Abstract
Aims:
To investigate the usefulness of contrast-enhanced ultrasonography for diagnosing renal cell carcinoma (RCC) in dialysis patients.
Material and methods:
Of 1301 dialysis patients who underwent abdominal computed tomography (CT) between January 2012 and March 2017, 19 were suspected to have solid renal lesions; of these patients, 18 gave consent for and underwent contrast-enhanced ultrasonography with perflubutane in addition to CT; 13 underwent dynamic contrast-enhanced CT, and 5, who could not be administered iodinated contrast media, underwent unenhanced CT. The final diagnoses were based on histopathological findings or the presence/absence of enlargement of the lesion during follow-up.
Results:
Of the 19 lesions in 18 patients, 14 were diagnosed as RCC and 5 as benign cysts. CT facilitated accurate diagnosis in 10/19 lesions (52.6%) with obvious enhancement (≥20 Hounsfield units [HU]), while definitive diagnosis by CT was difficult in 9 lesions: 2 lesions showed ambiguous enhancement (10–20 HU), 1 lesion was an inflammatory cyst with obvious enhancement, and 6 lesions were assessed by unenhanced CT. Compared with CT, contrast-enhanced ultrasonography allowed more accurate diagnosis (McNemar test, P = .02) in 17/19 lesions (89.5%, 14 RCC and 3 cysts; including all lesions assessed by unenhanced CT and 2 with ambiguous enhancement on CT), with 1 false-positive (inflammatory cyst with hyper-enhancement) and 1 false-negative result due to deep location of the lesion.
Conclusions:
Contrast-enhanced ultrasonography was useful for the diagnosis of RCC in dialysis patients with suspected solid renal lesions especially when contrast enhancement was not obvious on CT or contrast-enhanced CT could not be performed.
Keywords: contrast media, dialysis, renal cell carcinoma, ultrasonography
1. Introduction
Dialysis patients are at a higher risk of developing renal cell carcinoma (RCC) compared with healthy individuals.[1] Specifically, the reported prevalence of RCC in hemodialysis patients with acquired cystic disease of the kidney (ACDK) is 3% to 7%.[1] It has been reported that the prevalence of ACDK increases with the duration of hemodialysis, and that 90% of patients with a hemodialysis duration of ≥5 years have ACDK.[1,2] Recently, although a tendency towards improved survival of patients after initiation of hemodialysis has been observed [3,4], and mortality due to cardiovascular disease in hemodialysis patients is decreasing, mortality due to malignant tumors in these patients has not decreased[3]; thus, early detection of RCC by imaging is important in hemodialysis patients.
At present, ultrasonography and computed tomography (CT) are routinely used to diagnose renal masses in dialysis patients. In particular, evaluation of the presence or absence of blood flow is useful to differentiate between benign and malignant masses, and therefore, dynamic contrast-enhanced CT is considered a useful modality in patients who can undergo this examination. However, the contrast enhancement of RCCs associated with ACDK is often lower than that of conventional RCC,[5] which sometimes makes it difficult to differentiate RCC from hemorrhagic cysts. Furthermore, CT cannot be used to assess intratumoral blood flow in patients with asthma or iodine hypersensitivity, who cannot receive iodinated contrast media.[6] Magnetic resonance imaging (MRI) can also be performed to assess intratumoral blood flow; however, it has been reported that administration of gadolinium-based contrast agents to patients with renal failure is associated with the development of nephrogenic systemic fibrosis, and currently, use of gadolinium-based contrast agents is avoided in patients with renal failure. The usefulness of diffusion-weighted MRI, which does not require the use of contrast medium, as a substitute, has also been reported[7]; however, the cost of MRI is higher than that of US and its accessibility is poor compared with US or CT.
In recent years, there are reports of clinical studies investigating the usefulness of contrast-enhanced ultrasonography in differentiating benign from malignant cystic and solid masses.[8–16] However, to the best of our knowledge, only 1 case involving imaging of an RCC in a hemodialysis patient using contrast-enhanced ultrasonography has been reported[11]; no clinical systematic studies have investigated the usefulness of contrast-enhanced ultrasonography for evaluating renal masses in dialysis patients. Furthermore, the use of perflubutane microbubbles as ultrasonographic contrast agents in the liver has been reported to be associated with high contrast enhancement sensitivity and a low incidence of allergy; in addition, it can be used in patients with asthma or renal function impairment.[17] However, to the best of our knowledge, no detailed study on the use of perflubutane microbubbles as ultrasonographic contrast agents to evaluate renal masses has been performed.
The purpose of this study was to investigate the usefulness of contrast-enhanced ultrasonography with perflubutane for the diagnosis of RCC in dialysis patients.
2. Material and methods
2.1. Patient population
Between December 2012 and March 2017, a total of 1301 dialysis patients underwent abdominal CT at our hospital. Of these dialysis patients, 19 with suspected solid renal tumors were provided an explanation regarding the research and procedures involved in the study; 18 of these patients provided written informed consent and underwent contrast-enhanced ultrasonography beside CT. This prospective study was conducted with the approval of the Ethics Committee.
Suspicion of a solid renal mass was based on the following findings: contrast enhancement ≥10 Hounsfield units (HU) (including equivocal enhancement) on contrast-enhanced CT (in 13 patients with 13 lesions) and trend towards increase in the size of the mass (30.0 ± 12.1% increase [range: 14.8%–46.2%]) during the period prior to involvement in the study (345 ± 103 days; range, 142–421 days) on unenhanced CT (in 5 patients with 6 lesions). Simple cysts with CT values of <20 HU on unenhanced CT, lesions with CT values of ≥20 HU on unenhanced CT but with contrast enhancement of <10 HU on contrast-enhanced CT, which are considered as cysts, and lesions with obvious fat areas diagnosed as angiomyolipoma were not included.
Of the 18 patients, 13 (72%) underwent dynamic contrast-enhanced CT and 5 (28%) underwent unenhanced CT. Of the latter 5 patients who underwent unenhanced CT, 3 were able to urinate by themselves and only unenhanced CT was performed to preserve the residual renal function, 1 had a history of adverse reactions to contrast agents, and 1 refused to undergo injection of iodinated contrast medium.
2.2. Scanning protocol
The following CT scanners were used: LightSpeed VCT, Discovery CT750 HD, Revolution CT (GE Healthcare, Waukesha, WI), Aquilion 64, and Aquilion ONE (Canon medical systems, Otawara, Japan). In both unenhanced CT and contrast-enhanced CT, only renal images were obtained at 120 kVp and 100 to 200 mA with 0.625 to 1 mm collimation. In dynamic contrast-enhanced CT, 50 to 80 mL (body weight × 0.6 mL/kg) of contrast medium was injected over a period of 25 seconds, and images were obtained at 40 seconds (corticomedullary phase), 90 seconds (nephrographic phase), and 180 seconds (excretory phase) after the contrast injection.
LOGIQ E9 (GE Healthcare) was used for contrast-enhanced ultrasonography. Before imaging, 0.5 mL/body weight of perflubutane microbubbles (Sonazoid, Daiichi-Sankyo Pharmaceuticals, Tokyo, Japan, and GE Healthcare) was administered by intravenous injection, followed by 20 mL of physiological saline. Images were obtained using a convex probe with a center frequency of 4.0 to 6.0 MHz, with focus on the lower border of the mass, and a mechanical index (MI) of 0.16 to 0.24 in the tissue harmonic imaging mode. Images were obtained during a 30-second breath-hold after contrast agent administration.
2.3. Data analysis
With regard to CT, contrast-enhanced CT images in the corticomedullary and nephrographic phases and unenhanced CT images with a slice thickness of 1.25 to 2 mm were evaluated. The maximum circular region of interest was set within the solid portion of the mass. CT values were measured in each phase, and the difference between the CT value at the highest contrast enhancement and the unenhanced CT value was defined as contrast enhancement. Images were evaluated by 2 board-certified radiologists, and the extent of contrast enhancement was determined by consensus: contrast enhancement of ≥20 HU was defined as obvious enhancement, contrast enhancement of 10 to 20 HU was defined as ambiguous enhancement, and contrast enhancement of <10 HU was defined as non-enhancement.
With regard to interpretation of the ultrasonographic images, 2 board-certified radiologists visually evaluated the contrast enhancement of the lesions: contrast enhancement equivalent to or more than that of the renal parenchyma was defined as hyper-enhancement, contrast enhancement lower than that of the renal parenchyma was defined as hypo-enhancement, and barely detectable contrast enhancement was defined as non-enhancement.
The final diagnoses were made based on:
-
(1)
histopathological examination,
-
(2)
the presence of enlargement of the lesion and contrast enhancement of ≥ 20 HU in the CT examination, or
-
(3)
an absence of enlargement of the lesion in the CT examination conducted at least 1 year later (1228 ± 616 days; range, 369–1909 days).
2.4. Statistical analysis
Continuous variables are presented as the mean ± standard deviation. McNemar test was used to compare the diagnostic accuracy for RCC between CT and contrast-enhanced ultrasonography. The significance level for the test was set at P < .05 (2-sided). All data were analyzed using commercially available software (JMP version 12; SAS Institute Inc, Cary, NC).
3. Results
3.1. Final diagnosis
Eighteen patients (15 males and 3 females; mean age, 61.3 ± 10.7 years [range: 45–79 days]) were suspected to have a solid mass in the kidney by CT examination. Nineteen lesions were identified in the 18 patients. Of these, 14 lesions were considered to be RCCs. Of the 14 lesions, 11 were histopathologically confirmed as RCCs (5 cases of clear-cell RCC, 1 case of multilocular cystic RCC, and 5 cases of acquired cystic disease [ACD]-associated RCC); 3 were clinically considered as RCC because of the enlargement of the lesion during the follow-up period (from 14.7 to 20.8 mm in average of long and short diameters during 1206 days, 3.7 to 5.2 mm during 1380 days, and 6.8 to 9.4 mm during 1830 days, respectively) and obvious enhancement (contrast enhancement of 35 HU, 169 HU, and 151 HU, respectively); and 2 were clinically considered as RCCs because of intense early enhancement of ≥100 HU on dynamic contrast-enhanced CT. There are very few benign tumors that show contrast enhancement of ≥100 HU,[18–20] and therefore, we considered these lesions as most likely to be RCC. The mean contrast enhancement of these 11 lesions on dynamic contrast-enhanced CT was 82.1 HU (standard deviation [SD], 49.4 HU). The remaining 5 lesions showed no enlargement during follow-up (mean follow-up duration 3.1 years: 1.0–5.2 years), and were diagnosed as cysts, including 1 inflammatory cyst.
3.2. Diagnostic performance of contrast-enhanced ultrasonography
Of the 13 lesions assessed by dynamic contrast-enhanced CT, 11 (77%) showed obvious enhancement (mean: 85.5 ± 45.8 HU, range: [25–151 HU]) (Fig. 1). Of these 11 lesions, 10 lesions showed hyper-enhancement on ultrasonography (Fig. 2). One lesion that was located at a depth of 9.8 cm from the body surface was difficult to detect by ultrasonography. Of the 10 lesions that showed hyper-enhancement on ultrasonography, 9 were considered to be RCCs, and the remaining 1 lesion was clinically diagnosed as an inflammatory cyst, considering that the lesion decreased in size during the follow-up despite of contrast enhancement by both CT and ultrasonography.
Figure 1.
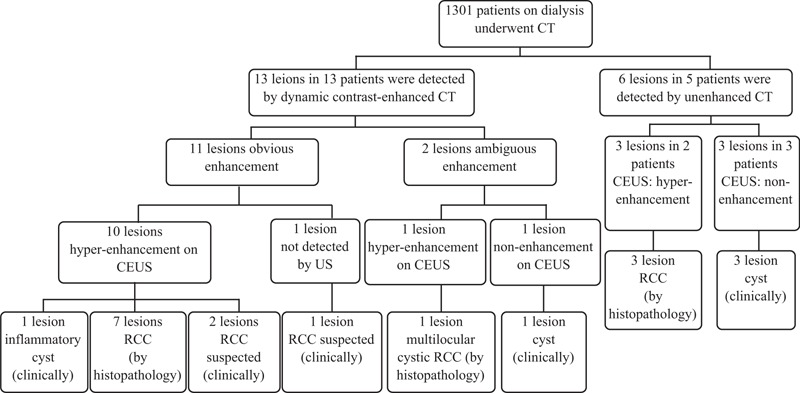
Patients on dialysis who were suspected to have renal cell carcinoma on abdominal computed tomography.
Figure 2.
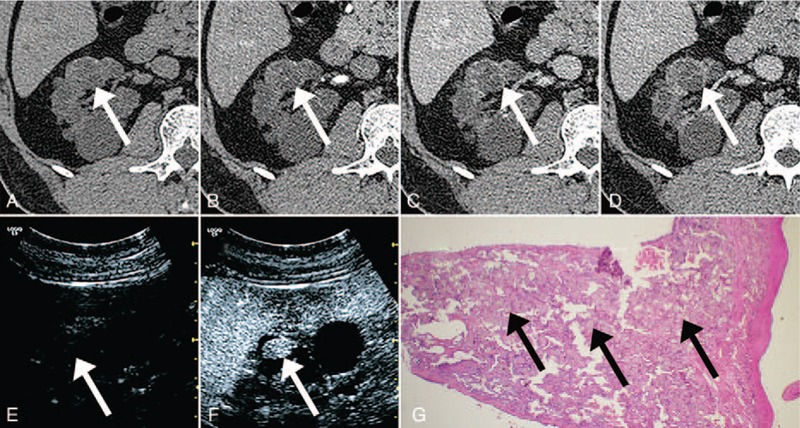
A 45-year-old male with acquired cystic disease-associated renal cell carcinoma confirmed by pathological examination. The mass (arrows) shows obvious enhancement (25 [HU] increase) on dynamic contrast-enhanced computed tomography (CT) and hyper-enhancement in contrast-enhanced ultrasonography. A, Unenhanced CT. B, Dynamic contrast-enhanced CT, early phase. C, Dynamic contrast-enhanced CT, parenchymal phase. D, Dynamic contrast-enhanced CT, delayed phase. E, Precontrast ultrasonography. F, Ultrasonography 30 seconds after the administration of contrast medium. G, Hematoxylin and eosin-stained slide. A papillary tumor appears to arise from the cystic wall. The tumor is composed of cells with eosinophilic cytoplasm, arranged in papillary and cribriform patterns. Arrows show calcium oxalate crystal depositions.
Of the 13 lesions assessed by dynamic contrast-enhanced CT, 2 (15%) showed ambiguous enhancement (10 and 15 HU). Of these 2 lesions, 1 showed hyper-enhancement on ultrasonography, and was diagnosed post-surgery as multilocular cystic RCC (Fig. 3). The other lesion showed no enhancement on contrast-enhanced ultrasonography and was diagnosed as a cyst; this lesion did not increase in size during subsequent follow-up (1471 days).
Figure 3.
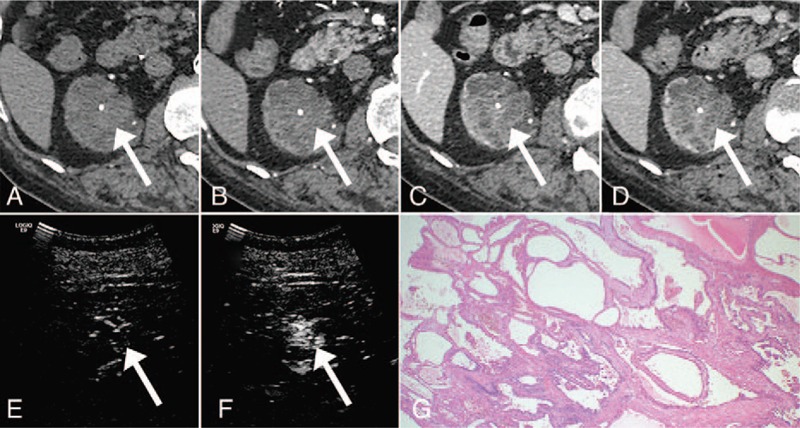
A 65-year-old male with multilocular cystic renal cell carcinoma (RCC). A, Unenhanced computed tomography (CT). B, Dynamic contrast-enhanced CT, early phase. C, Dynamic contrast-enhanced CT, parenchymal phase. D, Dynamic contrast-enhanced CT, delayed phase. E, Precontrast ultrasonography. F, Ultrasonography 30 seconds after the administration of contrast medium. The enhancement was ambiguous on the dynamic contrast-enhanced CT, while it was obvious on the contrast-enhanced US. G, Hematoxylin and eosin-stained slide. Multiple fibrous septa are lined by a single layer of low-grade tumor cells.
Of the 6 lesions in 5 patients assessed by unenhanced CT, 3 lesions in 2 patients showed hyper-enhancement on contrast-enhanced ultrasonography; these lesions were surgically resected, and were histopathologically confirmed as RCCs (double cancer of ACD-associated RCC and clear-cell RCC in 1 patient, and clear-cell RCC in 1 patient). The remaining 3 lesions in 3 patients were diagnosed as cysts by ultrasonography because they showed non-enhancement on contrast-enhanced ultrasonography. In fact, these lesions also did not increase in size during the subsequent follow-up (369, 634, and 1758 days, respectively) and were clinically diagnosed as cysts.
Overall, when the presence or absence of contrast enhancement (≥20 HU) on CT was used as a diagnostic criterion for RCC, 10 (52.6%) of the 19 lesions could be diagnosed by CT, while a definitive diagnosis by CT proved difficult in the remaining 9 lesions (2 lesions showing ambiguous enhancement on dynamic contrast-enhanced CT, 1 lesion being inflammatory cyst, and 6 lesions assessed by unenhanced CT). On the other hand, contrast-enhanced ultrasonography allowed accurate diagnosis of 17 of the 19 lesions (89.5%), with 1 false-positive result (inflammatory cyst with hyper-enhancement) and 1 false-negative result (lesion located at a depth of 9.8 cm from the body surface). There was a significant difference in the diagnostic accuracy between CT and contrast-enhanced ultrasonography (P = .02).
4. Discussion
This study demonstrated that in dialysis patients with suspected solid renal lesions on CT, contrast-enhanced ultrasonography was useful for differentiating between benign and malignant lesions, especially when contrast enhancement was not obvious on CT (2/2 lesions) or contrast-enhanced CT could not be performed (6/6 lesions in 5 patients). The current study also found that almost all lesions showing obvious enhancement on dynamic contrast-enhanced CT, also showed hyper-enhancement on ultrasonography using perflubutane microbubbles (10/11 lesions). These findings are important because when a definitive diagnosis by CT is difficult, contrast-enhanced ultrasonography could be used as an additional or alternative diagnostic tool to CT for evaluating renal masses in dialysis patients without additional radiation exposure.
To the best of our knowledge, this is the first detailed study to use perflubutane microbubbles as ultrasonographic contrast agents to evaluate renal masses. Among the second generation ultrasonographic contrast agents, perflubutane microbubbles reportedly show the strongest enhancement signal of first harmonic in scattered imaging.[21] In addition, perflubutane microbubbles are taken up or trapped by Kupffer cells of the liver in the post-vascular phase; they are also reported to be useful in the evaluation of hepatic masses.[17] In previous studies regarding other second generation ultrasonographic contrast agents, it has been reported that contrast-enhanced ultrasonography with sulfur hexafluoride microbubbles (SonoVue, Bracco, Milan, Italy) is comparable to contrast-enhanced CT in the evaluation of renal solid masses[16], and that contrast-enhanced ultrasonography with other second-generation contrast agents such as perflutren lipid microsphere (Definity, Lantheus Medical Imaging, North Billerica, United States) and perflutren protein-type A microspheres (Optison, Mallinckrodt St. Louis, United States and GE Healthcare, Waukesha, United States) has high sensitivity (94.7–100%) and specificity (93.9%–95%) for evaluating the indeterminate renal masses on CT or unenhanced ultrasonography.[14,22,23] Additionally, Paudice et al reported that, similar to contrast-enhanced CT, contrast-enhanced ultrasonography enabled differentiation of complex cysts from solid lesions in ACDK in renal transplant recipients.[24] Our study adds to the current literature, that is, our findings indicate that contrast-enhanced ultrasonography would also be useful in dialysis patients with suspected renal masses.
Of the 11 lesions that showed obvious enhancement on contrast-enhanced CT, 1 lesion was undetectable by contrast-enhanced ultrasonography because it was deep-seated (9.8 cm from the body surface), indicating that contrast-enhanced ultrasonography cannot assess blood flow in some deep-seated lesions. Further, it has been reported that deep-seated lesions located >6 cm from the body surface are difficult to assess quantitatively by contrast-enhanced liver ultrasonography[25]; this limitation of the contrast-enhanced ultrasonography is expected to be solved in the future by the technological progress of ultrasonographic equipment.
This study had some limitations. First, the number of patients was small, and prospective studies with a large number of patients are required. Second, a definitive pathological diagnosis was not made in all cases, and lesions assessed as benign or malignant based on clinical judgment were also included. However, we believe that the absence of enlargement of the lesion on follow-up of at least 1 year (1228 ± 616 days; range, 369–1909 days) indicated that the lesion was benign.
5. Conclusion
In dialysis patients with suspected solid renal lesions on CT, contrast-enhanced ultrasonography was useful for differentiating between benign and malignant lesions especially when contrast enhancement was not obvious on CT or contrast-enhanced CT could not be performed. Thus, contrast-enhanced ultrasonography could be used as an additional or alternative diagnostic tool to CT for evaluating renal masses in dialysis patients without additional radiation exposure.
Acknowledgments
The authors acknowledge the valuable assistance of Satoru Abe, Yuji Masuda, Chigusa Mochizuki, Shiho Tanaka, Keiichi Narita, MD, Mikio Nakamura, MD, Maki Ooi, and Kyoko Komatsu.
Author contributions
Conceptualization: Seishi Nakatsuka, Masahiro Jinzaki.
Data curation: Masahiro Hashimoto, Kiyoshi Ohkuma, Hirotaka Akita, Ryuichi Mizuno, Mototsugu Oya, Masahiro Jinzaki.
Formal analysis: Yoshitake Yamada.
Funding acquisition: Hirotaka Akita.
Investigation: Masahiro Hashimoto, Kiyoshi Ohkuma.
Methodology: Masahiro Hashimoto.
Supervision: Seishi Nakatsuka, Mototsugu Oya, Masahiro Jinzaki.
Writing – original draft: Masahiro Hashimoto, Yoshitake Yamada.
Writing – review & editing: Kiyoshi Ohkuma, Hirotaka Akita, Yoshitake Yamada, Seishi Nakatsuka, Ryuichi Mizuno, Mototsugu Oya, Masahiro Jinzaki.
Footnotes
Abbreviations: ACD = acquired cystic disease, ACDK = ACD of the kidney, CT = computed tomography, HU = Hounsfield units, MI = mechanical index, MRI = magnetic resonance imaging, RCC = renal cell carcinoma, SD = standard deviation.
How to cite this article: Hashimoto M, Ohkuma K, Akita H, Yamada Y, Nakatsuka S, Mizuno R, Oya M, Jinzaki M. Usefulness of contrast-enhanced ultrasonography for diagnosis of renal cell carcinoma in dialysis patients: comparison with computed tomography. Medicine. 2019;98:47(e18053).
This study was supported in part by JSPS KAKENHI; Grant Number JP15K19815.
The authors have no conflicts of interests to disclose.
References
- [1].Tickoo SK, dePeralta-Venturina MN, Harik LR, et al. Spectrum of epithelial neoplasms in end-stage renal disease - an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol 2006;30:141–53. [DOI] [PubMed] [Google Scholar]
- [2].Farivar-Mohseni H, Perlmutter AE, Wilson S, et al. Renal cell carcinoma and end stage renal disease. J Urol 2006;175:2018–20. discussion 2021. [DOI] [PubMed] [Google Scholar]
- [3].United States Renal Data System 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. 2016;Bethesda, MD:National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, https://www.usrds.org/2016/view/ [Google Scholar]
- [4].Nakai S, Masakane I, Shigematsu T, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2007). Ther Apher Dial 2009;13:457–504. [DOI] [PubMed] [Google Scholar]
- [5].Takebayashi S, Hidai H, Chiba T, et al. Using helical CT to evaluate renal cell carcinoma in patients undergoing hemodialysis: value of early enhanced images. AJR Am J Roentgenol 1999;172:429–33. [DOI] [PubMed] [Google Scholar]
- [6].Namasivayam S, Kalra MK, Torres WE, et al. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol 2006;12:210–5. [DOI] [PubMed] [Google Scholar]
- [7].Akita H, Jinzaki M, Akita A, et al. Renal cell carcinoma in patients with acquired cystic disease of the kidney: assessment using a combination of T2-weighted, diffusion-weighted, and chemical-shift MRI without the use of contrast material. J Magn Reson Imaging 2014;39:924–30. [DOI] [PubMed] [Google Scholar]
- [8].Chang EH, Chong WK, Kasoji SK, et al. Management of indeterminate cystic kidney lesions: review of contrast-enhanced ultrasound as a diagnostic tool. Urology 2016;87:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gerst S, Hann LE, Li D, et al. Evaluation of renal masses with contrast-enhanced ultrasound: initial experience. AJR Am J Roentgenol 2011;197:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Houtzager S, Wijkstra H, de la Rosette JJ, et al. Evaluation of renal masses with contrast-enhanced ultrasound. Curr Urol Rep 2013;14:116–23. [DOI] [PubMed] [Google Scholar]
- [11].Ishikawa I, Morita K, Hayama S, et al. Imaging of acquired cystic disease-associated renal cell carcinoma by contrast-enhanced ultrasonography with perflubutane microbubbles and positron emission tomography-computed tomography. Clin Exp Nephrol 2011;15:136–40. [DOI] [PubMed] [Google Scholar]
- [12].Kazmierski B, Deurdulian C, Tchelepi H, et al. Applications of contrast-enhanced ultrasound in the kidney. Abdom Radiol (NY) 2018;43:880–98. [DOI] [PubMed] [Google Scholar]
- [13].McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol 2012;67:909–22. [DOI] [PubMed] [Google Scholar]
- [14].Nicolau C, Bunesch L, Pano B, et al. Prospective evaluation of CT indeterminate renal masses using US and contrast-enhanced ultrasound. Abdom Imaging 2015;40:542–51. [DOI] [PubMed] [Google Scholar]
- [15].Oon SF, Foley RW, Quinn D, et al. Contrast-enhanced ultrasound of the kidney: a single-institution experience. Ir J Med Sci 2018;187:795–802. [DOI] [PubMed] [Google Scholar]
- [16].Wei SP, Xu CL, Zhang Q, et al. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: comparison with contrast-enhanced CT. Abdom Radiol (NY) 2017;42:2135–45. [DOI] [PubMed] [Google Scholar]
- [17].Moriyasu F, Itoh K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol 2009;193:86–95. [DOI] [PubMed] [Google Scholar]
- [18].Jinzaki M, Tanimoto A, Mukai M, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 2000;24:835–42. [DOI] [PubMed] [Google Scholar]
- [19].Pierorazio PM, Hyams ES, Tsai S, et al. Multiphasic enhancement patterns of small renal masses (≤4 cm) on preoperative computed tomography: utility for distinguishing subtypes of renal cell carcinoma, angiomyolipoma, and oncocytoma. Urology 2013;81:1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Young JR, Margolis D, Sauk S, et al. Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 2013;267:444–53. [DOI] [PubMed] [Google Scholar]
- [21].Shi WT, Forsberg F, Bautista R, et al. Image enhancement by acoustic conditioning of ultrasound contrast agents. Ultrasound Med Biol 2004;30:191–8. [DOI] [PubMed] [Google Scholar]
- [22].Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology 2014;271:133–42. [DOI] [PubMed] [Google Scholar]
- [23].Bertolotto M, Cicero C, Perrone R, et al. Renal masses with equivocal enhancement at CT: characterization with contrast-enhanced ultrasound. AJR Am J Roentgenol 2015;204:W557–65. [DOI] [PubMed] [Google Scholar]
- [24].Paudice N, Zanazzi M, Agostini S, et al. Contrast-enhanced ultrasound assessment of complex cystic lesions in renal transplant recipients with acquired cystic kidney disease: preliminary experience. Transplant Proc 2012;44:1928–9. [DOI] [PubMed] [Google Scholar]
- [25].Ignee A, Jedrejczyk M, Schuessler G, et al. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-Reliability and potential sources of errors. Eur J Radiol 2010;73:153–8. [DOI] [PubMed] [Google Scholar]


