ABSTRACT
Japanese encephalitis is a mosquito-borne arbo-viral disease with seasonal occurrence. Since 2009, AES/JE cases have been reported from Malkangiri district of Odisha State, India at an interval of one year.In the current study, the entomological parameters of known JE vector mosquito species were assessed for one year in Malkangiri district. Mosquito collections were done fortnightly in the index villages from August 2015 to July 2016 to record the density, their breeding habitats, feeding behaviour, parity, dusk index (DI) and infection status with JE virus. A total of 2347 JE vector mosquitoes belonging to nine species were collected from dusk collections. Culex vishnui (38.3%) was the predominant species followed by Cx. whitmorei (17.3%), Cx. fuscocephalus (13.6%), Cx. tritaeniorhynchus (11.1%), Cx. bitaeniorhynchus (6.1%), Anopheles subpictus (4.8%), An. barbirostris (4.4%), Cx. quinquefasciatus (2.3%) and Cx. gelidus (2.2%). The average DI of Cx. vishnui was 0.37 which was highest among all JE vector species and varied between 0.02 (April) and 0.9 (November). The human blood indexof Cx. vishnui was 0.026. A total of 1835 JE vector mosquitoes were screened for the isolation of JE virus, but none was found positive. Presence of paddy fields and ponds, abundance of JE vectors and their human feeding habit indicate the risk of JE transmission in the study area. Detection of JE virus in Cx. vishnui during 2016 outbreak in Malkangiri district further confirms that there would be a threat of JE transmission during the favourable period.
KEYWORDS: Culex vishnui, entomological factors, Japanese encephalitis virus, Malkangiri, Odisha
Introduction
Japanese encephalitis (JE) is one of the key forms of viral encephalitis, mostly prevalent in southern and eastern Asia[1] and transmitted to human beings by mosquitoes [2,3]. In India, the first endemic case of Japanese encephalitis was identified in the state of Tamil Nadu in 1955 [4]. From 1955 to 1966, about 65 JE cases were reported in South India [4]. Since 1973, epidemics of JE were reported from different parts of India, predominantly in West Bengal, Bihar, Uttar Pradesh, Assam, Andhra Pradesh, Karnataka, Tamil Nadu, Maharashtra, Haryana, Kerala, Odisha and union territories of Goa and Pondicherry [5–7]. Seasonal outbreaks of acute encephalitis syndrome (AES) among children have been reported in the country causing high morbidity and mortality [8]. During 2015, a total of 9854 cases and 1210 deaths due to AES, 1730 cases and 291 deaths due to JE were recorded by the National Vector Borne Disease Control Programme (NVBDCP) in India [8]. During the same year, in Odisha State, a total of 660 cases and two AES deaths and 33 cases and two deaths due to JE were reported [8].
Malkangiri, the southernmost district of Odisha State, India recorded 9 deaths in 2009 due to AES and 38 deaths of children in 2012 due to JE/AES [9,10]. In 2014, eight deaths of children occurred due to AES in Korkunda and Kalimela Community Health Centers (CHCs) of this district [10]. JE virus is the main causative agent for AES outbreaks [2,3]. Though the etiology of AES outbreak in 2014 could not be established, the seasonality, geographical distribution and clinical manifestations pointed toward the epidemiological features of JE. But there was no entomological information available for establishing the JE transmission in the district. In view of the recurring JE/AES outbreaks with life loss in the district, such information is highly essential to implement appropriate vector control measures to prevent the disease. Therefore, a study was undertaken in the JE/AES reported villages in Malkangiri district for a period of 1 year (August 2015 to July 2016) to assess the mosquito species composition, relative and seasonal abundance of JE vectors, parity, dusk index (DI), human blood index (HBI) and their infection status.
Material & methods
Study area
There are seven CHCs in Malkangiri district with 108 Gram Panchayats (GPs) and 1,045 villages. The district has a population of 641,385 living in 109,483 households; population density was 106 inhabitants/km2 (2014 census conducted by the health department). Three villages viz., Potrel (18°.15732ʹ N latitude and 82°.01143ʹ E longitude), Uskapali (18°.14297ʹ N latitude and 82°.02108ʹ E longitude) and Kodeiguda (18°.24809ʹ N latitude and 82°.03391ʹ E longitude) of Korkunda CHC, in which more number of suspected JE/AES cases occurred during 2014, were selected as index villages. The Korkunda CHC has a population of 143,867 living in 29,667 households. The population of the three index villages was 359, 555 and 134 and the number of human dwellings was 79, 105 and 29, respectively (2014 census conducted by the health department).
The three prevailing seasons in the district were summer (March–June), rainy (July–October) and winter (November–February). During the last 8 years, the maximum temperature ranged from 42-47° C and the minimum temperature from 13-16° C. The relative humidity (RH) varied from 60-97%, remained high during monsoon and post–monsoon months. The annual rainfall from 2010 to 2015 was 1852.9 mm, 1103.9 mm, 1715.2 mm, 1783.3 mm, 1509.6 mm and 1771.02, respectively.
In Korkunda block, there were 75,772 cows, 4,811 buffalos, 9,470 sheep, 22,431 goats, 10,007 pigs, 34,168 chickens and 2,034 ducks during 2012. In the index villages, the cattle population was considerably high. There were 3,471 cows, 60 buffaloes, 23 sheep, 582 goats, 241 pigs and 1567 chickens in Potrel village and 808 cows, 174 sheep, 293 goats, 170 pigs and 513 chickens in Uskapali village, 800 cows, 22 buffaloes, 11 sheep, 179 goats, 53 pigs and 202 chickens in Kodeiguda village (Source: Office of the Chief District Veterinary Officer, Malkangiri). Regarding the presence of migratory birds, no information was available in the district.
The total cultivated area in Korkunda block was 36,933 ha and paddy was cultivated in 26,255 ha during khariff (rainy) and in 800 ha during rabi (winter/summer) season. In the rainy season, paddy depends on rainwater, and in the summer season, it depends on canal irrigation, lift irrigation and stream water.
Mosquito collections
Both immature and adult surveys were carried out fortnightly in three index villages from August 2015 to July 2016 covering all the three seasons prevailing in the study area.
Immature survey
The available three breeding sites (paddy fields, ponds and pits) were surveyed at fortnightly intervals and the presence of immature of the known JE vectors in different breeding habitats was recorded to verify the prevalence of the vector species. A total of 100 dips each were taken from paddy fields and ponds and 50 dips from pits during each survey to estimate the immature density in these habitats. Immature collected from the field were brought to the laboratory, reared to adults and identified to species.
Adult survey
Dusk collections of resting adults were carried out fortnightly in the index villages from indoors (12 human dwellings, 12 cattle sheds and 12 pigsties from each index village) and outdoor sites (18 sites such as bushes, standing crops, plantations, root-intricacies and around pigsties/goat sheds in each index village) using oral aspirators (hand catches) spending 10 min in each site; thereby spending a total of 9 man hours in each village. The collected mosquitoes were identified to species level [11]. Per man-hour density (PMHD), i.e. number collected per man hour of each species collected from both indoors and outdoors was calculated and recorded every month. Trapping methods are unbiased and reliable and have been used successfully in India and abroad, to monitor the density of Culex vectors of viral encephalitis [12]. Hence, attempts were made to collect mosquitoes using light traps. Two traps, one each in cattle shed and pigsties, were fixed in each village and each trap was run for 3 hours from dusk for mosquito collection. The average number of mosquitoes collected per trap/night is expressed as per trap density (PTD). The known JE vector species collected from both indoors and outdoors were dissected and ovarioles were examined for dilatations and parous rate was calculated. The monthly dusk index (DI) of JE vector species was calculated by multiplying the PMHD and the proportion of parous female mosquitoes [13].
Blood meal analysis
Smears of blood meals of the fully fed JE vector mosquitoes from dusk collections were prepared on Whatman No. 1 filter papers and analyzed using Agar-gel diffusion method to know the source of blood feeding [14]. The reagents were obtained from MP Biomedicals, (Solon, OH). Human and bovine blood indices (HBI and BBI) for each tested species were calculated from the proportion that fed on human and bovine, respectively.
Vector infection with JE virus
After dissection, the unfed and fully gravid female mosquitoes were pooled and kept in sterile 1.5 ml eppendorf tubes. One pool containing five numbers of mosquitoes (from same species/habitat) was kept in each tube and stored in liquid nitrogen and was transported to Indian Council of Medical Research-Vector Control Research Center (ICMR-VCRC) laboratory. The mosquito samples were stored at −80° C for JE virus (JEV) isolation.
The pool-wise mosquito samples were homogenized and extraction of RNA was done using TRI reagent method (Molecular Research Center, Inc. USA). The amplification of RNA (from five pools-each PCR pool contains RNA from 25 mosquitoes) was done using primers specific to capsid pre-membrane region [15] of JE virus genome using transcriptor one-step RT-PCR kit (Roche Applied Science, Penzberg, Germany). RNA from 25 mosquitoes in a pool was pre-standardized in the laboratory. RT-PCR was performed with negative and positive controls. After the amplification, separation of 675 bp PCR amplicons was done in 1.5% agarose gel and results were documented using gel documentation system (Gelstan Medicae, India). The infection rate of JE virus was calculated from the number of positive pools.
Results
Immature survey
A total of 4750 dips were taken from ponds and 9.3% dips were found with mosquito breeding (Table 1). Among the positive dips, 128 (29.0%) dips were found positive for anophelines, 119 (26.9%) were culicines and 195 (44.1%) were both anophelines and culicines. A total of 828 immature were collected from ponds; 53.5% (n = 443) were anophelines and 46.5% (n = 385) were culicines. Per dip immature density of anopheline and culicine in the pond was 0.09 and 0.08, respectively. A total of 256 mosquitoes emerged from the ponds and 145 (56.6%) were anophelines comprising 10 species and 111 (43.4%) culicines comprising seven JE vector species viz., Culex fuscocephalus (38.7%), Cx. vishnui (27.0%), Cx quinquefasciatus (19.8%), Cx. whitmorei (7.2%), Cx. tritaeniorhynchus (3.6%), Cx. bitaeniorhynchus (2.7%) and Cx. gelidus (0.9%). Among the 10 anopheline species emerged from ponds, two were JE vector species viz., Anopheles subpictus (7.6%) and An. barbirostris (2.8%).
Table 1.
Emergence of culicines and anophelines from different breeding habitats.
| S.No. | Breeding habitat | No of dips taken | Dips positive | Total immature | Total emergence | culicines emerged (%) | anophelines emerged (%) |
|---|---|---|---|---|---|---|---|
| 1. | Pond | 4750 | 442 | 828 | 256 | 43.4 | 56.6 |
| 2. | Paddy field | 2400 | 328 | 637 | 159 | 57.2 | 42.8 |
| 3. | Pit | 250 | 37 | 82 | 23 | 34.8 | 65.2 |
Out of 2,400 dips taken from the paddy fields, 328 (13.7%) were positive for the breeding of mosquitoes. Among the positive dips, 60 (18.3%) dips were found positive for only anophelines, 100 (30.5%) for only culicines and 168 (51.2%) for both anophelines and culicines. A total of 637 immature were collected from the paddy fields and among them 47.7% were anophelines and 52.3% were culicines. Per dip immature density of anopheline and culicine was 0.13 and 0.14, respectively. A total of 159 mosquitoes emerged from the immature collected from the paddy fields and among them, 42.8% were anophelines comprising of seven species and 57.2% were culicines belonging to seven species (Table 1). The culicine species were Cx. quinquefasciatus (14.3%), Cx. tritaeniorhynchus (3.3%), Cx. gelidus (1.1%), Cx. vishnui (44.0%), Cx. bitaeniorhynchus (5.5%), Cx. fuscocephalus (24.2%), Cx. whitmorei (7.7%). Out of seven anopheline species emerged; one was JE vector viz., An. subpictus (10.3%).
A total of 250 dips were taken from pits and among them 10.8% dips were positive for anophelines, 27.0% culicines and 62.2% for both anophelines and culicines. A total of 82 larvae were collected from the pits and among them, 57.3% were anophelines and 42.7% culicines (Table 1). Per dip immature density of anopheline and culicine in the pit was 0.19 and 0.14, respectively. A total of eight anophelines comprising four species and 15 culicines comprising three species emerged from the pits. The JE vector species emerged were Cx. fuscocephalus (37.5%), Cx. quinquefasciatus (37.5%) and Cx. vishnui (25.0%).
Adult collections
Species composition
A total of 3,085 adult mosquitoes were collected, belonging to 24 species of two genera; Culex (8 species) and Anopheles (16 species), out of which 2132 (69.1%) were Culex mosquitoes and 953 (30.9%) were Anopheles mosquitoes. Among Culex species, Cx. vishnui (42.1%) was predominant followed by Cx. whitmorei (19.0%), Cx. fuscocephalus (15.0%), Cx. tritaeniorhynchus (12.2%), Cx. bitaeniorhynchus (6.7%), Cx. quinquefasciatus (2.5%) and Cx. gelidus (2.4%). There was only one Cx. fuscitarsis collected during the study period. Out of the 16 Anopheles species, An. nigerrimus (48.3%) was predominant followed by An. vagus (13.3%), An. subpictus (11.8%), An. barbirostris (10.9%), An. pallidus (5.5%), An. culicifacies (3.6%), An. splendidus (2.2%), An. annularis (1.9%), An. jeyporiensis (0.8%), An. jamesii (0.6%), An. maculatus (0.4%), An. ramsayi (0.2%), An. varuna (0.2%), An. tessellatus (0.1%), An. theobaldi (0.1%) and An. aconitus (0.1%).
Among 3,085 mosquitoes collected from dusk collections, 2347 mosquitoes belong to nine JE vector species, which include seven Culex and two Anopheles. A total of 1629 JE vector species were obtained indoors, 544 outdoors and 174 from light traps. Among the vector mosquitoes collected indoors, 282, 1111 and 236 were obtained from human dwellings, cattle sheds and pigsties, respectively. Out of 174 vector mosquitoes collected from light traps, 131 were from cattle sheds and 43 from pigsties.
Relative abundance of the vector species
Out of 2347 JE vectors collected from dusk collections, Cx. vishnui (38.3%) was predominant followed by Cx. whitmorei (17.3%), Cx. fuscocephalus (13.6%), Cx. tritaeniorhynchus (11.1%), Cx. bitaeniorhynchus (6.1%), An. subpictus (4.8%), An. barbirostris (4.4%), Cx. quinquefasciatus (2.3%) and Cx. gelidus (2.2%). The average yearly and month-wise PMHD of JE vectors collected are shown in Figures 1 and 2, respectively. Among the nine JE vector species collected, the PMHD of Cx. vishnui was found to be highest both indoors (1.44) and outdoors (0.98). The light trap collections also yielded all the nine JE vector species. The PTD of Cx. vishnui (0.22) was highest among the JE vector species followed by Cx. whitmorei (0.17), An. barbirostris (0.07), Cx. fuscocephalus (0.05), Cx. bitaeniorhynchus (0.04), Cx. tritaeniorhynchus (0.04), An. subpictus (0.02), Cx. gelidus (0.01) and Cx. quinquefasciatus (0.01).
Figure 1.
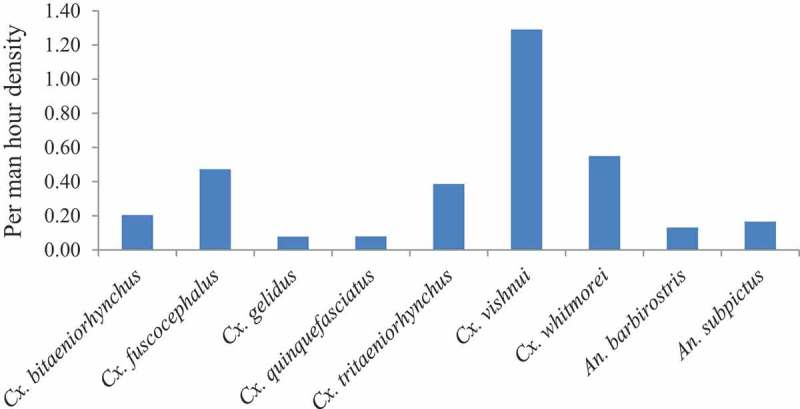
Average per man hour density of JE vectors during the study period.
Figure 2.
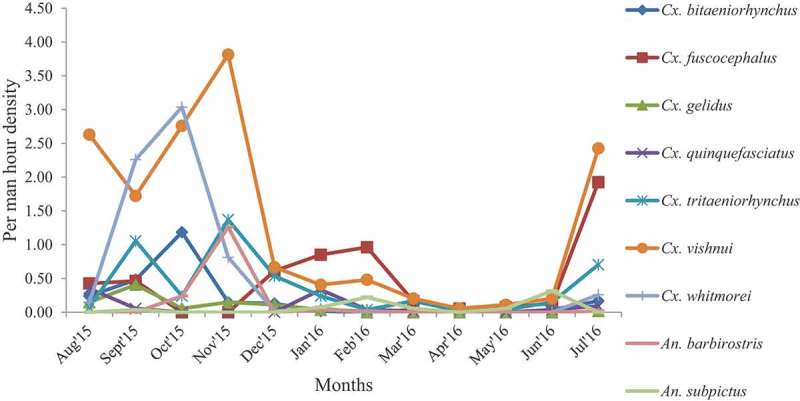
Per man hour density of JE vectors in different months.
Seasonal prevalence of JE vectors
The PMHD of JE vectors in different seasons is shown in Table 3. The PMHD of Cx. vishnui in the rainy season was 2.38 followed by 1.34 and 0.14 in the winter and summer months, respectively (Table 2). The monthly analysis of PMHD showed that the density of Cx. vishnui was highest in November (3.81) and lowest in April (0.06).
Table 3.
Parous rate of JE vector mosquitoes in different seasons.
| S.No. | Species | Parous rate |
||
|---|---|---|---|---|
| Summer | Rainy | Winter | ||
| 1. | Cx. bitaeniorhynchus | 25.0 | 25.0 | 31.3 |
| 2. | Cx. fuscocephalus | 52.2 | 27.9 | 38.2 |
| 3. | Cx. gelidus | NA | 29.4 | 18.8 |
| 4. | Cx. quinquefasciatus | 50.0 | 16.7 | 30.0 |
| 5. | Cx. tritaeniorhynchus | 35.0 | 29.5 | 19.5 |
| 6. | Cx. vishnui | 35.5 | 28.4 | 27.6 |
| 7. | Cx. whitmorei | NA | 21.9 | 18.8 |
| 8. | An. barbirostris | NA | 28.6 | 14.1 |
| 9. | An. subpictus | 54.2 | 50.7 | 41.7 |
NA: not available.
Table 2.
Per man hour density (PMHD) of JE vector mosquitoes in three seasons.
| S.No. | Species | PMHD |
||
|---|---|---|---|---|
| Summer | Rainy | Winter | ||
| 1. | Cx. bitaeniorhynchus | 0.02 | 0.52 | 0.07 |
| 2. | Cx. fuscocephalus | 0.11 | 0.70 | 0.61 |
| 3. | Cx. gelidus | 0.00 | 0.16 | 0.07 |
| 4. | Cx. quinquefasciatus | 0.02 | 0.13 | 0.09 |
| 5. | Cx. tritaeniorhynchus | 0.09 | 0.52 | 0.55 |
| 6. | Cx. vishnui | 0.14 | 2.38 | 1.34 |
| 7. | Cx. whitmorei | 0.00 | 1.43 | 0.22 |
| 8. | An. barbirostris | 0.00 | 0.06 | 0.33 |
| 9. | An. subpictus | 0.11 | 0.31 | 0.07 |
Parous rate
Overall, the parous rate of An. subpictus (50.5) was highest among all JE vector species followed by Cx. fuscocephalus (34.6), Cx. quinquefasciatus (30.0), Cx. gelidus (26.0), Cx. vishnui (28.4), Cx. tritaeniorhynchus (25.2), Cx. bitaeniorhynchus (25.8), Cx. whitmorei (21.5) and An. barbirostris (16.5). The parous rate of An. subpictus was 54.2, 50.7 and 41.7 in summer, rainy and winter seasons, respectively, and the parous rate in each season was comparatively higher than all other JE vector species (Table 3).
Dusk index
The average DI of Cx. vishnui (0.37) was the highest among all the JE vector species followed by Cx. fuscocephalus (0.16), Cx. whitmorei (0.12), Cx. tritaeniorhynchus (0.1), An. subpictus (0.08), Cx. bitaeniorhynchus (0.05), An. barbirostris (0.02), Cx. gelidus (0.02) and Cx. quinquefasciatus (0.02). The DI of Cx. vishnui was highest in the month of November (0.9) and lowest in the month of April (0.02). The DI of all the vector species was found higher during the rainy season except Cx. fuscocephalus, which was higher in the winter season (Table 4).
Table 4.
Dusk index of JE vector mosquitoes in different seasons.
| S.No. | Species | Dusk index |
||
|---|---|---|---|---|
| Summer | Rainy | Winter | ||
| 1. | Cx. bitaeniorhynchus | 0.005 | 0.13 | 0.02 |
| 2. | Cx. fuscocephalus | 0.05 | 0.2 | 0.23 |
| 3. | Cx. gelidus | 0.0 | 0.05 | 0.01 |
| 4. | Cx. quinquefasciatus | 0.009 | 0.02 | 0.03 |
| 5. | Cx. tritaeniorhynchus | 0.03 | 0.15 | 0.1 |
| 6. | Cx. vishnui | 0.05 | 0.68 | 0.37 |
| 7. | Cx. whitmorei | 0.0 | 0.31 | 0.04 |
| 8. | An. barbirostris | 0.0 | 0.02 | 0.05 |
| 9. | An. subpictus | 0.06 | 0.16 | 0.03 |
Human blood index
A total of 468 blood samples of nine JE vector species were tested and out of that 459 (98.1%) were positive for bovine antisera and the remaining 9 (1.9%) blood samples belonging to four species were positive for human antisera. The HBI of Cx. quinquefasciatus was 0.167 followed by Cx. fuscocephalus (0.034), Cx. vishnui (0.026) and Cx. bitaeniorhynchus (0.02) (Table 5).
Table 5.
Source of blood meal of JE vector mosquitoes.
| S.No. | Species | Total tested | Human | HBI | Bovine | BBI |
|---|---|---|---|---|---|---|
| 1. | Cx. bitaeniorhynchus | 49 | 1 | 0.02 | 48 | 0.98 |
| 2. | Cx. fuscocephalus | 88 | 3 | 0.034 | 85 | 0.966 |
| 3. | Cx. gelidus | 13 | 0 | 0.0 | 13 | 1.00 |
| 4. | Cx. quinquefasciatus | 6 | 1 | 0.167 | 5 | 0.833 |
| 5. | Cx. tritaeniorhynchus | 59 | 0 | 0.0 | 59 | 1.00 |
| 6. | Cx. vishnui | 153 | 4 | 0.026 | 149 | 0.974 |
| 7. | Cx. whitmorei | 24 | 0 | 0.0 | 24 | 1.00 |
| 8. | An. barbirostris | 63 | 0 | 0.0 | 63 | 1.00 |
| 9. | An. subpictus | 13 | 0 | 0.0 | 13 | 1.00 |
| Total | 468 | 9 | 0.019 | 459 | 0.981 |
HBI: human blood index; BBI: bovine blood index.
Infection status
RT-PCR assay was performed with 1835 JE vector mosquitoes belonging to the nine vector species collected during the study period, but none was positive.
Discussion
JE occurrence has been reported in India since 1952 [16]. A total of 44,097 cases and 5,728 deaths due to AES were reported in the country during 2008–2014 [8]. In Odisha State, it was first reported in Rourkela city during 1988 [17] and several outbreaks occurred in that city during the year 1992 and 1995 [18,19]. At present, out of 30 districts of Odisha State, 17 districts are endemic for JE (Source: NVBDCP, Odisha, 2018). Recently, Malkangiri district of Odisha State reported JE/AES outbreaks in 2009, 2012 and 2014 [10]. In 2016, a severe outbreak of JE and AES with high case fatality was reported in the district affecting 336 children with 103 deaths [10]. Out of the 103 deaths during the outbreak, 37 deaths were due to JE and the remaining 66 were due to AES [10]. The transmission of JEV in the district was confirmed by the detection of JE virus RNA from cerebrospinal fluid (CSF) samples and JE virus IgM from serological samples collected from the suspected cases during 2012 outbreak [19]. However, there was a lack of systematic entomological information to support those findings. This manuscript discusses the results of systematic year-round entomological study pertaining to the abundance (density), HBI, parity, DI and infection status of known JE vector species.
JE disease is predominantly found in agricultural localities, particularly in rice cultivation areas, where vector mosquitoes proliferate in close association with pigs, wading birds and ducks [20]. The current study villages were surrounded by paddy fields thereby, providing favorable breeding places for the JE vector mosquitoes. Among the three breeding habitats surveyed, paddy field was found as the most potential breeding habitat for JE vector species. Although immature were collected from pits and ponds throughout the year, paddy fields were found breeding with high intensity during rainy season. Cx. vishnui subgroup of mosquitoes breed in water with ample vegetation, mainly in paddy fields and their abundance may be related to their breeding in rice fields [20,21]. From the surveyed breeding habitats, seven culicine JE vector species emerged. Among them, Cx. vishnui (44.0%) was the predominant JE vector species emerged from the paddy fields. Similar to our findings, a study conducted in Gorakhpur, India reported that the paddy fields are mostly contributing the increase of population of the JE vector species [21]. Other studies at Cuddalore, South Arcot and Madurai districts of Tamil Nadu and Mandya district of Karnataka [22–25] also showed that Cx. vishnui subgroup mosquitoes were found more abundant during the paddy cultivation period.
Cx. vishnui has been recognized as the major vector of JE for many years and it plays an important role in the epidemiology of JE in different parts of India [23,26]. The maximum isolation of JEV was obtained from the Cx. vishnui subgroup [26–30] and was also shown to be capable of transmitting the JE virus in the laboratory [31]. Only two members of Cx. vishnui subgroup viz., Cx. vishnui and Cx. tritaeniorhynchus constituted 49.4% of total adult JE vectors were collected during the current study. The PMHD and the DI of these two vector species were relatively higher compared to other JE vector species. Although Cx. bitaeniorhynchus, Cx. quinquefasciatus and Cx. gelidus also play a role in JE virus transmission in India [23,27,30,32], the three species together contributed only 10.6% of the total JE vectors. An. subpictus was also regarded as JE vector in India and played role in JE transmission in Karnataka [33], Kerala [29] and Tamil Nadu [34]. An. barbirostris was found involved in JE transmission in Bengal [30]. In the current study, both An. subpictus and An. barbirostris together constituted 9.1% of total adult JE vectors. Though none of the JE vector mosquito was found infected with JE virus (JEV) in the current study, subsequent entomological study conducted during 2016 JE outbreak period (September 2016 – November 2016) in Malkangiri district showed that Cx. vishnui was involved in JE transmission as JE virus was isolated from this vector and the minimum infection rate of Cx. vishnui was 0.88% [10].
The density of the JE vector species in the current study was found relatively lower in comparison to the density observed during the outbreak periods (2012 & 2016) in Malkangiri district [10,19]. While the PMHD of Cx. vishnui in the district was 33.0 and 24.0 in 2012 and 2016, respectively, the density obtained in the current study was only 1.29 [10,19]. While the DI of Cx. vishnui was found to be 0.37 in the current study, the same was much higher (7.62) during 2016 outbreak in the district [10]. An earlier surveillance of JE in two villages of Tamil Nadu recorded 168.9 and 141.0 dusk indices of Cx. vishnui group [13], which was much higher than our present study. Further, it has been observed that all the JE/AES outbreaks in Malkangiri district from 2009 to 2016 occurred during the rainy months. This has been substantiated by the current entomological study that the PMHD and DI of major JE vector species (Cx. vishnui) was much higher in rainy months (PMHD = 2.38, DI = 0.68) in comparison to winter (PMHD = 1.34, DI = 0.37) and summer months (PMHD = 0.14, DI = 0.05). The HBI of Cx. vishnui in the current study was 0.026 indicating that a small proportion of this vector species feeds on human. The mosquito blood meal analysis for pig and other animals could not be carried out in the current study due to lack of logistics; which was a limitation of the study. Overall, the low density, HBI and DI of JE vector species observed during the current study might be due to the intense vector control interventions undertaken by local health administration such as indoor residual spraying with DDT and fogging indoors and outdoors with malathion in the study areas during 2014 outbreak period. Due to the extensive JE vector control program undertaken in the district, the major JE vectors; Cx. vishnui and Cx. tritaeniorhynchus were also found resistant to DDT, malathion and deltamethrin [35]. Further, the study showed that pig populations have reduced to a minimal level (142 pigs during 2015 in comparison to 1580 during 2012) due to the awareness program conducted by the health department. There was no migration of bird population to the study villages observed during the study period.
Conclusion
This was the first longitudinal entomological study covering all the three seasons conducted in the repeated occurrence of JE affected district in Odisha State. Data on seasonal variations in entomological parameters (PMHD, proportion parous, HBI and DI) of different JE vectors were generated in the study. Due to the reinforced vector control operations, the PMHD and DI of JE vectors were found to be relatively lower in the study villages compared to the entomological studies carried out in the same district [10,19] and elsewhere in India [13]. Abundance of vector breeding habitats such as paddy fields, ponds and pits surrounding the villages, availability of JE vectors, their human feeding habit and rearing of pigs as a source of economy in the tribal villages indicate the risk of JE transmission in the district. The geographic features and the environmental conditions such as prevailing temperature and rainfall also favors the spread of JEV in this district. Detection of JEV in Cx. vishnui during the 2016 outbreak in the study district further confirms that there would be a risk of transmission of JE during the favorable period. Since the district is endemic for falciparum malaria [10] and habitats such as rice fields and ponds support the breeding of the vectors of malaria and JE, integrated vector management (IVM) approach selecting appropriate insecticide needs to be adopted for the prevention/control of both the vector-borne diseases.
Funding Statement
The study was funded by National Vector Borne Disease Control Programme, Odisha.
Acknowledgments
We acknowledge the facilities and support provided by the Director, ICMR-Vector Control Research Center, Puducherry. The authors thank NVBDCP, Bhubaneswar, Government of Odisha for financing this study. We also thank the Health authorities of Malkangiri district for extending their cooperation for carrying out the study. The authors are highly grateful to Dr. K. Gunasekaran, Head of the Vector Biology and Control Division, ICMR-VCRC, Puducherry for his constant support and encouragement. We acknowledge the staff members of the ICMR-Vector Control Research Center, Field Station, Koraput, for their technical assistance in laboratory and field works.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Ghosh D, Basu A.. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl Trop Dis. 2009;3(9):e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alatoom A. An overview of arboviruses and bunyaviruses. Labmedicine. 2009;40(4):237–240. [Google Scholar]
- [3].Buescher EL. Arthropod-borne encephalitides in Japan and Southeast Asia. Am J Public Health Nations Health. 1956;46(5):597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carey DE, Myers RM, Pavri KM. Japanese encephalitis studies in Vellore, South India. II. Antibody response of patients. Indian J Med Res. 1968;56(9):1319–1329. [PubMed] [Google Scholar]
- [5].Kabilan L, Rajendran R, Arunachalam N, et al. Japanese encephalitis in India: an overview. Indian J Pediatr. 2004;71(7):609–615. [DOI] [PubMed] [Google Scholar]
- [6].Vrati S. Comparison of the genome sequences and the phylogenetic analyses of the GP78 and the Vellore P20778 isolates of Japanese encephalitis virus from India. J Biosci. 2000;25(3):257–262. [DOI] [PubMed] [Google Scholar]
- [7].Kumar A, Kumar R, Kaur J. Japanese encephalitis: medical emergency in India. Asian J Pharm Clin Res. 2012;5(3):9–12. [Google Scholar]
- [8].National Vector Borne Disease Control Programme. Japanese encephalitis vectors in India. National Vector Borne Disease Control Programme; 2018. Available from: https://www.nvbdcp.gov.in/WriteReadData/l892s/69175758181557490094.pdf.
- [9].Nayak P, Papanna M, Shrivastava A, et al. Unexplained neurological illness in children, Malkangiri district, Odisha, India 2014. 17th international congress on infectious diseases. Int J Infect Dis. 2016;45(S):305. [Google Scholar]
- [10].Sahu SS, Dash S, Sonia T, et al. Entomological investigation of Japanese encephalitis outbreak in Malkangiri district of Odisha State, India. Mem Inst Oswaldo Cruz Rio De Janeiro. 2018;113(6):e170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barraud PJ. The fauna of British India including Ceylon and Burma. Diptera. Vol. 5. Family Culicidae. Tribes Megarhinini and Culicini London: Taylor and Francis; 1934. p. 463. [Google Scholar]
- [12].World Health Organization. Manual on practical entomology in malaria. Part II. Geneva: World Health Organization; 1975. p. 15–38. Available from: https://apps.who.int/iris/bitstream/handle/10665/42481/WHO_OFFSET_13_%28part2%29.pdf?sequence=2&isAllowed=y. [Google Scholar]
- [13].Mani TR, Rao CVRM, Rajendran R, et al. Surveillance for Japanese encephalitis in villages near Madurai, Tamil Nadu, India. Trans R Soc Trop Med Hyg. 1991;85(2):287–291. [DOI] [PubMed] [Google Scholar]
- [14].Crans WJ. An agar- gel diffusion method for identification of mosquito blood meal. Mosq News. 1969;29(4):563–566. [Google Scholar]
- [15].Liang GD. Molecular epidemiological analysis of JEV in China. J Gen Virol. 2007;88(Pt3):885–894. [DOI] [PubMed] [Google Scholar]
- [16].Work TH, Shah KV. Serological diagnosis of Japanese B type of encephalitis in North Arcot district of Madras state, India, with epidemiological notes. Indian J Med Sci. 1956;10(8):582–586. [Google Scholar]
- [17].Vajpayee A, Mukherjee MK, Chakraborty AK, et al. Investigation of a outbreak of Japanese encephalitis in Rourkela city (Orissa) during 1989. J Commun Dis. 1991;23(1):18–21. [PubMed] [Google Scholar]
- [18].Dash AP, Chhotray GP, Mahapatra N, et al. Retrospective analysis of epidemiological investigation of Japanese encephalitis outbreak occurred in Rourkela, Orissa, India. Southeast Asian J Trop Med Public Health. 2001;32(1):137–139. [PubMed] [Google Scholar]
- [19].Dwibedi B, Mohapatra N, Rathorea SK, et al. An outbreak of Japanese encephalitis after two decades in Odisha, India. Indian J Med Res. 2015;142:30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Indian Council of Medical Research. Japanese encephalitis virus infection in mosquitoes and its epidemiological implications Indian council of medical research. ICMR bull. 2000;30(4). [Google Scholar]
- [21].Kanojia PC, Shetty PS, Geevarghese G. A long-term study on vector abundance & seasonal prevalence in relation to the occurrence of Japanese encephalitis in Gorakhpur district, Uttar Pradesh. Indian J Med Res. 2003;117:104–110. [PubMed] [Google Scholar]
- [22].Ramesh D, Muniaraj M, Samuel PP, et al. Seasonal abundance and role of predominant Japanese encephalitis vectors Culex tritaeniorhynchus & Cx. gelidus Theobald in Cuddalore district, Tamil Nadu. Indian J Med Res. 2015;142:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gajanana A, Rajendran R, Samuel PP, et al. Japanese encephalitis in South Arcot district, Tamil Nadu: a three-year longitudinal study of vector abundance and infection frequency. J Med Entomol. 1997;34:651–659. [DOI] [PubMed] [Google Scholar]
- [24].Geevarghese G, Mishra AC, Jacob PG, et al. Studies on the mosquito vectors of Japanese encephalitis virus in Mandya district, Karnataka, India. Southeast Asian J Trop Med Public Health. 1994;25:378–382. [PubMed] [Google Scholar]
- [25].Mishra AC, Jacob PG, Ramanujam S, et al. Mosquito vectors of Japanese encephalitis epidemic (1983) in Mandya district (India). Indian J Med Res. 1984;80:377–389. [PubMed] [Google Scholar]
- [26].Mariappan T, Samuel PP, Thenmozhi V, et al. Entomological investigations into an epidemic of Japanese encephalitis (JE) in northern districts of West Bengal, India (2011-2012). Indian J Med Res. 2014;139(5):754–761. [PMC free article] [PubMed] [Google Scholar]
- [27].Mourya DT, Ilkal MA, Mishra AC, et al. Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans R Soc Trop Med Hyg. 1989;83:550–552. [DOI] [PubMed] [Google Scholar]
- [28].Chakravarthy SK, Sarkar JK, Chakravarthy MS, et al. The first epidemic of Japanese encephalitis studied in India-Virological studies. Indian J of Med Res. 1973;63(1):77–82. [PubMed] [Google Scholar]
- [29].Dhanda V, Thenmozhi V, Kumar NP, et al. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala in 1996. Indian J of Med Res. 1997;106::4–6. [PubMed] [Google Scholar]
- [30].Banerjee K, Deshmukh PK, Ilkal MA, et al. Transmission of Japanese encephalitis virus by Culex bitaeniorhynchus Giles. Indian J of Med Res. 1978;67:889–893. [PubMed] [Google Scholar]
- [31].Soman RS, Rodrigues FM, Guttikar SN, et al. Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in ardeid birds. Indian J Med Res. 1977;66:709–718. [PubMed] [Google Scholar]
- [32].Thenmozhi V, Balaji T, Selvam A, et al. A longitudinal study on abundance and infection frequency of Japanese encephalitis vectors in Tirunelveli district, Tamil Nadu, India. Int J Mosq Res. 2015;2(3):166–169. [Google Scholar]
- [33].George S, George JP, Rao JA. Isolation of Japanese encephalitis and West Nile viruses from mosquitoes collected in Kolar district of Karnataka state during 1977-79. Indian J Med Res. 1987;85:235–238. [PubMed] [Google Scholar]
- [34].Thenmozhi V, Rajendran R, Ayanar K, et al. A long-term study on Japanese encephalitis virus infection in Anopheles subpictus in Cuddalore district, Tamil Nadu, South India. Trop Med Int Health. 2006;11(3):288–293. [DOI] [PubMed] [Google Scholar]
- [35].Sahu SS, Sonia T, Dash S, et al. Insecticide resistance status of three vectors of Japanese encephalitis in east central India. Med Vet Entomol. 2018;33(2):213–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- National Vector Borne Disease Control Programme. Japanese encephalitis vectors in India. National Vector Borne Disease Control Programme; 2018. Available from: https://www.nvbdcp.gov.in/WriteReadData/l892s/69175758181557490094.pdf.


