Abstract
It is very challenging to conduct a pharmacokinetic (PK) study on mice due to the limited amount of plasma one can obtain, which is also true for some clinical studies. Here, we developed and validated a simple, sensitive and robust LC-MS/MS method for measuring the prodrug valacyclovir (VACV) and its metabolite acyclovir (ACV) in mouse and human plasma. This assay utilized an acetonitrile protein precipitation method with isotope-labeled internal standards (IS) and enabled precise and accurate quantification of VACV and ACV from 10 μl plasma samples with a nine min gradient. The analytes were separated on a Waters Atlantis T3 C18 column. The precursor-product ion transitions for VACV (m/z 325.2 > 152.1), ACV (m/z 226.2 > 152.1), VACV-D4 (m/z 329.2 > 152.1, IS) and ACV-D4 (m/z 230.2 > 152.1, IS) were detected in a multiple reaction monitoring (MRM) positive ion mode using an API4000 LC-MS/MS system. The lower limit of quantification (LLOQ) was 2 nM for both VACV and ACV. The linear range was validated over the concentration ranges of 2 – 200 nM and 200 – 5000 nM for both compounds. The matrix effect and stability of VACV and ACV were also evaluated. This assay was successfully applied to a PK study in mice.
Keywords: valacyclovir, acyclovir, LC-MS/MS, mouse, plasma
1. Introduction
Mice are one of the most commonly used animal models for preclinical pharmacokinetic (PK) studies in drug discovery and development. Conventionally, composite blood sampling is often used due to the small body size of mouse, i.e. each data point was taken from multiple mice in order to collect sufficient volume of sample for measurement. However, composite sampling significantly increases animal and material usage, and inevitably introduces inter-animal variabilities [1]. One strategy is to develop a highly sensitive assay for the target compound, thus, requiring less sample volume and enabling one PK profile to be generated from a single mouse. In addition to mouse study, this strategy can also be applied to some clinical studies where the blood sample volume is limited, such as in pediatric/neonatal patient populations.
Valacyclovir (VACV) is a widely used antiviral drug for the treatment of herpes simplex, herpes zoster, and herpes B infections [2, 3]. It is designed as the L-valyl ester prodrug of acyclovir (ACV) with three to five-fold greater bioavailability relative to ACV [2]. After oral administration, VACV is rapidly absorbed and hydrolyzed to ACV in the small intestine and liver [4–6]. ACV is then converted to the active metabolite ACV triphosphate, a nucleoside analog that competitively inhibits viral DNA polymerase without affecting the normal cellular processes [2, 7].
To date, biphenyl hydrolase-like protein (BPHL) is the only identified hydrolase responsible for the conversion of VACV to ACV [6]. However, the in vivo effect of BPHL enzyme on the VACV metabolism and PK remains unclear. We intend to investigate the role of BPHL enzyme in the VACV metabolism and PK using wildtype and Bphl knockout mouse models. In order to depict one PK profile for each mouse (i.e., with serial blood sampling), a highly sensitive and selective assay is needed.
Previously, two LC-MS/MS methods have been reported to simultaneously quantify both VACV and ACV in human plasma and tsetse flies, respectively [8, 9]. However, a relatively large volume of sample (250 μL for human plasma in one study) was used in their workflow. For our mouse study, only 10 μL plasma would be available at each time point in order to have enough data points. In addition, the LLOQ of the previous methods is not low enough for the mouse PK study based on our pilot experiment. Therefore, the purpose of this study was to develop a simple, sensitive and robust LC-MS/MS assay for the quantification of VACV and ACV in mouse and human plasma.
2. Materials and methods
2.1. Reagents and animals
ACV, VACV, formic acid, and acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO). ACV-D4 and VACV-D4 were obtained from Toronto Research Chemicals (Toronto, Canada). Ammonium acetate was from Fisher Scientific Co. (Pittsburgh, PA). Water was prepared from a Milli-Q water purification system. Blank human plasma was obtained from Innovative Research Inc. (Novi, MI). Wildtype (C57BL/6) mice for PK studies were bred and housed in a temperature-controlled environment with 12-hour light and dark cycles, and received a standard diet and water ad libitum. Studies in mice were approved by the Institutional Animal Care and Use Committee of the University of Michigan. All other chemicals were of analytical grade and commercially available.
2.2. Solution Preparation
All stock solutions (4 mM ACV, 4 mM VACV, 3 mM ACV-D4 and 2 mM VACV-D4) were prepared in water. Working solutions containing both VACV and ACV were prepared by diluting ACV and VACV stock solution in acetonitrile at the following concentrations: 100 μM, 40 μM, 20 μM, 10 μM, 4 μM, 2 μM, 1 μM, 0.4 μM, 0.2 μM, 0.1 μM, and 0.04 μM. All stock and working solutions were stored at −80 °C until use. Calibrator samples were prepared by spiking 0.5 μl of working solutions to 9.5 μl of blank mouse or human plasma. The final concentrations of calibrators were 5000, 2000, 1000, 500, 200, 100, 50, 20, 10, 5, and 2 nM for both ACV and VACV. Quality control (QC) samples were prepared at concentrations of 2000, 200 and 10 nM. ACV and VACV were separately prepared for selectivity and stability verification.
2.3. Mouse PK study
A preliminary PK study was performed after oral and intravenous (IV) administrations of VACV in mice. In brief, 25 nmol/g body weight (BW) of VACV in 200 μL water per 20 g BW was administrated by oral gavage, and 25 nmol/g BW of VACV in 100 μL saline per 20 g BW was administrated by tail IV injection. About 20 μL blood samples were collected via tail nicks at 2, 5, 15, 30, 45, 60, 90, 120, 180, 240 and 360 min after oral dosing; and at 2, 5, 15, 30, 60, 90, 120, 180, 240 and 360 min after IV injection. The blood sample was then mixed with 1 μL EDTA-K3 and centrifuged at 3000g for 5 min at 4°C. Ten microliters plasma was collected at each time point and frozen at −80°C until use.
2.4. Plasma sample preparation
A direct protein precipitation method was used to extract both ACV and VACV from plasma. Ten microliters plasma samples, QC samples, or calibrators were mixed with 4-fold volume acetonitrile containing the internal standards (IS) 200 nM VACV-D4 and ACV-D4, then vortexed for 5 min and centrifuged at 17,000g for 10 min at 4 °C to remove the precipitated proteins. The supernatant was transferred to an LC vial for LC-MS/MS analysis.
2.5. LC-MS/MS conditions
The LC–MS/MS analysis was performed on a Shimadzu HPLC system (Shimadzu, Tokyo, Japan) coupled with an Applied Biosystems API 4000 triple quadrupole/linear ion trap (QTRAP) mass spectrometer (Foster City, CA).
Ten microliters of sample solution were injected into LC-MS/MS. Analytes were separated on a Waters Atlantis T3 C18 column (5um, 150×2.1mm, Dublin, Ireland). The mobile phase consisted of water containing 2 mM ammonium acetate and 0.2% formic acid (v/v) (phase A) and acetonitrile containing 0.2% formic acid (v/v) (phase B), and was delivered at a flow rate of 0.2 ml/min. A gradient elution was applied for the separation with the time program set as follows: phase B was started at 2% for 2 min, then increased to 4% during the time period of 2 to 4 min, further increased to 50% during 4 to 6 min, then returned to 2% at 6.5 min, and maintained until the end of run (9 min).
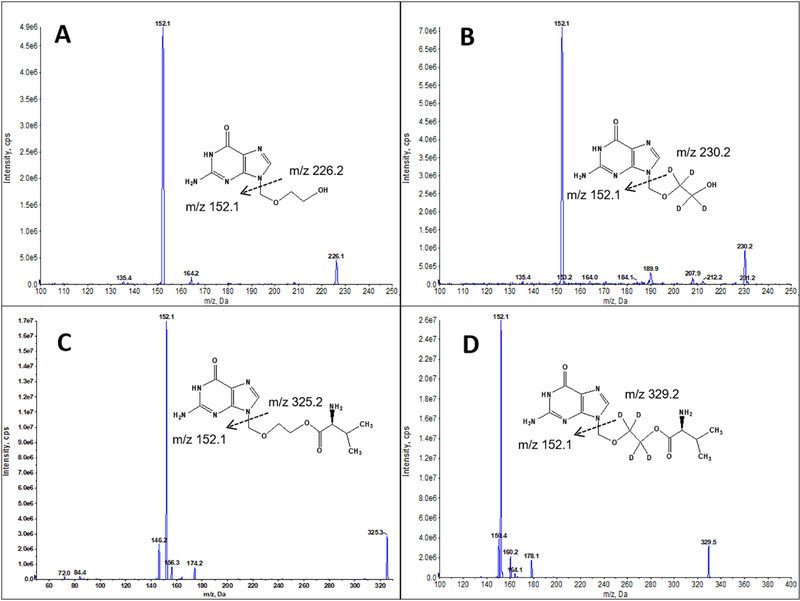
MS was operated in a positive ion mode using turbo electrospray ionization. As shown in Figure 1, the following transitions were monitored on a positive multiple reaction monitoring (MRM) mode: VACV, 325.2 > 152.1; ACV, 226.2 > 152.1; VACV-d4 (IS), 329.2 > 152.1; ACV-d4 (IS), 230.2 > 152.1. The source-dependent parameters were set as follows: curtain gas 20, ionspray voltage 4800, temperature 500, gas 1 60 and gas 2 60. The analyte-specific MS parameters were summarized in Table 1.
Figure 1:
Mass spectra showing both the precursor and product ions of acyclovir (ACV) (A), valacyclovir (VACV) (C), and their isotope-labeled internal standards ACV-D4 (B) and VACV-D4 (D).
Table 1.
Summary of analyte-specific MS parameters. ACV: acyclovir; VACV: valacyclovir.
| Compound | Parent Ion (m/z) | Product Ion (m/z) | Declustering Potential | Entrance Potential | Collision Energy | Collision Cell Exit Potential |
|---|---|---|---|---|---|---|
| VACV | 325.2 | 152.1 | 56 | 9 | 23 | 8.8 |
| ACV | 226.2 | 152.1 | 60 | 6 | 18 | 8.8 |
| VACV-D4 | 329.2 | 152.1 | 56 | 9 | 23 | 8.8 |
| ACV-D4 | 230.2 | 152.1 | 60 | 6 | 18 | 8.8 |
2.6. Data analysis
Analyst® software version 1.6 (AB Sciex, Foster City, CA) was used for LC-MS/MS data acquisition and processing. GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) was used to draw the PK plasma concentration-time profiles.
3. Results and discussion
3.1. Method validation
3.1.1. Selectivity
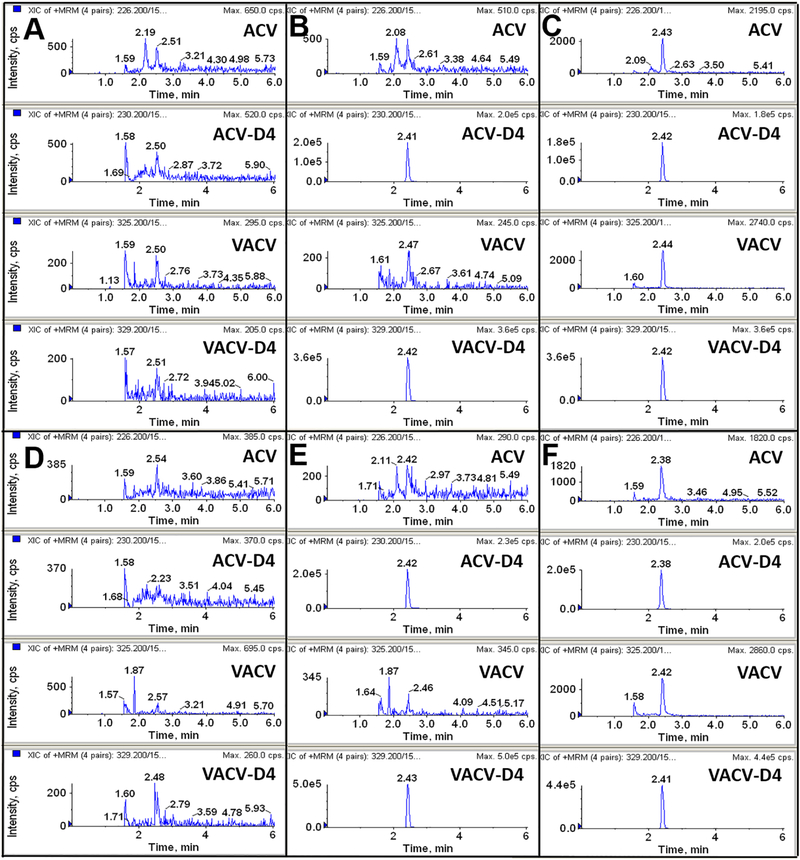
Selectivity was evaluated by analyzing blank plasma, plasma spiked with 200 nM ISs, and plasma spiked with ACV and VACV at LLOQ (2 nM). Both mouse and human blank plasma were obtained from five different sources. As shown in Figure 2, some minor unspecific peaks were observed in adjacent to ACV and VACV in mouse and human plasma, however, none of those interference was more than 20% of the respective LLOQ in peak height.
Figure 2:
Representative LC-MS/MS chromatograms of blank plasma (A for mouse, D for human), plasma spiked with internal standards valacyclovir-D4 (VACV-D4) and acyclovir-D4 (ACV-D4) (B for mouse, E for human), and plasma at the lower limit of quantification (LLOQ) (C for mouse, F for human).
3.1.2. Linearity and LLOQ
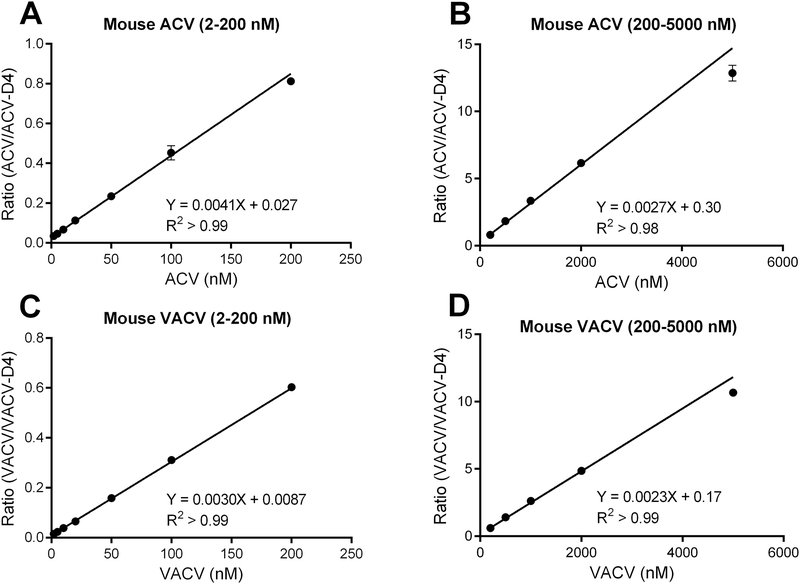
The assay linearity was determined using VACV and ACV calibration curves from 2 to 5000 nM. Better accuracy was achieved when the concentrations were separated into two smaller ranges, i.e., from 2–200 nM and 200–5000 nM (Figure 3, mouse data were shown). As shown in Table 2, most of the coefficient of correlation (R2) values for VACV and ACV, in both concentration ranges, were more than 0.99. The relative standard deviation for slope and intercept was in the range of 2.3–7.5% and 5.5%−24.4%, respectively. In addition, the slope and intercept for ACV and VACV were comparable in mouse and human plasma. The LLOQ was 2 nM for both ACV and VACV in both plasma at a signal to noise ratio >10.
Figure 3:
Calibration curves for valacyclovir (VACV) and acyclovir (ACV) over the concentrations ranging from 2 to 200 nM (A, C) and from 200 to 5000 nM (B, D) in mouse plasma (n = 5).
Table 2:
Summary of the linear equation for valacyclovir (VACV) and acyclovir (ACV) over the concentration range from 2 to 5000 nM in mouse and human plasma. Better accuracy was achieved when the range was separated into two small range. The weighing factor of 1/x2 was used. Y is the peak area ratio of the analyte to internal standard and x the concentration of the analyte.
| Compound | Conc. range (nM) | Mouse plasma (n= 5) | Human plasma (n= 5) |
|---|---|---|---|
| ACV | 2–200 | y = 0.0041 (± 0.00031) x + 0.027 (± 0.0034), R2 > 0.99 | y = 0.0042(± 0.00013) x + 0.0035(± 0.00085), R2 > 0.99 |
| 200–5000 | y = 0.0027(± 0.000085) x + 0.30 (± 0.017), R2 > 0.98 | y = 0.0026(± 0.000063) x + 0.34(± 0.032), R2 > 0.99 | |
| VACV | 2–200 | y = 0.0030(± 0.000070) x + 0.0087(± 0.0014), R2 > 0.99 | y = 0.0031 (± 0.000090) x + 0.0034(± 0.00057), R2 > 0.99 |
| 200–5000 | y = 0.0023(± 0.000052) x + 0.17(± 0.018), R2 > 0.99 | y = 0.0022(± 0.000071) x + 0.21 (± 0.024), R2 > 0.99 |
3.1.3. Accuracy and precision
Three concentrations (10, 200 and 2000 nM) of QC samples in five replicates were used to validate the accuracy and precision of the assay. Although the QC low and high concentrations deviated slightly from the ones recommended by the FDA guidance, the QC low (10 nM) and high (1000 nM) were reasonable representatives of the low and high concentrations of the analytes in the mouse pharmacokinetic study.
As shown in Table 3, the accuracy for intra-day and inter-day analyses ranged from 94.8% to 108.7%, and the precision (coefficient of variation, CV%) varied from 2.0% to 8.9%. All accuracy and precision results met the requirement in the FDA bioanalytical method validation guidance [10].
Table 3:
Summary of intra-day and inter-day accuracy and precision of LC-MS/MS assay for the determination of valacyclovir (VACV) and acyclovir (ACV) in mouse and human plasma. CV: coefficient of variation.
| Analyte and Matrix | Concentrations (nM) | Intra-day (n=5) |
Inter-day (n=5) |
||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (CV%) | Accuracy (%) | Precision (CV%) | ||
| ACV in mouse plasma | 10; 200; 2000 | 99.4%; 97.3%; 101.9% | 5.5%; 8.0%; 7.6% | 98.9%; 96.5%; 104.1% | 8.9%; 5.9%; 6.5% |
| ACV in human plasma | 10; 200; 2000 | 108.1%; 94.8%; 105.8% | 4.2%; 2.6%; 5.0% | 107.8%; 96.0%; 104.0% | 3.8%; 3.0%; 5.5% |
| VACV in mouse plasma | 10; 200; 2000 | 102.9%; 106.8%; 100.6% | 5.2%; 3.7%; 2.1% | 105.4%; 104.4%; 102.2% | 5.4%; 4.1%; 3.7% |
| VACV in human plasma | 10; 200; 2000 | 104.1%; 102.4%; 101.6% | 5.9%; 2.0%; 4.2% | 108.7%; 101.1%; 102.1% | 6.4%; 2.8%; 4.5% |
3.1.4. Matrix effects
Five different lots of blank mouse and human plasma were used to assess the matrix effects. Two sets of samples were prepared. Set A: phosphate-buffered saline spiked with ACV (10, 2000 nM), VACV (10, 2000 nM) and ISs (200 nM); Set B: extracted plasma mixed with ACV (10, 2000 nM), VACV (10, 2000 nM) and ISs (200 nM). The percentage of matrix effect was calculated as (b/a) *100, where a and b are the peak areas of ACV, VACV, and IS in sets A and B, respectively. As shown in Table 4, the mean matrix effect ranged from 98.0–112.6%, and the lot-to-lot variation was lower than 11.1%, indicating a minimal matrix effect.
Table 4:
Matrix effect of valacyclovir (VACV) and acyclovir (ACV) in mouse and human plasma (n=5).
| Analyte and Matrix | Concentrations (nM) | Matrix effect (%, Mean ± S.D.) |
|---|---|---|
| ACV in mouse plasma | 10; 2000 | 106.1 ± 8.5; 102.3 ± 8.7 |
| ACV in human plasma | 10; 2000 | 112.6 ± 8.7; 105.3 ± 11.1 |
| VACV in mouse plasma | 10; 2000 | 100.9 ± 6.5; 98.0 ± 5.0 |
| VACV in human plasma | 10; 2000 | 104.3 ± 8.1; 101.6 ± 8.8 |
| ACV-D4 in mouse plasma | 200 | 100.7 ± 5.5 |
| ACV-D4 in human plasma | 200 | 106.1 ± 6.4 |
| VACV-D4 in mouse plasma | 200 | 105.6 ± 8.0 |
| VACV-D4 in human plasma | 200 | 103.4 ± 7.4 |
3.1.5. Stability
The stability of ACV and VACV in mouse and human plasma was assessed by analyzing low and high concentration QC samples (10, 2000 nM) with four replicates under different conditions, including benchtop, autosampler, freeze-thaw, and long-term (−80 °C) stability. The results were compared with that of freshly prepared QC samples (Table 5). Deviation of the tested results from the reference concentrations should not exceed by 15%. Benchtop stability was tested by exposing the QC samples at room temperature or on ice for 4 hr. ACV was determined to be within 95.3–99.5% of that in freshly prepared QC samples. VACV was stable on ice in both mouse and human plasma (94.5–99.4%). However, we found that degradation occurred for VACV at room temperature in the plasma, especially in mouse (76.1–79.0% in mouse plasma vs 91.1–94.0% in human plasma after 4 hr exposure, compared to that of freshly prepared QC samples), which is consistent with previous reports [8]. The autosampler stability was assessed by measuring the processed QC samples after being stored in autosampler (4 °C) for 24 hr. Freeze-thaw stability was evaluated after three successive cycles of freezing (−80 °C) and thawing (room temperature). The long-term stability was determined by freezing the QC samples at −80 °C for 30 days. No significant degradation was observed in the autosampler, freeze-thaw and long-term (−80 °C) stability studies. Therefore, all the plasma samples obtained from the following mouse PK study were immediately put on ice, then transferred to −80°C freezer within 4 hr or directly prepared for LC-MS/MS analysis.
Table 5:
Stability of valacyclovir (VACV) and acyclovir (ACV) in mouse and human plasma under different conditions (n=4). QC: quality control; SD: standard deviation.
| ACV/VACV stability | Relative mean ± SD (%) of QC samples (10, 2000 nM) |
|||
|---|---|---|---|---|
| ACV in mouse plasma | ACV in human plasma | VACV in mouse plasma | VACV in human plasma | |
| Bench (4 hr at room temperature) | 98.3 ± 5.4; 99.5 ± 7.8 | 95.3 ± 2.9; 96.5 ± 8.5 | 76.1 ± 4.9; 79.0 ± 6.7 | 91.1 ± 4.7; 94.0 ± 9.0 |
| Bench (4 hr on ice) | 97.6 ± 5.4; 96.8 ± 2.5 | 98.6 ± 2.4; 95.7 ± 6.8 | 94.5 ± 5.6; 99.4 ± 6.4 | 95.0 ± 3.1; 98.4 ± 3.5 |
| Autosampler (4 °C for 24 hr) | 103.0 ± 8.6; 99.4 ± 4.3 | 104.2 ± 2.6; 100.1 ± 4.8 | 102.1 ± 4.1; 99.4 ± 3.5 | 104.2 ± 2.6; 100.1 ± 4.8 |
| Freeze-thaw | 94.8 ± 4.1; 101.2 ± 3.1 | 96.0 ± 7.4; 101.3 ± 2.3 | 93.6 ± 1.7; 98.2 ± 3.7 | 94.8 ± 3.7; 101.8 ± 3.6 |
| 30 days at −80 °C | 96.5 ± 4.6; 95.3 ± 7.1 | 101.9 ± 2.8; 103.0 ± 5.8 | 90.8 ± 7.1; 96.0 ± 4.0 | 93.3 ± 5.0; 96.0 ± 4.6 |
3.1.6. Dilution integrity
Dilution integrity was determined by preparing mouse and human plasma samples at concentrations of 10 μM and 20 μM for both ACV and VACV, and diluting them with blank plasma. Three replicates were prepared for each concentration. The 10 μM and 20 μM samples were diluted by 5 times and 10 times with blank plasma, respectively. The accuracy of the diluted samples ranged from 91.6% to 104.7% with precision less than 10%.
3.2. Application in a mouse PK study
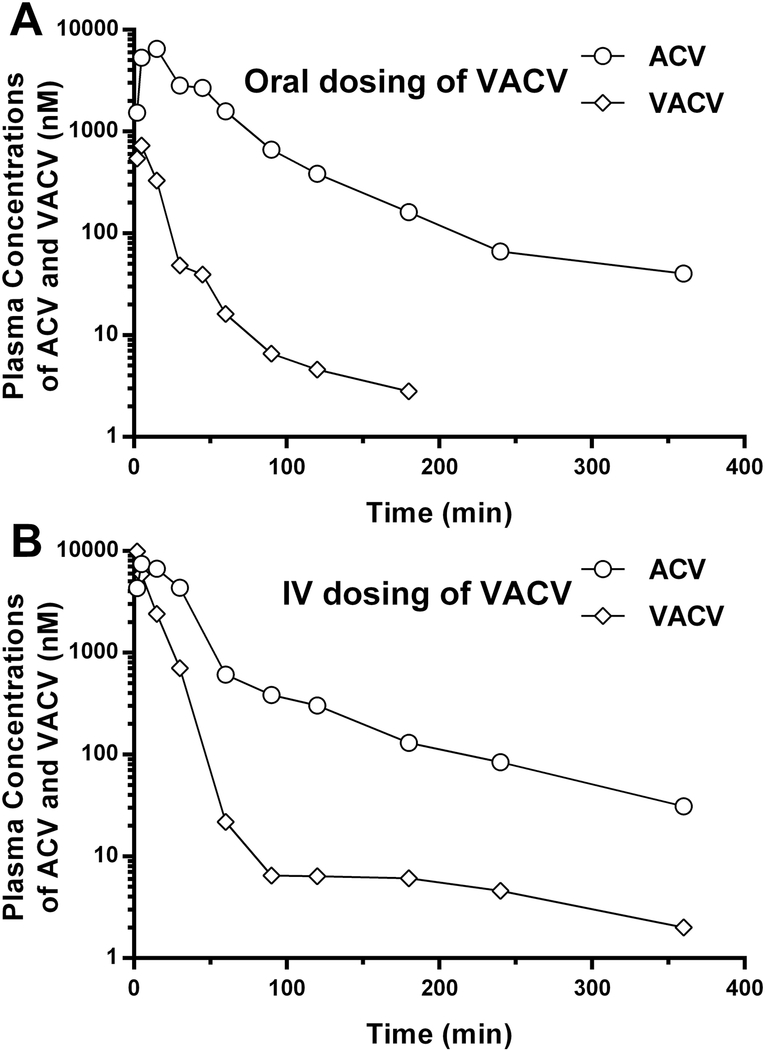
The validated method was successfully applied to a preliminary PK study in mice after oral and IV administrations of 25 nmol/g BW VACV. As shown in Figure 4, VACV was rapidly absorbed and promptly converted to ACV after both oral and IV administrations of VACV. The method enables precise quantification of VACV and ACV in mouse plasma up to 360 min (and 180 min for VACV after oral dosing) after the given doses of VACV. Analysis, however, of the metabolite ACV would be possible for a much longer time given its plasma concentrations relative to the LLOQ. Although limited to one mouse per administration route, our analysis indicated that the systemic exposure of ACV was markedly greater than that of VACV because of essentially complete presystemic (and subsequent) hydrolysis in the gastrointestinal tract and liver.
Figure 4:
The plasma concentration-time profiles of valacyclovir (VACV) and acyclovir (ACV) in mice after oral (A) and intravenous (IV) (B) administrations of 25 nmol/g body weight VACV (n = 1 per administration route).
It should be noted that the current method does not permit technical replicates for samples collected at each time point due to the limited volume of blood.
3.3. Comparison of the current method with previously published LC-MS/MS methods
There are several unique advantages of the current assay as compared to previous methods of analysis [8, 9]. First, direct protein precipitation in the sample preparation is faster and more cost-effective than the solid phase extraction method used by Yadav et al for human plasma samples [8] and the solid phase dispersion method by Sasanya et al for tsetse flies samples [9]. Second, the isotope-labeled IS can minimize the variations introduced during sample preparation and LC-MS/MS analysis (due to their similar physicochemical characteristics), thus, resulting in more robust and reliable quantification [11, 12]. Furthermore, a lower LLOQ (2 nM for both VACV and ACV) was achieved with our assay, which other assay methods could not obtain (i.e., 15 and 211 nM for VACV and ACV, respectively, by Yadav et al. [8]; 617 and 888 nM for VACV and ACV, respectively, by Sasanya et al. [9]). Comparing to the two previous LC-MS/MS methods, a similar triple quadruple MS (API 4000 vs API 4000 and Waters Quattro Micro) and identical MRM transitions were utilized for ACV and VACV quantification. However, some MS parameters were different. The instrument was manually tuned with infusion pump to optimize these parameters. For the source-dependent MS parameters, higher heater temperature (500 °C vs 400 °C) and different ionspray voltage (4800 V vs 5500 V and 3290 V) were used. For the analyte-specific MS parameters, different collision energy (VACV: 23 V vs 50 V and 15 V, ACV: 18 V vs 25 V and 12 V), declustering potential (VACV: 56 V vs 60 V and 32 V, ACV: 60 V vs 21 V and 40 V) were applied. All these optimized parameters may have contributed to the improved sensitivity.
4. Conclusion
In the present study, we developed and validated a sensitive and robust LC-MS/MS method for the analysis of the prodrug VACV and its metabolite ACV in small volumes of mouse and human plasma. Using the acetonitrile protein precipitation method and isotope-labeled ISs, this method enables precise and accurate quantification of VACV and ACV from 10 μl plasma samples with 2 nM LLOQ in a nine min gradient. This method was found to be readily applicable to a mouse PK study. It would also be a promising method for human PK studies, especially when sample volume is a limiting factor.
Acknowledgement
NIH National Institute of General Medical Sciences grant R01GM115481 (to DES).
Abbreviations
- ACV
acyclovir
- BPHL
biphenyl hydrolase-like protein
- BW
body weight
- CV
coefficient of variation
- IS
internal standard
- IV
intravenous
- LLOQ
lower limit of quantification
- MRM
multiple reaction monitoring
- PK
pharmacokinetics
- QC
quality control
- VACV
valacyclovir
Footnotes
Conflict of Interest
The authors have declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Joyce AP, Wang M, Lawrence-Henderson R, Filliettaz C, Leung SS, Xu X, O’Hara DM, One mouse, one pharmacokinetic profile: quantitative whole blood serial sampling for biotherapeutics, Pharmaceutical research, 31 (2014) 1823–1833. [DOI] [PubMed] [Google Scholar]
- [2].Beutner KR, Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy, Antiviral research, 28 (1995) 281–290. [DOI] [PubMed] [Google Scholar]
- [3].Ormrod D, Goa K, Valaciclovir: a review of its use in the management of herpes zoster, Drugs, 59 (2000) 1317–1340. [DOI] [PubMed] [Google Scholar]
- [4].Burnette TC, de Miranda P, Metabolic disposition of the acyclovir prodrug valaciclovir in the rat, Drug Metab Dispos, 22 (1994) 60–64. [PubMed] [Google Scholar]
- [5].de Miranda P, Burnette TC, Metabolic fate and pharmacokinetics of the acyclovir prodrug valaciclovir in cynomolgus monkeys, Drug Metab Dispos, 22 (1994) 55–59. [PubMed] [Google Scholar]
- [6].Kim I, Chu XY, Kim S, Provoda CJ, Lee KD, Amidon GL, Identification of a human valacyclovirase: biphenyl hydrolase-like protein as valacyclovir hydrolase, The Journal of biological chemistry, 278 (2003) 25348–25356. [DOI] [PubMed] [Google Scholar]
- [7].Alrabiah FA, Sacks SL, New antiherpesvirus agents. Their targets and therapeutic potential, Drugs, 52 (1996) 17–32. [DOI] [PubMed] [Google Scholar]
- [8].Yadav M, Upadhyay V, Singhal P, Goswami S, Shrivastav PS, Stability evaluation and sensitive determination of antiviral drug, valacyclovir and its metabolite acyclovir in human plasma by a rapid liquid chromatography-tandem mass spectrometry method, J Chromatogr B Analyt Technol Biomed Life Sci, 877 (2009) 680–688. [DOI] [PubMed] [Google Scholar]
- [9].Sasanya JJ, Abd-Alla AM, Parker AG, Cannavan A, Analysis of the antiviral drugs acyclovir and valacyclovir-hydrochloride in tsetse flies (Glossina pallidipes) using LC-MSMS, J Chromatogr B Analyt Technol Biomed Life Sci, 878 (2010) 2384–2390. [DOI] [PubMed] [Google Scholar]
- [10].U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), C.f.V.M. (CVM), Guidance for Industry: Bioanalytical Method Validation, 2013.
- [11].Stokvis E, Rosing H, Beijnen JH, Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not?, Rapid communications in mass spectrometry : RCM, 19 (2005) 401–407. [DOI] [PubMed] [Google Scholar]
- [12].Wieling J, LC-MS-MS experiences with internal standards, Chromatographia, 55 (2002) S107–S113. [Google Scholar]