Abstract
Background:
The existence of fibrocartilage, bone-like tissues, nerves, and blood vessels in the anulus fibrosus during intervertebral disc degeneration has been well documented. Migration of differentiated cells from outside the intervertebral disc has been hypothesized as a possible mechanism for the formation of these tissues. We hypothesized that the normal anulus fibrosus tissue contains multipotent progenitor cells, which are able to differentiate into cartilage and/or fibrocartilage cells, osteoblasts, neurons, and blood vessel cells.
Methods:
We isolated anulus fibrosus cells from the nondegenerative intervertebral discs of adolescent (thirteen to sixteen-year-old) patients with idiopathic scoliosis and cultured the cells in vitro in induction media containing different stimuli. Immunophenotypic analysis of cell surface markers was performed by flow cytometry. Expression of markers of adipogenesis, osteogenesis, chondrogenesis, neurogenesis, and differentiation into endothelial lineages was determined with use of immunostaining, cytohistological staining, and reverse transcription-polymerase chain reaction.
Results:
Anulus fibrosus cells expressed several of the cell surface antigens that are sometimes associated with mesenchymal stem cells, including CD29, CD49e, CD51, CD73, CD90, CD105, CD166, CD184, and Stro-1, and two neuronal stem cell markers, nestin and neuron-specific enolase. Furthermore, varying the stimulants added to the induction media determined whether anulus fibrosus cells differentiated into adipocytes, osteoblasts, chondrocytes, neurons, or endothelial cells.
Conclusions:
Anulus fibrosus cells isolated from nondegenerative intervertebral discs can differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells in vitro.
Clinical Relevance:
Our results, by offering new insights into the biology of anulus fibrosus cells, may assist in future strategies to treat intervertebral disc diseases.
Chronic low-back pain is one of the most common human diseases and is associated with high costs because of both the medical care and the economic implications of the patient's decreased ability to work. It has been estimated that up to 80% of the population experiences some form of chronic low-back pain over the course of their lives, making this a leading health concern1,2. Although the precise cause of chronic low-back pain has yet to be determined, intervertebral disc degeneration appears to be the leading cause3,4. Pathological changes, classified as degeneration, have been recognized as early as the second decade of life on the basis of histological analysis and magnetic resonance imaging5. Disc degeneration is a multifactorial process that involves both environmental and genetic contributions6.
A better understanding of normal cell development will allow us to comprehend and perhaps correct the errors that cause these medical conditions. Thus far, adults have been found to have stem cells in several mesoderm-derived tissues, including bone marrow, fat, skin, dura mater, and tendons7-9. The human intervertebral disc consists of three regions—the anulus fibrosus, the nucleus pulposus, and the end plates. While the nucleus pulposus is considered to be the remnant of the notochord, the anulus fibrosus is thought to be derived from mesoderm. The intact disc is avascular and is generally aneural except in the outermost part of the anulus fibrosus; however, during the moderate and end stages of disc degeneration, fibrocartilage-like tissue, bone formation, and nerve and blood vessel growth are found in the intervertebral disc10-13. These tissues are commonly thought to be derived from cells that migrate into the degenerating intervertebral disc14,15, but we hypothesize that the normal anulus fibrosus tissue contains multipotent progenitor cells, which are able to differentiate into cartilage and/or fibrocartilage cells, osteoblasts, neurons, and blood vessel cells.
Materials and Methods
Cell Isolation and Culture
Following the approved guidelines set by the U.S. National Institutes of Health Office of Human Subjects Research for use of surgical waste, we obtained human intervertebral disc samples from six individuals (thirteen to sixteen years old) who had undergone discectomy for surgical management of scoliosis at the University of Virginia Hospital. Samples from this source are likely to be more normal than those obtained from patients with degenerative disc disease. Following an anterior approach to the spine, an anulotomy was performed with a number-11 blade. A sharp Cobb elevator was then used to separate the disc from the osseous end plate so that as much of the disc as possible could be removed. As the disc samples were obtained from an anterior approach, no extraneous ligaments were attached. The outer layer of anulus fibrosus, rich in fibers and low in cells, was dissected in order to avoid the contamination of ingrown cells from nerves and small blood vessels. The poorly structured, gelatinous parachordal inner anulus fibrosus lies adjacent to the nucleus pulposus, and as it is difficult to discern nucleus pulposus from anulus fibrosus, these regions were removed to rule out nucleus pulposus contamination. The remaining transition zone containing solely anulus fibrosus tissue was then cut into small pieces and digested with 0.01% collagenase (Crescent Chemical, Islandia, New York) at 37°C for two to four hours. At the end of this time, the aqueous portion was carefully removed and centrifuged at 500 g for ten minutes. The resulting cell pellet was resuspended in Dulbecco's Modified Eagle Medium (DMEM; Gibco BRL, Grand Island, New York) with erythrocyte lysis buffer (160 mM of NH4Cl), agitated at room temperature for ten minutes, and recentrifuged to obtain a pellet. The cells were then resuspended and placed along with undigested anulus fibrosus tissue into DMEM/F12 (DMEM: Nutrient Mixture F-12; Gibco BRL) containing 10% fetal bovine serum (Gibco Invitrogen, Carlsbad, California) and 1% penicillin-streptomycin, and then were plated in a 100-mm tissue-culture dish and were maintained at 37°C in a humidified incubator with 5% CO2. Culture media was changed every other day. Cells were maintained at subconfluent levels and were passaged sequentially with use of trypsin-EDTA (Gibco BRL).
Flow Cytometry
To characterize the anulus fibrosus population, flow cytometry was performed with a BD FACSCalibur (Becton Dickinson, Franklin Lakes, New Jersey) as previously described16. Sources of antibodies are listed in a table in the Appendix. Among these antibodies are cell surface antigens expressed in adipose-derived stem cells or mesenchymal stem cells, for instance, CD29, CD49e, CD51, CD73, CD90, CD105, CD166, CD184, and Stro-1; cell surface antigens for hematopoietic and endothelial lineages, such as CD31, CD34, CD45, CD106, CD117, and CD13314,16,17; and neuronal stem cell markers nestin and neuron-specific enolase; CD24 is a surface marker for nucleus pulposus cells, but it is weakly expressed in anulus fibrosus cells18. Briefly, approximately 3 to 5 × 105 anulus fibrosus cells were stained with phycoerythrin (PE) or fluorescein isothiocyanate (FITC)-conjugated antibodies and isotype-matched controls (isotype control IgG1 [immunoglobulin G1] PE or isotype control IgG1 FITC) and were incubated in the dark for thirty minutes at 4°C. After incubation, cells were washed three times with buffer and resuspended in 0.25 mL of cold, protein-free phosphate-buffered saline with 0.25 mL cold formaldehyde (2%) solution as a preservative.
Adipogenic Differentiation
Adipogenic differentiation was induced with use of the Adipogenesis Assay Kit (Chemicon International, Temecula, California) as per the manufacturer's instructions. Briefly, second to fourth-passage 100% confluent anulus fibrosus cells were incubated with Adipogenesis Induction Media (DMEM/F12 supplemented with 10% fetal bovine serum, 0.5 M 1-methyl-3-isobutylxanthine, 10 μg/mL insulin, 1 μM dexamethasone, and 100 μM indomethacin) for six days at 37°C and 5% CO2. The Adipogenesis Initiation Media was replaced with the Adipogenesis Maintenance Media for two days. Cells were then induced with Adipogenesis Induction Media for an additional six days, Adipogenesis Maintenance Media for two days, and finally Adipogenesis Induction Media for another five days.
Osteogenic Differentiation
To induce osteogenic differentiation, second to fourth-passage 100% confluent anulus fibrosus cells were treated with osteogenic induction media for four weeks. Osteogenic induction media consists of DMEM/F12 supplemented with 0.01 μM 1,25-dihydroxyvitamin D3 (R & D Systems, Minneapolis, Minnesota), 50 μM ascorbate-2-phosphate, and 10 mM β-glycerophosphate, as previously reported19.
Chondrogenic Differentiation
Chondrogenesis of anulus fibrosus cells was induced in a pellet (micromass) cell culture system as previously described20. Briefly, 2 × 105 anulus fibrosus cells that had undergone between two and four passages were gently centrifuged for five minutes at 500 g in a 15-mL polypropylene tube to form a pellet at the bottom of the tube and then were treated with chondrogenic induction media for three weeks. Chondrogenic induction media consisted of DMEM/F12 supplemented with 1% fetal bovine serum, 10 nM dexamethasone, 10 ng/mL transforming growth factor β1 (BD Biosciences, Franklin Lakes, New Jersey), 1% ITS-Premix (6.25 g/mL insulin, 6.25 g/mL transferrin, 6.25 ng/mL selenous acid, 1.25 mg/mL bovine serum albumin, and 5.35 mg/mL linoleic acid; Collaborative Biomedical, Becton Dickinson, Bedford, Massachusetts), and 37.5 g/mL ascorbic-2-phosphate.
Neurogenic Differentiation
Neurogenic differentiation was induced with a multistep protocol with use of the NeuroCult NS-A Proliferation Kit and NeuroCult NS-A Differentiation Kit (STEMCELL Technologies, Vancouver, British Columbia, Canada) as per the manufacturer's instructions. Briefly, second to fourth-passage anulus fibrosus cells were seeded at a density of 5 × 104 viable cells/cm2 in 4 mL “Complete” NeuroCult NS-A Proliferation Medium in a six-well plate. Media was replenished (2 mL) at days 2, 4, and 6 after plating, and the neurosphere cultures were ready for neurogenic differentiation once the diameter of the spheres had reached 100 μm. For the neurogenic differentiation, single cells were dissociated from neurospheres at days 7 to 10. The cells were resuspended in “Complete” NeuroCult NS-A Differentiation Medium at a cell density of 1 × 105 cells/cm2 and were plated in eight-well BioCoat Poly-D-Lysine Culture Slides (Becton Dickinson). Half of the medium was replaced with fresh medium every two days, and neurogenesis was assessed on days 7 and 10. The “Complete” NeuroCult NS-A Proliferation Medium consisted of NeuroCult NS-A Basal Medium supplemented with 1/10 volume of NeuroCult NS-A Proliferation Supplements, 20 ng/mL human epidermal growth factor, 10 ng/mL human fibroblast growth factor-b, and 2 μg/mL heparin. The “Complete” NeuroCult NS-A Differentiation Medium consisted of NeuroCult NS-A Basal Medium supplemented with 1/10 volume of NeuroCult NS-A Differentiation Supplements.
Endothelial Cell Differentiation
Anulus fibrosus cells were plated in eight-well BioCoat Poly-D-Lysine Culture Slides at a density of approximately 5000 cells/cm2 and induced with Endothelial Cell Growth Medium MV 2 (PromoCell, Heidelberg, Germany), which consists of 5% fetal bovine serum, 5.0 ng/mL epidermal growth factor, 0.2 μg/mL hydrocortisone, 0.5 ng/mL vascular endothelial growth factor, 10 ng/mL basic fibroblast factor, 20 ng/mL R3 insulin-like growth factor-1, and 1 μg/mL ascorbic acid. Endothelial cell differentiation was assessed at days 3, 5, and 7.
Cytohistological Staining
Oil-Red-O Staining:
Cells were fixed with 4% formaldehyde, stained with oil-red-O solution (Chemicon International) for fifty minutes, and then counterstained with hematoxylin solution (Chemicon International) for fifteen minutes. Lipid droplet formation was quantified by ultraviolet-visible spectrometry, which revealed the OD520 value of oil red O-stained lipids in lysates.
Alizarin-Red-S Staining:
For evaluation of mineralized matrix, cells were fixed with ice-cold 70% ethanol for one hour, stained with Alizarin Red S Solution (Chemicon International) for thirty minutes, and viewed under a light microscope.
Safranin-O Staining:
Chondrogenic differentiation was evaluated after pellets were fixed with 10% buffered formalin for four hours. The samples were dehydrated by treatment with a series of graded alcohols and embedded in paraffin. Samples were then cut into 5-μm sections, rehydrated, and stained with safranin O for detection of proteoglycan.
Type-II Collagen Immunostaining and Immunofluorescence
Immunochemical staining for type-II collagen was performed with use of a collagen staining kit (Chondrex, Redmond, Washington). For immunofluorescence staining of intracellular proteins, cells growing on the glass coverslips were fixed with 4% formaldehyde for ten minutes and incubated with one of the mouse primary antibodies, including those against human aggrecan (1:50; RDI, Flanders, New Jersey), human β-tubulin (1:150; Invitrogen, Carlsbad, California), human microtubule-associated protein 2 (1:150; Santa Cruz Biotechnology, Santa Cruz, California), human neurofilament light chain (1:150; Invitrogen), and von Willebrand factor (vWF) (1:150; Santa Cruz Biotechnology), overnight at 4°C. This was followed by fluorescein or phycoerythrin-coupled goat antimouse IgG secondary antibody for one hour. Cells were counterstained with the fluorescent dye YOYO-1 iodide (1:3000; Invitrogen) for nucleic acids.
Gene Expression Analysis
Total RNA from cultured cells or cell pellets was obtained with use of the RNeasy Mini Kit (QIAGEN, Valencia, California) according to the manufacturer's instructions. cDNA was then synthesized from the total RNA with use of the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, California) according to the manufacturer's instructions. Polymerase chain reaction was performed with iQ Supermix (Bio-Rad), and real-time polymerase chain reaction was then performed with iTaq SYBR Green Supermix with ROX (Bio-Rad). Amplification primers are listed in a table in the Appendix.
Statistical Analysis
Statistical evaluation was performed with use of the analysis-of-variance test followed by a post hoc Student t test. A p value of <0.05 was considered significant. Data are expressed as the mean and standard deviation.
Source of Funding
The authors did not receive grants or outside funding in support of this research.
Results
Human Anulus Fibrosus Cell Expression of Mesenchymal and Neuronal Stem Cell Markers
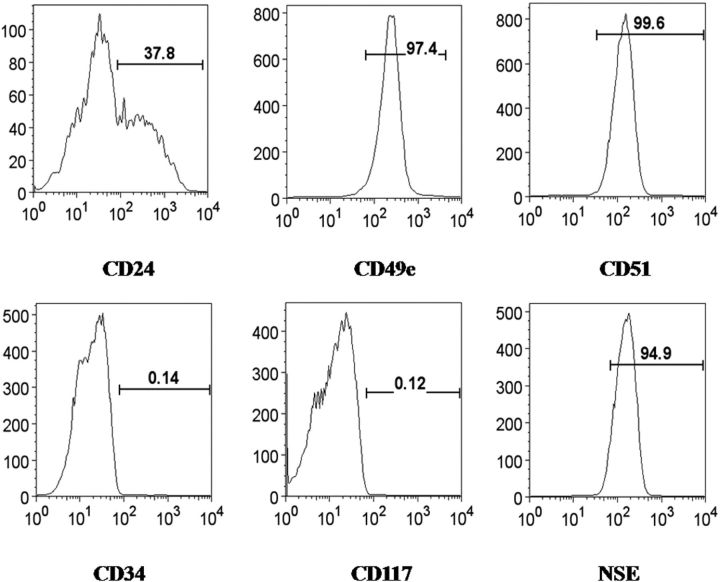
We investigated the expression of eighteen different cell markers, including CD29, CD31, CD34, CD45, CD49e, CD51, CD73, CD90, CD105, CD106, CD117, CD133, CD166, CD184, nestin, neuron-specific enolase, and Stro-1 in human anulus fibrosus cells as well as a marker for nucleus pulposus cells, CD24. In addition, dead cells were excluded with use of 7-aminoactinomycin D, with approximately 98% of the total cells negative for 7-aminoactinomycin D, indicating very high cell viability during flow cytometric analysis. Specificity of the secondary antibody was confirmed by negatively staining cells with the PE-labeled mouse IgG alone, with no staining of >99% of the total cells. The immunophenotype of the markers in cultured anulus fibrosus cells is illustrated in Figure 1. Positively stained markers included the majority of the cell surface antigens expressed in adipose-derived stem cells and mesenchymal stem cells, i.e., CD29, CD49e, CD51, CD73, CD90, CD105, CD166, and CD184, as well as the neuronal stem cell markers nestin and neuron-specific enolase. By contrast, cell surface antigens for hematopoietic and endothelial lineages were either only partially present or no binding was identified at all. Furthermore, 37.8% and 29% of cells were positive for CD24 and Stro-1, respectively.
Fig. 1.
Representative flow cytometry analysis of the expression of cell markers in cultured anulus fibrosus cells isolated from a human intervertebral disc. The cell markers CD29, CD49e, CD51, CD73, CD90, CD105, CD166, and CD184, as well as the neuronal markers nestin and neuron-specific enolase (NSE) were all expressed, whereas other markers were negative or partially positive. The horizontal axis represents the parameter's signal value in channel numbers, and the vertical axis represents the number of events per channel number. The number in each panel shows the percentage of cells expressing the specific cell marker.
Adipogenesis of Human Anulus Fibrosus Cells
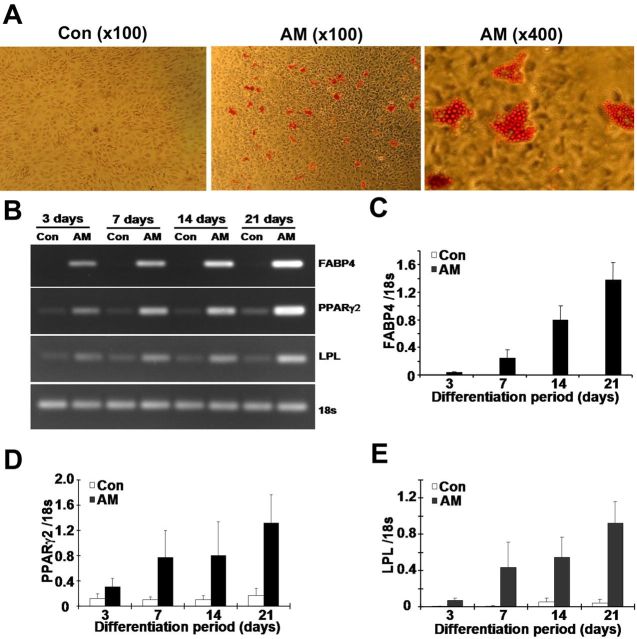
We next investigated adipogenesis of human anulus fibrosus cells. When cultured in the presence of adipogenic media, there was extensive formation of lipid droplets in human anulus fibrosus cells compared with unsupplemented cells as revealed by staining with oil red O (Fig. 2, A). Lipid droplet formation was quantified by ultraviolet-visible spectrometry, which revealed that the OD520 value of oil-red-O-stained lipids in lysates increased from 0.090 ± 0.002 for uninduced cells to 0.151 ± 0.007 for induced cells (p < 0.05). The induced cells also expressed the adipogenesis-specific genes, fatty acid binding protein 4, peroxisome proliferator-activated receptor-gamma 2, and lipoprotein lipase, as revealed by reverse transcription-polymerase chain reaction (Fig. 2, B) and real-time reverse transcription-polymerase chain reaction (Fig. 2, C, D, and E). Expression of these genes was markedly increased in induced cells with mRNA levels at twenty-one days that were approximately four, fifteen, and fiftyfold those at three days for peroxisome proliferator-activated receptor-gamma 2 (Fig. 2, D), lipoprotein lipase (Fig. 2, E), and fatty acid binding protein 4 (Fig. 2, C), respectively. On the other hand, in cells that were cultured in the unsupplemented medium, expression of lipoprotein lipase and peroxisome proliferator-activated receptor-gamma 2 remained unchanged, and no expression of fatty acid binding protein 4 was detected during twenty-one-day culture.
Fig. 2.
Adipogenic differentiation of cultured human anulus fibrosus cells was induced with use of the commercial adipogenic supplements in five samples. (A) Oil-red-O staining showing formation of lipid droplets within induced cells. Reverse transcription-polymerase chain reaction (B) and real-time reverse transcription-polymerase chain reaction (C, D, and E) analysis of cellular mRNA levels of several adipogenesis-specific proteins, with 18S rRNA as an internal control. The gene expression levels of fatty acid binding protein 4 (FABP4) (C), peroxisome proliferator-activated receptor-gamma 2 (PPARγ2) (D), and lipoprotein lipase (LPL) (E) are increased in anulus fibrosus cells treated with adipogenic media (AM) compared with control cells (Con) cultured in basal media. Error bars represent the standard deviation.
Osteogenesis of Human Anulus Fibrosus Cells
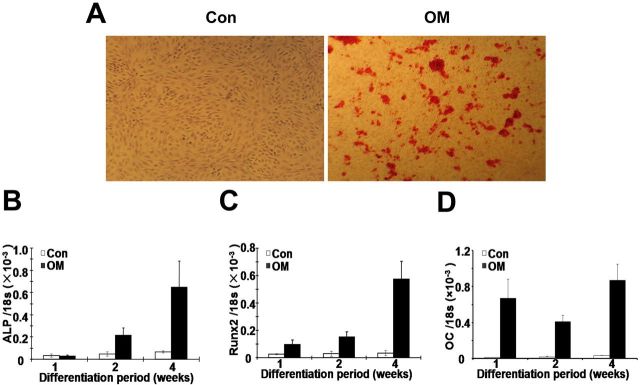
When human anulus fibrosus cells were cultured in an osteogenic medium for four weeks, an obvious increase in mineralization was revealed by alizarin-red-S staining (Fig. 3, A). Moreover, real-time reverse transcription-polymerase chain reaction (Fig. 3, B, C, and D) revealed that the osteogenic supplements stimulated expression of the osteogenesis-specific genes alkaline phosphatase, runt-related transcription factor 2, and osteocalcin. The mRNA levels at four weeks were approximately twenty and sixfold those at one week for alkaline phosphatase (Fig. 3, B) and runt-related transcription factor 2 (Fig. 3, C), respectively. Expression of osteocalcin was greatly elevated at one week after the addition of induction media and continued at a high level thereafter (Fig. 3, D). However, in uninduced cells, expression of each gene remained unchanged during four-week culture (Fig. 3, B, C, and D).
Fig. 3.
Osteogenic differentiation of cultured human anulus fibrosus cells was induced with use of the osteogenic supplements. Once cells had reached 100% confluence, they were induced for up to four weeks and then were stained with alizarin red S or assayed for gene expression. Mineralization in induced cells is revealed by alizarin red S (×40) (A). Real-time reverse transcription-polymerase chain reaction analysis of osteogenesis-specific genes alkaline phosphatase (ALP) (B), runt-related transcription factor 2 (Runx2) (C), and osteocalcin (OC) (D) was performed, with 18S rRNA as an internal control (Con). The expression of all three genes was upregulated following the induction protocol in five samples. Error bars represent the standard deviation. OM = osteogenic media.
Chondrogenesis of Human Anulus Fibrosus Cells
After treatment of human anulus fibrosus cells with chondrogenic supplements for three weeks, cells showed marked staining with safranin O (Fig. 4, A) compared with human anulus fibrosus pellets that had been cultured in basal medium and were weakly stained. Immunocytochemical staining also revealed that the chondrogenesis-specific extracellular matrix proteins, aggrecan and type-II collagen, were enriched in the induced cells (Fig. 4, A). This elevated expression was also confirmed by assaying the cellular mRNA levels with use of reverse transcription-polymerase chain reaction (Fig. 4, B) and real-time reverse transcription-polymerase chain reaction (Fig. 4, C, D, and E). After induction with chondrogenic stimuli for one week, levels of both type-II collagen and aggrecan had approximately doubled and their expression continued to increase after longer periods of induction (Fig. 4, D and E). The levels of expression of aggrecan and type-II collagen were not increased in the absence of the chondrogenic stimuli. By contrast, the expression of type-I collagen increased in the absence of chondrogenic stimuli, with a large elevation occurring between weeks 1 and 2 and the levels remaining constant from week 2 to week 3 (Fig. 4, C). However, in the presence of chondrogenic stimuli, there was no significant variation in the expression levels of type-I collagen throughout the three weeks in culture (Fig. 4, C). At one week, expression of type-I collagen in the induced group was about ninefold that in the uninduced group (Fig. 4, C).
Fig. 4.
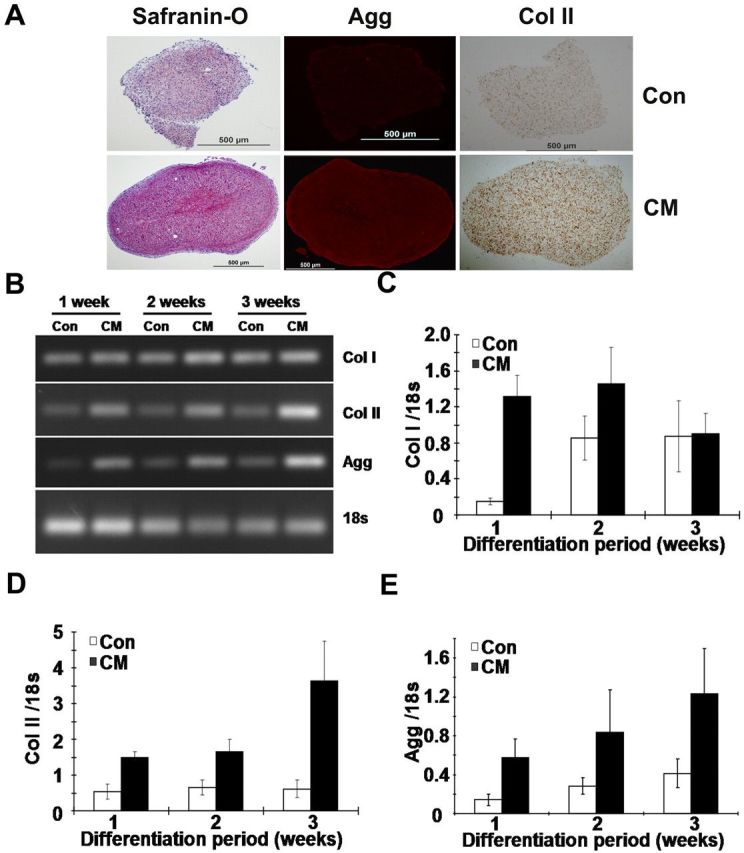
Chondrogenic differentiation of human anulus fibrosus cells treated with the chondrogenic supplements. Cells were induced for three weeks in a pellet cell culture system, and then the expression of several extracellular matrix proteins was assayed by cytochemical and reverse transcription-polymerase chain reaction analysis. Safranin-O staining of proteoglycan and immunocytochemical staining of aggrecan (Agg) and type-II collagen (Col II) (A). Reverse transcription-polymerase chain reaction (B) and real-time reverse transcription-polymerase chain reaction analysis of type-I (Col I) (C) and type-II collagen (D) and aggrecan (E) mRNA levels, with 18S rRNA as an internal control (Con). Expression of type-II collagen and aggrecan was substantially enhanced after chondrogenic induction in five samples. Error bars represent the standard deviation. CM = chondrogenic media.
Neurogenesis of Human Anulus Fibrosus Cells
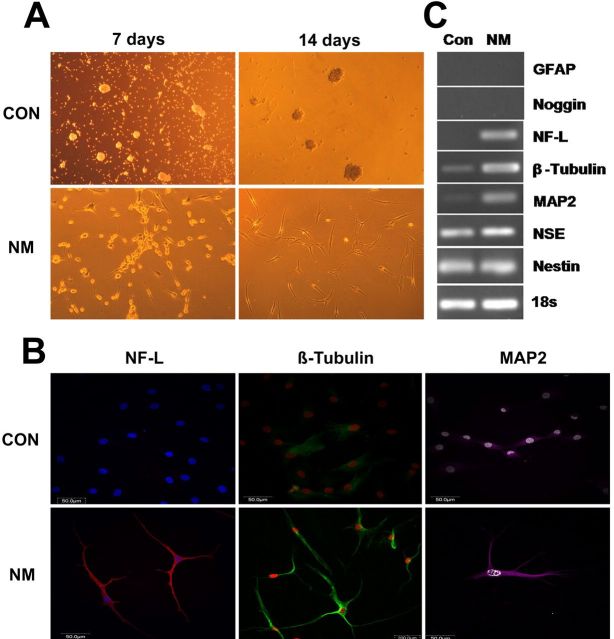
Neurogenesis of human anulus fibrosus cells was performed by initially expanding them with use of a sphere culture technique and then culturing them in the presence of neurogenic stimuli. Neurite-bearing cells were clearly visible after induction for seven or fourteen days, whereas no such signs of neuronal cells appeared when cells were cultured for seven or fourteen days in the absence of the neurogenic stimuli (Fig. 5, A). Expression of the neuronal markers β-tubulin, microtubule-associated protein 2, and neurofilament light chain was assayed by immunofluorescent staining and was positive in the cells that had been cultured in the presence of the neurogenic stimuli (Fig. 5, B). Elevated expression of these proteins was also confirmed by reverse transcription-polymerase chain reaction analysis (Fig. 5, C). However, levels of the other neuronal markers that were assayed, i.e., glial fibrillary acidic protein, noggin, neuron-specific enolase, and nestin, were found to be similar whether the cells had or had not been cultured in the presence of the neurogenic stimuli. Interestingly, although glial fibrillary acidic protein and noggin were not expressed whether the cells were or were not stimulated, neuron-specific enolase and nestin were both expressed at very high levels in both stimulated and unstimulated cells (Fig. 5, C).
Fig. 5.
Neurogenic differentiation of human anulus fibrosus cells by a sphere culture method with use of the NeuroCult NS-A Proliferation Kit and NeuroCult NS-A Differentiation Kit. After induction, several neuronal markers were examined by immunofluorescent staining and reverse transcription-polymerase chain reaction analysis. A: Cell morphology under a light microscope (×100). Cells displayed a neuron-like appearance after they were induced for fourteen days. B: Immunofluorescent staining of neurofilament light chain (NF-L), β-tubulin, and microtubule-associated protein 2 (MAP2). C: Reverse transcription-polymerase chain reaction analysis of glial fibrillary acidic protein (GFAP), noggin, neurofilament light chain, β-tubulin, microtubule-associated protein 2, neuron-specific enolase (NSE), and nestin mRNA levels, with 18S rRNA as an internal control (Con). Expression of β-tubulin, microtubule-associated protein 2, and neurofilament light chain was promoted after induction. NM = neurogenic media.
Induction of Human Anulus Fibrosus Cells to Differentiate into an Endothelial Cell Lineage
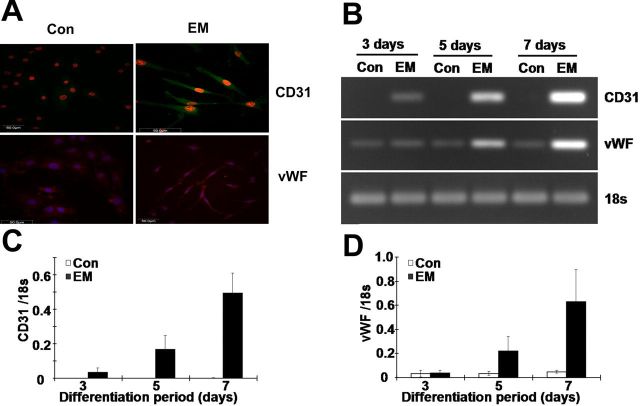
Human anulus fibrosus cells were grown in a commercially sourced, endothelial-inducing medium. The degree of differentiation into an endothelial cell lineage was assessed by assaying for the endothelial cell markers CD31 and vWF by a fluorescent immunostaining-based reverse transcription-polymerase chain reaction method and by real-time reverse transcription-polymerase chain reaction analysis. Both CD31 and vWF stained positively in induced cells, whereas they were negatively or weakly stained in noninduced cells (Fig. 6, A). By day 7 after induction, CD31 and vWF expression had increased by approximately tenfold in comparison with day 3 after induction (Fig. 6, B, C, and D).
Fig. 6.
Endothelial lineage differentiation of cultured human anulus fibrosus cells. Cells were incubated for up to seven days in Endothelial Cell Growth Medium MV 2, and then the expression of endothelial-specific genes was examined by immunofluorescent staining and reverse transcription-polymerase chain reaction. Cell morphology and immunofluorescent staining of CD31 and von Willebrand factor (vWF) (A). Reverse transcription-polymerase chain reaction (B) and real-time reverse transcription-polymerase chain reaction analysis of the levels of the mRNA for CD31 (C) and vWF (D) mRNA, with 18S rRNA as an internal control (Con). The expression of both genes was upregulated following induction in six samples. EM = endothelial media.
Discussion
In this study, we describe the isolation and monolayer culture of human anulus fibrosus cells isolated from patients with adolescent scoliosis prior to the development of clinical, radiographic, and magnetic resonance imaging evidence of degenerative changes in the intervertebral discs. We also characterize the phenotype and pluripotency of anulus fibrosus cells. The anulus fibrosus cells expressed the majority of cell surface antigens expressed in mesenchymal stem cells and two neuronal stem cell markers (Fig. 1). Moreover, they demonstrated a high level of multipotency as they could be induced to display phenotypic characteristics of adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells in the presence of the appropriate stimuli (Figs. 2 through 6). These data indicated the presence of multipotent progenitor cells in cultures of human anulus fibrosus cells, implying that the anulus fibrosus, similar to other tissues such as bone marrow and placenta9,21, is potentially a source of pluripotent mesenchymal stem cells. In a recent study, Risbud et al. showed evidence of skeletal progenitor cells in the degenerated human intervertebral disc, but their investigation focused on the pathways of disc cell differentiation into fat cells, osteoblasts, and chondrocytes22. They did not investigate differentiation of disc cells into neuronal cells and endothelial cells.
Many mesoderm-derived tissues, such as the stroma of the bone marrow, fat, tendon, and dura mater, contain putative stem cells. The nucleus pulposus is composed of cells of notochordal origin, whereas the anulus fibrosus develops from the sclerotomal and/or mesodermal cells that remain after division of the region of the sclerotome during resegmentation. We isolated human anulus fibrosus cells from nondegenerated intervertebral discs and demonstrated that they expressed nine cell surface antigens expressed in mesenchymal stem cells and two neuronal stem cell markers. Notably, expression of nestin has previously been demonstrated to be very important for allowing mesenchymal stem cells derived from adult bone marrow to commit to an astrocytic or neuronal fate23-25. In line with this, our present data demonstrate that nestin is expressed in anulus fibrosus cells, which could explain why these cells are able to undergo neurogenic differentiation in the presence of the appropriate stimuli (Fig. 5).
It has been previously demonstrated that disc cells cultured in a monolayer are composed of a heterogeneous population, displaying both morphological differences26 and differences in the immunophenotype of their surface antigens22. The existence of different subsets of cells in the disc cell monolayer cultures is further demonstrated by the fact that 29% of the total population in the current study expressed Stro-1. Cells derived from adult human bone marrow and trabecular bone that express Stro-1 can differentiate into multiple mesenchymal lineages, including hematopoiesis-supportive stromal cells with a vascular smooth muscle-like phenotype, adipocytes, osteoblasts, and chondrocytes27. It will be useful to know whether the subset of cells that expresses Stro-1 represents the multipotent population that can differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells.
While being maintained in a monolayer culture, some differentiated cells, including hepatocytes and articular cartilage chondrocytes, lose their cellular phenotypes and undergo the process of dedifferentiation28-30. The anulus fibrosus has many similarities to fibrocartilage, and it is therefore feasible that such dedifferentiation is also occurring in the monolayer cultures of disc cells, thereby resulting in the multipotent progenitor cells identified in the in vitro culture. This hypothesis is partly supported by the fact that both anulus fibrosus and nucleus pulposus cells undergo morphological and biochemical change during primary monolayer culture31. However, it is also possible that the multipotent progenitor cells are present in the original discs and are amplified while in the monolayer culture27.
Irrespective of how the multipotent progenitor cells in anulus fibrosus monolayer culture are derived, characterization of the cells will still provide useful information regarding the physiology of human intervertebral discs. It is widely recognized that the types, structures, and functions of the cellular components of intervertebral discs vary greatly with aging and degeneration32,33. Using a rabbit disc degeneration model, we previously demonstrated that injection of a 30-kDa fragment from the N terminus of fibronectin led to the formation of osteophytes in the anterior region of the anulus fibrosus, which consists of a peripheral region of cartilaginous tissue with a central region of osteoid and cancellous bone34. One possible explanation for this is the growth of stem cells around the degenerated disc into the disc area through the needle injury. Our demonstration of multipotent progenitor cells in the anulus fibrosus would provide another possible explanation for the osteophyte formation, as a result of differentiation of multipotent progenitor cells into chondrocytes or osteoblasts responding to an environmental change in the stem cell niche because of fibronectin-fragment injection.
In addition, as degeneration of the intervertebral disc and chronic low-back pain progress, nerves and blood vessels are increasingly found in the inner part of the anulus fibrosus11,32,35. Innervation often occurs when pain is associated with the chronic low-back pain and has been causally linked to the production of nerve growth factor in the degenerative intervertebral disc12. Abe et al. found that human anulus fibrosus and nucleus pulposus cells constitutively expressed nerve growth factor, whereas the proinflammatory cytokines IL-1β (interleukin-1β) and TNF-α (tumor necrosis factor-α) stimulated the production of nerve growth factor36. Early depletion of glycosaminoglycan was strongly associated with increased nerve and blood vessel ingrowth into the anulus fibrosus region in an ovine disc degeneration model35. An in vitro study revealed that aggrecan, the major type of glycosaminoglycan isolated from the anulus fibrosus and nucleus pulposus of a human intervertebral disc, inhibited cell adhesion and migration of two human endothelial cell lines HMEC-1 and EAhy-92637. While the exact mechanisms involved in these pathophysiological processes are not yet understood, innervation and vascularization are currently considered to result from the migration of cells from outside the intervertebral disc11,12,35,37. However, we speculate that multipotent progenitor cells in the anulus fibrosus also contribute to innervation and vascularization of the degenerative intervertebral disc through differentiation into various cell types. The isolation and characterization of stem cells in the anulus fibrosus will provide a new tool in the study of basic intervertebral disc biology.
Appendix
Tables listing the antibodies used in the flow cytometry analysis and the primers used for reverse transcription-polymerase chain reaction are available with the electronic version of this article on our web site at jbjs.org (go to the article citation and click on “Supporting Data”).
Acknowledgments
Note: The authors thank Dr. Sarah A. De La Rue of Readable Science, United Kingdom, for her assistance with this manuscript.
Footnotes
Investigation performed at the Orthopaedic Research Laboratories, University of Virginia School of Medicine, Charlottesville, Virginia
Disclosure: The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1. Diamond S Borenstein D. Chronic low back pain in a working-age adult. Best Pract Res Clin Rheumatol. 2006;20:707-20. [DOI] [PubMed] [Google Scholar]
- 2. Luo X Pietrobon R Sun SX Liu GG Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976). 2004;29:79-86. [DOI] [PubMed] [Google Scholar]
- 3. Battié MC Videman T Levalahti E Gill K Kaprio J. Heritability of low back pain and the role of disc degeneration. Pain. 2007;131:272-80. [DOI] [PubMed] [Google Scholar]
- 4. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-5. [DOI] [PubMed] [Google Scholar]
- 5. Walker MH Anderson DG. Molecular basis of intervertebral disc degeneration. Spine J. 2004;4:158S-66S. [DOI] [PubMed] [Google Scholar]
- 6. Anderson DG Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5:260S-6S. [DOI] [PubMed] [Google Scholar]
- 7. Pittenger MF Mackay AM Beck SC Jaiswal RK Douglas R Mosca JD Moorman MA Simonetti DW Craig S Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7. [DOI] [PubMed] [Google Scholar]
- 8. Deans RJ Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875-84. [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y Jahagirdar BN Reinhardt RL Schwartz RE Keene CD Ortiz-Gonzalez XR Reyes M Lenvik T Lund T Blackstad M Du J Aldrich S Lisberg A Low WC Largaespada DA Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-9. [DOI] [PubMed] [Google Scholar]
- 10. Inoue G Ohtori S Aoki Y Ozawa T Doya H Saito T Ito T Akazawa T Moriya H Takahashi K. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 2006;31:1433-8. [DOI] [PubMed] [Google Scholar]
- 11. Freemont AJ Peacock TE Goupille P Hoyland JA O'Brien J Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-81. [DOI] [PubMed] [Google Scholar]
- 12. Freemont AJ Watkins A Le Maitre C Baird P Jeziorska M Knight MT Ross ER O'Brien JP Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286-92. [DOI] [PubMed] [Google Scholar]
- 13. Coppes MH Marani E Thomeer RT Oudega M Groen GJ. Innervation of annulus fibrosis in low back pain. Lancet. 1990;336:189-90. [DOI] [PubMed] [Google Scholar]
- 14. Peng B Chen J Kuang Z Li D Pang X Zhang X. Expression and role of connective tissue growth factor in painful disc fibrosis and degeneration. Spine (Phila Pa 1976). 2009;34:E178-82. [DOI] [PubMed] [Google Scholar]
- 15. Roberts S Evans H Trivedi J Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88 Suppl 2:10-4. [DOI] [PubMed] [Google Scholar]
- 16. Katz AJ Tholpady A Tholpady SS Shang H Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412-23. [DOI] [PubMed] [Google Scholar]
- 17. Strem BM Hicok KC Zhu M Wulur I Alfonso Z Schreiber RE Fraser JK Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132-41. [DOI] [PubMed] [Google Scholar]
- 18. Fujita N Miyamoto T Imai J Hosogane N Suzuki T Yagi M Morita K Ninomiya K Miyamoto K Takaishi H Matsumoto M Morioka H Yabe H Chiba K Watanabe S Toyama Y Suda T. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890-6. [DOI] [PubMed] [Google Scholar]
- 19. Zuk PA Zhu M Ashjian P De Ugarte DA Huang JI Mizuno H Alfonso ZC Fraser JK Benhaim P Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schutze N Noth U Schneidereit J Hendrich C Jakob F. Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun Signal. 2005;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Battula VL Bareiss PM Treml S Conrad S Albert I Hojak S Abele H Schewe B Just L Skutella T Bühring HJ. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007;75:279-91. [DOI] [PubMed] [Google Scholar]
- 22. Risbud MV Guttapalli A Tsai TT Lee JY Danielson KG Vaccaro AR Albert TJ Gazit Z Gazit D Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976). 2007;32:2537-44. [DOI] [PubMed] [Google Scholar]
- 23. Tropel P Platet N Platel JC Noël D Albrieux M Benabid AL Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868-76. [DOI] [PubMed] [Google Scholar]
- 24. Wislet-Gendebien S Leprince P Moonen G Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295-302. [DOI] [PubMed] [Google Scholar]
- 25. Wislet-Gendebien S Hans G Leprince P Rigo JM Moonen G Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392-402. [DOI] [PubMed] [Google Scholar]
- 26. Horner HA Roberts S Bielby RC Menage J Evans H Urban JP. Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine (Phila Pa 1976). 2002;27:1018-28. [DOI] [PubMed] [Google Scholar]
- 27. Song L Young NJ Webb NE Tuan RS. Origin and characterization of multipotential mesenchymal stem cells derived from adult human trabecular bone. Stem Cells Dev. 2005;14:712-21. [DOI] [PubMed] [Google Scholar]
- 28. Elaut G Henkens T Papeleu P Snykers S Vinken M Vanhaecke T Rogiers V. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 2006;7:629-60. [DOI] [PubMed] [Google Scholar]
- 29. Diaz-Romero J Gaillard JP Grogan SP Nesic D Trub T Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731-42. [DOI] [PubMed] [Google Scholar]
- 30. Diaz-Romero J Nesic D Grogan SP Heini P Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol. 2008;214:75-83. [DOI] [PubMed] [Google Scholar]
- 31. Kluba T Niemeyer T Gaissmaier C Gründer T. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine (Phila Pa 1976). 2005;30:2743-8. [DOI] [PubMed] [Google Scholar]
- 32. Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18-44. [DOI] [PubMed] [Google Scholar]
- 33. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004;29:2691-9. [DOI] [PubMed] [Google Scholar]
- 34. Greg Anderson D Li X Tannoury T Beck G Balian G. A fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine (Phila Pa 1976). 2003;28:2338-45. [DOI] [PubMed] [Google Scholar]
- 35. Melrose J Roberts S Smith S Menage J Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976). 2002;27:1278-85. [DOI] [PubMed] [Google Scholar]
- 36. Abe Y Akeda K An HS Aoki Y Pichika R Muehleman C Kimura T Masuda K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976). 2007;32:635-42. [DOI] [PubMed] [Google Scholar]
- 37. Johnson WE Caterson B Eisenstein SM Roberts S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine (Phila Pa 1976). 2005;30:1139-47. [DOI] [PubMed] [Google Scholar]