Abstract
Rationale:
Helicobacter pylori infection causes atrophic gastritis in childhood, but atrophic gastritis due to H pylori infection is extremely rare in infancy. The relationship between H pylori infection and chronic diarrhea without protein leakage remains controversial.
Patient concerns:
An 8-month-old male infant presented to our hospital with severe watery diarrhea, erythema, and failure to thrive from approximately 1 month after birth. Blood, stool, esophagogastroduodenoscopy, total colonoscopy, and H pylori urease analysis results were positive, thereby suggesting atrophic gastritis.
Diagnoses:
Atrophic gastritis and chronic diarrhea due to H pylori infection.
Interventions:
We performed H pylori eradication therapy using triple therapy with vonoprazan (6 mg/kg), amoxicillin (300 mg/d), and clarithromycin (120 mg/kg) for 7 days.
Outcomes:
From approximately 1 week after the H pylori eradication therapy, the frequency of defecation had decreased, stool shape had improved, and body weight had gradually increased.
Lessons:
H pylori infection can cause atrophic gastritis and chronic diarrhea even in infancy. Early eradication therapy for H pylori infection may be useful for prevention of gastric cancer and improvement in growth disorders.
Keywords: atrophic gastritis, chronic diarrhea, eradication, Helicobacter pylori, infancy
1. Introduction
Helicobacter pylori (H. pylori) infection is generally established in the family at ≤5 years of age in Japanese children[1] and is known to cause atrophic gastritis in childhood.[2–5] Atrophic gastritis due to H pylori infection is extremely rare in infants; therefore, only few studies have reported this condition in infancy to early childhood.[5]H pylori infection is also known to be one of the causes of Ménétrier disease[6,7] and protein-losing gastroenteropathy,[8] which leads to protein leakage and diarrhea. There are a few studies that have reported the correlation between Ménétrier disease and H pylori infection, chronic diarrhea, and malnutrition.[9] Conversely, H pylori infection has not been shown to cause diarrhea without protein leakage.[10]
Here we report a case of an infant with atrophic gastritis and chronic diarrhea due to H pylori infection who was successfully treated with eradication therapy, with recovered growth.
2. Case presentation
An 8-month-old male infant presented to our hospital with severe watery diarrhea and erythema of the face, neck, and trunk (Fig. 1A and B). From approximately 1 month after birth, the patient had severe watery diarrhea and poor body weight gain. At 3 months of age, he was suspected of having a milk allergy and was hydrolyzed milk; however, his diarrhea did not improve and erythema gradually appeared. The results of the milk-specific immunoglobulin E, allergen-specific lymphocyte stimulation tests for casein, lactoferrin, and lactalbumin as well as the stool eosinophil test performed by the previous doctor were normal, but symptoms worsened and the patient was referred to our hospital for closer examination of his digestive tract.
Figure 1.
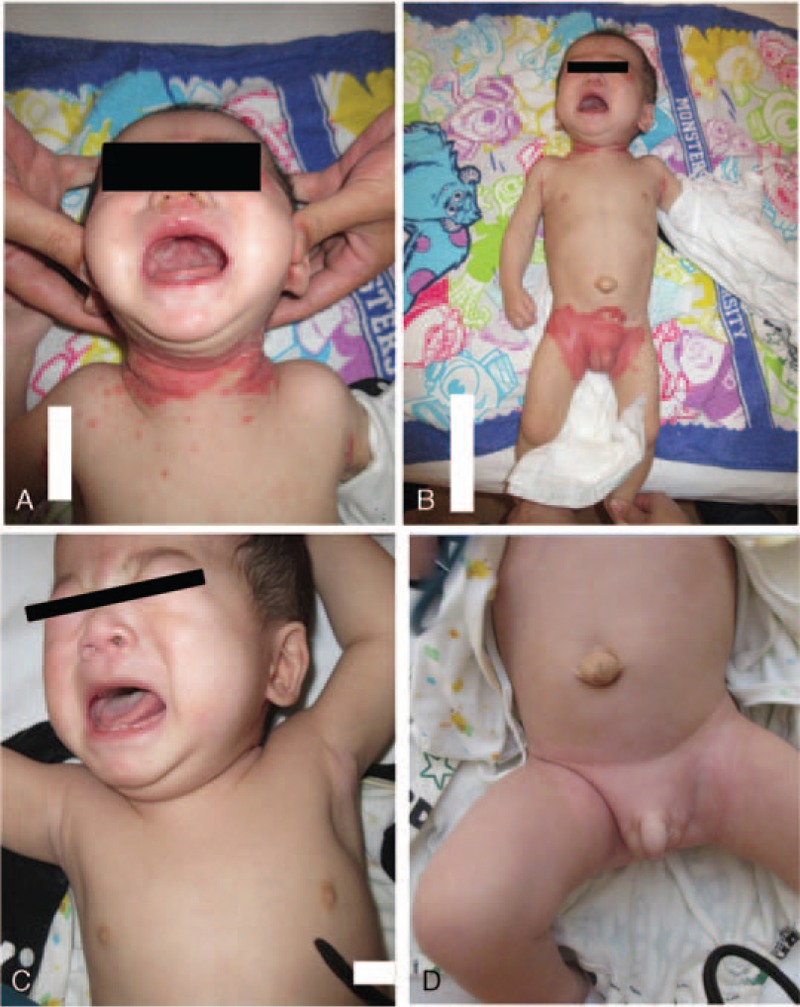
(A, B) Physical examination revealed erythema and crust around the opening area of mouth, eyelid rim, nostril, anal, and penis on admission. The trunk was barely thin. (C, D) After biotin administration, the erythema and crust disappeared quickly and the skin appeared normal.
On admission, the patient had more than 8 episodes of diarrhea per day. His height was 63.0 cm (−3.1 standard deviation [SD]) and body weight was 5.6 kg (−3.0 SD), demonstrating a failure to thrive (Fig. 2). His vital signs were normal: body temperature was 37.4°C, heart rate was 115 beats/min, and blood pressure was 92/44 mm Hg. Physical examination revealed erythema and crust around the opening area of his mouth, eyelid rim, nostril, anus, and penis His hair was normal and slightly thin, and neurological development was generally normal. There were no obvious abnormal findings in the chest and abdomen except for an umbilical hernia.
Figure 2.
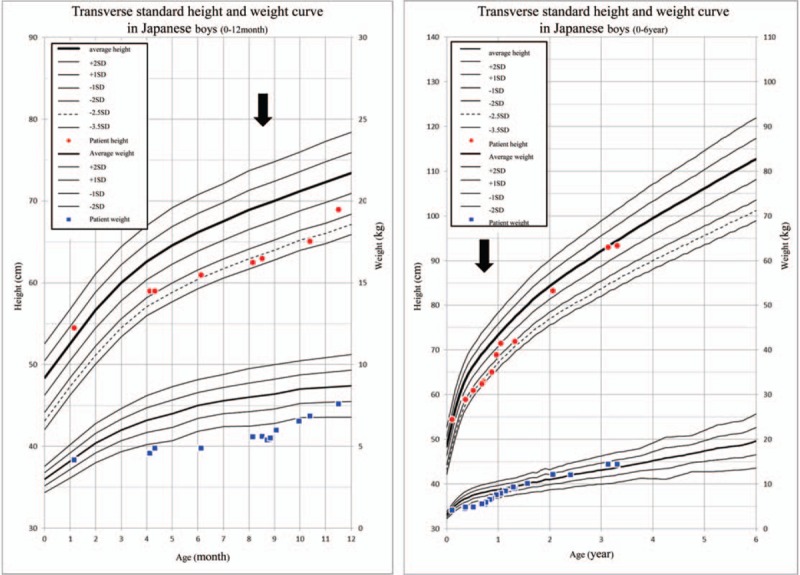
At admission, the height and body weight of the patient were below − 2.0 standard deviations. After Helicobacter pylori eradication therapy, his growth improved drastically in a short time. H pylori eradication therapy; SD = standard deviation Black arrow.
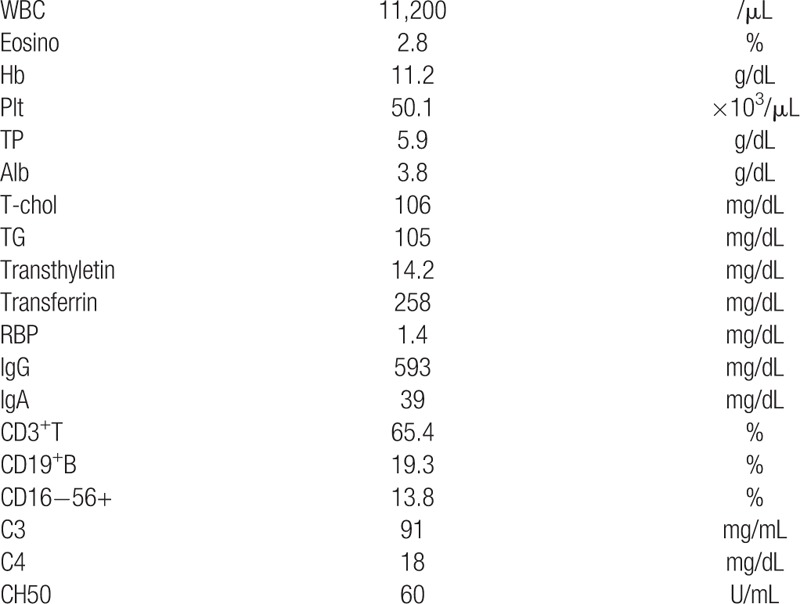
Table 1 shows the blood test results. The patient's white blood cell count was 11,200/μL (normal range: 3300–8600/μL), eosinophil percentage was normal (<5.6%), and hemoglobin level was 11.2 g/dL (normal range >10.5 g/dL). His laboratory data were as follows: total protein level was 5.9 g/dL (normal range 4.3–7.3 g/dL), serum albumin was 3.8 g/dL (normal range 2.5–4.5 g/dL), total cholesterol was 106 mg/dL (normal range 130–220 mg/dL), triglyceride was 105 mg/dL (normal range 36–130 mg/dL), transthyretin was 14.2 mg/dL (normal range 22.0–40.0 mg/dL), transferrin was 258 mg/dL (normal range 240–400 mg/dL), retinol binding protein was 1.4 mg/dL (normal range 2.4–7.0 mg/dL), immunoglobulin G was 593 mg/dL (normal range >234 mg/dL), CD3+T was 65.4% (normal range 50%–77%), CD19+B was 19.3% (normal range 13%–35%); CD16−56+ was 13.8% (normal range 2%–14%), C3 was 91 mg/mL (normal range 65–135 mg/mL), C4 was 18 mg/dL (normal range 13–40 mg/dL), and CH50 was 60 U/mL (normal range 25–48 U/mL). The patient's liver function, kidney function, and electrolyte data were in the normal range. Serum and urine H pylori antibody tests were negative. Urinalysis and urine sediment test results were normal. The stool bacterial culture test did not detect any pathological bacteria, and eosinophils in feces were negative. The concentration of alpha-1 antitrypsin in stool was 10.9 mg/dL (normal range <33 mg/dL). The stool antigen test for H pylori was negative. Chest and abdomen radiography, head plain computed tomography (CT), and abdominal enhanced CT revealed no obvious abnormal findings.
Table 1.
Blood test results at the time of admission.
To observe the presence of digestive tract diseases, we performed esophagogastroduodenoscopy (EGD) and total colonoscopy (TCS). Figure 3 presents the findings of EGD. No abnormal findings were found in the esophagus, but the stomach showed atrophic mucosa localized from the antrum to the upper corner. The degree of atrophy according to the Kimura–Takemoto classification system for atrophic gastritis was grade C-2.[11] No obvious abnormal findings were observed from the duodenal bulb to the second portion. The only pathological findings of the duodenal mucosa were mild edema and infiltration of inflammatory cells in the lamina propria (Fig. 4A and B). Electronic microscopic images showed no abnormal villi (Fig. 4C and D). The rapid urease test using gastric mucosa was positive. TCS revealed no obvious localized lesions from the terminal ileum to the rectum (Fig. 5). The only pathological findings of the terminal ileum and rectum mucosa were mild edema and infiltration of inflammatory cells in the lamina propria (Fig. 6A and B). Electronic microscopic images showed no abnormal villi, similar to that for the duodenum (Fig. 6C and D).
Figure 3.
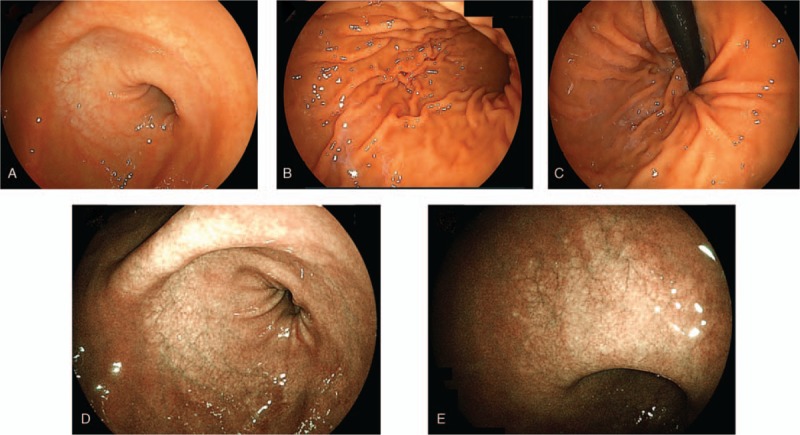
Esophagogastroduodenoscopy revealed gastric mucosal atrophy localized from the antrum to the upper corner. The atrophic grade was C-2 according to the Kimura–Takemoto classification system. The esophagus and duodenum did not present any abnormality. Linked color imaging findings for (A) antral zone, (B) gastric body, (C) cardia and fornix, (D) antral zone, (E) gastric angle.
Figure 4.
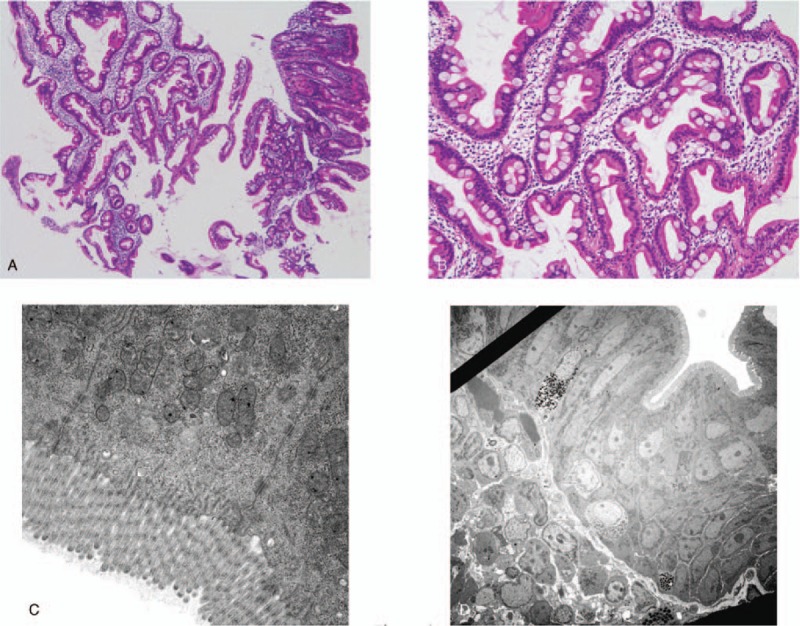
(A, B) The only pathological findings of the duodenal mucosa were mild edema and infiltration of inflammatory cells in the lamina propria (HE stain). (C, D) The electronic microscopic images showed no abnormal villi. HE = hematoxylin and eosin.
Figure 5.
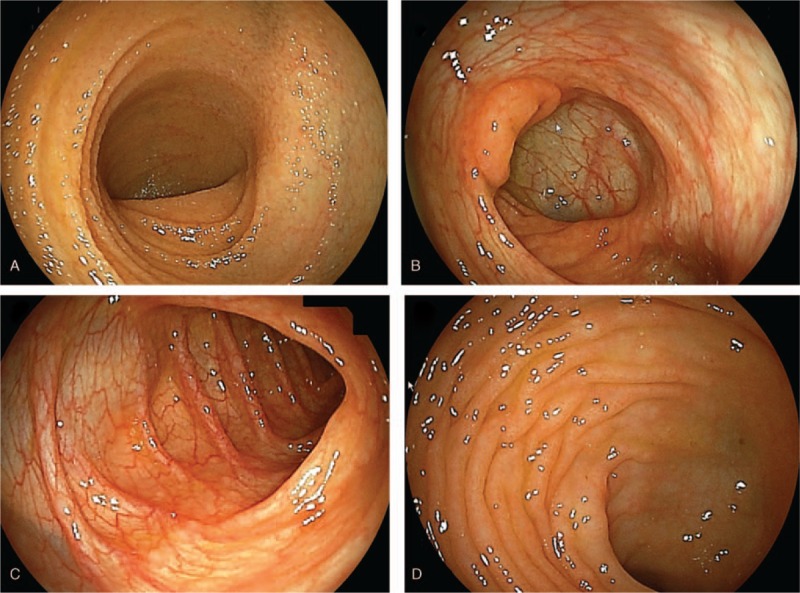
Total colonoscopy revealed no obvious localized lesions from the terminal ileum to the rectum. (A) terminal ileum, (B) ileocecum, (C) descending colon, (D) rectum.
Figure 6.
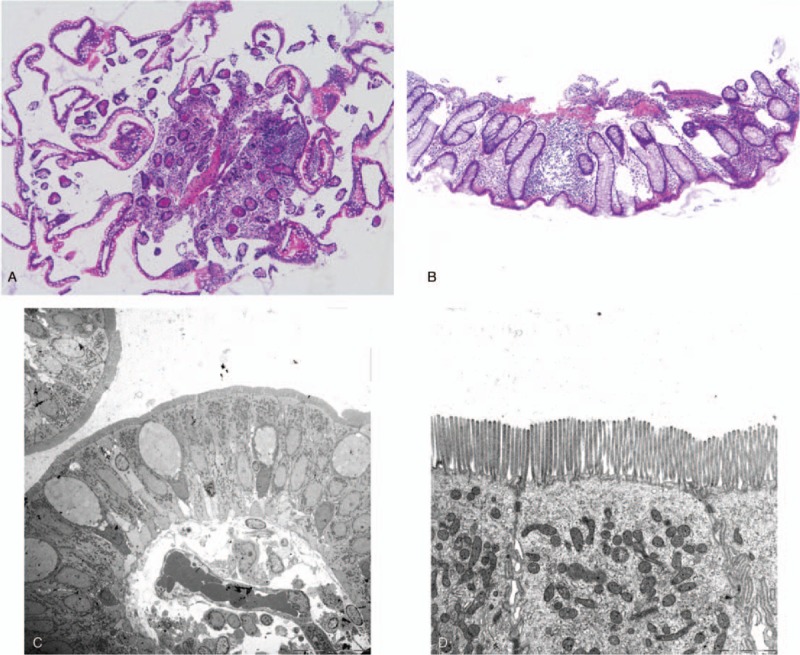
(A, B) The only pathological findings of the terminal ileum and rectum mucosa were mild edema and infiltration of inflammatory cells in the lamina propria (HE stain). (C, D) The electronic microscopic images showed no abnormal villi, similar to that for the duodenum. HE = hematoxylin and eosin.
There were no obvious abnormal findings for the causative agent of diarrhea and poor weight gain other than the positive H pylori urease test. Therefore, we performed H pylori eradication triple therapy using vonoprazan (6 mg/kg), amoxicillin (300 mg/d), and clarithromycin (120 mg/kg) for 7 days. There were no side effects during or after this eradication treatment. From approximately 1 week after H pylori eradication therapy, the frequency of defecation was decreased, stool shape was improved, and body weight was gradually increased. The only other treatment in addition to H pylori eradication therapy was that including coping treatments such as nutrition administration, hydration, vitamin supplementation, and probiotics. After treatment, the diarrhea had completely disappeared and the body weight had steadily increased, reaching the mean value for transverse standard height and weight curve in Japanese boys 1 year later. Skin erythema was rapidly improved by biotin administration because it was thought to be due to the long-term consumption of allergy milk containing no biotin (Fig. 1C and D).
At 3 years and 3 months of age, EGD was performed for the purpose of follow-up (Fig. 7). Atrophic gastric mucous spreading from the entire vestibular area to the side of the comet was observed; it was rated as grade C-2 according to the Kimura–Takemoto classification system, which was the same as the previous test. Figure 8 presents the pathological findings. Atrophy of the crypt epithelium and infiltration of the chronic inflammatory cells into the stroma were noted in both the antrum (Fig. 8A–C) and stomach body (Fig. 8D–F). The pathological findings were suggestive of atrophic gastritis. The rapid urease test for H pylori was negative, serum pepsinogen I was 34.1 ng/mL, and pepsinogen I/II ratio was 4.2. At present, no diarrhea symptoms are observed, and height and body weight has remained within the normal range for Japanese boys
Figure 7.
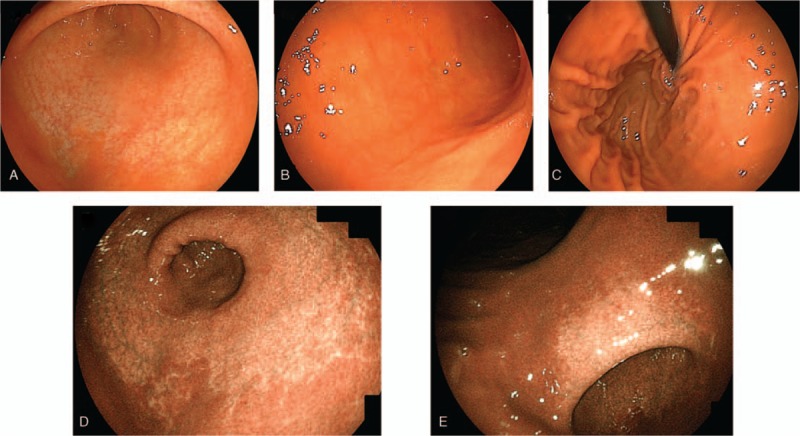
At 2 yr and 6 mo after Helicobacter pylori eradication therapy, esophagogastroduodenoscopy revealed that the degree of gastric mucosal atrophy was unchanged. Linked color imaging findings for (A) antral zone, (B) gastric body, (C) cardia and fornix, (D) antral zone, (E) gastric angle.
Figure 8.
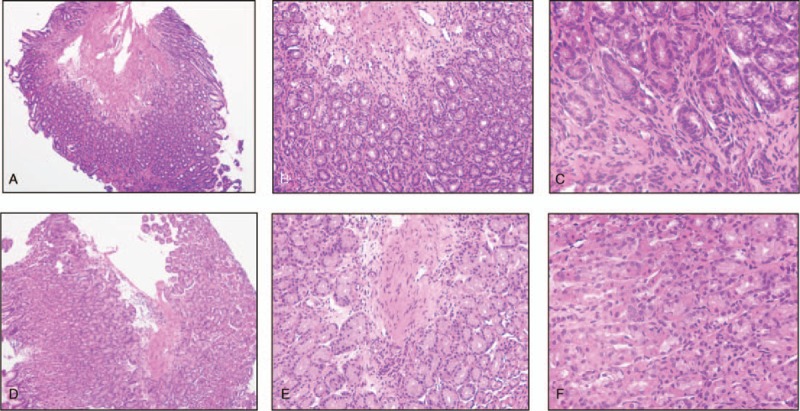
The pathological findings showed atrophy of the crypt epithelium and infiltration of chronic inflammatory cells into the stroma in both the antrum (A–C) and stomach body (D–F).
3. Discussion
The clinical course of the patient's disease provided 2 important clinical suggestions. First, H pylori infection can cause atrophic gastritis even in early infancy. Several studies have suggested that H pylori eradication in young children before gastric mucosa atrophy can effectively prevent new gastric cancers.[12,13] This work lead to clinical trials for screening and treatment of H pylori infection among junior high school students as a prevention strategy against gastric cancer in Japan.[14,15] However, H pylori eradication therapy for children at <15 years of age is not covered by insurance in Japan. Severe gastric mucosal atrophy and submucosal inflammation are rarely observed in children[16–18]; moreover, H pylori eradication therapy is not recommended in junior high school students because children have a low risk of differentiated gastric cancer.[16,17] In our study, atrophic gastritis was recognized in early childhood and gastric mucosal atrophy persisted for >2 years after eradication. Because gastric mucosal atrophy is a cause of gastric cancer,[19] we believe that H pylori eradication must be performed as soon as possible.
Second, H pylori infection can cause chronic diarrhea without protein leakage as well as growth failure. The patient in our study presented with chronic diarrhea with no apparent protein leakage in the stool. No abnormal findings were observed except the positive H pylori urease test; therefore, H pylori eradication therapy drastically improved the diarrhea. Although the relationship between H pylori infection and chronic diarrhea without protein leakage remains controversial, chronic diarrhea positive for H pylori infection should be considered an indication for eradication treatment. Although H pylori infection may play a protective role against bacterial diarrhea in children,[20] it is known to affect the gastric and intestinal microbiota[21–23] and may cause chronic diarrhea due to any cause other than infection. In our study, we found that long exposure to H pylori infection possibly decreased the growth rate, which adversely affected the patient's height and body weight. Regardless of the improvement in diarrhea, H pylori eradication itself may contribute to improvement in growth disorders. We believe that H pylori infection must be considered as a cause of chronic diarrhea and growth disorders even in early childhood.
In conclusion, H pylori infection can cause atrophic gastritis and chronic diarrhea without protein leakage as well as growth failure even in early infancy. We believe that early H pylori eradication therapy must be promoted for prevention of gastric cancer and improvement in growth disorders.
Acknowledgments
The authors would like to thank the patient and his parents for consenting and allowing us to write and publish this case report.
Author contributions
Conceptualization: Toshihiko Kakiuchi.
Data curation: Toshihiko Kakiuchi, Aiko Nakayama.
Formal analysis: Toshihiko Kakiuchi.
Investigation: Toshihiko Kakiuchi, Aiko Nakayama, Ryo Shimoda, Muneaki Matsuo.
Methodology: Toshihiko Kakiuchi.
Project administration: Muneaki Matsuo.
Supervision: Muneaki Matsuo.
Validation: Toshihiko Kakiuchi.
Writing – original draft: Toshihiko Kakiuchi, Aiko Nakayama.
Writing – review and editing: Muneaki Matsuo.
Toshihiko kakiuchi orcid: 0000-0002-9995-5522.
Footnotes
Abbreviations: CT = computed tomography, EGD = esophagogastroduodenoscopy, H pylori = Helicobacter pylori, TCS = total colonoscopy.
How to cite this article: Kakiuchi T, Nakayama A, Shimoda R, Matsuo M. Atrophic gastritis and chronic diarrhea due to Helicobacter pylori infection in early infancy: a case report. Medicine. 2019;98:47(e17986).
Informed written consent was obtained from the patient's parents for publishing this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Konno M, Fujii N, Yokota S, et al. Five-year follow-up study of mother-to-child transmission of Helicobacter pylori infection detected by a random amplified polymorphic DNA fingerprinting method. J Clin Microbiol 2005;43:2246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ozawa K, Kato S, Sekine H, et al. Gastric epithelial cell turnover and mucosal protection in Japanese children with Helicobacter pylori infection. J Gastroenterol 2005;40:236–46. [DOI] [PubMed] [Google Scholar]
- [3].Brigic E, Hadzic D, Mladina N. Childhood and coress model of carcinogenesis. Med Arch 2012;66:375–7. [DOI] [PubMed] [Google Scholar]
- [4].Boukthir S, Aouididi F, Mazigh Mrad S, et al. Chronic gastritis in children. Tunis Med 2007;85:756–60. [PubMed] [Google Scholar]
- [5].Yu Y, Su L, Wang X, et al. Association between Helicobacter pylori infection and pathological changes in the gastric mucosa in Chinese children. Intern Med 2014;53:83–8. [DOI] [PubMed] [Google Scholar]
- [6].Lepore MJ, Smith FB, Bonanno CA. Campylobacter-like organisms in patient with Menetrier's disease. Lancet 1988;1:466. [DOI] [PubMed] [Google Scholar]
- [7].Huh WJ, Coffey RJ, Washington MK. Ménétrier's disease: its mimickers and pathogenesis. J Pathol Transl Med 2016;50:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sato T, Chiguchi G, Inamori M, et al. Protein-losing gastroenteropathy and gastric polyps: successful treatment by Helicobacter pylori eradication. Digestion 2007;75:99. [DOI] [PubMed] [Google Scholar]
- [9].Sullivan PB, Thomas JE, Wight DG, et al. Helicobacter pylori in Gambian children with chronic diarrhoea and malnutrition. Arch Dis Child 1990;65:189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Castro-Rodriguez JA, Leon-Barua R, Penny M. Helicobacter pylori is not a determinant factor of persistent diarrhoea or malnutrition in Peruvian children. Trans R Soc Trop Med Hyg 1999;93:537–9. [DOI] [PubMed] [Google Scholar]
- [11].Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology 1972;63:584–92. [PubMed] [Google Scholar]
- [12].Kato S, Kikuchi S, Nakajima S. When does gastric atrophy develop in Japanese children? Helicobacter 2008;13:278–81. [DOI] [PubMed] [Google Scholar]
- [13].Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kusano C, Gotoda T, Ishikawa H, et al. The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer 2017;20: Suppl 1: 16–9. [DOI] [PubMed] [Google Scholar]
- [15].Kakiuchi T, Matsuo M, Endo H, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: a preliminary report. J Gastroenterol 2019;54:699–707. [DOI] [PubMed] [Google Scholar]
- [16].Kato S, Nakajima S, Nishino Y, et al. Association between gastric atrophy and Helicobacter pylori infection in Japanese children: a retrospective multicenter study. Dig Dis Sci 2006;51:99–104. [DOI] [PubMed] [Google Scholar]
- [17].Dimitrov G, Gottrand F. Does gastric atrophy exist in children? World J Gastroenterol 2006;12:6274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ricuarte O, Gutierrez O, Cardona H, et al. Atrophic gastritis in young children and adolescents. J Clin Pathol 2005;58:1189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Take S, Mizuno M, Ishiki K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J Gastroenterol 2007;42: Suppl 17: 21–7. [DOI] [PubMed] [Google Scholar]
- [20].Monajemzadeh M, Abbasi A, Tanzifi P, et al. The relation between Helicobacter pylori infection and acute bacterial diarrhea in children. Int J Pediatr 2014;2014:191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Llorca L, Perez-Perez G, Urruzuno P, et al. Characterization of the gastric microbiota in a pediatric population according to Helicobacter pylori status. Pediatr Infect Dis J 2017;36:173–8. [DOI] [PubMed] [Google Scholar]
- [22].Brawner KM, Kumar R, Serrano CA, et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol 2017;10:1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Benavides-Ward A, Vasquez-Achaya F, Silva-Caso W, et al. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res Notes 2018;11:468. [DOI] [PMC free article] [PubMed] [Google Scholar]


