Abstract
Serological and proteomic biomarkers can help clinicians diagnose rheumatic diseases earlier and assess disease activity more accurately. These markers have been incorporated into the recently revised classification criteria of several diseases to enable early diagnosis and timely initiation of treatment. Furthermore, they also facilitate more accurate subclassification and more focused monitoring for the detection of certain disease manifestations, such as lung and renal involvement. These biomarkers can also make the assessment of disease activity and treatment response more reliable. Simultaneously, several new serological and proteomic biomarkers have become available in the routine clinical setting—for example, a protein biomarker panel for rheumatoid arthritis and a myositis antibody panel for dermatomyositis and polymyositis. This review will focus on commercially available antibody and proteomic biomarkers in rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis (scleroderma), dermatomyositis and polymyositis, and axial spondyloarthritis (including ankylosing spondylitis). It will discuss how these markers can facilitate early diagnosis as well as more accurate subclassification and assessment of disease activity in the clinical setting. The ultimate goal of current and future biomarkers in rheumatic diseases is to enable early detection of these diseases and their clinical manifestations, and to provide effective monitoring and treatment regimens that are tailored to each patient’s needs and prognosis.
Introduction
The National Institutes of Health biomarkers definition working group defines a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”1 Biomarkers are increasingly used for early detection of disease, prognostication of disease course, and prediction of response to treatment.
Serological and proteomic markers have been incorporated into the classification criteria of several rheumatic diseases.2 3 4 Furthermore, they are increasingly used to subclassify diseases and assess disease activity. These advances have enabled earlier diagnosis, which can facilitate more timely initiation of treatment and ultimately better prognosis. Thus, more accurate subclassification and assessment of disease activity enable a more focused approach to the monitoring and treatment of rheumatic diseases. The number of commercially available serological and proteomic markers has substantially increased in recent years, enabling them to be used in routine clinical settings. This review focuses on the use of these commercially available biomarkers in several rheumatic diseases and discusses how they can help clinicians in the diagnosis and assessment of disease activity.
Sources and selection criteria
We identified the references used in this review by performing a PubMed and Cochrane Database search of articles published between January 2000 and January 2015. We also searched the reference lists of the articles identified. The search terms consisted of the names of the rheumatic diseases (for example, rheumatoid arthritis), biomarkers, classification criteria, disease activity, and antibodies. We reviewed all clinical trials, meta-analyses, prospective and retrospective case series, and clinical registries. We prioritized biomarkers that are used for early diagnosis and more accurate monitoring of disease activity. We focused on serological and proteomic markers that are commercially available in the United States.
Rheumatoid arthritis
Evidence shows that early diagnosis combined with the timely use of disease modifying anti-rheumatic drugs (DMARDs) improves the disease course of rheumatoid arthritis.5 6 7 The challenge is therefore to identify the best markers to diagnose and monitor the disease. Leading laboratory markers that have been clinically useful include autoantibodies, acute phase reactants, bone and cartilage markers, and various cytokines.
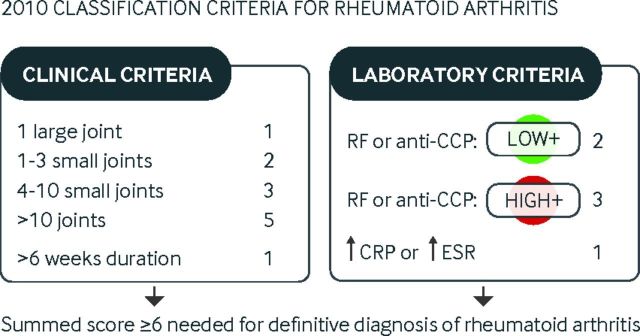
The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) proposed new classification criteria for rheumatoid arthritis in 2010, which were better equipped to diagnose early disease than the 1987 criteria, which were tailored towards the diagnosis of chronic erosive disease.2 The 2010 criteria factor in the number and site of joints (score range 0-5), disease duration (range 0-1), serum autoantibodies (range 0-3), and raised acute phase response (range 0-1), as summarized in fig 1.
Fig 1.
American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) 2010 classification criteria for rheumatoid arthritis
Autoantibodies
At least two broad classes of autoantibodies have been useful in the clinical management of rheumatoid arthritis, including rheumatoid factors and antibodies to citrullinated antigens, such as anti-CCP (cyclic citrullinated peptide) antibodies.
Rheumatoid factor
Rheumatoid factors are antibodies that are directed against IgG isotype antibodies. They are detected in the serum of 70-80% of patients with rheumatoid arthritis but they are not specific for rheumatoid arthritis. They can be detected in other systemic diseases, such as Sjögren’s syndrome and systemic infections, and in about 10% of healthy people.
As well as having a role in diagnostics, rheumatoid factor can also be used in prognosis. In a meta-analysis of available high quality studies (assessed by technical quality of the rheumatoid factor test, application of the reference or index test, blinding of observers, description of the study sample, and cohort assembly), rheumatoid factor had a sensitivity and specificity of 69% and 85%, respectively, for the diagnosis of rheumatoid arthritis.8 Several studies have shown that higher levels of rheumatoid factor are associated with more severe disease marked by disease progression, rheumatoid nodules, and various extra-articular manifestations.6 7 Finally, rheumatoid factor may also reflect the response to treatment, as exemplified in treatment studies using gold salts and DMARDs.9 10
Anti-cyclic citrullinated protein antibodies
Anti-cyclic citrullinated protein (CCP) antibodies are autoantibodies that react with a wide array of citrullinated peptides and proteins, including anti-perinuclear factor (APF), keratin, the Sa antigen, fibrin, fibrinogen, α enolase, eukaryotic translation initiation factor 4G1, vimentin, collagens, and synthetic CCPs.
Among these, anti-CCP antibodies are the most specific for rheumatoid arthritis, with 67% (95% confidence interval 62% to 72%) sensitivity and specificity of 95% (94% to 97%).8 It has been described as a complementary marker, because it is useful in rheumatoid factor negative patients with rheumatoid arthritis in the early phase of the disease.11 12 In other studies, the combination of rheumatoid factor and anti-CCP antibodies significantly increased sensitivity and specificity for the diagnosis of rheumatoid arthritis.13 A meta-analysis of 85 studies found that positivity for both anti-CCP antibodies and rheumatoid factor yielded a positive predictive value (PPV) close to 100%, significantly better than the PPV of either test alone. This dual positivity also identified patients with a more severe disease course.14
Abbreviations
ACR: American College of Rheumatology
ANA: Anti-nuclear antibody
ASAS: Assessment of Spondyloarthritis International Society
BAFF: B cell activating factor
CCP: Cyclic citrullinated peptide
CRP: C reactive protein
CTX-I/II: Telopeptides of type I/II collagen
DAS28: Disease activity score 28
DLCO: Diffusing capacity of the lung for carbon monoxide
DMARDs: Disease modifying anti-rheumatic drugs
dsDNA: Double stranded DNA
EGF: Epidermal growth factor
ELISA: Enzyme linked immunosorbent assay
ESR: Erythrocyte sedimentation rate
EULAR: European League Against Rheumatism
ILD: Interstitial lung disease
IIM: Idiopathic inflammatory myositis
MMP-3: Matrix metalloproteinase 3
MRI: Magnetic resonance imaging
NTproBNP: N-terminal pro-brain natriuretic peptide
OPG: Osteoprotegerin
PPV: Positive predictive value
RANKL: Receptor activator of nuclear factor κ B ligand
RNP: Ribonuclear protein
SLE: Systemic lupus erythematosus
SLICC: Systemic Lupus Erythematosus Collaborating Clinics
SSA/Ro: Sjögren’s syndrome related antigen A, also called Ro
SSB/La: Sjögren’s syndrome type B antigen, also known as La
TNF: Tumor necrosis factor
VCAM-1: Vascular cell adhesion molecule 1
VEGF: Vascular endothelial growth factor
Anti-CCP antibodies emerge as early as 10-14 years before the onset of symptoms.15 16 Interestingly, in a multivariate logistic regression model analysis of 937 patients with rheumatoid arthritis, only anti-CCP antibodies were independently associated with the development of ischemic heart disease.17
Finally, evidence about whether anti-CCP antibodies can be used to monitor the response to treatment is conflicting. Some studies reported a commensurate drop in anti-CCP antibodies after treatment, whereas others have failed to substantiate this finding.7
Acute phase reactants
Two acute phase reactants, erythrocyte sedimentation rate (ESR) and C reactive protein (CRP), have been widely studied in rheumatoid arthritis. An increases in these acute phase reactants correlates with disease outcomes such as joint erosion, Health Assessment Questionnaire Disability Index (HAQ-DI) scores, radiographic progression, and functional outcomes.18 19 20 It is therefore no surprise that inclusion of these acute phase reactants in composite disease activity indices such as the disease activity score 28 (DAS28) reliably predicts disease course.21 22 An increase in either of these acute phase reactants contributes one point towards a definitive diagnosis in current classification criteria for rheumatoid arthritis (fig 1).
However, the sensitivity and specificity of these markers is lower than that of autoantibodies. In a study published in 2014 of more than 9000 patients with rheumatoid arthritis, neither acute phase reactant was raised in 46% of patients who had high disease activity, while at least one of these acute phase reactants was raised in 33% of people with low disease activity.23 Raised CRP concentrations may indicate an increased risk of cardiovascular disease in patients with rheumatoid arthritis.24 Finally, it is important to note that inflammation or infection anywhere in the body can result in increased CRP and ESR values.
Bone and cartilage turnover markers
Although synovial, cartilage, and bone derived markers have limited value in diagnosing rheumatoid arthritis they may be useful in predicting and monitoring disease progression and response to treatment.25
Synovial markers
Matrix metalloproteinase 3 (MMP-3) has emerged as a promising biomarker because concentrations are raised in the joints in patients with active disease, serum MMP-3 is predictive of future joint destruction in early rheumatoid arthritis, serum MMP-3 concentrations decrease after treatment, and monitoring of MMP-3 improves therapeutic outcome.25 A recent multivariate analysis of 118 patients with rheumatoid arthritis identified baseline anti-CCP and MMP-3 as the strongest independent predictors of radiographic disease eight years later.26
Cartilage markers
Telopeptides of type II collagen (CTX-II) and cartilage oligomeric matrix protein (COMP) have shown promise as potential biomarkers in rheumatoid arthritis. Both are raised in active disease, are predictive of joint destruction, and are useful in monitoring response to treatment.25 27 Urinary N-telopeptide of type I collagen crosslinked peptide (uNTX) values were also increased in patients with rheumatoid arthritis (especially in female patients), correlate with functional class,28 29 and decrease after treatment with methotrexate.30
Bone derived markers
Of bone derived markers, telopeptide of type I collagen (CTX-I), a catabolic bone marker, and OPG/RANKL (osteoprotegerin/receptor activator of nuclear factor κ B ligand) are particularly noteworthy. A longitudinal study of 238 patients with rheumatoid arthritis monitored various bone and cartilage biomarkers over 10 years.31 Of the different markers tracked, baseline CTX-I was the most accurate predictor of disease progression and radiographic damage over the 10 years (β=16.4; interquartile range 5.7-27.1).31 CTX-I has also been shown to track treatment response after treatment with tumor necrosis factor (TNF) blockers.25
The OPG-RANKL-RANKL ligand signaling pathway maintains a fine balance between the activity of osteoblasts, which form bone, and osteoclasts, which resorb bone. In early untreated rheumatoid arthritis, the baseline RANKL:OPG ratio predicted long term bone damage, independent of other predictors (Spearman’s rho=−0.38; P=0.001 and R 2=19-21, P<0.001).32 33 34 In a more recent study of multiple biomarkers in early active untreated rheumatoid arthritis, baseline RANKL:OPG ratio, CTX-I and CTX-II emerged as the strongest independent predictors of damage progression over an 11 year period. The prediction of annual radiological progression was strongest when the RANKL:OPG ratio and CTX-I or CTX-II were all included in the prediction model (36-39% explained variance).34 As noted for CTX-I, serum RANKL is also useful for tracking the response to TNF blockade.35
Finally, inhibition of osteoclasts with denosumab, a humanized antibody to RANKL, reduces erosions and peri-articular bone loss without affecting synovial inflammation.32
Cytokines
Cytokines represent the fastest growing field of all the rheumatoid arthritis biomarkers. More than 40 cytokines have been examined for their diagnostic and prognostic potential in rheumatoid arthritis, including inflammatory cytokines, chemokines, and various growth factors.7 36 Research groups have carried out broad scale multiplexed screens (simultaneous determination of several proteins in the same well using solid phase assays) and have identified about 20 cytokines and related mediators that are raised in rheumatoid arthritis.37 38 39 40
An offshoot of these types of studies is the use of a novel commercially available protein biomarker panel (comprising vascular cell adhesion molecule 1 (VCAM-1), epidermal growth factor (EGF), vascular endothelial growth factor A (VEGF-A), IL-6 (interleukin 6), TNF receptor 1, MMP-1, MMP-3, YKL-40 (a secreted glycoprotein), leptin, resistin, serum amyloid A (SAA), and CRP) to monitor disease activity in rheumatoid arthritis. This panel incorporates various cytokines, chemokines, adhesion molecules, adipokines, and synovial or skeletal markers and has been shown to track disease activity in rheumatoid arthritis.41 42 43
Clearly, these newer panels warrant further validation alongside the biomarkers described above. More importantly, the composition of these panels will probably evolve rapidly as research into additional cytokines and serum proteins continues worldwide.
Finally, ongoing research in genomics, proteomics, and metabolomics will provide additional biomarker candidates for systematic validation.44 With these future developments, the laboratory criteria for diagnosing rheumatoid arthritis (fig 1) and tracking disease activity and treatment response are likely to evolve in the coming years.
Composite indices and biomarkers
As shown in fig 1, the ACR-EULAR classification criteria for rheumatoid arthritis include clinical measures and laboratory markers such as autoantibodies and acute phase reactants. Similarly, several composite indices have been reported for the routine assessment of disease status at each hospital visit.
DAS28 is a commonly used composite disease activity index that factors in the numbers of swollen or tender joints (28 joints included), acute phase reactants (ESR or CRP), and the patient’s “global assessment of health.”45
Another clinical index is the “ACR score,” which is used to measure changes in symptoms.46 Different degrees of improvement are referred to as ACR20, ACR50, ACR70, and so on. This score is more often used for assessing treatment response in clinical trials and represents a “common standard” between researchers across different clinical trials.
As for other indices described above, changes in acute phase reactants are also factored into the ACR scores. Currently, acute phase reactants and autoantibodies are the only laboratory assessed markers that are included in composite indices used to diagnose or monitor rheumatoid arthritis. However, as more biomarkers are validated, composite indices that incorporate newer laboratory assessed biomarkers and that have better clinical performance will hopefully become available.
Systemic lupus erythematosus (SLE)
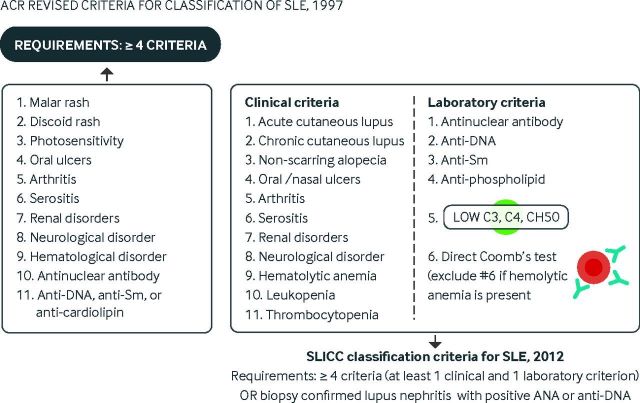
SLE is currently diagnosed by the presence of at least four of 11 criteria outlined by the ACR.47 48 In an attempt to improve the clinical relevance of these criteria, the Systemic Lupus Erythematosus Collaborating Clinics (SLICC) group revised the classification criteria in 2012 (fig 2).3
Fig 2.
Comparison of the American College of Rheumatology revised classification criteria for systemic lupus erythematosus (1997) and the Systemic Lupus Erythematosus Collaborating Clinics classification criteria (2012). ANA=anti-nuclear antibodies
The SLICC classification system resulted in fewer misclassifications and had higher sensitivity than the ACR classification system.3 Both schemes use the same raw criteria but weigh them differently. Important differences pertaining to the SLICC scheme include:
Hematological disorders can contribute as many as three points
Anti-DNA, anti-Sm, anti-phospholipid antibodies, and reduced levels of complement can each be counted as independent criteria
At least one clinical criterion and one laboratory criterion is needed
The presence of biopsy confirmed lupus nephritis and a positive anti-nuclear antibody (ANA) or anti-DNA test is sufficient for the diagnosis of SLE
The absence of photosensitivity as a separate diagnostic criterion may sometimes change classification results.
The relatively non-specific symptoms of SLE coupled with the need to meet four ACR or SLICC criteria often delays diagnosis.49 Hence, better diagnostic and prognostic markers are clearly needed in this field. Key biomarkers that are currently in clinical use or are likely to be used in the near future are summarized below.
Autoantibodies
Time course before development of SLE
Autoantibodies can be the earliest manifestations of SLE and the first diagnostic clue to emerge. This insight came from three longitudinal studies that carefully mapped the evolution of various autoantibody specificities over time.50 51 52 In the first study of 130 people with SLE, autoantibodies evolved in an ordered progression—ANAs, anti-phospholipid antibodies, anti-SSA/Ro (anti-Sjögren’s syndrome related antigen A, also called Ro), and anti-La antibodies emerged early (more than three years before diagnosis), whereas anti-double stranded DNA (anti-dsDNA) antibodies appeared about two years before diagnosis. Antibodies to Sm and U1 ribonucleoprotein (U1 RNP) were the last to surface, appearing about a year before diagnosis.50
In a second study of North Europeans, autoantibodies against nuclear antigens were detected on average 5.6 years before the onset of symptoms and 8.7 years before the diagnosis of SLE.51 Anti-SSA/Ro antibodies appeared earliest, at a mean of 6.6 years before the onset of symptoms.
In the third study, anti-cardiolipin antibodies emerged an average of three years before the diagnosis of SLE.52 Thus, in patients who do not meet the requirements for a diagnosis of SLE, the presence of any of these autoantibodies (especially those that are more specific than ANA) may be a prelude to SLE, and such patients should be followed up closely for the development of SLE.
Anti-nuclear antibodies
The ANA test, which measures the presence and titer of ANAs, is a broad screening test—the presence of many different autoantibody types can lead to a positive result. This test has sensitivities exceeding 98% for the diagnosis of SLE and lupus nephritis.53 54 However, the test lacks specificity because ANA can be positive in other autoimmune diseases, thyroid diseases, hepatic diseases, cancers, chronic infections, and elderly people.53 54
Specificity of different ANAs
Among the different ANAs, the dsDNA reactive anti-DNA antibodies are more specific for SLE.53 Although enzyme linked immunosorbent assays (ELISAs) are often used to measure this antibody, ELISAs are less specific but more sensitive than the gold standard immunofluorescence assay using Crithidia luciliae. In a study of 158 ANA positive people, anti-dsDNA ELISA was 79% sensitive and 73% specific for the diagnosis of SLE, whereas the C luciliae assay was 41% sensitive and 99% specific.55
Association with disease activity
Antibody titers vary with disease activity and flares, and they are also associated with the presence of lupus nephritis.56 57 58 59 A more recent study found that anti-dsDNA antibody levels were raised as early as four years before the diagnosis of proliferative lupus nephritis, and that they rose by about 1 IU/mL per year.60 Similarly, monitoring of anti-dsDNA antibody levels has helped guide early aggressive treatment and thereby prevented disease flares (both renal and otherwise).61 62 63 The utility of anti-dsDNA in predicting and monitoring treatment response has been re-validated in more recent clinical trials aimed at depleting B cells using rituximab or blocking the B cell activating factor (BAFF) pathway.64 65 66 67
Anti-Sm and anti-U1 RNP
Anti-Sm is also useful for the diagnosis of SLE (fig 1). It is more specific than anti-dsDNA antibodies because it has not been described in other rheumatic diseases, but it is less sensitive, being present in only 20-30% of patients.
Anti-U1 RNP is strongly associated with a related overlap disease termed mixed connective tissue disease, marked by the presence of other connective tissue diseases such as inflammatory myositis or systemic sclerosis. The specificity of anti-U1 RNP for mixed connective tissue disease ranges from 85% to greater than 99%.68 Unlike anti-DNA, there is little evidence for the clinical utility of measuring titers of anti-Sm or anti-U1 RNP when monitoring disease activity or treatment responses.
Anti-SSA
Another anti-nuclear antibody of clinical interest in SLE is anti-SSA/Ro. Although this antibody is highly associated with Sjögren’s syndrome and its associated symptoms (dry mouth and dry eyes), it is also present in 30% of patients with SLE and 70-90% of those with subacute cutaneous lupus erythematosus. Furthermore, it is also seen in mothers of children with neonatal lupus erythematosus and congenital heart block.53
In adults, anti-SSA antibodies correlate with photosensitivity, cutaneous vasculitis (palpable purpura), and hematological disorders (anemia, leukopenia, and thrombocytopenia). Although the levels of these antibodies seem to fluctuate with disease, more work is needed to substantiate this. Interestingly, these antibodies are among the earliest to appear, as early as six years before the diagnosis of SLE, even before anti-DNA antibodies appear.50 51
Anti-SSB/La
A related antibody, anti-SSB/La (Sjögren’s syndrome type B antigen, also known as La (Lupus)), is present in 10% of patients with SLE, 30% of those with subacute cutaneous lupus erythematosus, and 90% of patients with neonatal lupus and congenital heart block. The presence of these antibodies in patients with SLE might be associated with less severe renal and neurological manifestations.69 Similar to anti-SSA, anti-SSB antibodies are also strongly associated with Sjögren’s syndrome.
Anti-phospholipid antibodies
Anti-phospholipid antibodies (including anti-cardiolipin antibodies and the lupus anticoagulant) are present in a third of patients with SLE. Of these, one third go on to develop livedo reticularis and skin ulcers as well as clinical features of anti-phospholipid syndrome, including venous thrombosis, arterial thrombosis, recurrent pregnancy loss, thrombocytopenia, and hemolytic anemia.70 These antibodies are also associated with cerebral vascular disease and focal damage in neuropsychiatric SLE. Longitudinal studies have shown that these specificities emerge about 7.6 years before the diagnosis of SLE.52
Other antibodies
Several other antibodies have been described in association with various manifestations of SLE.53 Of particular interest is the high degree of association of anti-histone antibodies with drug induced lupus (sensitivity of 96-100%), of anti-ribosomal P antibodies with neuropsychiatric lupus, and of anti-C1q antibodies with renal lupus.
Autoantibodies in SLE arise several years before the diagnosis can be made. Those patients with autoantibodies who do not yet meet the criteria for a definitive diagnosis of SLE have long been classified as having “incomplete lupus erythematosus.”71 Recent studies report a 14-21% conversion rate from incomplete lupus erythematosus to SLE over a two to six year period.72 73 Although many of these patients are treated conservatively with hydroxychloroquine or low dose corticosteroids, no data are available to guide any particular drug regimen that can dampen the progression to SLE.
Reduced complement
Because autoantibodies play a prominent role in the pathogenesis of SLE and lupus nephritis, it is not surprising that complement proteins are consumed during disease. Several early studies showed that reduced complement C3 and C4 levels are associated with more severe disease in SLE, including renal flares.74 75 76 In recent longitudinal studies, baseline reductions in complement C3 or C4 were shown to predict disease flares two months or even a year before they occurred.67 77 Finally, the utility of reduced complement in predicting and monitoring treatment response has also been validated in recent clinical trials aimed at depleting B cells using rituximab or blocking the BAFF pathway.64 65 66 67
CRP and ESR
Although CRP is often raised in SLE, these increases are inconsistently related to disease activity or flares. Instead, they seem to reflect active infections, serositis, cardiovascular risk or certain polymorphisms in the gene encoding CRP.78 79 80 81 82 83
Another traditional marker of inflammation, ESR, has also been evaluated for its potential as a biomarker. In a longitudinal study, raised ESR was associated with disease activity or damage accrual in SLE,84 independent of the presence of anti-dsDNA antibodies. A subsequent analysis of 1000 serial measurements of ESR made at bimonthly intervals in 71 patients with lupus nephritis showed that increased ESR was an independent predictor of renal flares two months later and a marker of concurrent renal flare.77 Similar findings were noted in a study published in 2013—increased ESR correlated with concurrent SELENA-SLEDAI (SLE disease activity index), the physician global assessment, renal disease, joint disease, rash, serositis, hematological visual analogue scale scores, fatigue, hematuria and proteinuria, although future disease course could not be predicted.85
Given these promising findings the utility of ESR warrants continued assessment and study.
The interferon signature
At the RNA level, multiple gene expression studies have examined the peripheral blood cells in SLE. Interestingly, the overexpressed RNA molecules that overlap among these studies belong mainly to the type 1 interferon pathway.86 At the DNA level, several of the susceptibility genes implicated in SLE also map to this pathway, including IRF5, IRF7, IFIH1, STAT4, and TYK2. Similarly, at the protein level, several molecules related to this cytokine pathway have also been shown to be raised in SLE, often in association with disease activity; these include Siglec-1, galectin-3-binding protein, and interferon driven chemokines.87 88 89 90
Further research is needed to establish whether the interferon gene signature or related serum proteins have clinical utility as biomarkers for monitoring disease activity or treatment response in SLE.
Emerging biomarkers
Many recent publications have documented specific serum proteins as potential biomarkers of SLE or specific clinical manifestations associated with SLE. These include circulating levels of β2 microglobulin, syndecan-1, BAFF, fatty acid binding protein 4, ficolins, high mobility group protein B1, human neutrophil peptides 1-3, insulin-like growth factor 1, IL-6, IL-23, milk fat globule epidermal growth factor 8, oxidized low density lipoprotein, resistin, various oxidative stress markers, calcium binding proteins S100A8/A9 and S100A12, thiols, soluble Mer protein, urokinase plasminogen activator receptor, colony stimulating factor 1, receptor for advanced glycation end products, Toll-like receptor 2, E-selectin, and VCAM-1.
Few studies have screened serum proteins in an unbiased, non-hypothesis driven manner so that large numbers of protein biomarker candidates can be investigated in one scan. One study that examined 52 different soluble mediators, including cytokines, chemokines, and soluble receptors, used validated multiplex bead based or ELISAs in plasma from patients with SLE.91 Several soluble mediators were raised pre-flare, including T helper 1 (Th1)-type, Th2-type, and Th17-type cytokines; soluble TNF receptor I and II; Fas and Fas ligand; and CD40 ligand.
Most of the studies to date have used cross sectional datasets. Several ongoing assessments in longitudinal datasets will probably better define the best serum or urine markers of impending renal involvement and which protein markers would best complement current diagnostic criteria.
For many rheumatic diseases soluble protein biomarkers in body fluids may hold potential as therapeutic targets as well as disease markers. Examples of such protein targets that are raised in rheumatoid arthritis or SLE include TNF-α, IL-1, IL-6, BAFF, and interferon 1. Raised circulating levels and the availability of drugs that target these molecules underscore the pathogenic relevance of these molecules in rheumatic diseases.
Systemic sclerosis
Systemic sclerosis is a multisystem, autoimmune disease characterized by immune dysregulation, vasculopathy, and fibrosis of skin and internal organs. It is associated with high morbidity and mortality.92 Pulmonary involvement including interstitial lung disease (ILD) and pulmonary hypertension have replaced scleroderma renal crisis as the primary cause of disease related mortality.93 94
Systemic sclerosis related autoantibodies
Systemic sclerosis is associated with specific mutually exclusive autoantibodies.95 Three antibodies—anti-centromere, anti-topoisomerase I, and anti-RNA polymerase III antibodies—are included in the 2013 ACR/EULAR classification criteria for this disease.2 3 96 According to these new criteria, bilateral skin thickening of fingers that extends proximally to the metacarpophalangeal joints is sufficient for the diagnosis (sufficient criterion). Patients who do not fulfill this criterion are classified using a scoring system that includes sclerodactyly or puffy fingers, digital tip ulcers or pitting scars, telangiectasia, ILD or pulmonary arterial hypertension, Raynaud’s phenomenon, and the presence of one of the systemic sclerosis specific antibodies.
Systemic sclerosis is classified clinically on the basis of the extent of skin involvement into limited and diffuse subtypes—patients with skin involvement proximal to the elbows and knees are categorized as having diffuse cutaneous involvement.97 ANAs are present in 96% of patients with systemic sclerosis.98
Evidence suggests that systemic sclerosis related antibodies provide additional prognostic information beyond the limited/diffuse subclassification.99 100 101 Table 1 shows clinical correlates of these antibodies. Anti-topoisomerase I antibodies (also called anti-Scl-70) are more common in patients with diffuse cutaneous involvement. Furthermore, patients with these antibodies are at risk of developing progressive ILD and should be closely monitored for development of this complication.95 101 102 By contrast, anti-centromere antibodies are associated with limited cutaneous involvement, absence of clinically significant ILD, and better survival.95 100 101 103
Table 1.
Systemic sclerosis related antibodies and their demographic and clinical correlates
| Antibody | Prevalence* | Demographic and clinical associations |
|---|---|---|
| Anti-topoisomerase I (Scl-70) | 9-39% | Diffuse cutaneous involvement, interstitial lung disease |
| Anti-centromere | 16-39% | Limited cutaneous involvement, better survival |
| Anti-RNA polymerase III | 2-25% | Diffuse cutaneous involvement, scleroderma renal crisis, gastric antral vascular ectasia |
| Anti-Pm-Scl | 5-9% | Limited cutaneous involvement, polymyositis, and dermatomyositis |
| Anti-U3 RNP (anti-fibrillarin) | 5-18% | African-American race, pulmonary arterial hypertension |
*Prevalence varies widely according to ethnicity and geographic area.
RNP=ribonuclear protein.
RNA polymerase III
Anti-RNA polymerase III is another systemic sclerosis specific antibody that is associated with diffuse cutaneous involvement and is strongly linked to the occurrence of scleroderma renal crisis (odds ratios ranging from 5 to 17.5).104 105 106 Therefore, patients with systemic sclerosis and anti-RNA polymerase III antibodies should be monitored closely for new onset hypertension. In addition, oral glucocorticoids should be used with caution in patients with systemic sclerosis, especially those with anti-RNA polymerase III antibodies, because these drugs have been linked to the development of scleroderma renal crisis.107 108
Recently anti-RNA polymerase III antibodies have been linked to gastric antral vascular ectasia (also known as watermelon stomach) (odds ratio 4.6, 1.2 to 21.1), which can lead to gastrointestinal bleeding and severe anemia is patients with systemic sclerosis.109 If detected, bleeding gastric antral vascular ectasia lesions can be treated by endoscopic cauterization. Four cohort studies reported a higher prevalence of contemporaneous onset of cancer and systemic sclerosis in patients with anti-RNA polymerase III antibodies compared with the other two autoantibody groups.106 110 111 112
In addition, increased expression of nucleolar RNA polymerase III was found in tumor tissue samples from patients with anti-RNA polymerase III antibodies but not in patients with the other autoantibodies.110 Moreover, genetic alterations of the gene encoding polymerase III polypeptide A was found in the tumor tissue of six out of eight patients with anti-RNA polymerase III antibodies but none of eight patients without these antibodies, supporting the hypothesis that there may be a link between cancer and the systemic sclerosis specific immune response in this serological subset.113
Anti-PM-Scl and anti-U3-RNP antibodies
Two less common systemic sclerosis related antibodies that show anti-nucleolar staining pattern on ANA testing are anti-PM-Scl and anti-U3-RNP antibodies (also called anti-fibrillarin antibodies). PM-Scl antibodies are associated with overlap with other connective tissue diseases (particularly polymyositis-dermatomyositis), limited cutaneous involvement, and better survival.114 115
Anti-U3-RNP antibodies are found in about 5% of white patients with systemic sclerosis and 18-27% of African-American patients.116 117 These antibodies are associated with the development of pulmonary arterial hypertension.101 116
CRP
CRP is an easily obtainable general inflammatory marker and is increasingly used as a marker of prognosis and disease activity. In two recent studies, raised CRP was associated with the diffuse cutaneous disease type and more severe skin and lung involvement. More importantly, higher CRP was associated with progressive ILD and shorter survival.118 119
Serological markers for early detection of pulmonary arterial hypertension
In a recently completed, large, multicenter, cross sectional study of patients with systemic sclerosis, high serum urate and N-terminal pro-brain natriuretic peptide (NTproBNP) values were predictive of the presence of pulmonary arterial hypertension in 466 patients who underwent right heart catheterization. The entry criteria for this study were disease duration greater than three years and diffusing capacity of the lung for carbon monoxide (DLCO) less than 60%.
This study proposed a two step approach for referral of patients for right heart catheterization. In step 1, a prediction score based on the presence of anti-centromere antibodies, telangiectasias, right axis deviation on electrocardiography, forced vital capacity:DLCO ratio, high serum urate, and NTproBNP is developed for referral to echocardiography. In step 2, a prediction score is calculated on the basis of the prediction score from step 1 and the presence of two echocardiographic variables (right atrium area and tricuspid regurgitation velocity) for referral to right heart catheterization. This algorithm had a sensitivity of 96% and specificity of 48% for detecting pulmonary arterial hypertension. Interestingly, dyspnea and physical findings related to the right heart and World Health Organization functional class were not sufficiently predictive to be included in the final prediction algorithm.120
Emerging biomarkers
Patients with systemic sclerosis have a distinct blood and skin gene expression profile.121 122 Similar to patients with SLE, they have a distinct type 1 interferon gene expression profile that correlates with the severity of skin, lung, and muscle involvement.123 124 Furthermore, patients can be categorized on the basis of their skin gene expression profile, including a subgroup with a prominent inflammatory signature.125 Emerging evidence suggests that this subgroup might have a better response to immunosuppression.126 Further longitudinal studies are needed to determine whether these gene expression profiles can help clinical decision making and lead to more focused and effective treatments.
Idiopathic inflammatory myositis
Idiopathic inflammatory myositis (IIM) comprises a group of diseases that cause bilateral muscle weakness as a result of muscle inflammation. Polymyositis and dermatomyositis are two typical IIMs. Although several classification criteria have been proposed for these two diseases, none has been validated.127 128 Despite its limitations, the following criteria are widely used to diagnose polymyositis and dermatomyositis129 130:
The diagnosis of polymyositis is considered probable when three of the muscular criteria are present and definite when four of them are present. The diagnosis of dermatomyositis is considered probable when the cutaneous criterion (necessary criterion) is present in addition to two muscular criteria and definite when the cutaneous criterion and three muscular criteria are present. Other causes of muscle weakness, such as muscular dystrophy or metabolic and endocrine myopathies, must be ruled out before using these criteria.
Myositis antibodies
Antibodies associated with IIM are subdivided into myositis related antibodies and myositis specific antibodies. Table 22 lists the commercially available myositis antibodies, as well as their prevalence and clinical correlates.
Table 2.
Myositis antibodies and their clinical correlates
| Antibody | Prevalence in DM135 | Prevalence in PM135 | Clinical association |
|---|---|---|---|
| Myositis specific antibodies | |||
| Anti-SRP | 1% | 5% | PM, severe, treatment resistant disease |
| Anti-Mi2 | 9% | 1% | Classic DM, good treatment response |
| Anti-synthetase antibodies: | Extramuscular manifestations: ILD, arthralgias, fever, Raynaud’s phenomenon, and mechanic’s hands; associated with anti-SSA antibodies | ||
| Anti-Jo1 | 11% | 21% | |
| Anti-PL12 | 3% | 2% | |
| Anti-PL7 | 2% | 5% | |
| Anti-EJ | 1% | 1% | |
| Anti-OJ | 1% | 1% | |
| Myositis related antibodies | |||
| Anti-SSA | 13% | 12% | Overlap with other connective tissue diseases |
| Anti-U1 RNP | 6% | 5% | Mixed connective tissue disease |
| Anti-PM-Scl | 9% | 6% | Overlap with SSC and SLE |
| Anti-Ku | 1% | 2% | Overlap with SSC and SLE |
DM=dermatomyositis, PM=polymyositis, ILD=interstitial lung disease, SSC=systemic sclerosis, SLE=systemic lupus erythematosus.
Myositis related antibodies
These are associated with the presence of other systemic rheumatic diseases such as SLE and systemic sclerosis. In addition to previously discussed myositis related antibodies (anti-U1 RNP, anti-PM-Scl, and anti-SSA antibodies), anti-Ku also falls into this category and is associated with overlap between polymyositis and other connective tissue diseases.131 132
Myositis specific antibodies
The most common myositis specific antibodies are anti-synthetase antibodies. These antibodies target specific amino acid-tRNA synthetases that are an important component of the protein translation machinery. Each enzyme catalyzes the esterification of one amino acid to its cognate tRNA. The most common anti-synthetase antibody is anti-Jo-1, which targets histidyl-tRNA synthetase.133 134 Between 10% and 18% of patients with IIM have anti-Jo-1 antibodies.133 135
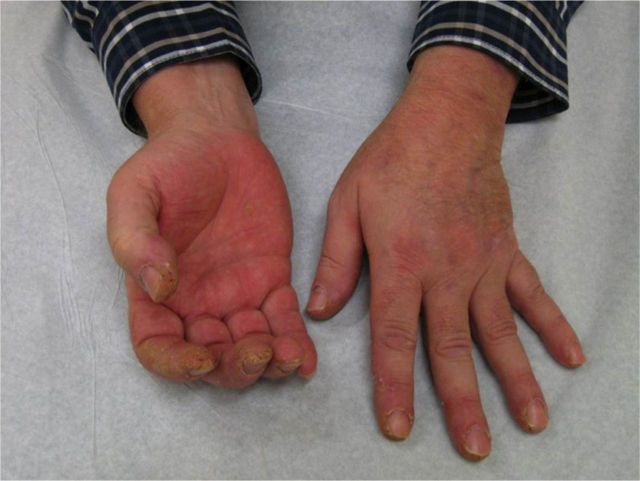
Other anti-synthetase antibodies such as anti-PL12, anti-PL7, anti-OJ, and anti-EJ have much lower prevalences (1-5%).135 Anti-synthetase antibodies are usually mutually exclusive but they can co-occur with anti-SSA antibodies.136 Extramuscular manifestations—namely ILD, arthralgias, fever, Raynaud’s phenomenon, and mechanic’s hands (fig 3)—are common in patients with IIM and anti-synthetase antibodies.135
Fig 3.
Hyperkeratotic mechanic hands seen commonly in patients with anti-synthetase syndrome
Besides anti-synthetase antibodies, there are two commercially available myositis specific antibodies: anti-signal recognition particle (anti-SRP)138 and anti-Mi-2. Anti-SRP is present in about 5% of patients with polymyositis and 1% of those with dermatomyositis.135 Patients with anti-SRP experience severe and treatment resistant disease. Contrary to anti-synthetase antibodies, extramuscular manifestations are uncommon in patients with IIM and anti-SRP antibodies.135 138
Anti-Mi2 antibodies are present in 9% of patients with dermatomyositis but in only 1% of those with polymyositis.135 These antibodies are associated with the classic manifestations of dermatomyositis and a good response to treatment.139 Similar to anti-SRP antibodies, patients with anti-Mi2 antibodies usually lack extramuscular manifestations, with the exception of the cutaneous signs of dermatomyositis.135 Although ILD is rare in patients with anti-SRP and anti-Mi2 antibodies, not all patients with IIM and lung involvement have anti-synthetase antibodies. In a single center study of all consecutive patients with IIM and ILD, only 53% of patients had anti-synthetase antibodies.140
Antibodies to melanoma differentiation associated gene 5 (MDA5) have recently been associated with dermatomyositis. Patients with this antibody have mild or no myositis but have an increased risk of developing digital ulcers, oral ulcers, and ILD.141 142
Muscle enzymes
Creatine kinase, aldolase, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase are muscle enzymes that can be raised in IIM. In a case series study from a single center, all patients with IIM had an elevation of at least one muscle enzyme. Creatine kinase and aldolase were raised in 96% and 86% of patients, respectively.143 This indicates that a small subset of patients with active IIM can have active myositis without a rise in creatine kinase. Increases in aspartate aminotransferase and alanine aminotransferase are often muscular in origin in patients with IIM and do not require extensive hepatic investigation, such as liver biopsy.
A correlation may be seen between creatine kinase concentrations and the degree of muscle weakness, although in longstanding disease with advanced muscle loss the degree of muscle dysfunction may be much greater than creatine kinase values suggest. Furthermore, the rise in creatine kinase might not be accompanied by discernible muscle weakness in early disease. Creatine kinase values are expected to decline within weeks of initiation of immunosuppressive treatment. However, dose adjustment should not focus solely on normalizing creatine kinase because this can lead to overtreatment.144 Work is under way to develop validated myositis response criteria to treatment in IIM.
Axial spondyloarthritis
Axial spondyloarthritis is a chronic inflammatory condition that affects the axial skeleton. It causes chronic back pain (pain present on most days for more than three months) and characteristically starts before the age of 45 years. Axial spondyloarthritis can also be associated with enthesitis; inflammation in peripheral joints; and extra-articular manifestations such as uveitis, psoriasis, and inflammatory bowel disease.
Ankylosing spondylitis is the prototype of axial spondyloarthritis. Signs of sacroiliitis on plain radiographs are needed for the diagnosis of ankylosing spondylitis.145 However, inflammation and pain precede the development of radiographic features of sacroilliitis by several years.146 Therefore, a second category of non-radiographic axial spondyloarthritis has been defined, the diagnosis of which is based on evidence of active inflammation in the sacroiliac joints on magnetic resonance imaging (MRI) or a combination of other findings. New criteria have been proposed by the Assessment of Spondyloarthritis International Society (ASAS) to facilitate early diagnosis of axial spondyloarthritis.147 Using these criteria, patients with chronic back pain and age of onset under 45 years can be classified as having axial spondyloarthritis on the basis of the presence of HLA-B27 and two features of spondyloarthritis or sacroiliitis on imaging (plain radiograph or MRI) plus one feature of spondyloarthritis (fig 4).
Fig 4.
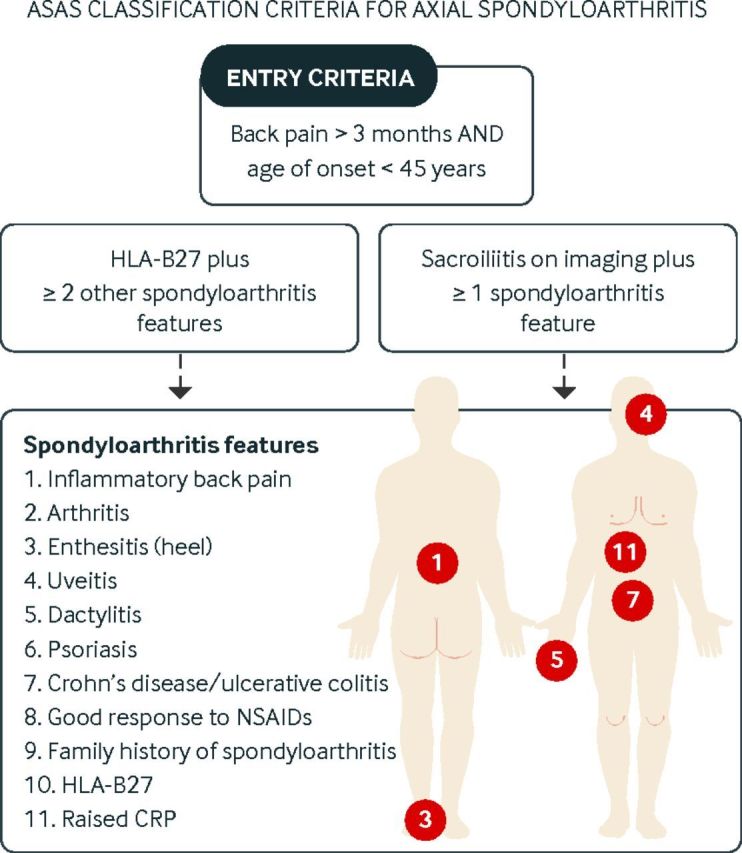
ASAS classification criteria for axial spondyloarthritis
HLA-B27
Although HLA-B27 was not included in the classification criteria for ankylosing spondylitis,145 it is an integral part of the new ASAS criteria for axial spondyloarthritis.147 The prevalence of HLA-B27 varies among different ethnic groups. In a recent US national survey, prevalence was 7.5% in non-Hispanic white people, 4.6% in Mexican Americans, and 1.1% in non-Hispanic black people.148
Although 85-95% of white patients with ankylosing spondylitis have HLA-B27,149 only 6% of HLA-B27 carriers in the general population develop the condition.150 Thus, a positive test for HLA-B27 is not sufficient for the diagnosis of ankylosing spondylitis. Similarly, HLA-B27 positivity is not necessary for the diagnosis of ankylosing spondylitis. The prevalence of HLA-B27 in patients with ankylosing spondylitis varies with ethnicity. For example, it is substantially lower in African-American patients with ankylosing spondylitis (50% v 85-95% in white patients).149
In patients with ankylosing spondylitis, HLA-B27 positivity is associated with younger age at disease onset, development of anterior uveitis, and positive family history of spondyloarthritis, but it is not associated with increased structural damage on radiography.151 152 153
HLA-B27 is particularly useful for diagnosing non-radiographic spondyloarthritis. In a longitudinal study with a mean follow-up of 7.7 years, the combination of severe sacroiliitis on MRI with HLA-B27 positivity was an excellent predictor of future development of ankylosing spondylitis (likelihood ratio 8).146 The usefulness of HLA-B27 in determining who would benefit from rheumatological evaluation in primary care was shown in a recent multicentre prospective study. Patients with chronic back pain (>3 months) and age of onset before 45 years who had either HLA-B27 positivity, inflammatory back pain, or sacroiliitis on MRI were referred to rheumatologists for further evaluation. Using this simple referral strategy, 35% of patients were diagnosed with spondyloarthritis. This result was not significantly different from that seen for a more complicated referral strategy (two of: inflammatory back pain, HLA-B27, sacroiliitis on imaging, family history of spondyloarthritis, good response to non-steroidal anti-inflammatory drugs, extra-articular manifestations).154
CRP and ESR
Raised CRP is included in the ASAS classification criteria for axial spondyloarthritis.147 CRP or ESR is raised in 40-50% of patients with ankylosing spondylitis,153 so a normal ESR or CRP does not rule out this condition. Levels of both of these acute phase reactants are higher in patients with ankylosing spondylitis than in those with non-radiographic axial spondyloarthritis.155
Raised CRP is also associated with increased radiographic changes on spinal radiographs153 and signs of inflammation on sacroiliac MRI.156 Furthermore, raised CRP and ESR predict future radiographic progression in sacroiliac joints and the spine in patients with ankylosing spondylitis.157 158 159
The ASAS has also developed a new ankylosing spondylitis disease activity score that combines patient reported assessment with these acute phase reactants.160 In this activity measure, CRP is combined with patient reported back pain, the duration of morning stiffness, peripheral joint problems, and patient global assessment. ESR can also be used (with slightly different weighting) if CRP is not available. This score helps discriminate patients with different levels of disease activity and is sensitive to change over time, which makes it appropriate for assessing treatment effect.160
Emerging biomarkers
The cartilage turnover marker CTX-1 (C-terminal crosslinking telopeptide of type II collagen) was associated with higher inflammation scores on MRI in sacroiliac joints and the spine.161 Several bone formation markers including sclerostin, Dickkopf-1,162 and bone alkaline phosphatase have also been investigated recently as biomarkers. Among these markers, bone alkaline phosphatase levels can be measured in the clinical laboratory. However, data on the utility of this serum marker are not consistent.163 Two serum markers, VEGF and calprotectin, were recently shown to predict radiographic progression in patients with ankylosing spondylitis, independently of baseline CRP values.164 165 The clinical utility of these markers needs to be confirmed in independent studies.
Looking ahead to future utilization of biomarkers in rheumatic diseases
Serological markers have already been incorporated in the classification criteria of several rheumatic diseases, enabling earlier diagnosis of these conditions.2 3 96 In addition to established markers of disease activity such as CRP and ESR, novel protein markers are becoming commercially available for more accurate monitoring of disease activity.41 42 43
As discussed in the emerging biomarker sections, several candidate transcript and protein markers can improve the monitoring of disease activity. However, sufficiently powered longitudinal studies in well characterized cohorts are needed to examine the utility of these novel markers beyond the currently available clinical and serological information. For example, several connective tissue diseases such as SLE, systemic sclerosis, dermatomyositis, and polymyositis have a prominent interferon transcript signature.123 166 167 Future longitudinal studies are needed to elucidate the role of this gene expression signature for monitoring disease activity and predicting response to treatment.89 90 124 168 169
It is difficult to predict response to a particular treatment with the currently available clinical and serological information, partly because of substantial molecular heterogeneity within each of the rheumatic diseases. Basically, each rheumatic disease is made up of various imperfectly defined molecular endotypes,120 125 166 170 which might respond differently to drugs that target specific biological pathways.
Emerging data suggest that transcript and protein biomarkers can more accurately define these disease endotypes and predict response to treatment.44 126 171 This can refine the design of clinical trials by enabling more informed enrichment strategies and decreasing the number of patients that need to be enrolled. Ultimately, more informative predictive biomarkers can lead to a more focused and effective treatment of rheumatic diseases.
Conclusion
Revised classification criteria that incorporate serological and proteomic markers have been put forward in several rheumatic diseases.2 3 96 This development has enabled earlier diagnosis and the timely initiation of treatment. Furthermore, these markers have led to more accurate activity assessment and disease sub-setting at the molecular level, which can facilitate clinical decisions about monitoring and treatment regimens. This review focuses on commercially available serological and proteomic markers; however, several other gene expression and protein based biomarkers are currently under development for accurate diagnosis and course prediction in rheumatic diseases. Further research is needed to validate them in independent cohorts and to examine their clinical utility beyond the currently available markers. The ultimate goal of developing biomarkers is to enable early detection of rheumatic diseases and their clinical manifestations, and to provide more effective monitoring and treatment regimens that are tailored to the individual’s needs and prognosis.
Contributors: CM and SA both contributed to conception of the work, literature search, data interpretation, manuscript preparation, and approval of the final version of the manuscript. SA is guarantor.
Funding: This review is supported by funds from the National Institutes of Health K23-AR-061436 (SA) and the Scleroderma Foundation Collaborative Research Grant.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: none.
Provenance and peer review: Commissioned; externally peer reviewed.
Cite this as: BMJ 2015;351:h5079
References
- 1.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95. [DOI] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747-55. [DOI] [PubMed] [Google Scholar]
- 5.Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004;97:421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeough CM, Berrar D, Wright G, et al. Killer immunoglobulin-like receptor and human leukocyte antigen-C genotypes in rheumatoid arthritis primary responders and non-responders to anti-TNF-alpha therapy. Rheumatol Int 2012;32:1647-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Sajitharan D, Mohan C. Biomarkers of rheumatoid arthritis: recent progress. Expert Opin Med Diagn 2010;4:293-305. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007;146:797-808. [DOI] [PubMed] [Google Scholar]
- 9.Lemm G, Ruschen S, Warnatz H. An ELISA for IgA-IgG and IgM-RF measurement. II. RF in several disease and control groups and under gold therapy in RA. Scand J Rheumatol Suppl 1988;75:256-60. [DOI] [PubMed] [Google Scholar]
- 10.Bobbio-Pallavicini F, Caporali R, Alpini C, et al. Predictive value of antibodies to citrullinated peptides and rheumatoid factors in anti-TNF-alpha treated patients. Ann N Y Acad Sci 2007;1109:287-95. [DOI] [PubMed] [Google Scholar]
- 11.Raza K, Breese M, Nightingale P, et al. Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol 2005;32:231-8. [PMC free article] [PubMed] [Google Scholar]
- 12.Van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci 2008;1143:268-85. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Song W, Li Y, et al. Diagnostic value of anti-cyclic citrullinated peptide antibodies in northern Chinese Han patients with rheumatoid arthritis and its correlation with disease activity. Clin Rheumatol 2010;29:413-7. [DOI] [PubMed] [Google Scholar]
- 14.Taylor P, Gartemann J, Hsieh J, et al. A systematic review of serum biomarkers anti-cyclic citrullinated peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011;2011:815038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741-9. [DOI] [PubMed] [Google Scholar]
- 16.Nielen MM, van SD, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380-6. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Longo FJ, Oliver-Minarro D, de la Torre I, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum 2009;61:419-24. [DOI] [PubMed] [Google Scholar]
- 18.Plant MJ, Williams AL, O’Sullivan MM, et al. Relationship between time-integrated C-reactive protein levels and radiologic progression in patients with rheumatoid arthritis. Arthritis Rheum 2000;43:1473-7. [DOI] [PubMed] [Google Scholar]
- 19.Aman S, Paimela L, Leirisalo-Repo M, et al. Prediction of disease progression in early rheumatoid arthritis by ICTP, RF and CRP. A comparative 3-year follow-up study. Rheumatology (Oxford) 2000;39:1009-13. [DOI] [PubMed] [Google Scholar]
- 20.Van Leeuwen MA, van Rijswijk MH, van der Heijde DM, et al. The acute-phase response in relation to radiographic progression in early rheumatoid arthritis: a prospective study during the first three years of the disease. Br J Rheumatol 1993;32(suppl 3):9-13. [DOI] [PubMed] [Google Scholar]
- 21.Prevoo ML, van’t Hof MA, Kuper HH,. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44-8. [DOI] [PubMed] [Google Scholar]
- 22.Devlin J, Gough A, Huissoon A, et al. The acute phase and function in early rheumatoid arthritis. C-reactive protein levels correlate with functional outcome. J Rheumatol 1997;24:9-13. [PubMed] [Google Scholar]
- 23.Kay J, Morgacheva O, Messing SP, et al. Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res Ther 2014;16:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graf J, Scherzer R, Grunfeld C, et al. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One 2009;4:e6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denarie D, Constant E, Thomas T, Marotte H. Could biomarkers of bone, cartilage or synovium turnover be used for relapse prediction in rheumatoid arthritis patients? Mediators Inflamm 2014;2014:537324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houseman M, Potter C, Marshall N, et al. Baseline serum MMP-3 levels in patients with rheumatoid arthritis are still independently predictive of radiographic progression in a longitudinal observational cohort at 8 years follow up. Arthritis Res Ther 2012;14:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson ML, Svensson B, Petersson IF, et al. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet Disord 2013;14:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamoto J, Takeda T, Ichimura S. Urinary cross-linked N-telopeptides of type I collagen levels in patients with rheumatoid arthritis. Calcif Tissue Int 2003;72:491-7. [DOI] [PubMed] [Google Scholar]
- 29.Al-Awadhi A, Olusi S, Al-Zaid N, et al. Serum concentrations of interleukin 6, osteocalcin, intact parathyroid hormone, and markers of bone resorption in patients with rheumatoid arthritis. J Rheumatol 1999;26:1250-6. [PubMed] [Google Scholar]
- 30.Torikai E, Kageyama Y, Takahashi M, et al. The effect of methotrexate on bone metabolism markers in patients with rheumatoid arthritis. Mod Rheumatol 2006;16:350-4. [DOI] [PubMed] [Google Scholar]
- 31.Syversen SW, Goll GL, van der Heijde D, et al. Cartilage and bone biomarkers in rheumatoid arthritis: prediction of 10-year radiographic progression. J Rheumatol 2009;36:266-72. [DOI] [PubMed] [Google Scholar]
- 32.Geusens PP. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2012;4:225-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geusens PP, Landewé RB, Garnero P, et al. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum 2006;54:1772-7. [DOI] [PubMed] [Google Scholar]
- 34.Van Tuyl LH, Voskuyl AE, Boers M, et al. Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann Rheum Dis 2010;69:1623-8. [DOI] [PubMed] [Google Scholar]
- 35.Vis M, Havaardsholm EA, Haugeberg G, et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:1495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm 2014;2014:545493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hueber W, Robinson WH. Proteomic biomarkers for autoimmune disease. Proteomics 2006;6:4100-5. [DOI] [PubMed] [Google Scholar]
- 38.Kokkonen H, Soderstrom I, Rocklov J, et al. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum 2010;62:383-91. [DOI] [PubMed] [Google Scholar]
- 39.Meyer PW, Hodkinson B, Ally M, et al. Circulating cytokine profiles and their relationships with autoantibodies, acute phase reactants, and disease activity in patients with rheumatoid arthritis. Mediators Inflamm 2010;2010:158514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McArdle A, Flatley B, Pennington SR, et al. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther 2015;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eastman PS, Manning WC, Qureshi F, et al. Characterization of a multiplex, 12-biomarker test for rheumatoid arthritis. J Pharm Biomed Anal 2012;70:415-24. [DOI] [PubMed] [Google Scholar]
- 42.Bakker MF, Cavet G, Jacobs JW, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis 2012;71:1692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken ) 2012;64:1794-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SL, Plant D, Eyre S, et al. The potential use of expression profiling: implications for predicting treatment response in rheumatoid arthritis. Ann Rheum Dis 2013;72:1118-24. [DOI] [PubMed] [Google Scholar]
- 45.Van der Heijde DM, van’t Hof MA, van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis 1990;49:916-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35. [DOI] [PubMed] [Google Scholar]
- 47.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271-7. [DOI] [PubMed] [Google Scholar]
- 48.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 49.Ozbek S, Sert M, Paydas S, et al. Delay in the diagnosis of SLE: the importance of arthritis/arthralgia as the initial symptom. Acta Med Okayama 2003;57:187-90. [DOI] [PubMed] [Google Scholar]
- 50.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526-33. [DOI] [PubMed] [Google Scholar]
- 51.Eriksson C, Kokkonen H, Johansson M, et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClain MT, Arbuckle MR, Heinlen LD, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum 2004;50:1226-32. [DOI] [PubMed] [Google Scholar]
- 53.Cozzani E, Drosera M, Gasparini G, et al. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis 2014;2014:321-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996;156:1421-5. [PubMed] [Google Scholar]
- 55.Haugbro K, Nossent JC, Winkler T, et al. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann Rheum Dis 2004;63:386-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alba P, Bento L, Cuadrado MJ, et al. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis 2003;62:556-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis P, Percy JS, Russell AS. Correlation between levels of DNA antibodies and clinical disease activity in SLE. Ann Rheum Dis 1977;36:157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ter Borg EJ, Horst G, Hummel EJ, et al. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum 1990;33:634-43. [DOI] [PubMed] [Google Scholar]
- 59.Cortes-Hernandez J, Ordi-Ros J, Labrador M, et al. Antihistone and anti-double-stranded deoxyribonucleic acid antibodies are associated with renal disease in systemic lupus erythematosus. Am J Med 2004;116:165-73. [DOI] [PubMed] [Google Scholar]
- 60.Olson SW, Lee JJ, Prince LK, et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol 2013;8:1702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bootsma H, Spronk P, Derksen R, et al. Prevention of relapses in systemic lupus erythematosus. Lancet 1995;345:1595-9. [DOI] [PubMed] [Google Scholar]
- 62.Tseng CE, Buyon JP, Kim M, et al. The effect of moderate-dose corticosteroids in preventing severe flares in patients with serologically active, but clinically stable, systemic lupus erythematosus: findings of a prospective, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:3623-32. [DOI] [PubMed] [Google Scholar]
- 63.Petri M, Singh S, Tesfasyone H, et al. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol 2009;36:2476-80. [DOI] [PubMed] [Google Scholar]
- 64.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215-26. [DOI] [PubMed] [Google Scholar]
- 65.Van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012;71:1343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012;64:2328-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petri MA, Van Vollenhoven RF, Buyon J, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum 2013;65:2143-53. [DOI] [PubMed] [Google Scholar]
- 68.Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol 2012;26:61-72. [DOI] [PubMed] [Google Scholar]
- 69.Malik S, Bruner GR, Williams-Weese C, et al. Presence of anti-La autoantibody is associated with a lower risk of nephritis and seizures in lupus patients. Lupus 2007;16:863-6. [DOI] [PubMed] [Google Scholar]
- 70.Chu P, Pendry K, Blecher TE. Detection of lupus anticoagulant in patients attending an anticoagulation clinic. BMJ 1988;297:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greer JM, Panush RS. Incomplete lupus erythematosus. Arch Intern Med 1989;149:2473-6. [PubMed] [Google Scholar]
- 72.Olsen NJ, Li QZ, Quan J, et al. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther 2012;14:R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al DM, Massarotti EM, Fine A, et al. Development of SLE among “potential SLE” patients seen in consultation: long-term follow-up. Int J Clin Pract 2014;68:1508-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricker DM, Hebert LA, Rohde R, et al. Serum C3 levels are diagnostically more sensitive and specific for systemic lupus erythematosus activity than are serum C4 levels. The Lupus Nephritis Collaborative Study group. Am J Kidney Dis 1991;18:678-85. [DOI] [PubMed] [Google Scholar]
- 75.Ho A, Barr SG, Magder LS, et al. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum 2001;44:2350-7. [DOI] [PubMed] [Google Scholar]
- 76.Moroni G, Radice A, Giammarresi G, et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 2009;68:234-7. [DOI] [PubMed] [Google Scholar]
- 77.Birmingham DJ, Irshaid F, Nagaraja HN, et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus 2010;19:1272-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertoli AM, Vila LM, Reveille JD, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): LXI. Value of C-reactive protein as a marker of disease activity and damage. J Rheumatol 2008;35:2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ter Borg EJ, Horst G, Limburg PC, et al. C-reactive protein levels during disease exacerbations and infections in systemic lupus erythematosus: a prospective longitudinal study. J Rheumatol 1990;17:1642-8. [PubMed] [Google Scholar]
- 80.Barnes EV, Narain S, Naranjo A, et al. High sensitivity C-reactive protein in systemic lupus erythematosus: relation to disease activity, clinical presentation and implications for cardiovascular risk. Lupus 2005;14:576-82. [DOI] [PubMed] [Google Scholar]
- 81.Firooz N, Albert DA, Wallace DJ, et al. High-sensitivity C-reactive protein and erythrocyte sedimentation rate in systemic lupus erythematosus. Lupus 2011;20:588-97. [DOI] [PubMed] [Google Scholar]
- 82.Morrow WJ, Isenberg DA, Parry HF, et al. C-reactive protein in sera from patients with systemic lupus erythematosus. J Rheumatol 1981;8:599-604. [PubMed] [Google Scholar]
- 83.Bertouch JV, Roberts-Thompson PJ, Feng PH, et al. C-reactive protein and serological indices of disease activity in systemic lupus erythematosus. Ann Rheum Dis 1983;42:655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vila LM, Alarcon GS, McGwin G Jr, et al. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXIX. Elevation of erythrocyte sedimentation rate is associated with disease activity and damage accrual. J Rheumatol 2005;32:2150-5. [PubMed] [Google Scholar]
- 85.Stojan G, Fang H, Magder L, et al. Erythrocyte sedimentation rate is a predictor of renal and overall SLE disease activity. Lupus 2013;22:827-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arriens C, Mohan C. Systemic lupus erythematosus diagnostics in the “omics” era. Int J Clin Rheumtol 2013;8:671-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rose T, Grutzkau A, Hirseland H, et al. IFNalpha and its response proteins, IP-10 and SIGLEC-1, are biomarkers of disease activity in systemic lupus erythematosus. Ann Rheum Dis 2013;72:1639-45. [DOI] [PubMed] [Google Scholar]
- 88.Nielsen CT, Lood C, Ostergaard O, et al. Plasma levels of galectin-3-binding protein reflect type I interferon activity and are increased in patients with systemic lupus erythematosus. Lupus Sci Med 2014;1:e000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bauer JW, Baechler EC, Petri M, et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med 2006;3:e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bauer JW, Petri M, Batliwalla FM, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum 2009;60:3098-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Munroe ME, Vista ES, Guthridge JM, et al. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol 2014;66:1888-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elhai M, Meune C, Avouac J, et al. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2011;51:1017-26. [DOI] [PubMed] [Google Scholar]
- 93.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 2007;66:940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809-15. [DOI] [PubMed] [Google Scholar]
- 95.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 2005;35:35-42. [DOI] [PubMed] [Google Scholar]
- 96.Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College Of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747-55. [DOI] [PubMed] [Google Scholar]
- 97.Leroy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202-5. [PubMed] [Google Scholar]
- 98.Salazar GA, Assassi S, Wigley F, et al. Antinuclear antibody-negative systemic sclerosis. Semin Arthritis Rheum 2015;44:680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med 2005;118:2-10. [DOI] [PubMed] [Google Scholar]
- 100.Assassi S, Del JD, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009;61:1403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625-35. [DOI] [PubMed] [Google Scholar]
- 102.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010;12:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jacobsen S, Halberg P, Ullman S, et al. A longitudinal study of pulmonary function in Danish patients with systemic sclerosis. Clin Rheumatol 1997;16:384-90. [DOI] [PubMed] [Google Scholar]
- 104.Bunn CC, Denton CP, Shi-Wen X, et al. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol 1998;37:15-20. [DOI] [PubMed] [Google Scholar]
- 105.Nguyen B, Mayes MD, Arnett FC, et al. HLA-DRB1*0407 and *1304 are risk factors for scleroderma renal crisis. Arthritis Rheum 2011;63:530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nikpour M, Hissaria P, Byron J, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther 2011;13:R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steen VD, Medsger TA Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum 1998;41:1613-9. [DOI] [PubMed] [Google Scholar]
- 108.DeMarco PJ, Weisman MH, Seibold JR, et al. Predictors and outcomes of scleroderma renal crisis: the high-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis trial. Arthritis Rheum 2002;46:2983-9. [DOI] [PubMed] [Google Scholar]
- 109.Ghrenassia E, Avouac J, Khanna D, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol 2014;41:99-105. [DOI] [PubMed] [Google Scholar]
- 110.Shah AA, Rosen A, Hummers L, et al. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum 2010;62:2787-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moinzadeh P, Fonseca C, Hellmich M, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther 2014;16:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Airo P, Ceribelli A, Cavazzana I, et al. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. J Rheumatol 2011;38:1329-34. [DOI] [PubMed] [Google Scholar]
- 113.Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014;343:152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koschik RW, Fertig N, Lucas MR, et al. Anti-PM-Scl antibody in patients with systemic sclerosis. Clin Exp Rheumatol 2012;30(2 suppl 71):S12-6. [PubMed] [Google Scholar]
- 115.D’Aoust J, Hudson M, Tatibouet S, et al. Clinical and serologic correlates of anti-PM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheumatol 2014;66:1608-15. [DOI] [PubMed] [Google Scholar]
- 116.Aggarwal R, Lucas M, Fertig N, et al. Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum 2009;60:1112-8. [DOI] [PubMed] [Google Scholar]
- 117.Sharif R, Fritzler MJ, Mayes MD, et al. Anti-fibrillarin antibody in African American patients with systemic sclerosis: immunogenetics, clinical features, and survival analysis. J Rheumatol 2011;38:1622-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muangchan C, Harding S, Khimdas S, et al; Canadian Scleroderma Research Group. Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 2012;6:1405-14. [DOI] [PubMed] [Google Scholar]
- 119.Liu X, Mayes MD, Pedroza C, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis. Arthritis Care Res (Hoboken ) 2013;65:1375-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coghlan JG, Denton CP, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan FK, Zhou X, Mayes MD, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 2006;45:694-702. [DOI] [PubMed] [Google Scholar]
- 122.Whitfield ML, Finlay DR, Murray JI, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci U S A 2003;100:12319-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Assassi S, Mayes MD, Arnett FC, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum 2010;62:589-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Mayes MD, Tan FK, et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum 2013;65:226-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milano A, Pendergrass SA, Sargent JL, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One 2008;3:e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hinchcliff M, Huang CC, Wood TA, et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol 2013;133:1979-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev 2014;13:367-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iaccarino L, Ghirardello A, Bettio S, et al. The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun 2014;48-49:122-7. [DOI] [PubMed]
- 129.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [DOI] [PubMed] [Google Scholar]
- 130.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403-7. [DOI] [PubMed] [Google Scholar]
- 131.Rozman B, Cucnik S, Sodin-Semrl S, et al. Prevalence and clinical associations of anti-Ku antibodies in patients with systemic sclerosis: a European EUSTAR-initiated multi-centre case-control study. Ann Rheum Dis 2008;67:1282-6. [DOI] [PubMed] [Google Scholar]
- 132.Cavazzana I, Ceribelli A, Quinzanini M, et al. Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases. Lupus 2008;17:727-32. [DOI] [PubMed] [Google Scholar]
- 133.Brouwer R, Hengstman GJ, Vree EW, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001;60:116-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arnett FC, Hirsch TJ, Bias WB, et al. The Jo-1 antibody system in myositis: relationships to clinical features and HLA. J Rheumatol 1981;8:925-30. [PubMed] [Google Scholar]
- 135.Lega JC, Fabien N, Reynaud Q, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev 2014;13:883-91. [DOI] [PubMed] [Google Scholar]
- 136.Hamaguchi Y, Fujimoto M, Matsushita T, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 2013;8:e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sundaragiri PR Vallabhajosyula S, Kanaan JP. Interstitial lung disease in antisynthetase syndrome without clinical myositis. BMJ Case Rep 2014;2014; doi: 10.1136/bcr-2014-204296. [DOI] [PMC free article] [PubMed]
- 138.Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum 1990;33:1361-70. [DOI] [PubMed] [Google Scholar]
- 139.Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991;70:360-74. [DOI] [PubMed] [Google Scholar]
- 140.Johnson C, Connors GR, Oaks J, et al. Clinical and pathologic differences in interstitial lung disease based on antisynthetase antibody type. Respir Med 2014;108:1542-8. [DOI] [PubMed] [Google Scholar]
- 141.Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 2011;65:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hamaguchi Y, Kuwana M, Hoshino K, et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol 2011;147:391-8. [DOI] [PubMed] [Google Scholar]
- 143.Hochberg MC, Feldman D, Stevens MB. Adult onset polymyositis/dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Semin Arthritis Rheum 1986;15:168-78. [DOI] [PubMed] [Google Scholar]
- 144.Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol 2013;40:550-64. [DOI] [PubMed] [Google Scholar]
- 145.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361-8. [DOI] [PubMed] [Google Scholar]
- 146.Bennett AN, McGonagle D, O’Connor P, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413-8. [DOI] [PubMed] [Google Scholar]
- 147.Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777-83. [DOI] [PubMed] [Google Scholar]
- 148.Reveille JD, Hirsch R, Dillon CF, et al. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 2012;64:1407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khan MA, Braun WE, Kushner I, et al. HLA B27 in ankylosing spondylitis: differences in frequency and relative risk in American Blacks and Caucasians. J Rheumatol Suppl 1977;3:39-43. [PubMed] [Google Scholar]
- 150.Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998;41:58-67. [DOI] [PubMed] [Google Scholar]
- 151.Joshi R, Reveille JD, Brown MA, et al. Is there a higher genetic load of susceptibility loci in familial ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feldtkeller E, Khan MA, van der HD, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61-6. [DOI] [PubMed] [Google Scholar]
- 153.Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum 2009;60:717-27. [DOI] [PubMed] [Google Scholar]
- 154.Sieper J, Srinivasan S, Zamani O, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis 2013;72:1621-7. [DOI] [PubMed] [Google Scholar]
- 155.Wallis D, Haroon N, Ayearst R, et al. Ankylosing spondylitis and nonradiographic axial spondyloarthritis: part of a common spectrum or distinct diseases? J Rheumatol 2013;40:2038-41. [DOI] [PubMed] [Google Scholar]
- 156.Bredella MA, Steinbach LS, Morgan S, et al. MRI of the sacroiliac joints in patients with moderate to severe ankylosing spondylitis. AJR Am J Roentgenol 2006;187:1420-6. [DOI] [PubMed] [Google Scholar]
- 157.Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012;64:1388-98. [DOI] [PubMed] [Google Scholar]
- 159.Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369-74. [DOI] [PubMed] [Google Scholar]
- 160.Van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811-8. [DOI] [PubMed] [Google Scholar]
- 161.Pedersen SJ, Sorensen IJ, Lambert RG, et al. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor alpha inhibitors: a study of radiographic progression, inflammation on magnetic resonance imaging, and circulating biomarkers of inflammation, angiogenesis, and cartilage and bone turnover. Arthritis Rheum 2011;63:3789-800. [DOI] [PubMed] [Google Scholar]
- 162.Heiland GR, Appel H, Poddubnyy D, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:572-4. [DOI] [PubMed] [Google Scholar]
- 163.Coiffier G, Bouvard B, Chopin F, et al. Common bone turnover markers in rheumatoid arthritis and ankylosing spondylitis: a literature review. Joint Bone Spine 2013;80:250-7. [DOI] [PubMed] [Google Scholar]
- 164.Poddubnyy D, Conrad K, Haibel H, et al. Elevated serum level of the vascular endothelial growth factor predicts radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis 2014;73:2137-43. [DOI] [PubMed] [Google Scholar]
- 165.Turina MC, Sieper J, Yeremenko N, et al. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis 2014;73:1746-8. [DOI] [PubMed] [Google Scholar]
- 166.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 2003;100:2610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baechler EC, Bauer JW, Slattery CA, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 2007;13:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chiche L, Jourde-Chiche N, Whalen E, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol 2014;66:1583-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang B, Higgs BW, Chang L, et al. Pharmacogenomics and translational simulations to bridge indications for an anti-interferon-alpha receptor antibody. Clin Pharmacol Ther 2013;93:483-92. [DOI] [PubMed] [Google Scholar]
- 170.Gregersen PK, Oswald M. Editorial: the power of a modular approach to transcriptional analysis. Arthritis Rheumatol 2014;66:1418-20. [DOI] [PubMed] [Google Scholar]
- 171.Streicher K, Morehouse CA, Groves CJ, et al. The plasma cell signature in autoimmune disease. Arthritis Rheumatol 2014;66:173-84. [DOI] [PubMed] [Google Scholar]