Abstract
The aim of this research is to investigate the application value of TTE in the diagnosis of the anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA).
The echocardiographic findings of 11 patients with ALCAPA confirmed by surgery in our hospital from October 2007 to December 2018 were retrospectively analyzed and compared with the preoperative computed tomography angiography (CTA) diagnosis and intraoperative diagnosis.
Surgery was performed in all of the patients to establish the dual coronary artery system. Four underwent the Takeuchi procedure and 7 had re-implantation of the anomalous left coronary artery. The CTA diagnoses of the 11 patients were consistent with the surgical diagnoses, and the diagnostic accuracy was 100% (11/11). Echocardiographic diagnosis showed consistent results in 10 cases, while one case was misdiagnosed as endocardial fibroelastosis; the diagnostic accuracy was 90.9% (10/11). The echocardiographic features of these patients with ALCAPA included: abnormal left coronary ostium arising from the pulmonary trunk with retrograde coronary artery flow in 10 patients; enlargement of the right coronary artery in 8 patients; abundant intercoronary septal collaterals in 6 patients; and moderate and significant mitral regurgitation in 7 patients. Echocardiography showed that the left ventricular end-diastolic diameter and left ventricular end-systolic diameter before surgery were significantly different from those after surgery (P < .05) and that the left ventricular ejection fraction and fractional shortening before surgery were not significantly different from those after surgery (P > .05).
Transthoracic echocardiography can diagnose ALCAPA in a timely, accurate, and noninvasive manner, and it could be of great significance in guiding clinical operations and in predicting prognosis.
Keywords: echocardiography, anomalous origin of the left coronary artery from the pulmonary artery
1. Introduction
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital cardiovascular malformation in which the left coronary artery originates from the pulmonary artery (PA) instead of the left coronary sinus. The incidence is 1 in 300,000 live births and accounts for 0.25% to 0.5% of congenital heart disease cases.[1,2] The natural course of the disease has a poor prognosis, and it is often complicated by myocardial ischemia or infarct heart disease. The mortality rate of children without a timely diagnosis and treatment can reach more than 90% within the first year of life. Surgery is the only means to cure the disease.[3] Therefore, early definitive diagnosis and timely surgical intervention are very important. With the development of ultrasonic imaging, transthoracic echocardiography (TTE) serves as a noninvasive method for diagnosis of ALCAPA by providing important information including the anatomic, functional, and hemodynamic evaluation of the cardiovascular system.[4,5] Thus, clinicians, especially ultrasonography doctors, should have a comprehensive understanding of the echocardiographic manifestations of ALCAPA to reduce misdiagnoses and missed diagnoses. This study retrospectively analyzed the echocardiographic findings, computed tomography angiography (CTA) findings, and intraoperative diagnostic results of 11 patients with surgically confirmed ALCAPA admitted to our hospital to investigate the diagnostic value of echocardiography for the disease and to improve its diagnostic efficacy.
2. Materials and methods
2.1. Patients
A retrospective review of clinical database from October 2007 to December 2018 was performed and 12 patients were identified with ALCAPA. The inclusion criteria were
-
(1)
availability of echocardiographic data in all of the patients;
-
(2)
patients with ALCAPA with CTA data for comparison;
-
(3)
surgery done to determine the diagnostic accuracy of these two imaging modalities in all of the patients.
Patients with ALCAPA without echocardiographic data or without surgery done to confirm the diagnosis were excluded. One patient with ALCAPA were excluded because of the following reasons: without echocardiographic data (n = 1). After exclusion, 11 cases were included in the analysis. Written informed consent was obtained from the participants, and the study was approved by the Hospital Ethics Committee.
2.2. Imaging protocols of transthoracic echocardiography
A Philips IE33 color Doppler ultrasonic diagnostic system (Philips Medical Systems, Andover, MA) was used in this study. This ultrasound system is equipped with a 2.0 to 4.0 MHz transducer.
TTE was carried out in all of the patients within the preoperative and postoperative week. Uncooperative children were sedated by lavation with 10% chloral hydrate at 50 mg/kg or by intramuscular injection with luminal at 5 mg/kg. TTE was performed with the patient in the supine or lateral position and was monitored by electrocardiography. Multisection scanning was performed of the parasternal, apical, subxiphoid, and suprasternal fossae. The origins, travel, and inner diameters of the left and right coronary arteries and the relationship between the pulmonary artery and the left coronary artery were studied on multiple planes with two-dimensional ultrasound. Color Doppler ultrasound was used to focus the observation on the direction of blood flow in the left and right coronary arteries and abnormal blood flow in the pulmonary artery, the collateral circulation between the left and right coronary arteries, and the presence or absence of abnormal retrograde blood flow of the coronary artery in the trunk of the pulmonary artery. Segmental wall-motion abnormalities of the left ventricular wall, endocardial and papillary muscle echogenicity, and valvular regurgitation were evaluated. Conventional measurements of the left ventricle (LV) in the parasternal left ventricle long-axis view using M-mode image included: left ventricular end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS). Postoperatively, the patients were evaluated mainly for left ventricular morphology and function, anastomotic blood flow, changes in coronary collateral vessels, and valvular regurgitation. The infantile and adult ultrasound diagnostic criteria were mainly distinguished based on the collateral circulation of the interventricular septum, with the former lacking collateral circulation, while the latter has abundant collateral blood flow. TTE examinations were performed by at least two echocardiography physicians who both had at least 8 years of experience in echocardiographic imaging. Any discrepancy between the observers was resolved by consensus.
2.3. CTA
CT coronary angiography was performed using a 64 slice CT scanner (Acquilion 64 Toshiba, Japan). Scan parameters were as follows: Slice collimation, 64 × 0.5 mm; rotation time, 0.40 minutes; tube voltage, 80 kV; tube current, 300 mA; and pitch, 0.2. The scan parameters were modified to decrease the patient dose. CT angiography was triggered automatically by the arrival of the main contrast bolus (automatic bolus tracking). We injected 50 ml nonionic contrast medium (Iomeron 350/ml; Iomeprol, Bracco, Italy) at a flow rate of 3 ml/s in the left antecubital vein. This was followed by a 30 ml saline chaser bolus at a flow rate of 3 ml/s to wash out contrast from the right ventricle. All images were deidentified and transferred to a separate workstation (Vitrea2, Vital Images) for further image processing and analysis.
2.4. Surgical control
Eleven patients underwent surgical treatment. Echocardiographic diagnosis and CTA diagnosis were compared with intraoperative findings.
2.5. Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL) software package. Measurement data are expressed as the mean ± standard deviation (X ± s). Count data were expressed as numbers of cases and percentages. The t test was used to compare various parameters before and after surgery. The Chi-square test was used to compare the rates. P < .05 was considered statistically significant.
3. Results
3.1. Clinical presentation
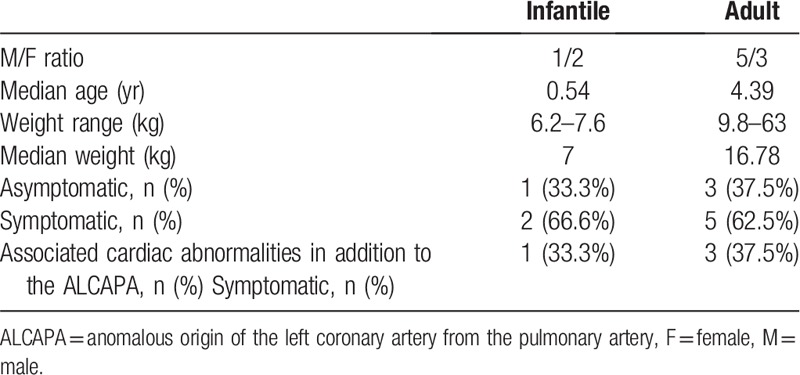
Of the 11 patients with surgically confirmed ALCAPA, 5 cases were the infantile type, and 6 were the adult type. The 11 patients included 5 men and 6 women with a mean age ± SD of 13.2 ± 18.6 years (range, 6 months–73 years). Four (36.36%) patients had other associated cardiac abnormalities in addition to the ALCAPA, which included: atrial septal defect (ASD) in one patient, patent foramen ovale (PFO) in 2 patients, and rheumatic heart disease in another patient (Table 1).
Table 1.
Characteristics of patients.
3.2. Echocardiography
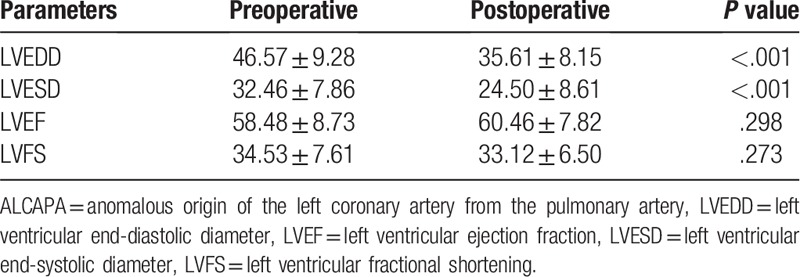
Echocardiographic diagnosis showed consistent results in 10 cases, while 1 case was misdiagnosed as endocardial fibroelastosis; the diagnostic accuracy was 90.9% (10/11). The echocardiographic features of these patients with ALCAPA included: abnormal left coronary ostium arising from the pulmonary trunk with retrograde coronary artery flow in 10 patients; enlargement of the right coronary artery in 8 patients; abundant intercoronary septal collaterals in 6 patients; and moderate and significant mitral regurgitation in 7 patients (Figs. 1 and 2). Echocardiography showed that the left ventricular end-diastolic diameter and left ventricular end-systolic diameter before surgery were significantly different from those after surgery (P < .05) and that the left ventricular ejection fraction and fractional shortening before surgery were not significantly different from those after surgery (P > .05) (Table 2).
Figure 1.
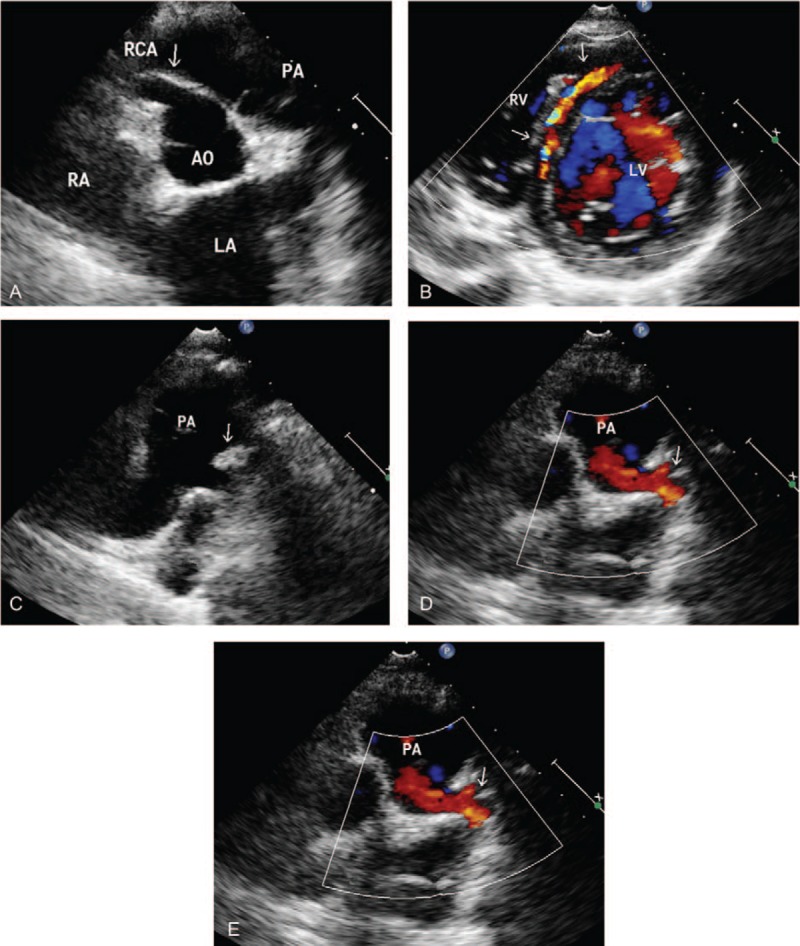
ALCAPA in a 8-year-old male. (A) Transthoracic echocardiography shows a dilated right coronary artery (arrows). (B) Color Doppler flow image reveals the collateral vessel in the interventricular septum (arrow). (C) Parasternal short-axis view shows the left coronary artery originating from pulmonary artery (arrow). (D) Color Doppler reveals a retrograde flow from the ostium of the LCA to the PA (arrow). (E) Pulse Doppler showed the retrograde red shunt a lowvelocity, prominently diastolic flow spectrum. LA = left atrium, LV = left ventricle, RA = right atrium, AO = aortic, PA = pulmonary artery, RCA = right coronary artery.
Figure 2.
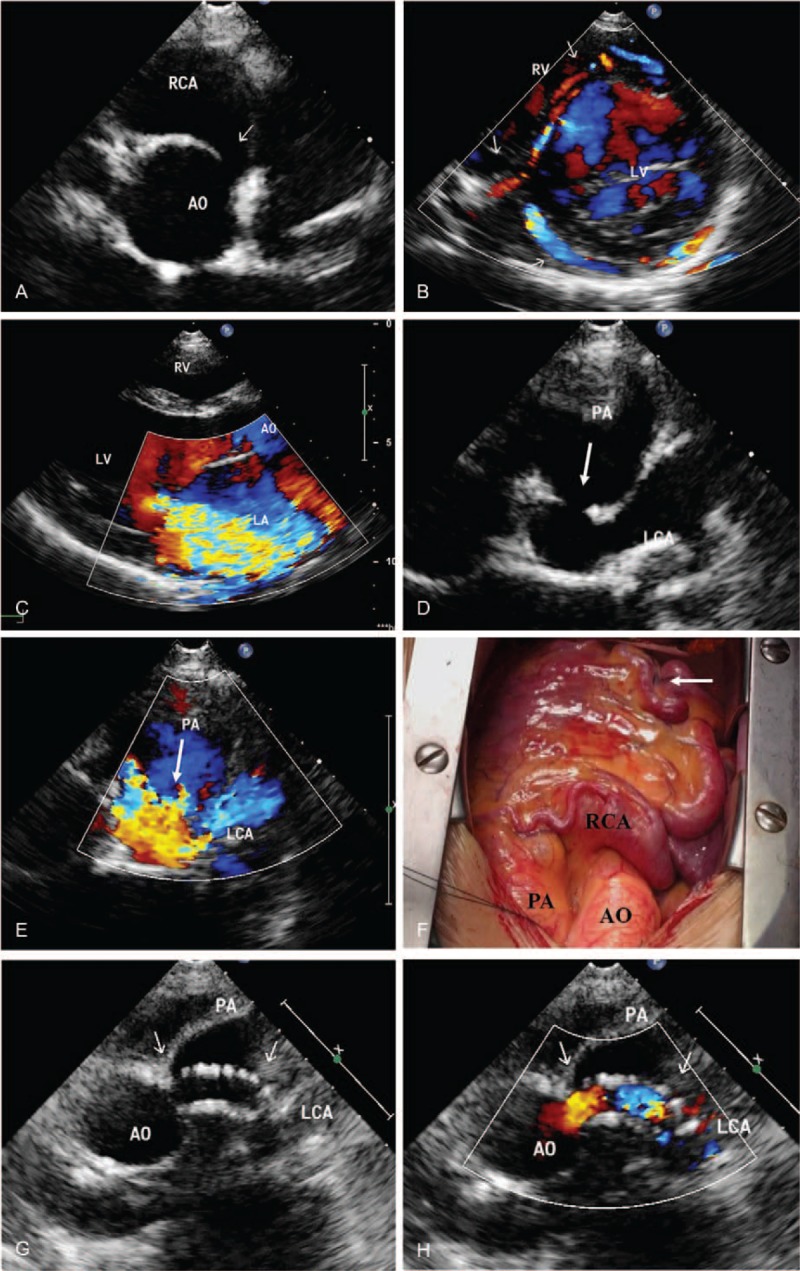
ALCAPA in a 43-year-old female. (A) Transthoracic echocardiography shows a dilated right coronary artery (arrows). (B) Color Doppler flow image reveals the collateral vessel in the interventricular septum (arrow). (C) The parasternal long-axis view showed severe mitral regurgitation. (D) Parasternal short-axis view shows the left coronary artery originating from pulmonary artery (arrow). (E) Color Doppler reveals a retrograde flow from the ostium of the LCA to the PA (arrow). (F) Intraoperatively, marked dilatation of the right coronary artery was observed, with abundant collateral circulation forming distally (arrow). (G) Postoperative reexamination ultrasound showed the establishment of tunnels in the pulmonary artery. (H) Color Doppler ultrasound showed smooth flow of blood in the tunnels of the pulmonary artery. LA = left atrium, LV = left ventricle, RA = right atrium, AO = aortic, PA = pulmonary artery, RCA = right coronary artery, LCA = left coronary artery. ALCAPA = anomalous origin of the left coronary artery from the pulmonary artery, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVESD = left ventricular end-systolic diameter, LVFS = left ventricular fractional shortening.
Table 2.
Comparison of the echocardiographic parameters preoperative and postoperative.
Ultrasound findings of infantile ALCAPA: ① The left coronary artery ostium was not detected in the left coronary sinus, and the trunk of the left coronary artery was directly connected to the pulmonary artery. Color Doppler ultrasound showed that the left coronary artery blood had a retrograde flow into the pulmonary artery. ② The left ventricle was significantly dilated, and the left ventricular systolic function was reduced. ③ The right coronary artery had a normal origin, but the inner diameter was widened. ④ Sparse collateral circulation was observed between the left and right coronary arteries. ⑤ The chordae tendineae of the mitral valve, papillary muscle, and local endocardium had fibrosis with increased echo-intensity. ⑥ The mitral valve showed a moderate to severe regurgitation signal.
Ultrasound findings of adult-type ALCAPA: ① The left coronary artery ostium was not detected in the left coronary sinus, and the trunk of the left coronary artery was directly connected to the pulmonary artery. Color Doppler ultrasound showed that the left coronary artery blood had a retrograde flow into the pulmonary artery. ② The left ventricle was significantly dilated, and the left ventricular wall had segmental wall-motion abnormalities. ③ The right coronary artery had a normal origin, but the inner diameter was widened. ④ Abundant collateral circulation was observed between the left and right coronary arteries. ⑤ The mitral valve showed a mild to moderate regurgitation signal.
3.3. Diagnostic performance
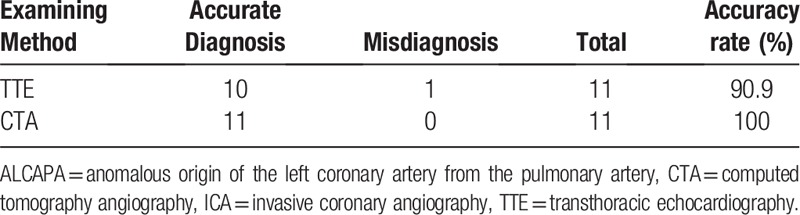
The CTA diagnoses of the 11 patients were consistent with the surgical diagnoses, and the diagnostic accuracy was 100%. There was no statistical difference between TTE and CTA for diagnosing ALCAPA (P > .05) (Table 3).
Table 3.
Diagnostic performance of TTE and CTA in comparison to intraoperative findings (n = 11).
3.4. Intraoperative findings
Of 11 patients who were treated by surgical repairs, Takeuchi procedure was performed on 4 patients (Fig. 2G and H) and re-implantation of the anomalous coronary into the aorta was done on 7 cases. Mitral valvuloplasty for severe mitral regurgitation was performed on six patients with ALCAPA re-implantation. One patient with ALCAPA re-implantation underwent mitral valve replacement for rheumatic mitral valve disease.
4. Discussion
ALCAPA is a rare congenital heart disease first reported by Bland, White, and Garland in 1933, and it is also known as “Bland-White-Garland” syndrome.[6] As a result of poor separation or rotation of the arterial trunk and pulmonary septum or malposition of the anlagen of the coronary artery during the embryonic period, the left coronary artery does not arise normally from the left coronary sinus but originates from the root of the pulmonary artery.[7,8] According to the degree of collateral circulation between the left and right coronary arteries, ALCAPA can be divided into infantile and adult types in infantile ALCAPA. Therefore, infants are less likely to be symptomatic during the first 2 months of life. Two months after birth, pulmonary vascular resistance and pulmonary artery pressure gradually decrease to normal levels, the blood supply to the left ventricle from the pulmonary artery through the coronary artery is reduced, and the pulmonary artery oxygen content also gradually decreases, while only a few or no collateral vessels are established between the left and right coronary arteries, so severe myocardial ischemia and necrosis, cardiac insufficiency, and papillary muscle ischemia and atrophy occur, leading to mitral valve incompetence. Without timely surgical correction, most of these patients (80% to 90%) will die within 1 year.[9] In adult ALCAPA, once effective collateral circulation is established, and the state of myocardial ischemia is improved. However, left ventricular dilatation, subendocardial ischemia, mitral regurgitation, and heart failure can gradually occur clinically due to the “blood stealing” phenomenon of the left coronary artery, induced by the decrease in pulmonary artery pressure. The median survival of adult patients without surgical treatment is 35 years old, with 80% to 90% dying from sudden cardiac death.[10,11]
These data show that the natural prognosis of ALCAPA is not ideal, and there is a risk of sudden death, so regardless of whether there are clinical symptoms, the diagnosis should be confirmed as soon as possible, and timely surgical treatment should be performed to improve the prognoses of patients. The aim of surgical treatment is to restore the blood supply to the coronary artery system and to increase the blood supply to the left ventricular myocardium.[12,13] Currently, there are two main surgical methods to treat ALCAPA at home and abroad:
-
(1)
coronary artery grafting, which directly transplants the abnormal coronary artery originating from the pulmonary artery to the aortic root; and
-
(2)
the Takeuchi procedure, which constructs a tunnel in the pulmonary artery to connect the ostium of the abnormal coronary artery originating from the pulmonary artery to the aorta.[14]
Presently, re-implantation has become the procedure of choice whenever possible with an excellent mediumterm survival rate and a low morbidity rate. Our study findings are in accordance with the literature as 63.6% of our patients (7/11) underwent this procedure. If the artery cannot be mobilized sufficiently, another option for the repair of ALCAPA is the intrapulmonary tunnel (Takeuchi) procedure, which was performed in four patients in our study. In this study, the left ventricular diameter showed improvement after the first week of operation.
ICA is recognized as the “gold standard” for diagnosis of coronary artery disease because of its excellent spatial and temporal resolution.[15–17] However, the procedure is invasive with ionizing radiation exposure and the use of expensive potentially nephrotoxic contrast media which are inappropriate for patients with allergic history to iodine in the contrast media. CTA has been increasingly used to assess the coronary anatomy in recent years with very good diagnostic accuracy and is considered as a reliable alternative to ICA for diagnostic evaluation of the coronary anatomy. Eleven patients in whom coronary CTA flow, and expensiveness. Furthermore, the post-processing of coronary CTA images is quite complex and rather time consuming.
Echocardiography is economical, convenient, fast, safe, and noninvasive, and it can be repeatedly detected. It displays the heart chamber geometry, valve activity, heart function, and hemodynamics in real time; allows for observation of the coronary artery ostium and travel; and has become the preferred examination method for the diagnosis of ALCAPA.[18] All 11 patients with ALCAPA confirmed by surgery underwent preoperative echocardiography and CTA. All of the CTA results were consistent with the intraoperative diagnoses, and while the ultrasound diagnoses of 10 cases were consistent with the intraoperative diagnoses, for one case, the ultrasound diagnosis was not consistent. One case was misdiagnosed as endocardial fibroelastosis by ultrasound. The ultrasound showed left heart enlargement, endocardial thickening, echo enhancement, and a decrease in left ventricular function, but the abnormality of the coronary artery origin was not detected, whereas CTA examination and intraoperative diagnosis confirmed that the left coronary trunk originated from the primary pulmonary artery. The reason for misdiagnosis was that ultrasound indicated that the origin of the left and right coronary arteries was normal, and the transverse pericardial sinus could be mistakenly considered the left coronary artery opening at the left coronary sinus of the aorta. The transverse pericardial sinus was also one of the important factors affecting ultrasound diagnosis of ALCAPA. A strip-shaped anechoic area could be detected on the short axis section of large arteries, similar to the width of a normal left coronary artery. The transverse pericardial sinus was easily mistaken for the left coronary artery opening at the left coronary sinus of the aorta. Attention should be paid to the absence of branching and blood flow signals in the transverse pericardial sinus for differential diagnosis.[19,20]
ALCAPA must be differentiated from coronary-pulmonary artery fistula and primary endocardial fibroelastosis. The lumen of a coronary artery fistula opening into the pulmonary artery is often single, rather than a convergence formed by branches. The origin of the left and right coronary arteries is normal, there is a small amount of coronary collateral flow in the interventricular septum, there is no significant left ventricular enlargement or segmental wall motion abnormalities in infants and children, and there is no thickening or calcification of the left ventricular endocardium, papillary muscle, mitral valve, or subvalvular chordae tendineae.[21,22] Echocardiography of primary endocardial fibroelastosis showed enlargement of cardiac chambers, decreased ventricular systolic function, mitral regurgitation, thickening of the left ventricular endocardium, and increased echogenicity. Two-dimensional echocardiography images of primary endocardial fibroelastosis were similar to those of ALCAPA; thus, ALCAPA was easily misdiagnosed as primary endocardial fibroelastosis. It has been reported in the literature that approximately 5.8% of ALCAPA cases are misdiagnosed as primary endocardial fibroelastosis,[23,24] so caution should be exercised. The origin, travel, and inner diameter of the coronary arteries were normal in pure primary endocardial fibroelastosis; the left coronary artery blood flow was forward; and no abnormal blood flow was observed in the pulmonary artery lumen.[25]
5. Conclusions
In summary, the echocardiographic findings of patients with ALCAPA have some typical features, but there are differences between the ultrasound findings of the infantile and adult types. Echocardiography has high diagnostic accuracy; it can be used as the preferred, reliable and noninvasive imaging diagnostic method in clinical practice; and it is of great significance in guiding clinical surgical treatment and in predicting prognosis.
Acknowledgments
We greatly appreciate the assistance of the Department of Thoracic and Cardiovascular Surgery, The First Affiliated Hospital of Nanchang University, and thank them for their efforts.
Author contributions
Conceptualization: Xin-Chun Yuan, Jia Hu, Xi Zeng, Ai-Yun Zhou, Li Chen.
Data curation: Xin-Chun Yuan, Jia Hu, Xi Zeng, Ai-Yun Zhou, Li Chen.
Methodology: Xin-Chun Yuan, Jia Hu.
Writing – original draft: Xin-Chun Yuan, Jia Hu, Xi Zeng.
Writing – review & editing: Xin-Chun Yuan.
Footnotes
Abbreviations: ALCAPA = anomalous origin of the left coronary artery from the pulmonary artery, AO = aortic, ASD = atrial septal defect, CTA = computed tomography angiography, F = female, ICA = invasive coronary angiography, LA = left atrium, LCA = left coronary artery, LV = left ventricle, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVESD = left ventricular end-systolic diameter, LVFS = left ventricular fractional shortening, M = male, PA = pulmonary artery, PA = pulmonary artery, PFO = patent foramen ovale, RA = right atrium, RCA = right coronary artery, TTE = transthoracic echocardiography.
How to cite this article: Yuan Xc, Hu J, Zeng X, Zhou Ay, Chen L. Echocardiographic diagnosis of anomalous origin of the left coronary artery from the pulmonary artery. Medicine. 2019;98:47(e18046).
This study was approved by the ethical review committee of The First Affiliated Hospital of Nanchang University, and written informed consent was obtained from the patient. All authors declare that they have no conflicts of interest.
References
- [1].Edwards JE. The direction of blood flow in coronary arteries arising from the pulmonary trunk. Circulation 1964;29:163–6. [DOI] [PubMed] [Google Scholar]
- [2].Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28–40. [DOI] [PubMed] [Google Scholar]
- [3].Hauser M. Congenital anomalies of the coronary arteries. Heart 2005;91:1240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frommelt MA, Miller E, Williamson J, et al. Detection of septal coronary collaterals by color flow Doppler mapping is a marker for anomalous origin of a coronary artery from the pulmonary artery. J Am Soc Echocardiogr 2002;15:259–63. [DOI] [PubMed] [Google Scholar]
- [5].Coles DR, Smail MA, Negus IS, et al. Comparion of radiation dose form multislise computed tomography coronary angiography and conventional diagnostic angiography. J Am Coll Cardiol 2006;47:1840–5. [DOI] [PubMed] [Google Scholar]
- [6].Sun Z, Choo GH, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol 2012;85:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in paediatrics and associated radiation exposure and estimated cancer risk. JAMA Paediatr 2013;167:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shapiro MD, Dodd JD, Kalva S, et al. A comprehensive electrocardiogram-gated 64-slice multidetector computed tomography imaging protocol to visualize the coronary arteries, thoracic aorta, and pulmonary vasculature in a single breath hold. J Comput Assist Tomogr 2009;33:225–32. [DOI] [PubMed] [Google Scholar]
- [9].Yau JM, Singh R, Halpern EJ, et al. Anomalous origin of the left coronary artery from the pulmonary artery in adults: a comprehensive review of 151 adult cases and a new diagnosis in a 53-year-old woman. Clin Cardiol 2011;34:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Silverman NH. Echocardiographic presentation of anomalous origin of the left coronary artery from the pulmonary artery. Cardiol Young 2015;25:1512–23. [DOI] [PubMed] [Google Scholar]
- [11].Cohen MS, Herlong RJ, Silverman NH. Echocardiographic imaging of anomalous origin of the coronary arteries. Cardiol Young 2010;20:26–34. [DOI] [PubMed] [Google Scholar]
- [12].Meinel FG, Bayer IIRR, Zwerner PL, et al. Coronary computed tomographic angiography in clinical practice: state of the art. Radio Clin N Am 2015;53:287–96. [DOI] [PubMed] [Google Scholar]
- [13].Yang YL, Nanda NC, Wang XF, et al. Echocardiographic diagnosis of anomalous origin of the left coronary artery from the pulmonary artery. Echocardiography 2007;24:405–11. [DOI] [PubMed] [Google Scholar]
- [14].Alva C, Gomez FD, Jimenez-Arteaga S, et al. Anomalous origin of the left coronary artery from the pulmonary artery. Echocardiographic diagnosis. Arch Cardiol Mex 2009;79:274–8. [PubMed] [Google Scholar]
- [15].Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol 1998;29:689–95. [DOI] [PubMed] [Google Scholar]
- [16].Bunton R, Jonas R, Lang P, et al. Anomalous origin of left coronary artery from pulmonary artery: ligation versus establishment of a two coronary artery system. J Thorac Cardiovasc Surg 1987;93:103–8. [PubMed] [Google Scholar]
- [17].Ramirez S, Curi-Curi PJ, Calderon-Colmenero J, et al. Outcomes of coronary reimplantation for correction of anomalous origin of left coronary artery from pulmonary artery. Rev Esp Cardiol 2011;64:681–7. [DOI] [PubMed] [Google Scholar]
- [18].Kottayil BP, Jayakumar K, Dharan BS, et al. Anomalous origin of left coronary artery from pulmonary artery in older children and adults: direct aortic implantation. Ann Thorac Surg 2011;91:549–53. [DOI] [PubMed] [Google Scholar]
- [19].Manoly I, Karangelis D, Viola N, et al. Repair of Bland-White-Garland syndrome via a modified technique. Tex Heart Inst J 2014;41:48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rodriguez-Gonzalez M, Tirado AM, Hosseinpour R, et al. Anomalous origin of the left coronary artery from the pulmonary artery: diagnoses and surgical results in 12 pediatric patients. Tex Heart Inst J 2015;42:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].KrexiL, Sheppard MN. Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), a forgotten congenital cause of sudden death in the adult. Cardiovasc Pathol 2013;22:294–7. [DOI] [PubMed] [Google Scholar]
- [22].Lardhi AA. Anomalous origin of left coronary artery from pulmonary artery: A rare cause of myocardial infarction in children. J Family Community Med 2010;17:113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen S, Chen Z, Zheng B. Diagnostic usefulness of quantitative tissue velocity imaging and anatomic M-mode echocardiography for coronary artery disease: a pilot study. J Clin Ultrasound 2014;43:346–52. [DOI] [PubMed] [Google Scholar]
- [24].Manlhiot C, Millar K, Golding F, et al. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol 2010;31:242–9. [DOI] [PubMed] [Google Scholar]
- [25].Patel SG, Frommelt MA, Frommelt PC, et al. Echocardiographic diagnosis, surgical treatment, and outcomes of anomalous left coronaryartery from the pulmonary artery. J Am Soc Echocardiogr 2017;30:896–903. [DOI] [PubMed] [Google Scholar]


