Abstract
PURPOSE
Kaposi sarcoma (KS) is an HIV-associated skin cancer that is highly prevalent in Botswana and associated with significant morbidity and mortality. Histopathology-confirmed diagnosis is required for chemotherapeutic interventions in Botswana, which creates barriers to care because of limited biopsy and pathology services. We sought to understand the role a dermatology specialist can play in improving KS care through quality improvement (QI) initiatives to reduce histologic turnaround times (TATs) for KS.
METHODS
Employment of a dermatology specialist within a public health care system that previously lacked a local dermatologist generated quality improvements in KS care. Retrospective review identified patients diagnosed with KS by skin biopsy in the predermatology QI interval (January 1, 2015, to December 31, 2015) versus the postdermatology QI interval (January 1, 2016, to November 31, 2017). Histology TATs and clinical characteristics were recorded. A t test compared the median histology TATs in the pre- and post-QI intervals.
RESULTS
A total of 192 cases of KS were diagnosed by skin biopsy. Nearly all (98.4%) were HIV-positive; and 52.8% of patients were male with a median age of 39 years. Median TAT in the postdermatology QI interval was 11 days (interquartile range, 12-23 days) compared with 32 days in the predermatology QI interval (interquartile range, 24-56 days; P < .00).
CONCLUSION
Dermatology-led QI initiatives to improve multispecialty care coordination can significantly decrease histology TATs for KS. The reduction of diagnostic delays is a key first step to decreasing the morbidity and mortality associated with this cancer in resource-limited settings.
INTRODUCTION
Kaposi sarcoma (KS) is an HIV-associated skin cancer that is etiologically linked to human herpesvirus 8 and estimated to be one of the most incident cancers across many regions of Africa.1,2 The disease typically presents on the skin as violaceous patches, plaques, or nodules but can involve internal organs, leading to a mortality risk of up to 70% at 1 year for people with untreated HIV.3,4
Since the introduction of combined antiretroviral treatment (ART) in developed countries, the incidence of KS has declined markedly in patients with HIV infection, but the disease continues to disproportionately affect the African continent, where an estimated 85% of all cases worldwide occur.2 Studies that analyzed data between 2003 and 2010 from the International Epidemiology Databases to Evaluate AIDS and the Botswana National Cancer Registry, a period during which ART coverage in Botswana increased from 7.3% to 82.3%, demonstrated only a modest decrease in KS incidence despite these remarkable improvements in ART access.5,6 Of note, 78% of patients in the Botswana National Cancer Registry required oncologic intervention with chemotherapy and/or radiation in addition to ART for KS. This frequent need for oncologic treatment adds another layer of complexity and a source for delays in KS care systems.5
Successful management of KS in Botswana requires care coordination among multiple departments from initial referral by primary care or an HIV clinic through the dermatology and pathology departments for diagnosis to oncology departments for treatment. Research has indicated that a number of psychological, socioeconomic, and health system factors can contribute to cancer care delays at multiple points from initial presentation to treatment.7-9 Because tissue biopsy is the gold standard for KS diagnosis and histopathologic confirmation is required before chemotherapeutic treatment of KS in Botswana, KS care in our health care system is limited by the capacity for biopsy and histologic diagnosis.10-14 In Botswana, there is currently only one dermatology clinic in the public health care system, at Princess Marina Hospital (PMH), that can consistently perform point-of-care punch biopsies for KS diagnosis. This clinic is served by the National Health Laboratory (NHL) in the capital city of Gaborone, which which has only 1-2 pathologists who read skin histology slides despite having no specialized dermatopathology expertise. Because the majority of the population depends on the free health care services provided by the Ministry of Health and Wellness (MoHW), a diagnosis of KS in Botswana relies on the services of the dermatology clinic at PMH and histopathology readings at NHL.
CONTEXT
Key Objective
To understand the role a dermatologist can play in improving Kaposi sarcoma (KS) care through quality improvement initiatives to reduce diagnostic delays for this high-prevalence cancer in Botswana.
Knowledge Generated
Employment of a new dermatology specialist within a public health care system that previously lacked a local dermatologist generated quality improvements in multispecialty collaboration and histopathology workflow, which led to a 21-day decrease in median histology turnaround time.
Relevance
Multispecialty care coordination is a critical component of cancer care, particularly in low- and middle-income countries where patients face increased barriers to care. A dermatologist can provide key leadership and expertise needed to facilitate rapid histologic diagnosis, which is a key limiting factor for KS treatment in Botswana. The creation of a multispecialty collaborative workflow and monitoring system for KS can serve as a model to improve cancer care in similar low- and middle-income country environments.
This article focuses on overcoming the problem of diagnostic delays for KS, one of the most common cancers in Botswana, by describing processes that have been implemented to reduce histology turnaround times (TATs). We describe the early quality improvement (QI) outcomes that a full-time dermatologist can spearhead, including improved multispecialty collaboration and histopathology workflow, to reduce diagnostic histology TATs for patients with KS in this resource-limited public health care system.
METHODS
Through a collaborative effort across dermatology, oncology, and pathology departments at PMH, University of Botswana, and University of Pennsylvania (UPenn) and NHL employees, a number of interventions were implemented to improve the management of patients with KS in Botswana. These were initiated after the hiring of a full-time dermatologist in January 2016, author V.L.W., as the only dermatology specialist employed through the Botswana MoHW at the time. Subsequently, she focused on assessing the quality of care of patients with KS. Through discussions with the oncology, infectious disease, and pathology departments at PMH as well as with referring primary care clinics around the country, major concerns were expressed, including patients being referred to oncology without a confirmed KS diagnosis, which lead to significant care delays and wasted resources; difficulty getting patients with KS into the dermatology clinic for diagnostic confirmation; and slow TATs for biopsy diagnosis, which caused delays in treatment intervention. Thus, diagnostic delay was a major cause of care flow disruption in patients with KS in Botswana. Because the dermatology clinic at PMH is the only public sector clinic with consistent capacity to perform rapid bedside punch biopsies, this is the main source of KS diagnosis in Botswana and thus the biggest potential limiting factor in KS care flow.
Next, V.L.W. assessed the PMH outpatient department for barriers to clinical care in dermatology, started working closely with the pathology department to perform a comprehensive analysis of inadequacies in the histopathology laboratory, and then used a multidisciplinary approach to find solutions to these challenges. Pathologists from UPenn assisted with workflow analysis and problem-solving strategies. A number of interventions were implemented in the dermatology and pathology departments to improve the diagnostic workflow and thus quality of care for patients with KS. After these actions, histology TAT for KS skin biopsy specimens was identified as an outcome that could be measured to assess the impact of QI interventions on diagnostic delays for patients with KS diagnosed at PMH.
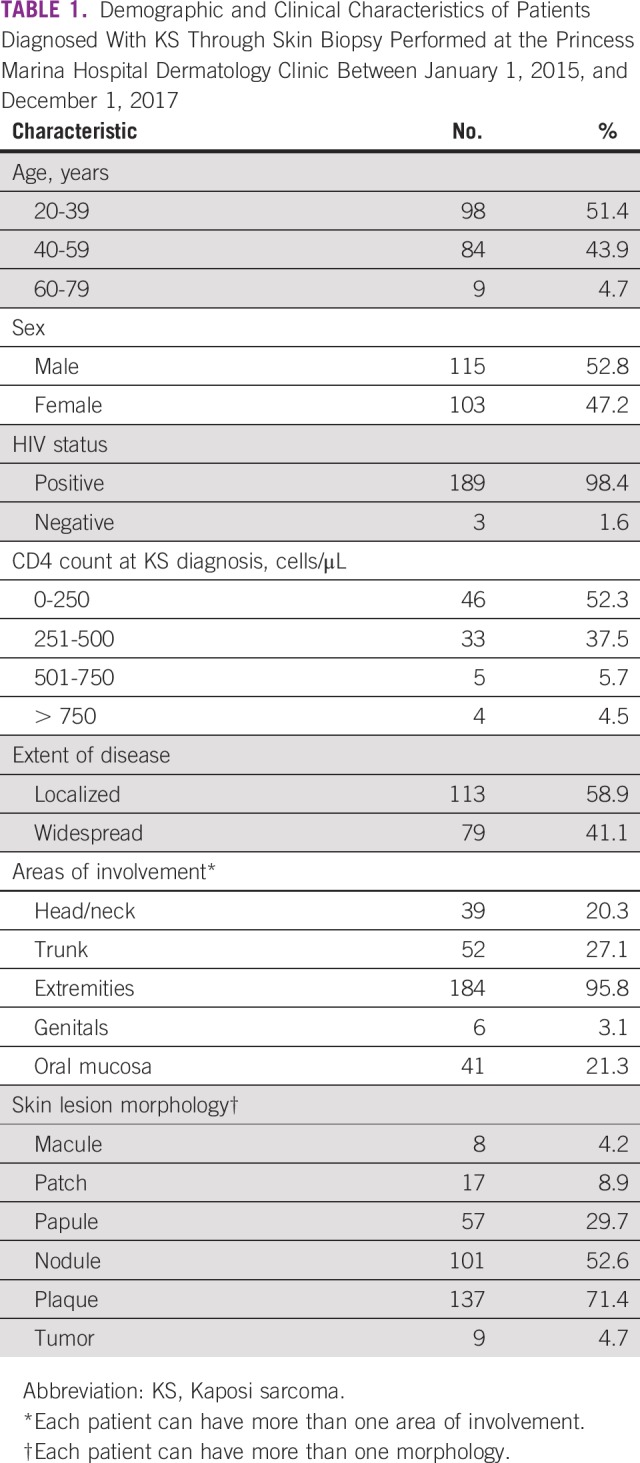
Retrospective review of dermatology biopsy logs identified patients diagnosed with KS by skin biopsy from January 1, 2015, to November 31, 2017. Medical records were assessed to determine the date biopsy specimens were collected and the final date a diagnosis of KS was made through histopathologic sign-out. The number of days from skin biopsy to histopathologic sign-out was evaluated to determine diagnostic histology TAT. Data were separated into two intervals. From January 1, 2015, to December 31, 2015, represented the time before a dermatologist was employed at PMH and was labeled as the predermatology QI interval. From January 1, 2016, to November 31, 2017, represented the postdermatology QI interval at PMH. Demographics and clinical characteristics were gathered from dermatology patient logs, including age, sex, HIV status, CD4 count, extent of disease, body areas of involvement, and lesion morphology (Table 1). A t test was performed to compare the median diagnostic histology TAT from the pre- and postdermatology QI intervals.
TABLE 1.
Demographic and Clinical Characteristics of Patients Diagnosed With KS Through Skin Biopsy Performed at the Princess Marina Hospital Dermatology Clinic Between January 1, 2015, and December 1, 2017
RESULTS
QI Workflow Implementation for Dermatology Clinic and Dermatopathology
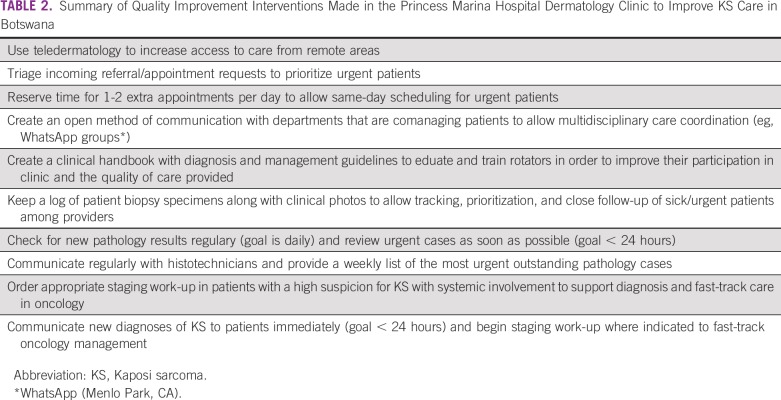
Dermatology clinical interventions implemented at PMH (Table 2) included coordination with the outpatient department and other specialties (eg, oncology, infectious disease) to allow prioritized, same-day scheduling and immediate skin biopsy for patients with suspected KS. A teledermatology service was initiated to allow clinicians across the country to have a standardized mechanism to refer patients with suspected KS to dermatology. Patient visits and skin biopsies were tracked through Excel-based logs (Microsoft Corporation, Redmond, WA). Each week, a list of outstanding dermatology histologic KS specimens and other urgent cases was generated to help histotechnicians prioritize specimen processing. The dermatology team began to actively check the NHL daily for new processed slides to allow urgent slides to be immediately reviewed by a pathologist. The dermatology and pathology departments agreed on a goal of straightforward KS cases being signed out within 1 workday from receipt of a slide. For complicated or unclear cases, dermatology sought urgent telepathology consultation from a remote dermatopathologist with a goal to sign out the case within 1 week. Immediate attempts were made to notify patients with KS of their results within 1 workday of the histologic sign-out, and staging work-ups were initiated whenever indicated to fast-track management. Telepathology was used through a whole-slide imaging system that was purchased by the MoHW in 2013 but remained unused in the NHL until 2016 when the new dermatologist intervened to get the machine properly connected and functional.
TABLE 2.
Summary of Quality Improvement Interventions Made in the Princess Marina Hospital Dermatology Clinic to Improve KS Care in Botswana
The dermatology clinical team consisted of a dermatology specialist (V.L.W.); a nurse; a monthly rotating dermatology resident from North America; and various rotating local trainees from the University of Botswana, which could include medical students and internal medicine, pediatric, or family medicine residents. Challenges faced by the dermatology team included difficulty in accommodating the growing patient load experienced when KS referrals increased. It was also challenging for a single specialist to undertake training monthly rotating dermatology residents to understand and contribute to the dermatology care systems. V.L.W. overcame these challenges by creating a clinical handbook and treatment guidelines to standardize and simplify training of rotators while also improving their clinical education and participation. She also trained a local medical officer to be independently proficient in basic dermatology skills to increase the patient capacity of the dermatology clinic, assist in training rotators, and build capacity for sustainable dermatology care in Botswana.
The NHL histology section underwent a number of workflow changes to address delays in histology TATs (Table 3). These included the creation of a triage system to allow tissue specimens for diagnosis of KS or other urgent tumors to be pushed up the grossing list for more rapid processing. Additional changes included standardizing a tissue processing workflow; implementing a numerical organizational method for storing tissue blocks and slides; and convening a daily 10-minute huddle. These huddle meetings allowed the NHL team to strategize how to deal with daily workloads and challenges, review the progress made in outstanding cases, improve communication among laboratory staff, delegate responsibilities, and emphasize appropriate accountability for job duties. Instead of being burdened by an increase in KS specimens from dermatology, the NHL histology section benefited from the concurrent QI initiatives to improve laboratory functioning and experienced an overall increase in productivity with a general reduction in TATs across all specimens.
TABLE 3.
Summary of Quality Improvement Interventions Made in Pathology at the National Health Laboratory of Botswana to Reduce Turnaround Times for Kaposi Sarcoma Diagnosis
Histology TATs for KS Before and After Dermatology Clinic and Dermatopathology QI Interventions
KS was diagnosed in 192 patients seen by the PMH dermatology clinic between January 1, 2015, and December 1, 2017. A majority of patients (189; 98.4%) were HIV-positive. Median age at diagnosis was 39 years (range, 21-73 years) with a male-to-female ratio of 1.5:1. Of patients with a CD4 count available proximal to diagnosis (n = 88), 52.3% had < 250 cells/μL. Most patients presented with localized disease (58.9%), and plaques (71.4%) were the most frequent lesion morphology. Table 1 lists additional details of this cohort.
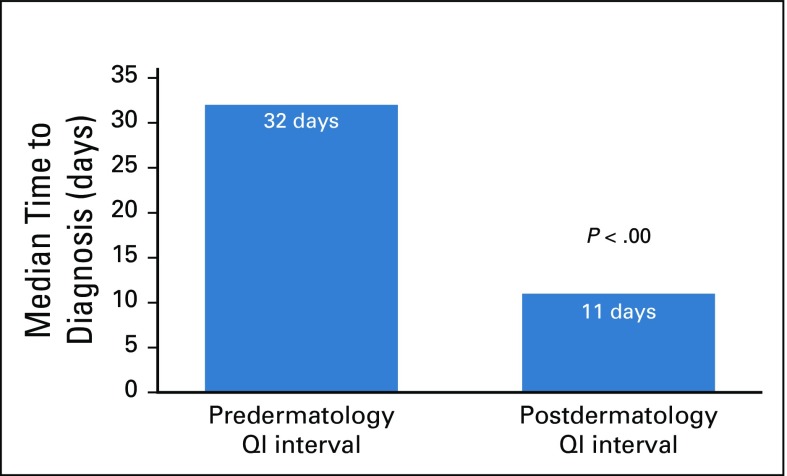
Seventy-three patients were diagnosed with KS in the predermatology QI interval, and 119 were diagnosed in the postdermatology QI interval. A t test was performed to compare the median diagnostic histology TAT in the pre- and postdermatology QI intervals. TAT was significantly decreased from 32 days (interquartile range, 24-56 days) in the predermatology QI interval v 11 days (interquartile range, 12-23 days) in the postdermatology QI interval (P < .00; Fig 1).
FIG 1.
Comparison of median diagnostic histology turnaround times for Kaposi sarcoma before and after dermatology-driven quality improvement (QI) interventions at the National Health Laboratory of Botswana.
DISCUSSION
Although experienced physicians can identify KS clinically, tissue diagnosis is the standard of care for confirmation of diagnosis and initiation of treatment.10-14 Thus, patient outcomes for KS rely on rapid clinical evaluation and expedient histopathologic diagnosis. However, specialty care services needed for KS, particularly dermatology and pathology services, are often lacking in low- and middle-income countries (LMICs).10,11 Until 2009, Botswana did not have its own medical school although a number of residency programs have since been developed, there continues to be no program for training local dermatologists. Historically, there have been 2-3 private dermatologists working in the capital of Botswana. However, long wait times and fees have made them inaccessible and rarely used by the majority of the population that relies on the public healthcare system. Botswana has struggled to maintain dermatology services in the public sector because of comparatively low salaries and restrictions against working outside the public sector to allow specialists to supplement their salaries. There have been multiple gaps without any local dermatology specialist in the public sector, most recently from 2013 to 2015. During these gaps, care was offered by visiting Cuban dermatologists and rotating dermatology residents and faculty from North America. Through a partnership between the American Academy of Dermatology’s Resident International Grant and the Botswana UPenn Partnership, dermatology residents have been able to rotate through the PMH dermatology clinic for 4-6 weeks at a time to allow dermatology care to be continuously provided to Botswana since 2008, even during periods where a local dermatology supervisor was absent. While this system offered patients much-needed dermatology care and access to biopsy services, it was difficult to maintain continuity of care and ensure timely follow-up of histopathology results without a full-time local supervisor in the dermatology clinic. The rapid turnover of volunteer dermatology clinicians also limited opportunities for collaborative relationships to form between dermatology and other departments. Recruitment of a dermatologist was a low priority for the Botswana MoHW because of the limited budget available for specialists and, most likely, the common misconception that dermatology is a less vital part of the health care system. In 2016, the Botswana-UPenn Partnership assisted by recruiting an American dermatologist (V.L.W.) into a position funded by the Botswana MoHW but under a joint employment contract with Botswana-UPenn Partnership. Joint employment structures can be an innovative method to recruit specialists to work in highly underserved areas. Shared funding to supplement local salaries can be most beneficial to recruit specialists to resource-limited settings, but in this case, even a joint contract was advantageous to allow additional academic benefits above what a government position offered.
Accurate and timely pathologic diagnosis is a challenge faced by many developing countries in Africa.15,16 As a result of being short-staffed and overburdened, histology TATs had been highly variable at the NHL and a growing cause for concern in Botswana. Martei et al17 reported that the mean TAT for breast biopsy specimens in Botswana between 2011 and 2015 was 35.7 days, and it extended up to 100.2 days for specimens that required immunohistochemistry. Although processes had begun to help to address these delays, including installation of rapid automated tissue processing equipment and increasing the number of pathologists at NHL from 2 to 4, diagnostic delays were still problematic. Having a dermatologist available to assist with review of skin specimens and directly explain how delays were adversely affecting patient care helped to create the impetus needed for NHL staff to focus on improving workflow processes to prioritize the evaluation of KS specimens.
The arrival of a full-time dermatologist at PMH spearheaded efforts to increase cooperation between the dermatology, oncology, and pathology departments and NHL staff to create an organized workflow and monitoring system for KS biopsy specimens in Botswana. Before our QI interventions, time to KS diagnosis was highly variable and delayed for a number of reasons, the most important of which being the lack of a consistent physician in place to triage patients into the clinic for skin biopsy and to follow-up dermatopathology specimens. The presence of a dermatology specialist at PMH and implementation of a multispecialty-coordinated approach to KS diagnosis was associated with a statistically significant decrease in the median diagnostic histology TATs of KS specimens from 32 to 11 days. This reduction in TAT has substantial clinical implications. Because oncologic treatment of KS cannot be initiated until a histologic diagnosis is confirmed, the histology TAT is a direct contributor to treatment delays in Botswana. Instead of waiting for > 1 month, patients with KS can be seen, diagnosed, and potentially started on treatment within 2 weeks, a drastic improvement, which in some patients has allowed for the administration of lifesaving chemotherapy. The reduction of histologic TAT is a key first step to decreasing the morbidity and mortality associated with this highly prevalent cancer. A similar approach to care coordination in the PMH gynecology clinic achieved a notable reduction in treatment delays for patients with cervical cancer through a dedicated multidisciplinary clinical team.18
This study illustrates the critical role dermatology can play in multiple parts of the care flow of patients with KS. It is important to have clinicians trained to evaluate and biopsy skin lesions concerning for KS, but it is equally as important to have a supervising physician in place who can triage the evaluation of urgent specimens, liaise with pathology teams, and follow up to obtain timely results. Rapid turnover of physicians in resource-limited settings can lead to fragmented care, diagnostic delays, and thus, treatment delays. Having a dermatology specialist within a health care system can provide significant benefits to quality of care. For patients with KS, dermatologists are in a unique position to take the lead in facilitating cooperation between clinical and pathologic services to streamline care. Dermatologists are one of the few specialists who receive dedicated training in both clinical care and histopathology. Thus, they can expertly serve as a key intermediary to bridge the gaps in communication, cooperation, and understanding between hospital-based clinical care services and laboratory histopathologic services.
Direct coordination between dermatology and other services can play an important role in the management of diseases that rely on tissue diagnosis, such as KS and other skin cancers. Employment of a dermatology specialist is often not a top priority within limited-resource settings, but it should be viewed as a critical part of the health care system. This study provides evidence of the pivotal role a dermatologist can play in leading QI initiatives to streamline care coordination and fast-track diagnosis, particularly for skin cancers. In addition, the QI changes that we implemented can serve as a model for other public health care systems that are looking to reduce diagnostic delays for KS and other skin cancers. Tables 2 and 3 list interventions that can be emulated to improve KS care within any dermatology or pathology department. We highlight the utility of creating a clinical handbook with treatment guidelines so that successful, sustainable changes can be passed on to other clinicians.
Limitations of this study include its retrospective nature and the inability to directly link the interventions described to improved KS outcomes. Future studies will aim to evaluate wait times for consultation in oncology and time to treatment in relation to patient outcomes for KS. Also important will be the investigation of other patient factors that may contribute to KS care delays, including stigma, financial constraints, and limited health literacy.
In summary, multispecialty care coordination is a critical component of cancer care, particularly in LMICs where patients face increased barriers to timely care. A dermatologist can provide key leadership and expertise needed to facilitate rapid histologic diagnosis, which is a limiting factor for KS treatment in Botswana. The creation of a multispecialty collaborative workflow and monitoring system for KS demonstrated by our study can serve as a model to improve cancer care in similar LMIC environments.
ACKNOWLEDGMENT
We thank Carrie Kovarik, MD, and the numerous dermatology residents who rotated at PMH dermatology clinic and played an integral role in patient care to make these study achievements possible. We thank the Kramer Family and the Amercian Academy of Dermatology for their continued support of the Resident International Grant Program and the Botswana UPenn Partnership Dermatology Program. We also thank the team of pathologists who contributed greatly to the QI initiatives at the NHL including Pierre A. Russo, MD; Kumarasen Cooper, MBChB, DPhil; Tricia Bhatti, MD; Mariarita Santi, MD, PhD; and Vincent Falck, MD. Additional thanks to William Rate, MS.
AUTHOR CONTRIBUTIONS
Conception and design: Victoria L. Williams, Thapelo Bale, Koorileng Kesalopa, Mukendi A. Kayembe, Surbhi Grover
Administrative support: Victoria L. Williams, Surbhi Grover
Provision of study material or patients: Victoria L. Williams, Koorileng Kesalopa, Mukendi A. Kayembe, Surbhi Grover
Collection and assembly of data: Victoria L. Williams, Olaf Rodriguez, Karen Mosojane
Data interpretation and analysis: Victoria L. Williams, Mohan Narasimhamurthy, Mukendi A. Kayembe, Surbhi Grover
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Victoria L. Williams
Consulting or Advisory Role: Patient Discovery
No other potential conflicts of interest were reported.
REFERENCES
- 1.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer: GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. https://www.iarc.fr/news-events/latest-world-cancer-statistics-globocan-2012-estimated-cancer-incidence-mortality-and-prevalence-worldwide-in-2012/
- 3.Mwanda OW, Fu P, Collea R, et al. Kaposi’s sarcoma in patients with and without human immunodeficiency virus infection, in a tertiary referral centre in Kenya. Ann Trop Med Parasitol. 2005;99:81–91. doi: 10.1179/136485905X19928. [DOI] [PubMed] [Google Scholar]
- 4.Olweny CLM, Borok M, Gudza I, et al. Treatment of AIDS-associated Kaposi’s sarcoma in Zimbabwe: Results of a randomized quality of life focused clinical trial. Int J Cancer. 2005;113:632–639. doi: 10.1002/ijc.20606. [DOI] [PubMed] [Google Scholar]
- 5. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al: Cancer Incidence following expansion of HIV treatment in Botswana. PLoS One 10:e0135602, 2015 [Erratum: PloS One 10:e0138742, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohner E, Valeri F, Maskew M, et al. Incidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in Southern Africa: A prospective multicohort study. J Acquir Immune Defic Syndr. 2014;67:547–554. doi: 10.1097/QAI.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boer C, Niyonzima N, Orem J, et al. Prognosis and delay of diagnosis among Kaposi’s sarcoma patients in Uganda: A cross-sectional study. Infect Agent Cancer. 2014;9:17. doi: 10.1186/1750-9378-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwivedi AK, Dwivedi SN, Deo S, et al. An epidemiological study on delay in treatment initiation of cancer patients. Health. 2012;4:66–79. [Google Scholar]
- 9.Price AJ, Ndom P, Atenguena E, et al. Cancer care challenges in developing countries. Cancer. 2012;118:3627–3635. doi: 10.1002/cncr.26681. [DOI] [PubMed] [Google Scholar]
- 10.Amerson E, Woodruff CM, Forrestel A, et al. Accuracy of clinical suspicion and pathologic diagnosis of Kaposi sarcoma in East Africa. J Acquir Immune Defic Syndr. 2016;71:295–301. doi: 10.1097/QAI.0000000000000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DN, Langan SM, Freeman EE. Task shifting in dermatology: A call to action. JAMA Dermatol. 2017;153:1179–1180. doi: 10.1001/jamadermatol.2017.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laker-Oketta MO, Wenger M, Semeere A, et al. Task shifting and skin punch for the histologic diagnosis of Kaposi’s sarcoma in sub-Saharan Africa: A public health solution to a public health problem. Oncology. 2015;89:60–65. doi: 10.1159/000375165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slaught C, Williams V, Grover S, et al: A retrospective review of patients with Kaposi’s sarcoma in Botswana Int J Dermatol 58:707-712, 2019. [DOI] [PMC free article] [PubMed]
- 14.https://oncolife.com.ua/doc/nccn/kaposi_sarcoma.pdf National Comprehensive Cancer Network: AIDS-Related Kaposi Sarcoma: Version I.2018 November 3, 2017. [DOI] [PubMed]
- 15.Masamba LPL, Mtonga PE, Kalilani Phiri L, et al. Cancer pathology turnaround time at Queen Elizabeth Central Hospital, the largest referral center in Malawi for oncology patients. J Glob Oncol. 2017;3:734–739. doi: 10.1200/JGO.2015.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muvugabigwi G, Nshimiyimana I, Greenberg L, et al. Decreasing histology turnaround time through stepwise innovation and capacity building in Rwanda. J Glob Oncol. 2018;4:1–6. doi: 10.1200/JGO.17.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martei YM, Narasimhamurthy M, Prabhakar P, et al. Breast cancer pathology turnaround time in Botswana. J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.17.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grover S, Chiyapo SP, Puri P, et al. Multidisciplinary gynecologic oncology clinic in Botswana: A model for multidisciplinary oncology care in low- and middle-income settings. J Glob Oncol. 2017;3:666–670. doi: 10.1200/JGO.2016.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]