ABSTRACT
Mosquito-borne diseases, including arbovirus-related diseases, make up a large proportion of infectious disease cases worldwide, causing a serious global public health burden with over 700,000 deaths annually. Mosquito-borne arbovirus outbreaks can range from global to regional. In the East African Community (EAC) region, these viruses have caused a series of emerging and reemerging infectious disease outbreaks. Member states in the EAC share a lot in common including regional trade and transport, some of the factors highlighted to be the cause of mosquito-borne arbovirus disease outbreaks worldwide. In this review, characteristics of 24 mosquito-borne arboviruses indigenous to the EAC are reviewed, including lesser or poorly understood viruses, like Batai virus (BATV) and Ndumu virus (NDUV), which may escape their origins under perfect conditions to establish a foothold in new geographical locations. Factors that may influence the future spread of these viruses within the EAC are addressed. With the continued development observed in the EAC, strategies should be developed by the Community in improving mosquito and mosquito-borne arbovirus surveillance to prevent future outbreaks.
KEYWORDS: East African Community, mosquito-borne arboviruses, mosquito vector, public health, Africa
Graphical Abstract
Introduction
Globally, mosquitoes are regarded as a nuisance as they are perceived to cause more harm than good to humans. These very small vectors belong to the family Culicidae, which has approximately 3,500 mosquito species distributed in 41 genera [1]. Mosquitoes are vectors for a lot of disease pathogens which have been the cause of more than 700,000 deaths yearly across the globe [2]. Some of these deaths are caused by viruses of which mosquitoes harbor a great number. Viruses vectored by mosquitoes can be separated into two groups: mosquito-specific viruses and mosquito-borne viruses. This review will focus on the latter which in this context will be referred to as mosquito-borne arboviruses. Arboviruses are viruses that are transmitted by arthropod vectors like mosquitos, ticks, sandflies, midges, and insect bugs [3]. With the global distribution of arboviruses worldwide, some vectors and viruses are establishing a foothold in new geographical locations where they become endemic post initial spread [4]. The geographical distribution of mosquito-borne arboviruses can range from global to regional. Viruses like dengue (DENV), Chikungunya virus (CHIKV), and West Nile virus (WNV) can be located nearly everywhere in the world. While in South and Central Asia, mosquito-borne Japanese encephalitis virus is widespread, majorly because of factors promoting amplification of the virus in favorable hosts [4]. Mosquito-borne arboviruses replicate in the host mosquito immune system before being transmitted into an appropriate vertebrate cell. This transmission has led to a series of global emerging and reemerging infectious disease outbreaks [2]. Mosquito-borne arboviruses pathogenic to humans can be classified into one of the following families: Togaviridae, Bunyaviridae, and Flaviviridae [4]. Each of these families is made up of diverse genera that contain many different viruses. These viruses can cause a wide range of human diseases like dengue fever, chikungunya virus disease, yellow fever (YF), Rift Valley fever (RVF), and Zika virus (ZIKV). This review will focus on mosquito-borne arboviruses that can cause disease in humans within the East African Community (EAC).
The East African Community is an intergovernmental organization made up of six countries; namely Kenya, Uganda, Tanzania, Rwanda, Burundi, and South Sudan [5–7]. Mosquito-borne arbovirus research completed by both governmental and non-governmental research institutes in these countries has been published online. However, no paper has tried to review the common viruses within these countries that share a common language and passport which allows them freedom of transport and trade; some of the factors that have been highlighted to be the cause of serious emerging and reemerging infectious diseases [8–10]. This review will provide a synoptic overview of medically important mosquito-borne arboviruses present within the EAC countries, and also highlight a few factors that could contribute to the future spread of these viruses within the EAC.
The East African community partner states
The East African Community (EAC) was first established by Kenya, Uganda, and Tanzania in 1967 and dissolved by the three states a decade later (1977). In the year 2000, the three founder states reconstituted the EAC; Burundi and Rwanda joined later in 2007 [6,7,11], which made South Sudan the sixth and final member joining the EAC in 2016 [12–14]. The six EAC member states are governed by a governing institutional structure, five specialized institutions, and several other commissions as highlighted by Ogolla et.al [7]. The Great Lakes Region that includes Kenya, Uganda, Tanzania, Rwanda, and Burundi cover a total surface area of 1,817.7 thousand square kilometers; however, the EAC member states vary in size [6,7] as shown in Figure 1. Tanzania is the largest of the member states and also home to the headquarters of the East African Community. In this review, some of the viruses are stated to have been isolated in Sudan and not South Sudan. This is majorly because South Sudan used to be part of Sudan until 9 July 2011, when South Sudan (currently the Republic of South Sudan) gained independence and became Africa’s 54th country [15,16].
Figure 1.
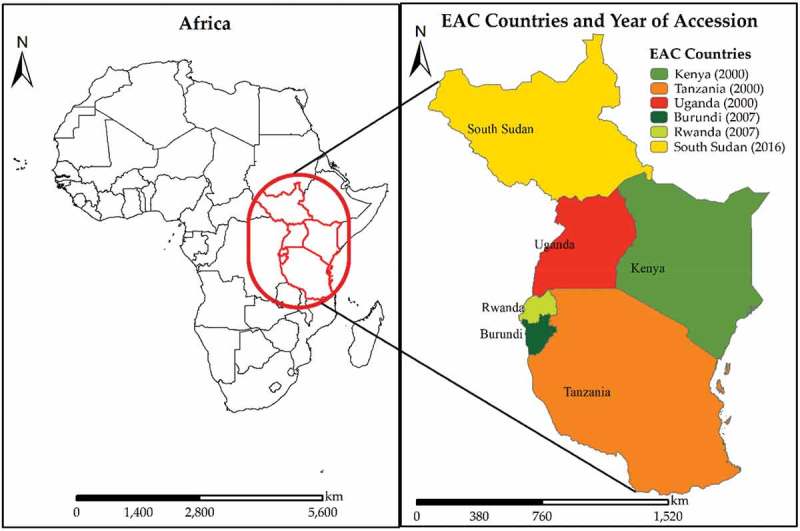
Location of the EAC in Africa and the countries that make up the Community. The legend shows the country names and their year of accession into the EAC in bracket.
An overview of medically important mosquito-borne arboviruses in the EAC
Mosquito-borne arboviruses of human significance in the EAC can be grossly summarized into three families, the Bunyaviridae (whose primary genus is Orthobunyavirus with a few other important families like the Phlebovirus), Togaviridae (composed of the genus Alphavirus), and the Flaviviridae (genus Flavivirus). An overview of known mosquito-borne arboviruses in these families is summarized below. Table 1 provides a summary of individual viruses including lesser or poorly understood viruses, their characteristics, and location in the EAC. The arrangement of this overview will be alphabetical similar to that of Table 1.
Table 1.
Summary of some characteristics of mosquito-borne arboviruses within the EAC.
| Family/Genus | Virus | Known mosquito host species | Location in the EAC a | Disease symptoms in humans | Known/Likely amplifying host | Reference |
|---|---|---|---|---|---|---|
| Bunyaviridae | ||||||
| Orthobunyavirus | Batai (BATV) | Aedes simpsoni, Culex gelidus, Anopheles barbirostris, An. maculipennis, Aedes punctor | Uganda, Sudan | Flu-like illness | N/A | [50,58,59,242] |
| Bunyamwera (BUNV) | Ae. circumluteolus, Ae. mcintoshi, An. funestus | Kenya, Uganda | Fever, headache, joint pain, rash | Geese and other water birds | [4,32,58,88] | |
| Bwamba (BWAV) | Ae. furcifer, An. coustani, An. funestus, An. gambiae, Mansonia uniformis | Kenya, Rwanda, Tanzania, Uganda | Fever, headache, backache, fatal hemorrhagic complications | N/A | [36–40,46,78,88] | |
| Germiston (GERV) | Cx. rubinotus | Uganda | Fever, headache, lower back pain | N/A | [4,188] | |
| Ilesha (ILEV) | An. Gambiae | Uganda | Fever, headache, myalgia, fatal meningo-encephalitis | N/A | [4,17,18] | |
| Ngari (NRIV) | Ae. mcintoshi, Ae. simpsoni, An. funestus | Kenya, Sudan, Tanzania | Fatal hemorrhagic fever | N/A | [32,49,58] | |
| Nyando (NDV) | An. funestus | Kenya, Uganda | Moderate to severe febrile illness | N/A | [19,20] | |
| Pongola (PGAV) | Ae. circumluteolus, Ae. mcintoshi | Uganda, Kenya | Fever, headache, joint pains | N/A | [21,133] | |
| Witwatersrand (WITV) | Cx. robinotus | Uganda | N/A | Birds suspected | [4,188] | |
| Phlebovirus | Rift Valley fever (RVFV) | Ae. aegypti, Ae. caballus, Ae. cumminsii, Ae. circumluteolus, Ae. dentatus, Ae. juppi, Ae. mcintoshi, Ae. ochraceus, Ae. pembaensis, An. squamosus, Cx. bitaeniorhyncus, Cx. quinquefasciatus, Cx. poicilipes, Cx. theileri, Cx. univittatus, Cx. zombaensis, Eretmapodites quinquevittatus, Mansonia africana, Mansonia uniformis | Kenya, Rwanda, Tanzania, Uganda, South Sudan. | Mild febrile illness with malaise, headache, myalgia, nausea, severe symptoms can be fatal | Cattle, Sheep, goats | [4,22–24,69,70,75] |
| Flaviviridae | ||||||
| Flavivirus | Banzi (BANV) | Cx. rubinotus | Kenya, Tanzania | Febrile illness | Rodents likely | [85,88,90,218] |
| Dengue (DENV) | Ae. aegypti, Ae. albopictus | Kenya, Rwanda, Sudan, Tanzania, Uganda | Arthlagia, fever, severe headache, retro-orbital pain, myalagia, and maculopapular rash. For DHF, bleeding, high fever, persistent vomiting, and intense abdominal pains | Originally non-human primates, but currently humans | [98,100,108,111,113,114,201] | |
| Ntaya (NTAV) | Culex spp. | Kenya, Uganda | Headache, fever, rigors, myalgia | N/A | [121–123] | |
| Uganda S (UGSV) | Ae. longipalpis | Uganda | N/A | N/A | [92,93] | |
| Usutu (USUV) | Ae. albopictus, Ae. caspius, An. maculipennis, Coquellittidia aurites, Cx. neavei, Cx. perexiguus, Cx. perfuscus, Cx. quinquefasciatus, Mansonia africana, Cx. pipiens, Cx. univittatus, | Kenya, Uganda | Fever, rash, jaundice, neuroinvassion | Birds, horses, and possibly bats | [4,130,132] | |
| West Nile (WNV) | Cx. modestus, Cx. neavei, Cx. perexiguus, Cx. pipiens, Cx. quinquefasciatus, Cx. univittatus, Cx. theileri | Kenya, Rwanda, South Sudan, Tanzania, Uganda | Febrile illness associated with fever, headache, rash, muscle pains, myalgia, joint pains and arthralgia; severe infections may result in paralysis, seizures, or cerebellar ataxia that can cause neurological impairment | Birds | [142–150] | |
| Yellow fever (YFV) | Ae. Aegypti, Ae. africanus, Ae. albopictus, Ae. furcifer/taylori, Ae. leucocelaenus, Ae. luteocephalus, Ae. metallicus, Ae. opok, Ae. simpsoni Complex, Ae. vittatus, Haemagogus capricorni, Haemagogus equines, Haemagogus janthinomys, Haemagogus leucocelanus, Haemagogus mesodentatus, Haemagogus spegazzinii, Sabethes chloropterus | Burundi, Kenya, Rwanda, South Sudan, Uganda | Mild symptoms are nonspecific and include fever, headache and constitutional problems. Severe cases include, fever, headache, lower back pain, chills, malaise, nausea, generalized myalgia, and dizziness, often manifesting Faget’s sign (increasing temperature with decreasing pulse rate) | Monkeys, chimpanzees, mangabey, baboons, bush babies, capuchin, marmosets, spiders, howler, and possibly hedgehogs | [4,8,153,156–158,160,163,165,167] | |
| Zika (ZIKV) | Ae. aegypti, Ae. africanus, Ae. albopictus, Ae. furcifer, Ae. jamoti, Ae. opok, Ae. flavicollis, Ae. grahami, Ae. taeniorostris, Ae. tarsalis, Ae. vittatus, Ae. dalziella, Ae. fowleri, Ae. luteocephalus, Ae. metallicus, Ae. minimus, Ae. neoafricanus, An. gambiae, Eretmapodites inornatus, Eretmapodites quinquevittatus, Mansonia uniformis | Burundi, Kenya, Rwanda, Sudan, Tanzania, Uganda | Common symptoms include, arthralgia, myalgia, fever, rash, fatigue, edema, conjunctivitis, and headache | Different species on non-human primates | [4,25,26,149,174,177–179] | |
| Togaviridae | ||||||
| Alphavirus | Babanki (BBKV) | Ae. africanus, Ae. mcintoshi, Ae. ochraceus, Ae. simpsoni, Culex spp. | Kenya, Uganda | Febrile illness accompanied by rash and arthritis | N/A | [55,133,188,189] |
| Chikungunya (CHIKV) | Ae. aegypti, Ae. africanus, Ae. albopictus, Ae. cordellieri, Ae. furcifer, Ae. fulgens, Ae. luteocephalus, Ae. neoafricanus, Ae. taylori, Ae. vittatus | Burundi, Kenya, South Sudan, Tanzania, Uganda | Common symptoms: fever, headache, rash, arthralgia, and myalgia. Less common symptoms: vomiting, diarrhea, hemorrhage, and inflammation of the ear and ocular disease. Severe manifestations may be fatal and include, myocarditis, neurological disease and multiorgan failure. |
Non-human primates, humans | [115,149,192,195–197,200–202] | |
| Ndumu (NDUV) | Ae. circumluteolus, Ae. mcintoshi, Ae. ochraceus, Ae. sudanensis Ae. tricholabis, Cx. rubinotus, Mansonia uniformis | Kenya, Uganda | N/A | N/A | [133,214,219] | |
| O’nyong-nyong (ONNV) | An. funestus, An. gambiae, Mansonia uniformis | Kenya, Sudan, Tanzania, Uganda, | Acute fever, joint pains, skin rash, lymphadenopathy, immobilization for up to 4 days, arthralgia | N/A | [220–222,226] | |
| Semliki Forest (SFV) | Ae. abnormalis-group, Ae. argenteopunctatus | Uganda | Mild febrile illness, encephalitis | N/A | [229,230,232,233] | |
| Sindbis (SINV) | Cx. univittatus, Cx. neavei, Cx. pipiens, Cx. torrentium, Culiseta morsitans, Coquillettidia fuscopennata, Mansonia fuscopennata | Kenya, Uganda | Febrile illness, fever, arthritis, maculo-papular rash | Birds | [4,236,237,244] |
aViruses with Sudan as a country were islolated before South Sudan gained independence in 9 July 2011
Bunyaviridae
The family name Bunyaviridae originated from Bunyamwera in Western Uganda, which is one of the countries in the EAC [27]. Viruses in the family Bunyaviridae are the largest RNA viruses with three distinct single-stranded, linear, negative or ambisense RNA [28]. This family is composed of more than 300 named member viruses distributed into five genera: Phlebovirus, Nairovirus, Orthobunyavirus, Hantavirus and Tospovirus. Majority of Bunyaviridae viruses are arboviruses and they have been documented to be the cause of new or emerging viral diseases. Apart from hantaviruses which are spread by specific rodents, arthropod-borne viruses are transmitted by a variety of vectors including midges, biting flies, ticks, or specific mosquitoes [27,29]. Mosquito-borne arboviruses in this family that are of medical significance can be found in the genus Orthobunyavirus and Phlebovirus.
Viruses in the genus orthobunyavirus (bunyaviridae)
The majority of mosquito-borne viruses in this Bunyaviridae family that are of medical importance occur in the genus Orthobunyavirus and are mostly transmitted by Culex mosquitoes. Other mosquito species like Aedes and Anopheles constitute an important group of vectors in the genus [4]. Viruses in this genus include:
Bunyamwera virus (BUNV)
Bunyamwera virus was first isolated in 1943 from the Aedes mosquito species found in an uninhabited area of Semliki Forest in Western Uganda known as Bunyamwera. During this time there were more than 20 known arboviruses including the Yellow fever virus which were unrelated to the virus, hence it was named Bunyamwera [30,31]. The virus has spread across many regions over the past decades and remains an important virus due to its ability to cause human diseases. Symptoms of human infections with BUNV include mild fever with rash [31]. A study in Kenya showed the presence of the virus still in circulation in the Northern part of the country [32]. In 1955 a different strain of the virus was isolated from Aedes circumluteolus mosquitoes in the northern coastal area of KwaZulu-Natal Province in South Africa [33]. Subsequent isolation of this virus in the same locality of South Africa from a patient who was an adult mosquito catcher presenting severe headache, fever and stiffness followed [34], including another study in the same KwaZulu-Natal Province resulting in 54% seropositivity in adult humans [35].
Bwamba virus (BWAV)
Bwamba virus was first isolated in the 1930s by Smithburn et.al. from 9 febrile road construction workers working in the Bwamba Forest located in Uganda [36–38]. The Ugandan patients presented a rapid onset of the virus including symptoms like fever, headache and backache. It was also reported that no fatalities were recorded at the time, with the symptoms persisting for 5–7 days and later clearing without sequelae [39]. Human fatalities have however been recently reported to occur on Rwandan refugees, with the affected victims showing hemorrhagic complications (bleeding from the oral mucosa into the gastrointestinal tract) that suggest the virus to be a cause of severe disease manifestations [38,40]. Another study has also reported that BWAV is fatal to mice when injected intra-cerebrally and not intra-peritoneally [36]. BWAV virus has also been isolated from various regions within the EAC and beyond. African countries that have been reported to have the virus include: Kenya, Tanzania [39], Nigeria [37,41], Cameroon, South Africa [42], Mozambique [43], and Central African Republic [44]. BWAV is spread by mosquito vectors, primarily by Anopheles funestus and Anopheles gambiae [45]. Other mosquito vectors that spread BWAV include; Mansonia uniformis, Anopheles funestus, Anopheles gambiae, Aedes furcifer, Anopheles coustani, and Aedes (Neomelanoconion) spp [38,46,47].
Ngari virus (NRIV) and Batai virus (BATV)
The first cases of both isolation of the NRIV virus in humans (1993), and the vector Aedes simpsoni (1979) were in Senegal [48,49]. In the following years (1997 and 1998), large-scale outbreaks of the virus have occurred in the East African countries including Kenya, Tanzania and Somalia, with the outbreaks being incorrectly described by other publications to be closely related to Batai virus (BATV) [32,50]. Studies on NRIV genome have classified the virus to be a reassortment between Bunyamwera virus and Batai virus. NRIV virus genome is composed of 3 segments; two of which are believed to originate from BUNV (S and L segments), and one from BATV (M segment) [50,51]. NRIV has been said to be more virulent than BUNV and BATV even though studies have said it is a reassortment originated from the two viruses [49].
BATV has been said to be one of the most widespread orthobunyaviruses with initial isolations and serological evidence across the globe in various countries like Russia [52], India [53], Slovakia [54], South-East Asia [55], and also in the East African countries [50]. In Africa, serological evidence of viruses closely related to BATV was documented in Uganda in 1967 [56], and 1988 in Sudan [57]. From this, scientists wanted to answer the question as to if the M segment of the Sudanese isolate KV-141 and the Ugandan isolate UgMP-6830 closely matched that of the Kenyan NRIV than those of BATV available in other distant geographic locations. Sequence analysis results revealed that KV- 141 and UgMP-6830 M segments were closely related to the Kenyan NRIV. Partial S and L segment sequence of KV-141 (Sudan) were also shown to be identical to that of NRIV (Kenya). From these studies, it was concluded that the Batai-related virus isolated from Ugandan mosquitoes [56] are real isolates of BATV, unlike the isolates from the 1988 Sudan febrile disease outbreak which were actually NRIV [50]. BATV causes a mid-flu like illness in humans while NRIV has been associated with fatal hemorrhagic fever in humans [58]. Mosquito vectors where BATV has been isolated from include Aedes simpsoni, Culex gelidus, Anopheles barbirostris, A. maculipennis and Aedes punctor [58].
From the 1988 Sudan outbreak, a total of 77,500 patient cases were recorded with 18% of the cases being diagnosed as malaria, a small portion of 7% were reported to be due to NRIV after testing 195 sera serologically [4,59]. Following the outbreak in Kenya and Somalia, which is estimated to have affected about 89,000 people in the year 1997–1998, a total of about 250 deaths were recorded. This outbreak was considered to be a Rift Valley fever (RVF) outbreak; however, some patients recorded hemorrhagic fever and further investigation on these cases reported that 27% of the cases had evidence of acute NRIV [50,51].
Viruses within the EAC in this genus with less information online are summarized in Table 1.
Viruses in the genus phlebovirus (Bunyaviridae)
The genus Phlebovirus consists of 38 distinct viruses that show varying degrees of cross-reactivity using standard serological tests [60]. Arboviruses in this genus can taxonomically be divided into; dipteran- and tick-borne arboviruses. Phlebotomus sandflies account for the majority of dipteran-borne phleboviruses, with the Rift Valley fever virus (RVFV) representing an outlier due to its associations with mosquito species [60–62].
Rift Valley fever virus (RVFV)
The first description of Rift Valley fever (RVF) was done in 1931 by Daubney [63] and his colleagues following an outbreak that caused sudden deaths and abortions in sheep located along the shores of Lake Naivasha in the Great Rift Valley of Kenya [63]. Daubney also later stated that there might have been a possibility of much earlier outbreaks of RVFV occurring in the region that may have been undocumented due to various reasons [64]. Other papers have also highlighted that RVFV might have been existent from as early as 1911, with some speculations as to whether the virus would have been the fifth plague documented biblically in the book of Exodus which described an epizootic disease affecting livestock [65–67]. The geographical distribution of the virus has grown since then to include many countries in Africa and beyond [68]. RVF disease is an enzootic and epizoonotic disease that mainly affects animals through mosquito-borne transmission cycles. Human beings may be affected indirectly during associations with infected animals especially during outbreaks [66]. In the EAC, RVFV was first reported in Kenya in the 1930s [63]. Since then, the virus has sparked a lot of concern in regards to human and animal health with outbreaks occurring in different EAC countries. In the years 1997–1998, a large outbreak occurred in the northeastern part of Kenya following the El Niño rains and heavy flooding that resulted in many deaths of both humans and livestock [69]. During this period, human infections were majorly linked to contact with livestock and animal body fluids [70]. No outbreak was recorded again in the EAC until the 2006–2007 outbreak. In the 2006–2007 outbreak that occurred in Kenya, Somalia, Tanzania, and Sudan, also a large number of humans and animals were affected [71,72]. In other EAC countries like Uganda, large-scale outbreaks of RVF have not been reported since 1968, whereby 7 cases were recorded in Entebbe Uganda [73]. From then, it was only in March 2016 that 2 acute human RVF cases were reported in Kabale district located in southwestern parts of Uganda [74]. A Serological study done in livestock in Uganda near the Rwandan border provided evidence of a possible spillover of the virus between the two countries due to livestock exchange in this transportation corridor [70]. A serosurvey of circulating RVFV done in livestock located in Rwandan borders showed 16.8% seropositivity, with high seropositivity being observed in districts close to the Tanzanian border than in the 2 districts near the Ugandan border [75]. In East Africa, the important mosquito vectors that are responsible for the transmission of RVF include Aedes ochraceus, Aedes mcintoshi, Aedes dalzieli, Culex pipiens, and Aedes vexans [76]. Detection of infected vector species A. ochraeus in Garissa, Kenya, represented a new RVFV-vector in the EAC [77]. The vector A. ochraeus is known to be distributed in West Africa [78], with documentations in Somalia and Sudan which are neighboring countries of Kenya [79,80]. The different mosquito species have been suggested to be the cause of complex epidemiological RVFV patterns in East Africa [81].
Flaviviridae
Africa has been suggested to be the ancestral origin of all mosquito and tick-borne flaviviruses based on phylogenetic classification trees [82,83]. Members of the Flavivirus genus are globally distributed and composed of many species that have been taxonomically identified (currently there are more than 50, with more than 40 human pathogens) [10]. Viruses in the genus Flavivirus can be divided into four ecological groups that include two mosquito-borne groups, a tick-borne group, and a group of no-known vectors or non-vectored flaviviruses [83,84]. Pathogenic mosquito or tick-borne flaviviruses can cause an array of clinical diseases with disease symptoms including rash, mild/severe febrile illness, and in some cases encephalitis or hemorrhagic disease [10]. Mosquito-borne arboviruses of medical significance in this family include:
Banzi virus (BANV) and Uganda S virus (UGSV)
Banzi virus was first isolated from a 9-year-old boy with febrile illness in Tongaland, South Africa in 1956 [85]. Subsequent isolation of the virus in Tanzania, Kenya and many southern African countries followed [85–88]. Serological studies have shown that BANV is closely related to the Uganda S and Yellow fever virus with the virus being classified as belonging to the Uganda S serocomplex [89]. The primary vector believed to spread the virus is Culex rubinotus [87,90], with rodents being said to be the virus’ natural host [91].
Just like in the case of BANV little is known about the epidemics of Uganda S virus. The first case of isolation of the virus was in Uganda from a pool of wild-caught Aedes mosquitoes [92]. 5.8% of the total 121 human sera screened by neutralization tests demonstrated antibodies to Uganda S virus with only a single serum out of 6 testing positive for UGSV in wild monkeys. UGSV virus is highly neurotropic for mice only and no clinical signs and symptoms are produced by other laboratory animals [92]. Boorman et.al. highlighted a study done previously that showed the presence of UGSV in Ilobi, a village 30 miles to the north of Lagos, Nigeria [93]. The study suggested that human infections with the virus were not uncommon. Boorman et.al [93]. also demonstrated that under experimental conditions UGSV can last in the vector Ae. aegypti for a period of up to 79 days and the vector could transmit the virus by biting after only a short incubation time [93].
Dengue virus (DENV)
Literature citing the history of Dengue suggests that the virus may have existed for centuries [94]. The virus is believed to have existed as early as the Chin Dynasty (AD 265 to 420) where reports of some illness similar to dengue fever were recorded in a Chinese ‘encyclopedia of disease symptoms and remedies’ [95]. The records were later edited through the following dynasties; Tang Dynasty (AD 610), and the Northern Sung Dynasty (AD 992) [96]. In the following centuries and decades, the virus was believed to have spread across the globe especially during migration, trade and urbanization activities [95]. However blurred the history of Dengue is, the first epidemics were said to have occurred across three continents: Asia, Africa and North America between the period 1779–1780 [97]. In Africa, Dengue was reported in the late 19th and early 20th centuries [98] in various countries including Zanzibar (1823, 1870), Egypt (1887, 1927), and Senegal (1927–1928) [97–99]. DENV is composed of four serotypes (DENV 1–4) that have antigenic cross-reactivity. Infection from one of the serotypes does not guarantee cross-protection from other serotypes [100], this means that people living in a DENV endemic region can get infected with all four serotypes in a lifetime [97]. DENV can be transmitted among humans through mosquito vectors Ae. aegypti and Ae. albopictus, with the former being mostly found in urban settings while the latter being located in peri-urban and rural locations [101–104]. Worldwide 50–100 million cases of dengue fever and 250–500,000 cases of DHF are reported annually [94]. Dengue can cause more illness and death than other human arbovirus diseases [105]. Patients suffering from DF may show symptoms which include arthralgia, fever, severe headache, retro-orbital pain, myalgia, and maculopapular rash. For DHF, bleeding, high fever, persistent vomiting, and intense abdominal pains, which may deteriorate to failure of the circulatory system and altered mental status are common symptoms [4].
In the EAC, the first four outbreaks occurred in Tanzania in the period between 1823 and 1926 [106], with the 1826 and 1870 outbreaks being considered more of a Chikungunya virus outbreak [107,108]. The 1870 dengue pandemic started in Tanzania and spread toward various countries including Egypt, India, Saudi Arabia, Yemen, Indonesia and China [109]. Between 1952–1953 another dengue outbreak was reported at the Makonde Plateau of Tanzania [110,111]. In Kenya, the first dengue outbreak was reported in Malindi and Kilifi in the years 1860 to 1868 [106], with the first laboratory-confirmed dengue outbreak being reported in 1982 around the same coastal towns of Malindi and Kilifi [112]. In 1985–1986, outbreaks were reported in the Eastern part of Port Sudan city involving 17 cases [113]. In 2004–2005 DENV-3 reemerged again in the same port with 312 DHF cases being reported and a total of 12 lives lost. The virus later spread to the Northern parts of Kenya that border Sudan [114]. In 2010, reemergence of Dengue occurred in two countries with reports of 100 cases in Tanzania [115] and 3,765 cases in Sudan (Port Sudan city) [116]. In 2011, another outbreak occurred also in South Kordofan (Sudan) which involved 299 cases resulting in 71 deaths [100]. Interestingly also in May and October 2011, an outbreak was confirmed in Somalia (Mogadishu) among African Union Mission Soldiers (AMISON) working there [100,117]. Somalia borders Mandera town (located in the Northern parts of Kenya) where dengue positive virus samples were reported by the Kenyan Ministry of Health (MoH) [112]. More cases of Dengue were reported in 2013 in Tanzania [118]. In the same year, cases were reported in Mandera/Wajir (Kenya), with the outbreak later spreading to the coastal region of Kenya (Mombasa town) [119,120]. Dengue presence has been reported in 34 countries across Africa including all countries in the East African Community with no data available for Burundi [4,98].
Ntaya virus (NTAV)
Ntaya virus was originally isolated in 1951 from mice inoculated intracerebrally with a mixed pool of mosquitoes captured at Ntaya swamp located in Western Uganda [121]. Twenty different mosquito species were identified in the pool of 1,318 mosquitoes collected, and of the 20 species, 1,284 belonged to the Culex genus [4,121]. At this time, all 43 sera tested from persons living near the Ntaya swamp tested negative for neutralization of the virus [121]. This meant that the natural host for the virus remained unknown. Antibodies against the virus have been found in human sera in various African countries, specifically Kenya, Uganda, Cameroon, Central African Republic and Nigeria [122]. NTAV symptoms have been associated with headache, fever, rigors and myalgia [122]. A study of the full genome sequence of NTAV revealed molecular characteristics of the virus that can help in the development of molecular diagnostic tests [123]. According to a study in Romania, NTAV can also be found in migratory birds and domestic animals (1.6% to 13.9% in cattle, sheep, goats, pigs) [124–126].
Usutu virus (USUV)
Usutu virus was first isolated in Natal (South Africa) from a female adult Culex neavie mosquito in January 1959 [127]. The virus has been shown to infect birds across Europe and Africa mostly leading to the death of some bird species with observed symptoms like: neural necrosis, hepatosplenomegaly, and necrotic changes of the liver, spleen, and heart [128]. African birds have been said to show some level of resistance to the virus compared to the European species, with less mortality rates compared to those located in Europe [129]. Phylogenetic analysis shows the possible distribution of the virus by migratory birds via various routes connecting to Europe; an east Atlantic pathway linking Africa to Western Europe (Spain), a Black Sea/Mediterranean route to central Europe, and East Africa/West Asian route [4]. Human infections of the virus have been documented both in Africa and Europe [130,131]. The two patients of Europe were both immunocompromised showing the virus’ ability to infect immunocompromised humans [132]. Two human cases were also reported in Africa with very little clinical information. The first case was reported in 1981 from a patient in the Central African Republic who had fever and rash, the second case was reported in Burkina Faso from a 10-year-old patient with symptoms of fever and jaundice in 2004 [130]. The geographical distribution of USUV shows the virus to be located in most African countries including countries in East Africa like Kenya, Uganda and Tunisia [130]. In the EAC, USUV has been isolated in Kenya in a study done between 2007–2012 from Culex pipens [133], and in Uganda from Coquillettidia azurites in 1962 [134] and Culex sp in 1968 [73]. More seroprevalence studies should be done across the EAC and Africa to acquire more data on infections and epidemics of USUV.
West Nile virus (WNV)
In 1937 WNV was isolated from one sample taken from a febrile 37-year-old female patient from Omogo, West Nile district in the Northern Province of Uganda [135]. Multiple lineages of WNV have been identified based on sequencing, with the two major lineages 1 and 2 being identified in Africa following the first isolation of WNV in Uganda [136,137]. Since this first isolation in Uganda, the virus has spread globally and some highlight that the virus’ introduction, re-introduction, and spread across Europe [138,139] and the Americas [140,141] was majorly aided by migratory birds. WNV is a mosquito-borne arbovirus that can affect both humans and animals, with ticks also being implicated to be a vector of WNV even though their importance has not been established [137]. Human WNV infections develop symptoms of febrile illness associated with fever, headache, rash, muscle pains, myalgia, joint pains and arthralgia; severe infections may result in paralysis, seizures, or cerebellar ataxia that can cause neurological impairment [142–144]. In the EAC the only case of WNV isolation was reported in Uganda, the other countries only document serological evidence of the virus from various mosquito species and humans. The virus was serologically identified from a male Cx. univittatus mosquito for the first time in Kenya during a fieldwork investigation, which also suggested the vertical transmission nature of WNV to humans [145]. In Rwanda, the first evidence of WNV was reported by Seruyange et.al [146] in 2017 when they screened 874 serum samples from human blood donors in Rwanda using commercially available kits. In the study, 10.4% of the sera tested positive for virus-specific immunoglobulin G antibodies [146]. The virus has also been serologically detected in human sera in Tanzania [147]. Serological detection has been reported in South Sudan [148–150], with also no available data of the virus in Burundi [137].
Yellow fever virus (YFV)
The virus yellow fever derived its name from the human clinical characteristics of jaundice (typical yellowing of the skin) associated with liver dysfunction [151]. Historically, the virus is believed to have originated in Africa and later spread to other regions of the world including Europe and the Americas via slave trade [8,152]. A review on the virus evolution done by Mutebi et.al [153] highlighted that the nucleotide sequence and phylogenetic analysis of YFV may place the virus’ origin in East and Central Africa, extending to West Africa, and finally transported from West Africa to South America [153]. The disease yellow fever (YF) caused by YFV is primarily transmitted to humans by mosquitoes [154]. The transmission cycle of YFV shows that the virus can infect both humans and non-human primates in tropical regions of Africa and the Americas. This cycle occurs between forest mosquitoes and wild primates, with secondary human transmission occurring in three cycles involving sylvatic, intermediate, and urban cycles [155,156]. The sylvatic transmission cycle in Africa is aided primarily by the vectors Ae. metallicus, Aedes africanus, Ae. furcifer/taylori, Ae. opok, Ae. luteocephalus, and Ae. vittatus [153,157,158]. The African sylvatic hosts involve a variety of primates including monkeys, baboons, mangabey, hedgehogs, chimpanzees, bush babies, and possibly hedgehogs [4]. The urban cycle has been highlighted to be the deadliest of the three cycles occurring in Africa due to human to human transmission via the mosquito Ae. aegypti [153] which is located in most African countries. YF is still a major public health problem globally, with 90% of the total cases of infection occurring in Sub-Saharan Africa [153,159], where it is approximated that 51,000 to 380,000 cases occur yearly with about 19,000 to 180,000 deaths [160,161]. Symptoms of the disease have been reviewed extensively in many papers [151,156,160,162,163]. These symptoms range from mild to severe with mild symptoms often being nonspecific, manifested as fever, headache and constitutional problems. In such scenarios, patients recover after a few days with no further sequelae. In severe cases, however, patients show symptoms of fever, headache, lower back pain, chills, malaise, nausea, generalized myalgia, and dizziness, often manifesting Faget’s sign (increasing temperature with decreasing pulse rate) [156]. In East Africa and the EAC, the disease is endemically maintained in a monkey- Ae. (Stegomyia) africanus jungle transmission cycle and can periodically be seen emerging in intermediate sylvatic or urban cycles that involve human to human transmissions [162]. Evidence of YF in East Africa was discovered in the mid-1930s where no known outbreaks or human diseases of YF were known, and since this time many outbreaks have been reported in East Africa [164]. The transmission of YFV by Ae. (Stegomyia) africanus cycle was first described in an outbreak in Uganda in the year 1948 and since then the virus has been seen to circulate in forests all over Africa [163]. In the EAC, outbreaks of YF have been reported in Kenya [165], Uganda [163], and South Sudan [166,167] with little evidence of the virus being reported in Rwanda and Burundi [4]. Even though the greatest taxonomic diversity of YF vectors are located in East and Central Africa, it is difficult to establish which mosquito species are YF-competent vectors in nature [168].
Zika virus (ZIKV)
Zika virus was first isolated in the year 1947 from a febrile rhesus macaque monkey, and later from Aedes africanus mosquitoes both located in Zika Forest of Uganda [169]. In 1954, the first 3 reported human cases occurred in Nigeria [170] with the virus spreading to other countries in Africa, Asia, and Oceania [171]. Phylogenetic analysis of Zika virus show distinct African and Asian lineages: East African lineage (MR766 prototype cluster), West African (Nigerian cluster), and Asian lineages [172]. All the lineages of ZIKV are believed to have emerged from the East African lineage in the late 1800s or early 1900s [173]. ZIKV is primarily transmitted by the Aedes mosquito species. The virus can be spread by 17 different Aedes mosquito species including the malaria vector Anopheles gambiae, two Eretmapodites species, and Mansonia uniformis mosquitoes [174]. Approximately 80% of ZIKV infections are asymptomatic [175,176]. If symptoms occur they can be mild, self-limiting, and nonspecific common to other arbovirus infections [171]. The common symptoms include arthralgia, myalgia, fever, rash, fatigue, edema, conjunctivitis, and headache [177,178]. Severe cases are often rare and need hospitalization with fewer fatalities [4].
In the EAC no outbreak of the virus has been reported. Only serological investigations have been reported in the EAC countries including cases of isolation of the virus in human sera. A review on the epidemiology of Zika virus 1947–2007 reported by Posen et.al [179] highlights the percentage of sera in many countries including countries in the EAC. In this review, the percentage sera in Kenya was 14.9% (3719 sera tested), Tanzania 12.7% (1063 sera tested), Uganda 6.3% (4236 sera tested), Burundi 1.4% (623 sera tested), and Sudan 0% (109 sera tested) [179]. Human infections were also reviewed and in the EAC, an incident was reported in Burundi between 1980–1982 [180], 4 article reports in Kenya between 1961–1962 and 1966–1969, 3 article reports in Tanzania between 1945–1948, 1961–1962 and 1967–1969, a single report in Sudan in 1976, and eight article reports in Uganda between 1945–1948, 1961–1962, 1967–1969, 1970, and 1984 that can be seen in the review by Posen et.al [179]. The only evidence seen online about ZIKV in Rwanda was highlighted in a conference proceeding on December 14–15, 2017 by Seruyange et.al whereby 874 viral-specific immunoglobulin G from blood donors were screened using commercial ELISA kits [146]. From this study, the seroprevalence of ZIKV was said to be low at 1.4% despite the virus being isolated in its neighboring countries. A review written by Anna et.al also highlights the virus’ presence in the EAC member states [171].
Togaviridae
The term Togavirus was first suggested by Lwoff and Tournier to be used in the classification of enveloped RNA viruses having nucleocapsids with cubical symmetry [181]. At this time the term was not formally accepted [182]. Following the use of the term as a jargon name describing group A and group B arboviruses [183], the group proposed the family name Togaviridae in 1978 which became formally accepted and approved [184–186]. Initially, the Togaviridae group contained two genera: Alphavirus (from group A arboviruses) and Flavivirus (from the group B arboviruses), and later on two more genera were approved: Rubivirus and Pestivirus [185] yielding a total of 4 genera. From that period until now the classification of the virus has changed. Currently, only two genera of the virus are maintained and accepted: Alphavirus and Rubivirus. The genus Rubivirus has a single species which is the Rubella virus that is of less importance in this review since it is not transmitted by mosquito vectors. The Alphavirus genus, however, has more than 30 species most of which are mosquito-borne arboviruses transmitted between mosquito vectors and vertebrate hosts including humans, rodents, birds, reptiles, pigs, amphibians, equids, and non-human primates [187]. A summary of the important alphaviruses spread by mosquito vectors include:
Babanki virus (BBKV)
This virus species is poorly understood and little information is available about it online. However, online sources indicate that Babanki virus was first isolated in Northwest Cameroon in 1969. The virus is closely related to the Sindbis virus which will be discussed later [133]. BBKV is said to be a strain of Sindbis virus and has been recovered from humans suffering from febrile illness accompanied by rash and arthritis in various African countries including Cameroon, Madagascar, and the Central African Republic [55,133]. The virus is usually associated with migratory birds that nest next to lakes during breeding times [133]. In Kenya, the virus was isolated from Culex species mosquitoes located around lake Naivasha and Victoria [133]. In Uganda however, the first isolation of the virus was in a study from late 2008 and early 2009 (Sempaya, Kikusa, and Nkokonjeru) [188]. BBKV was isolated from six mosquito pools in the study: five pools of Cx. perfuscus and a single pool of Culex species. The virus sequence in this study showed a similarity to BBKV of 98–99.8% [188]. In Uganda also, the virus was isolated in two wild bats from a study of 323 bats caught from various locations in the country [189].
Chikungunya virus (CHIKV)
According to the local Tanzanian dialect (Makonde), Chikungunya is a term that means a disease that bends up the joints [190]. The first outbreak of CHIKV was reported in the Newala and Masisi districts located in the Southern Province of the former Tanganyika territory currently known as Tanzania, from July 1952 to March 1953. The incidence rate at this time was said to be 23% and the disease was considered as a febrile disease that caused an onset of crippling joint pain with occasional rash symptoms [191–193]. Clinical manifestations of CHIKV have been documented in many papers [190,194–196] and they include fever, rash, myalgia, and headache. More symptoms including severe cases and diagnostics have been documented by Sam et.al [197]. The virus has been considered not to be life-threatening in the past until an outbreak accompanied by mother-to-child transmission, neurologic and hemorrhagic manifestations and mortality occurred in La Reunion in 2006 [198,199]. CHIKV belongs to the Semliki Forest complex and is closely related to O’nyong-nyong virus, another virus in the same genus Alphavirus identified in the preceding year, 1959, in Ugandan patients presenting symptoms similar to those of CHIKV [191,192]. CHIKV genotypes have been identified and they include the East/Central/South African (ECSA), West African and Asian genotypes [192,193]. Transmission of CHIKV is maintained in enzootic cycles among non-human primates and is transmitted by Aedes mosquito species. Human infections always occur after a bite from infected mosquitoes [200]. Transmission of CHIKV in Africa is maintained in a sylvatic transmission cycle that involves a variety of mosquito species including: Ae. aegypti, A. africanus in East Africa; Ae. furcifer, Ae. taylori, Ae. dalzieli, Ae. luteocephalus, and Ae. vittatus in West Africa; and Ae. taylori and Ae. cordellieri in South Africa and non-human primates [192,201,202]. Outbreaks of CHIKV have occurred across the globe [1] since the first outbreak in Tanzania. The first major outbreaks, however, occurred in Thailand and India in the 1960s and 70s [203]. From this period no other major outbreaks were recorded globally up until 2004, when a large outbreak occurred in Lamu located along the Kenyan coast and about 13,500 cases were reported [204]. In 2005, the outbreak subsequently spread to the La Reunion and Comoros Islands along the Indian Ocean with over 500,000 cases being reported, which included fatalities [205,206].
Reported cases and outbreaks of CHIKV have been recorded in all countries across the East African Community. In all the countries the ECSA lineage is common. The first case is in Tanzania where the first outbreak was also reported worldwide in 1952. Another combined outbreak of DENV and CHIKV was also reported in Tanzania in 2011–2014 [115,207,208]. In Burundi, the first outbreak was recorded between 1980–1982 [195]. In the same period, the first outbreak was also reported in Kenya in 1982, followed by the large epidemic of 2004 in Lamu and Mombasa with an attack rate of 75% [204]. More than 150 Kenyans have succumbed to the disease [194]. Another combined outbreak of CHIKV and DENV was also recorded in Kenya in 2013 similar to Tanzania [208]. Outbreaks have also been reported in Mandera county of Kenya in 2016 that borders Somalia, where reported outbreaks of CHIKV were ongoing at the same time. The most recent outbreak was reported after 2016 in the coastal city of Mombasa in 2018 [209]. In Uganda, several outbreaks of the virus have been reported between 1985–1971 [210,211]. In Sudan, the first case was reported in 1989 where 12% of the population tested were positive for CHIKV. Subsequent outbreaks in Sudan have been recorded in 2005, 2012, and 2013 [212]. Another report on Mongolian peacekeepers deployed to South Sudan highlighted that the virus is common in South Sudan and its borders [149]. Rwanda however, has had no reported outbreaks despite outbreaks being reported all over its neighboring countries. However serological evidence was reported in a study in 2018. In this study 874 Rwandan sera were screened using commercially available ELISA kits, the seroprevalence was reported to be high at 47% [146].
Ndumu virus (NDUV)
Ndumu virus was first isolated in the northern KwaZulu-Natal area of the eastern seaboard of South Africa from Mansonia uniformis in 1959 [213]. Since then, the virus has spread to other parts of Africa. NDUV infections have not been studied much but the virus is said to affect humans with no morbidities [214]. The virus has shown morbidity in mice experimentally infected with the virus [213]. The virus has also been shown to cause no morbidity in non-human primates. A study done in a nonimmune verevet monkey inoculated intracerebrally showed viremia with no signs of illness [55,213]. Serological evidence provided evidence of the widespread nature of the virus in South Africa where the virus was isolated from mosquito species Mansonia uniformis and Aedes circumluteolus [213]. NDUV has been isolated from livestock in domestic settings including the first isolation of the virus in pigs located in Uganda [214] with the suggestion of possible isolation of the virus in goats [215]. The virus was also isolated from other vertebrates in Kenya including Rhipicephalus pulchellus collected from cattle, and warthogs [216]. In Kenya, the virus has been isolated from more than 10 mosquito species including: Ae. sudanensis, Ae. mcintoshi, Ae. ochraceus, and Cx. rubinotus [133,215,217]. NDUV is highly isolated in the Cx. rubinotus mosquito species [133], this may mean that the mosquito that occurs in abundance in riverine lakes [218] may play an important role in the maintenance and transmission of the virus [219].
O’nyong-nyong virus (ONNV)
Similar to the way Tanzanians described CHIKV as a ‘disease that bends up the joints’ [190], the Acholi people of northwestern Uganda called the disease ‘O’nyong-nyong’ a term in their local dialect to mean ‘very painful weakening of joints’ [220]. ONNV was first isolated during a major epidemic that began in Gulu, located in north-west Uganda in 1959 and spread regionally to adjacent countries including Congo, Sudan, Kenya, Tanzania and along the shores of Lake Victoria to southeastern Africa by 1962 [220,221]. Another epidemic of ONNV occurred during 1996–1997 in south-central Uganda with high infection rates of up to 48% [221]. During the outbreak, people infected showed symptoms that included: acute fever, joint pains, skin rash, lymphadenopathy, immobilization for up to 4 days, arthralgia that lasted for 6 days, and viremia [221,222]. ONNV is a member of Semliki Forest antigenic complex and together with CHIKV they form a monophyletic group within this complex [192]. ONNV has got a variety of strains including the 1996 Uganda strain [223], and 2001 ONNV Kenya strain that has got a nearly identical sequence to that of Uganda Gulu strain from 1959 [224,225]. ONNV is transmitted by mosquitoes and is unique among other alphaviruses in that it can be transmitted primarily by night-feeding anopheline mosquitoes; Anopheles funestus and Anopheles gambiae which can also transmit malaria parasites [220,226]. The lack of ONNV in one particular area in Uganda lacking the two anopheline mosquitoes gives strong proof of the capabilities of the two anthropophilic mosquitoes in transmitting ONNV [192]. The virus has also been isolated from Mansonia uniformis [227]. In East Africa, ONNV is said to have caused large epidemics infecting millions of persons and disappearing for a long period of time before reemerging. It is speculated that during this time, the virus is probably being maintained through an enzootic cycle, whose hosts remain unknown [225]. Among the EAC countries, isolations and outbreaks have been reported in Kenya, Uganda, Tanzania, and Sudan [220,226,228].
Semliki Forest virus (SFV)
Semiliki Forest virus was first isolated in Uganda from the Aedes abnormalis group of mosquitoes captured in 1942 [229]. Subsequent isolation of the virus followed in many African countries including from Ae. argenteopunctatus species from central Mozambique in 1959 [230]. Since this time the virus has been used as a model for studying the molecular biology of RNA viruses because it grows well with high titers in cells [231]. Laboratory injected rabbits with SFV developed fever and paralysis [229], while mice developed hind-leg paralysis and convulsions which were followed by death [229,230]. Rhesus infected monkeys, on the other hand, did not develop any symptoms [229], but vervet monkeys showed fever with no other symptoms manifesting [230]. The only outbreak of SFV recorded online occurred in Bangui (found in the Central African Republic) during October-December 1987, where patients including French soldiers working there showed mild febrile illness symptoms [232]. The virus was classified to be a Biosafety Level 3 virus in USA after a person died with suspicion of SFV infection in the laboratory with human encephalitis symptoms [233]. In other European countries, the virus is classified as a Biosafety Level 2 virus [231]. Two strains of the virus exist: the original virulent isolate of SFV designated L10 [231] and the avirulent strain designated A7 isolated from mosquitoes in Mozambique [230]. Uganda is the only country within the EAC that the virus has been isolated, other African countries where the virus has been isolated include Mozambique, South Africa, Cameroon, Central African Republic, Senegal, Congo, and Nigeria [4,234].
Sindbis virus (SINV)
Sindbis virus was first isolated in 1952 from a group of 63 Culex mosquitoes captured in Sindbis Health district, located north of Cairo, Egypt [235]. The first human signs and symptoms of SINV were however not reported until 1961 when humans in Uganda presented symptoms of the viral disease [236]. Human infections cause symptoms that include: febrile illness, fever, arthritis, and maculopapular rash [237]. Sindbis virus is said to be the most widely distributed arbovirus [238]. The spread of SINV internationally has been attributed to migratory birds [239]. Seasonal migration of these birds has indeed shown the correlation of migration, with geographical distribution of SINV genotypes [240]. SINV is grouped into six genotypes (SINV I-IV) that are antigenically distinct based on their difference in the E2 glycoprotein gene. The distribution of the virus genotypes includes SINV-I circulating mainly in Africa, Europe, and the Middle East; SINV-II in Australia and Malaysia; SINV-III in India and Philippines; SINV-IV in Azerbaijan and China; SINV-V in new Zealand; and finally SINV-VI in south-west Australia [241]. Transmission of the virus to birds occurs through ornithophilic mosquitoes (Culex and Culiseta spp.), while in humans the virus is spread from opportunistically feeding bridge vectors [242]. More about the virus strain and transmission has been reviewed by Adouchief et.al [241]. In South Africa, outbreaks of the virus have occurred concurrently with the outbreak of West Nile virus in the years 1974 [90], 1983 and 1984, where hundreds of Sindbis human cases were reported [4]. In the EAC, the virus has only been isolated in Uganda and Kenya. In Uganda, the virus was isolated from humans and the mosquito Mansonia (Coquillettidia) fuscopennata [236,243]. In Kenya however, the virus has only been isolated from a wide range of Culex and Aedes mosquito species captured from different regions around Kenya [133,217]. A study done by Sigei et.al on the evolutionary traits of Sindbis virus strains isolated from mosquitoes captured in Kenya between 2007 and 2013 confirmed that there is circulation of SINV-I genotype in Kenya that has been associated with human diseases in South Africa and northeastern Europe [244].
Viruses within the EAC in this family with less information online are summarized in Table 1.
Factors that may contribute to the future spread of mosquito-borne arboviruses within the East African Community
Transport
Transportation has been cited to be the cause of emerging and reemerging infectious diseases across the globe. This is the case also with mosquito-borne arboviruses. A lot of research has highlighted that transportation has played a key role in the spread of mosquito vectors and their associated arboviruses into new locations leading to epidemics of emerging and reemerging arboviral infections [70,98,245]. Different transport routes exist throughout the EAC. Some of these routes involve corridors that link landlocked countries to the busy seaport of Mombasa where mosquito vectors that contain arboviruses in this review have been isolated. Reported outbreaks of arboviruses have also been recorded including CHIKV and DENV outbreaks [120,201]. The Port of Mombasa located in Kenya serves as a gateway to East and Central Africa, and is one of the largest and busiest Ports along the East African coastline. The only other country with a port in the EAC is Tanzania with the Port of Dar Es Salaam [246]. In regards to roads linking the EAC countries, the Northern corridor is the most important as it links all the 6 countries within the EAC [5]. The Northern Corridor is the largest in the EAC and serves as a transportation corridor linking landlocked countries of the Great Lakes region like the Democratic Republic of Congo, Rwanda, Burundi, and Uganda to the port of Mombasa in Kenya. The corridor is 2,000 km and serves other countries in the EAC including Tanzania and South Sudan [5,247]. Railway lines also exist including the Kenya-Uganda Railway and the ongoing construction of the Standard Gauge Railway (a joint project between countries in the EAC with the Chinese One Belt One Road initiative) that aims to link the seaport of Mombasa to other countries in East and Central Africa [248,249]. Citizens of the various countries within the EAC can now travel freely within countries in the EAC due to the countries agreement to share a common passport. These transportation facts may play a role in the spread of arboviruses within the EAC.
Trade
From this review, some of the arboviruses have been said to be spread across the continent from one region to the other due to trade. A classic example includes the Yellow fever virus that was said to have originated in Africa and later spread to other parts of the world due to slave trade that dated back as far as 1650 [8,157,191]. Large numbers of slaves were imported from Africa who were said to be YFV-infected and along with them the Ae. aegypti mosquito was also transported, this caused infections mainly to nonimmune white people in the Caribbean [4]. In a review done by Pfeffer and Dobler, they highlighted the spread of different viruses from one location to another and the important roles of humans and animals in arbovirus spread because of trade [9]. A customs trade union exists within the EAC that ensures freedom of moving goods produced within the community imposing some form of barrier to external trade [14]. Among the commodities that are traded within the EAC include livestock. Livestock found in different countries within the EAC are known to be associated with mosquito-borne viruses including Rift Valley fever, and Ndumu virus [75,214]. The transportation corridors discussed above serve also as routes for trade within the EAC. Some of the countries within the EAC like Uganda are landlocked and rely on access to seaports of Mombasa (a region with high mosquito-borne arbovirus outbreaks like CHIKV and DENV) [119,201] and Tanzania (Dar-es-Salam) (also endemic to mosquito borne arboviruses) for trade [246].
Climate
In their review of the impact climate change has had on the spread of arboviruses globally, Gould and Higgs highlighted various possible factors that have influenced the emergence of arboviruses in the past 50 years [250]. They state that climate is a major factor in determining: the geographic and temporal distribution of arthropods; characteristics of arthropod life cycles; dispersal patterns of associated arboviruses; the evolution of arboviruses; and the efficiency by which arboviruses are transmitted from arthropods to vertebrate hosts [250]. Meanwhile, other literature have associated arboviral spread with fluctuating climatic conditions [4,9,68,250]. The EAC member states experience climatic conditions that are atypical to equatorial regions [246]. These conditions are surprisingly cool and dry for its latitude despite it having a generally high altitude, and the rain shadow of the westerly monsoon winds from the Ethiopian Highlands and Ruwenzori Mountains. In East Africa, moderate temperatures are experienced, except for the hot and humid coastal belt. Different altitudes experience different temperatures: altitudes of up to 1,500 meters have a temperature range between highs of 25°C (77°F) and lows of 15°C (59°F), while above 2,500 meters frost is common in dry seasons with maximum temperatures of about 21°C (70°F) or less [7]. In the EAC, periodic outbreaks of mosquito-borne arboviruses like DENV, CHIKV, RVFV, Ngari virus have seen outbreaks that were preceded by heavy rainfall and floods that were said to be good breeding sites of mosquitoes [4,77,112,198].
Conclusion
From this review, it is evident that a substantial number of mosquito-borne arboviruses exist within the East African Community partner states as shown in Figure 2. Some of these viruses like RVFV, ZIKV, and WNV have originally been isolated for the first time in the EAC and spread to other countries worldwide where they become endemic and cause devastating public health outbreaks post initial spread. It is also clear that the majority of viruses are located in Uganda and Kenya with less evidence of virus isolations in Rwanda and Burundi respectively. This raises concerns that can only be addressed by further research to establish the true prevalence of mosquitoes and mosquito-borne arboviruses in these countries. The future spread of mosquito-borne arboviruses from country to country within the EAC may be facilitated with the rapid expansion in trade, transport and fluctuating environmental conditions. Already transport routes like the Northern corridor and other routes linking the seaport of Mombasa to other landlocked countries like Uganda and Burundi exist. All these factors may provide a ‘perfect environment’ for future arbovirus outbreaks within the EAC. Surely, all it takes is the introduction of vectors or viruses by humans into new locations where they become quiescent until under perfect sylvatic conditions they can spread and cause devastating outbreaks. We, therefore, recommend that the EAC governing structure: 1) make it a priority to increase vigilance of the spread of mosquito vectors and also monitor the introduction/spread of new arboviruses within the EAC; 2) come up with routine arbovirus surveillance activities especially with regards to less studied arboviruses like BBKV, SFV, and BATV; 3) and if possible set up a joint research institution for arbovirus research in the EAC. Since an incredible number of novel viruses have been isolated in the EAC, it is also probable that other novel mosquito-borne arboviruses still exist within the Community waiting for the perfect conditions to cause devastating outbreaks.
Figure 2.
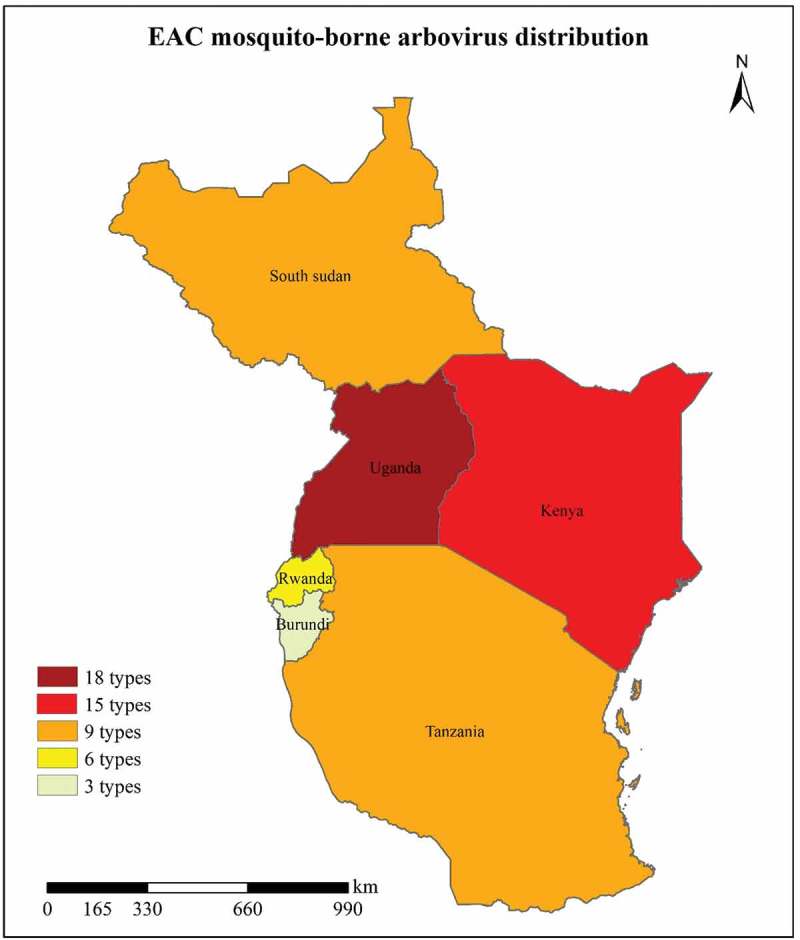
Distribution of mosquito-borne arboviruses across the East African Community. A total of 24 different mosquito-borne arboviruses (Table 1) are located within the EAC with high prevalence in Uganda, Kenya, Tanzania and South Sudan.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors acknowledge Jean de Dieu Nambajimana from the Key Labaratory of Mountain Surface Processes and Ecological Regulation, Institute of Mountain Hazards and Environment, Chinese Academy of Sciences, for helping in designing of the maps.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Xia H, Wang Y, Atoni E, et al. Mosquito-associated viruses in China. Virol Sin. 2018;33:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ketkar H, Herman D, Wang P.. Genetic determinants of the re-emergence of arboviral diseases. Viruses. 2019;11 DOI: 10.3390/v11020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burt FJ, Goedhals D, Mathengtheng L.. Arboviruses in southern Africa: are we missing something? Future Virol. 2014;9:993–1008. [Google Scholar]
- [4].Braack L, Gouveia De Almeida AP, Cornel AJ, et al. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasites Vectors. 2018;11 DOI: 10.1186/s13071-017-2559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gichaga FJ. The impact of road improvements on road safety and related characteristics. IATSS Res. 2017;40:72–75. [Google Scholar]
- [6].McAuliffe MC, Saxena MSC, Yabara MM. The East African Community: prospects for sustained growth. Washington (DC): International Monetary Fund; 2012. [Google Scholar]
- [7].Ogola FO, Njenga GN, Mhando PC, et al. A profile of the East African Community. Africa J Manag. 2015;1:333–364. [Google Scholar]
- [8].Bryant JE, Holmes EC, Barrett ADT. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLOS Pathog. 2007;3:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pfeffer M, Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit Vectors. 2010;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moureau G, Cook S, Lemey P, et al. New insights into flavivirus evolution, taxonomy and biogeographic history, extended by analysis of canonical and alternative coding sequences. PLoS One. 2015;10:e0117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schlumberger CE, Weisskopf N. Opportunities and challenges for LCC development: the case of East Africa. Washington (DC): The World Bank. 2014. DOI:10.1596/978-1-4648-0282-9_ch5 [Google Scholar]
- [12].Firehiwot G, Suk P. Corruption and economic growth in east African countries. 한국아프리카학회지. 2013;39:3–24. [Google Scholar]
- [13].Wandera C, Niyibizi A. Potential benefits of adoption of laser materials processing in East Africa’s manufacturing industry. 3rd DeKUT International Conference on STI&E NYERI, KENYA. 2018:125–141. Available from: https://scholar.google.com/scholar?cluster=17788851847486531760&hl=en&as_sdt=2005 [Google Scholar]
- [14].Hamad H. “Neo-functionalism”: relevancy for East African Community political integration. Africology: The Journal of Pan African Studies. 2016;9:69–81. [Google Scholar]
- [15].LeRiche M, Arnold M. South Sudan: from revolution to independence. Oxford, UK: Oxford University Press; 2013. [Google Scholar]
- [16].Rolandsen ØH. Another civil war in South Sudan: the failure of Guerrilla government? J East Afr Stud. 2015;9:163–174. [Google Scholar]
- [17].Christian KA, Iuliano AD, Uyeki TM, et al. What we are watching—top global infectious disease threats, 2013–2016: an update from CDC’s global disease detection operations center. Heal Secur. 2017;15:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morvan JM, Digoutte JP, Marsan P, et al. Ilesha virus: a new aetiological agent of haemorrhagic fever in Madagascar. Trans R Soc Trop Med Hyg. 1994;88:205. [DOI] [PubMed] [Google Scholar]
- [19].Williams MC, Woodall JP, Corbet PS. Nyando virus: A hitherto undescribed virus isolated fromAnopheles funestus giles collected in Kenya. Arch Gesamte Virusforsch. 1965;15:422–427. [DOI] [PubMed] [Google Scholar]
- [20].Digoutte JP, Gagnard VJM, Brès P, et al. Infection à virus Nyando chez l’homme. Bull La Société Pathol Exot. 1972;65:751–758. [PubMed] [Google Scholar]
- [21].Kalunda M, Lwanga-Ssozi C, Lule M, et al. Isolation of Chikungunya and Pongola viruses from patients in Uganda. Trans R Soc Trop Med Hyg. 1985;79:567. [DOI] [PubMed] [Google Scholar]
- [22].Tchouassi DP, Okiro ROK, Sang R, et al. Mosquito host choices on livestock amplifiers of rift valley fever virus in Kenya. Parasit Vectors. 2016;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mansfield KL, Banyard AC, McElhinney L, et al. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33:5520–5531. [DOI] [PubMed] [Google Scholar]
- [24].Dar O, McIntyre S, Hogarth S, et al. Rift Valley fever and a new paradigm of research and development for zoonotic disease control. Emerg Infect Dis. 2013;19:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552–1563. [DOI] [PubMed] [Google Scholar]
- [26].Musso D, Nilles EJ, Cao‐Lormeau V. Rapid spread of emerging Z ika virus in the P acific area. Clin Microbiol Infect. 2014;20:O595–6. [DOI] [PubMed] [Google Scholar]
- [27].Chapter 22 - Bunyaviridae In: MacLachlan NJ, Dubovi EJBT-FVV, editors Fifth E Fenner’s veterinary virology. Boston: Academic Press; 2017. p. 411–424. DOI: 10.1016/B978-0-12-800946-8.00022-2 [DOI] [Google Scholar]
- [28].Briese T, Calisher CH, Higgs S. Viruses of the family Bunyaviridae: are all available isolates reassortants? Virology. 2013;446:207–216. [DOI] [PubMed] [Google Scholar]
- [29].Burrell CJ, Howard CR, Murphy FA. Chapter 29 - Bunyaviruses In: Burrell CJ, Howard CR, Murphy -FABT-F, WMV , Fifth E, editors. London: Academic Press; 2017. p. 407–424. DOI: 10.1016/B978-0-12-375156-0.00029-1 [DOI] [Google Scholar]
- [30].Mahaffy AF, Haddow AJ, Smithburn KC. A neurotropic virus isolated from aedes mosquitoes caught in the semliki forest. Am J Trop Med Hyg. 1946;s1–26:189–208. [DOI] [PubMed] [Google Scholar]
- [31].Porterfield JS. Bunyaviridae, infection and immunity. Encycl Immunol. 1998;390–393. DOI: 10.1006/RWEI.1999.0104 [DOI] [Google Scholar]
- [32].Odhiambo CO, Venter M, Swanepoel R, et al. Circulation, evolution and transmission of ngari and bunyamwera orthobunya viruses in Northern Kenya. Int J Infect Dis. 2014;21:230. [Google Scholar]
- [33].KOKERNOT RH, HEYMANN CS, MUSPRATT J, et al. Studies on arthropod-borne viruses of Tongaland. V. isolation of Bunyamwera and Rift Valley Fever viruses from mosquitoes. S Afr J Med Sci. 1957;22:71–80. [PubMed] [Google Scholar]
- [34].Smithburn KC, Paterson HE, Kokernot RH, et al. Isolation of Bunyamwera virus from a naturally infected human being and further isolations from aedes (Banksinella) Circumluteolus Theo. 1. Am J Trop Med Hyg. 1958;7:579–584. [DOI] [PubMed] [Google Scholar]
- [35].Smithburn KC, Heymann CS, Weinbrein MP, et al. Neutralizing antibodies for certain viruses in the sera of human beings residing in northern Natal. S Afr Med J. 1959;33:555–61. [PubMed] [Google Scholar]
- [36].Black FL, Clarke DH. The interaction of Bwamba virus with homologous immune serum. J Immunol. 1955;74:411LP– 417. [PubMed] [Google Scholar]
- [37].Tomor O, Monath TP, Lee V, et al. Bwamba virus infection: A sero-survey of veterbrates in five ecological zones in Nigeria. Trans R Soc Trop Med Hyg. 1974;68:461–465. [DOI] [PubMed] [Google Scholar]
- [38].Lutwama JJ, Rwaguma EB, Nawanga PL, et al. Isolations of Bwamba virus from south central Uganda and north eastern Tanzania. Afr Health Sci. 2002;2:24–28. [PMC free article] [PubMed] [Google Scholar]
- [39].Smithburn KC, Mahaffy AF, Paul JH. Bwamba Fever and Its Causative Virus. Am J Trop Med Hyg. 1941;s1–21:75–90. [Google Scholar]
- [40].Groseth A, Mampilli V, Weisend C, et al. Molecular characterization of human pathogenic bunyaviruses of the nyando and bwamba/pongola virus groups leads to the genetic identification of Mojuí dos Campos and Kaeng Khoi Virus. PLoS Negl Trop Dis. 2014;8:e3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moore DÁ, Causey OR, Carey DE, et al. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol. 1975;69:49–64. [DOI] [PubMed] [Google Scholar]
- [42].Woodall JP. Human infections with arboviruses of the Bunyamwera group 1969.
- [43].Brès P. Données récentes apportées par les enquêtes sérologiques sur la prévalence des arbovirus en Afrique, avec référence spéciale à la fièvre jaune. Bull World Health Organ. 1970;43:223–267. [PMC free article] [PubMed] [Google Scholar]
- [44].Gonzalez J-P. Arbovirus discovery in Central African Republic (1973–1993): zika, Bozo, Bouboui, and more. Ann Infect Dis Epidemiol. 2017;2:1022. [Google Scholar]
- [45].Gonzalez J-P, Georges AJ. Buyamweral fevers: bunyamwera, Ilesha, Germiston, Bwanba and Tataguine. In: Monath T, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton (FL): CRC press. 1988.2:87–98. [Google Scholar]
- [46].Lee VH, Monath TP, Tomori O, et al. Arbovirus studies in nupeko forest, a possible natural focus of yellow fever virus in Nigeria II. Entomological investigations and viruses isolated. Trans R Soc Trop Med Hyg. 1974;68:39–43. [DOI] [PubMed] [Google Scholar]
- [47].Karabastos N. Intemational catalogue of arboviruses. Atlanta GA Inf Subcomm Exch OC Am Comm Arthrupod-Bome Viruses. Am Soc Trop Med Hyg. 1985;3:361–368. [Google Scholar]
- [48].Zeller HG, Diallo M, Angel G, et al. Ngari virus (Bunyaviridae: bunyavirus). First isolation from humans in Senegal, new mosquito vectors, its epidemiology. Bull Soc Pathol Exot. 1996;89:12–16. [PubMed] [Google Scholar]
- [49].Eiden M, Vina-Rodriguez A, Mamy BO E, et al. Ngari virus in goats during Rift Valley fever outbreak, Mauritania, 2010. Emerg Infect Dis J. 2014;20:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Briese T, Bird B, Kapoor V, et al. Batai and Ngari Viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa. J Virol. 2006;80:5627LP– 5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gerrard SR, Li L, Barrett AD, et al. Ngari virus is a Bunyamwera virus reassortant that can be associated with large outbreaks of hemorrhagic fever in Africa. J Virol. 2004;78:8922LP– 8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].GAIDAMOVICH SY, Obukhova VR, Vinograd AI, et al. Olyka-an arbovirus of the Bunyantwera group in the USSR. Acta Virol. 1973;17:444. [PubMed] [Google Scholar]
- [53].Singh KRP, PAVRI KM. Isolation of Chittoor virus from mosquitoes and demonstration of serological conversions in sera of domestic animals at Manjri, Poona, India. Indian J Med Res. 1966;54:220–224. [PubMed] [Google Scholar]
- [54].Bardos V, Cupkova E. The Calovo virus-the second virus isolated from mosquitoes in Czechoslovakia. J Hyg Epidemiol Microbiol Immunol. 1962;6:186–192. [PubMed] [Google Scholar]
- [55].Karabatsos N, edited by. International catalogue of arboviruses, including certain other viruses of vertebrates. Third edition ed. San Antonio, Texas: American Society of Tropical Medicine and Hygiene for The Subcommittee on Information Exchange of the American Committee on Arthropod-borne Viruses; 1985. [Google Scholar]
- [56].Henderson BE, Kirya GB, Hewitt LE. Serological survey for arboviruses in Uganda, 1967–69. Bull World Health Organ. 1970;42:797. [PMC free article] [PubMed] [Google Scholar]
- [57].Hunt AR, Calisher CH. Relationships of bunyamwera group viruses by neutralization. Am J Trop Med Hyg. 1979;28:740–749. [PubMed] [Google Scholar]
- [58].Dutuze MF, Nzayirambaho M, Mores CN, et al. A review of Bunyamwera, Batai, and Ngari Viruses: understudied orthobunyaviruses with potential one health implications. Front Vet Sci. 2018;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nashed NW, Olson JG, El-Tigani A. Isolation of Batai virus (Bunyaviridae: bunyavirus) from the blood of suspected malaria patients in Sudan. Am J Trop Med Hyg. 1993;48:676–681. [DOI] [PubMed] [Google Scholar]
- [60].Tesh RB. The genus Phlebovirus and its vectors. Annu Rev Entomol. 1988;33:169–181. [DOI] [PubMed] [Google Scholar]
- [61].Wuerth JD, Weber F. Phleboviruses and the type I interferon response. Viruses. 2016;8 DOI: 10.3390/v8060174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54–74. [DOI] [PubMed] [Google Scholar]
- [63].Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east africa. J Pathol Bacteriol. 1931;34:545–579. [Google Scholar]
- [64].Daubney R, Hudson JR. RIFT VALLEY FEVER. Lancet. 1932;219:611–612. [Google Scholar]
- [65].Flick R, Bouloy M. Rift Valley fever virus. Curr Mol Med. 2005;5:827–834. [DOI] [PubMed] [Google Scholar]
- [66].Khan AS, Smith CV. Rift Valley fever: still an emerging infection after 3500 years. Lancet Glob Heal. 2016;4:e773–4. [DOI] [PubMed] [Google Scholar]
- [67].Davies FG. The historical and recent impact of Rift Valley fever in Africa. Am J Trop Med Hyg. 2010;83:73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pepin M, Bouloy M, Bird BH, et al. Rift Valley fever virus(Bunyaviridae: phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Woods CW, Karpati AM, Grein T, et al. An outbreak of Rift Valley fever in northeastern Kenya, 1997–98. Emerg Infect Dis. 2002;8:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nyakarahuka L, de St. Maurice A, Purpura L, et al. Prevalence and risk factors of Rift Valley fever in humans and animals from Kabale district in Southwestern Uganda, 2016. PLoS Negl Trop Dis. 2018;12:e0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].WHO 18 May 2007, vol. 82, 20 (pp 169–180). WHO; 2012. [Google Scholar]
- [72].Adam AA, Karsany MS, Adam I. Manifestations of severe Rift Valley fever in Sudan. Int J Infect Dis. 2010;14:e179–80. [DOI] [PubMed] [Google Scholar]
- [73].Henderson BE, McCrae AWR, Kirya BG, et al. Arbovirus epizootics involving man, mosquitoes and vertebrates at Lunyo, Uganda 1968. Ann Trop Med Parasitol. 1972;66:343–355. [DOI] [PubMed] [Google Scholar]
- [74].Maurice A de S. Notes from the field: rift valley fever response—kabale District, Uganda, March 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1200–1201. [DOI] [PubMed] [Google Scholar]
- [75].Umuhoza T, Berkvens D, Gafarasi I, et al. Seroprevalence of Rift Valley fever in cattle along the Akagera–nyabarongo rivers, Rwanda. J S Afr Vet Assoc. 2017;88:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ogoma SB, Lweitoijera DW, Ngonyani H, et al. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl Trop Dis. 2010;4:e773–e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Himeidan YE, Kweka EJ, Mahgoub MM, et al. Recent outbreaks of rift valley fever in East Africa and the middle East. Front Public Health. 2014;2:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fontenille D, Traore-Lamizana M, Diallo M, et al. New vectors of Rift Valley fever in west Africa. Emerg Infect Dis. 1998;4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Edwards FW. Mosquitoes of the Ethiopian region III-culicine adults and pupae. London, United Kingdom: Br Museum Natural Hist; 1941. (n.d.). [Google Scholar]
- [80].White GB. Notes on a catalogue of Culicidae of the Ethiopian region. Mosq Syst. 1975;7:303–344. [Google Scholar]
- [81].Sang R, Kioko E, Lutomiah J, et al. Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am J Trop Med Hyg. 2010;83:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gould EA, de Lamballerie X, Paolo M de A, et al. Evolution, epidemiology, and dispersal of flaviviruses revealed by molecular phylogenies. Academic Press. 2001;57:71–103. [DOI] [PubMed] [Google Scholar]
- [83].Gould EA, Holmes EC. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv Virus Res. 2003;59:277–314. [DOI] [PubMed] [Google Scholar]
- [84].Holbrook MR. Historical perspectives on flavivirus research. Viruses. 2017;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Smithburn KC, Paterson HE, Heymann CS, et al. An agent related to Uganda S virus from man and mosquitoes in South Africa. S Afr Med J. 1959;33:959–962. [PubMed] [Google Scholar]
- [86].Kokernot RH, Szlamp EL, Levitt J, et al. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of the Caprivi Strip and Bechuanaland Protectorate. Trans R Soc Trop Med Hyg. 1965;59:553–562. [DOI] [PubMed] [Google Scholar]
- [87].McIntosh BM, Dos Santos ISL, Meenehan GM. Culex (Eumelanomyia) Rubinotus Theobald as Vector of Banzi, Germiston and Witwatersrand Viruses III. Transmission of virus to hamsters by wild-caught infected C. rubinotus. J Med Entomol. 1976;12:645–646. [DOI] [PubMed] [Google Scholar]
- [88].Metselaar D, Henderson BE, Kirya GB, et al. Isolation of arboviruses in Kenya, 1966–1971. Trans R Soc Trop Med Hyg. 1974;68:114–123. [DOI] [PubMed] [Google Scholar]
- [89].Calisher CH, Karabatsos N, Dalrymple JM, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37–43. [DOI] [PubMed] [Google Scholar]
- [90].McIntosh BM, Jupp PG, Dos Santos I, et al. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as vector. S Afr J Sci. 1976;72:295–300. [Google Scholar]
- [91].McIntosh BM. Susceptibility of some African wild rodents to infection with various arthropod-borne viruses. Trans R Soc Trop Med Hyg. 1961;55:63–68. [DOI] [PubMed] [Google Scholar]
- [92].Dick GWA, Haddow AJ. Uganda S virus. A hitherto unrecorded virus isolated from mosquitoes in Ugnada. (I). Isolation and pathogenicity. Trans R Soc Trop Med Hyg. 1952;46:600–618. [DOI] [PubMed] [Google Scholar]
- [93].Boorman JPT. Transmission of Uganda S virus by Aedes (Stegomyia) Aegypti Linn. Trans R Soc Trop Med Hyg. 1958;52:383–388. [DOI] [PubMed] [Google Scholar]
- [94].Wilder-Smith A, Murray QM. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gubler DJ. Dengue/dengue haemorrhagic fever : history and current status. New York (John Wiley): Chichester. 2006;277:3–22. DOI:10.1002/0470058005.ch2 [Google Scholar]
- [96].Nobuchi H. The symptoms of a dengue-like illness recorded in a Chinese medical encyclopedia. Kanpo Rinsho. 1979;26:422–425. [Google Scholar]
- [97].Amer- N, Arabia S. Dengue/dengue hemorrhagic fever : the emergence of a global health problem. Emerg Infect Dis. 1995;1:55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Amarasinghe A, Kuritsk JN, Letson GW, et al. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Organization WH Monograph on dengu. WHO Regional Office for South-East Asia; 1993. [Google Scholar]
- [100].Baba M, Villinger J, Masiga DK. Repetitive dengue outbreaks in East Africa: A proposed phased mitigation approach may reduce its impact. Rev Med Virol. 2016;26:183–196. [DOI] [PubMed] [Google Scholar]
- [101].Semenza JC, Sudre B, Miniota J, et al. International dispersal of dengue through air travel: importation risk for Europe. PLoS Negl Trop Dis. 2014;8:e3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Benedict MQ, Levine RS, Hawley WA, et al. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007;7:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Diallo M, Sall AA, Moncayo AC, et al. Potential role of sylvatic and domestic African mosquito species in dengue emergence. Am J Trop Med Hyg. 2005;73:445–449. [PubMed] [Google Scholar]
- [104].Dhar-Chowdhury P, Haque CE, Lindsay R, et al. Socioeconomic and ecological factors influencing Aedes aegypti prevalence, abundance, and distribution in Dhaka, Bangladesh. Am J Trop Med Hyg. 2016;94:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gubler DJ, Zaki SR. Dengue and other viral hemorrhagic fevers. Pathol Emerg Infect. 1998;2:43–71. [Google Scholar]
- [106].Van Kleef E, Bambrick H, Hales S. The geographic distribution of dengue fever and the potential influence of global climate change. Trop Net. 2010. Available from: http://journal.tropika.net/scielo.php?script=sci_arttext&pid=S2078-86062010005000001&lng=en [Google Scholar]
- [107].Carey DE. Chikungunya and dengue: a case of mistaken identity? J Hist Med Allied Sci. 1971;26:243–262. [DOI] [PubMed] [Google Scholar]
- [108].Gratz NG, Knudsen AB, Organization WH The rise and spread of dengue, dengue haemorrhagic fever and its vectors: a historical review (up to 1995). Geneva: World Health Organization; 1996. [Google Scholar]
- [109].Hirsch A. Handbook of geographical and historical pathology. Vol. 1 London: New Sydenham Society; 1883. [Google Scholar]
- [110].Ulmann E. The geographical distribution of dengue up to 1957. World Atlas Epidemic Dis Part III Falk-Verlag[Links] 1957.
- [111].Robinson MC. All epidemic of a dengue-like fever in the southern province of Tanganyika. Cent Afr J Med. 1956;2:394–396. [PubMed] [Google Scholar]
- [112].Obonyo M, Fidhow A, Ofula V. Investigation of laboratory confirmed dengue outbreak in North-eastern Kenya, 2011. PLoS One. 2018;13:e0198556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hyams KC, Oldfield EC, Scott RM, et al. Evaluation of febrile patients in Port Sudan, Sudan: isolation of dengue virus. Am J Trop Med Hyg. 1986;35:860–865. [DOI] [PubMed] [Google Scholar]
- [114].Malik A, Earhart K, Mohareb E, et al. Dengue hemorrhagic fever outbreak in children in Port Sudan. J Infect Public Health. 2011;4:1–6. [DOI] [PubMed] [Google Scholar]
- [115].Vairo F, Nicastri E, Meschi S, et al. Seroprevalence of dengue infection: a cross-sectional survey in mainland Tanzania and on Pemba Island, Zanzibar. Int J Infect Dis. 2012;16:e44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Seidahmed OME, Siam HAM, Soghaier MA, et al. Dengue vector control and surveillance during a major outbreak in a coastal Red Sea area in Sudan. EMHJ-Eastern Mediterranean Health Journal. 2012;18:1217–1224. [PubMed] [Google Scholar]
- [117].Bosa HK, Montgomery JM, Kimuli I, et al. Dengue fever outbreak in Mogadishu, Somalia 2011: co-circulation of three dengue virus serotypes. Int J Infect Dis. 2014;21:3. [Google Scholar]
- [118].Mboera LEG, Mweya CN, Rumisha SF, et al. The risk of dengue virus transmission in Dar es Salaam, Tanzania during an epidemic period of 2014. PLoS Negl Trop Dis. 2016;10:e0004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lutomiah J, Barrera R, Makio A, et al. Dengue outbreak in Mombasa City, Kenya, 2013–2014: entomologic Investigations. PLoS Negl Trop Dis. 2016;10:e0004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ellis EM, Neatherlin JC, Delorey M, et al. A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis. 2015;9:e0003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Smithburn KC, Haddow AJ. Ntaya virus. a hitherto unknown agent isolated from mosquitoes collected in Uganda. Proc Soc Exp Biol Med. 1951;77:130–133. [DOI] [PubMed] [Google Scholar]
- [122].Woodruff AW, Bowen ET, Platt GS. Viral infections in travellers from tropical Africa. Br Med J. 1978;1:956–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dilcher M, Sall AA, Hufert FT, et al. Full-length genome sequence of Ntaya virus. Virus Genes. 2013;46:162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Antipa C, Girjabu E, Iftimovici R, et al. Serological investigations concerning the presence of antibodies to arboviruses in wild birds. Virologie. 1984;35:5–9. [PubMed] [Google Scholar]
- [125].Drăgănescu N, Girjabu E, Iftimovici R, et al. Investigations on the presence of antibodies to alphaviruses, flaviviruses, Bunyavirus and Kemerovo virus in humans and some domestic animals. Virologie. 1978;29:107–111. [PubMed] [Google Scholar]
- [126].Drăgănescu N, Iftimovici R, Iacobescu V, et al. Investigations on the presence of antibodies to several flaviviruses in humans and some domestic animals in a biotope with a high frequency of migratory birds. Virologie. 1975;26:103–108. [PubMed] [Google Scholar]
- [127].McIntosh BM. Usutu (SA Ar 1776), nouvel arbovirus du groupe B. Int Cat Arboviruses. 1985;3:1059–1060. [Google Scholar]
- [128].Chvala S, Bakonyi T, Bukovsky C, et al. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet Microbiol. 2007;122:237–245. [DOI] [PubMed] [Google Scholar]
- [129].Pauli G, Bauerfeind U, Blümel J, et al. Usutu VIrus. Transfus Med Hemother. 2014;41(1):73–82. DOI:10.1159/000357106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Nikolay B, Diallo M, Boye C, et al. Usutu virus in Africa. Vector-Borne and Zoonotic Diseases. 2011;11 DOI: 10.1089/vbz.2011.0631. [DOI] [PubMed] [Google Scholar]
- [131].Weissenböck H, Kolodziejek J, Url A, et al. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. 2002;8:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cavrini F, Gaibani P, Longo G, et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill Bull Eur Sur Les Mal Transm Eur Commun Dis Bull. 2009;14:pii: 19448. [PubMed] [Google Scholar]
- [133].Ochieng C, Lutomiah J, Makio A, et al. Mosquito-borne arbovirus surveillance at selected sites in diverse ecological zones of Kenya; 2007 – 2012. Virol J. 2013;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Simpson DIH, Haddow AJ, Knight EM. The isolation of west nile virus from man and of usutu virus from the bird-biting mosquito Mansonia Aurites (Theobald) in the Entebbe Area of Uganda AU - Williams, M. C. Ann Trop Med Parasitol. 1964;58:367–374. [DOI] [PubMed] [Google Scholar]
- [135].Smithburn KC, Hughes TP, Burke AW, et al. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;1:471–492. [Google Scholar]
- [136].Donadieu E, Bahuon C, Lowenski S, et al. Differential virulence and pathogenesis of West Nile viruses. Viruses. 2013;5:2856–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sule WF, Oluwayelu DO, Hernández-Triana LM, et al. Epidemiology and ecology of West Nile virus in sub-Saharan Africa. Parasit Vectors. 2018;11:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Figuerola J, Soriguer R, Rojo G, et al. Seroconversion in wild birds and local circulation of West Nile virus, Spain. Emerg Infect Dis. 2007;13:1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].López G, Jiménez-Clavero MÁ, Tejedor CG, et al. Prevalence of West Nile virus neutralizing antibodies in Spain is related to the behavior of migratory birds. Vector-Borne Zoonotic Dis. 2008;8:615–622. [DOI] [PubMed] [Google Scholar]
- [140].Dusek RJ, McLean RG, Kramer LD, et al. Prevalence of West Nile virus in migratory birds during spring and fall migration. Am J Trop Med Hyg. 2009;81:1151–1158. [DOI] [PubMed] [Google Scholar]
- [141].Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–483. [DOI] [PubMed] [Google Scholar]
- [142].Hayes EB, Sejvar JJ, Zaki SR, et al. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Fros JJ, Miesen P, Vogels CB, et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Heal. 2015;1:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. Jama. 2013;310:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Miller BR, Nasci RS, Godsey MS, et al. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley Province, Kenya. Am J Trop Med Hyg. 2000;62:240–246. [DOI] [PubMed] [Google Scholar]
- [146].Seruyange E, Twagirumugabe T, Bosco Gahutu J, et al. Chikungunya and West Nile viruses among blood donors in Rwanda: seroprevalence and distribution of mosquito vectors. 2018;1 DOI: 10.11604/pamj.cp.2017.4.43.248 [DOI] [Google Scholar]
- [147].Manson-Bahr PEC, Downie AW. Persistence of Tanapox in Tana River Valley. Br Med J. 1973;2:151LP– 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rodhain F, Ardoin P, Metselaar D, et al. An epidemiologic and serologic study of arboviruses in Lake Rudolf basin. Trop Geogr Med. 1975;27:307–312. [PubMed] [Google Scholar]
- [149].Enkhtsetseg A, Davadoorj R, Fernandez S, et al. Seroconversion to causes of febrile illness in mongolian peacekeepers deployed to South Sudan. Am J Trop Med Hyg. 2016;95:1469–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Depoortere E, Kavle J, Keus K, et al. Outbreak of West Nile virus causing severe neurological involvement in children, Nuba Mountains, Sudan, 2002. Trop Med Int Heal. 2004;9:730–736. [DOI] [PubMed] [Google Scholar]
- [151].Klitting R, Gould EA, Paupy C, et al. What does the future hold for yellow fever virus? (I). Genes (Basel). 2018;9:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Barrett ADT, Monath TP. Epidemiology and ecology of yellow fever virus. Advances in virus research. 2003;61:291–317. [DOI] [PubMed] [Google Scholar]
- [153].Mutebi J-P, Barrett ADT. The epidemiology of yellow fever in Africa. Microbes Infect. 2002;4:1459–1468. [DOI] [PubMed] [Google Scholar]
- [154].Reed W, Carroll J, Agramonte A, et al. The etiology of yellow fever—a preliminary note. Public Health Pap Rep. 1900;26:37. [PMC free article] [PubMed] [Google Scholar]
- [155].Monath TP. Facing up to re-emergence of urban yellow fe ver. Lancet. 1999;353:1541. [DOI] [PubMed] [Google Scholar]
- [156].Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. 2010;30:237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Huang Y-J, Higgs S, Horne K, et al. Flavivirus-mosquito interactions. Viruses. 2014;6:4703–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Germain M, Cornet M, Mouchet J, et al. Recent advances in research regarding sylvatic yellow fever in West and Central Africa. Bull Inst Pasteur. 1982;80:315–330. [Google Scholar]
- [159].Tomori O. Yellow fever: the recurring plague. Crit Rev Clin Lab Sci. 2004;41:391–427. [DOI] [PubMed] [Google Scholar]
- [160].Garske T, Van Kerkhove MD, Yactayo S, et al. Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014;11:e1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Kraemer MUG, Sinka ME, Duda KA, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. 2015;2:150035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ellis B, Barrett ADT. The enigma of yellow fever in East Africa. 2008;18 DOI: 10.1002/rmv.584. [DOI] [PubMed] [Google Scholar]
- [163].Smithburn KC, Haddow AJ, Lumsden WHR. An outbreak of sylvan yellow fever in Uganda with Aedes (Stegomyia) africanus Theobald as principal vector and insect host of the virus. Ann Trop Med Parasitol. 1949;43:74–89. [DOI] [PubMed] [Google Scholar]
- [164].Sawyer WA, Whitman L. The yellow fever immunity survey of North, East and South Africa. Trans R Soc Trop Med Hyg. 1936;29:397–412. [Google Scholar]
- [165].Sanders EJ, Marfin AA, Tukei PM, et al. First recorded outbreak of yellow fever in Kenya, 1992–1993. I. Epidemiologic investigations. Am J Trop Med Hyg. 1998;59:644–649. [DOI] [PubMed] [Google Scholar]
- [166].Onyango CO, Grobbelaar AA, Gibson GVF, et al. Yellow fever outbreak, southern Sudan, 2003. Emerg Infect Dis. 2004;10:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Onyango CO, Ofula VO, Sang RC, et al. Yellow fever outbreak, Imatong, southern Sudan. Emerg Infect Dis. 2004;10:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Hanley KA, Monath TP, Weaver SC, et al. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Dick GWA, Kitchen SF, Haddow AJ. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. [DOI] [PubMed] [Google Scholar]
- [170].Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. [DOI] [PubMed] [Google Scholar]
- [171].Plourde AR, Bloch EM. A literature review of zika virus. Emerg Infect Dis J. 2016;22:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Faye O, Freire CCM, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl Trop Dis. 2014;8:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97:269–273. [DOI] [PubMed] [Google Scholar]
- [174].Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Duffy MR, Chen T-H, Hancock WT, et al. Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med. 2009;360:2536–2543. [DOI] [PubMed] [Google Scholar]
- [176].Ioos S, Mallet H-P, Goffart IL, et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44:302–307. [DOI] [PubMed] [Google Scholar]
- [177].Haddow AD, Woodall JP. Distinguishing between Zika and Spondweni viruses. Bull World Health Organ. 2016;94:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Paixão ES, Barreto F, Da Glória Teixeira M, et al. History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health. 2016;106:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Posen HJ, Keystone JS, Gubbay JB, et al. Epidemiology of Zika virus, 1947–2007. BMJ Glob Heal. 2016;1:e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Rodhain F, Carteron B, Laroche R, et al. Human arbovirus infections in Burundi: results of a seroepidemiologic survey, 1980–1982. Bull Soc Pathol Exot Filiales. 1987;80:155–61. [PubMed] [Google Scholar]
- [181].Lwoff A, Tournier P. The classification of viruses. Annu Rev Microbiol. 1966;20:45–74. [DOI] [PubMed] [Google Scholar]
- [182].Porterfield JS, Casals J, Chumakov MP, et al. Togaviridae. Intervirology. 1978;9:129–148. [DOI] [PubMed] [Google Scholar]
- [183].Andrewes C. Viruses of vertebrates. Viruses Vertebr 1964.
- [184].Fenner F. The classification and nomenclature of viruses. J Gen Virol. 1976;31:463–470. [DOI] [PubMed] [Google Scholar]
- [185].Fenner F, Pereira HG, Porterfield JS, et al. Family and generic names for viruses approved by the international committee on taxonomy of viruses, june 1974. Intervirology. 1974;3:193–198. [DOI] [PubMed] [Google Scholar]
- [186].Westaway EG, Brinton MA, Gaidamovich SY, et al. Togaviridae. Intervirology. 1985;24:125–139. [DOI] [PubMed] [Google Scholar]
- [187].Chen R, Mukhopadhyay S, Merits A, et al. ICTV virus taxonomy profile: togaviridae. J Gen Virol. 2018;99:761–762. [DOI] [PubMed] [Google Scholar]
- [188].Mossel EC, Crabtree MB, Mutebi J-P, et al. Arboviruses isolated from mosquitoes collected in Uganda, 2008–2012. J Med Entomol. 2017;54:1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Kading RC, Kityo RM, Mossel EC, et al. Neutralizing antibodies against flaviviruses, Babanki virus, and Rift Valley fever virus in Ugandan bats. Infect Ecol Epidemiol. 2018;8:1439215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Zeller H, Van Bortel W, Sudre B. Chikungunya: its history in Africa and Asia and its spread to new regions in 2013–2014. J Infect Dis. 2016;214:S436–40. [DOI] [PubMed] [Google Scholar]
- [191].Monath TP. The arboviruses: epidemiology andEcology. Boca Raton (FL): CRC Press. 2019;2. [Google Scholar]
- [192].Powers AM, Brault AC, Tesh RB, et al. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. [DOI] [PubMed] [Google Scholar]
- [193].Weaver SC. Arrival of Chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Negl Trop Dis. 2014;8:e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wahid B, Ali A, Rafique S, et al. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017;58:69–76. [DOI] [PubMed] [Google Scholar]
- [195].Cavrini F, Gaibani P, Pierro AM, et al. Chikungunya: an emerging and spreading arthropod-borne viral disease. J Infect Dev Ctries. 2009;3:744–752. [DOI] [PubMed] [Google Scholar]
- [196].Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008;36:2536–2541. [DOI] [PubMed] [Google Scholar]
- [197].Sam I-C, Kümmerer BM, Chan Y-F, et al. Updates on chikungunya epidemiology, clinical disease, and diagnostics. Vector-Borne Zoonotic Dis. 2015;15:223–230. [DOI] [PubMed] [Google Scholar]
- [198].Borgherini G, Poubeau P, Staikowsky F, et al. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis. 2007;44:1401–1407. [DOI] [PubMed] [Google Scholar]
- [199].Ramful D, Carbonnier M, Pasquet M, et al. Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J. 2007;26:811–815. [DOI] [PubMed] [Google Scholar]
- [200].Caglioti C, Lalle E, Castilletti C, et al. Chikungunya virus infection: an overview. New Microbiol. 2013;36:211–227. [PubMed] [Google Scholar]
- [201].Konongoi SL, Nyunja A, Ofula V, et al. Human and entomologic investigations of chikungunya outbreak in Mandera, Northeastern Kenya, 2016. PLoS One. 2018;13:e0205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Diallo M, Thonnon J, Traore-Lamizana M, et al. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281–286. [DOI] [PubMed] [Google Scholar]
- [203].Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. [DOI] [PubMed] [Google Scholar]
- [204].Sergon K, Njuguna C, Kalani R, et al. Seroprevalence of chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. Am J Trop Med Hyg. 2008;78:333–337. [PubMed] [Google Scholar]
- [205].Sergon K, Yahaya AA, Brown J, et al. Seroprevalence of chikungunya virus infection on Grande Comore Island, union of the Comoros, 2005. Am J Trop Med Hyg. 2007;76:1189–1193. [PubMed] [Google Scholar]
- [206].Renault P, Solet J-L, Sissoko D, et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–731. [PubMed] [Google Scholar]
- [207].Hertz JT, Munishi OM, Ooi EE, et al. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Chipwaza B, Mugasa JP, Selemani M, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8:e3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Berry IM, Eyase F, Pollett S, et al. Recent outbreaks of chikungunya virus (CHIKV) in Africa and Asia are driven by a variant carrying mutations associated with increased fitness for Aedes aegypti. BioRxiv. 2018;373316. [Google Scholar]
- [210].Weinbren MP, Haddow AJ, Williams MC. The occurrence of chikungunya virus in Uganda I. Isolation from mosquitoes. Trans R Soc Trop Med Hyg. 1958;52:253–262. [DOI] [PubMed] [Google Scholar]
- [211].McCrae AWR, Henderson BE, Kirya BG, et al. Chikungunya virus in the Entebbe area of Uganda: isolations and epidemiology. Trans R Soc Trop Med Hyg. 1971;65:152–168. [DOI] [PubMed] [Google Scholar]
- [212].Adam A, Seidahmed OME, Weber C, et al. Low seroprevalence indicates vulnerability of eastern and central Sudan to infection with chikungunya virus. Vector-Borne Zoonotic Dis. 2016;16:290–291. [DOI] [PubMed] [Google Scholar]
- [213].Kokernot RH, McIntosh BM, Worth CB. Ndumu virus, a hitherto unknown agent, isolated from culicine mosquitoes collected in northern Natal, Union of South Africa. Am J Trop Med Hyg. 1961;10:383–386. [DOI] [PubMed] [Google Scholar]
- [214].Masembe C, Michuki G, Onyango M, et al. Viral metagenomics demonstrates that domestic pigs are a potential reservoir for Ndumu virus. Virol J. 2012;9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lutomiah J, Omondi D, Masiga D, et al. Blood meal analysis and virus detection in blood-fed mosquitoes collected during the 2006–2007 Rift Valley fever outbreak in Kenya. Vector-Borne Zoonotic Dis. 2014;14:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Lwande OW, Lutomiah J, Obanda V, et al. Isolation of tick and mosquito-borne arboviruses from ticks sampled from livestock and wild animal hosts in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2013;13:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Crabtree M, Sang R, Lutomiah J, et al. Arbovirus surveillance of mosquitoes collected at sites of active Rift Valley fever virus transmission: kenya, 2006–2007. J Med Entomol. 2009;46:961–964. [DOI] [PubMed] [Google Scholar]
- [218].Jupp PG, McIntosh BM, Anderson D. Culex (Eumelanomyia) Rubinotus Theobald as Vector of Banzi, Germiston and Witwatersrand Viruses IV. Observations on the biology of C. rubinotus. J Med Entomol. 1976;12:647–651. [DOI] [PubMed] [Google Scholar]
- [219].Lutomiah J, Ongus J, Linthicum KJ, et al. Natural vertical transmission of Ndumu virus in culex pipiens (Diptera: culicidae) mosquitoes collected as larvae. J Med Entomol. 2014;51:1091–1095. [DOI] [PubMed] [Google Scholar]
- [220].Haddow AJ, Davies CW, Walker AJ. O’nyong-nyong fever: an epidemic virus disease in East Africa 1. Introduction. Trans R Soc Trop Med Hyg. 1960;54:517–522. [Google Scholar]
- [221].Sanders EJ, Rwaguma EB, Kawamata J, et al. O’nyong-nyong fever in south-central Uganda, 1996–1997: description of the epidemic and results of a household-based seroprevalence survey. J Infect Dis. 1999;180:1436–1443. [DOI] [PubMed] [Google Scholar]
- [222].Kiwanuka N, Sanders EJ, Rwaguma EB, et al. o’nyong-nyong fever in South-central Uganda, 1996—1997: clinical features and validation of a clinical case definition for surveillance purposes. Clin Infect Dis. 1999;29:1243–1250. [DOI] [PubMed] [Google Scholar]
- [223].Lanciotti RS, Ludwig ML, Rwaguma EB, et al. Emergence of epidemic O’nyong-nyong fever in Uganda after a 35-year absence: genetic characterization of the virus. Virology. 1998;252:258–268. [DOI] [PubMed] [Google Scholar]
- [224].Volk SM, Chen R, Tsetsarkin KA, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84:6497–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Rezza G, Chen R, Weaver SC. O’nyong-nyong fever: a neglected mosquito-borne viral disease. Pathog Glob Health. 2017;111:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Johnson BK, Gichogo A, Gitau G, et al. Recovery of o’nyong-nyong virus from anopheles funestus in Western Kenya. Trans R Soc Trop Med Hyg. 1981;75:239–241. [DOI] [PubMed] [Google Scholar]
- [227].Lutwama JJ, Kayondo J, Savage HM, et al. Epidemic O’Nyong-Nyong fever in southcentral Uganda, 1996–1997: entomologic studies in Bbaale village, Rakai District. Am J Trop Med Hyg. 1999;61:158–162. [DOI] [PubMed] [Google Scholar]
- [228].Atoni E, Rumberia C, Karungu S, et al. Arboviruses of human health significance in Kenya. Afr J Health Sc. 2018;31:121–141. [Google Scholar]
- [229].Smithburn KC, Haddow AJ. Semliki forest virus. I. isolation and pathogenic properties. J Immunol. 1944;49:141–157. [Google Scholar]
- [230].McIntosh BM, Worth CB, Kokernot RH. Isolation of semliki forest virus from Aedes (Aedimorphus) argenteopunctatus (theobald) collected in Portuguese East Africa. Trans R Soc Trop Med Hyg. 1961;55:192–198. [DOI] [PubMed] [Google Scholar]
- [231].Atkins GJ, Sheahan BJ, Liljeström P. The molecular pathogenesis of Semliki Forest virus: a model virus made useful? J Gen Virol. 1999;80:2287–2297. [DOI] [PubMed] [Google Scholar]
- [232].Mathiot CC, Grimaud G, Garry P, et al. An outbreak of human Semliki Forest virus infections in Central African Republic. Am J Trop Med Hyg. 1990;42:386–393. [DOI] [PubMed] [Google Scholar]
- [233].Willems WR, Kaluza G, Boschek CB, et al. Semliki Forest virus: cause of a fatal case of human encephalitis. Science. 1979;203:1127–1129. [DOI] [PubMed] [Google Scholar]
- [234].Hubálek Z, Rudolf I, Nowotny N. Arboviruses pathogenic for domestic and wild animals. Adv Virus Res. 2014;89: 201–275. Elsevier. [DOI] [PubMed] [Google Scholar]
- [235].Taylor RM, Hurlbut HS, Work TH, et al. Sindbis virus: a newly recognized arthropod-transmitted virus1. Am J Trop Med Hyg. 1955;4:844–862. [DOI] [PubMed] [Google Scholar]
- [236].Woodall J, Williams M, Ellice J. Sindbis infection in man. East Afr Virus Res Inst Rep. 1962;12:17. [Google Scholar]
- [237].Laine M, Luukkainen R, Toivanen A. Sindbis viruses and other alphaviruses as cause of human arthritic disease. J Intern Med. 2004;256:457–471. [DOI] [PubMed] [Google Scholar]
- [238].Tesh RB. Arthritides caused by mosquito-borne viruses. Annu Rev Med. 1982;33:31–40. [DOI] [PubMed] [Google Scholar]
- [239].Hubálek Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J Wildl Dis. 2004;40:639–659. [DOI] [PubMed] [Google Scholar]
- [240].Lundström JO, Pfeffer M. Phylogeographic structure and evolutionary history of Sindbis virus. Vector-Borne Zoonotic Dis. 2010;10:889–907. [DOI] [PubMed] [Google Scholar]
- [241].Adouchief S, Smura T, Sane J, et al. Sindbis virus as a human pathogen—epidemiology, clinical picture and pathogenesis. Rev Med Virol. 2016;26:221–241. [DOI] [PubMed] [Google Scholar]
- [242].Francy DB, Jaenson TGT, Lundström JO, et al. Ecologic studies of mosquitoes and birds as hosts of Ockelbo virus in Sweden and isolation of Inkoo and Batai viruses from mosquitoes. Am J Trop Med Hyg. 1989;41:355–363. [PubMed] [Google Scholar]
- [243].Woodall JP, Williams MC, Corbet PS, et al. The isolation of Sindbis virus from the mosquito Mansonia (Coquillettidia) fuscopennata (Theobald) in Uganda. Ann Trop Med Parasitol. 1964;58:383–389. [DOI] [PubMed] [Google Scholar]
- [244].Sigei F, Nindo F, Mukunzi S, et al. Evolutionary analyses of Sindbis virus strains isolated from mosquitoes in Kenya. Arch Virol. 2018;163:2465–2469. [DOI] [PubMed] [Google Scholar]
- [245].Gould E, Pettersson J, Higgs S, et al. Emerging arboviruses: why today? One Heal. 2017;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].McIntyre MMA. Trade integration in the East African Community: an assessment for Kenya. International Monetary Fund;2005. [Google Scholar]
- [247].Ferguson AG, Morris CN. Mapping transactional sex on the Northern Corridor highway in Kenya. Health Place. 2007;13:504–519. [DOI] [PubMed] [Google Scholar]
- [248].Chege SM, Wang D, Suntu SL, et al. Influence of technology transfer on performance and sustainability of standard gauge railway in developing countries. Technol Soc. 2018;56:79–92. [Google Scholar]
- [249].Wissenbach U, Wang Y. African politics meets Chinese engineers: the Chinese-built standard gauge railway project in Kenya and East Africa 2017.
- [250].Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009;103:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


