Abstract
Peripherally inserted central catheter (PICC) is often applied in chemotherapy patients and commonly causes upper extremity venous thrombosis (UEVT), which should be prevented.
To assess the preventive effects of the anticoagulants rivaroxaban and low molecular weight heparin (LMWH) on UEVT in patients receiving chemotherapy through PICCs.
A total of 423 chemotherapy patients with continuous PICC use between January 2014 and June 2015 at the Oncology Department of Dongying People's Hospital were divided into 3 groups: rivaroxaban (10 mg/day, orally), LMWH (Enoxaparine, 4000 anti-Xa IU/day, subcutaneous injection), and control (no anticoagulant). UEVT incidence and other complications during PICC use were observed and recorded.
The rivaroxaban, LMWH, and control groups included 138 (79 males; 54.9 ± 11.0 years), 144 (76 males; 56.0 ± 10.9 years), and 141 (71 males; 53.3 ± 10.9 years) patients, (P = .402 and P = .623 for age and sex respectively). There were no differences in cancer location (P = .628), PICC implantation site (P > .05), body mass index (BMI) (P = .434), blood pressure (all P > .05), blood lipids (5 laboratory parameters included, all P > .5), smoking (P = .138), history of lower limb venous thrombosis (P = .082), and 10 other associated comorbidities (all P > .5). Twenty-nine patients withdrew from the study (5 from the rivaroxaban, 12 from the LMWH, and 12 from the control groups, respectively), and 394 patients were analyzed. There were significant differences in the rivaroxaban group and the LMWH group compared to the control group (P = .010 and P = .009, respectively), but no significant difference was observed between the rivaroxaban group and the LMWH group (P = .743).
Anticoagulants such as rivaroxaban and LMWH may reduce the incidence of PICC-related UEVT in patients receiving chemotherapy.
Keywords: chemotherapy, low molecular weight heparin, peripherally inserted central catheter, rivaroxaban, upper extremity venous thrombosis
1. Introduction
Peripherally inserted central catheter (PICC) greatly decreases pain and difficulty associated with frequent punctures, protects peripheral veins, and reduces complications, and is widely used in patients receiving chemotherapy.[1] One of the complications associated with PICC is catheter-related upper extremity venous thrombosis (UEVT).[2] The incidence of PICC-related UEVT in cancer patients varies from 6% to 15%.[3,4] Effective prevention of PICC-related UEVT remains a clinical challenge.
Previous studies assessing the pathogenesis of PICC-related UEVT mostly analyzed the 3 parameters of Virchow's triad, and suggested that PICC causes vascular endothelial injury and exposes subendothelial collagen to blood, slows blood flow, and aggravates stasis, inducing thrombosis.[5] It was recently proposed that PICC-related UEVT is a catheter-related thrombus. As an intravascular foreign body, PICC directly activates factor XII and thrombosis initiated by factor XIIa is considered as a contact pathway for thrombus formation.[6] In addition, because of PICC-associated vascular wall injury and vascular endothelial damage caused by chemotherapeutic drugs, exposed platelet tissue factor (or factor III) and factor VIIa in circulation form the tissue factor-factor VIIa complex, which activates the coagulation system and eventually result in thrombus formation. That is the classic tissue factor pathway (also called endogenous pathway for thrombus formation) involved in PICC-related UEVT.[7] Hence, PICC-related UEVT is caused by multiple factors and pathways, including both contact and endogenous pathways for thrombus formation.
Low molecular weight heparins (LMWHs) are preferentially used to treat patients with venous thrombosis,[8] and are also used to prevent venous thrombosis in multiple myeloma patients treated with thalidomide and chemotherapy.[9] Rivaroxaban is an oral direct factor Xa inhibitor with high selectivity that addresses the shortcomings of traditional anticoagulant drugs.[10] Nevertheless, the preventive effects of LMWH and rivaroxaban on PICC-related UEVT during chemotherapy are largely unknown.
We hypothesized that treatment with anticoagulants may help prevent UEVT in patients with indwelled PICCs. The aim of the present comparative study was to assess the preventive effects of the anticoagulants rivaroxaban and LMWH on UEVT in chemotherapy patients with PICC insertion.
2. Materials and methods
2.1. Patients
Patients from the Department of Oncology of Dongying People's Hospital and treated between January 2014 and June 2015 were enrolled in this comparative study. The study was approved by the hospital's ethics committee. Signed informed content was provided by all participants.
The inclusion criteria were:
-
(1)
≥18 years of age;
-
(2)
diagnostic of gastric, lung, esophageal, breast, colorectal, or ovarian cancer; and
-
(3)
scheduled for treatment via PICC insertion.
The exclusion criteria were:
-
(1)
overt predisposition to bleeding having any of the following: platelets < 60 × 109 /L, prothrombin time > 16 seconds (normal 12–13 seconds), activated partial thromboplatin time > 50 seconds (normal 30–40 seconds), thrombin time>22 seconds (normal 15–19 seconds), fibrinogen < 2.0 g/L (normal 2–4 g/L);
-
(2)
history of thrombosis with previous thrombolytic therapy;
-
(3)
obvious heart, liver, or kidney dysfunction; or
-
(4)
failure of PICC indwelling.
2.2. Grouping
The patients were grouped according to the thromboprophylaxis they received: rivaroxaban, LMWH, and control groups. The selection of thromboprophylaxis was made according to the physicians’ experience and after discussion with the patient.
2.3. PICC indwelling
PICCs indwelling and routine maintenance in all patients were carried out by a PICC team of five people, who have all participated in the PICC training class of the Provincial Nursing Association. The 4Fr PICC catheters were from BD Biosciences (USA).
2.4. Administration of anticoagulant drugs
At present, there is no clear scheme for the prevention of PICC-related UEVT, including the length of treatment and drug dosage. Due to the persistent presence of prothrombotic factors in chemotherapy patients with PICCs insertion, anticoagulants were used throughout the whole course of chemotherapy to prevent UEVT. Considering safety and efficacy, the doses of LMWH and rivaroxaban recommended in the guidelines for the prevention of deep venous thrombosis after hip or knee replacement were referenced and adopted.[11] The LMWH group was treated with Enoxaparin Sodium Injection (Hangzhou Jiuyuan Gene Engineering Co., Ltd, China; 4000 anti-Xa IU per day, subcutaneous injection). The rivaroxaban group received rivaroxaban (Bayer Schering Pharma AG, Germany; 10 mg per day, oral). Control patients were administered no anticoagulant or placebo.
2.5. Follow-up and data collection
All patients were followed until PICC removal. The complications that occurred during the entire course of PICC indwelling were recorded. All complications were managed according to the hospital protocols.
2.6. Diagnosis of UEVT
Venography is the gold standard for the diagnosis of venous thrombosis, but it is an invasive and costly procedure, which easily causes contrast load and radiation damage. Meanwhile, ultrasound is non-invasive, safe, fast, and inexpensive, representing an ideal diagnostic method. The main diagnostic criteria for UEVT were: the vein could not be compressed, solid mass in the lumen, filling defect of blood flow signal observed in the lumen, and phase change lost in blood flow spectrum.[3,12] Thrombosis ipsilateral to the PICC was considered as PICC-related UEVT.[13] PICC-related UEVT was diagnosed in the presence and also in the absence of local symptoms and signs.[14,15]
2.7. Ultrasound
Routine upper extremity venous ultrasound examination was performed in all patients at 1, 3, 7, and 14 days after catheter placement, as well as before and after each subsequent chemotherapy. In case of upper limb swelling, pain, and/or other symptoms, ultrasound examination was performed for assessment. In patients with lower limb swelling, pain, positive Nouhof and Homans, lower extremity venous ultrasound examination using a Philips IE33 system (Philips, Netherlands) was performed. In patients that develop UEVT or lower extremity deep venous thrombosis (DVT), treatment was performed according to the hospital protocols. Patients with suspected pulmonary embolism underwent computed tomography pulmonary angiography.
Routine dynamic monitoring of biochemical indexes such as blood routine tests (especially platelets), coagulation function, D-dimer, and liver and kidney functions, were carried out.
2.8. Other complications and patient management
PICC removal was carried out in case of catheter-related infection (including thrombotic phlebitis). In case of lower extremity venous thrombosis, thrombus treatment was performed. Chemotherapy was discontinued when platelets were <60 × 109/L or hemorrhagic event occurred. Occurrence of any severe complication (e.g., pulmonary embolism) resulted in chemotherapy discontinuation.
2.9. Statistical analysis
SPSS13.0 (SPSS Inc., Chicago, IL) was used for statistical analyses. After confirmation of normal distribution using the Kolmogorov-Smirnov test, continuous data were presented as means ± standard deviation and were analyzed using one-way analysis of variance (ANOVA), with thepost-hoc Tukey's test. Categorical data are presented as frequencies and were analyzed using the chi-square test or the Fisher exact test, as appropriate. P values <.05 were considered statistically significant.
3. Results
3.1. Patient baseline characteristics
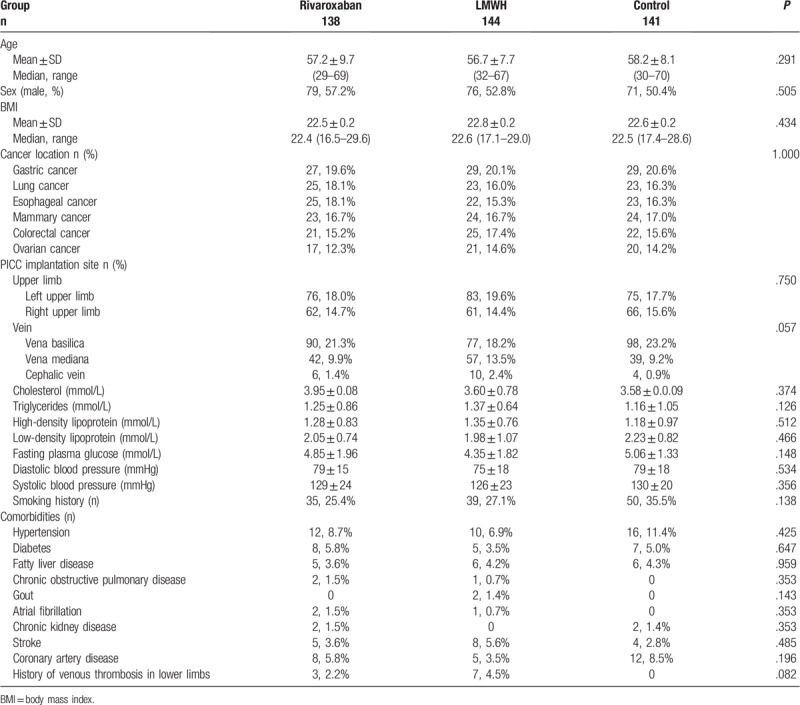
A total of 423 patients on chemotherapy were enrolled. There were 138 patients in the rivaroxaban group (79 males and 59 females; aged between 29 and 69 years; 54.9 ± 11.0 years); there were 27 patients with gastric cancer, 25 with lung carcinoma, 25 with esophageal cancer, 23 with breast carcinoma, 21 with colorectal cancer, and 17 with ovarian cancer. There were 144 patients in the LMWH group (76 males and 68 females, aged from 32 to 67 years; 56.0 ± 10.9 years); there were 29 patients with gastric cancer, 22 with lung carcinoma, 23 with esophageal cancer, 24 with breast carcinoma, 25 with colorectal cancer, and 21 with ovarian cancer. There were 141 patients in the control group (71 males and 70 females; aged from 30 to 70 years; 53.31 ± 10.88 years); there were 29 patients with gastric cancer, 23 with lung carcinoma, 23 with esophageal cancer, 22 with breast carcinoma, 20 with colorectal cancer, and 24 with ovarian cancer. There were 265, 138, and 20 patients with indwelling in the vena basilica, vena mediana, and cephalic vein, respectively. There were 234 and 189 patients with indwelling in the left and right upper limbs, respectively. All catheters were implanted under bedside ultrasound guidance. There were 409 patients with successful catheter indwelling on the first try (96.7%); the remaining 14 patients had successful indwelling on the second attempt.
The three groups showed no significant differences in sex (P = .623), age (P = .402), cancer location (P = .628), PICC implantation site (P > .05), body mass index (BMI) (P = .434), blood pressure (all P > .05), blood lipids (5 laboratory parameters included, all P > .5), smoking (P = .138), history of lower limb venous thrombosis (P = .082), and ten other associated comorbidities (all P > .5) (Table 1).
Table 1.
Patient characteristics.
3.2. Adverse events and study withdrawal
A total of 423 PICC catheters were implanted in 423 patients, and lasted 1 to 160 days (Table 2). In the rivaroxaban group, one patient developed lower extremity DVT, three did not tolerate chemotherapy, one died of severe infection, and 5 withdrew from the study. In the LMWH group, one patient developed thrombocytopenia (heparin-induced thrombocytopenia [HIT] was considered), 1 developed hemoptysis, three had lower extremity DVT, 4 did not tolerate chemotherapy, 3 did not tolerate injection pain, and 12 withdrew from the study. In the control group, five patients developed DVT of the lower extremity, 2 developed myocardial infarction (2 deaths), one had PICC-related infection after indwelling and the PICC was removed, four did not tolerate chemotherapy, and 12 withdrew from the study. Therefore, 394 patients (133 in the rivaroxaban group, 132 in the LMWH group, and 129 in the control group) were included in the final analysis. These data are summarized in Figure 1.
Table 2.
Clinical outcomes of the patients.
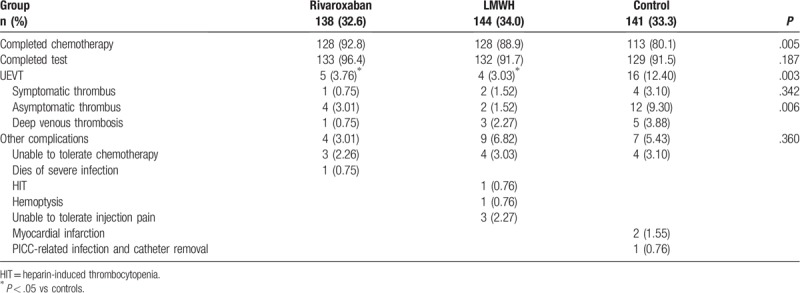
Figure 1.
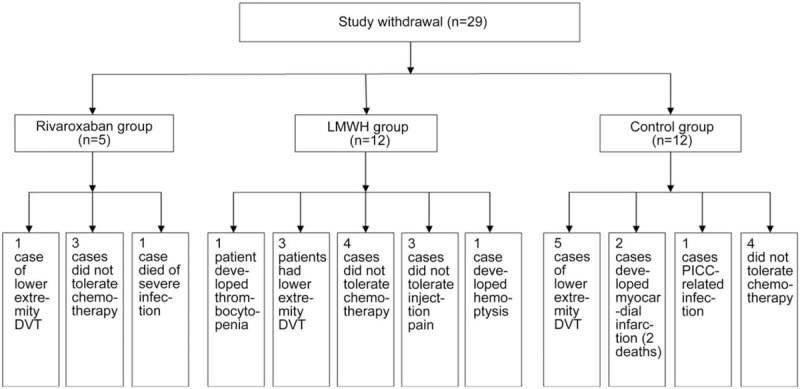
Occurrence of adverse events in the rivaroxaban, low molecular weight heparin (LMWH), and control group.
3.3. Treatment with anticoagulants decreases PICC-related UEVT and thrombus occurrence
In the 394 patients, the incidence of PICC-related UEVT was 6.35% (25/394) (Table 2). Pairwise comparison of UEVT occurrence showed a significant difference between the Rivaroxaban and control groups (P = .010); there was a significant difference between the LMWH group and controls as well (P = .004). Meanwhile, no significant difference was found between the Rivaroxaban and LMWH groups (P = .743).
3.4. Other complications
Additional complications were recorded in 29 patients. Pulmonary embolism was diagnosed in 3 patients, including 1 case in the rivaroxaban and 2 cases in control group. No conclusion can be drawn regarding the incidence or the prevalence of pulmonary embolism because computed tomography was not performed in all patients. Hemorrhagic events (hemoptysis) occurred in one patient of the LMWH group.
Among the remaining 394 patients, the earliest PICC-related UEVT occurred the day after catheter placement, while the longest time to occurrence was 110 days after indwelling. Precisely, times for the occurrence of PICC-related UEVTs were P50 = 15.0 days, P75 = 35.0 days, P90 = 53.0 days, and P95 = 100.0 days, respectively (Fig. 2).
Figure 2.
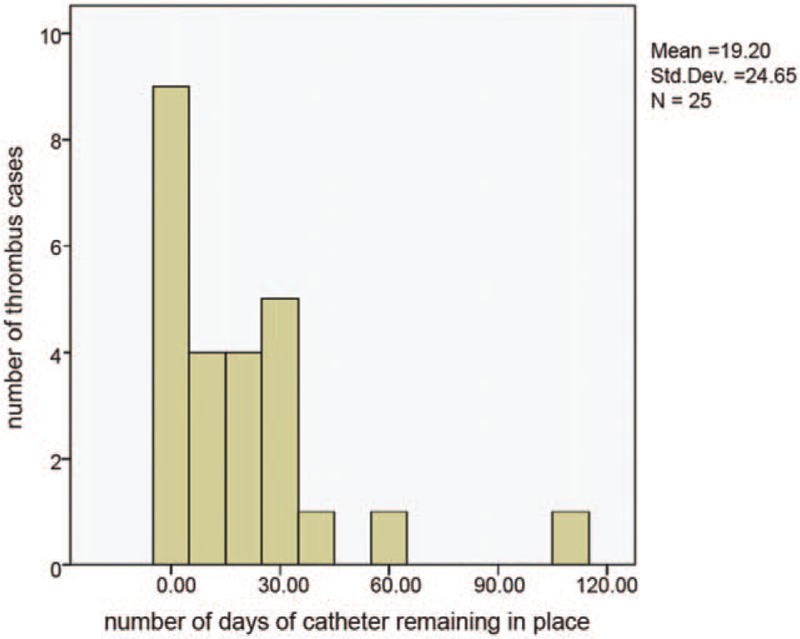
Number of cases and time distribution for PICC-related upper extremity venous thrombosis (UEVT) in all groups.
4. Discussion
This study demonstrated that rivaroxaban and LMWH reduce the incidence of PICC-related UEVT in cancer patients on chemotherapy. Although PICC chemotherapy has many advantages and is considered relatively safe,[16] it is still accompanied by a high incidence of PICC-related UEVT. Aw et al[17] evaluated 340 patients treated with chemotherapy via PICC, and found 19 cases of UEVT (5.6%; 95% CI 3.6–8.6). Cortelezzi et al[18] reported an incidence of symptomatic PICC-related UEVT of 25.7%, while Liem et al[19] found that the incidence of UEVT was 35% (54/154). In the present study, the occurrence of PICC-related UEVT was 6.35%, similar to Aw,[17] but much lower than Cortelezzi[18] and Liem.[19] The reasons for discrepancy may include the types of cancer, the types of chemotherapy, thromboprophylaxis, method for detection, etc.
Similar to lower extremity DVT, asymptomatic thrombus is very common in patients with PICC-related UEVT. Abdullah et al[20] assessed 26 patients treated with PICC who underwent routine upper extremity venography before extubation, and found an incidence of PICC-related UEVT of 38.5%, including 85.7% with complete venous thrombosis and only one patient with thrombus symptoms. Dubois et al[15] evaluated 214 children (<18 years of age) who underwent ultrasound examination at 2, 4, 7, 14, 21 and 28 days after PICC indwelling as well as once per month afterwards until removal, and reported a thrombus incidence of 9.35%; only 1 patient had thrombus-related symptoms. Periard et al[21] assessed 31 patients treated with PICC who underwent B-ultrasound examination, and reported that 19.4% developed UEVT, with no patient showing thrombus signs. In the present study, ultrasound was performed after PICC indwelling and during chemotherapy in all patients, regardless of complaints. The occurrence of PICC-related UEVT in the control group was 12.4% (16/129), and symptomatic thrombus accounted for 25% (4/16). Thus, PICC-related UEVT has a high risk and affects cancer treatment.
We next evaluated the occurrence timing of PICC-related UEVT. Of the 394 patients, 50% developed thrombus 15.0 days after catheter indwelling (P50 = 15.0 days), and most individuals developed thrombus within 2 months of indwelling (P90 = 53.0 days), indicating that within 2 months, especially within 3 weeks, of catheter indwelling, high thrombus incidence was obtained, corroborating previous reports. Indeed, Walshe et al[22] reported removal caused by symptomatic PICC-related UEVT, with 70% occurring 1 week after catheter indwelling, while the remaining 30% were noted two weeks after indwelling. In addition, Sperry et al[23] performed an observational study of 798 catheters indwelled in 670 patients; average time of PICC-related UEVT was 13.6 days. Furthermore, studies by King et al[24] and Ong et al[25] revealed that the average time to the occurrence of PICC-related UEVT is 15 days. Al-Asadi et al[26] showed that the average time to thrombosis was 13 days after PICC indwelling. On the other hand, Tran et al[27] evaluated 899 catheters implanted in 498 patients treated with chemotherapy and found a median time of UEVT of 26 days after catheter placement. Madabhavi et al[28] showed that the median time of PICC use was 152 days, which is much longer than in the present study. The above findings emphasize that thromboprophylaxis for UEVT should be carried out during PICC use.
Nevertheless, the application of anticoagulants in the prevention of PICC-related UEVT remains controversial. Studies reported that the application of prophylactic anticoagulants does not reduce the risk of PICC-related UEVT.[24,29] Meanwhile, a recent study indicated that prophylactic anticoagulant therapy can effectively reduce the risk and re-hospitalization rate of PICC-related UEVT.[30]
Of the 394 cancer patients assessed in this work, PICC-related UEVT occurred in 6.35% (25/394). Interestingly, thrombus incidence after application of the anticoagulants rivaroxaban or enoxaparine was 3.40% (9/265), while 12.4% (16/129) was observed in the control group without anticoagulant treatment. These data clearly suggest that the application of anticoagulants could significantly reduce the occurrence of PICC-related UEVT in cancer patients. In addition, few related adverse reactions were observed. Hemorrhagic events (hemoptysis) occurred in only one patient of the LMWH group. Safety in thrombus prevention for rivaroxaban and LMWH was comparable.
4.1. Limitations
The main limitation of this study was that no risk assessment in patients was performed. Routine upper extremity venous ultrasound examination was performed in all patients at various times, which imposed an important burden on them. Because multiple studies reported that PICC-related UEVT events often occur within the first 2 months after catheter placement,[22,23] ultrasound examination at this frequency was adopted in the present study. Another limitation of this study was its relatively small sample size. Finally, there was no randomization, and treatment was decided by the physicians. Therefore, further well-designed trials with large sample size are wanted to confirm the current findings.
4.2. Future directions
Whether the patients with moderate or high risk according to clinical grading (e.g., Caprini Score and the American society of clinical oncology clinical practice guideline for venous thrombosis prophylaxis and treatment in patients with cancer)[31] should receive more appropriate thromboprophylaxis should be evaluated in the future.
5. Conclusion
PICC-related UEVT has a high occurrence rate. Anticoagulants such as rivaroxaban and LMWH may reduce the incidence of PICC-related UEVT in patients receiving chemotherapy.
5.1. Clinical implications
The Factor Xa antagonist rivaroxaban is well known for preventing thrombosis after hip and knee surgery. Recently, it has been increasingly used to treat thrombosis during chemotherapy for tumors.[32] Nonetheless, its application in preventing thrombosis in cancer patients, especially for the prevention of PICC-related UEVT, has not been reported yet. Rivaroxaban represents a new generation of oral anticoagulants that can be used to prevent PICC-related UEVT, and thus play an important role in preventing systemic VTE events in cancer patients. Compared with LMWH, rivaroxaban has the advantages of oral administration, convenience of application outside hospitals, and avoidance of heparin-induced thrombocytopenia. Additional studies are necessary to examine the advantages of rivaroxaban for the prevention of PICC-related UEVT.
Author contributions
Conceptualization: Shoutian Lv, Yongmei Liu, Xuehui Zhang.
Data curation: Shoutian Lv, Yongmei Liu, Gang Wei, Xueyan Shi, Shaoping Chen.
Formal analysis: Shoutian Lv, Yongmei Liu, Gang Wei, Xueyan Shi, Shaoping Chen, Xuehui Zhang.
Project administration: Xuehui Zhang.
Writing – original draft: Shoutian Lv, Yongmei Liu.
Writing – review & editing: Gang Wei, Xueyan Shi, Shaoping Chen, Xuehui Zhang.
Footnotes
How to cite this article: Lv S, Liu Y, Wei G, Shi X, Chen S, Zhang X. The anticoagulants rivaroxaban and low molecular weight heparin prevent PICC-related upper extremity venous thrombosis in cancer patients. Medicine. 2019;98:47(e17894).
Abbreviations: DVT = deep venous thrombosis, HIT = heparin-induced thrombocytopenia, LMWH = low molecular weight heparin, PE = pulmonary embolism, PICC = peripherally inserted central catheter, UEVT = upper extremity venous thrombosis, VTE = venous thromboembolism.
SL and YL contributed equally to this work.
The authors declare that they have no conflict of interest.
References
- [1].Johansson E, Hammarskjöld F, Lunderg D, et al. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol 2013;52:886–92. [DOI] [PubMed] [Google Scholar]
- [2].Jones D, Wismayer K, Bozas G, et al. The risk of ven-ous thromboembolism associated with peripherally inserted central catheters in ambulant cancer patients. Thromb J 2017;15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kang JR, Long LH, Yan SW, et al. Peripherally inserted central catheter-related vein thrombosis in patients with lung cancer. Clin Appl Thromb Hemost 2017;23:181–6. [DOI] [PubMed] [Google Scholar]
- [4].Catalano O, de Lutio di Castelguidone E, Sandomenico C, et al. Central venous device-related thrombosis as imaged with MDCT in oncologic patients: prevalence and findings. Acta Radiol 2011;52:148–54. [DOI] [PubMed] [Google Scholar]
- [5].Kim HJ, Yun J, Kim HJ, et al. Safety and effectiveness of central venous catheterization in patients with cancer: prospective observational study. J Korean Med Sci 2010;25:1748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gentile A, Petit L, Masson F, et al. Subclavian central venous catheter-related thrombosis in trauma patients: incidence, risk factors and influence of polyurethane type. Crit Care 2013;17:R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol 2011;73:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wharin C, Tagalakis V. Management of venous thromboembolism in cancer patients and the role of the new oral anticoagulants. Blood Rev 2014;28:1–8. [DOI] [PubMed] [Google Scholar]
- [9].Minnema MC, Breitkreutz I, Auwerda JJ, et al. Prevention of venous thromboembolism with low molecular-weight heparin in patients with multiple myeloma treated with thalidomide and chemotherapy. Leukemia 2004;18:2044–6. [DOI] [PubMed] [Google Scholar]
- [10].Imberti D, Benedetti R. Primary prophylaxis of VTE in cancer outpatients. Thromb Res 2016;140: Suppl 1: S103–108. [DOI] [PubMed] [Google Scholar]
- [11].Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost 2011;105:444–53. [DOI] [PubMed] [Google Scholar]
- [12].Marnejon T, Angelo D, Abu Abdou A, et al. Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access 2012;13:231–8. [DOI] [PubMed] [Google Scholar]
- [13].Kang J, Sun W, Li H, et al. Peripherally inserted central catheter-related vein thrombosis in breast cancer patients. J Vasc Access 2016;17:67–71. [DOI] [PubMed] [Google Scholar]
- [14].Ahh DH, Illum HB, Wang DH, et al. Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract 2013;9:e8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dubois J, Rypens F, Garel L, et al. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. CMAJ 2007;177:1185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Campagna S, Gonella S, Berchialla P, et al. Can peripherally inserted central catheters be safely placed in patients with cancer receiving chemotherapy? a retrospective study of almost 400,000 catheter-days. Oncologist 2019;pii: theoncologist.2018-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aw A, Carrier M, Koczerginski J, et al. Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res 2012;130:323–6. [DOI] [PubMed] [Google Scholar]
- [18].Cortelezzia A, Fracchiolla NS, Maisonneuve P, et al. Central venous catheter-related complications in patients with hematological malignancies: a retrospective analysis of risk factors and prophylactic measures. Leuk Lymphoma 2003;44:1495–501. [DOI] [PubMed] [Google Scholar]
- [19].Liem TK, Yanit KE, Moseley SE, et al. Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg 2012;55:761–7. [DOI] [PubMed] [Google Scholar]
- [20].Abdullah BJ, Mohammad N, Sangkar JV, et al. Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC). Br J Radiol 2005;78:596–600. [DOI] [PubMed] [Google Scholar]
- [21].Periard D, Monney P, Waeber G, et al. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J Thromb Haemost 2008;6:1281–8. [DOI] [PubMed] [Google Scholar]
- [22].Walshe LJ, Malak SF, Eagan J, et al. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002;20:3276–81. [DOI] [PubMed] [Google Scholar]
- [23].Sperry BW, Roskos M, Oskoui R. The effect of laterality on venous thromboembolism formation after peripherally inserted central catheter placement. J Vasc Access 2012;13:91–5. [DOI] [PubMed] [Google Scholar]
- [24].King MM, Rasnake MS, Rodriguez RG, et al. Peripherally inserted central venous catheter-associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J 2006;99:1073–7. [DOI] [PubMed] [Google Scholar]
- [25].Ong B, Gibbs H, Catchpole I, et al. Peripherally inserted central catheters and upper extremity deep vein thrombosis. Australas Radiol 2006;50:451–4. [DOI] [PubMed] [Google Scholar]
- [26].Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single Centre cohort study. Thromb J 2019;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tran H, Arellano M, Chamsuddin A, et al. Deep venous thromboses in patients with hematological malignancies after peripherally inserted central venous catheters. Leuk Lymphoma 2010;51:1473–7. [DOI] [PubMed] [Google Scholar]
- [28].Madabhavi I, Patel A, Sarkar M, et al. A study of the use of peripherally inserted central catheters in cancer patients: a single-center experience. J Vasc Nurs 2018;36:149–56. [DOI] [PubMed] [Google Scholar]
- [29].Levine MN. Prevention of thrombotic disorders in cancer patients undergoing chemotherapy. Thromb Haemost 1997;78:133–6. [PubMed] [Google Scholar]
- [30].Baser O, Liu X, Phatak H, et al. Venous thromboembolism prophylaxis and clinical consequences in medically ill patients. Am J Ther 2013;20:132–42. [DOI] [PubMed] [Google Scholar]
- [31].Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Laube ES, Mantha S, Samedy P, et al. Treatment of central venous catheter-associated deep venous thrombosis in cancer patients with rivaroxaban. Am J Hematol 2017;92:E9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


