Abstract
The present study aimed to investigate the safety and short-term outcome of laparoscopy-assisted distal radical gastrectomy in treating gastric cancer among obese patients.
Perioperative outcomes were compared between 67 gastric cancer patients with a body mass index (BMI) ≥25 kg/m2 (obese group) and 198 ones with BMI <25 kg/m2 (non-obese group). All the cases underwent laparoscopic radical resection between April 2009 and October 2013.
The value of BMI was 27.3 ± 2.67 kg/m2 in the obese group and 21.3 ± 2.64 kg/m2 in non-obese group. There were no significant differences between 2 groups in age, sex, presence of diabetes, tumor size, number of metastatic lymph nodes, or metastatic lymph node ratio. Postoperative complications did not differ between the 2 groups (P > .05). There were significant differences between the 2 groups in operation time (non-obese: [234.2 ± 67.1] minutes vs obese group: [259.4 ± 78.5]; P = .017), postoperative hospital stay (obese group [19.7 ± 14.8] day vs non-obese [15.4 ± 7.1], P = .002), and retrieved lymph nodes ([27.6 ± 11.0] day vs non-obese [31.9 ± 12.5] day, P = .002).
Obesity may prolong operation time and postoperative hospital stay, and cause less retrieved lymph nodes, but does not increase the incidence of postoperative complications. The experienced center can properly conduct laparoscopic assisted radical gastrectomy in obese patients.
Keywords: body mass index, gastric cancer, laparoscopy, lymph node dissection, obesity, postoperative complication
1. Introduction
For patients with gastric cancer (GC), obesity is associated with poor surgical outcomes, including longer operation time, fewer lymph node dissections, and more postoperative complications.[1] International Obesity Task Force recommends that obesity is defined as body mass index (BMI) ≥25 kg/m2 in Asians.[2] Anatomically, the stomach and surrounding blood vessels are complex. There are many lymphatic drainage pathways and station 2 lymph nodes locate around blood vessels. Besides, abdominal wall is more thicker in obese individuals. Operation difficulty and postoperative infection are increased in obese patients with hypertrophy abdominal walls. Therefore, obesity has been considered as a contraindication in laparoscopic surgery.
Currently, laparoscopically assisted distal gastrectomy is still the main option for cases with advanced gastric cancer.[3] In 1997, laparoscopy-assisted distal gastrectomy was firstly used to treat gastric cancer and achieved fine short-term efficacy.[4] Since then, this therapeutic method has been widely applied worldwide. It allows faster healing after surgery and fewer postoperative complications apart from shorter hospitalization period.[5]
Main objective of this study was to retrospectively analyze the safety and short-term efficacy of laparoscopic radical gastrectomy among obese patients with GC.
2. Material and methods
2.1. Patients’ collection
A total of 265 GC cases received minimally invasive surgery in our hospital from April 2009 to October 2013. All patients were diagnosed with gastric adenocarcinoma through gastroscope biopsy before surgery, and received blood routine examination, biochemistry, tumor markers, cardiopulmonary function, chest, abdomen, and pan computed tomography (CT) examinations. Inclusion criteria for GC patients: they could bear surgical treatment; without distant metastasis; tumor did not significantly invade surrounding tissues or organs; lymph nodes around the stomach were not surrounded by blood vessels; and they signed written informed consent in advance. Patients with following conditions were excluded from this study: showing liver and/or other metastases; suffering serious heart, lung, liver, or kidney diseases or those involving other important organs; and patients cannot tolerate surgery.
The patients were classified according to the 7th edition of the 2007 Union for International Cancer Control (UICC) staging system. Patients with BMI ≥25 kg/m2 were categorized in the obese group, while those with BMI <25 kg/m2 were in the non-obese group. There were no statistically significant differences in clinical indicators between the 2 groups. This study was approved by the Ethic Committee of Peking University Cancer Hospital & Institute and written informed consent was signed by every patient.
2.2. Surgical methods
Laparoscopic assisted distal radical gastrectomy was operated after general anesthesia, with patients lying supine and using leveling leg. Pneumoperitoneum was constructed via umbilical region, establishing observation holes, placing laparoscope so as to determine no visible invasion into surrounding tissue during the operation, no pelvic cavity transfer, and no ascites, and negative results from cytology test on peritoneal lavage fluid, suitable for D2 gastric cancer. Surgeon stood on the left side of patient, placed a stamped card through the 6-hole method, and used a liver blocker to lift the liver so as to fully expose operative field. Operating table was high at the head and low at the foot end, greater omentum was turned to the cephalic side, appendiculous ligament was cut with an ultrasonic knife, omentum was separated along transverse colon, and the spleen curve of the colon was reached along the left side and exposed at the proximal pancreatic tail while the root cut the left gastric plexus and bulged the stomach to the right side, cleaning NO.4sb and 4d. Stayed at the right side of the colon off the hepatic curvature of the greater omentum, entered into the gap before and after the transverse mesorectal leaf. Anatomical plane close to the surface of the pancreatic head was anatomized, right gastric and omental retinal artery and vein were clipped off at root, cleaning NO.6. Pancreatic dorsal membrane was isolated from the upper edge of the pancreas, and common hepatic artery was found along gastroduodenal artery and NO.8a was dissected. The ligation of the right gastric artery was cut off and NO.5 was dissected. In the upper edge of the pancreas along the splenic artery, NO.11p was dissected. The left gastric vessels were isolated, ligated, and cut at root to clean NO.7 and 9. Subsequently, the liver was retracted to expose hepatoduodenal ligament, and the anterior segment of the hepatoduodenal ligament was incised, so NO.12a was dissected. Cut small omentum until the right side of the fontanelle, cleaning NO.1. Naked gastric curvature of the gastric wall, cleaning NO.3. The whole stomach was dissatisfied, and the pneumoperitoneum was completed. The median incision on the abdomen was taken with a length of about 5 cm. The incision was made layer by layer and the abdomen was incised to protect the incision. Lift the antrum from the pylorus 3 cm transverse duodenum, purse placed into the stapler nail for anastomosis. 5 cm from the proximal end of the tumor, the linear incision closure crossed the stomach and the specimen was obtained for examination. Reconstruct the digestive tract. Place the drainage tube of the right upper abdomen routinely after surgery.
2.3. Perioperative nursing
During perioperative period, the patients of 2 groups experienced the same perioperative nursing: 24 hours before the operation, intravenous nutrition support was started; 8 hours fasting before the operation; prophylactic use of the first generation cephalosporin 1 hour before operation was discontinued 24 hours after operation; the first day after the operation, the catheter was removed and proper activity was allowed. Parenteral nutrition support was continued, with enteral nutrition gradually increased; and analgesic drug was routinely given within 2 days after operation. The characteristics and quantity of drainage fluid in peritoneal drainage tube were observed. Five to 7 days after operation, upper gastrointestinal angiography was implemented to appropriately remove abdominal drainage tube. A small amount of water and liquid food were allowed for patients without postoperative complications, who were discharged from the hospital 8 to 10 days later. No postoperative complication referred to complete enteral nutrition at the time of discharge and no obstruction after oral feeding, and certain degree of healing without fever, abdominal pain, vomiting, or other symptoms. Postoperative complications meant delayed discharge due to any symptoms requiring medication and/or surgery.
Observation indicators in surgery included intraoperative blood loss, blood transfusion rate, recovery and anal exhaust time, time to start liquid diets, total number of lymph node cleaned, number of lymph nodes metastasis, lymph node metastasis rate, postoperative complications, and recent survival.
2.4. Statistical analysis
SPSS 17.0 software was used to analyze data. Quantitative variables were expressed as mean ± standard deviation (SD) and analyzed by t test. Qualitative variables were analyzed by chi-square test. Kaplan–Meier curve was used to implement survival analysis. P < .05 was considered as statistically significant level.
3. Results
3.1. General features of patients in 2 BMI groups
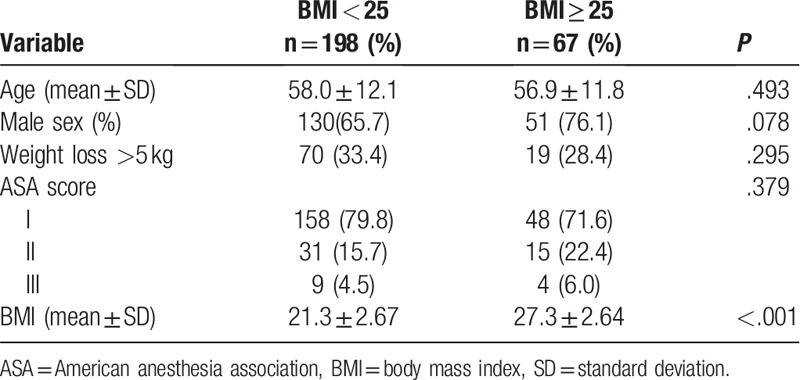
There were no statistically significant differences in general clinical features, either age, sex, or American Society of Anesthesiologists (ASA) score between non-obese and obese groups (Table 1, P > .05). BMI was significantly different between 2 groups. It suggested that the 2 groups were well matched and had fine comparability.
Table 1.
Clinical features of patients in different BMI groups.
3.2. Analysis for surgical features
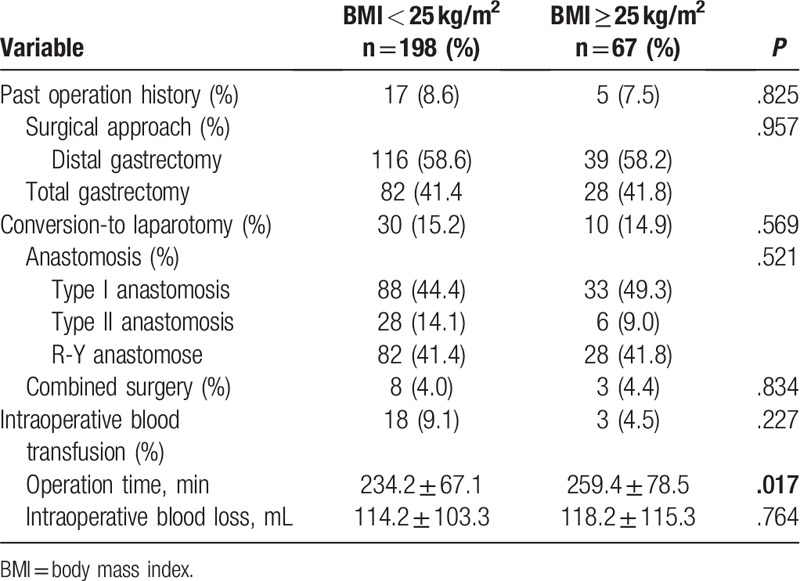
Comparison results on surgical features between non-obese and obese groups were recorded in Table 2. There was no significant difference between the 2 groups in surgical approach, laparotomy, anastomosis, combined surgery, intraoperative blood transfusion, or intraoperative blood loss (Table 2, P > .05). Nonetheless, obese patients experienced significantly longer operation time than non-obese ones (P = .017).
Table 2.
Comparison of surgical features between 2 groups.
3.3. Comparison on oncological and pathological results between obese and non-obese groups
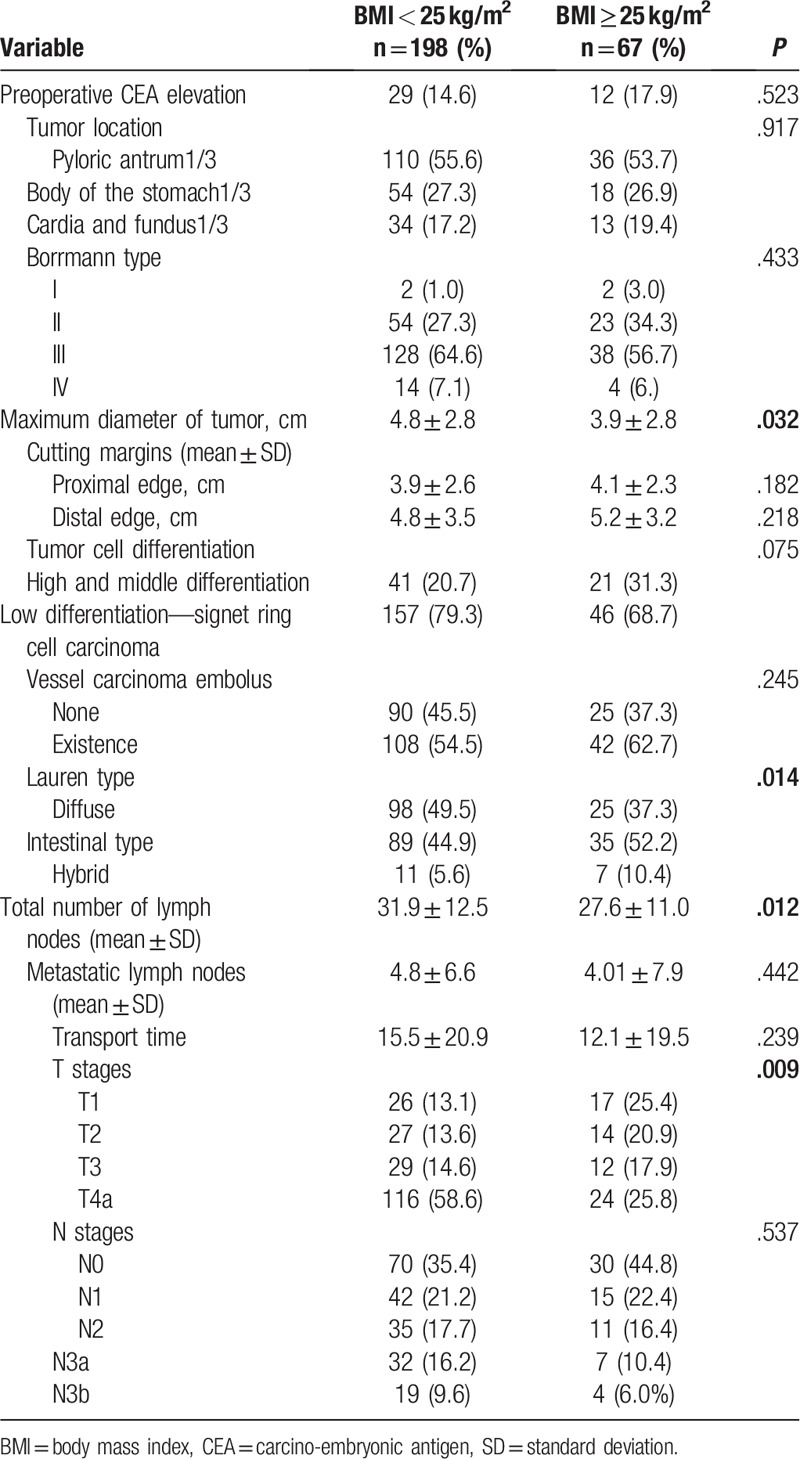
There was no significant difference in preoperative carcino-embryonic antigen (CEA), tumor location, borrmann type, cutting margins, tumor cell differentiation, vessel carcinoma embolus, metastatic lymph nodes, transport time, or lymph node metastasis (N stage) (Table 3, P > .05). Differences in tumor size, Lauren type, total number of lymph nodes, and depth of invasion (T stage) between obese and non-obese groups were statistically significant (Table 3). In the non-obese group, tumor size was larger (P = .032), infiltration was deeper (P = .009), total number of lymph nodes was more (P = .012), and Lauren diffuse type was more common (P = .014) than those in obese group.
Table 3.
Comparison of oncological and pathological results between the obese and non-obese groups.
3.4. Postoperative complications and perioperative mortality in the 2 groups
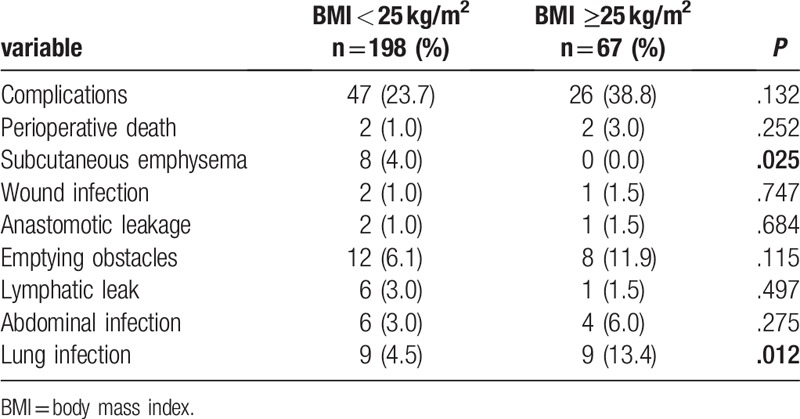
The incidence of complications such as perioperative death, wound infection, anastomotic leakage, emptying obstacles, lymphatic leakage, and abdominal infection were not statistically different between the 2 groups (Table 4, P > .05). The incidences of pulmonary infection and subcutaneous emphysema were statistically different between 2 groups (Table 4). Obese patients faced more lung infections (P = .012), and non-obese ones experienced increased risk of subcutaneous emphysema (P = .025). There was no significant difference in perioperative mortality between the 2 groups.
Table 4.
Postoperative complications and perioperative mortality in obese and non-obese groups.
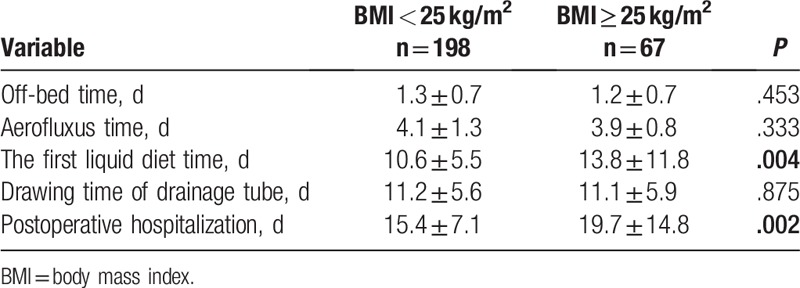
3.5. Postoperative recovery among the patients in 2 groups
Comparing the time of ambulation, ventilation time, and drainage tube removal time between the 2 groups, no statistically significant difference appeared (Table 5, P > .05). The postoperative hospital stay (P = .002) and postoperative infusion time (P = .004) were significantly different between the 2 groups. Obese patients experienced longer hospital stay and postponed feed.
Table 5.
Postoperative recovery of the patients in 2 groups.
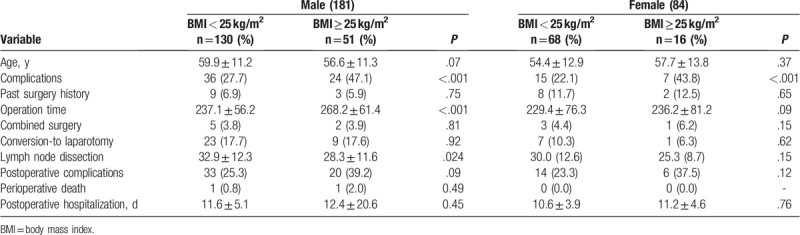
3.6. Sex-based subgroup analysis
Male obese patients had prolonged surgery (Table 6, P < .001) and fewer lymph node dissections (P = .024). There was no statistically significant difference in female patients between the 2 groups.
Table 6.
Gender-based subgroup analysis.
3.7. Recent survival of the patients in 2 groups
The disease-free survival time of GC patients in the obese group (group 2) was longer than those in the non-obese group (group 1) (Fig. 1), though the difference was not significant.
Figure 1.
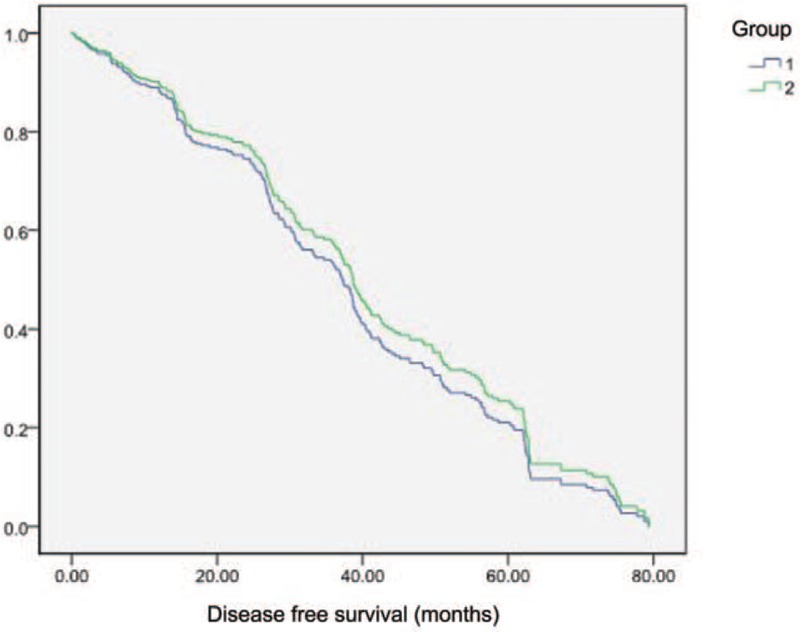
Survival analysis. Group 1, non-obese group; group 2, obese group.
4. Discussion
Compared with laparotomy, laparoscopic assisted radical gastrectomy is less traumatic, and also has other advantages such as faster postoperative recovery, fewer complications, as well as better short-term and long-term efficacy. Hu et al[6] showed that the safety of D2 radical gastrectomy for locally advanced gastric cancer was similar to that of laparotomy. Due to a large amount of adipose tissue in abdominal wall and abdominal cavity in obese patients, it is difficult to expose operative field in laparotomy. With regard to the feasibility of laparoscopic radical gastrectomy for obese patients, Lee et al[7] conducted a retrospective study on 1485 GC patients receiving laparoscopic assisted gastrectomy. Their results showed that there was no difference in postoperative complications or mortality between obese and non-obese patients, while obese patients tend to experience longer operation time and less dissection lymph nodes. Lymph nodes and major blood vessels in obese patients are covered by a large amount of adipose tissue and it is difficult to completely clean lymph nodes. Sometimes the area of lymph node dissection is appropriately narrowed, in order to avoid uncontrolled bleeding or prolonged operation. In addition, removing lymph nodes in surgical specimens is more difficult for obese patients than those non-obese patients. Interestingly, there is no significant reduction in the duration of surgery or the number of lymph nodes in obese women, which has been reported in several studies on laparoscopic radical gastrectomy.[8,9] The results may be related to the differences of fat distribution between men and women. Adipose tissues are mainly distributed in the trunk among men, especially in abdomen, while in the buttocks and thighs among women.
Clinical significance of operation time and lymph node dissection in GC should be taken seriously, because the prolongation of operative time and anesthesia time are frequently risk factors for postoperative complications.[9] Patients with complications had significantly longer operative time than uncomplicated ones. Therefore, surgeons should be fully aware that prolonged operation time will increase the incidence of complications, especially in elderly patients with more complications. A meta-analysis performed by Wu et al[10] showed that obese patients with GC faced significantly long operation time, increased anastomotic leakage, decreased total lymph node dissection, and prolonged postoperative hospitalization. Ojima et al[11] indicated that obese patients experienced significantly longer operation time, and more intraoperative blood loss than those non-obese patients, while postoperative hospitalization had no significant association with obesity. In the radical operation for GC, the number of lymph node dissection is >15, which can be accurate for N staging, while accurate N staging is more important than the number of lymph node dissection. In our study, there was no significant difference in the number of retrieved lymph nodes between non-obese and obese patients. The number of lymph node dissection in the 2 groups exceeded 27 in this study. The number of dissociated lymph nodes was obviously lower in obese patients than that in non-obese ones.
Many studies have shown that laparoscopic radical gastrectomy among obese patients does not increase surgical complications.[12–15] Laparoscopic cholecystectomy (LC) and other simple operations do not increase postoperative complications among obese patients, and many scholars believe that LC is more suitable for obese patients. Although laparoscopic gastrectomy for D2 lymph node dissection is complicated, our research showed that laparoscopic radical gastrectomy operated by experienced surgeons was safe for obese patients. Li et al[16] studied the learning curve of laparoscopic assisted radical gastrectomy and found that the different phases of the operation reflected the characteristics of the learning curve in terms of operation time and intraoperative bleeding. Different surgical methods and surgical teams could lead to varied learning curve rules. Literature reports on laparoscopic gastrectomy usually require 50 to 90 patients.[17] Kawamura et al[18] also reported that adverse effects of obesity on surgical operations were decreased with surgical experiences, and there was no significant difference in postoperative complications.
Certainly, some limitations in this study should be noted. Firstly, patients in this retrospective study were collected from a single institution. Secondly, sample size was not large enough, and the number of patients in 2 groups was unequal. Finally, the follow-up time was relatively short. Further studies with enlarger sample size, multiple institutions, and prolonged follow-up time should be performed to verify the present results.
In conclusion, laparoscopic radical gastrectomy does not increase the incidence of postoperative complications in obese patients. Obese patients only have 2 slight disadvantages in terms of operative time and total retrieved number of lymph nodes, but such disadvantages are smaller among female patients or when the operation is implemented by experienced surgeons. Experienced centers can perform laparoscopic radical gastrectomy for obese patients. However, laparoscopic gastrectomy among male obese patients should be discreetly performed to reduce intraoperative and postoperative complications.
Author contributions
Conceptualization: Maoxing Liu, Jiadi Xing, Ahmet Arslan, Fei Tan.
Data curation: Maoxing Liu, Jiadi Xing, Ahmet Arslan, Yingcong Fan, Kai Xu.
Formal analysis: Maoxing Liu, Jiadi Xing, Fei Tan, Yingcong Fan, Xinyu Qi, Hong Yang, Xiangqian Su.
Funding acquisition: Jiadi Xing, Fei Tan, Yingcong Fan, Kai Xu, Xinyu Qi, Zhendan Yao, Nan Zhang, Hong Yang, Ming Cui.
Investigation: Ahmet Arslan, Yingcong Fan, Kai Xu, Zhendan Yao, Hong Yang.
Methodology: Maoxing Liu, Ahmet Arslan, Fei Tan, Kai Xu, Zhendan Yao, Chenghai Zhang.
Writing – original draft: Xinyu Qi, Nan Zhang, Chenghai Zhang, Ming Cui, Xiangqian Su.
Writing – review & editing: Xinyu Qi, Nan Zhang, Chenghai Zhang, Ming Cui, Xiangqian Su.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, CEA = carcino-embryonic antigen, CT = computed tomography, GC = gastric cancer, LC = laparoscopic cholecystectomy, SD = standard deviation, UICC = Union for International Cancer Control.
How to cite this article: Liu M, Xing J, Arslan A, Tan F, Fan Y, Xu K, Qi X, Yao Z, Zhang N, Zhang C, Yang H, Cui M, Su X. Safety and efficacy of laparoscopic gastrectomy in obese patients with gastric cancer. Medicine. 2019;98:47(e17991).
ML and JX are co-first authors.
This study was supported by the National Natural Science Foundation of China (No.81672439 and 81272766, 81450028), Beijing Natural Science Foundation (No.7162039), Capital's Funds for Health Improvement and Research (CFH 2018-2-2153), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No.XM201309 and ZYLX201701).
The authors have no conflicts of interest to disclose.
References
- [1].Go JE, Kim MC, Kim KH, et al. Effect of visceral fat area on outcomes of laparoscopyassisted distal gastrectomy for gastric cancer: subgroup analysis by gender and parameters of obesity. Ann Surg Treat Res 2015;88:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Korsic M, Fister K, Ivankovic D, et al. [Visceral obesity]. Lijecnicki vjesnik 2011;133:284–7. [PubMed] [Google Scholar]
- [3].Wang Y, Zhao X, Song Y, et al. A systematic review and meta-analysis of robot-assisted versus laparoscopically assisted gastrectomy for gastric cancer. Medicine (Baltimore) 2017;96:e8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Naitoh T, Gagner M. Laparoscopically assisted gastric surgery using Dexterity Pneumo Sleeve. Surg Endosc 1997;11:830–3. [DOI] [PubMed] [Google Scholar]
- [5].Vinuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446–56. [DOI] [PubMed] [Google Scholar]
- [6].Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open d2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 2016;34:1350–7. [DOI] [PubMed] [Google Scholar]
- [7].Lee HJ, Kim HH, Kim MC, et al. The impact of a high body mass index on laparoscopy assisted gastrectomy for gastric cancer. Surg Endosc 2009;23:2473–9. [DOI] [PubMed] [Google Scholar]
- [8].Son SY, Jung DH, Lee CM, et al. Laparoscopic gastrectomy versus open gastrectomy for gastric cancer in patients with body mass index of 30 kg/m2 or more. Surg Endosc 2015;29:2126–32. [DOI] [PubMed] [Google Scholar]
- [9].Sugimoto M, Kinoshita T, Shibasaki H, et al. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg Endosc 2013;27:4291–6. [DOI] [PubMed] [Google Scholar]
- [10].Wu XS, Wu WG, Li ML, et al. Impact of being overweight on the surgical outcomes of patients with gastric cancer: a meta-analysis. World J Gastroenterol 2013;19:4596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ojima T, Iwahashi M, Nakamori M, et al. The impact of abdominal shape index of patients on laparoscopy-assisted distal gastrectomy for early gastric cancer. Langenbecks Arch Surg 2012;397:437–45. [DOI] [PubMed] [Google Scholar]
- [12].Kim MG, Yook JH, Kim KC, et al. Influence of obesity on early surgical outcomes of laparoscopic-assisted gastrectomy in gastric cancer. Surg Laparosc Endosc Percutan Tech 2011;21:151–4. [DOI] [PubMed] [Google Scholar]
- [13].Kanellos D, Kanellos I. Is laparoscopic gastrectomy safe for western patients with gastric cancer with high body mass index? Surg Endosc 2010;24:977–9. [DOI] [PubMed] [Google Scholar]
- [14].Ojima T, Iwahashi M, Nakamori M, et al. Influence of overweight on patients with gastric cancer after undergoing curative gastrectomy: an analysis of 689 consecutive cases managed by a single center. Arch Surg 2009;144:351–8. discussion 358. [DOI] [PubMed] [Google Scholar]
- [15].Hu L, Li C, Li W, et al. [Effect of body mass index on postoperative short-term outcomes of laparoscopy radical gastrectomy: a meta-analysis]. Zhonghua Wei Chang Wai Ke Za Zhi 2015;18:826–31. [PubMed] [Google Scholar]
- [16].Li S, Wu X, Zhang L. Preliminary study on learning curve of laparoscopic radical gastrectomy for gastric cancer. J Digest Oncol 2011;3:3. [Google Scholar]
- [17].Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc 2009;23:1259–64. [DOI] [PubMed] [Google Scholar]
- [18].Kawamura H, Tanioka T, Funakoshi T, et al. Surgical effects of obesity on laparoscopy-assisted distal gastrectomy. Surg Laparosc Endosc Percutan Tech 2011;21:155–61. [DOI] [PubMed] [Google Scholar]


