Abstract
The present study is aimed to clarify the utility of magnetic resonance cholangiopancreatography (MRCP) and the additional value of diffusion-weighted imaging (DWI) in diagnosing pancreatic ductal adenocarcinoma (PDAC) concomitant with intraductal papillary mucinous neoplasm (IPMN).
This retrospective study involved 38 patients with PDAC concomitant with IPMN and 114 patients (control) who were randomly selected from 320 patients with IPMN without PDAC and were matched with cases for magnetic resonance imaging (MRI) strength (1.5 T/3.0 T). Two radiologists reviewed the 2 MR image sets with relevant clinical information blinded, first MRCP alone and then combined MRI set including DWI. Diagnostic capability and interobserver agreement were assessed by using receiver operating characteristics curve (Az) analysis and weighted κ statistics.
Az values for the 2 observers were 0.834 and 0.821 for MRCP alone and 0.964 and 0.926 for the combined MRI (P < .001 and P < .001), respectively. The sensitivity of MRCP alone was 61% (23/38), with both observers failing to diagnose PDACs located at the end of tail or away from the pancreatic duct. Meanwhile, with combined MRI, sensitivity was significantly increased for both observers (61% to 92%, P = .002; 61% to 87%, P = .004). Moreover, the interobserver agreement was higher with combined MRI (κ = 0.85) than MRCP alone (κ = 0.59).
MRCP and DWI might be a superior option with a higher diagnostic capability of PDAC concomitant with IPMN than MRCP alone, especially for tumors away from the pancreatic duct.
Keywords: diffusion-weighted imaging (DWI), intraductal papillary mucinous neoplasm (IPMN), magnetic resonance cholangiopancreatography (MRCP), pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm (PDAC concomitant with IPMN)
1. Introduction
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are neoplasms that are characterized by pancreatic duct dilation, intraductal papillary growth and mucus secretion, and they present a wide spectrum of histological atypia ranging from low-grade dysplasia to invasive carcinoma.[1] There are two types of IPMN-related pancreatic carcinoma (PC). The first is malignant transformations of IPMN itself (PC derived from IPMN),[2–4] and the second is pancreatic ductal adenocarcinoma (PDAC) distinct from IPMN (PDAC concomitant with IPMN). Yamaguchi et al[3] examined 765 patients with IPMN and detected PDAC concomitant with IPMN in 31 (4.1%) patients. Tada et al[5] reported that patients with pancreatic cystic lesions including IPMN were at a high risk of PDAC, with a standardized incidence rate of 22.5. The carcinogenic risk of branch duct IPMN is reported to be 0.2% to 2% annually.[2,3,5–23] International guideline on IPMN[4] recommend that the progress of IPMN should be examined and monitored by magnetic resonance imaging (MRI) at specific intervals depending on the cyst diameter. However, this guideline only considers the risk of PC derived from IPMN and do not factor in the occurrence of PDAC concomitant with IPMN. The diagnostic value of MRI for PDAC concomitant with IPMN remains unclear.
MR cholangiopancreatography (MRCP) visualizes cystic lesions and the pancreatic duct, therefore, it excels at depicting progression of IPMN[4] and secondary findings of PDAC, such as pancreatic duct stenosis with upstream dilatation.[24–26] However, despite being highly sensitive to morphological changes in the pancreatic duct, in MRCP, it may be difficult to diagnose PDAC that develop distant from the main pancreatic duct. Diffusion-weighted imaging (DWI) that visualizes the thermally induced motion of water molecules in biological tissues, called Brownian motion, directly visualizes carcinomas.[27] Therefore, it can detect PDAC irrespective of the presence/absence of pancreatic duct infiltration.
The present study aimed to clarify the limitation of MRCP and the additional value of DWI in diagnosing PDAC concomitant with IPMN.
2. Materials and methods
This retrospective study was approved by the institutional review board of the University of Yamanashi. Based on the clinical database, we enrolled 38 patients who had been diagnosed with PDAC concomitant with IPMN between January 2006 and March 2017 at the Yamanashi University Hospital as the PDAC group.
To perform a comparison study, 320 patients with IPMN without PDAC who had undergone MRI were extracted from the clinical database. Of these 320 patients, 114 were randomly selected to match with cases for MRI strength (1.5 T/3.0 T) as the control group. IPMN was defined as branch duct dilatation (≥ 5 mm) communicating with the main pancreatic duct. PDAC concomitant with IPMN is defined as follows: IPMN is obviously distant from PDAC, according to the radiological images and macroscopic or microscopic findings. The diagnosis of PDAC was made with MRI and/or computed tomography and/or endoscopic ultrasound findings. The results were confirmed with histological examination of surgically resected specimens or endoscopic ultrasound-guided fine needle aspiration specimens. When histological diagnosis is not obtained, PDAC was diagnosed by clinical and image follow-up examinations for at least 3 months. The control subjects underwent clinical and MRI and/or computed tomography follow-up examination for at least 12 months, and no evidence of PDAC was detected in any of the control subjects during the follow-up period.
Two radiologists (S.I. and T.S. with 12 and 6 years of experience, respectively) independently reviewed the MRI images of a total of 152 patients (38 in the PDAC group and 114 in the control group) blindly with clinical diagnoses undisclosed.
The observers first reviewed MRCP alone for the likelihood of PDAC. Subsequently, they reviewed fat-saturated T1-weighted imaging (FST1WI) and DWI. They used a 5-point scale to assign the confidence level for PDAC. MRCP scale was categorized as
-
1.
normal pancreatic duct;
-
2.
pancreatic duct slight stenosis or mild dilatation;
-
3.
pancreatic duct slight stenosis and mild dilatation;
-
4.
pancreatic duct severe stenosis or dilatation; and
-
5.
pancreatic duct severe stenosis and dilatation.
FST1WI and DWI scales were categorized as
-
1.
no focal lesion on FST1WI and no signal on DWI;
-
2.
not applicable;
-
3.
localized atrophy on FST1WI and localized signal on DWI;
-
4.
low intensity lesion on FST1WI and no signal on DWI; and
-
5.
low intensity lesion on FST1WI and localized signal on DWI. A detection score of ≥3 was accepted as positive for the presence of PDAC. The combined MRI scores were given with FST1WI and DWI findings added to MRCP findings.
2.1. MR protocol
MRI studies were performed by using either 3.0 T system (Discovery 750 HD; GE Healthcare, Waukesha, WI) or one of the two 1.5 T systems (Signa Excite HD, GE Healthcare, Waukesha, WI; and Signa LX; GE Healthcare, Milwaukee, WS). Three types of sequences were acquired; MRCP, 2D-single shot fast spin echo sequence with slice thickness of 20 or 50 mm (1.5 T and 3 T) and 3D respiratory-triggered first recovery fast spin-echo sequence (3 T); FST1WI, 2D gradient echo (1.5 T) or 3D gradient echo imaging (3 T); DWI with b value of 1000 s/mm2 (1.5 T and 3 T).
2.2. Statistical analysis
All analyses were performed using the BellCurve for Excel software version 2.20 (Social Survey Research Information Co., Ltd., Tokyo, Japan). The PDAC group was compared with the control in terms of clinical data by using Mann–Whitney U test, Fisher Exact test and chi-square test. Receiver operating characteristic (ROC) curves were used to represent the performance of individual observers for PDAC detection. The diagnostic accuracy for each observer was determined by calculating the area under the ROC curve (Az). A detection score of ≥3 were accepted as positive for the presence of PDAC. The McNemar test was applied to evaluate the differences between MRCP alone and combined MRI in terms of detecting PDACs. In all statistical comparisons, a P value of < .05 were defined as statistically significant. The interobserver agreement among observers for PDAC detection was calculated with linear-weighted κ values. A κ value of < 0.20 indicated poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80 good agreement; 0.81–1.00, excellent agreement.
3. Results
3.1. Patient characteristics of PDAC and control groups
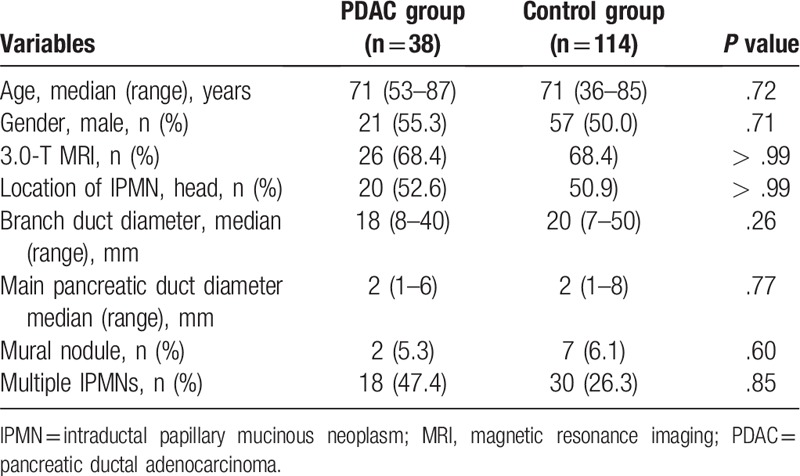
Table 1 shows the comparison between the patient characteristics of PDAC and control groups. In the PDAC group, the median age (range) was 71 years (53–87 years), 21 (55.3%) were men, 26 (68.4%) underwent MRI of 3.0 T, 20 (52.6%) had IPMN at the pancreatic head, the median branch duct diameter (range) was 18 mm (8–40 mm), median pancreatic duct diameter (range) was 2 mm (1–6 mm), mural nodules were observed in two patients (5.3%), and 18 (47.4%) had multiple IPMNs. In the control group, the median age (range) was 71 years (36–85 years), 57 (50%) were men, 78 (68.4%) had an MRI strength of 3.0 T, 58 (50.9%) had IPMN at the pancreatic head, the median branch duct diameter (range) was 20 mm (7–50 mm), the median pancreatic duct diameter (range) was 2 mm (1–8 mm), seven (6.1%) had mural nodules, and 30 (26.3%) exhibited multiple IPMNs. No significant difference was observed between the groups.
Table 1.
Patient characteristics.
3.2. Characteristics of PDAC concomitant with the IPMN
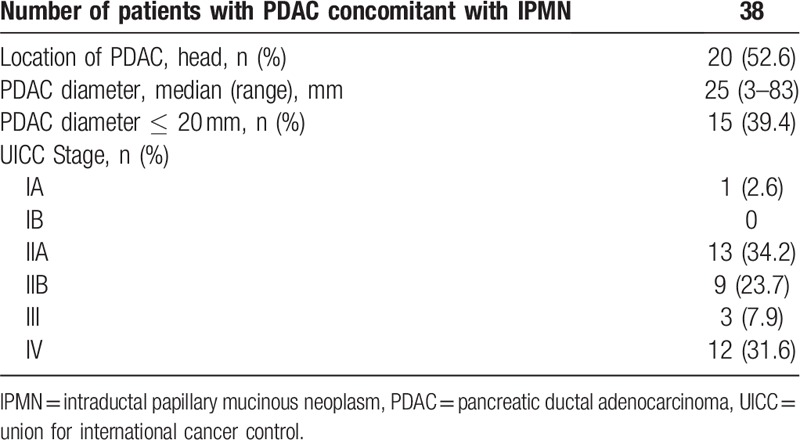
In the PDAC group, 20 (52.6%) had pancreatic carcinoma in the pancreatic head, the median tumor diameter (range) was 25 mm (3–83 mm), and 15 (39.4%) had a tumor ≤20 mm in diameter (Table 2). Thirty-two (84.2%) were confirmed with histopathology.
Table 2.
Characteristics of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm.
3.3. Diagnostic performance of MRCP alone
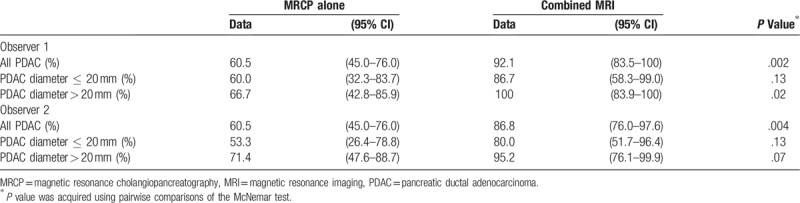
The sensitivity of MRCP was 60.5% (95% CI, 45.0–76.0%) for both observers and the specificity was 93.9% (95% CI, 87.1–97.0%), 97.4% (95% CI, 92.3–99.5%) and the accuracy was 85.5% (95% CI, 79.9–91.1%), 88.2% (95% CI, 83.1–93.3%) for observers 1 and 2, respectively (Table 3). Neither observer detected PDAC concomitant with IPMN with MRCP alone in 11 (28.9%) patients, of which five (45.5%) had PDAC in the pancreatic uncus, 2 (18.2%) in the end of the pancreatic tail and 2 (18.2%) predominant extra pancreatic growth (Fig. 1A).
Table 3.
Comparison diagnostic performance of magnetic resonance cholangiopancreatography alone and combined magnetic resonance imaging for the detection of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm.
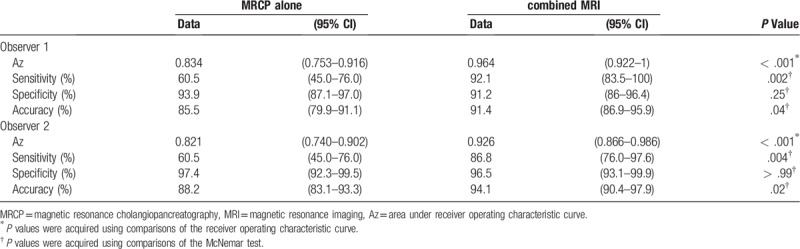
Figure 1.
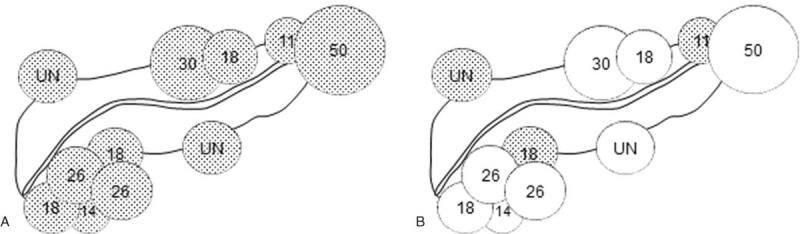
A. Illustration showing the location and diameter (mm) of PDAC which neither observer detected with MRCP alone. Five (45.5%) had PDAC in the pancreatic uncus, 2 (18.2%) in the end of the pancreatic tail and 2 (18.2%) predominant extra pancreatic tumor growth. The median tumor diameter was 18 mm (range, 11–50 mm). B. Of these 11 patients, eight (72.7%) became identifiable with combined MRI. ● = undetected PDAC, ○ = detected PDAC, UN = unmeasurable.
3.4. Additional value of FST1WI and DWI to MRCP
As summarized in Table 3, in the detection of PDAC, the sensitivity of combined MRI was significantly higher than that of MRCP alone for both observers (observer 1, 92.1% vs 60.5%, P = .002; observer 2, 86.8% vs 60.5%, P = .004). Of 11 patients who neither observer detected PDAC concomitant with IPMN with MRCP alone, eight (72.7%) became identifiable with combined MRI (Fig. 1B, Fig. 2).
Figure 2.
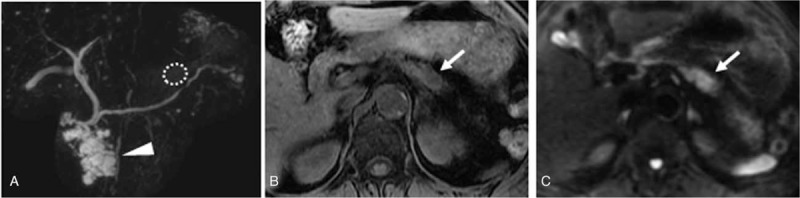
A–C. PDAC concomitant with IPMN. A. MRCP shows IPMN (arrowhead) measuring 40 mm in pancreatic head with normal main pancreatic duct. B. Fat suppressed T1-weighted imaging shows low intensity mass (arrow) in the tail of pancreas. C. DWI with b value of 1000 s/mm2 shows hyper intensity (arrow) in the tail of pancreas.
In addition, both observers showed significantly higher Az values when interpreting combined MRI compared with MRCP alone (observer 1, 0.964 [95% CI, 0.922–1] vs 0.834 [95% CI, 0.753–0.916], P < .001; observer 2, 0.926 [95% CI, 0.866–0.986] vs 0.821 [95% CI, 0.740–0.902], P < .001) (Table 3, Fig. 3). In the diagnosis of PDAC concomitant with IPMN, in which the tumor diameter was ≤ 20 mm, the sensitivity of combined MRI was higher than that of MRCP alone; however, no significant differences were observed (observer 1, 86.7% vs 60%, P = .13; observer 2, 80% vs 53.3%, P = .13) (Table 4).
Figure 3.
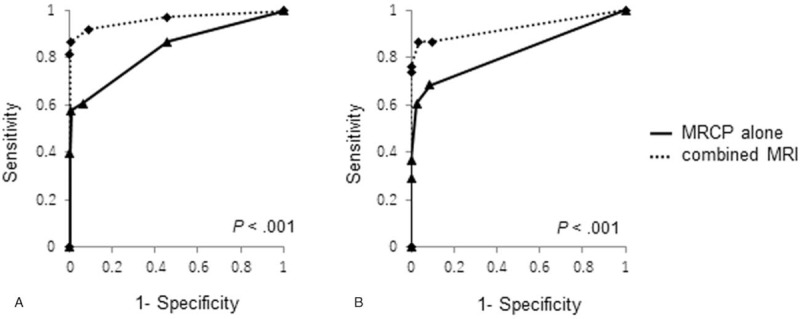
Receiver operating characteristic curves were used to evaluate diagnostic performance of MRCP alone and combined MRI for detection of PDAC concomitant with IPMN. A. For observer 1, area under the receiver operating characteristic curve (Az) was significantly improved in the combined MRI (0.964; 95% CI 0.922–1.006) compared with MRCP alone (0.834; 95% CI 0.753–0.916) (P < .001). B. For observer 2, Az value was significantly improved in the combined MRI (0.926; 95% CI 0.866–0.986) compared with MRCP alone (0.821; 95% CI 0.740–0.926) (P < .001).
Table 4.
The sensitivity of magnetic resonance cholangiopancreatography alone and combined magnetic resonance imaging for the detection of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of which diameter was ≤20 mm.
The interobserver agreement was higher with combined MRI (κ = 0.85, excellent agreement) than with MRCP alone (κ = 0.59, moderate agreement).
4. Discussion
International guidelines on IPMN[4] recommend the MRCP, which excels at visualizing cysts and the pancreatic duct,[28] for the diagnosis and follow-up observation of IPMN. However, no previous studies have reported on the effectiveness of MRCP for diagnosing PDAC concomitant with IPMN. In the present study, the sensitivity of MRCP alone was low (60.5%) and observers failed to detect PDACs located at the end of tail or away from the pancreatic duct. Meanwhile, with combined MRI including DWI, the sensitivity and Az values were significantly increased for both observers. In addition, the interobserver agreement was higher with combined MRI (κ = 0.85, excellent agreement) than with MRCP alone (κ = 0.59, moderate agreement).
IPMN frequently complicated by PDAC distant from IPMN lesion, namely PDAC concomitant with IPMN.[2,3,5–23] Accordingly, it is necessary to factor in the risk of PDAC concomitant with IPMN during follow-up observation for IPMN.
MRCP showed a high diagnostic value (84–95% sensitivity and 82–97% specificity) of ordinary PDAC by evaluation of secondary findings, such as pancreatic duct stenosis with upstream dilatation.[25,26] However, despite highly sensitive to morphological changes in the pancreatic duct, it may be difficult to diagnose PDAC that develop distant from the main pancreatic duct.[29,30] Kamata et al[16] followed patients with branch duct IPMN and found seven cases of PDAC concomitant with IPMN. EUS detected all PDACs, whereas MRI detected only 3 of 7 PDACs (sensitivity, 43%). In the present study, both observers yielded low sensitivity with MRCP alone, failing to detect 40% of PDACs concomitant with IPMN that were located distant from the main pancreatic duct. According to the above results, we should recognize that there is a limit to MRCP for the diagnosis of PDAC concomitant with IPMN, given the presence of lesions that scarcely affect the main pancreatic duct.
Diffusion-weighted imaging, which visualizes the thermally induced motion of water molecules in biological tissues, called Brownian motion, directly visualizes carcinomas.[27] Previous studies have found that ordinary PDAC appears hyperintense compared with the surrounding pancreas parenchyma on DWI.[31–33] However, one study has reported that DWI failed to delineate 47% of ordinary PDACs,[34] and obstructive pancreatitis due to pancreatic duct obstruction might be responsible for the failed detection. In the present study, the sensitivity of combined MRI was significantly improved compared with that of MRCP alone for both observers. MRCP had a limitation because its detectability depends upon the tumor location, however, adding DWI which directly visualizes carcinoma significantly improved the diagnostic performance and interobserver agreement. MRCP and DWI play a complementary role and contribute to improving the diagnostic performance of PDAC concomitant with IPMN.
The present study has several limitations. The First, retrospectively design might have caused selection bias. The second is that the actual complication rate of PDAC concomitant with IPMN is 0.2% to 2% annually, whereas in present study, the number of control group was set to be three times that of the PDAC group. This artificially high rate of complications of PDAC concomitant with IPMN may have affected the diagnostic values. The third is that only 15 patients had PDAC with diameter ≤ 20 mm, making it impossible to reveal the diagnostic capability at early stages. Larger-scale studies are warranted.
In Conclusion, MRCP has limitations in diagnosing PDAC concomitant with IPMN, which was located at the end of tail or away from the pancreatic duct. MRCP with DWI has higher diagnostic performance of PDAC concomitant with IPMN.
Author contributions
Conceptualization: Mitsuharu Fukasawa.
Data curation: Tatsuya Shimizu, Shintaro Ichikawa, Shinichi Takano, Makoto Kadokura, Hiroko Shindo, Ei Takahashi, Sumio Hirose, Yoshimitsu Fukasawa, Hiroshi Hayakawa, Yasuhiro Nakayama, Tatsuya Yamaguchi, Taisuke Inoue, Hiromichi Kawaida.
Formal analysis: Satoshi Kawakami.
Investigation: Tatsuya Shimizu, Shintaro Ichikawa.
Methodology: Tatsuya Shimizu, Shintaro Ichikawa, Utaroh Motosugi.
Project administration: Satoshi Kawakami.
Supervision: Hiroshi Onishi, Nobuyuki Enomoto.
Writing – original draft: Satoshi Kawakami.
Writing – review & editing: Mitsuharu Fukasawa, Tadashi Sato, Shinya Maekawa.
Footnotes
Abbreviations: DWI = diffusion-weighted imaging, FST1WI = fat suppressed T1-weighted imaging, IPMN = intraductal papillary mucinous neoplasm, MRCP = magnetic resonance cholangiopancreatography, MRI = magnetic resonance imaging, PDAC = pancreatic ductal adenocarcinoma, ROC = receiver operating characteristic.
How to cite this article: Kawakami S, Fukasawa M, Shimizu T, Ichikawa S, Sato T, Takano S, Kadokura M, Shindo H, Takahashi E, Hirose S, Fukasawa Y, Hayakawa H, Nakayama Y, Yamaguchi T, Inoue T, Maekawa S, Kawaida H, Motosugi U, Onishi H, Enomoto N. Diffusion-weighted image improves detectability of magnetic resonance cholangiopancreatography for pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm. Medicine. 2019;98:47(e18039).
The authors have no conflicts of interest to disclose.
References
- [1].Adsay NV, Fukushima N, Furukawa T, et al. Intraductal neoplasms of the pancreas. WHO Classification of Tumours of the Digestive System 2010;304–13. [Google Scholar]
- [2].Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas 2011;40:364–70. [DOI] [PubMed] [Google Scholar]
- [3].Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 2011;40:571–80. [DOI] [PubMed] [Google Scholar]
- [4].Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–53. [DOI] [PubMed] [Google Scholar]
- [5].Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol 2006;4:1265–70. [DOI] [PubMed] [Google Scholar]
- [6].Yamaguchi K, Ohuchida J, Ohtsuka T, et al. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2002;2:484–90. [DOI] [PubMed] [Google Scholar]
- [7].Hanada K, Amano H, Hino F, et al. Management strategies for branch duct intraductal papillary-mucinous neoplasms. Digest Endosc 2006;18:S68–72. [Google Scholar]
- [8].Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 2008;57:1561–5. [DOI] [PubMed] [Google Scholar]
- [9].Ikeuchi N. Prognosis of cancer with branch duct type IPMN of the pancreas. World J Gastroenterol 2010;16: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg 2010;251:70–5. [DOI] [PubMed] [Google Scholar]
- [11].Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol 2010;45:952–9. [DOI] [PubMed] [Google Scholar]
- [12].Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy 2010;42:1077–84. [DOI] [PubMed] [Google Scholar]
- [13].Tanno S, Nakano Y, Sugiyama Y, et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology 2010;10:173–8. [DOI] [PubMed] [Google Scholar]
- [14].Ohno E, Itoh A, Kawashima H, et al. Malignant transformation of branch duct-type intraductal papillary mucinous neoplasms of the pancreas based on contrast-enhanced endoscopic ultrasonography morphological changes: focus on malignant transformation of intraductal papillary mucinous neoplasm itself. Pancreas 2012;41:855–62. [DOI] [PubMed] [Google Scholar]
- [15].Ohtsuka T, Kono H, Tanabe R, et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg 2012;204:44–8. [DOI] [PubMed] [Google Scholar]
- [16].Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014;46:22–9. [DOI] [PubMed] [Google Scholar]
- [17].Mandai K, Uno K, Yasuda K. Does a family history of pancreatic ductal adenocarcinoma and cyst size influence the follow-up strategy for intraductal papillary mucinous neoplasms of the pancreas? Pancreas 2014;43:917–21. [DOI] [PubMed] [Google Scholar]
- [18].Kawada N, Uehara H, Nagata S, et al. Imaging morphological changes of intraductal papillary mucinous neoplasm of the pancreas was associated with its malignant transformation but not with development of pancreatic ductal adenocarcinoma. Pancreatology 2015;15:654–60. [DOI] [PubMed] [Google Scholar]
- [19].Sahora K, Crippa S, Zamboni G, et al. Intraductal papillary mucinous neoplasms of the pancreas with concurrent pancreatic and periampullary neoplasms. Eur J Surg Oncol 2016;42:197–204. [DOI] [PubMed] [Google Scholar]
- [20].Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut 2017;66:495–506. [DOI] [PubMed] [Google Scholar]
- [21].Hisada Y, Nagata N, Imbe K, et al. Natural history of intraductal papillary mucinous neoplasm and non-neoplastic cyst: long-term imaging follow-up study. J Hepatobiliary Pancreat Sci 2017;24:401–8. [DOI] [PubMed] [Google Scholar]
- [22].Date K, Ohtsuka T, Nakamura S, et al. Surveillance of patients with intraductal papillary mucinous neoplasm with and without pancreatectomy with special reference to the incidence of concomitant pancreatic ductal adenocarcinoma. Surgery 2018;163:291–9. [DOI] [PubMed] [Google Scholar]
- [23].Ikegawa T, Masuda A, Sakai A, et al. Multifocal cysts and incidence of pancreatic cancer concomitant with intraductal papillary mucinous neoplasm. Pancreatology 2018;18:399–406. [DOI] [PubMed] [Google Scholar]
- [24].Barish MA, Yucel EK, Ferrucci JT. Magnetic resonance cholangiopancreatography. New Engl J Med 1999;341:258–64. [DOI] [PubMed] [Google Scholar]
- [25].Adamek HE, Albert J, Breer H, et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet 2000;356:190–3. [DOI] [PubMed] [Google Scholar]
- [26].Lopez Hanninen E, Amthauer H, Hosten N, et al. Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology 2002;224:34–41. [DOI] [PubMed] [Google Scholar]
- [27].Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 2000;43:828–36. [DOI] [PubMed] [Google Scholar]
- [28].Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7:754–73. [DOI] [PubMed] [Google Scholar]
- [29].Lopez Hänninen E, Ricke J, Amthauer H, et al. Magnetic resonance cholangiopancreatography: image quality, ductal morphology, and value of additional T2- and T1-weighted sequences for the assessment of suspected pancreatic cancer. Acta Radiologica 2004;46:117–25. [DOI] [PubMed] [Google Scholar]
- [30].Li B, Zhang L, Zhang ZY, et al. Differentiation of noncalculous periampullary obstruction: comparison of CT with negative-contrast CT cholangiopancreatography versus MRI with MR cholangiopancreatography. Eur Radiol 2015;25:391–401. [DOI] [PubMed] [Google Scholar]
- [31].Ichikawa T, Erturk SM, Motosugi U, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol 2007;188:409–14. [DOI] [PubMed] [Google Scholar]
- [32].Kartalis N, Lindholm TL, Aspelin P, et al. Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol 2009;19:1981–90. [DOI] [PubMed] [Google Scholar]
- [33].Takakura K, Sumiyama K, Munakata K, et al. Clinical usefulness of diffusion-weighted MR imaging for detection of pancreatic cancer: comparison with enhanced multidetector-row CT. Abdom Imaging 2011;36:457–62. [DOI] [PubMed] [Google Scholar]
- [34].Fukukura Y, Takumi K, Kamimura K, et al. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology 2012;263:732–40. [DOI] [PubMed] [Google Scholar]


