Abstract
Introduction:
Although stress is a well-establish risk factor for the development of chronic musculoskeletal pain, the underlying mechanisms, specifically the contribution of neuroendocrine stress axes, remain poorly understood.
Objective:
To evaluate the hypothesis that psychological stress-induced activation of the sympathoadrenal stress axis prolongs the muscle pain observed after strenuous exercise.
Methods:
Adult male Sprague-Dawley rats were exposed to unpredictable sound stress and eccentric exercise. The involvement of the sympathoadrenal stress axis was evaluated by means of surgical interventions, systemic administration of epinephrine, and intrathecal β2-adrenergic receptor antisense.
Results:
Although sound stress alone did not modify nociceptive threshold, it prolonged eccentric exercise-induced mechanical hyperalgesia. Adrenal medullectomy (ADMdX) attenuated, and administration of stress levels of epinephrine to ADMdX rats mimicked this effect of sound stress. Knockdown of β2-adrenergic receptors by intrathecal antisense also attenuated sound stress-induced prolongation of eccentric exercise-induced hyperalgesia.
Conclusion:
Together, these results indicate that sympathoadrenal activation, by unpredictable sound stress, disrupts the capacity of nociceptors to sense recovery from eccentric exercise, leading to the prolongation of muscle hyperalgesia. This prolonged recovery from ergonomic pain is due, at least in part, to the activation of β2-adrenergic receptors on muscle nociceptors.
Keywords: Delayed-onset muscle soreness, β2-adrenergic receptor, Adrenal medulla, Nociceptor, Rat
1. Introduction
Chronic musculoskeletal pain, a leading cause of physical disability and decreased quality of life, produces huge direct and indirect economic losses.24 Although the pathophysiology of musculoskeletal pain is still poorly understood, a sizeable body of evidence indicates that psychological stress plays an important role in its induction and aggravation.9,16,17,54 For instance, epidemiological studies have shown a tight relationship between psychological stress and musculoskeletal pain in diverse populations.10,36,38,49 And, clinical studies have emphasized the role of persistent stress in the induction of chronic widespread musculoskeletal pain, identifying a key role of nociceptive afferent inputs in the maintenance of primary and secondary muscle hyperalgesia.55–58 Preclinical evidence has also shown a contribution of nociceptor sensitization to stress-induced muscle pain, revealed by increased excitability of muscle nociceptors after exposure to psychological stress.11,26 However, the mechanism by which stress induces musculoskeletal pain persistence remains to be established.
Single nucleotide polymorphisms in the β2-adrenergic receptor16,17,52 and enzymes mediating catecholamine metabolism8,17,42 determine increased susceptibility to develop persistent musculoskeletal pain after exposure to stress and physical injury. In rodent models of stress-induced muscle hyperalgesia, there is a concomitant persistent increase in plasma epinephrine.32,33 This stress-induced hyperalgesia is prevented by excision of the adrenal medulla,3,32,33 whereas systemic administration of stress levels of epinephrine in adrenal-medullectomized rats recapitulates the pain phenotype observed in rats exposed to unpredictable sound stress.32,33 Finally, systemic administration of stress levels of epinephrine, alone, aggravates muscle hyperalgesia produced in rats previously submitted to neonatal stress.3 Although these observations support the suggestion that the sympathoadrenal axis is involved in stress-induced muscle pain, the underlying mechanism remains unknown.
We have recently demonstrated that physical resilience, the intrinsic capacity to recover from tissue injury, exhibits a cytokine signature, which is detected by muscle nociceptors.2 Disruption of a nociceptor's capacity to detect this signature prevents the recovery of baseline nociceptive threshold.2 The fact that nociceptors also respond to the sympathoadrenal mediator epinephrine12,35 suggests that stress can also modulate the function of nociceptors as sensors of the physical resilience status. In line with this, there is evidence that exposure to stressful events delays the recovery from muscle soreness after strenuous resistance60 or eccentric27 exercise. Therefore, we evaluated the hypothesis that stress-induced activation of sympathoadrenal stress axis disrupts the nociceptor capacity to act as sensors of physical resilience, delaying the recovery of normal muscle nociceptive threshold.
2. Materials and methods
2.1. Animals
Experiments were performed on adult male Sprague-Dawley rats (Crl:CD), weighing 250 to 400 g (approximately 8–12 weeks old), obtained from Charles River (Hollister, CA). Rats were housed 3 per cage in an AAALAC International accredited animal facility, the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12-hour light/dark cycle (lights on 7 am–7 pm; room temperature 21–23°C), with water and food available ad libitum. On completion of experiments, rats were euthanized using CO2-induced asphyxia followed by bilateral thoracotomy. Animal care and use conformed to NIH guidelines. The University of California, San Francisco Institutional Animal Care and Use Committee approved all experimental protocols. Concerted effort was made to reduce the suffering and number of animals used.
2.2. Eccentric exercise
The method used to eccentrically exercise the rat hind limb has been described previously.4,31 Briefly, rats were anesthetized with 2.5% isoflurane (Henry Schein Animal Health, Dublin, OH) and placed in supine position, on a heating pad, to maintain body core temperature at 37°C. The right hind paw was affixed to the foot bracket of an exercise apparatus (Model RU-72; NEC Medical Systems, Tokyo, Japan) with surgical paper tape (Micropore; 3M Health Care, St. Paul, MN), such that the angle of both the knee and ankle joints were ∼90° (the paw 30° from vertical). After clipping and disinfecting the skin on the calf, the gastrocnemius muscle was stimulated using subcutaneously (s.c.) inserted needle electrodes (25 G × 5/8″ Becton; Dickinson & Co, Franklin Lakes, NJ), with a Model DPS-07 stimulator (Dia Medical System, Inc, Tokyo, Japan) that delivered trains of rectangular pulses (100 Hz, 700 ms, 3 V) every 3 seconds, to elicit a total of 300 contractions. During each stimulus-induced contraction of the gastrocnemius muscle, an electronic motor rotated the foot to produce extension of the contracting gastrocnemius muscle.
2.3. Sound stress protocol
Exposure to sound stress occurred over 4 days, as described previously.3,32 Animals were placed 3 per cage and the cage placed 25 cm from a speaker that emitted 4 pure tones (5, 11, 15, and 19 kHz), whose amplitudes varied randomly from 20 to 110 dB sound pressure level at random times each minute, lasting 5 or 10 seconds.51,59 Sham stressed animals were placed in the sound chamber for 30 minutes, but without exposure to the sound stimulus. After sound stress or sham treatment, rats were returned to their home cages, in the animal care facility. Animals were exposed to the stressor on days 1, 3, and 4.3,32,59
2.4. Adrenal medullectomy
Rats were injected with meloxicam 2 mg/kg s.c. (Metacam; Boehringer Ingelheim Vetmedica, St. Joseph, MO), anesthetized with 2.5% isoflurane in oxygen, and the incision site infiltrated with 0.25% bupivacaine (Marcaine; Hospira, Lake Forest, IL). Adrenal glands were then located through bilateral incisions in the abdominal wall, their capsules incised, and the medullae excised3,44,62; the fascia and skin were then sutured separately. Rats were provided with 0.45% saline to drink (ad libitum) for the first 7 days after surgery and received an additional injection of meloxicam 24 hours after surgery. To allow for maximum recovery of hypothalamic–pituitary–adrenal axis function, adrenal medullectomy was performed at least 5 weeks before additional experimental procedures.62
2.5. Chronic administration of epinephrine
Chronic administration of epinephrine was performed by osmotic minipumps (ALZET model 2004; DURECT Corporation, Cupertino, CA) implanted in the interscapular region. Rats were injected with meloxicam (2 mg/kg s.c.), anesthetized with 2.5% isoflurane in oxygen, and the incision site infiltrated with 0.25% bupivacaine. Stress levels of epinephrine were induced by filling the osmotic minipumps, so that they delivered 5.4 μg/0.25 μL/h of epinephrine.3,32,34 Nociceptive threshold was measured in epinephrine-implanted, adrenal-medullectomized (ADMdX) rats, 14 days after surgery.
2.6. Intrathecal antisense oligodeoxynucleotides
To evaluate the role of nociceptor β2-adrenergic receptors (β2-AR) in stress-induced prolongation eccentric exercise-induced muscle hyperalgesia, we attenuated its expression by means of intrathecal (i.t.) injections of antisense oligodeoxynucleotides (AS ODN) directed against β2-AR mRNA.19 The AS ODN sequence, 5′-AAA GGC AGA AGG ATG TGC-3′, was directed against a unique sequence of the rat β2-AR mRNA (GeneBank accession number NM_012492). This AS ODN sequence has been previously reported to produce specific β2-AR protein knockdown in the peripheral nervous system and behavioral changes consistent with such a decrease in protein expression in the rat.19,22 The mismatch (MM) ODN sequence, 5′-ATA GCC TGA TGG AAG TCC-3′, was designed by mismatching 6 bases (denoted by bold typeface) of the AS sequence.
Oligodeoxynucleotides were synthesized by Invitrogen (San Francisco, CA), dissolved in sterile 0.9% NaCl (B. Braun Medical, Inc, Irvine, CA) to a concentration of 10 µg/µL, and stored in 20-µL aliquots at −20°C until use. Immediately before injection, aliquots of ODNs were further diluted in sterile 0.9% NaCl to a concentration of 2 µg/µL, and injected i.t. (40 µg/20 µL) daily for 3 consecutive days under brief anesthesia with 2.5% isoflurane as previously reported.3,4,19,32 Thereafter, rats were injected with ODN every other day, to ensure sustained knockdown of nociceptor β2-AR. Tail flick was systematically checked to ensure proper i.t. injections.43
2.7. Measurement of muscle mechanical nociceptive threshold
Mechanical nociceptive threshold in the right gastrocnemius muscle was quantified using a digital force transducer (Chatillon DF2; Amtek, Inc, Largo, FL) with a 7 mm-diameter probe.2–5 Rats were lightly restrained in cylindrical acrylic holders with lateral slats that allow for easy access to the hind limb for application of the force transducer probe to the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in millinewtons, required to produce a flexion reflex in the hind limb. The results are expressed as percentage change from baseline mechanical nociceptive threshold. All behavioral testing was conducted between 10 am and 4 pm Behavioral data collection was performed unblinded to treatment condition.
2.8. Statistics
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made using two-way repeated-measures analysis of variance followed by Bonferroni multiple-comparisons post hoc test. Statistical significance was set at P < 0.05. Statistical software Prism 8.0 (GraphPad Software, Inc, La Jolla, CA) was used to perform data analysis and graph plotting.
3. Results
3.1. Sound stress prolongs eccentric exercise-induced muscle hyperalgesia
Two protocols of unpredictable sound stress followed by eccentric exercise were studied (Fig. 1A). As previously reported,2,4 naive (control) rats submitted to eccentric exercise exhibit a significant decrease in nociceptive threshold (ie, mechanical hyperalgesia) in the gastrocnemius muscle. Hyperalgesia reached a peak 48 hours after eccentric exercise (n = 6/group, P < 0.05; Fig. 1B) and fully recovered by day 5 (n = 6/group, P < 0.05; Fig. 1B). By contrast, rats submitted to unpredictable sound stress either 1 or 14 days before displayed muscle hyperalgesia that persisted for up to 20 days (n = 6/group, P < 0.05; Fig. 1B). Exposure to unpredictable sound stress 14 days before eccentric exercise produced a small, albeit significant, increase in peak hyperalgesia compared with naive rats (n = 6/group, P < 0.05; Fig. 1B). Given that rats submitted to sound stress with the last exposure either 1 or 14 days before eccentric exercise displayed similar duration of muscle hyperalgesia (Fig. 1B), we performed subsequent experiments using the eccentric exercise one day after sound stress.
Figure 1.
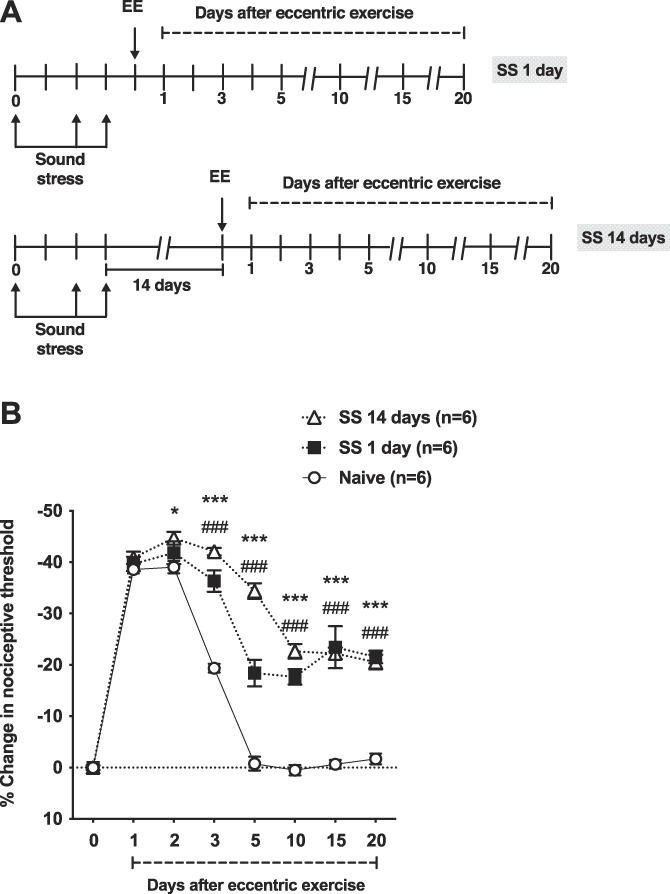
Preexposure to sound stress prolongs eccentric exercise-induced mechanical hyperalgesia. (A) The experimental protocol used to study muscle nociceptive threshold in rats submitted to unpredictable sound stress on days 1, 3, and 4. After assessment of baseline nociceptive threshold sound stress (SS) rats and naive (control) rats were submitted to eccentric exercise 1 (SS 1 day) or 14 (SS 14 days) days after the last sound stress session. The nociceptive threshold was then measured in the ipsilateral gastrocnemius muscle, up to day 20 after eccentric exercise. (B) Two-way ANOVA showed significant effects for treatment (F2,15 = 61.07, P < 0.001), time (F7,105 = 446.9, P < 0.001), and treatment by time interaction (F14,105 = 30.54, P < 0.001). Post hoc analysis revealed significant differences between control rats and SS 1 day rats from day 3 (P < 0.05) to day 20 (P < 0.001) after eccentric exercise. Significant differences between control rats and SS 14 days rats were observed, from day 2 (P < 0.05) to day 20 (P < 0.001) after eccentric exercise. Significant differences between SS 1 day and SS 14 days rats were observed only in days 3 (P < 0.05) and 5 (P < 0.001) after eccentric exercise. *P < 0.05; ***P < 0.001 (Naive vs SS 14 days); ###P < 0.001 (Naive vs SS 1 day). ANOVA, analysis of variance.
3.2. Adrenal medullectomy attenuates stress-induced prolongation of exercise-induced muscle hyperalgesia
To explore the contribution of the sympathoadrenal neuroendocrine stress axis to prolongation of eccentric exercise-induced muscle hyperalgesia produced by sound stress, the effect of ADMdX or a sham procedure was studied (Fig. 2A, B). ADMdX alone did not produce a change in nociceptive threshold, compared with control (sham) rats, at baseline (n = 6/group, P > 0.05; Fig. 2B), or 1 day after the last sound stress (n = 6/group, P > 0.05; Fig. 2B). Importantly, ADMdX alone (no sound stress) had no effect on eccentric exercise-induced muscle hyperalgesia (n = 6/group, P > 0.05; Fig. 2B). The ADMdX plus sound stress rats, however, experienced significantly less muscle hyperalgesia compared with sham controls, on days 1 to 20, after eccentric exercise (n = 6/group, P < 0.05; Fig. 2B).
Figure 2.
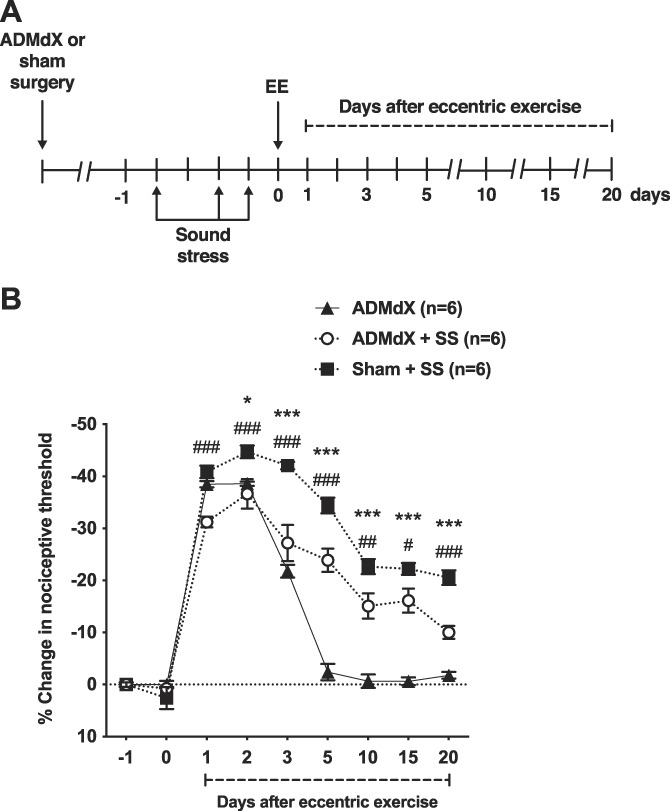
Adrenal medullectomy attenuates the prolongation of eccentric exercise-induced muscle hyperalgesia by sound stress. (A) Timing of the experimental protocol used to study muscle nociceptive threshold after surgical excision of the adrenal medulla (ADMdX) or sham procedure in control rats. Five weeks after surgery, after assessment of baseline nociceptive threshold measured in the ipsilateral gastrocnemius muscle (time point −1), rats were submitted to unpredictable sound stress on days 1, 3, and 4. One day after the last SS session, nociceptive threshold was assessed (time point 0) and thereafter rats were submitted to eccentric exercise and the nociceptive threshold measured up to day 20 after eccentric exercise. (B) Two-way ANOVA showed significant effects for treatment (F2,15 = 48.35, P < 0.001), time (F8,120 = 423.95, P < 0.001), and treatment by time interaction (F16,120 = 27.96, P < 0.001). Post hoc analysis revealed significant differences between control (sham) rats + sound stress (SS) and ADMdX + SS rats, from day 1 (P < 0.05) to day 20 (P < 0.001) after eccentric exercise. Of note, nociceptive responses of ADMdX rats are similar to those displayed by naive (control) rats (Fig. 1B), suggesting that excision of the adrenal medulla does not modify, by itself, the nociceptive responses to eccentric exercise. *P < 0.05; ***P < 0.001 (Sham + SS vs ADMdX); #P < 0.05, ##P < 0.01, ###P < 0.001 (Sham + SS vs ADMdX + SS). ANOVA, analysis of variance.
3.3. Sustained administration of stress levels of epinephrine prolongs eccentric exercise-induced muscle hyperalgesia
To determine whether epinephrine contributes to the prolongation of eccentric exercise-induced muscle hyperalgesia produced by sound stress, we studied its sustained administration in ADMdX rats (Fig. 3A). Administration of saline or epinephrine at stress levels to ADMdX rats, alone or in combination with sound stress, did not modify the nociceptive threshold compared with baseline (n = 6/group, P > 0.05; Fig. 3B). However, compared with saline, epinephrine treatment prolonged eccentric exercise-induced muscle hyperalgesia, producing a significant decrease in nociceptive threshold from day 3 to day 20 after eccentric exercise (n = 6/group, P < 0.05; Fig. 3B). There were no significant differences in the mechanical hyperalgesia of rats receiving epinephrine at stress levels and rats receiving epinephrine plus sound stress (n = 6/group, P > 0.05; Fig. 3B).
Figure 3.
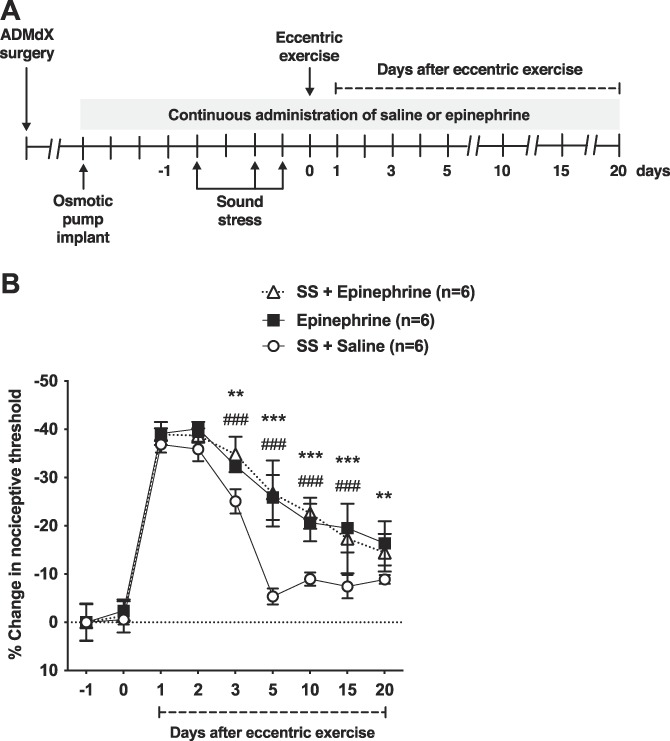
Administration of stress levels of epinephrine prolongs eccentric exercise-induced muscle hyperalgesia. (A) Experimental protocol used to study muscle nociceptive threshold after the administration of stress levels of epinephrine or saline, alone or in combination with unpredictable sound stress (SS), in ADMdX rats. Two weeks after osmotic pumps containing epinephrine or saline were implanted, ADMdX rats were submitted to sound stress (SS + Epinephrine; SS + Saline) or sham procedure (Epinephrine) on days 1, 3, and 4. One day after the last sound stress session, they were submitted to eccentric exercise and the nociceptive threshold measured in the ipsilateral gastrocnemius muscle up to day 20 after eccentric exercise. (B) Two-way ANOVA showed significant effects for time (F8,120 = 394.7, P < 0.001), treatment (F2,15 = 34.11, P < 0.001), or treatment by time interaction (F16,120 = 10.30, P = 0.7497). Post hoc analysis revealed significant differences between control rats receiving saline and rats receiving epinephrine alone (Epinephrine) from day 3 to day 20 after eccentric exercise (P < 0.001) and between control rats (SS + Saline) and rats receiving epinephrine plus sound stress (SS + Epinephrine) from day 3 to day 15 after eccentric exercise (P < 0.001). No significant differences between rats receiving epinephrine alone (Epinephrine) and rats receiving epinephrine plus sound stress (SS + Epinephrine) were observed at any time point after eccentric exercise (P > 0.05). **P < 0.01; ***P < 0.001 (SS + Saline vs Epinephrine); ###P < 0.001 (SS + Saline vs SS + Epinephrine). ANOVA, analysis of variance.
3.4. Knockdown of β2-AR attenuates sound stress-induced prolongation of exercise-induced muscle hyperalgesia
The role of nociceptor β2-AR in the prolongation of eccentric exercise-induced muscle hyperalgesia by preexposure to sound stress was evaluated by means of intrathecal AS ODN for β2-AR mRNA. The ODN treatment did not produce significant changes in nociceptive threshold, when measured before and immediately after sound stress (n = 6/group, P > 0.05; Fig. 4B). However, compared with control MM group, rats treated with AS ODN exhibited significant attenuation of muscle hyperalgesia from day 2 to 20 after eccentric exercise (n = 6/group, P < 0.05; Fig. 4B).
Figure 4.
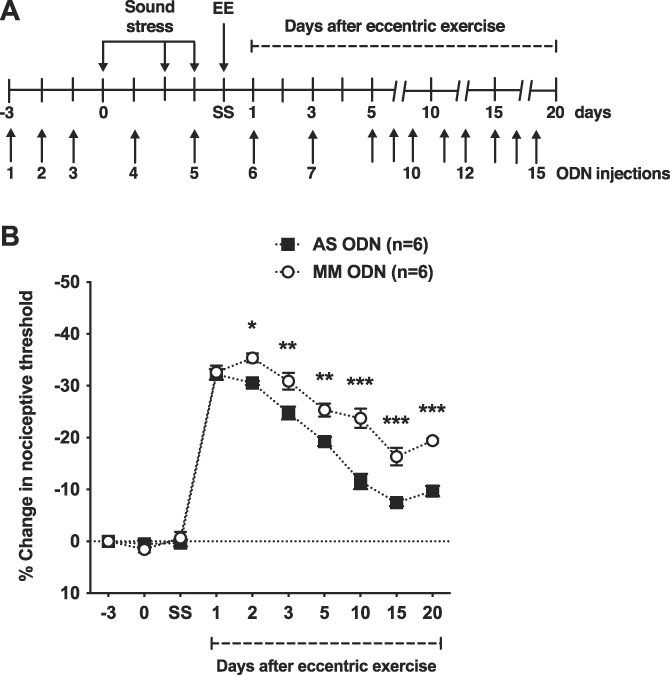
Nociceptor expression of the β2-AR is necessary for sound stress-induced prolongation of eccentric exercise-induced muscle hyperalgesia. (A) Timing of the experimental protocol used to assess muscle nociceptive threshold after antisense oligodeoxynucleotide (AS ODN) and mismatch oligodeoxynucleotide (MM ODN) treatments, followed by exposure to unpredictable sound stress and eccentric exercise. After measurement of baseline nociceptive threshold (time point −3), rats received daily i.t. injections of AS ODN or MM ODN, for 3 days and their nociceptive threshold assessed one day after the last ODN injection (time point 0) and submitted to sound stress. One day after the last sound stress session, rats' nociceptive thresholds were measured (time point SS) and they were submitted to eccentric exercise (EE). To provide continuous knockdown of β2-AR, after the third daily injection, ODN injections were administered every other day, for a total of 15 injections. (B) Although devoid of a significant effect on nociceptive threshold, the AS ODN treatment directed against β2-AR produced significant attenuation of the prolongation of eccentric exercise hyperalgesia produced by sound stress. Indeed, two-way ANOVA showed significant effects for treatment (F1,10 = 53.98, P = 0.0001), time (F9,90 = 331.79, P < 0.0001), and interaction (F9,90 = 10.12, P < 0.0001). The Bonferroni multiple-comparisons test revealed significant differences between AS ODN and MM ODN treated rats from day 2 to day 20 after eccentric exercise. *P < 0.05; **P < 0.01; ***P < 0.001. ANOVA, analysis of variance.
4. Discussion
Although it is well established that psychological stress plays an important role in the induction and aggravation of chronic musculoskeletal pain,41,54 the underlying mechanism remains to be elucidated. Furthermore, it is unclear how exposure to stressful psychological stimuli leads to muscle nociceptor mechanical sensitization.11,26 Therefore, the finding that the sympathoadrenal mediator epinephrine, acting at 2 adrenergic receptors on nociceptors, plays a central role in the prolongation of exercise-induced muscle pain produced by unpredictable stress provides insight into how stress can aggravate musculoskeletal pain.
4.1. Stress disrupts recovery of muscle nociceptive threshold after eccentric exercise
We have previously shown that rats submitted to a protocol of eccentric exercise display mechanical muscle hyperalgesia, with a return to baseline nociceptive threshold within 5 days.2,4 In the present experiment, we observed that this recovery was delayed by previous exposure to an intense stressor, unpredictable sound stress. The enhancement of muscle pain by stress was, however, rather limited to its recovery phase. Indeed, the main difference in muscle pain between rats submitted to sound stress and control rats started after the peak of hyperalgesia, and persisted for over 2 weeks after eccentric exercise, indicating that sound stress selectively affects the capacity to recover from the hyperalgesia induced by eccentric exercise, ie, stress affects physical resilience. Of note, sound stress does not, by itself, affect nociceptive threshold in the gastrocnemius muscle.3,33 These observations are consistent with reports in human volunteers where previous or concomitant exposure to stress aggravates muscle soreness observed after eccentric exercise or sport activities.27,60 For example, high levels of chronic stress, before pain onset, prolongs muscle pain after eccentric exercise in previously pain-free volunteers.27 Chronic psychological stress also impairs recovery from muscle soreness and produces increased fatigue, after a 4-day period of strenuous resistance exercise.60 Our previous observations in a rat model of early-life stress shows that subsequent exposure to unpredictable sound stress exacerbates ongoing muscle pain.3 Together, these findings support the suggestion that psychological stress is able to aggravate muscle pain, regardless of whether the exposure to stressful stimuli occurs before or after pain onset.
4.2. Stress level of epinephrine is necessary and sufficient to prolong muscle hyperalgesia after eccentric exercise
Adrenal medullectomy attenuated the sound stress-induced prolongation of muscle hyperalgesia, indicating that sympathoadrenal response is necessary for stress-induced impairment of recovery from eccentric exercise-induced hyperalgesia. And, the administration of epinephrine at stress levels mimicked the effect of sound stress, confirming that adrenal medulla-derived epinephrine is sufficient to produce the effect of sound stress. Indeed, systemic epinephrine induced 15% to 25% decrease in mechanical nociceptive threshold between days 5 and 20 after eccentric exercise, whereas complete return to baseline nociceptive threshold occurs at day 5 in normal control rats.
Our observations are in line with previous finding that sound stress produces persistently increased levels of sympathoadrenal catecholamines, including epinephrine.32,33 Of note, alone the excision of the adrenal medulla does not modify muscle, or cutaneous mechanical nociceptive threshold3,33 or, as shown by the present results, the response to eccentric exercise. These observations indicate that the effect of adrenal medullectomy is not due to a change in baseline nociceptive threshold but due to its enhancement by sound stress. Although we did not explore the source of the stress-induced prolongation of hyperalgesia that is resistant to adrenal medullectomy, the contribution of extra-adrenal sources of catecholamines might play a role. Indeed, sympathetic postganglionic neurons contribute to sound stress-induced hyperalgesia, as revealed by its attenuation after chemical or surgical sympathectomy, or systemic administration of an α − 1 adrenergic receptor antagonist.20
Besides its direct actions on nociceptors, catecholamines enhance the release of a number of proalgesic cytokines from mast cells13 and lymphocytes,53 cell types that play a central role in the induction and recovery of skeletal muscle injury.61 Indeed, a transient rise in catecholamine is observed after strenuous exercise, believed to act on the β2-AR expressed by skeletal muscle fibers, contributing to tissue regeneration.7,50 Furthermore, ex vivo exposure of skeletal muscle tissue to epinephrine induces the release interleukin-6,40 a well-established proalgesic mediator,18,39 which contributes to eccentric exercise-induced muscle pain.4 Thus, although our results suggest that nociceptors are prominent targets of sympathoadrenal catecholamines (see below), we cannot rule out the contribution of other players in the prolongation of muscle pain induced by sound stress.
4.3. Nociceptor β2-AR receptors are necessary for prolonged muscle hyperalgesia after eccentric exercise
Consistent with a role of nociceptor β2-AR, its knockdown by intrathecal antisense attenuated the prolonged muscle hyperalgesia after eccentric exercise. We have previously shown that this antisense sequence, administered intrathecally, produced a marked decrease in β2-AR in peripheral nerves19 and antinociceptive effects in stress-dependent pain.19,22 Because only sensory neurons were exposed to both antisense treatment and circulating catecholamines, nociceptor β2-ARs likely play a central role in the prolongation of muscle pain produced by sound stress. Sensory neurons express β2-AR45 and epinephrine induces nociceptor excitation and sensitization.1,12,35 The involvement of nociceptor β2-AR in visceral mechanical hyperalgesia after exposure to stressful stimuli has also been reported.48,63,64 These observations, and the fact that catecholamines do not cross the blood–brain barrier,37 further support the fact that the proalgesic effect of epinephrine observed here depends on peripheral targets such as nociceptors.
The finding that β2-AR antisense did not completely abolish the prolongation of exercise-induced hyperalgesia may be due to a number of factors. For instance, it has been observed in many physiological settings that glucocorticoids enhance the β2-AR expression.15 Indeed, in vitro exposure of colonic nociceptors to epinephrine and corticosterone produced upregulation of β2-AR, concomitant with enhanced nociceptor excitability.48 Given that the pronociceptive effect of exposure to unpredictable sound stress also involves the activation of the hypothalamic–pituitary–adrenal axis and the release of corticosterone,32 enhanced nociceptor expression of β2-AR in this model is possible, which could have limited the efficacy of antisense. Finally, the involvement of peripheral β1- and β3-AR has also been reported in preclinical studies where pain is associated with increased levels of catecholamines.14
4.4. Nonnociceptor mechanisms in the prolongation of exercise-induced muscle pain by sound stress
Although our findings point to a contribution of nociceptors in the prolongation of eccentric exercise-induced pain by sound stress, additional mechanisms may also be involved. For instance, a contribution of dysfunctional descending pain modulatory controls cannot be excluded. Indeed, we,21 and others,29 have demonstrated that exposure to repeated stress markedly attenuates the antinociceptive capacity of endogenous inhibitory pain circuits. Furthermore, repeated stress activates GABAergic neurons of the rostral ventromedial medulla that inhibit opioidergic/GABAergic neurons of the dorsal horn, acting as descending pain facilitatory (ie, pronociceptive) controls, selectively enhancing mechanical nociception.23 Moreover, clinical studies have shown attenuated diffuse noxious inhibitory controls in fibromyalgia patients who experience chronic widespread pain.30,47 And, peripheral sensitization induced by muscle contraction interacts with abnormal descending pain modulation leading to pain facilitation in fibromyalgia patients.25 Together, these observations support the suggestion that repeated stress also contributes to the prolongation of hyperalgesia induced by eccentric exercise, by disrupting normal function of supraspinal modulatory pain controls.
4.5. Stress disrupts the capacity of nociceptors to sense physical resilience status
Nociceptors are sensitized by signals generated in damaged tissue, observed as hyperalgesia, providing a motivational drive for adaptive/protective behaviors. We have demonstrated active suppression of the hyperalgesia induced by eccentric exercise,2 well before completion of structural recovery,6 allowing for resumption of physical activity. Thus, muscle nociceptors are posited to act as sensors of functional recovery after strenuous exercise.2 Indeed, a sizeable amount of evidence shows that nociceptors are sensitized by psychological stress.11,26,48,63,64 Importantly, nociceptors express a number of receptors that allow for detection of neuroendocrine stress axes signals, including by glucocorticoid19,32 and β2-adrenergic19,45 receptors. Also, as mentioned above, catecholamines can act on a number of cell types involved in skeletal muscle damage and repair, modulating their capacity to release cytokines that could modify nociceptor excitability. Thus, consistent with our previous observations,33 we postulate that persistent sympathoadrenal axis activation, by unpredictable sound stress, induces modifications in nociceptor excitability, and in the cytokine signature of the damaged tissue, likely disrupting the role of nociceptors as sensors of the status of functional recovery, potentially contributing to pain chronification.
4.6. Clinical considerations
Although pharmacological treatments provide only modest relief for chronic pain, including chronic musculoskeletal pain,28 therapeutic exercise has shown some promise. Aggravation of ongoing muscle pain by exercise can, however, limit its clinical usefulness.46 Our findings indicate that stress is likely detrimental to the success of exercise-based therapy. Thus, cognitive and behavioral treatments might be beneficial to minimize the pain induced by therapeutic exercise, underscoring the well-established importance of a multidisciplinary approach for the treatment of chronic musculoskeletal pain.
4.7. Conclusion
Exposure to unpredictable sound stress produces a long-lasting prolongation of the muscle hyperalgesia produced by eccentric exercise. This sympathoadrenal-dependent phenomenon involves β2-AR on the primary afferent nociceptor. Together, these findings suggest that stress can disrupt the capacity of nociceptors to sense tissue recovery after strenuous exercise, providing further insight into the mechanisms underlying chronic musculoskeletal pain.
Disclosures
The authors have no conflict of interest to declare.
This work was supported National Institutes of Health grant AR063312.
Acknowledgments
The authors thank Dr Oliver Bogen for technical help.
Author contributions: P. Alvarez designed and performed experiments, analyzed data, and wrote the manuscript; P.G. Green performed experiments and analyzed data; J.D. Levine analyzed experiments and supervised the study. All authors read and approved the manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 2001;21:6933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alvarez P, Bogen O, Green PG, Levine JD. Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. PAIN 2017;158:1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alvarez P, Green PG, Levine JD. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiatry 2013;74:688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci 2010;32:819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alvarez P, Levine JD, Green PG. Neonatal handling (resilience) attenuates water-avoidance stress induced enhancement of chronic mechanical hyperalgesia in the rat. Neurosci Lett 2015;591:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 1983;54:80–93. [DOI] [PubMed] [Google Scholar]
- [7].Beitzel F, Gregorevic P, Ryall JG, Plant DR, Sillence MN, Lynch GS. Beta2-adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J Appl Physiol (1985) 2004;96:1385–92. [DOI] [PubMed] [Google Scholar]
- [8].Borsa PA, Parr JJ, Wallace MR, Wu SS, Dai Y, Fillingim RB, George SZ. Genetic and psychological factors interact to predict physical impairment phenotypes following exercise-induced shoulder injury. J Pain Res 2018;11:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS, Holbrook D, Rathlev NK, Foley KA, Lee DC, Collette R, Domeier RM, Hendry PL, McLean SA. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. PAIN 2013;154:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Celik S, Celik K, Dirimese E, Taşdemir N, Arik T, Büyükkara İ. Determination of pain in musculoskeletal system reported by office workers and the pain risk factors. Int J Occup Med Environ Health 2018;31:91–111. [DOI] [PubMed] [Google Scholar]
- [11].Chen X, Green PG, Levine JD. Stress enhances muscle nociceptor activity in the rat. Neuroscience 2011;185:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen X, Levine JD. Epinephrine-induced excitation and sensitization of rat C-fiber nociceptors. J Pain 2005;6:439–46. [DOI] [PubMed] [Google Scholar]
- [13].Chi DS, Fitzgerald SM, Pitts S, Cantor K, King E, Lee SA, Huang SK, Krishnaswamy G. MAPK-dependent regulation of IL-1- and beta-adrenoreceptor-induced inflammatory cytokine production from mast cells: implications for the stress response. BMC Immunol 2004;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ciszek BP, O'Buckley SC, Nackley AG. Persistent catechol-O-methyltransferase-dependent pain is initiated by peripheral β-adrenergic receptors. Anesthesiology 2016;124:1122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cornett LE, Hiller FC, Jacobi SE, Cao W, McGraw DW. Identification of a glucocorticoid response element in the rat beta2-adrenergic receptor gene. Mol Pharmacol 1998;54:1016–23. [DOI] [PubMed] [Google Scholar]
- [16].Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins TJ, Sama S, Belfer I, Goldman D, Max MB, Weir BS, Maixner W. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet 2006;141B:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol 2013;9:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience 2008;152:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci 2008;27:83–92. [DOI] [PubMed] [Google Scholar]
- [20].Donello JE, Guan Y, Tian M, Cheevers CV, Alcantara M, Cabrera S, Raja SN, Gil DW. A peripheral adrenoceptor-mediated sympathetic mechanism can transform stress-induced analgesia into hyperalgesia. Anesthesiology 2011;114:1403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ferrari LF, Gear RW, Levine JD. Attenuation of activity in an endogenous analgesia circuit by ongoing pain in the rat. J Neurosci 2010;30:13699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferrari LF, Levine E, Levine JD. Independent contributions of alcohol and stress axis hormones to painful peripheral neuropathy. Neuroscience 2013;228:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 2017;93:822–39.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- [25].Ge HY, Nie H, Graven-Nielsen T, Danneskiold-Samsøe B, Arendt-Nielsen L. Descending pain modulation and its interaction with peripheral sensitization following sustained isometric muscle contraction in fibromyalgia. Eur J Pain 2012;16:196–203. [DOI] [PubMed] [Google Scholar]
- [26].Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. PAIN 2011;152:2549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hannibal K, Bishop M. (Abstract 318) Chronic stress, prior to pain onset, and persistent centrally-mediated pain sensitivity following an acute episode of delayed-onset muscle soreness. J Pain 2015;16:S55. [Google Scholar]
- [28].Häuser W, Walitt B, Fitzcharles MA, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther 2014;16:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Itomi Y, Tsukimi Y, Kawamura T. Impaired diffuse noxious inhibitory controls in specific alternation of rhythm in temperature-stressed rats. Eur J Pharmacol 2016;784:61–8. [DOI] [PubMed] [Google Scholar]
- [30].Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. PAIN 2005;114:295–302. [DOI] [PubMed] [Google Scholar]
- [31].Kano Y, Sampei K, Matsudo H. Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol Scand 2004;180:291–9. [DOI] [PubMed] [Google Scholar]
- [32].Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci 2008;28:5721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain 2009;10:1073–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. PAIN 2005;116:79–86. [DOI] [PubMed] [Google Scholar]
- [35].Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol 1999;81:1104–12. [DOI] [PubMed] [Google Scholar]
- [36].Kim YM, Cho SI. Work-life imbalance and musculoskeletal disorders among south Korean workers. Int J Environ Res Public Health 2017;14:1331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kostrzewa RM. The blood-brain barrier for catecholamines—revisited. Neurotox Res 2007;11:261–71. [DOI] [PubMed] [Google Scholar]
- [38].Malmberg-Ceder K, Haanpää M, Korhonen PE, Kautiainen H, Soinila S. Relationship of musculoskeletal pain and well-being at work—does pain matter. Scand J Pain 2017;15:38–43. [DOI] [PubMed] [Google Scholar]
- [39].Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. PAIN 2010;151:345–55. [DOI] [PubMed] [Google Scholar]
- [40].Mattingly AJ, Laitano O, Clanton TL. Epinephrine stimulates CXCL1 IL-1. Physiol Rep 20175:e13519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 2007;21:403–25. [DOI] [PubMed] [Google Scholar]
- [42].McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain 2011;12:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 1994;32:197–200. [DOI] [PubMed] [Google Scholar]
- [44].Miao FJ, Benowitz NL, Basbaum AI, Levine JD. Sympathoadrenal contribution to nicotinic and muscarinic modulation of bradykinin-induced plasma extravasation in the knee joint of the rat. J Pharmacol Exp Ther 1992;262:889–95. [PubMed] [Google Scholar]
- [45].Nicholson R, Dixon AK, Spanswick D, Lee K. Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci Lett 2005;380:316–21. [DOI] [PubMed] [Google Scholar]
- [46].Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012;15:ES205–13. [PubMed] [Google Scholar]
- [47].Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. J Clin Psychiatry 2011;72:219–24. [DOI] [PubMed] [Google Scholar]
- [48].Ochoa-Cortes F, Guerrero-Alba R, Valdez-Morales EE, Spreadbury I, Barajas-Lopez C, Castro M, Bertrand J, Cenac N, Vergnolle N, Vanner SJ. Chronic stress mediators act synergistically on colonic nociceptive mouse dorsal root ganglia neurons to increase excitability. Neurogastroenterol Motil 2014;26:334–45. [DOI] [PubMed] [Google Scholar]
- [49].Østerås B, Sigmundsson H, Haga M. Perceived stress and musculoskeletal pain are prevalent and significantly associated in adolescents: an epidemiological cross-sectional study. BMC Public Health 2015;15:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Silva MT, Wensing LA, Brum PC, Câmara NO, Miyabara EH. Impaired structural and functional regeneration of skeletal muscles from β2-adrenoceptor knockout mice. Acta Physiol (Oxf) 2014;211:617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Singh VB, Corley KC, Phan TH, Boadle-Biber MC. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Res 1990;516:66–76. [DOI] [PubMed] [Google Scholar]
- [52].Skouen JS, Smith AJ, Warrington NM, O' Sullivan PB, McKenzie L, Pennell CE, Straker LM. Genetic variation in the beta-2 adrenergic receptor is associated with chronic musculoskeletal complaints in adolescents. Eur J Pain 2012;16:1232–42. [DOI] [PubMed] [Google Scholar]
- [53].Slota C, Shi A, Chen G, Bevans M, Weng NP. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun 2015;46:168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Staud R. Is it all central sensitization? Role of peripheral tissue nociception in chronic musculoskeletal pain. Curr Rheumatol Rep 2010;12:448–54. [DOI] [PubMed] [Google Scholar]
- [56].Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. PAIN 2009;145:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Staud R, Robinson ME, Weyl EE, Price DD. Pain variability in fibromyalgia is related to activity and rest: role of peripheral tissue impulse input. J Pain 2010;11:1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Staud R, Weyl EE, Bartley E, Price DD, Robinson ME. Analgesic and anti-hyperalgesic effects of muscle injections with lidocaine or saline in patients with fibromyalgia syndrome. Eur J Pain 2014;18:803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci 2003;17:805–12. [DOI] [PubMed] [Google Scholar]
- [60].Stults-Kolehmainen MA, Bartholomew JB, Sinha R. Chronic psychological stress impairs recovery of muscular function and somatic sensations over a 96-hour period. J Strength Cond Res 2014;28:2007–17. [DOI] [PubMed] [Google Scholar]
- [61].Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol 2011;1:2029–62. [DOI] [PubMed] [Google Scholar]
- [62].Wilkinson CW, Shinsako J, Dallman MF. Return of pituitary-adrenal function after adrenal enucleation or transplantation: diurnal rhythms and responses to ether. Endocrinology 1981;109:162–9. [DOI] [PubMed] [Google Scholar]
- [63].Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 2010;138:294–304.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang C, Rui YY, Zhou YY, Ju Z, Zhang HH, Hu CY, Xiao Y, Xu GY. Adrenergic β2-receptors mediates visceral hypersensitivity induced by heterotypic intermittent stress in rats. PLoS One 2014;9:e94726. [DOI] [PMC free article] [PubMed] [Google Scholar]


