ABSTRACT
A source of comprehensive information on the prevalence of herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) can help researchers and policymakers address HSV related burden in the society. At the moment, this is not readily available. This study aims to fill this gap by attempting to estimate the seroprevalence of HSV-1 and HSV-2 infections in Nigeria on the basis of published data. A systematic review and meta-analysis including cross-sectional studies on HSV-1 and HSV-2 in Nigeria was conducted. Electronic databases including PubMed/MEDLINE, CENTRAL, African Journals Online (AJOL), ScienceDirect, WHO-Afro Library, WHO-IRIS and African Index Medicus were searched for English Language publications on HSV-1 and HSV-2. Seven relevant publications were identified. Seroprevalence measures of 3 and 23 for HSV-1 and HSV-2, respectively, were extracted. The pooled mean seroprevalences for HSV-1 and HSV-2 were 74.0% (37.4–99.4%) and 63.4% (56.1–70.4%) respectively. The mean seroprevalence of HSV-1 was higher among females, 82.4% (n = 509, CI, 36.6–100.0%), than males, 54.5% (n = 198, CI, 47.6–61.4%). The mean seroprevalence of HSV-2 were 51.8% (n = 1414, CI: 39.4–64.0%) and 86.5% (n = 162, CI: 80.8–91.3%) among healthy and clinical populations, respectively. The study was limited by the paucity of quality studies, variations in diagnostic methods and high heterogeneity in seroprevalence estimates. In conclusion, the seroprevalence of HSV-1 and HSV-2 remain high in Nigeria. Large and representative national epidemiological surveys covering all regions and specific groups are recommended.
KEYWORDS: Herpes simplex virus, seroprevalence, systematic review, meta-analysis, infection
1. Introduction
Herpes simplex virus types 1 and 2 are lifelong infections [1]. Following initial infection, lifetime latency is established within neural ganglions from which viruses can be reactivated periodically [2]. Globally, infections caused by herpes simplex virus types 1 and 2 are amongst the most common human viral infections. The transmission of HSV-2 is mainly through sexual means, while HSV-1 is transmitted non-sexually during infancy [2]. However, there is an increasing proportion of genital herpes infections caused by HSV-1 in the developed world. This is probably due to changes in sexual behavior, with oral-genital sex becoming very common [3]. Both types of the virus cause sub-clinical infection and thus many of those infected are oblivious of their infection status. When the patient becomes symptomatic however, there is a presence of episodic ulcerative lesions at the site of infection [1]. Globally, HSV-2 is also the most common sexually transmitted disease (STD) [4], and the most widespread cause of Genital Ulcer Disease (GUD) [5]. HSV-1 and HSV-2 infections sometimes lead to serious complications in infected individuals. These complications range from fatality in infants infected perinatally to corneal blindness, herpetic whitlow, gingivostomatitis, aseptic meningitis and encephalitis amongst others [6,7]. HSV also has a significant interaction with HIV, as HSV-2 infection quadruples the risk of transmitting HIV infection and also increases 2- to 3- fold the chances of acquiring the disease [1]. According to meta-analysis, HSV-2 seropositivity is associated with HIV acquisition risk ratio of 2.7 in men and 3.1 in women [8].
In order to adequately contain the spread of HSV, efficient serological testing is important [4]. ELISA can be used to detect type-specific IgG and IgM antibodies in the blood serum [4] because the existence of particular antibodies offers evidence of HSV-1, HSV-2, or HSV-1 and HSV-2 coinfection. In clinical management of HSV infection, Acyclovir, Famcyclovir and Pencyclovir have demonstrated increased efficacy [9]. Besides clinical management, public enlightenment to achieve prevention of infection of HSV should be mounted as currently there is low awareness of the infection among the Nigerian population. Prevention programs should, in particular, take into account the rapid increase in HSV-2 infection in adult life [10].
HSV-2 prevalence of 30–80% in women, and 10–50% in men has been reported in Sub-Saharan Africa [11]. In Nigeria, the seroprevalence of HSV-1 and HSV-2 has been reported in several populations such as female sex workers [12], pregnant women attending antenatal clinics [13,14], children and young adults [15,16], as well as individuals attending sexually transmitted infections clinic [17]. The prevalence of herpes simplex infection was estimated to range between 9.2% and 100%.
Currently, there is only limited information on the epidemiology of HSV in Nigeria. This is the first meta-analysis to be carried out to assess the overall prevalence of HSV-1 and HSV-2 in the Nigerian population, a country of approximately 190 million people. This meta-analysis will provide a clear and reliable understanding of the burden of herpes simplex infection in the country.
1.1. Objectives
To conduct a systematic review and meta-analysis of published data on the prevalence of herpes simplex virus type 1 and 2 infections in Nigeria.
2. Materials and methods
2.1. Data sources and search strategy
This meta-analysis was conducted as suggested by the Cochrane guidelines [18] and the review is being reported following the PRISMA guidelines [19]. A modification of an existing methodology and analysis plan [7] was applied. PUBMED/MEDLINE, ScienceDirect, African Journals Online (AJOL), African Index Medicus and CENTRAL databases were searched for relevant works in the English Language using a predefined holistic strategy based on the combination of relevant terms to ensure thorough retrieval of studies. Both text words and medical subject headings used in the literature search were adapted to fit each database. The main search strategy conducted in PubMed/MEDLINE is shown in Supplementary Table 2. The cutoff date for database search was 1 October 2018. Also, reference list of included publications, proceedings of conferences and other gray literature were searched for additional materials.
2.2. Study selection and inclusion/exclusion criteria
Two reviewers independently and sequentially screened the titles and abstracts of the publications identified through database searches after duplicates have been removed. The full text of publications that passed the initial title and abstract screening were retained. The screening of the full text was further independently performed by the two reviewers to assess for eligibility, consensually including and excluding publications based on the predefined inclusion and exclusion criteria. If the views of the two independent reviewers differed on a particular publication, its inclusion or exclusion was decided by a third reviewer.
The inclusion criteria were met by cross-sectional studies of participants residing in Nigeria and reporting the prevalence of HSV infection, or enough data to compute this estimate using type-specific diagnostic assays such as Enzyme Immunoassays (EIA) or enzyme-linked immunosorbent assays (ELISAs). Studies that reported the number of individuals on which the prevalence estimate was based were included.
Exclusion criteria included guidelines, perspectives, correspondences, systematic reviews and meta-analysis, vaccine efficacy trials, economic analyses, modeling, time series, KAP (Knowledge, Attitude and Practice) studies, qualitative studies, questionnaire-based studies, genotype or mutation analysis studies. We also excluded studies that sampled Nigerians not resident in Nigeria at the time of the study and studies reporting the prevalence of herpes infection caused by Human Herpes Virus (HHV) 3–8.
The delineation of publication, study and measure by Khadr et al. [7] was applied.
2.3. Quality assessment
All selected studies were rated using the quality checklist reported in a previous study [20]. This checklist was an adaptation of the STROBE checklist and included questions addressing sample size, type of study, study population, sampling methods, data collection methods and tools, variables definition, statistical methodology, study objectives and illustration of the results based on objective [21]. Each question in the checklist was assigned a unitary point and only studies scoring at least 8 points were included in the review [20]. The quality of the included studies was further assessed by conducting risk of bias (ROB) assessment (as outlined by the Cochrane approach) [18] and precision assessment. ‘The studies were categorized as high or low risk of bias and precision based on the differentiation employed in a previous study. Studies were categorized as low versus high ROB using two quality domains assessing the rigor of sampling method (probability based vs otherwise) and response rate (≥80% vs otherwise). A study was considered to have high (vs low) precision if the sample size was ≥100’ [7].
2.4. Data extraction and statistical analysis
The data extraction was done independently by two reviewers. Variables that were extracted include: study region, year(s) of data collection, prevalence of HSV-1 and HSV-2, diagnostic method used, study design, sampling method, study site and sample size. This information was entered into an Excel spreadsheet.
The pooled prevalence of HSV-1 and HSV-2 in the Nigerian population with 95% confidence intervals (CI) was estimated using DerSimonian-Laird random-effects models provided ≥1 measure was available. This method accounts for sampling variation and heterogeneity in effect size [22]. The Freeman-Turkey double-arcsine transformation was used for variance stabilization [23].
The Cochran Q statistic was calculated to assess the existence of heterogeneity in effect size (P< .10 indicated heterogeneity) [23]. The I2 heterogeneity measure was estimated to assess the percentage of between-study variation in effect size that is due to actual differences in effect size rather than chance [7].
Subgroup analyses were done by summarizing prevalence data by specific populations. For HSV-1, Age bracket (children, adult and mixed), sex and region-specific (North-West and North-Central) subgroup analyses were done. For HSV-2, healthy, clinical and other populations (HIV-infected patients and female sex workers, pregnant women), age group (≤20, 21–40, >40 and mixed years), sex (male, female and mixed) and region-specific (South–South, North-Central and South-West) subgroup analyses were carried out.
The meta-analyses were performed using MetaXL software v. 5.3 (EpiGear International Pty Ltd).
3. Results
3.1. Search results and scope of evidence
The search of the different databases identified 84 publications with 9 duplicates which were removed. After the screening of title and abstract, 48 publications were eliminated. One (1) publication was included after screening reference lists of retrieved publications. The full texts of 28 publications were scrutinized for eligibility, among which 21 were excluded. The reasons for the exclusion are illustrated in Figure 1. Overall, 7 publications reporting the outcome of 26 studies (HSV-1, n = 3; HSV-2, n = 23) were deemed eligible and thus included in the meta-analysis. The characteristics of each study are shown in Table 1.
Figure 1.
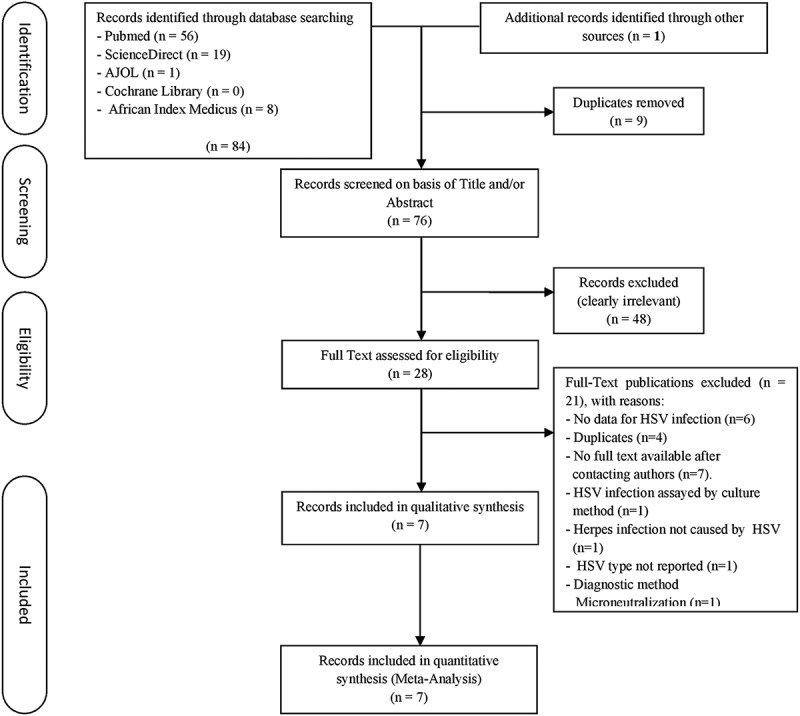
Process of identification and selection for inclusion in the review. HSV, Herpes Simplex Virus.
Table 1.
Studies reporting herpes simplex virus type 1 and 2 seroprevalence among different populations in Nigeria.
| Authors (Year) | Year (s) of Data Collection | Geopolitical Zone | Study Site | Study Design | Sampling Method | Population | Serological Assay | Sample Size, No. | HSV Seroprevalence, No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Herpes Simplex Virus Type 1 | |||||||||
| Healthy Children Populations (n = 2) | |||||||||
| Shiabu et al. [16] | 2011 | North-West | Hospital | CS | Conv. | 0-5-y-old male children | ELISA | 198 | 108 (54.5) |
| Shiabu et al. [16] | 2011 | North-West | Hospital | CS | Conv. | 0-5-y-old female children | ELISA | 179 | 110 (61.5) |
| Healthy Adult Populations (n = 1) | |||||||||
| Drisu et al. [24] | N/A | North-Central | Community | CS | Conv. | 15-49-y-old women | ELISA | 330 | 318 (96.4) |
| Herpes Simplex Virus Type 2 | |||||||||
| Healthy Adult Populations (n = 8) | |||||||||
| Kalu [13] | 2011–2012 | South-South | Hospital | Cohort | Conv. | 16-20-y-old pregnant women | ELISA | 7 | 2 (28.6) |
| Kalu [13] | 2011–2012 | South-South | Hospital | Cohort | Conv. | 21-25-y-old pregnant women | ELISA | 98 | 38 (38.8) |
| Kalu [13] | 2011–2012 | South-South | Hospital | Cohort | Conv. | 26-30-y-old pregnant women | ELISA | 242 | 96 (39.7) |
| Kalu [13] | 2011–2012 | South-South | Hospital | Cohort | Conv. | 31-35-y-old pregnant women | ELISA | 225 | 109 (48.4) |
| Kalu [13] | 2011–2012 | South-South | Hospital | Cohort | Conv. | 36-40-y-old pregnant women | ELISA | 86 | 59 (68.6) |
| Kalu [13] | 2011–2012 | South-South | Hospital | Cohort | Conv. | 41-45-y-old pregnant women | ELISA | 16 | 8 (50.0) |
| Drisu et al. [24] | N/A | North-Central | Community | CS | Conv. | 15-49-y-old women | ELISA | 330 | 254 (77.0) |
| Kalu [14] | 2011–2012 | South-South | Hospital | CS | Conv. | 15-45-y-old pregnant women | ELISA | 410 | 194 (47.3) |
| Clinical Adult Population (n = 7) | |||||||||
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 21-30-y-old male patients attending an STI clinic | EIA | 3 | 2 (66.6) |
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 21-30-y-old female patients attending an STI clinic | EIA | 78 | 65 (83.3) |
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 31-40-y-old male patients attending an STI clinic | EIA | 10 | 10 (100.0) |
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 31-40-y-old female patients attending an STI clinic | EIA | 42 | 38 (90.4) |
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 41-50-y-old male patients attending an STI clinic | EIA | 1 | 1 (100.0) |
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 41-50-y-old female patients attending an STI clinic | EIA | 11 | 10 (90.9) |
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 51-60-y-old female patients attending an STI clinic | EIA | 3 | 3 (100.0) |
| Clinical Mixed Age Population | |||||||||
| Agabi et al. [25] | N/A | North-Central | Hospital | CS | Conv. | 11-20-y-old female patients attending an STI clinic | EIA | 14 | 12(85.7) |
| Other Populations (n = 7) | |||||||||
| Dada et al. [12] | 1990–1991 | South-West | Community | CS | Conv. | >20-y-old female sex workers | ELISA | 796 | 470(59.0) |
| Odebisi-Omokanye et al. [17] | N/A | North-Central | Hospital | CS | Conv. | 11-20-y-old HIV patients | ELISA | 12 | 3(25.0) |
| Odebisi-Omokanye et al. [17] | N/A | North-Central | Hospital | CS | Conv. | 21-30-y-old HIV patients | ELISA | 27 | 22(81.5) |
| Odebisi-Omokanye et al. [17] | N/A | North-Central | Hospital | CS | Conv. | 31-40-y-old HIV patients | ELISA | 98 | 46(46.9) |
| Odebisi-Omokanye et al. [17] | N/A | North-Central | Hospital | CS | Conv. | 41-50-y-old HIV patients | ELISA | 65 | 39(60.0) |
| Odebisi-Omokanye et al. [17] | N/A | North-Central | Hospital | CS | Conv. | 51-60-y-old HIV patients | ELISA | 43 | 26 (60.5) |
| Odebisi-Omokanye et al. [17] | N/A | North-Central | Hospital | CS | Conv. | 61-70-y-old HIV patients | ELISA | 31 | 19 (61.3) |
Abbreviations: N/A, Not Available; CS, Cross-Sectional; Conv, Convenience; ELISA, Enzyme-Linked Immunosorbent Assay; HSV-1, Herpes Simplex Virus Type 1; HSV-2, Herpes Simplex Virus Type 2; EIA, Enzyme Immunoassay; HIV, Human Immunodeficiency Virus; STI, Sexually Transmitted Infection.
3.2. Seroprevalence overview
The earliest and latest measures were published in 1990 and 2018, respectively. All the measures were based on cross-sectional or cohort study designs. The estimated overall seroprevalence measures ranged from 54.5–96.4% for HSV-1 and 25.0–100.0% for HSV-2.
3.3. Pooled seroprevalence estimates
Table 2 shows the results of the HSV-1 seroprevalence meta-analyses. The mean seroprevalence of HSV-1 was highest among females. Subgroup seroprevalence could only be reported for two out of the six geopolitical regions in the country with both reported regions in Northern Nigeria. North-West region had a lower seroprevalence than the North-Central region. Figure 2 shows the forest plot of the overall pooled estimate of HSV-1 seroprevalence.
Table 2.
Pooled mean estimates for herpes simplex virus type 1 seroprevalence among different populations in Nigeria.
| Population Type | Outcome measures, Total No. | Samples, Total No. | HSV-1 Seroprevalence |
Pooled Mean HSV-1 Seroprevalence, Mean (95% CI) |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|
| Range | Median | I2(95 % CI), % | Q | P value | ||||
| Age bracket | ||||||||
| All Children | 2 | 377 | 54.5–61.5 | 57.9(51.1–64.6) | 93.6 (89.3–96.2) | 1.8 | 0.176 | |
| All Adult | 1 | 318 | 96.4 | - | - | - | - | - |
| Sex | ||||||||
| Male | 1 | 198 | - | - | 54.5 (47.6–61.4) | - | - | - |
| Female | 2 | 509 | 61.5–96.4 | 79.0 | 82.4 (36.6–100.0) | 99.0 (98.1–99.5) | 105.0 | 0.000 |
| Region-specific | ||||||||
| North-West | 2 | 377 | 54.5–61.5 | 58 | 57.9 (51.1–64.6) | 45.3 (0.0–0.0) | 1.8 | 0.176 |
| North-Central | 1 | 330 | - | - | 96.4 (94.0–98.1) | - | - | - |
| All Studies | 3 | 707 | 54.5–96.4 | 61.5 | 74.0(37.4–99.4) | 98.9(98.2–99.4) | 186.4 | 0.000 |
Figure 2.
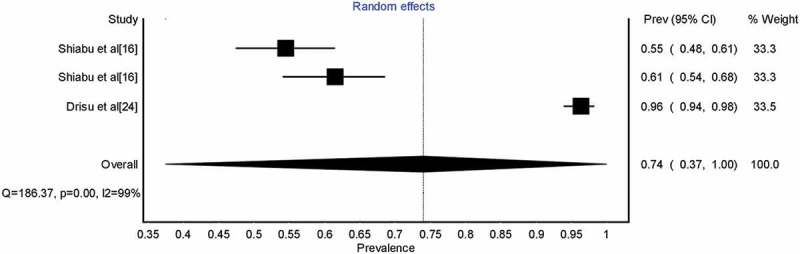
Forest plot showing overall Herpes Simplex Virus type 1 seroprevalence in Nigeria.
Table 3 shows the results of the HSV-2 seroprevalence meta-analyses. The seroprevalence of HIV-infected patients and female sex workers were higher than that of the healthy general population. The HSV-2 seroprevalence was higher in 21–40-year-old individuals compared to individuals >40 years, but the lowest seroprevalence among the different age groups was recorded in individuals ≤20 years. Unlike in the case of HSV-1, seroprevalence of HSV-2 was higher in males. For the subgroup analysis based on region, the North-Central region had higher seroprevalence compared to the South–South and South-West regions. Figure 3 shows the forest plot of the overall pooled estimate of HSV-2 seroprevalence.
Table 3.
Pooled mean estimates for herpes simplex virus type 2 seroprevalence among different populations in Nigeria.
| Population Type | Outcome measures, Total No. | Samples, Total No. | HSV-2 Seroprevalence |
Pooled Mean HSV-2 Seroprevalence, Mean (95% CI) |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|
| Range | Median | I2(95 % CI), % | Q | P value | ||||
| Healthy general population | 8 | 1414 | 28.6–68.6 | 47.9 | 51.8(39.4–64.0) | 94.5(91.3–96.5) | 127.2 | 0.000 |
| Pregnant women | 7 | 1084 | 28.6–68.6 | 47.3 | 47.6(40.4–54.8) | 76.4(50.5–88.8) | 25.4 | 0.000 |
| Clinical populations | 8 | 162 | 66.6–100 | 90.7 | 86.5(80.8–91.3) | 0.0(0.0–51.0) | 4.6 | 0.704 |
| Other populations | ||||||||
| HIV-infected patients | 6 | 276 | 25.0–81.5 | 60.3 | 57.5 (45.9–68.6) | 68.9 (29.4–87.1) | 16.6 | 0.005 |
| Female sex workers | 1 | 796 | - | - | 59.0 (55.6–62.4) | - | - | - |
| Age group | ||||||||
| ≤20 years | 3 | 33 | 25.0–85.7 | 28.6 | 48.3(6.8–91.0) | 82.8(48.1–94.4) | 11.7 | 0.003 |
| 21–40 years | 10 | 909 | 38.8–100.0 | 67.6 | 66.3 (52.9–78.5) | 92.9 (89.0–95.4) | 126.8 | 0.000 |
| >40 years | 7 | 170 | 50.0–100.0 | 61.3 | 63.8 (54.2–72.8) | 27.0 (0.0–68.3) | 8.2 | 0.223 |
| Mixed | 3 | 1536 | 47.3–77.0 | 59.0 | 61.7 (45.9–76.4) | 97.2 (94.4–98.6) | 71.4 | 0.000 |
| Sex | ||||||||
| Male | 3 | 14 | 66.6–100.0 | 100.0 | 89.5 (64.3–100.0) | 26.2 (0.0–92.3) | 2.7 | 0.258 |
| Female | 14 | 2358 | 28.6–100.0 | 63.8 | 63.3 (54.4–72.3) | 93.4 (90.6–95.4) | 198.0 | 0.000 |
| Mixed | 6 | 276 | 25.0–81.5 | 60.2 | 57.5(45.9–68.6) | 68.9(29.4–87.1) | 16.6 | 0.005 |
| Region-specific | ||||||||
| South-South | 7 | 1084 | 28.6–68.6 | 47.3 | 47.6 (40.4–54.8) | 76.4 (50.5–88.8) | 25.4 | 0.000 |
| North-Central | 15 | 768 | 25.0–100.0 | 81.5 | 73.2 (63.7–81.8) | 81.8 (71.0–88.5) | 76.9 | 0.000 |
| South-West | 1 | 746 | 63.0 | - | - | - | - | - |
| All Studies | 23 | 2648 | 25.0–100.0 | 66.7 | 63.4(56.1–70.4) | 90.4(86.9–92.9) | 228.3 | 0.000 |
Figure 3.
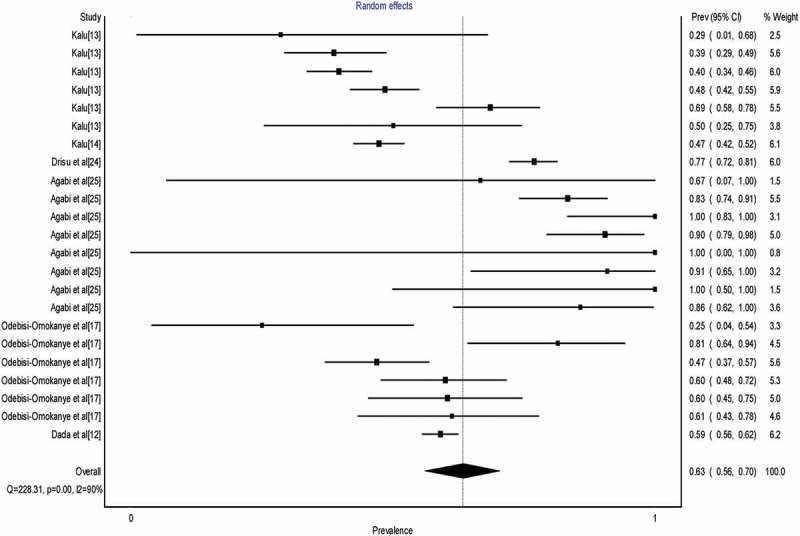
Forest plot showing overall Herpes Simplex Virus type 2 seroprevalence in Nigeria.
4. Discussion
This is the first systematic review and meta-analysis with a focus on HSV-1 and HSV-2 infections in Nigeria. It highlights the seroprevalence estimate of specific populations. Also, the impact of gender, age bracket, age group, geopolitical region and HIV co-infection were assessed. The information contained in this systematic review and meta-analysis will contribute to improved knowledge of HSV infection in Nigeria and Sub-Saharan Africa. It also can, in addition, serve as a guide in the mapping of intervention programs to tackle the menace. The pooled mean seroprevalences of HSV-1 and HSV-2 were 74.0% (37.4–99.4%) and 63.4% (56.1–70.4%) respectively.
The overall HSV-2 seroprevalence of 63.4% is higher than the HSV-2 prevalence of 26.6% observed in Kenya [26], 13.2% in China [27], 18.8% in Russia [28], 6.0% in Netherlands [29] and 15.7% in the United States [1]. These studies had approximately 2.5–10 times lower HSV-2 seroprevalence compared to Nigeria. Also, contrary to the findings of many studies [27,30–33], it was found that seroprevalence of HSV-2 was higher in males compared to females in Nigeria, but this observation should be interpreted with caution as only 3 studies with a total sample population of 14 were used to estimate the seroprevalence of HSV-2 among Nigerian males and as such the disparity may be due to sparseness of seroprevalence data amongst the Nigerian male population. The seroprevalence of 57.5% among HIV-infected patients is in tandem with the established trend of risk association between HSV-2 and HIV [34]. High-risk behavior among HIV-infected patients does not significantly predispose them to a higher probability of contracting HSV-2 compared to HIV-infected patients who do not engage in high-risk behaviors [35]. The high prevalence of HSV-2/HIV co-infection observed in this study can be attributed to the lower immunity in HIV positive individuals which increases their susceptibility to HSV infection. Also, there is an influx of HIV target cells due to HSV-2 replication in the genital mucosa [26]. Control strategies for HSV-2 should be incorporated as a strategy for HIV prevention and vice versa. The seroprevalence among female sex workers (FSW) (59.0%) observed in this study is comparable to 58.3% prevalence observed in FSW in the Hekuo district of China [36]. FSWs are a high-risk group and factors that influence the prevalence of HSV-2 in FSWs include older age, HIV infection, duration of sex work and oral sex [36]. Among the pregnant women, we observed an HSV-2 prevalence of 47.6% which is higher than prevalence estimates obtained among pregnant women in Tanzania [37], South Africa [38] and India [39] but lower than prevalence in Haiti [40] and Turkey [41]. The high prevalence of HSV-2 in Nigerian pregnant women can infer that there is a concomitant high probability of neonatal HSV-2 transmission and this is important because as much as 70% of neonatal HSV infection is caused by women who present no symptoms of HSV lesions at delivery [42]. Also, the prevalence data among pregnant women were obtained from only one state (Edo). HSV-2 seroprevalence also varied according to the geopolitical region; seroprevalence in North-Central region was 73.2%, compared to 47.6% and 63.0% for South–South and South-West regions, respectively. There were no clear reasons observed for the variability in seroprevalence among the regions but these estimates based on regions are also prone to paucity of adequate studies as there are no available data for the South–East, North–West and North-Eastern regions. Specific strategies should be implemented in the North-Central region where the prevalence is higher.
The overall HSV-1 seroprevalence observed in this meta-analysis (74.0%) is higher than the 25.7% reported in Iran [2], 53.9% in the United States [1] and the 67% global estimate [43]. However, the HSV-1 seroprevalence among Nigerian males (54.5%) and females (82.4%) estimated in this meta-analysis is lower than the 87% reported for both males and females in Africa [43]. The gender disparity in HSV-1 prevalence can be attributed to the external genitalia of women having a more vulnerable mucosal lining leading to them having a higher susceptibility to HSV-1 [44]. It should be pointed out that the North-Central region also has the highest HSV-1 seroprevalence at 96.4% (as was the case with HSV-2) compared to 57.9% for North-West. Data were unavailable for the South–East, South–South, South–West and North-Western regions. The North-Central region should be further studied to determine if the higher seroprevalence for both HSV-1 and HSV-2 was due to the inadequacy in seroprevalence studies or if it is indeed a case of heightened HSV-1 and HSV-2 infection. Studies on other sexually transmitted viral infections such as HBV [45,46] and HIV [45,47] in Nigeria have reported a higher prevalence of these infections in the North-Central region compared to other regions of the country.
Our estimates are hampered by small numbers of studies reporting prevalence of both HSV-1 and HSV-2, and by issues of small sample size (78.3% of studies for HSV-2 had low precision of prevalent measures, i.e. sample population <100), quality of data (all the studies had unclear risk of bias in at least one domain) and generalizability within studies. Also, data availability varied by states and region with only 7 states (Oyo, Kwara, Plateau, Edo, Kogi, Kwara, Kaduna and Lagos) out of 36 states and the Federal Capital Territory reporting seroprevalence studies. Seroprevalence showed high heterogeneity, I2 = 97.4 and 95.8 for overall HSV-1 and HSV-2 pooled seroprevalence, respectively (P= 0.000). Different diagnostic assays were used across studies, and assays may vary by sensitivity and specificity.
5. Conclusion
The seroprevalence of both HSV-1 and HSV-2 remains high in Nigeria. The sparseness of seroprevalence studies shows the virus is understudied among researchers in Nigeria. A large representative epidemiological study encompassing all the regions and specific groups is recommended for a more reliable prevalence of HSV-1 and HSV-2 infection in the Nigerian population. The findings of this study also highlight the need for an increase in the pace to develop vaccines for both HSV-1 and HSV-2 control.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors are very thankful to the College Station, Texas USA for sending us Stata 15 statistical software at no cost. We also wish to thank Prof. J. O. Ugwuanyi for proofreading the manuscript.
Author Contributions
Authors REE and IAC conceived the study. Authors REE and MSO carried out the initial search and review of literatures and ran the analysis. Author OINA served as a 3rd reviewer and performed an additional literature search. Author REE wrote the initial draft. Author IAC read the initial draft and provided general oversight of the study. All authors read and approved the final draft before submission.
Competing Interests
Authors declare no competing interests.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis. 2013;209:325–333. [DOI] [PubMed] [Google Scholar]
- [2].Malary M, Abedi G, Hamzehgardeshi Z, et al. The prevalence of herpes simplex virus type 1 and 2 infection in Iran: A meta-analysis. Int J Rep Biomed. 2016;14:615–624. [PMC free article] [PubMed] [Google Scholar]
- [3].Malkin JE. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes. 2004;11(Supp_1):S2A–23A. [PubMed] [Google Scholar]
- [4].Okonko IO, Cookey TI.. Seropositivity and determinants of immunoglobulin-G (IgG) antibodies against herpes simplex virus (HSV) types −1 and −2 in pregnant women in Port Harcourt, Nigeria. Afr Health Sc. 2015;15:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kroon S. Limiting the continued spread of genital herpes: recommendations from the IHMF management strategies workshop (management strategies in herpes). Surrey, UK: PPS Europe, 1994; 1–40. [Google Scholar]
- [6].Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Internal Med. 1998;98:958–972. [DOI] [PubMed] [Google Scholar]
- [7].Khadr L, Harfouche M, Omori R, et al. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2018;00:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kaul R, Nagelkerke NJ, Kimani J, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis. 2007;196:1692–1697. [DOI] [PubMed] [Google Scholar]
- [9].Abdulla AK. Seroprevalence of herpes simplex virus type 2 (HSV-2) in pregnant women and its relation to some blood cells and IL-2 in Kirkuk, Iraq. Mid East J Int Med. 2014;7:19–27. [Google Scholar]
- [10].Jaishankar D, Shukla D. Genital herpes: insights into sexually transmitted disease. Microb Cell. 2016;3:438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Supp_1):24A–25A. [PubMed] [Google Scholar]
- [12].Dada AJ, Ajayi AO, Diamondstone L, et al. A serosurvey of Haemophilus ducreyi, syphilis, and herpes simplex virus type 2 and their association with human immunodeficiency virus among female sex workers in Lagos, Nigeria. Sex Trans Dis. 1998;25:237–242. [DOI] [PubMed] [Google Scholar]
- [13].Kalu EI, Ojide CK, Chuku A, et al. Obstetric outcomes of human herpes virus-2 infection among pregnant women in Benin, Nigeria. Nig J Clin Pract. 2015;18:453–461. [DOI] [PubMed] [Google Scholar]
- [14].Kalu EI, Ojide KC, Fowotade A, et al. Sexual behavioral correlates with HSV-2 seroprevalence among pregnant women in Nigeria. J Infect Dev Ctries. 2014;8:1006–1012. [DOI] [PubMed] [Google Scholar]
- [15].Sogbetun AO, Montefiore D, Anong CN. Herpes hominis antibodies among children and young adults in Ibadan. Brit J Vener Dis. 1979;55:44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shaibu AM, Aminu M, Musa BOP, et al. Seroprevalence of IgG antibodies to herpes simplex virus type-1 in Nigerian children. Nig J Med. 2014;23:40–45. [PubMed] [Google Scholar]
- [17].Odebisi-Omokanye MB, Udeze AO, Akanbi K, et al. Serosurvey of herpes simplex virus type-2 infection among HIV infected individuals accessing a secondary health care facility in Kwara state, North Central Nigeria. Nig J Pure Appl Sc. 2017;30:3059–3065. [Google Scholar]
- [18].Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. [Cited 2018 September27].
- [19].Moher D, Liberati A, Tetzlaff J, et al., The Prisma Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moosazadeh M, Nekoei‐moghadam M, Emrani Z, et al. Prevalence of unwanted pregnancy in Iran: a systematic review and meta‐analysis. Int J Health Plann Manage. 2014;29:277–290. [DOI] [PubMed] [Google Scholar]
- [21].von Elm E, Altman DG, Egger M, et al., STROBE Initiative . Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- [22].Borenstein M, Hedges LV, Higgins JPT, et al. Identifying and quantifying heterogeneity In: Borenstein M, Hedges LV, Higgins JP, et al, editors. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons; 2009. p. 107–126. [Google Scholar]
- [23].Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21:607–611. [Google Scholar]
- [24].Drisu UI, Oronsaye EF, Adejumo GIB, et al. Seroprevalence, Type-specific of herpes simplex virus and associated risk factors among women of child bearing age in Kogi state, Nigeria. Health. 2018;10:1006–1017. [Google Scholar]
- [25].Agabi AY, Banwat EB, Mawak JD, et al. Seroprevalence of herpes simplex virus type-2 among patients attending the sexually transmitted infections clinic in Jos, Nigeria. J Infect Dev Ctries. 2010;4:572–575. [DOI] [PubMed] [Google Scholar]
- [26].Akinyi B, Odhiambo C, Otieno F, et al. Prevalence, incidence and correlates of HSV-2 infection in an HIV incidence adolescent and adult cohort study in Western Kenya. PLoS One. 2017;12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin H, He N, Su M, et al. Herpes simplex virus infections among rural residents in eastern China. BMC Infect Dis. 2011;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balaeva T, Grjibovski AM, Sidorenkov O, et al. Seroprevalence and correlates of herpes simplex virus type 2 infection among young adults in Arkhangelsk, Northwest Russia: a population-based cross-sectional study. BMC Infect Dis. 2016;16:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Woestenberg PJ, Tjhie JH, de Melker HE, et al. Herpes simplex virus type 1 and type 2 in the Netherlands: seroprevalence, risk factors and changes during a 12-year period. BMC Infect Dis. 2016;16:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carcamo CP, Campos PE, Garcia PJ, et al. Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: a national population-based survey. Lancet Infect Dis. 2012;12:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huai P, Li F, Li Z, et al. Seroprevalence and associated factors of HSV-2 infection among general population in Shandong Province, China. BMC Infect Dis. 2019;19:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brazzale AG, Russell DB, Cunningham AL, et al. Seroprevalence of herpes simplex virus type 1 and type 2 among the indigenous population of Cape York, Far North Queensland, Australia. Sex Health. 2010;7:453. [DOI] [PubMed] [Google Scholar]
- [33].McQuillan G, Moran-Kruszon D, Flagg EW, et al. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief. 2018;304;1–8. [PubMed] [Google Scholar]
- [34].Looker JK, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta- analysis. Lancet Infect Dis. 2017;17:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patel P, Bush T, Mayer KH, et al. The SUN study investigators. Prevalence and risk factors associated with herpes simplex virus-2 infection in a contemporary cohort of HIV-infected persons in the United States. Sex Transm Dis. 2017;39:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jie JW, Bin ZZ, Xi Y, et al. Herpes simplex virus type 2 risks in female sex workers in the China-Vietnam border county of Hekou. Biomed Envrion Sci. 2012;25:706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Malima-Yahya K, Olsen-Evjen B, Matee MI, et al. HIV-1, HSV-2 and syphilis among pregnant women in a rural area of Tanzania: prevalence and risk factors. BMC Infect Dis. 2008;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Abbai NS, Govender S, Nyirenda M. Herpes simplex virus-2 infections in pregnant women from Durban, South Africa: prevalence, risk factors and co-infection with HIV-1. S Afr J Infect Dis. 2018;1–7. [Google Scholar]
- [39].Biswas D, Borkakoty B, Mahanta J, et al. Seroprevalence and risk factors of herpes simplex virus type-2 infection among pregnant women in Northeast India. BMC Infect Dis. 2011;11:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Domercant JW, Louis FJ, Hulland E, et al. Seroprevalence of herpes simplex virus type-2 (HSV-2) among pregnant women who participated in a national HIV surveillance activity in Haiti. BMC Infect Dis. 2017;17:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dolar N, Serdaroglu S, Yilmaz G, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in Turkey. J Eur Acad Derma Vener. 2006;20:1232–1236. [DOI] [PubMed] [Google Scholar]
- [42].Anaedobe CG, Ajani TA. Sexual behavioural correlates of herpes simplex virus type 2 infections among pregnant women in South-western Nigeria. Inter J Com Med Pub Health. 2018;5:1274–1280. [Google Scholar]
- [43].Looker JK, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident of herpes simplex virus type 1 infections in 2012. PLoS ONE. 2015;10:e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marchi S, Trombetta CM, Gasparini R, et al. Epidemiology of herpes simplex virus type 1 and 2 in Italy: a seroprevalence study from 2000 to 2014. J Prev Med Hyg. 2017;58:E27–E33. [PMC free article] [PubMed] [Google Scholar]
- [45].Mbaawuaga EM, Iroegbu CU, Ike AC, et al. Studies on prevalence, co-infection and associated risk factors of hepatitis B virus (HBV) and human immunodeficiency virus (HIV) in Benue State, Nigeria. Sci J Pub Health. 2014;2:569–576. [Google Scholar]
- [46].Mac PA, Suleiman AC, Airiohuodion PE. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care in Central Nigeria. J Infect Dis Epidemiol. 2019;5:068. [Google Scholar]
- [47].FMO National HIV seroprevalence sentinel survey. 2010. [Cited 2018 November20]. Available from www.nigeriaiaids.org/documents/2010_National
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- FMO National HIV seroprevalence sentinel survey. 2010. [Cited 2018 November20]. Available from www.nigeriaiaids.org/documents/2010_National


