ACE2 expression level in skeletal muscle of Ad-ACE2-treated db/db mice. d: Analysis of intramuscular triglyceride concentration in skeletal muscle of Ad-ACE2-treated db/db mice. e: Relative gene expression levels of fatty acid oxidation-related genes (PGC-1α, PPARα, PPARγ, CPT-1α, and MCAD). f: Relative protein levels of lipid-metabolizing (SREBP-1c and ACCα). The data are presented as the mean ± SD of n = 3 independent experiments in C2C12 cells, n = 3 in Ad-ACE2-treated db/db mice. *P < 0.05 and **P < 0.01 versus Ad-GFP cell or Ad-GFP-treated mice by Student’s t test
ACE2 was overexpressed by adenovirus in the db/db mice to evaluate its role in skeletal muscle lipid metabolism in vivo. After injection of adenoviruses, RT-PCR result indicated the protein level of ACE2 was overexpressed (Fig. 2c). The content of TG in skeletal muscle of the Ad-ACE2-treated mice was slightly lower than that of Ad-GFP-treated mice (Fig. 2d). The mRNA levels of fatty acid oxidation-related genes, PGC-1α, PPARα, PPARγ, CPT-1α, and MCAD were up-regulated in the skeletal muscle of the Ad-ACE2-treated mice compared to the Ad-GFP-treated mice (Fig. 2e). Moreover, the Ad-ACE2-treated mice exhibited a significant reduction of protein level of SREBP-1c and ACCα in the skeletal muscle (Fig. 2f).
ACE2 ameliorates ER stress and mitochondrial function in vitro and in vivo
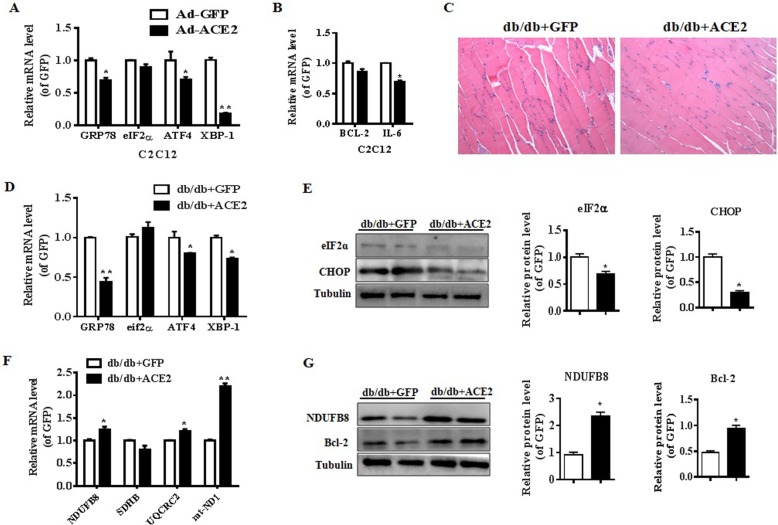
In the C2C12 cell, the mRNA levels of GRP78, ATF4 and XBP-1 were significantly decreased in the ACE2-overexpressing cells, but not eIF2α (Fig. 3a). Inflammation gene IL-6 was also reduced in the ACE2-overexpressing C2C12 cells (Fig. 3b).
Fig. 3.
ACE2 regulated ER stress and mitochondrial function in ACE2-overexpressing C2C12 cells and Ad-ACE2-treated db/db mice. a: Relative gene expression levels of ER stress-related genes (GRP78, Eif2α, ATF4 and XBP-1) in ACE2-overexpressing C2C12 cells. b: Relative gene expression levels of BCL-2 and IL-6 in ACE2-overexpressing C2C12 cells. c: H&E-stained histology of gastrocnemius muscle in the Ad-ACE2-treated and Ad-GFP-treated db/db mice (100× H&E). d: Relative gene expression levels of ER stress-related genes (GRP78, Eif2α, ATF4 and XBP-1). e: Relative protein levels of eIF2α and CHOP in the skeletal muscle of Ad-ACE2-treated db/db mice. f: Relative gene expression levels of NDUFB8, SDHB, UQCRC2 and mt-ND1 in the skeletal muscle of Ad-ACE2-treated db/db mice. g: Relative protein levels of NDUFB8 and Bcl-2 in the skeletal muscle of Ad-ACE2-treated db/db mice. The data are presented as the mean ± SD of n = 3 independent experiments in C2C12 cells, n = 3 in Ad-ACE2-treated db/db mice. *P < 0.05 and **P < 0.01 versus Ad-GFP cell or Ad-GFP-treated mice by Student’s t test
Meanwhile, pathological changes were observed in the H&E stained skeletal muscle sections. The Ad-ACE2-treated mice demonstrated less structural damage and consistent protection morphology compared to the Ad-GFP-treated mice (Fig. 3c). Next, we studied whether ACE2 altered the expression of ER stress and mitochondrial function-related genes. We found the mRNA levels of GRP78, ATF4, XBP-1 was depressed (Fig. 3d) and the protein levels of eIF2α and CHOP were inhibited in the Ad-GFP-treated db/db mice (Fig. 3e). Moreover, the mRNA levels of NDUFB8, UQCRC2, and mt-ND1 were increased in the skeletal muscle of the Ad-ACE2-treated mice (Fig. 3f). Additionally, the protein levels of NDUFB8 and Bcl-2 were reduced obviously in the Ad-GFP-treated mice (Fig. 3g). These data indicated ACE2 may significantly improve the skeletal muscle lipid metabolism in the db/db mice. These studies also suggested that ACE2 may regulate ER stress and mitochondrial function in the skeletal muscle.
ACE2 regulates the IKKβ/NFκB/IRS-1 pathway in muscle
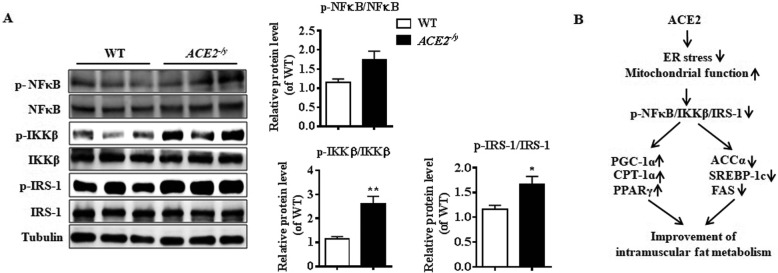
To explore a possible mechanism involvement of ACE2 in its beneficial effect against ER stress, we evaluated the expression of the IKKβ/NFκB pathway in the ACE2−/y mice and found the levels of phosphorylated NFκB and IKKβ markedly increased. Although the total amount of NFκB and IKKβ protein did not change (Fig. 4a). Consistently, IRS-1, which is another important marker of ER stress downstream of NFκB, was also significantly up-regulated in the ACE2−/y mice (Fig. 4a). These results suggested that ACE2-regulated ER stress in the skeletal muscle was also dependent on the IKKβ/NFκB/IRS-1 signaling pathway as in the liver.
Fig. 4.
Analysis of IKKβ/NFκB/IRS-1 pathway in skeletal muscle of ACE2−/y mice. a: Relative protein levels of NFκB (p-NFκB), IKKβ (p-IKKβ), and IRS-1 (Ser307) in skeletal muscle of ACE2−/y mice. b: Proposed model of intramuscular fat metabolism amelioration by the ACE2. The up- or down-regulation of metabolic pathways is indicated by arrows. (↑ for up-regulation and ↓ for down-regulation). The data are presented as the mean ± SD of n = 3 in ACE2−/y mice. *P < 0.05 versus WT by Student’s t test
Discussion
This study provided a novel avenue to treat skeletal muscle disease and even other ER stress or mitochondrial-associated pathologies. We demonstrated the amount of lipid accumulation was increased in the skeletal muscle of the ACE2 knockout mice, and the expression of ER stress and mitochondrial function related proteins in skeletal muscle were affected by the lack of ACE2. Moreover, ACE2 up-regulation improved skeletal muscle lipid metabolism and ER stress genes in the C2C12 cells. Importantly, ACE2 may significantly ameliorate skeletal muscle lipid metabolism, ER stress and mitochondrial function in the Ad-ACE2-treated db/db mice.
Increasing fatty acid oxidation while reducing intramuscular lipids is considered as an effective mean to improve insulin sensitivity. PGC-1α has been implicated in the regulation of skeletal muscle oxidative metabolism and mitochondrial biogenesis. As a transcriptional co-activator, PGC-1α activates several transcription factors to drive transcription of vast gene networks involved in many aspects of energy homeostasis, including glucose utilization and fatty acid oxidation, as well as mitochondrial biogenesis and function [15]. Some key transcriptional regulators, such as LXRα and SREBP-1c, coordinately control the lipogenesis, which increase the expression of key lipogenic genes, including those for FAS, SCD1 and ACC [16]. ACC1 converts acetyl-CoA to malonyl-CoA and inhibits fatty acid entry into the mitochondria reducing β -oxidation. In this study, we illustrated that skeletal muscle TG contents were significantly higher in the ACE2−/y mice than that in the WT mice. Subsequently, we also found the mRNA levels of fatty acid oxidation genes PGC-1α, PPARα, PPARγ, CPT-1α and MCAD decreased in the ACE2 KO mice, and increased in the ACE2-overexpressing C2C12 cells and the db/db mice. Meanwhile, the protein levels of lipogenesis proteins ACCα and SREBP-1C increased in the ACE2 KO mice, and decreased in the ACE2-overexpressing db/db mice. Accordingly, these results suggested that ACE2 may regulate intramuscular fat accumulate in mice.
It demonstrated the alterations in mitochondria and ER physical interactions contribute to insulin resistance [17]. Indeed, mitochondria and ER interact at contact points, called mitochondria-associated endoplasmic reticulum membranes, in order to exchange calcium (Ca2+) and lipids, thus regulating cell metabolism and fate [15]. In consequence of ER stress, an adaptive process named UPR is triggered. Three distinct branches of the UPR system can be initiated by transmembrane effector signal transduction proteins-protein kinase R (PKR)-like ER kinase (PERK), inositol requiring enzyme 1 alpha (IRE1α), and activating transcription factor 6 (ATF6). The UPR consists of three signaling branches which are initiated by signals such as the dissociation of BiP (GRP78) from the intracellular receptor domains of the ER. These signals activate combinations of the three stress sensors, protein kinase RNA-like PERK, ATF6 and inositol-requiring enzyme 1 α (IRE 1α). CHOP is a downstream effector of all the three branches of the UPR [18]. Enhanced CHOP expression has several effects on cells including alteration of the balance of pro- and anti-apoptotic proteins that act on mitochondria [18]. In RAS, Ang II and ACE2 have been reported to induce ER stress via GRP78/eIF2α/ATF4/CHOP axis in the liver [10, 19]. In the present study, the changes in GRP78/eIF2α/XBP-1/ATF4/CHOP expression suggested that ACE2 ameliorates ER stress in skeletal muscle and liver may through the same pathway. The mechanism underlying this process could involve the ability of ACE2 to regulate GRP78/ eIF2α/ XBP-1/ATF4/CHOP pathway.
Mitochondria regulate energy homeostasis by metabolizing nutrients, generating ATP and heat. In skeletal muscle, substantial pieces of evidence show that mitochondrial dysfunctions, in terms of number and functionality, are jointly liable for IR [20, 21]. The respiratory electron transport chain complexes I-IV which transfer the electron in the inner mitochondria membrane and the ATP-synthase enzyme (Complex V) play a critical role in mitochondrial oxidation [22]. Here, we showed that SDHB, NDUFB8 and UQCRC2 were down-regulated in the ACE2 KO mice. Consistently, NDUFB8 and UQCRC2 were down-regulated in the Ad-ACE2-treated db/db mice. These data suggested that ACE2 can improve skeletal muscle mitochondria function by regulating respiratory electron transport chain complexes.
Activation of the pro-inflammatory transcription NF-κB has been connected to the impairment of insulin signaling induced by the ER stress in skeletal muscle [23]. The UPR is mainly mediated by the activation of IRE-1α, PERK/ eIF2-α, and ATF6 signaling pathways. These three cellular pathways can interact with several inflammatory signals, including NF-κB [24]. It has been reported that UPR reduces the NF-κB inhibitor IκBα through several mechanisms [25, 26], which results in NF-κB activation and the regulation of genes involved in inflammation and insulin resistance [24]. Given that accumulation of intracellular lipid species can activate cellular signaling cascades conducive to NFκB activation [27], we check the IKKβ/NFκB signal pathway. Of interest, ACE2 down-regulation resulted in the increased expression of the phosphorylation of IKKβ and NFκB in the skeletal muscle of the ACE2 KO mice. The insulin-signaling pathway requires insulin receptor substrates (IRS-1 and IRS-2). IRS-1 has a major role in skeletal muscle [28]. Serine hyperphosphorylation of IRS-1 is a marker of IR in peripheral tissues, which leads to subsequent activation of PI3K pathway [29]. Akt is a key protein involved in PI3K pathway, its phosphorylation promotes the metabolic activities in skeletal muscle [30]. It’s remarkable that ACE2 down-regulation increased expression of the phosphorylation of IRS-1 in the skeletal muscle of the ACE2 KO mice. However, one previous study showed that phosphorylated Akt in the soleus muscle equally in the standard diet-fed ACE2KO mice compared with the WT mice [31]. These findings suggest that ACE2 mediate ER stress and mitochondria function in intracellular lipid metabolism, which was involved in the regulation of the IKKβ/NFκB/IRS-1 pathway, but not Akt pathway.
Conclusions
As shown in Fig. 4b, ACE2 ameliorates intramyocellular lipid metabolism could involve the ability of regulating ER stress and mitochondria function in skeletal muscle. The underlying mechanism is mediated partly through the activation of NFκB/ IKKβ/IRS-1 signaling pathway. This study may further provide a strategy for treating insulin resistance in skeletal muscle.
Acknowledgements
We sincerely thank the Xiu Tuo, Yi-Chen Zhang for technical assistance.
Abbreviations
- ACCα
Acetyl-CoA carboxylase α
- ACE2
Angiotensin-converting enzyme 2
- ACE2−/y
ACE2 knockout mice
- Ang-(1–7)
Angiotensin-(1–7)
- ATF4
Activating transcription factor-4
- ATF6
Activating transcription factor 6
- BiP
Binding immunoglobulin protein
- CHOP
C/EBP homologous protein
- CPT-1α
Carnitine palmitoyltransferase 1 α
- DM
Differentiation medium
- eIF2α
eukaryotic translation initiation factor 2α
- ER
Endoplasmic reticulum
- FAs
Fatty acids
- GM
Growth medium
- GRP78
Glucose regulated protein 78
- GTT
Glucose tolerance tests
- IRE 1α
Inositolrequiring enzyme1 α
- IRE1α
Inositol requiring enzyme 1 alpha
- LXRα
Liver X receptor-α
- MCAD
Medium chain acy-CoA dehydrogenase
- mt-ND1
mitochondrial encoded NADH dehydrogenase 1
- PERK
Pancreatic endoplasmic reticulum kinase
- PGC-1α
PPARγ coactivator 1α
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPARγ
Peroxisome proliferator-activated receptor gamma
- RAS
Renin-angiotensin system
- SDHB
Succinate dehydrogenase subunit B
- SREBP-1c
Sterol regulatory element-binding protein-1c
- TG
Triglyceride
- UPR
Unfolded protein response
- UQCRC2
Ubiquinol-cytochrome c reductase complex core protein 2
- WT
Wild-type
- XBP-1
X-box-binding protein-1
Authors’ contributions
Xi Cao conceived the idea for the study, designed the experiments, performed the experiments, and wrote and edited the manuscript. Xin-Meng Lu performed the experiments and edited the manuscript. Xiu Tuo, Jing-Yi Liu, Yi-Chen Zhang, and Li-Ni Song performed the experiments. Zhi-Qiang Cheng edited the manuscript. Jin-Kui Yang and Zhong Xin conceived the idea for the study, designed the experiments, and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Foundation of Precision Medicine Research, State Key Development Program, China (2017YFC0909600); National Natural Science Foundation of China (81670774); Beijing Hospitals Authority Youth Programme (QML20170205) and Scientific Research Fund of Beijing Tongren Hospital, Capital Medical University (2014-YJJ-GGL-040).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval
All animals were handled in accordance with the protocol approved by the Ethics Committee of Animal Research at Beijing Tongren Hospital, Capital Medical University, Beijing, China.
Consent for publication
Not applicable
Competing interests
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xi Cao and Xin-Meng Lu contributed equally to this work.
Contributor Information
Jin-Kui Yang, Email: jinkui.yang@gmail.com.
Zhong Xin, Email: xinz@medmail.com.cn.
References
- 1.Glimcher LH, Lee AH. From sugar to fat: how the transcription factor XBP1 regulates hepatic lipogenesis. Ann N Y Acad Sci. 2009;1173(Suppl 1):E2–E9. doi: 10.1111/j.1749-6632.2009.04956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41(3):197–239. doi: 10.1016/S0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 4.Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O'Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes. 2012;61(12):3094–3105. doi: 10.2337/db12-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 6.Seghieri M, Trico D, Natali A. The impact of triglycerides on glucose tolerance: lipotoxicity revisited. Diabetes Metab. 2017;43(4):314–322. doi: 10.1016/j.diabet.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG (mRen2)27(Ren2) rats. J Hepatol. 2008;49(3):417–428. doi: 10.1016/j.jhep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao X, Yang FY, Xin Z, Xie RR, Yang JK. The ACE2/Ang-(1-7)/mas axis can inhibit hepatic insulin resistance. Mol Cell Endocrinol. 2014;393(1–2):30–38. doi: 10.1016/j.mce.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216(2):R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 10.Cao X, Song LN, Zhang YC, Li Q, Shi TT, Yang FY, Yuan MX, Xin Z, Yang JK. Angiotensin-converting enzyme 2 inhibits endoplasmic reticulum stress-associated pathway to preserve nonalcoholic fatty liver disease. Diabetes Metab Res Rev. 2019;35(4):e3123. doi: 10.1002/dmrr.3123. [DOI] [PubMed] [Google Scholar]
- 11.Shi TT, Yang FY, Liu C, Cao X, Lu J, Zhang XL, Yuan MX, Chen C, Yang JK. Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic beta-cells. Biochem Biophys Res Commun. 2018;495(1):860–866. doi: 10.1016/j.bbrc.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 12.Sukumaran V, Veeraveedu PT, Gurusamy N, Lakshmanan AP, Yamaguchi K, Ma M, Suzuki K, Nagata M, Takagi R, Kodama M, Watanabe K. Olmesartan attenuates the development of heart failure after experimental autoimmune myocarditis in rats through the modulation of ANG 1-7 mas receptor. Mol Cell Endocrinol. 2012;351(2):208–219. doi: 10.1016/j.mce.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen H, Uhal BD. The unfolded protein response controls ER stress-induced apoptosis of lung epithelial cells through angiotensin generation. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L846–L854. doi: 10.1152/ajplung.00449.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echeverria-Rodriguez O, Del Valle-Mondragon L, Hong E. Angiotensin 1-7 improves insulin sensitivity by increasing skeletal muscle glucose uptake in vivo. Peptides. 2014;51:26–30. doi: 10.1016/j.peptides.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14(22):2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tubbs E, Rieusset J. Metabolic signaling functions of ER-mitochondria contact sites: role in metabolic diseases. J Mol Endocrinol. 2017;58(2):R87–R106. doi: 10.1530/JME-16-0189. [DOI] [PubMed] [Google Scholar]
- 18.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 19.Ha TS, Park HY, Seong SB, Ahn HY. Angiotensin II induces endoplasmic reticulum stress in podocyte, which would be further augmented by PI3-kinase inhibition. Clin Hypertens. 2015;21:13. doi: 10.1186/s40885-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 22.Lemarie A, Grimm S. Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer? Oncogene. 2011;30(38):3985–4003. doi: 10.1038/onc.2011.167. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108(3):437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med. 2012;18(10):589–598. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183(2):1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honma M, Sawada S, Ueno Y, Murakami K, Yamada T, Gao J, Kodama S, Izumi T, Takahashi K, Tsukita S, Uno K, Imai J, et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J Obes. 2018;42(9):1544–1555. doi: 10.1038/s41366-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boller S, Joblin BA, Xu L, Item F, Trub T, Boschetti N, Spinas GA, Niessen M. From signal transduction to signal interpretation: an alternative model for the molecular function of insulin receptor substrates. Arch Physiol Biochem. 2012;118(3):148–155. doi: 10.3109/13813455.2012.671333. [DOI] [PubMed] [Google Scholar]
- 30.Kubota H, Noguchi R, Toyoshima Y, Ozaki Y, Uda S, Watanabe K, Ogawa W, Kuroda S. Temporal coding of insulin action through multiplexing of the AKT pathway. Mol Cell. 2012;46(6):820–832. doi: 10.1016/j.molcel.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Takeda M, Yamamoto K, Takemura Y, Takeshita H, Hongyo K, Kawai T, Hanasaki-Yamamoto H, Oguro R, Takami Y, Tatara Y, Takeya Y, Sugimoto K, et al. Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice. Diabetes. 2013;62(1):223–233. doi: 10.2337/db12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.