Abstract
Background:
Knee joint morphology contributions to anterior cruciate ligament (ACL) loading are rarely considered in the injury prevention model. This may be problematic as the knee mechanical response may be influenced by these underlying morphological factors. The goal of the present study was to explore the relationship between posterior tibial slope (which has been recently postulated to influence knee and ACL loading), impact-induced anterior tibial acceleration, and resultant ACL strain during a simulated single-leg landing.
Methods:
Eleven lower limb cadaveric specimens from female donors who had had a mean age (and standard deviation) of 65 ± 10.5 years at the time of death were mounted in a testing apparatus to simulate single-limb landings in the presence of pre-impact knee muscle forces. After preconditioning, specimens underwent five impact trials (mean impact force, 1297.9 ± 210.6 N) while synchronous three-dimensional joint kinetics, kinematics, and relative anteromedial bundle strain data were recorded. Mean peak tibial acceleration and anteromedial bundle strain were quantified over the first 200 ms after impact. These values, along with radiographically defined posterior tibial slope measurements, were submitted to individual and stepwise linear regression analyses.
Results:
The mean peak anteromedial bundle strain (3.35% ± 1.71%) was significantly correlated (r = 0.79; p = 0.004; β = 0.791) with anterior tibial acceleration (8.31 ± 2.77 m/s-2), with the times to respective peaks (66 ± 7 ms and 66 ± 4 ms) also being significantly correlated (r = 0.82; p = 0.001; β = 0.818). Posterior tibial slope (mean, 7.6° ± 2.1°) was significantly correlated with both peak anterior tibial acceleration (r = 0.75; p = 0.004; β = 0.786) and peak anteromedial bundle strain (r = 0.76; p = 0.007; β = 0.759).
Conclusions:
Impact-induced ACL strain is directly proportional to anterior tibial acceleration, with this relationship being moderately dependent on the posterior slope of the tibial plateau.
Clinical Relevance:
Anterior tibial acceleration is associated with anteromedial bundle strain during simulated landings. The magnitude of the impact-induced accelerations governing the strain response is additionally correlated with the posterior slope of the tibial plateau. Additional exploration of the effect of other knee morphological variables on ACL strain during simulated high-risk landings appears warranted.
Anterior cruciate ligament (ACL) injuries remain prevalent and result in substantial short and longer-term knee disabililty1,2. Current interventions to prevent ACL injuries focus on modifying neuromuscular control strategies employed during landings, the rationale being that this control directly influences joint mechanics and is amenable to training3,4. In spite of early successes3,4, however, injury rates and their associated sex disparity have continued5,6. It appears that the current prevention model fails to address unrecognized risk factors associated with the non-contact ACL injury mechanism.
When one lands from a jump, the ground-reaction force induces transient segmental accelerations that are transmitted from the foot to the head7,8. These accelerations are attenuated by the resistance of bone as well as passive and active soft-tissue deformation8,9. When accelerations that are induced in a restraining structure cause deformations (strains) that approach the failure tolerance of the structure, the potential for injury increases10,11. Inadequate shock attenuation is commonly implicated in overuse injuries during repetitive high-impact running or jumping tasks, during which fatigued muscles provide inadequate damping12. Impact-induced tibiofemoral accelerations during a landing may similarly cause ACL strains approaching those sufficient to cause rupture. Specifically, in instances in which an ineffective overarching neuromuscular strategy prevails, it is feasible that impact-induced sagittal plane anterior tibial accelerations could be large enough to compromise the passive ligamentous restraints.
A number of knee morphologic variables have been identified as risk factors for ACL injury, with many also demonstrating sex dependence. A small femoral notch, higher-than-normal body-mass index (BMI), and increased joint and ACL laxity prospectively predicted ACL injury risk in United States military cadets13. Variations in lower limb alignment, such as increased anterior pelvic tilt14, femoral anteversion14,15, and genu recurvatum14,16 have been proposed to impact ACL loading and the resultant risk of ligament injury. Because morphologic contributions to injury risk are largely unmodifiable1, they have not been considered within the injury-prevention model. However, the underlying knee morphology directly influences joint mechanics17 and the potential for injurious load states.
The posterior tibial plateau slope has been identified as a risk factor for ACL injury18-20. Individuals who had previously sustained an ACL injury21, particularly females22, had larger posterior tibial slopes compared with matched controls. Speculation exists with regard to how posterior tibial slope plays a role in an ACL injury18,20,23. Recently, the execution of a “provocative” landing (decreased ankle plantar flexion and increased knee extension and hip flexion), as compared with a safe lower limb landing, was suggested to orient the tibial slope more vertically at impact, resulting in greater anterior tibial thrust23. From a biomechanical perspective, an increased posterior tibial slope may increase ACL strain during such movements by directly increasing the impact-induced anterior tibial accelerations with respect to the femur.
The goal of the present study was to test the primary hypothesis that, during a simulated dynamic single-limb landing, peak relative anteromedial bundle strain is directly associated with both posterior tibial slope and peak anterior tibial acceleration. We also tested a secondary hypothesis that the timings of the peak acceleration and strain magnitudes are highly correlated.
Materials and Methods
Hypotheses were tested in an in vitro repeated-measures laboratory experiment. A pre hoc power analysis employing regression coefficients obtained from the study by Withrow et al.24 showed that a minimum of ten same-sex specimens were required to achieve statistical power of 0.90 with a nominal alpha of 0.05. Data were therefore collected on eleven unembalmed lower limb cadaveric specimens from female donors who had had a mean age of 65 ± 10.5 years at the time of death. All specimens were procured from the University of Michigan Anatomical Donations Department and were fresh frozen at –20°C until twelve hours prior to testing24. Limbs were visually checked, and those presenting with scars, indications of surgery, deformities prior to procurement, radiographic abnormalities or osteophytes, cartilage erosion, exposed bone, and/or any ACL tears were discarded. Specimens were then dissected, with the joint capsule, ligaments, and other passive joint tissues being left intact. Muscle tissue from the quadriceps, medial and lateral hamstrings, and medial and lateral gastrocnemius tendons was also removed. Following dissection, specimens were cut transtibially and transfemorally, approximately 24 cm from the joint line, and were potted within two 7.6-cm-diameter polymethylmethacrylate cylinder blocks with a height of 5.1 cm.
Once potted, each knee specimen was mounted in the apparatus described by Withrow et al.24 to simulate, in the presence of muscle forces, the three-dimensional impulsive loads associated with a jump landing24 (Fig. 1). A double loop of nylon cord (diameter, ∼2 mm; tensile stiffness, ∼2 kN/cm) simulated the in vivo tensile stiffness of the quadriceps muscle-tendon unit under sudden stretch24,25. This cord was attached to the quadriceps tendon via a cryoclamp and was run along the quadriceps muscle line of action to the potted fixture end25,26. Constant-force springs simulated medial and lateral hamstrings and gastrocnemius muscle-tendon units. Pre-impact quadriceps tension was set to 180 N, with the tension in each hamstring and gastrocnemius muscle being set to 70 N, similar to those calculated27 previously for landing maneuvers. Specimens were mounted with an initial knee flexion angle of 15° to be consistent with typical single-leg landings in vivo28.
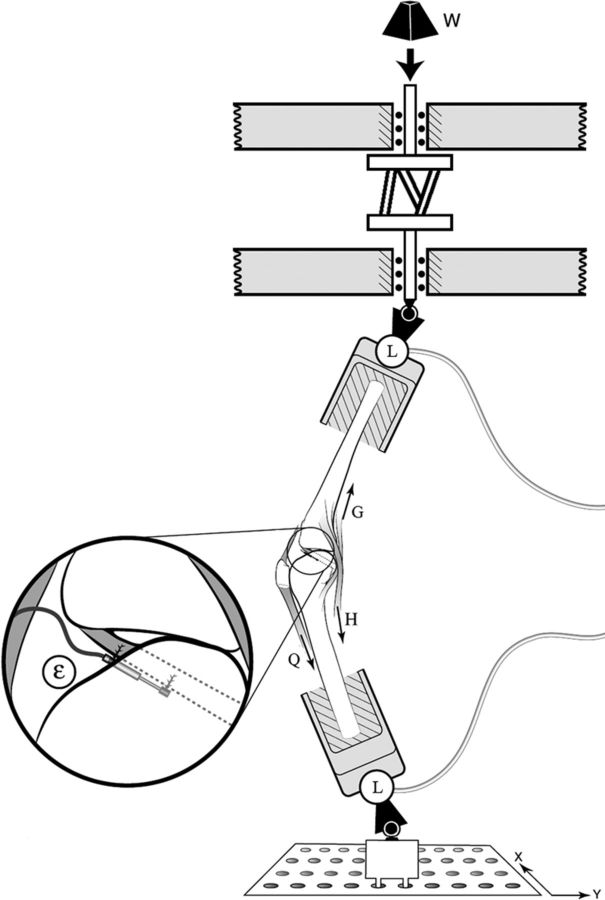
Fig. 1.
Schematic of the test setup, showing the knee mounted for testing as well as the applied impulsive loading (W) and two three-axis load cells (L). Lines of action of the quadriceps (Q), hamstrings (H), and gastrocnemius (G) muscle-tendon units are also visible. The inset shows the differential variable reluctance transducer attached to the anteromedial region of the anterior cruciate ligament in order to measure the relative strain (ɛ).
Each specimen underwent approximately ten consecutive simulated impact trials. The first five trials preconditioned the specimen25, with data from the next five successful trials being used in statistical analyses. To successfully simulate the impulsive knee loads, a weight of approximately 178 N was released vertically in line with the tibial end of the specimen from a height of between 7 and 9 cm. This release height was adjusted over the preconditioning trials to simulate a two-times-body-weight impact for each specimen. The weight struck the end of an impact rod in series with the distal part of the tibia with an impulsive compressive force averaging approximately 1200 N that peaked at approximately 50 ms25. This value is consistent with that estimated or quantified during in vivo landings27,29. In all trials, the knee specimen was oriented with the base of the mounting pot placed directly below the impact rod and the line of action of the impulsive distal tibial force acting 4 cm posterior to the knee joint center. This resulted in an impulsive compression and flexion moment being exerted about the knee joint, generating femoral and tibial moments and an increase in the knee flexion angle24-26. The three-dimensional forces and moments applied to the knee joint by the distal part of the tibia and proximal part of the femur were recorded for two seconds after impact via six-axis load cells (AMTI, Watertown, Massachusetts).
A miniature (3-mm stroke) differential variable reluctance transducer (DVRT; MicroStrain, Burlington, Vermont) was inserted into the distal third of the anteromedial bundle of the ACL25,26 to measure the change in length relative to the initial length. This initial length value corresponded to the pre-impact length of the differential variable reluctance transducer, as recorded with balanced pretensioned muscle and gravitational forces and the static knee flexion angle (15°)25. With use of this baseline measurement, changes in the length of the differential variable reluctance transducer were recorded for each trial over the same two-second post-impact period via a 16-bit analog-to-digital converter and were converted to relative anteromedial bundle strain.
Three-dimensional knee kinematics were recorded during each impact trial via three infrared-emitting diodes that were rigidly attached to the tibial plateau and the femoral condyle. A Certus system (Northern Digital, Waterloo, Ontario, Canada) acquired the three-dimensional location of each infrared-emitting diode at 400 Hz for two seconds immediately after impact with a resolution of 0.1 mm, from which three-dimensional tibiofemoral kinematics were quantified to the nearest degree and millimeter25. The knee was assigned six degrees of freedom, corresponding to motion along or about three orthogonal axes30 passing through a fixed joint center (the midpoint of the femoral condyles at the height of the most proximal point on the intercondylar notch)24. Kinematic data recorded during each impact trial were expressed relative to the specimen's pre-impact (neutral) posture.
Following testing, the specimen was removed from the loading frame and true lateral radiographs were made. Specifically, images were made at a fixed distance of 2 m, with the tibia aligned vertically and the primary ray focused at the height of the tibial surface, perpendicular to tibial long axis. From the resulting radiographic images, tibial slope measures were made in a blinded fashion on the basis of previous methods18,31. The anterior and posterior cortices of the tibial shaft were first determined at points approximately 4 cm apart on the distal radiograph (Fig. 2). The midpoints of the two lines connecting these points were then quantified, and the sagittal plane longitudinal axis was constructed such that it passed through each midpoint18. The peak anterior and posterior points of the medial tibial plateau as observed on the radiograph (points A and B) were subsequently defined. The slope angle was calculated as the enclosed angle between the line passing through these two points and the line perpendicular to the sagittal plane longitudinal axis18.
Fig. 2.
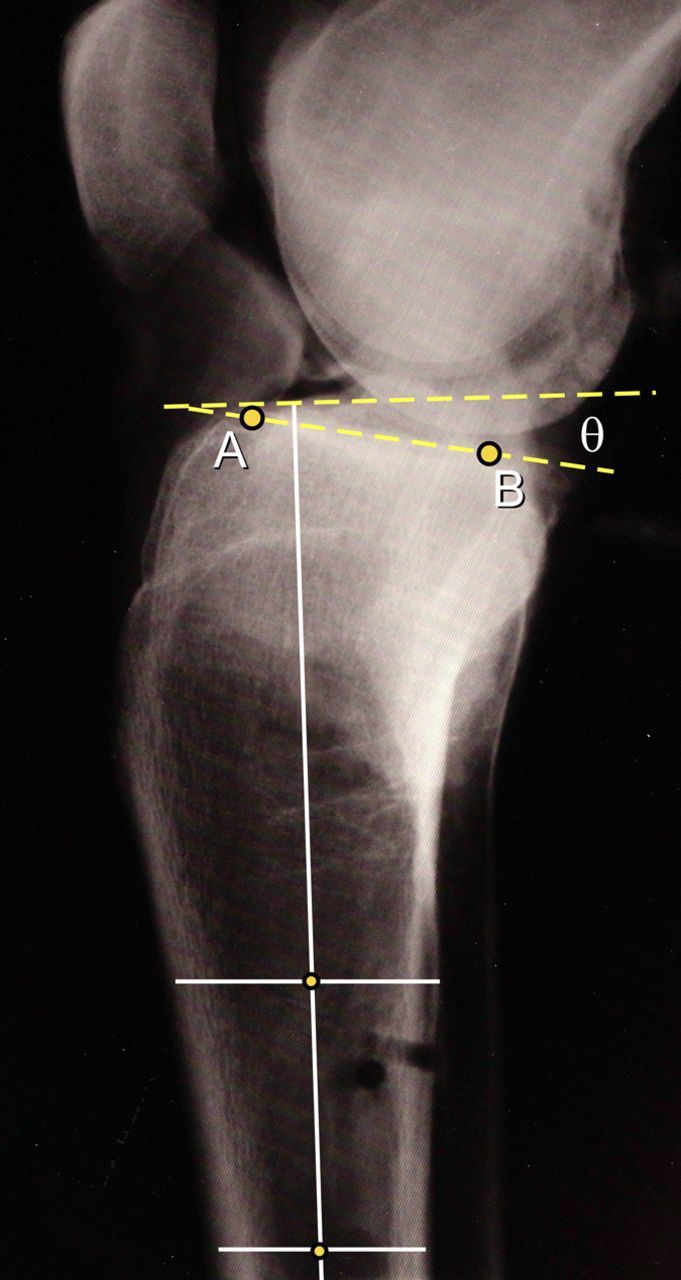
Lateral radiograph illustrating the method used to calculate the posterior slope angle of the tibial plateau. A line passing through the midpoints of two lines connecting the anterior and posterior cortices of the tibial shaft, located approximately 4 cm apart, defined the sagittal plane longitudinal axis of the tibia. The peak anterior (A) and posterior (B) points on the medial tibial plateau were then identified, with the slope angle defined as the enclosed angle between the line joining points A and B and the line perpendicular to the longitudinal axis.
Statistical Methods
The relative three-dimensional translations and rotations between the femur and the tibia were calculated over the duration of each trial20,32. Anterior tibial displacement data were then low-pass filtered with a cubic smoothing spline with a 50-Hz cutoff frequency33 and were double differentiated to provide accelerations. From these data, the peak anterior tibial acceleration over the first 200 ms after impact was recorded. The peak relative anteromedial bundle strain was similarly determined for each trial over this same time period. To assess tibial slope measurement reliability, three researchers initially quantified slope angles in each specimen on three consecutive days. Intraclass correlation coefficients were subsequently quantified within a two-way mixed model. Intraclass correlation coefficients values were classified as poor (<0.4), fair to good (0.4 to 0.74), or excellent (≥0.75)34. Intraclass correlation coefficients that were calculated for intra-rater and inter-rater agreement regarding tibial slope measures were >0.914, suggesting that they could be submitted to statistical treatment with confidence. Posterior tibial slope values were thus averaged across the three measurements obtained for each specimen and were submitted to the analyses. To test the primary research hypothesis, individual regression analyses were first conducted to examine potential associations between both peak anterior tibial acceleration and posterior tibial slope and resultant peak anteromedial bundle strain. Based on the outcomes of these preliminary analyses, a multiple linear stepwise regression model was adopted to examine the extent to which specimen-based peak anteromedial bundle strain measures were predicted by posterior tibial slope, peak tibial acceleration, and the interaction between these terms. Significance levels for inclusion and exclusion within this model were set at p < 0.05 and p < 0.1, respectively. To test our secondary hypothesis, the relative timings of specimen-based peak anteromedial bundle strain and peak anterior tibial acceleration were also submitted to a simple linear regression model. Regression coefficients for all treatments were regarded as significant at p < 0.05. All analyses were performed with use of SPSS software version 17.0 (SPSS, Chicago, Illinois).
Source of Funding
This study was funded, in part, through the National Institutes of Health (AR054821-01). This funding was used to assist in specimen procurement, building the impact-loading device, purchasing equipment/materials used in the cadaveric testing, and the funding of graduate students working on this project.
Results
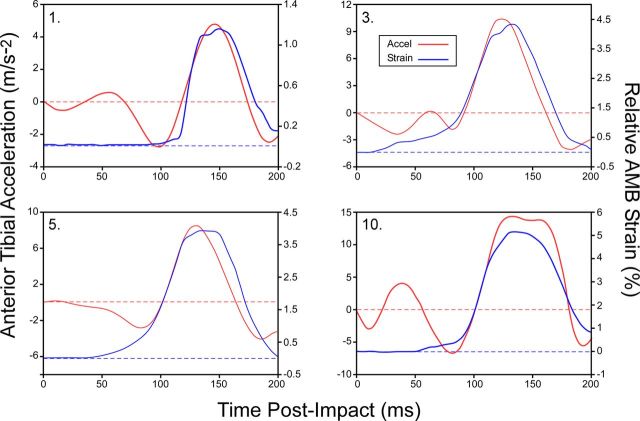
Following testing, each joint was visually examined for damage. No gross morphologic changes were found in the ACL of any specimen, with each ligament remaining functionally intact. The mean peak impact force (and standard deviation) applied to the distal part of the tibia was 1297.9 ± 210.6 N (range, 1002.5 to 1783.1 N), occurring 50.6 ± 2.8 ms (range, 46 to 56 ms) following impact. Time-series data depicting impact-induced anterior tibial acceleration and associated anteromedial bundle strain measurements over the first 200 ms of the impact phase are presented for a random sample of four specimens in Figure 3. The mean duration of the anterior tibial acceleration pulse was 37.2 ± 6.0 ms. Definitive peaks were evident in both anterior tibial accelerations (mean, 8.31 ± 2.77 m/s-2; range, 5.19 m/s-2 to 14.45 m/s-2) and anteromedial bundle strain magnitudes (mean, 3.35% ± 1.71%; range, 1.15% to 6.67%) for all specimens, occurring at a mean of 66 ± 7 ms (range, 51 to 76 ms) and 66 ± 4 ms (range, 60 to 73 ms) after impact, respectively (Fig. 3) (see Appendix). The mean posterior tibial slope across specimens was 7.6° ± 2.1° (range, 4.7° to 10.5°).
Fig. 3.
Line plots showing impact-induced anterior tibial acceleration and associated anteromedial bundle (AMB) strain measurements over the first 200 ms of the impact phase for a random sample of four specimens.
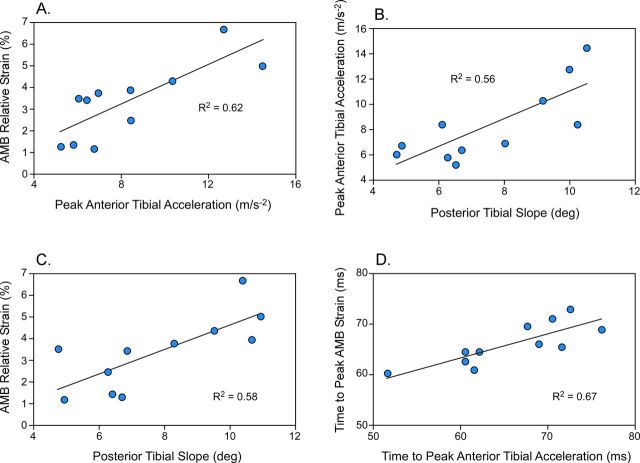
In testing the primary hypothesis, peak anteromedial bundle strain was significantly correlated (r = 0.79; p = 0.004) with peak anterior tibial acceleration (Fig. 4, A), explaining 62% of the associated variance (β = 0.791). Specifically, for every 1-m/s-2 increase in peak anterior tibial acceleration, the anteromedial bundle will experience a 0.4% greater peak relative strain. Peak impact-induced anterior tibial acceleration was also significantly correlated (r = 0.75; p = 0.004) with posterior tibial slope (Fig. 4, B), which explained 56% of the variance (β = 0.786). Here, for every 1° increase in tibial slope, the peak anterior tibial acceleration increased by 1.11 m/s-2.
Fig. 4.
Regression plots showing associations between mean subject-based peak impact-induced anterior tibial acceleration and peak relative anteromedial bundle (AMB) strain (A), posterior tibial slope angle and impact-induced anterior tibial acceleration (B), posterior tibial slope angle and peak relative anteromedial bundle strain (C), and the respective timings of peak impact-induced anterior tibial acceleration and peak relative anteromedial bundle strain (D).
In testing the secondary hypothesis, the timing of the peak anterior tibial acceleration was significantly correlated (r = 0.82; p = 0.001) with the timing of peak anteromedial bundle strain (Fig. 4, D), explaining 67% of the variance (β = 0.818).
Peak relative anteromedial bundle strain was also significantly correlated (r = 0.76; p = 0.007) with posterior tibial slope (Fig. 4, C), explaining 58% of the variance (β = 0.759). For every 1° increase in posterior tibial slope, the anteromedial bundle experienced 0.6% greater peak relative strain. Including both peak anterior tibial acceleration and posterior tibial slope within the full stepwise linear regression model did not significantly improve peak relative anteromedial bundle strain predictions (p = 0.304). Specifically, the posterior tibial slope term only explained an additional 4.9% of the variance observed in peak relative anteromedial bundle strain beyond that explained by peak anterior tibial acceleration alone.
Discussion
To our knowledge, this is the first study to demonstrate significant associations between impact-induced anterior tibial acceleration, posterior tibial slope, and anteromedial bundle-ACL strain for dynamic high-impact jump landings. Understanding the relationships between knee morphology and resultant mechanics during such tasks is ultimately critical to improved neuromuscular-based ACL injury prevention methods. We did not induce ACL injury in any trial, and a future study may examine the relationships between peak anterior tibial acceleration, tibial slope, and ACL rupture.
A relatively large anterior tibial acceleration peak was evident in all specimens soon after impact, coinciding directly with peaks in the anteromedial bundle strain response. Furthermore, increases in the magnitude of the acceleration peak predicted similar increases in anteromedial bundle strain. It has been well documented that the ACL is the primary passive restraint to anterior tibial translations and loads35,36. Acceleration transients at the proximal part of the tibia during abrupt deceleration tasks have similarly been shown to generate tibiofemoral shear forces that load the passive knee structures37,38. A feasible explanation for our observed relationship is obtained when one considers Newton's second law of motion (F = ma). Greater anterior tibial acceleration will result in greater force being placed on the ACL. This increased force will in turn increase the relative strain experienced within the ligament, which attempts to restrain the accelerating tibia. Using the same impact loading apparatus, Withrow et al.24 demonstrated that anteromedial bundle strain also correlates well with the knee flexion moment, flexion angle, and quadriceps force. Our study extends their initial findings by showing that these parameters may affect anteromedial bundle strain via their contributions to the large transient tibial acceleration pulse, lasting >35 ms in duration and peaking at 65 ms after landing impact, coinciding almost exactly with the instant of peak anteromedial bundle strain.
The fact that our peak strain magnitudes were consistent with those measured during movements eliciting substantially smaller knee joint accelerations39,40 may initially seem counterintuitive. Considering that strain was measured “locally” in each instance, outcome comparisons may be compromised because of the sensitivity of the strain response to placement of the differential variable reluctance transducer along the ligament tissue. The extent to which the strain behavior of the anteromedial bundle varies along the length of this structure remains unknown and appears to be worthy of further exploration. It should be noted, however, that our peak strain measurements were extremely consistent with those measured previously in vivo for similar high-impact landing scenarios41. We are confident that our observed strain outcomes and resultant anatomical-mechanical relations are correct. Regardless, the above inconsistencies strongly suggest that greater insight into how the complex interactions between knee joint and ACL geometric, structural, and mechanical factors dictate the ACL strain response under dynamic loading is warranted.
Impact-induced peak tibial acceleration magnitudes varied over a wide range across specimens. With both impact load and pre-impact muscle forces being constant, between-specimen variations in acceleration and strain profiles must have been dictated by additional joint-related factors. On the basis of previously demonstrated links to ACL injury risk21,22, we had initially posited posterior tibial slope to be one such factor. It has been suggested that the posterior tibial slope induces potentially hazardous ACL loads during landing tasks by producing a larger anterior tibial translation and/or thrust23. Explicit relationships between tibial slope, knee kinematics, and ACL load had only been quantified under clinically relevant joint-loading conditions (e.g., the anterior drawer test and Lachman test)18,42,43. Furthermore, the tibial translation and ACL strain profile observed in these instances was not affected by slope under constrained anterior-posterior load applications. Our results suggest that, at least for high-impact landings under the same external load constraints, tibial acceleration and resultant ACL strain are sensitive to the posterior tibial slope. A larger-than-normal slope orients the ACL more in an anterior-posterior direction44 and shifts the tibiofemoral contact point anteriorly43. This latter adjustment is posited to increase the anterior tibial shear load component of the tibial compressive force when transitioning from non-weight-bearing to weight-bearing18,45, causing greater ACL loads46. A similar chain of events may prevail during high-impact landing scenarios, in which high-rate compressive joint loading transfers to an equally high anterior tibial shear load rate, culminating in a greater anterior tibial acceleration pulse and ACL strain. These relationships may precipitate even greater tibial accelerations and ACL strains for an individual exhibiting the “provocative” landing postures, during which the effect of tibial slope may be even greater23. Others have shown that, at least for clinical load states, the posterior tibial slope transfers a component of axial compressive tibial loading into anterior tibial translation and ACL strain43. Additional work is necessary to determine whether tibial slope contributions to high-impact three-dimensional knee joint loading similarly extrapolate to cause actual ACL injury.
We defined tibial slope as the angle of the medial tibial plateau, measured indirectly on a standard lateral knee radiograph31,47. These measurements were also significantly correlated (R2 = 0.97; p = 0.00001) with slope measurements made directly on each specimen on the basis of recently published methods48. This establishment of relationships between slope and both acceleration and strain with use of this simple indirect radiographic technique supports its en masse screening potential. However, Hashemi et al.20 recently demonstrated that links between posterior tibial slope and ACL injury may be more complex, with the medial tibial slope magnitude and its depth of concavity presenting with additional risk. Delineation of these different slopes, including cartilage contributions to slope variations49,50, is not possible on radiographs, so three-dimensional computed tomography or magnetic resonance imaging may be necessary18,20,51.
Compressive joint loads (approximately two times body weight) during the impact simulations were less than those of landings in which ACL injury is common20,29,52,53. As noted, however, associations between posterior tibial slope, tibial acceleration, and ACL strain appear to be governed by standard mechanical principles. Hence, larger ACL strains in the presence of larger impact loads are likely. A larger impact load for a knee with an increased posterior tibial slope will similarly induce a larger anterior shear component of the tibial compressive force, tibial acceleration, and ACL strain. This relationship between impact load, tibial acceleration, and ACL strain profiles also may explain why a larger BMI prospectively predicts ACL injury risk13. We also examined relationships between slope, kinematics, and ACL strain within the sagittal plane only. While it has been suggested that ACL injury is possible via a purely sagittal plane loading mechanism29,54, it is increasingly speculated that a more complex three-dimensional scenario prevails55. Hence, the tibial slope-acceleration mechanism could contribute to injury risk without being the only critical factor.
Our study supports the concept that the posterior tibial slope is an important risk factor for ACL injury20,23,56. In particular, a steeper slope increases the propensity for potentially traumatic impact-induced anterior tibial accelerations and resultant ACL strains. A simulation of actual injury scenarios within our cadaveric model would provide important additional insights here, and we will take such steps in our future work. Current outcomes may also provide immediate improvements to large-scale ACL injury risk assessment methods. In addition to possible radiologic assessments, ongoing advances in body-worn sensor technologies57,58 with integrated accelerometry may ultimately afford improved risk screening based on safe, noninvasive segment acceleration measures. The success of targeted training interventions similarly may be evaluated by determining whether large impact-induced tibial accelerations can be consistently reduced for a variety of landing scenarios in which ACL injuries are common.
The present study had several limitations. The radiographs did not allow for the measurement of the tibial plateau depth of concavity, a known predictor of ACL injury27. Another potential limitation relates to the reliability of our tibial slope measurement method. Several measurement methods have been proposed20,59, and it is unclear whether the associations between slope, acceleration, and strain would exist if other methods were used. With slope definitions appearing reasonably correlated, however59,60, similar outcomes seem likely. The high reliability that we observed for these measurements also adds strength to their efficacy. While slope measurements were reliable, study outcomes may have been compromised if these and anteromedial bundle strain measurements were inaccurate, considering the relatively small between-specimen variations in each of these parameters. We measured both slope and strain data with use of well-established techniques, although there are few data available regarding their true accuracies. Considering the strengths of association observed between slope, tibial acceleration, and anteromedial bundle strain measurements, we believe that our findings make an important contribution to the understanding of knee morphologic-mechanical interactions.
Another limitation, common in this type of testing, was the inability to quantify the true anteromedial bundle resting length with the specimen positioned within the loading frame61,62. We defined length changes in terms of relative anteromedial bundle strain, with use of the length of the differential variable reluctance transducer for the static muscle pre-load state as the reference value24. In addition, only local ACL-anteromedial bundle strain was recorded; attaching even a miniature gauge on the posterolateral bundle of the ACL risks compromising the posterior joint capsule63 or incurring measurement artifact24. However, the strain behavior of the ACL-anteromedial bundle does provide a reasonable representation of the entire ligament strain response39,64. The recent work by Mizuno et al.19 and Kanamori et al.65 supports this contention, with general agreement between local anteromedial bundle strain and ACL in situ loads observed under clinical load applications. A final limitation is the extent to which data obtained from aged cadaveric specimens can be used to infer ACL injury causality in a young, healthy population. Although advancing age is known to impact knee joint and ACL tissue properties66, we would expect results in younger specimens to exhibit similar qualitative trends with different absolute values.
In conclusion, impact-induced ACL strain is directly proportional to anterior tibial acceleration, with both of these factors also being dependent on the posterior slope of the tibial plateau.
Appendix
A table showing specimen-based peak impact-induced anterior tibial acceleration magnitudes and associated peak relative anteromedial bundle strain measures quantified during simulated single leg landings is available with the online version of this article at jbjs.org.
Investigation performed at the School of Kinesiology and Department of Mechanical Engineering, University of Michigan, Ann Arbor, Michigan
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by the authors of this work are available with the online version of this article at jbjs.org.
References
- 1. Griffin LY Albohm MJ Arendt EA Bahr R Beynnon BD Demaio M Dick RW Engebretsen L Garrett WE Jr Hannafin JA Hewett TE Huston LJ Ireland ML Johnson RJ Lephart S Mandelbaum BR Mann BJ Marks PH Marshall SW Myklebust G Noyes FR Powers C Shields C Jr Shultz SJ Silvers H Slauterbeck J Taylor DC Teitz CC Wojtys EM Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512-32. [DOI] [PubMed] [Google Scholar]
- 2. Renstrom P Ljungqvist A Arendt E Beynnon B Fukubayashi T Garrett W Georgoulis T Hewett TE Johnson R Krosshaug T Mandelbaum B Micheli L Myklebust G Roos E Roos H Schamasch P Shultz S Werner S Wojtys E Engebretsen L. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42:394-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hewett TE Lindenfeld TN Riccobene JV Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27:699-706. [DOI] [PubMed] [Google Scholar]
- 4. Myklebust G Engebretsen L Braekken IH Skjølberg A Olsen OE Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13:71-8. [DOI] [PubMed] [Google Scholar]
- 5. Agel J Arendt EA Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33:524-30. [DOI] [PubMed] [Google Scholar]
- 6. Mihata LC Beutler AI Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34:899-904. [DOI] [PubMed] [Google Scholar]
- 7. Derrick TR. The effects of knee contact angle on impact forces and accelerations. Med Sci Sports Exerc. 2004;36:832-7. [DOI] [PubMed] [Google Scholar]
- 8. Lafortune MA Lake MJ Hennig EM. Differential shock transmission response of the human body to impact severity and lower limb posture. J Biomech. 1996;29:1531-7. [PubMed] [Google Scholar]
- 9. Coventry E O'Connor KM Hart BA Earl JE Ebersole KT. The effect of lower extremity fatigue on shock attenuation during single-leg landing. Clin Biomech (Bristol, Avon). 2006;21:1090-7. [DOI] [PubMed] [Google Scholar]
- 10. Moran KA Marshall BM. Effect of fatigue on tibial impact accelerations and knee kinematics in drop jumps. Med Sci Sports Exerc. 2006;38:1836-42. [DOI] [PubMed] [Google Scholar]
- 11. Voloshin AS Mizrahi J Verbitsky O Isakov E. Dynamic loading on the human musculoskeletal system—effect of fatigue. Clin Biomech (Bristol, Avon). 1998;13:515-20. [DOI] [PubMed] [Google Scholar]
- 12. Elvin NG Elvin AA Arnoczky SP. Correlation between ground reaction force and tibial acceleration in vertical jumping. J Appl Biomech. 2007;23:180-9. [DOI] [PubMed] [Google Scholar]
- 13. Uhorchak JM Scoville CR Williams GN Arciero RA St Pierre P Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831-42. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen AD Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37:389-98. [DOI] [PubMed] [Google Scholar]
- 15. Bråten M Terjesen T Rossvoll I. Femoral anteversion in normal adults. Ultrasound measurements in 50 men and 50 women. Acta Orthop Scand. 1992;63:29-32. [DOI] [PubMed] [Google Scholar]
- 16. Trimble MH Bishop MD Buckley BD Fields LC Rozea GD. The relationship between clinical measurements of lower extremity posture and tibial translation. Clin Biomech (Bristol, Avon). 2002;17:286-90. [DOI] [PubMed] [Google Scholar]
- 17. Amiri S Cooke D Kim IY Wyss U. Mechanics of the passive knee joint. Part 2: interaction between the ligaments and the articular surfaces in guiding the joint motion. Proc Inst Mech Eng H. 2007;221:821-32. [DOI] [PubMed] [Google Scholar]
- 18. Hashemi J Chandrashekar N Gill B Beynnon BD Slauterbeck JR Schutt RC Jr Mansouri H Dabezies E. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizuno K Andrish JT van den Bogert AJ McLean SG. Gender dimorphic ACL strain in response to combined dynamic 3D knee joint loading: implications for ACL injury risk. Knee. 2009;16:432-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashemi J Chandrashekar N Mansouri H Gill B Slauterbeck JR Schutt RC Jr Dabezies E Beynnon BD. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38:54-62. [DOI] [PubMed] [Google Scholar]
- 21. Brandon ML Haynes PT Bonamo JR Flynn MI Barrett GR Sherman MF. The association between posterior-inferior tibial slope and anterior cruciate ligament insufficiency. Arthroscopy. 2006;22:894-9. [DOI] [PubMed] [Google Scholar]
- 22. Todd MS Lalliss S Garcia E DeBerardino TM Cameron KL. The relationship between posterior tibial slope and anterior cruciate ligament injuries. Am J Sports Med. 2010;38:63-7. [DOI] [PubMed] [Google Scholar]
- 23. Boden BP Breit I Sheehan FT. Tibiofemoral alignment: contributing factors to noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2009;91:2381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Withrow TJ Huston LJ Wojtys EM Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am J Sports Med. 2006;34:269-74. [DOI] [PubMed] [Google Scholar]
- 25. Withrow TJ Huston LJ Wojtys EM Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;90:815-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Withrow TJ Huston LJ Wojtys EM Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon). 2006;21:977-83. [DOI] [PubMed] [Google Scholar]
- 27. Pflum MA Shelburne KB Torry MR Decker MJ Pandy MG. Model prediction of anterior cruciate ligament force during drop-landings. Med Sci Sports Exerc. 2004;36:1949-58. [DOI] [PubMed] [Google Scholar]
- 28. Kernozek TW Torry MR Iwasaki M. Gender differences in lower extremity landing mechanics caused by neuromuscular fatigue. Am J Sports Med. 2008;36:554-65. [DOI] [PubMed] [Google Scholar]
- 29. Yu B Lin CF Garrett WE. Lower extremity biomechanics during the landing of a stop-jump task. Clin Biomech (Bristol, Avon). 2006;21:297-305. [DOI] [PubMed] [Google Scholar]
- 30. Grood ES Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136-44. [DOI] [PubMed] [Google Scholar]
- 31. Genin P Weill G Julliard R. [The tibial slope. Proposal for a measurement method]. J Radiol. 1993;74:27-33. French. [PubMed] [Google Scholar]
- 32. DeGoede KM Ashton-Miller JA. Fall arrest strategy affects peak hand impact force in a forward fall. J Biomech. 2002;35:843-8. [DOI] [PubMed] [Google Scholar]
- 33. Woltring HJ Huiskes R de Lange A Veldpaus FE. Finite centroid and helical axis estimation from noisy landmark measurements in the study of human joint kinematics. J Biomech. 1985;18:379-89. [DOI] [PubMed] [Google Scholar]
- 34. Portney LG Watkins MP. Foundations of clinical research: applications to practice. 2nd ed Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- 35. Butler DL. Anterior cruciate ligament: its normal response and replacement. J Orthop Res. 1989;7:910-21. [DOI] [PubMed] [Google Scholar]
- 36. Markolf KL Burchfield DM Shapiro MM Shepard MF Finerman GA Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13:930-5. [DOI] [PubMed] [Google Scholar]
- 37. Bryant AL Newton RU Steele J. Successful feed-forward strategies following ACL injury and reconstruction. J Electromyogr Kinesiol. 2009;19:988-97. [DOI] [PubMed] [Google Scholar]
- 38. Lafortune MA. Three-dimensional acceleration of the tibia during walking and running. J Biomech. 1991;24:877-86. [DOI] [PubMed] [Google Scholar]
- 39. Beynnon BD Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31:519-25. [DOI] [PubMed] [Google Scholar]
- 40. Beynnon BD Fleming BC Johnson RJ Nichols CE Renström PA Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24-34. [DOI] [PubMed] [Google Scholar]
- 41. Cerulli G Benoit DL Lamontagne M Caraffa A Liti A. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: case report. Knee Surg Sports Traumatol Arthrosc. 2003;11:307-11. [DOI] [PubMed] [Google Scholar]
- 42. Fening SD Kovacic J Kambic H McLean S Scott J Miniaci A. The effects of modified posterior tibial slope on anterior cruciate ligament strain and knee kinematics: a human cadaveric study. J Knee Surg. 2008;21:205-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giffin JR Vogrin TM Zantop T Woo SL Harner CD. Effects of increasing tibial slope on the biomechanics of the knee. Am J Sports Med. 2004;32:376-82. [DOI] [PubMed] [Google Scholar]
- 44. Li G Papannagari R DeFrate LE Yoo JD Park SE Gill TJ. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop. 2006;77:267-74. [DOI] [PubMed] [Google Scholar]
- 45. Beynnon BD Fleming BC Labovitch R Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20:332-7. [DOI] [PubMed] [Google Scholar]
- 46. Petersen W Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res. 2007;454:35-47. [DOI] [PubMed] [Google Scholar]
- 47. Yoo JH Chang CB Shin KS Seong SC Kim TK. Anatomical references to assess the posterior tibial slope in total knee arthroplasty: a comparison of 5 anatomical axes. J Arthroplasty. 2008;23:586-92. [DOI] [PubMed] [Google Scholar]
- 48. de Boer JJ Blankevoort L Kingma I Vorster W. In vitro study of inter-individual variation in posterior slope in the knee joint. Clin Biomech (Bristol, Avon). 2009;24:488-92. [DOI] [PubMed] [Google Scholar]
- 49. Hudek R Schmutz S Regenfelder F Fuchs B Koch PP. Novel measurement technique of the tibial slope on conventional MRI. Clin Orthop Relat Res. 2009;467:2066-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Espregueira-Mendes JD da Silva MV. Anatomy of the proximal tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2006;14:241-9. [DOI] [PubMed] [Google Scholar]
- 51. Simon RA Everhart JS Nagaraja HN Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43:1702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McLean SG Samorezov JE. Fatigue-induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc. 2009;41:1661-72. [DOI] [PubMed] [Google Scholar]
- 53. Blackburn JT Padua DA. Sagittal-plane trunk position, landing forces, and quadriceps electromyographic activity. J Athl Train. 2009;44:174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeMorat G Weinhold P Blackburn T Chudik S Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32:477-83. [DOI] [PubMed] [Google Scholar]
- 55. McLean SG Huang X Su A Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech (Bristol, Avon). 2004;19:828-38. [DOI] [PubMed] [Google Scholar]
- 56. McLean SG Lucey SM Rohrer S Brandon C. Knee joint anatomy predicts high-risk in vivo dynamic landing knee biomechanics. Clin Biomech (Bristol, Avon). 2010;25:781-8. [DOI] [PubMed] [Google Scholar]
- 57. Bergmann JH Mayagoitia RE Smith IC. A portable system for collecting anatomical joint angles during stair ascent: a comparison with an optical tracking device. Dyn Med. 2009;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Favre J Aissaoui R Jolles BM de Guise JA Aminian K. Functional calibration procedure for 3D knee joint angle description using inertial sensors. J Biomech. 2009;42:2330-5. [DOI] [PubMed] [Google Scholar]
- 59. Han HS Chang CB Seong SC Lee S Lee MC. Evaluation of anatomic references for tibial sagittal alignment in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2008;16:373-7. [DOI] [PubMed] [Google Scholar]
- 60. Brazier J Migaud H Gougeon F Cotten A Fontaine C Duquennoy A. [Evaluation of methods for radiographic measurement of the tibial slope. A study of 83 healthy knees]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82:195-200. French. [PubMed] [Google Scholar]
- 61. Fleming BC Beynnon BD Tohyama H Johnson RJ Nichols CE Renström P Pope MH. Determination of a zero strain reference for the anteromedial band of the anterior cruciate ligament. J Orthop Res. 1994;12:789-95. [DOI] [PubMed] [Google Scholar]
- 62. Hashemi J Breighner R Jang TH Chandrashekar N Ekwaro-Osire S Slauterbeck JR. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: an in vitro simulation. Knee. 2010;17:235-41. [DOI] [PubMed] [Google Scholar]
- 63. Bach JM Hull ML. Strain inhomogeneity in the anterior cruciate ligament under application of external and muscular loads. J Biomech Eng. 1998;120:497-503. [DOI] [PubMed] [Google Scholar]
- 64. Butler DL Kay MD Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech. 1986;19:425-32. [DOI] [PubMed] [Google Scholar]
- 65. Kanamori A Woo SL Ma CB Zeminski J Rudy TW Li G Livesay GA. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: a human cadaveric study using robotic technology. Arthroscopy. 2000;16:633-9. [DOI] [PubMed] [Google Scholar]
- 66. Woo SL Hollis JM Adams DJ Lyon RM Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217-25. [DOI] [PubMed] [Google Scholar]