Abstract
Background:
This study aimed to explore the effect of YHJD (Yiqi Huayu Jiedu decoction) in patients with stages II and III gastric cancer.
Methods:
A multicenter, prospective, cohort study was conducted in Jiangsu Province Hospital of Traditional Chinese Medicine, Jiangsu Cancer Hospital, Nanjing Drum Tower Hospital, People's Liberation Army Bayi Hospital, Changzhou Traditional Chinese Medicine Hospital, Changzhou Tumor Hospital, Traditional Chinese Medicine Hospital of Kunshan, Yangzhou Hospital of Traditional Chinese Medicine, and Yixing Tumor Hospital. A total of 489 patients with stage II or III gastric cancer were enrolled after radical gastrectomy. Among them, 238 were included in the chemotherapy group (received chemotherapy alone) and 251 in the YHJD group (received chemotherapy combined with YHJD). The DFS (disease-free survival) rate, 5-year survival rate, quality of life, and traditional Chinese medicine (TCM) symptoms of the 2 groups were compared.
Results:
The DFS curve of the YHJD group was higher than that of the chemotherapy group (P = .0042). The HR (hazard ratio) was 0.672, and its corresponding 95% CI (confidence interval) was 0.511 to 0.884. For stage II patients, the P value was .8323, which indicated that the difference was not significant. The risk HR was 0.938, and the corresponding 95% CI was 0.521 to 1.689. For stage III patients, the P value was .0072, indicating a statistically significant difference. The HR was 0.653, and the corresponding 95% CI was 0.477 to 0.893. The 5-year survival rate of the YHJD group was 85.29%, which was higher than that of the chemotherapy group (71.05%). Compared with the chemotherapy group, the YHJD group had better quality of life and lower TCM symptom scores.
Conclusion:
YHJD decoction is effective in improving DFS rate in patients with gastric cancer stage III after radical gastrectomy. Moreover, it can reduce the risk of recurrence and metastasis and improve the quality of life in patients with gastric cancer stage II or III after radical gastrectomy.
Keywords: cohort trial, gastric cancer, traditional Chinese medicine, Yiqi Huayu Jiedu decoction
1. Introduction
The techniques used in gastric cancer resection and chemotherapy have significantly improved; moreover, some new agents have demonstrated efficacy against gastric cancer.[1,2] However, the long-term survival rate of gastric cancer patients has not significantly improved, and the tumor recurrence and metastasis is the primary cause of death. Clinical data showed that gastric cancer recurrence and metastasis commonly occur 2 years after radical gastrectomy, with a total incidence rate of >70%.[3] Decreasing this incidence remains a hot and difficult issue in medical research.
In China, Traditional Chinese medicine (TCM) plays an important role in the treatment of gastric cancer, and the efficacy and safety of TCM have been widely reported.[4,5] However, the clinical data of large samples and results of long-term follow-up are still lacking.
The YHJD, which was made according to the TCM theory and by Professor Liu Shenlin from Jiangsu Province Hospital of Traditional Chinese Medicine, has demonstrated good clinical efficacy.[6] In order to provide reliable statistical evidence, from 2009 to 2016, the Jiangsu Province Hospital of TCM combined with other 8 hospitals conducted a multicenter, large sample clinical trial to determine whether chemotherapy combined with TCM treatment, using YHJD, had better curative effect than chemotherapy alone.
2. Clinical information
2.1. General information
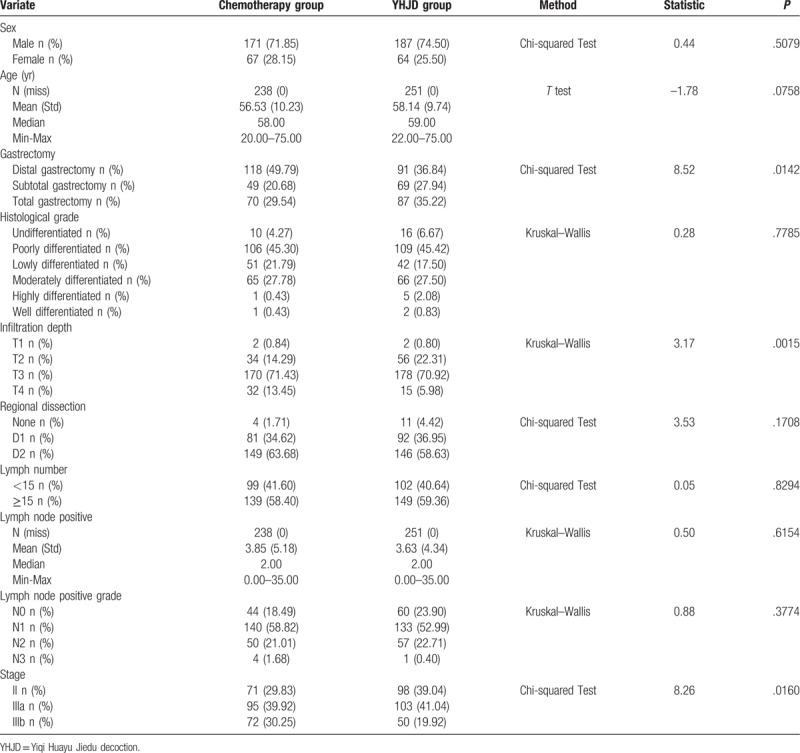
From August 2008 to September 2013, 489 patients with stage II or III gastric cancer treated in Jiangsu Province Hospital of Traditional Chinese Medicine, Jiangsu Cancer Hospital, Nanjing Drum Tower Hospital, People's Liberation Army Bayi Hospital, Changzhou Traditional Chinese Medicine Hospital, Changzhou Tumor Hospital, Traditional Chinese Medicine Hospital of Kunshan, Yangzhou Hospital of Traditional Chinese Medicine, and Yixing Tumor Hospital were divided in to the following groups: YHJD group (received chemotherapy combined with YHJD) and chemotherapy group (received chemotherapy alone). As shown in Table 1, the YHJD group consisted of 251 patients, of whom 187 were men and 64 were women, with a median age of 59 years (range: 22–75 years); in this group, 98 (39.04%) had stage II gastric cancer, while 153 (60.96%) had stage III. Meanwhile, the chemotherapy group consisted of 238 patients, of whom 171 were men and 67 were women, with a median age of 58 years (range: 20–75 years). In this group, 71 (29.83%) had stage II gastric cancer, while 167 (70.17%) had stage III. There were statistically significant differences between the 2 groups in terms of surgical method (P = .0142), infiltration depth (P = .0015), and stage (P = .0160), but no statistically significant differences were observed in other variables between the 2 groups (P > .05).
Table 1.
Baseline comparison of basic information between chemotherapy group and YHJD group.
2.2. Diagnostic criteria
The diagnostic criteria for gastric cancer and staging criteria were based on the “NCCN (National Comprehensive Cancer Network) Guideline for Gastric Cancer”.[7]
2.3. Inclusion criteria
Patients aged 18 years or older but younger than 75 years; with pathologically confirmed gastric cancer; with stage II or III gastric cancer and had undergone curative surgery; whose TCM syndrome differentiation is mainly spleen deficiency; with qi stagnation or cold deficiency, stomach yin deficiency, spleen yang failure, or liver and stomach irritability[8]; with a Karnofsky performance status score of 60 points or more; with expected survival of more than 6 months; and had signed an informed consent form were included.
2.4. Exclusion criteria
Patients with metastatic gastric cancer; whose histopathological or radiological examination results showed local recurrence or distant metastasis; who are pregnant or breastfeeding; had severe primary cardiovascular disease, liver disease, kidney disease, or hematological disorders; with disabilities (blind, deaf, dumb, mental retardation, mental disorders, and physical disabilities); with suspected or history of alcohol or drug abuse; with allergies to 2 or more medications or food allergies; and who are allergic to the ingredients of YHJD were excluded.
2.5. Statement of ethical standards
All procedures were performed in accordance with the standards of the Ethical Committee of Jiangsu Province Hospital of Traditional Chinese Medicine (IRB number: 2009NL-022-01) on human experimentation and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients. The identifying information of all included patients will not be published.
3. Treatment method
3.1. Chemotherapy group
This group consisted of patients who received chemotherapy alone after undergoing radical surgery.
3.1.1. Chemotherapy
Chemotherapy doses administered were based on the “NCCN Clinical Practice Guide for Gastric Cancer”.[7]
-
(1)
DCF regimen: Docetaxel 75 mg/m2, intravenous (iv), day 1, every 21 days; oxaliplatin, 160 mg/m2, iv, day 1, every 21 days; 5-Fu 750 mg/(m2/day), 24-hour continuous intravenous drip, days 1 to 5, every 21 days; administered in 4 to 6 cycles.
-
(2)
FOLFOX4 regimen: Oxaliplatin 85 to 100 mg/(m2/day), iv every 2 hours, day 1, every 21 days; leucovorin 200 mg/(m2/day), iv every 2 hours, days 1 to 2, every 21 days; 5-Fu 400 mg/m2, intravenous injection, days 1 to 2, every 21 days; 5-Fu 600 mg/(m2/day), 22 hours continuous intravenous drip, days 1 to 2, every 21 days; administered in 4 to 6 cycles.
3.2. YHJD group
This group consisted of patients who received chemotherapy combined with TCM after undergoing radical surgery. The chemotherapy regimen employed in this group was the same as that of the chemotherapy group.
3.2.1. TCM treatment methods
The basic TCM decoction was YHJD. The hospitals have standards for purchasing Chinese medicines and a unified preparing process. The following ingredients were used to create the decoction: Astragalus membranaceus, 15 g; Codonopsis pilosula, 15 g; Atractylodes macrocephala, 10 g; Angelica, 10 g; Radices paeoniae alba, 10 g; Pericarpium citri reticulatae, 6 g; Pinellia, 10 g; Rhizoma sparganii, 10 g; Curcuma zedoary, 10 g; Chinese sage herb, 30 g; Hedyotis diffusa, 30 g; and Radix glycyrrhizae preparata, 5 g. Supplement (based on syndrome differentiation[8]): In order to treat qi deficiency and stagnation and abdominal distension, 10 g of Caulis perillae and 10 g of Fructus aurantii are added; for cold deficiency and stomach pain, 5 g of Cassia twig and 5 g of Galangal; for stomach yin deficiency and burning pain, 15 g of Radix glehniae and 12 g of Radix ophiopogonis; for problems with spleen yang transportation and loose stool, 3 g of Blast-fried ginger and 5 g of Nutmeg; for liver and stomach qi stagnation and vomiting and acid regurgitation, 3 g of Coptis chinensis, 2 g of Evodia rutaecarpa, and 30 g of Calcined concha arcae. The abovementioned medicines were used to prepare a 400-ml decoction.
3.3. Dosage method and course of treatment
Chemotherapy was administered after surgery. In combination with or after chemotherapy, Chinese medicine was administered in the YHJD group, based on the syndrome differentiation, 2 times per day, at a dose of 200 mL, for more than 6 months.
4. Curative effect observation
4.1. Curative effect index
The DFS rate, 5-year survival rate, quality of life scores, and TCM symptom scores of the 2 groups were compared.
4.2. Evaluation method of curative effect
Judgement of recurrence and metastasis: Gastroscopy and pathological examinations were performed to determine whether local recurrence occurred; chest and abdominal computed tomography (or ultrasound), ECT (emission computed tomography) or local tissue biopsy, and other examinations were performed to determine whether distant metastasis occurred.
Judgement of quality of life: EORTC QLQ-C30 was used to assess patients’ quality of life.[9]
Judgement of TCM symptoms: TCM symptoms were rated according to the “Guiding Principles for Clinical Research of New TCM Drugs”.[10]
4.3. Following up method
Patients were followed up every 3 weeks for the first 18 weeks after enrollment, and every 6 weeks after 18 weeks until recurrence or metastasis. This was a follow-up study; hence, some patients may be lost to follow-up, and the incomplete data were referred to as tail data. For this purpose, the exact date of death should be carefully recorded. For patients who withdrew early from the study, the visit week recorded in their last case report was adjusted. For example, the last normal follow-up date was week 6, and the maximum visit time of the case was recorded as 6+ weeks if the patient could not be traced at week 9.
4.4. Data processing method
Numerical variables were expressed as mean, standard deviation, median, and ranges. Categorical variables were expressed as counts and proportions.
Inter-group comparison of numerical variables: If the variables were normally distributed, a t test is used to compare the 2 groups, while a paired t test is used to compare the 2 groups before and after treatment. If variables were not normally distributed, Mann–Whitney U test (Wilcoxon rank-sum test) is used, and Wilcoxon signed-rank test would be used to compare the two groups before and after treatment.
Inter-group comparison of categorical or ordinal variables: Binary variables were compared using a chi-square test for 2-by-2 contingency table or Fisher exact test, while a chi-square test for 2-by-m contingency table analysis and Kruskal–Wallis test were used to compare multicategorical variables.
The Kaplan–Meier method, also named Product Limit Method, was applied to compare the DFS of the 2 groups. Z test was used to compare the survival rate at each follow-up point, while Log-rank test was applied to provide an overall comparison of the 2 groups.
All statistical test results had test statistics and corresponding exact P values. All statistical tests were performed using a 2-tailed test with a .05 significance level. All statistical results were directly exported from SAS 9.1 (or above).
5. Treatment results
5.1. Comparison of DFS rates
The DFS curve of the YHJD group and the chemotherapy group were determined using the Kaplan–Meier method. As shown in Figure 1, during the study, 90 (36%) patients in the YHJD group developed recurrence, while 118 (50%) patients in the chemotherapy group had recurrence. The DFS curve of the YHJD group was higher than that of the chemotherapy group, and logarithmic rank test generated a P value of .0042, which indicates that the difference between the 2 groups was statistically significant. Using Cox regression, the hazard ratio (HR) was 0.672 (chi-square = 8.07, P = .0045), and the corresponding 95% CI was 0.511 to 0.884. The results showed that the recurrence rate of the YHJD group was only 0.672 times higher than that of the chemotherapy group.
Figure 1.
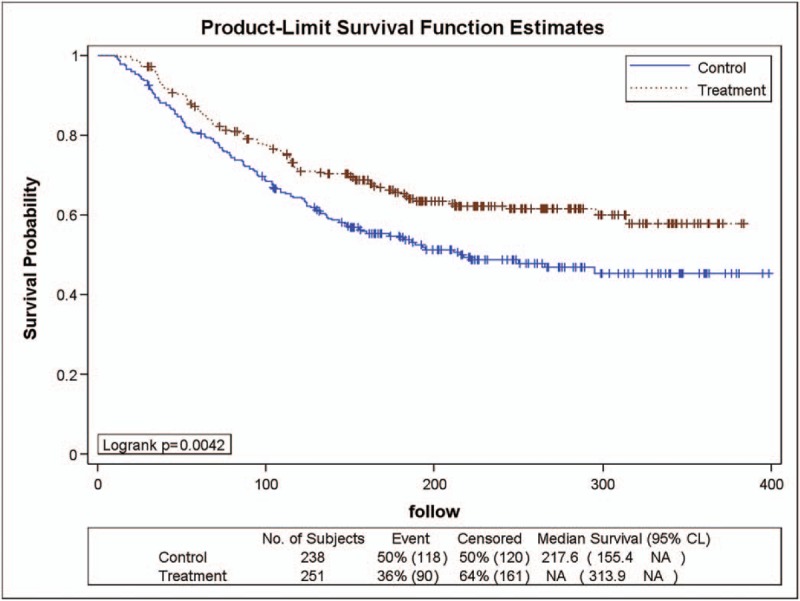
Product-limit survival function estimates in 2 groups.
As the proportion of stage II and III patients was inconsistent at baseline, the DFS rates of stage II and III patients were compared using a log-rank test.
As shown in Figure 2, 98 patients in the YHJD group had stage II gastric cancer, of whom 25 (26%) had recurrence. Of the 71 patients in the chemotherapy group, 20 (28%) had recurrence. The logarithmic rank test generated a P value of .8323, which indicated no significant difference. Cox regression analysis showed that the risk HR was 0.938 (chi-square = 0.05, P = .8316), and the corresponding 95% CI was 0.521 to 1.689, which indicated that the recurrence rate of the YHJD group was 0.938 times higher than that of the chemotherapy group, and the difference between the 2 groups was not significant.
Figure 2.
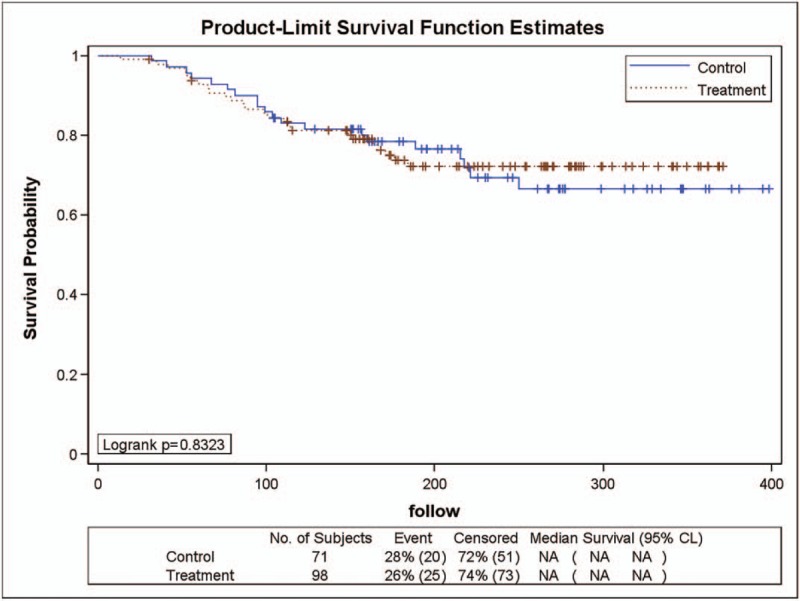
Product-limit survival function estimates for stage II patients.
As shown in Figure 3, 153 patients in the YHJD group had stage III gastric cancer, of whom 65 (42%) had recurrence. In the chemotherapy group, 167 patients had stage III gastric cancer, of whom 98 (59%) had recurrence. A logarithmic rank test was performed and revealed a P value of .0072, which indicated that there is a significant difference in DFS rate between the 2 groups. The HR was 0.653 (chi-square = 7.10, P = .0077) and the corresponding 95% CI was 0.477–0.893, indicating that the recurrence rate of the YHJD group was only 0.653 times higher than that of the chemotherapy group, and the difference was significant. The results showed that YHJD had effectively improved the DFS rate of gastric cancer patients with stage III after radical gastrectomy.
Figure 3.
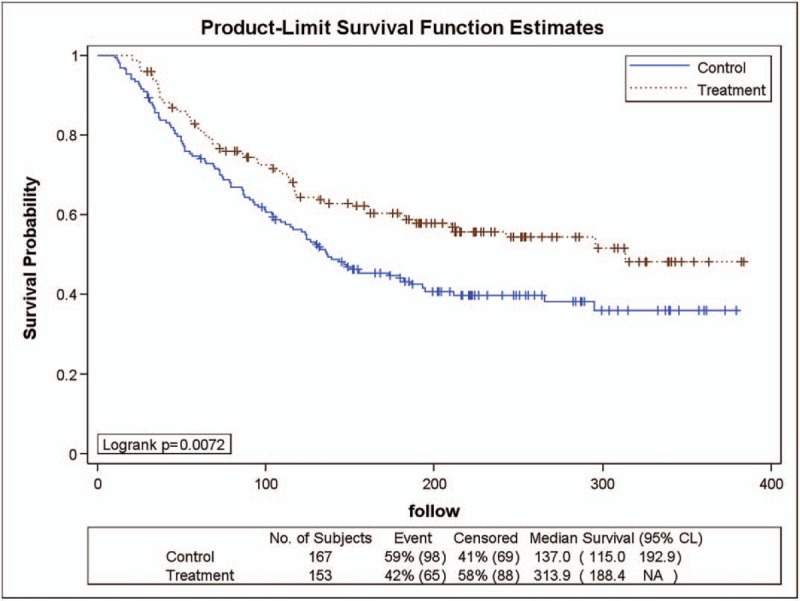
Product-limit survival function estimates for stage III patients.
As shown in Figure 4, 41 (40%) patients with stage IIIa gastric cancer in the YHJD group had recurrence. In the chemotherapy group, 56 (59%) patients developed recurrence. The DFS curve of the YHJD group was higher than that of the chemotherapy group, and the logarithmic rank test revealed a P value of .0319. Cox regression analysis showed that the risk HR was 0.646 (chi-square = 4.53, P = .0334), and its corresponding 95% CI was 0.431 to 0.966, indicating that the recurrence rate of the YHJD group was 0.646 times higher than that of the chemotherapy group, and the difference between the 2 groups was significant.
Figure 4.
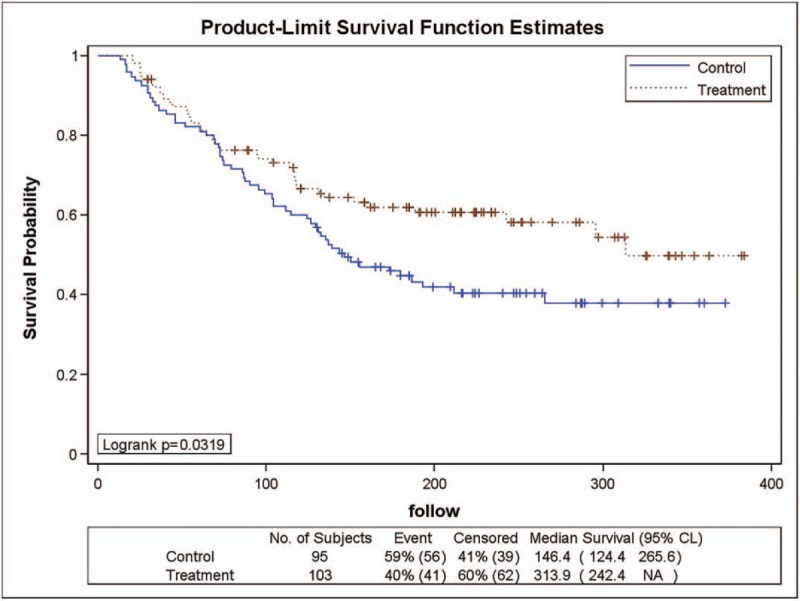
Product-limit survival function estimates for stage III a patients.
As shown in Figure 5, 50 patients in the YHJD group had stage IIIb gastric cancer, of whom 24 (48%) developed recurrence. Of the 72 patients in the chemotherapy group, 42 (58%) had recurrence. A logarithmic rank test was performed and revealed a P value of .1571, which indicated that there was no statistically significant difference between the 2 groups. Cox regression analysis showed that the risk HR was 0.697 (chi-square = 1.98, P = .1594), and the corresponding 95% CI was 0.422 to 1.152, indicating that the recurrence rate of the YHJD group was 0.697 times higher than that of the chemotherapy group, but the difference was not statistically significant.
Figure 5.
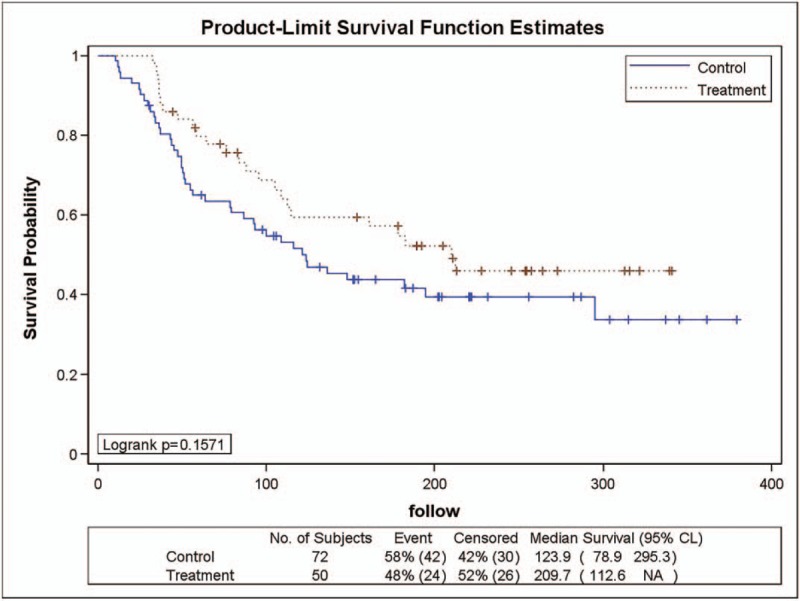
Product-limit survival function estimates for stage III b patients.
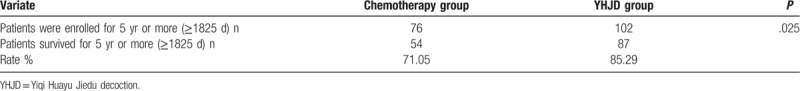
5.2. 5-year survival rates of the 2 groups
Five-year survival rate refers to the proportion of cancer patients that survive for more than 5 years after various comprehensive treatments. It is an index that doctors use to evaluate the effect of surgery and other treatments. As shown in Table 2, the 5-year survival rate was higher in the YHJD group (85.29%) than in the chemotherapy group (71.05%); as indicated by Fisher exact test (P = .025), the 5-year survival rates are statistically significantly different between 2 groups.
Table 2.
Comparison of 5-year survival rates between 2 groups.
5.3. Comparison of quality of life and TCM syndromes
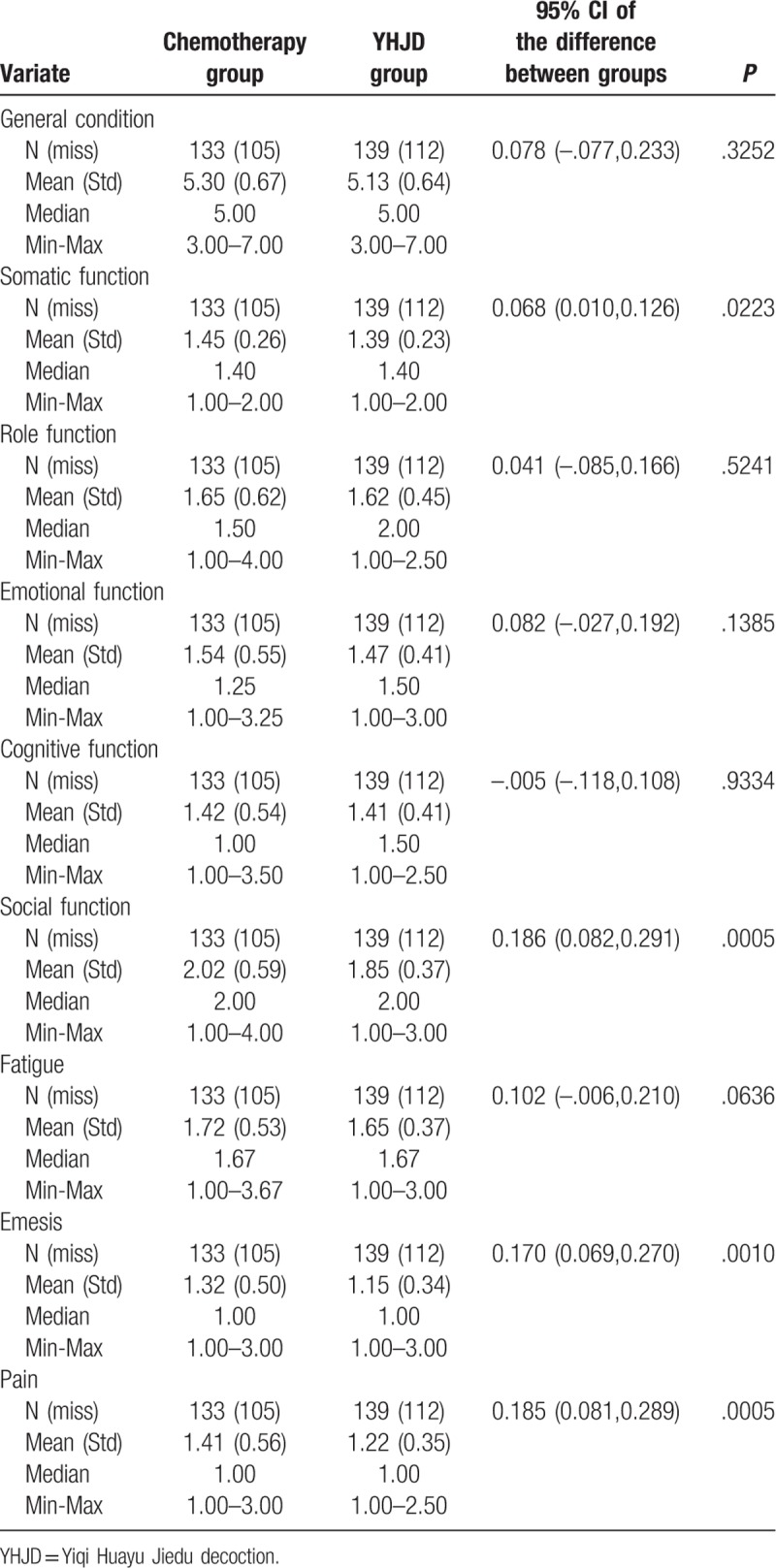
We determined the patients’ quality of life scores. A higher general condition score indicates improvement in the patients’ quality of life. If the patients garnered a higher score in other variables, it indicates a more serious problem. At the beginning of the trial, data showed that except general condition (P < .0001) and fatigue (P = .0020), no statistical difference was observed between the other variables (P > .05) (Table 3). The general condition score of the chemotherapy group was higher than that of the YHJD group, while the fatigue score of the chemotherapy group was lower than that of the YHJD group. After 18 weeks of treatment, significant differences were observed in the somatic function, social function, emesis, and pain between the two groups (P < .05) (Table 4), and the score of the YHJD group was lower than that of the chemotherapy group.
Table 3.
Baseline comparison of Quality of Life scores between 2 groups.
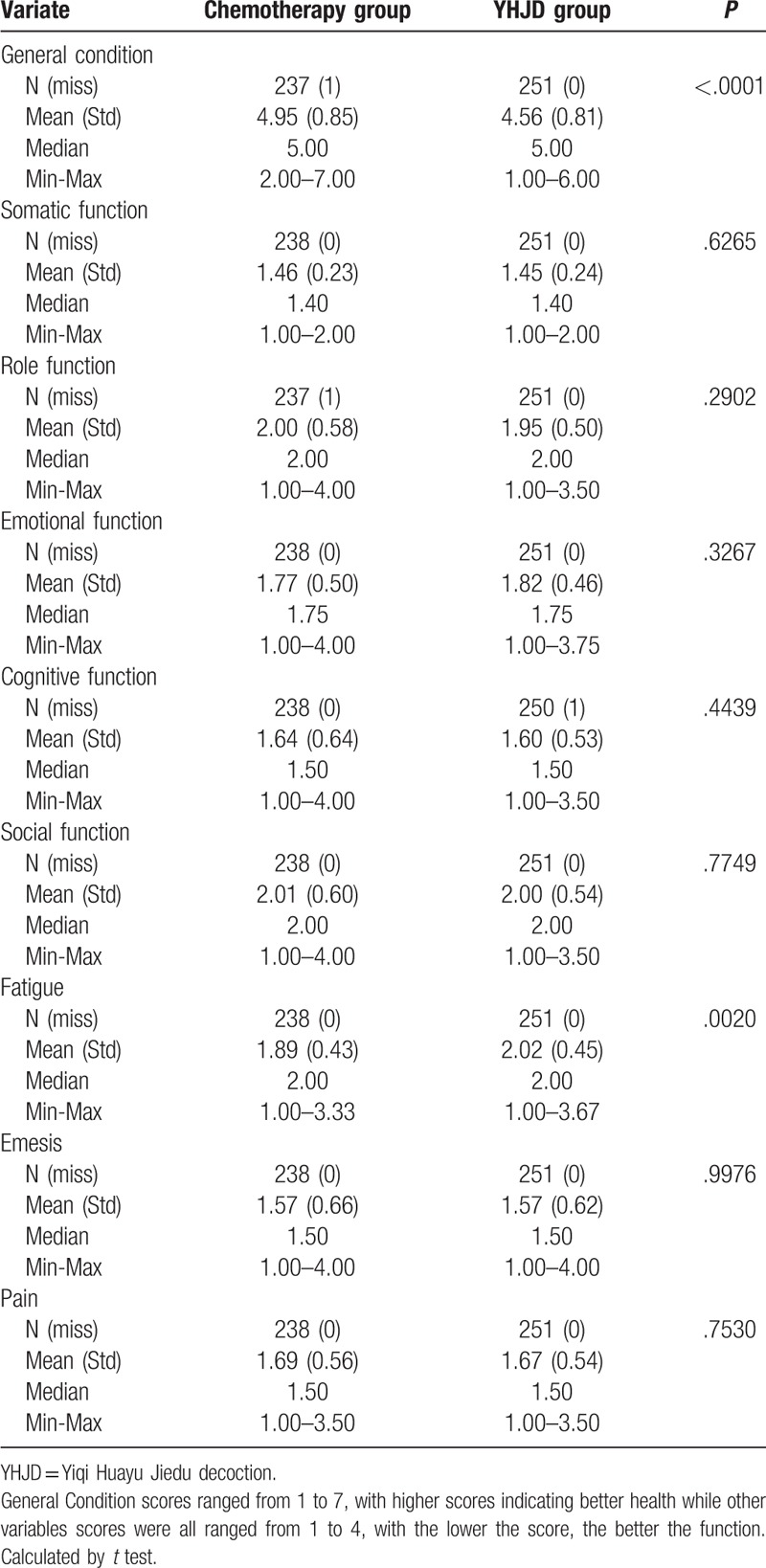
Table 4.
Comparison of Quality of Life scores between 2 groups after 18 weeks.
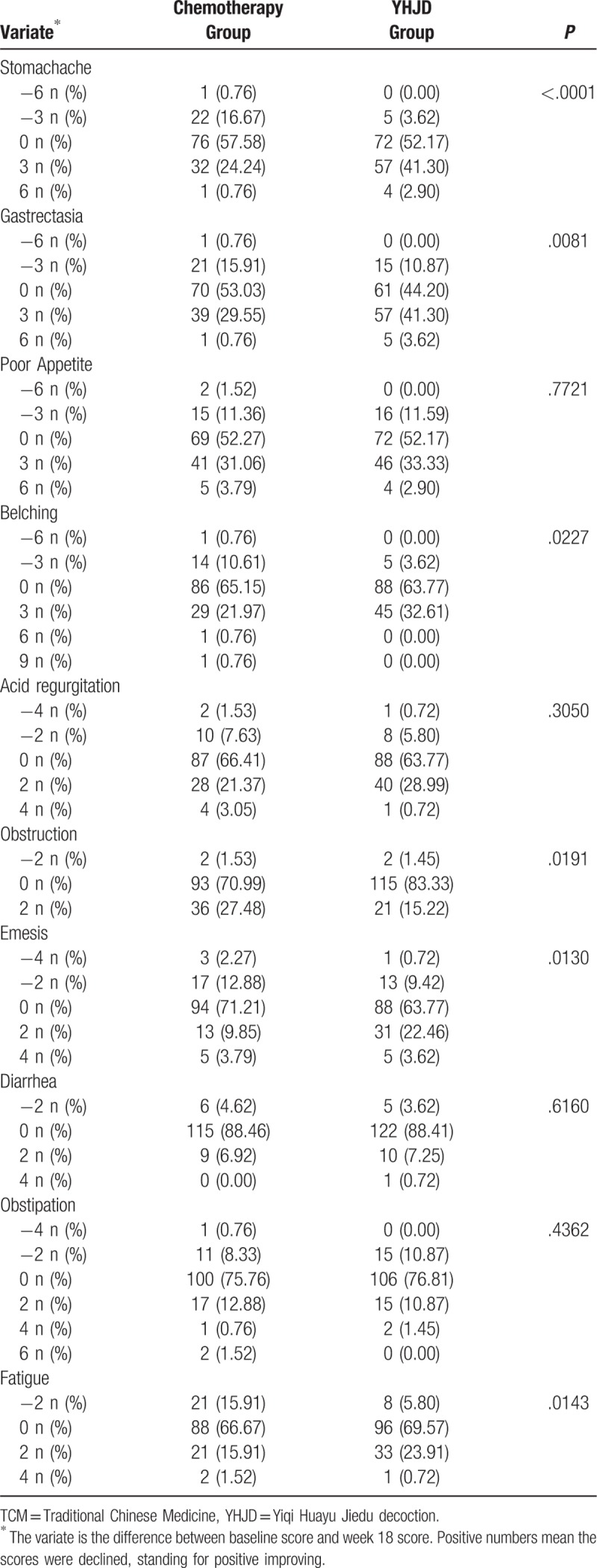
We used scores to assess the patients’ TCM symptoms. The higher the scores, the more severe the corresponding symptoms. As shown in Table 5, the results of the baseline comparison of TCM symptoms scores showed that the P values of the 6 variables (stomachache, gastrectasia, obstruction, emesis, and fatigue) were all less than .05, indicating that there were statistical differences among the 6 variables, while no statistical differences were found among the other variables (P > .05). Since the baseline scores of some variables were inconsistent, we determined the difference between the baseline score and the score after 18 weeks of treatment of each variable. The differences were then used to compare the 2 groups. After 18 weeks of treatment, significant differences were observed in the frequency of stomachache, gastrectasia, belching, obstruction, emesis, and fatigue (P < .05; Table 6), except obstruction, the positive differences of the YHJD group were higher than that of the chemotherapy group. However, there was no significant difference in the frequency of poor appetite, acid regurgitation, diarrhea, and obstipation (P > .05).
Table 5.
Baseline comparison of TCM syndromes scores between 2 groups.
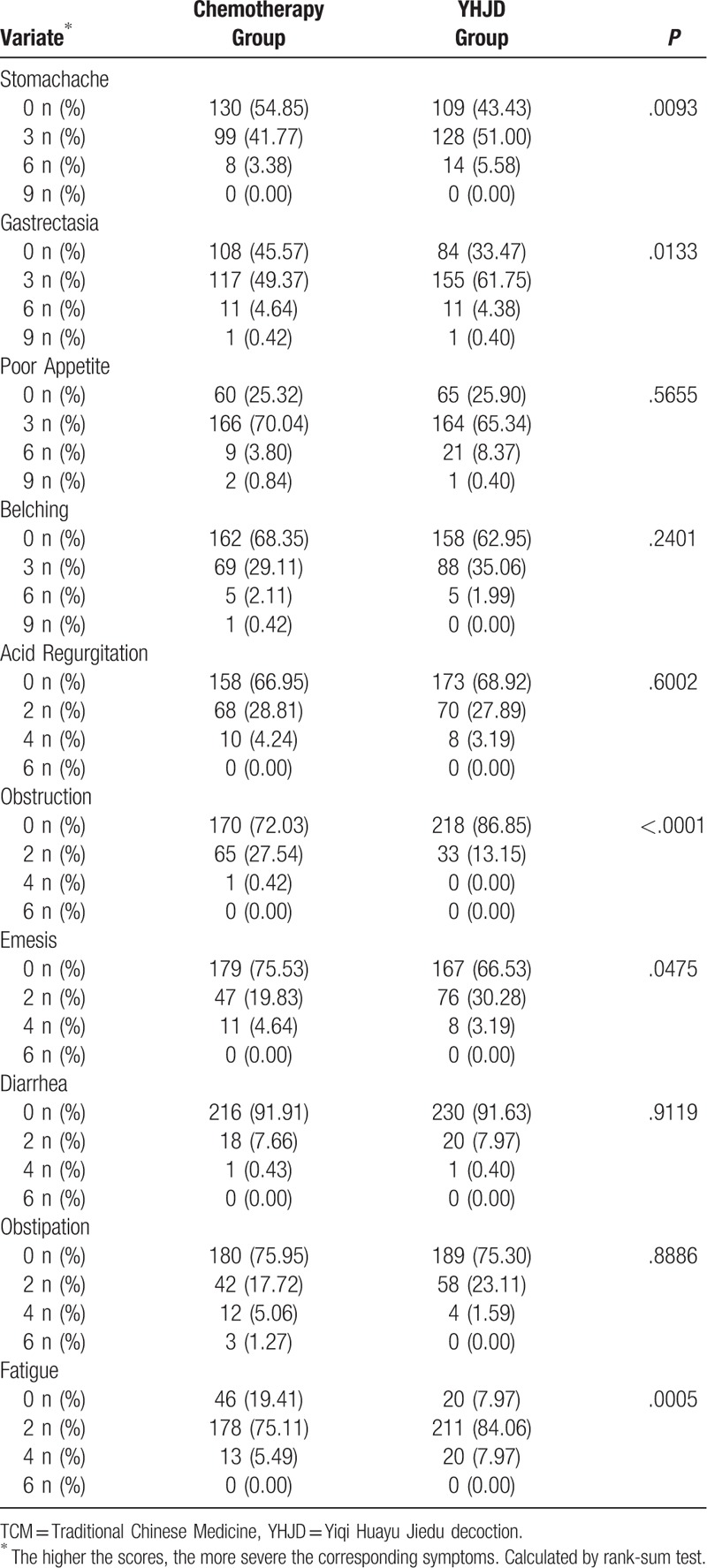
Table 6.
Comparison of TCM syndromes between 2 groups after 18 weeks.
6. Discussion
The Jiangsu Province Hospital of Traditional Chinese Medicine was used by the State Administration of Traditional Chinese Medicine to conduct a clinical research, and gastric cancer is one of the key diseases. Since the 1960s, our hospital has treated a large number of gastric cancer patients using Chinese medicine and accumulated a wealth of clinical experience, especially in preventing recurrence and metastasis.
In Chinese medicine, “spleen deficiency, stasis, and cancerous toxins” were the primary causes of gastric cancer. On the basis of spleen deficiency, high adhesion state of blood and the formation of tumor emboli can trigger gastric cancer recurrence and metastasis, with the passing of time and presence of “stasis and cancerous toxins” as the key factors. Absorbing qi and resolving blood stasis are important treatment methods. Hence, Professor Liu Shenlin created the YHJD. On the contrary, the syndrome differentiation is the primary characteristic of Chinese medicine, affects the patients’ postoperative prognosis, needs concrete analysis based on clinical practice, and is important for determining the appropriate treatment. Therefore, in the application process, different herbs should be used in the YHJD according to the patients’ conditions. Moreover, TCM holds that a disease is a dynamic process, and the signs and symptoms of patients vary during the course of the disease. When a patient comes for a follow-up visit, the drug composition was adjusted according to the patient's condition at that time, which was a more realistic personalized TCM treatment.
This study lasted almost 8 years. Through the collaborative efforts of Chinese and Western medicine experts in multiple centers, 489 patients with advanced gastric cancer had received long-term intervention and follow-up. The results showed that the DFS rate of the YHJD group was significantly higher than that of the chemotherapy group. This finding indicates that chemotherapy combined with YHJD could effectively reduce the risk of recurrence and metastasis in patients with advanced gastric cancer, especially in those with stage III. Moreover, the YHJD was effective in attenuating patients’ symptoms and improving their quality of life. Nevertheless, due to the limited number of patients with the abovementioned medical conditions and the complexity of multicenter collaboration, our research has some limitations. If a randomized and blind trial was conducted, the results would have been more convincing. We are still following up and expanding the number of cases observed. Additional studies are warranted in order to obtain more data. Moreover, when conditions are in place, we can conduct a randomized and blinded trial.
Acknowledgment
We thank the patients and their caregivers and all members of our team. We also thank the staff from Jiangsu Cancer Hospital, Nanjing Drum Tower Hospital, People's Liberation Army Bayi Hospital, Changzhou Traditional Chinese Medicine Hospital, Changzhou Tumor Hospital, Traditional Chinese Medicine Hospital of Kunshan, Yangzhou Hospital of Traditional Chinese Medicine, and YiXing Tumor Hospital.
Author contributions
Conceptualization: Peng Shu, Shenlin Liu.
Data curation: Peng Shu, Shenlin Liu.
Formal analysis: Peng Shu, Huijuan Tang.
Funding acquisition: Shenlin Liu.
Investigation: Peng Shu, Huijuan Tang, Jie Shao, Minghao Qi.
Methodology: Peng Shu, Bin Zhou, Ruiping Wang, Yuanyuan Xu.
Project administration: Shenlin Liu.
Software: Huijuan Tang.
Supervision: Shenlin Liu.
Visualization: Peng Shu, Huijuan Tang, Minghao Qi, Wenjie Huang.
Writing – original draft: Huijuan Tang.
Writing – review & editing: Peng Shu, Yun Xia, Shenlin Liu.
Huijuan Tang orcid: 0000-0003-3810-1256.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, ECT = emission computed tomography, HR = hazard ratio, NCCN = National Comprehensive Cancer Network, TCM = Traditional Chinese Medicine, YHJD = Yiqi Huayu Jiedu decoction.
How to cite this article: Shu P, Tang H, Zhou B, Wang R, Xu Y, Shao J, Qi M, Xia Y, Huang W, Liu S. Effect of Yiqi Huayu Jiedu decoction on stages II and III gastric cancer: a multicenter, prospective, cohort study. Medicine. 2019;98:47(e17875).
PS and HT contributed equally to this work and are first authors.
Supported by State Administration of Traditional Chinese Medicine of the People's Republic of China (CN), (No. 200807022, 2008–2016).
The authors have no conflicts of interests to disclose.
Statement of significance: First multicenter, large sample, precise and reliable clinical trial of Chinese medicine effect on gastric cancer lasted 8 years follow-up was conducted in 9 hospitals.
References
- [1].Kondoh C, Kadowaki S, Komori A, et al. Salvage chemotherapy with the combination of oxaliplatin, leucovorin, and 5-fluorouracil in advanced gastric cancer refractory or intolerant to fluoropyrimidines, platinum, taxanes, and irinotecan. Gastric Cancer 2018;21:1050–7. [DOI] [PubMed] [Google Scholar]
- [2].Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [4].Liu X, Xiu LJ, Jiao JP, et al. Traditional Chinese medicine integrated with chemotherapy for stage IV non-surgical gastric cancer: a retrospective clinical analysis. J Integr Med 2017;15:469–75. [DOI] [PubMed] [Google Scholar]
- [5].Hung KF, Hsu CP, Chiang JH, et al. Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in Taiwan: a nationwide retrospective matched-cohort study. J Ethnopharmacol 2017;199:168–74. [DOI] [PubMed] [Google Scholar]
- [6].Shu P, Wu W. Clinical study of Jianpi Yangwei Formula combined with chemotherapy on postoperative intervention effects of gastric cancer at II and III phases. J Clin Med Pract 2013;3:16–8. (in Chinese). [Google Scholar]
- [7].National Comprehensive Cancer Network. Gastric Cancer (Version 1.2008). Available at: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [access date June 01, 2008]. [Google Scholar]
- [8].Jiangsu Administration of Traditional Chinese medicine, Zhejiang Administration of Traditional Chinese medicine, Shanghai HB, et al. Criteria of diagnosis and therapeutic effect of internal diseases and syndromes in Traditional Chinese Medicine. State Administration of Traditional Chinese Medicine. 1994. ZY/T 001.1-94. (in Chinese). [Google Scholar]
- [9].European Organisation for Research and Treatment of Cancer. EORTC QLQ-C30 version 3.0. 1995. [DOI] [PubMed] [Google Scholar]
- [10].Zheng X. Guiding Principles for Clinical Research of New TCM Drugs. Beijing:China Medical Science and Technology Press; 2002. [Google Scholar]


