Abstract
Background:
Single intra-articular injections of local anesthetics are commonly used clinically. Recent in vitro studies have demonstrated chondrotoxic effects of local anesthetics, with the greatest emphasis on bupivacaine toxicity. This in vivo study was conducted to determine whether a single intra-articular injection of 0.5% bupivacaine results in chondrocyte morbidity and rapid chondrolysis.
Methods:
Forty-eight Sprague-Dawley rats received a 100-μL injection of sterile 0.9% saline solution (negative control) into one stifle joint and 100 μL of either preservative-free 0.5% bupivacaine (experimental group) or 0.6 mg/mL monoiodoacetate (positive control) into the contralateral joint. The rats were killed at one week, four weeks, twelve weeks, or six months. Live and dead cells were quantified with use of three-dimensional confocal reconstructions of fluorescent-stained tissues at standardized locations on the distal part of the femur. Histological findings were graded with use of a modified Mankin score, and cell density was quantified with use of custom image-analysis software.
Results:
In the specimens injected with bupivacaine, the chondral surfaces remained intact as seen with gross and histological examination. No differences in superficial chondrocyte viability or modified Mankin scores were observed between the saline-solution and bupivacaine groups at any location or time point (p > 0.05). Quantitative histological analysis of the bupivacaine-treated knees at six months revealed an up to 50% reduction in chondrocyte density compared with that of the saline-solution-treated knees (p ≤ 0.01). Monoiodoacetate injection resulted in death of up to 87% of the superficial chondrocyte cells at one week and chondrolysis at six months. Despite severe histological abnormalities by four weeks after monoiodoacetate injection, cartilage injury was not evident on gross inspection until six months.
Conclusions:
This in vivo study showing reduced chondrocyte density without cartilage tissue loss six months after a single intra-articular injection of 0.5% bupivacaine suggests bupivacaine toxicity. The effects of bupivacaine were milder than those of an injection of 0.6% monoiodoacetate, which resulted in chondrolysis over the same time period.
Clinical Relevance:
This study shows that the in vivo effects of a single injection of intra-articular bupivacaine on articular cartilage are subtle. The data in the monoiodoacetate group show that substantial subsurface pathological effects may not be obvious on gross inspection and suggest that any potential toxic effects of bupivacaine following a single injection would be difficult to detect clinically.
Recent clinical case reports and basic-science studies have raised concern regarding potential toxicity to articular chondrocytes with prolonged exposure to local anesthetics used either alone or in combination with other substances such as corticosteroids1-8. Bupivacaine has been found to be particularly chondrotoxic in vitro2,6. Several studies have shown a dose and time-dependent chondrotoxicity of local anesthetics, including bupivacaine1,8. These data suggest that chondrocytes may tolerate brief exposure to low doses of bupivacaine.
The primary means by which chondrocytes are potentially exposed to bupivacaine clinically is through intra-articular administration. Bupivacaine has been commonly administered into the joint as a single bolus injection either as a local anesthetic for arthroscopy or in the perioperative setting for pain control. Local anesthetics are also widely used in combination with corticosteroids for symptomatic treatment of joint pain, inflammatory arthritis, and osteoarthritis.
Pumps have been used for continuous intra-articular infusion of local anesthetics only relatively recently, and this method has been associated clinically with chondrolysis of the shoulder5. Chondrolysis has been defined as the disappearance of articular cartilage as the result of lysis or dissolution of the cartilage matrix and cells9. The causes of chondrolysis are frequently unknown. The clinical onset of bupivacaine-related chondrolysis in the shoulder was reported to be rapid, occurring within one year after continuous intra-articular infusion of bupivacaine with epinephrine5. In vitro data showing a dose and time-dependent toxicity of local anesthetics to articular chondrocytes1,8 support the hypothesis that chondrocyte necrosis may be a factor in the rapid onset of chondrolysis related to bupivacaine.
While continuous intra-articular administration protocols can be expected to result in more prolonged exposure of chondrocytes to larger doses of bupivacaine than are associated with single-injection therapy, the in vivo effects of a single intra-articular injection of bupivacaine remain controversial. An in vivo rabbit study showed histological evidence of inflammation of articular cartilage ten days following a single intra-articular injection of 0.5% bupivacaine10. In vivo studies of the effects of 0.5% bupivacaine on porcine articular cartilage showed transient suppression of proteoglycan synthesis over the three-day study period11,12. Clinically, bupivacaine has been administered widely as a single intra-articular injection for decades.
The present six-month in vivo study was performed to test the hypothesis that a single intra-articular injection of 0.5% bupivacaine into the mammalian knee joint does not induce chondrocyte necrosis or rapid chondrolysis.
Materials and Methods
Injection Procedure
All animal injections were performed with use of a protocol approved by the University of Pittsburgh institutional animal care and use committee. Forty-eight male Sprague-Dawley rats that were twelve to thirteen weeks of age and weighed ∼300 g received experimental intra-articular injections13. In preliminary studies in which methylene blue was injected into cadaver rats of the same age and sex, we determined that a 100-μL injection both distended the capsule and could be reaspirated. The rats were assigned to two groups, one of which was injected with bupivacaine and the other, with monoiodoacetate. The left knees of the twenty-four rats in the bupivacaine group received a single 100-μL injection of sterile 0.5% bupivacaine. The left knees of the twenty-four rats in the monoiodoacetate group received a single 100-μL injection of sterile-filtered 0.6% (0.6 mg/mL) monoiodoacetate. All forty-eight rats received a single 100-μL injection of sterile saline solution into the right knee. Four rats, two in the bupivacaine group and two in the monoiodoacetate group, did not survive until the date on which the animals were to be killed, leaving a total of forty-four rats (eighty-eight knees). A minimum of five rats per group were killed at one week, four weeks, twelve weeks, and six months after the injection, and the distal parts of the femora were harvested for further analysis.
Monoiodoacetate injection was used for the comparison group because it is a well-established method for inducing chondrolysis with an injection14-16. Monoiodoacetate induces chondrolysis by killing articular chondrocytes through inhibition of glyceraldehyde-3-phosphate dehydrogenase activity, which is needed for glycolysis17. The use of monoiodoacetate as a positive control provides a reference time line for the development of injection-induced chondrolysis in this animal model and provides additional confirmation of the success of the intra-articular injection technique.
Chondrocyte Viability Staining
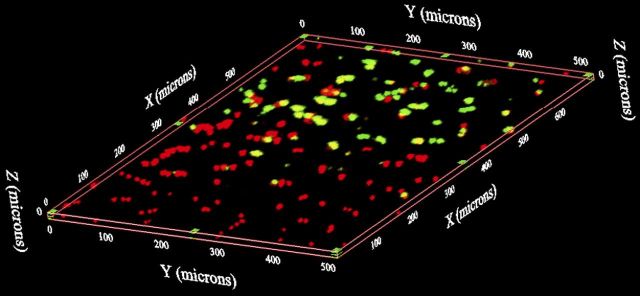
The distal portions of freshly harvested femora were placed into prewarmed staining solution consisting of 0.1% propidium iodide (Invitrogen, Carlsbad, California) and 0.1% 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen) for one hour at 37°C. Samples were then washed and imaged with use of confocal microscopy (IX81; Olympus, Center Valley, Pennsylvania) and fluorescent stereomicroscopy (MVX; Olympus). Fluorescent stereomicroscopy was used to assess the distribution of live and dead cells. For quantitation of live and dead cells at and near the articular surface, the centers of the medial femoral condyle, lateral femoral condyle, and trochlear groove were identified, and confocal microscopy was used to generate a three-dimensional volume measuring 720 × 540 μm and 25 μm deep (Fig. 1). Image analysis with Image-Pro Plus (Media Cybernetics, Bethesda, Maryland) was performed to determine the percentages of live and dead cells within each three-dimensional reconstruction. Mean cell viability was analyzed with statistical software (SPSS, Chicago, Illinois); the paired t test was used for within-animal comparisons, with significance defined as p < 0.05.
Fig. 1.
Quantification of viable chondrocytes with use of confocal microscopy. Following fluorescent staining with propidium iodide (red, indicating dead cells) and CMFDA (green, indicating live cells), a serial z-axis image stack 720 × 540 μm was acquired from the articular surface of the center of the medial and lateral femoral condyles and the center of the trochlear groove to a depth of 25 μm. Image stacks were then reconstructed in three-dimensional space. The percent cell viability was counted in each three-dimensional image.
Gross Morphological Analysis
The distal part of the femur was stained with India ink and imaged with a stereomicroscope. The trochlear groove and the femoral condyles were graded with use of a previously published scale for gross assessment of articular cartilage18. Grade 1 indicated an intact articular surface; grade 2, minimal fibrillation; grade 3, overt fibrillation; and grade 4, erosion with exposed bone.
Histological Analysis
The distal part of the femur was fixed, decalcified, embedded in paraffin, and cut into 6-μm sections (in the sagittal plane for the femoral condyles and in the axial plane for the trochlear groove). Serial sections were then stained with hematoxylin and eosin and safranin O-fast green. Histological interpretation was performed by a board-certified pathologist and an experienced orthopaedic surgeon. Scoring with a modification of the system described by Mankin et al.19 was independently performed by two additional observers. The total mean modified Mankin scores were analyzed with statistical software, with use of the paired t test for within-animal comparisons (saline solution versus bupivacaine) and one-way analysis of variance for three-way intergroup comparisons. Significance was defined as p < 0.05.
Quantitative Histological Analysis
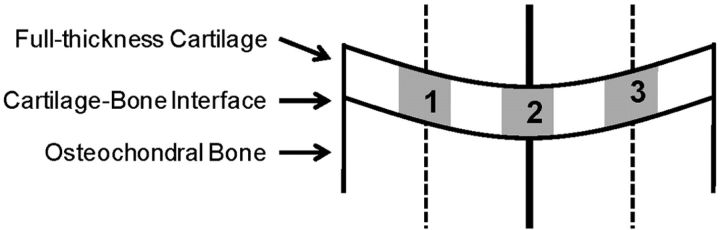
Five hundred and sixty-six sections stained with hematoxylin and eosin were imaged and segmented with use of customized histomorphometric analysis software (Visiopharm, Hørsholm, Denmark). Standardized regions of interest were obtained by first identifying the center of the trochlea, the medial femoral condyle, or the lateral femoral condyle on each section. A line was drawn at the center, perpendicular to the articular surface, dividing the image into two halves. Each image half was again bisected by a line drawn parallel to the central line (Fig. 2). The software was then used to generate three full-thickness cartilage regions of interest of approximately equal size (∼300 μm wide), centered on each line. The software was used to calculate a mean cell density within each region of interest, and the densities were averaged for each histological section. Statistical comparisons with use of one-way analysis of variance for three-way intergroup comparisons among the saline-solution, bupivacaine, and monoiodoacetate-injected knees were performed with significance defined as p < 0.05. Data were checked for normal distribution, and within-animal comparisons, with use of the paired t test and significance set at p < 0.05, were additionally performed between the saline-solution-injected right knees and the bupivacaine-injected left knees.
Fig. 2.
Computerized quantification of histological sections. Following segmentation of the articular cartilage with use of customized histomorphometric software, the center of the trochlea, the medial femoral condyle, or the lateral femoral condyle was identified on each section. A line was drawn at the center, perpendicular to the cartilage surface, dividing the image into two halves (solid line). Each half section was again bisected, and a second line was drawn (dashed lines). The software was then used to generate three full-thickness cartilage regions of interest, centered on each line, of approximately equal size (∼300 μm wide). The mean cell density was then calculated within each region of interest, and the densities were averaged for each histological section.
Source of Funding
This study was funded by the Albert Ferguson Endowed Chair (CRC), Department of Orthopaedic Surgery, University of Pittsburgh.
Results
Gross and Stereomicroscopic Assessment
The articular cartilage surface was grossly normal in all groups at one, four, and twelve weeks after injection.
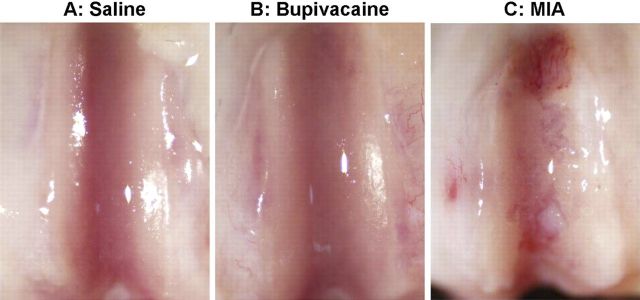
India ink staining and stereomicroscopy revealed no signs of fibrillation or erosion (grade 1) in the twelve-week period following the saline-solution or bupivacaine intra-articular injection. Six months after injection, the articular surfaces in the saline-solution and bupivacaine groups continued to appear smooth, glistening, and intact (Fig. 3, A and B). Similarly, India ink staining and stereomicroscopy showed no changes in the gross cartilage appearance twelve weeks after monoiodoacetate injection. In contrast, the monoiodoacetate-injected specimens appeared highly abnormal at six months (Fig. 3, C). Sixty percent of the monoiodoacetate-treated knees exhibited severe fibrillation, increased vascularization, and cartilage erosion visible on inspection with the naked eye (grade 4). The remaining 40% showed fibrillation and smaller patches of erosion (grade 3).
Fig. 3.
Gross assessment of chondral surfaces. The articular surfaces were smooth and glistening, with no visible surface irregularities, six months after a single intra-articular injection of normal saline solution (A) or 0.5% bupivacaine (B). In contrast, a single injection of 0.6% monoiodoacetate (MIA) (C) resulted in visible cartilage erosions.
Chondrocyte Viability After Injection
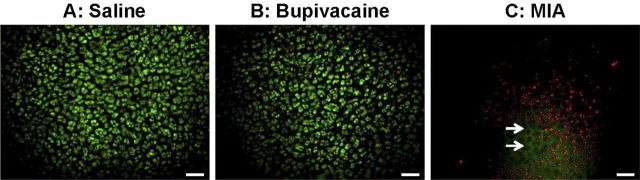
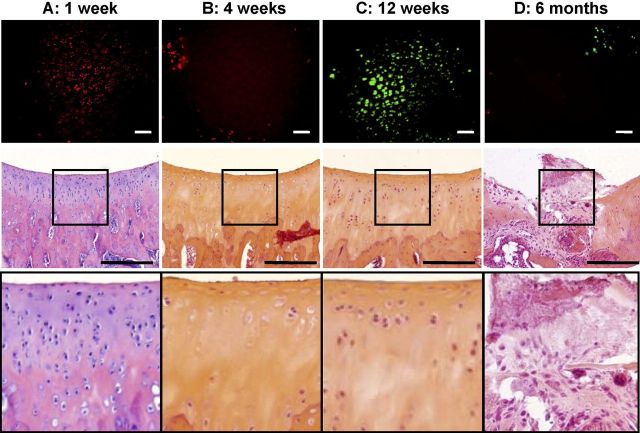
As compared with the saline-solution group, the bupivacaine group showed no differences in chondrocyte viability on the articular cartilage surface at one week, four weeks, twelve weeks, or six months (p > 0.05; Table I and Fig. 4). In contrast, the single injection of monoiodoacetate decreased chondrocyte viability (p < 0.04; Fig. 4). When compared with the saline-solution group, the monoiodoacetate group demonstrated an up to 87% decrease in chondrocyte viability in the femoral condyles (p < 0.04) and trochlear grooves (p < 0.001; Fig. 5, A) at one week. At four weeks after monoiodoacetate injection, areas devoid of stained cells were observed and empty lacunae were visible on confocal microscopy (p < 0.03; Fig. 5, B). At twelve weeks, viable cells, in numbers comparable with those in the saline-solution-injected specimens (p > 0.05), were seen in the trochlear grooves, medial femoral condyles, and lateral femoral condyles (Fig. 5, C). The number of observable viable cells in the medial femoral condyles decreased again six months after monoiodoacetate injection (p = 0.017; Table I and Fig. 5, D).
Fig. 4.
Surface chondrocyte viability. No significant loss of surface chondrocyte viability was seen one week after a single intra-articular injection of normal saline solution (A) or 0.5% bupivacaine (B) (p > 0.05 for the comparison between the two groups). In contrast, a single injection of 0.6% monoiodoacetate (MIA) (C) resulted in up to 87% cell death in the femoral condyles and trochlear groove (p < 0.05 compared with the saline-solution group). Viability was assessed with red/green staining and examination with three-dimensional confocal microscopy (living cells stained green [CMFDA] and dead cells stained red [propidium iodide]). The arrows indicate empty lacunae, seen only in the monoiodoacetate group with confocal microscopy. Scale bar = 60 μm.
Fig. 5.
Histological appearance and surface chondrocyte viability following monoiodoacetate injection. No difference in the total modified Mankin scores was observed, at any of the study time points, between specimens given a single intra-articular injection of normal saline solution and those given a single intra-articular injection of 0.5% bupivacaine. Injection of 0.6% monoiodoacetate resulted in higher total modified Mankin scores, indicative of increasing degeneration, by four weeks (p < 0.01 compared with those in the saline-solution group), with the highest scores obtained at six months (p < 0.001 compared with those in the saline-solution group). Comparison of the results of the viability imaging and histological assessment of the monoiodoacetate-injected specimens demonstrated consistent and complementary information. A: One week after monoiodoacetate injection, viability assessment showed substantial chondrocyte necrosis despite a normal histological appearance. B: At four weeks, both viability imaging and histological examination showed acellular tissue with empty lacunae and chondrocyte drop-out from superficial to deep zones. C: At twelve weeks, partial cellular repopulation was observed, especially in the superficial zone, with both viability imaging and histological analysis. D: At six months, scattered live and dead cells were seen with viability imaging. Histological examination revealed full-thickness cartilage loss and fibrosis. Scale bars on fluorescent stereomicroscopy images = 40 μm. Scale bars on histological images = 100 μm.
TABLE I.
Mean Cell Viability*
| 1 Week |
4 Weeks |
12 Weeks |
6 Months |
|||||||||
| Saline Solution (6 Knees) | Bupivacaine (6 Knees) | Monoiodoacetate (6 Knees) | Saline Solution (5 Knees) | Bupivacaine (5 Knees) | Monoiodoacetate (6 Knees) | Saline Solution (5 Knees) | Bupivacaine (5 Knees) | Monoiodoacetate (5 Knees) | Saline Solution (6 Knees) | Bupivacaine (6 Knees) | Monoiodoacetate (5 Knees) | |
| Lat. fem. condyle | 80 ± 6 | 91 ± 2 | 13 ± 12A | 89 ± 6 | 95 ± 3 | 23 ± 14B | 83 ± 5 | 92 ± 4 | 91 ± 4 | 97 ± 1 | 98 ± 1 | 74 ± 13 |
| Med. fem. condyle | 88 ± 3 | 85 ± 4 | 14 ± 11B | 93 ± 4 | 92 ± 3 | 21 ± 17A | 77 ± 6 | 88 ± 3 | 94 ± 3 | 97 ± 1 | 96 ± 1 | 49 ± 13A |
| Trochlea | 86 ± 4 | 86 ± 3 | 20 ± 9C | 92 ± 4 | 86 ± 7 | 79 ± 10 | 87 ± 6 | 86 ± 6 | 97 ± 2 | 94 ± 5 | 98 ± 1 | 92 ± 7 |
The values are given, in percentages, as the mean and standard error of the mean. A = p < 0.05, B = p < 0.01, and C = p < 0.001 as compared with the saline-solution group (paired t test).
Qualitative Histological Assessment of Cartilage
Subtle histological changes in the articular cartilage, including altered cell distribution, altered cell density, and less distinct cartilage subsurface layers, were observed at different time points in both the saline-solution-injected and bupivacaine-injected specimens. Overall, the histological appearance of the bupivacaine-injected knees appeared similar to that of the saline-solution controls, with the exception of intermittent hypercellularity at four weeks and hypocellularity at six months. The tidemark remained intact after bupivacaine and saline-solution injection.
Comparison of viability imaging with histological findings demonstrated consistent and complementary information regarding the monoiodoacetate-injected specimens (Fig. 5). One week after monoiodoacetate injection, viability assessment showed substantial chondrocyte necrosis without structural evidence of cell death in sections stained with hematoxylin and eosin (Fig. 5, A). At four weeks, cell-free zones at the surface and subsurface were readily apparent as evidenced by both the near absence of cells on viability imaging and near full-thickness chondrocyte drop-out on histological assessment, with empty lacunae superficially and marked hypocellularity deeper (Fig. 5, B). At twelve weeks, viability imaging showed surface cell repopulation, which was similarly observed in histological sections (Fig. 5, C). By six months after monoiodoacetate injection, viable cells were again observed with fluorescent and confocal microscopy. However, histological examination revealed nearly complete loss of articular cartilage with replacement by fibrous repair tissues intermingled with hypocellular areas of residual articular cartilage (Fig. 5, D). A continuum of abnormal histological patterns was observed six months after monoiodoacetate injection. Changes ranged from retention of full-thickness cartilage structure, but with either marked hypocellularity with full-thickness loss of chondrocytes and empty surface and subsurface lacunae, to complete loss of cartilage structure, increased repair tissue, and alterations in subchondral bone structure including complete fibrosis. Forty percent of the monoiodoacetate-injected specimens showed histological signs of osteoarthritis with complete loss of recognizable articular cartilage and severe subchondral bone changes.
Mankin Degeneration Scores
The total modified Mankin scores for the bupivacaine-injected specimens were similar to those for the saline-solution-injected specimens throughout the six-month study period (p > 0.05 at all time points). In contrast, specimens from the monoiodoacetate-injection group showed a steady increase in the total Mankin scores, indicative of increasing degeneration over six months (p < 0.05 at twelve weeks and six months). At six months after monoiodoacetate injection, the total modified Mankin scores (mean and standard error of the mean, 9.5 ± 0.2 for the lateral femoral condyles, 9.7 ± 0.3 for the medial femoral condyles, and 12.3 ± 0.3 for the trochlear grooves) were significantly higher than the corresponding scores in the saline-solution and bupivacaine groups, which ranged from 6.3 ± 0.3 to 6.8 ± 0.5 (p < 0.001).
Quantitative Histological Analysis
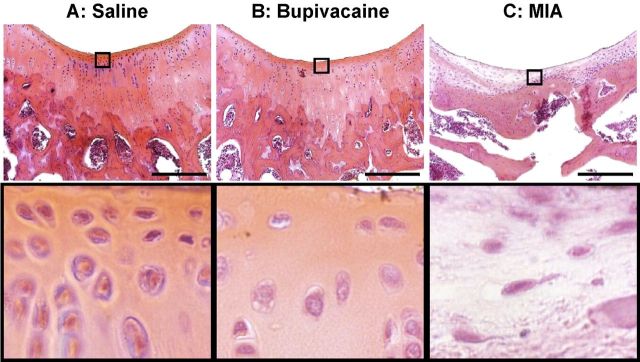
Computerized quantitative analysis of histological sections showed reduced chondrocyte density, compared with that in the saline-solution-injected specimens, at six months following the single intra-articular injection of bupivacaine. The cell density in the bupivacaine-injected specimens was similar to that in the saline-solution-injected specimens one week after injection. At four weeks, a 43% increase in cell density was observed in the trochlear grooves (p = 0.026). Chondrocyte density fluctuated and was again similar to that in the saline-solution group at the twelve-week time point. Chondrocyte density decreased between twelve weeks and six months after bupivacaine injection, with a 43% reduction observed in the trochlear grooves (p = 0.011). Chondrocyte density in the medial femoral condyles declined to 50% of that in the saline-solution-injected group by six months after the bupivacaine injection (p = 0.006; Fig. 6 and Table II).
Fig. 6.
Chondrocyte density. Quantitative image analysis of histological specimens showed that chondrocyte density in specimens injected with a single intra-articular injection of 0.5% bupivacaine, while similar to that in the saline-solution group at one week after injection, was 50% less than that in the saline-solution group at six months after injection (p = 0.017). Histological images show the density of chondrocytes in the saline-solution-injected specimen (A) to be higher than that in the bupivacaine-injected specimen (B) at six months. Six months after injection with 0.6% monoiodoacetate (MIA) (C), replacement with fibrous repair tissue can be seen. Scale bar = 250 μm.
TABLE II.
Mean Cell Density*
| 1 Week |
4 Weeks |
12 Weeks |
6 Months |
|||||||||
| Saline Solution (6 Knees) | Bupivacaine (6 Knees) | Monoiodoacetate (6 Knees) | Saline Solution (5 Knees) | Bupivacaine (5 Knees) | Monoiodoacetate (6 Knees) | Saline Solution (5 Knees) | Bupivacaine (5 Knees) | Monoiodoacetate (5 Knees) | Saline Solution (6 Knees) | Bupivacaine (6 Knees) | Monoiodoacetate (5 Knees) | |
| Lat. fem. condyle | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.5 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 0.7 ± 0.1 | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.5 ± 0.0D |
| Med. fem. condyle | 1.9 ± 0.2 | 1.7 ± 0.1 | 1.3 ± 0.1D | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 0.1D | 2.1 ± 0.3 | 1.9 ± 0.2 | 1.6 ± 0.2 | 1.2 ± 0.1 | 0.6 ± 0.1B | 1.0 ± 0.1 |
| Trochlea | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.2 | 1.1 ± 0.1 | 1.9 ± 0.1A | 1.0 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.1 | 1.9 ± 0.2 | 1.1 ± 0.1A | 0.9 ± 0.1F |
The values (× 10−3 cells per region of interest) are given as the mean and standard error of the mean. A = p < 0.05, B = p < 0.01, and C = p < 0.001 as compared with the saline-solution group (paired t test). D = p < 0.05, E = p < 0.01, and F = p < 0.001 as compared with the saline-solution group (one-way analysis of variance).
Significant decreases in cell density were observed at one week, four weeks, and six months after monoiodoacetate injection. As early as one week after monoiodoacetate injection, the cell density in the medial femoral condyles was decreased by 30% as compared with that in the saline-solution group (p = 0.017). A further decline was observed at four weeks. At twelve weeks after monoiodoacetate injection, the cell density was similar to that in the saline-solution controls (p > 0.05). By six months, cell density was 50% of that in the saline-solution controls in the lateral femoral condyles (p = 0.001) and 48% of that in the saline-solution controls in the trochlear grooves (p = 0.004, Table II).
Discussion
This in vivo study showed a significant reduction in chondrocyte density six months after a single intra-articular injection of 0.5% bupivacaine. The articular surfaces of bupivacaine-injected joints remained intact on gross and histological evaluation. In contrast, joints injected with 0.6% monoiodoacetate exhibited extensive chondrocyte necrosis and full-thickness pathological changes followed by grossly visible loss of articular cartilage during the same time period. These results show that, while rapid chondrolysis was induced by monoiodoacetate, signs of chondrocyte toxicity following a single injection of 0.5% bupivacaine were subtle and did not result in tissue loss or surface damage during the same time period.
Chondrolysis is a catastrophic joint condition defined as the disappearance of articular cartilage as the result of disintegration or dissolution of the cartilage matrix and cells9. This condition has been most frequently reported clinically in several joints, most notably the hip and shoulder5,20-22. Intra-articular exposure of cartilage to chondrotoxic substances such as chlorhexidine has been implicated in chondrolysis of the knee, and intra-articular exposure of cartilage to bupivacaine has been implicated in chondrolysis of the shoulder5,23. Degenerative arthritis and autofusion of the joint are readily appreciable radiographically and are expected undesirable secondary clinical outcomes following chondrolysis21,24,25.
While loss of articular cartilage is grossly observable in chondrolysis, this study highlights that an intact articular surface can mask severe pathological changes. While the articular surface of the monoiodoacetate-injected specimens remained intact on gross inspection through twelve weeks, fluorescent and confocal microscopy revealed an up to 87% loss of superficial chondrocytes at one week and areas with the absence of observable chondrocytes at four weeks. Similarly, histological study revealed severe abnormalities such as full-thickness loss of chondrocytes, subchondral bone necrosis, and fibrosis as early as four weeks after monoiodoacetate injection. In this small-animal model, evidence of cellular repopulation and fibrous repair responses was noted at twelve weeks. These reparative responses, however, were insufficient to prevent the onset of cartilage loss and joint degeneration six months following monoiodoacetate injection. Cartilage damage was not evident on gross inspection until the development of these advanced changes related to chondrolysis.
Pathological changes of the articular cartilage following single intra-articular injections of 0.5% bupivacaine were not observed with fluorescent or confocal microscopy or with conventional histological examination. The chondral surfaces remained grossly and histologically intact throughout the study period. While the modified Mankin degeneration score was sensitive enough to show differences between the monoiodoacetate and saline-solution groups, no differences in the modified Mankin scores were noted between the bupivacaine and saline-solution groups. This finding is consistent with the qualitative analysis showing few histological signs of degeneration in the bupivacaine-injected knees. Consequently, many of the parameters considered when calculating the Mankin score, such as depth of surface fissuring, chondrocyte cloning, or subchondral bone changes, were not observed. Because of a reliance on subjective serial assessment of hypocellularity and hypercellularity, both qualitative evaluation and the Mankin scoring system are limited for evaluation of cell density, an important outcome measure of chondrotoxicity. To address this limitation, customized histomorphometric analysis software was used to systematically calculate chondrocyte density in standardized regions of interest. Quantitative analysis of chondrocyte density showed some hypercellularity compared with the cell density in the saline-solution controls at four weeks. Consistent with the evidence of repair seen in the monoiodoacetate-injected specimens, this transient hypercellularity likely reflects a reparative response to injury in this small-animal model. Ultimately, chondrocyte homeostasis did not fully recover, and a marked reduction in chondrocyte density, compared with that in the saline-solution group, was measured at six months after bupivacaine injection.
The fact that early chondrocyte necrosis was not observed in vivo following a single intra-articular injection of 0.5% bupivacaine could be explained by the dose and time-dependent chondrotoxicity reported in vitro2,6. A previous in vitro study showed 0.5% bupivacaine to be highly toxic to articular chondrocytes. That study further showed that, while the toxicity of 0.25% bupivacaine was less than that of 0.5% bupivacaine, chondrotoxicity increased proportionally to both the duration of bupivacaine exposure and the time after bupivacaine exposure. Significant chondrocyte toxicity was not observed following exposure to 0.125% bupivacaine1.
The range of doses and time of exposure of chondrocytes after a single joint injection would be reduced in vivo by a variety of factors, including joint fluid, bleeding, articular cartilage integrity, and bupivacaine absorption and clearance. The maximal blood concentration of bupivacaine has been observed clinically to occur thirty to forty-five minutes after a bolus injection into the knee26. These data indirectly show that systemic absorption alone substantially reduces the effective intra-articular concentration of bupivacaine within this time frame. In contrast to continuous infusion protocols that overcome in vivo dilutional effects4, these factors may have reduced the effective concentration following a single injection of 0.5% bupivacaine to a level that did not result in observable immediate chondrocyte death.
The marked reduction in chondrocyte density observed six months following bupivacaine injection, however, suggests substantial residual chondrocyte morbidity. The longer-range impact of a 50% reduction in chondrocyte density, even with no additional cell loss, is unknown. It is reasonable to postulate that, with fewer chondrocytes to repair and maintain the matrix, hypocellular cartilage may have a reduced tolerance to mechanical loading or subsequent injury27.
The monoiodoacetate data from this study are consistent with the findings in others that death and morbidity of articular chondrocytes are implicated in the pathogenesis of cartilage loss and joint degeneration28-30. The critical threshold at which chondrocyte loss leads to the development of progressive cartilage degeneration in humans remains unknown. Animal studies cannot precisely answer this question.
While animal studies permit use of advanced laboratory techniques to evaluate the in vivo effects of intra-articular bupivacaine injection on articular cartilage, no animal model precisely mimics the thickness, structure, size, biomechanics, repair potential, loading conditions, and kinematics of the articular cartilage of a human joint. Human articular cartilage is much thicker than rat articular cartilage. This means that substances injected into joints that enter the cartilage by diffusion would potentially affect a much smaller proportion of the tissue in humans1.
The finding of substantial chondrocyte morbidity without cartilage tissue loss following a single injection of 0.5% bupivacaine in this in vivo study is consistent with in vitro evidence that bupivacaine is toxic to articular chondrocytes. A reduction in chondrocyte density is not detectable clinically on radiographs, magnetic resonance imaging, or arthroscopy31,32. As highlighted by the monoiodoacetate data, severe chondrocyte necrosis and subsurface pathological changes leading to chondrolysis can be present in cartilage that has retained an intact articular surface. Any development of chondrosis or joint degeneration due to milder changes such as a progressive reduction in chondrocyte density is probably multifactorial and may take many years. This study shows that the chondrotoxic effects of a single intra-articular injection of 0.5% bupivacaine are subtle and would be difficult to detect clinically.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania
Disclosure: The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1. Chu CR Izzo NJ Coyle CH Papas NE Logar A. The in vitro effects of bupivacaine on articular chondrocytes. J Bone Joint Surg Br. 2008;90:814-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chu CR Izzo NJ Papas NE Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693-9. [DOI] [PubMed] [Google Scholar]
- 3. Dragoo JL Korotkova T Kanwar R Wood B. The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med. 2008;36:1484-8. [DOI] [PubMed] [Google Scholar]
- 4. Gomoll AH Kang RW Williams JM Bach BR Cole BJ. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813-9. [DOI] [PubMed] [Google Scholar]
- 5. Hansen BP Beck CL Beck EP Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35:1628-34. [DOI] [PubMed] [Google Scholar]
- 6. Piper SL Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91. [DOI] [PubMed] [Google Scholar]
- 7. Seshadri V Coyle CH Chu CR. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25:337-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karpie JC Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7. [DOI] [PubMed] [Google Scholar]
- 9. The American Heritage Stedman's medical dictionary. Updated 2nd ed. Boston: Houghton Mifflin; 2007. p xxxii, 909. [Google Scholar]
- 10. Dogan N Erdem AF Erman Z Kizilkaya M. The effects of bupivacaine and neostigmine on articular cartilage and synovium in the rabbit knee joint. J Int Med Res. 2004;32:513-9. [DOI] [PubMed] [Google Scholar]
- 11. Nole R Munson NM Fulkerson JP. Bupivacaine and saline effects on articular cartilage. Arthroscopy. 1985;1:123-7. [DOI] [PubMed] [Google Scholar]
- 12. Fulkerson JP Damiano P. Effect of prostaglandin E2 on adult pig articular cartilage slices in culture. Clin Orthop Relat Res. 1983;179:266-9. [PubMed] [Google Scholar]
- 13. Luc M Pham T Chagnaud C Lafforgue P Legré V. Placement of intra-articular injection verified by the backflow technique. Osteoarthritis Cartilage. 2006;14:714-6. [DOI] [PubMed] [Google Scholar]
- 14. Guingamp CP Gegout-Pottie P Philippe L Terlain B Netter P Gillet P. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40:1670-9. [DOI] [PubMed] [Google Scholar]
- 15. Kuwata K Sato S Era S Sogami M Kida K Iwama T Kato K Matsunaga T Watari H. Cross-relaxation times of normal and biochemically induced osteoarthritic rabbit knee cartilages. Jpn J Physiol. 1997;47:291-7. [DOI] [PubMed] [Google Scholar]
- 16. Pulichino AM Rowland S Wu T Clark P Xu D Mathieu MC Riendeau D Audoly LP. Prostacyclin antagonism reduces pain and inflammation in rodent models of hyperalgesia and chronic arthritis. J Pharmacol Exp Ther. 2006;319:1043-50. [DOI] [PubMed] [Google Scholar]
- 17. Grossin L Etienne S Gaborit N Pinzano A Cournil-Henrionnet C Gerard C Payan E Netter P Terlain B Gillet P. Induction of heat shock protein 70 (Hsp70) by proteasome inhibitor MG 132 protects articular chondrocytes from cellular death in vitro and in vivo. Biorheology. 2004;41:521-34. [PubMed] [Google Scholar]
- 18. Yoshioka M Coutts RD Amiel D Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87-98. [DOI] [PubMed] [Google Scholar]
- 19. Mankin HJ Dorfman H Lippiello L Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523-37. [PubMed] [Google Scholar]
- 20. Bojescul JA Wilson G Taylor DC. Idiopathic chondrolysis of the ankle. Arthroscopy. 2005;21:224-7. [DOI] [PubMed] [Google Scholar]
- 21. Good CR Shindle MK Kelly BT Wanich T Warren RF. Glenohumeral chondrolysis after shoulder arthroscopy with thermal capsulorrhaphy. Arthroscopy. 2007;23:797, e1-5. [DOI] [PubMed] [Google Scholar]
- 22. Korula RJ Jebaraj I David KS. Idiopathic chondrolysis of the hip: medium- to long-term results. ANZ J Surg. 2005;75:750-3. [DOI] [PubMed] [Google Scholar]
- 23. van Huyssteen AL Bracey DJ. Chlorhexidine and chondrolysis in the knee. J Bone Joint Surg Br. 1999;81:995-6. [DOI] [PubMed] [Google Scholar]
- 24. Rachinsky I Boguslavsky L Cohen E Hertzanu Y Lantsberg S. Bilateral idiopathic chondrolysis of the hip: a case report. Clin Nucl Med. 2000;25:1007-9. [DOI] [PubMed] [Google Scholar]
- 25. Sivanantham M Kutty MK. Idiopathic chondrolysis of the hip: case report with a review of the literature. Aust N Z J Surg. 1977;47:229-31. [DOI] [PubMed] [Google Scholar]
- 26. Convery PN Milligan KR Quinn P Sjövall J Gustafsson U. Efficacy and uptake of ropivacaine and bupivacaine after single intra-articular injection in the knee joint. Br J Anaesth. 2001;87:570-6. [DOI] [PubMed] [Google Scholar]
- 27. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;325:10-8. [DOI] [PubMed] [Google Scholar]
- 28. Aigner T Kurz B Fukui N Sandell L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol. 2002;14:578-84. [DOI] [PubMed] [Google Scholar]
- 29. Sandell LJ Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldring MB Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626-34. [DOI] [PubMed] [Google Scholar]
- 31. Chu CR Izzo NJ Irrgang JJ Ferretti M Studer RK. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. J Biomed Opt. 2007;12:051703. [DOI] [PubMed] [Google Scholar]
- 32. Chu CR Lin D Geisler JL Chu CT Fu FH Pan Y. Arthroscopic microscopy of articular cartilage using optical coherence tomography. Am J Sports Med. 2004;32:699-709. [DOI] [PubMed] [Google Scholar]