Abstract
Objective:
To evaluate the efficacy and safety of carbetocin for prevention of postpartum hemorrhage in women undergoing vaginal delivery compared with oxytocin.
Methods:
We conducted a systemic literature search in PubMed, the Cochrane Library, and Embase without language restrictions from inception of each of database to November 18th, 2018. Randomized controlled trials with outcome measure of blood loss ≥500 ml were eligible if they compared carbetocin with oxytocin to prevent postpartum hemorrhage during the third stage of labor in women undergoing vaginal delivery.
Results:
This meta-analysis of 5 randomized controlled trials (30,314 women) indicated that there was no significant difference between carbetocin and oxytocin in blood loss ≥500 ml in women undergoing vaginal delivery (relative risks (RRs), 0.52; 95% confidence intervals (CIs), 0.24 to 1.15; P = .11; I2 = 69%). Sensitivity analyses showed the same results. No significant differences were found in blood loss ≥1000 ml, use of additional uterotonic agents, blood transfusion, uterine massage, flushing, vomiting, abdominal pain, nausea, dizziness, headache, palpitation, itching, and shivering.
Conclusions:
This meta-analysis showed that carbetocin was as effective and safe as oxytocin for prevention of postpartum hemorrhage in women undergoing vaginal delivery, and the choice of carbetocin for routine prophylaxis will depend on cost-effectiveness.
Keywords: carbetocin, postpartum hemorrhage, vaginal delivery
1. Introduction
Although great effort has been made to reduce maternal mortality, postpartum hemorrhage remains to be the largest direct cause of maternal death, accounting for nearly one-fourth death worldwide and contributes to long term disability and severe maternal morbidity such as blood transfusion, emergency surgery, and admission to intensive care unit.[1,2] Postpartum hemorrhage is defined as blood loss at least 500 ml after vaginal delivery and blood loss more than 1000 ml after cesarean section.[3] The most common cause of postpartum hemorrhage is uterine atony, which results from poor contraction of the uterus after childbirth.[3] The incidence of postpartum hemorrhage has been increasing in developed countries including the USA and Europe for the past 15 years.[4]
Currently, the World Health Organization (WHO) recommends active management of the third stage of labor for prevention of postpartum hemorrhage.[3] Prophylactic administration of uterotonic agents is identified as the most important component of active management of the third stage of labor, which has reduced the incidence of postpartum hemorrhage nearly by 50%.[5] Oxytocin, which has a short half-life and duration of action, is the current standard therapy for the prevention of postpartum hemorrhage. However, as it is susceptible to heat, its efficacy cannot be assured in many low- and middle- countries where access to cold-chain transport and storage is unavailable, and quality issues such as impurity and insufficient active ingredients also compromise its efficacy.[6] In contrast, carbentocin, which is a long-acting oxytocin analogue, has been widely used in preventing postpartum hemorrhage since 1997, and heat-stable carbentocin, and has been shown to maintain active for more than 36 months at 30 °C and 75% relative humidity.[7]
Clinical trials of carbetocin for postpartum hemorrhage prevention have focused mainly on cesarean delivery. A recent systematic review showed that carbetocin was more effective than oxytocin for reducing the need for additional uterotonic drugs and the need for uterine massage after cesarean delivery.[8] However, it is unclear whether carbetocin is more effective and tolerable than oxytocin for prevention of postpartum hemorrhage following vaginal delivery. Therefore, we found it necessary to evaluate the efficacy and safety of carbetocin in preventing postpartum hemorrhage in women undergoing vaginal delivery compared with oxytocin.
2. Methods
2.1. Search strategy
This meta-analysis was performed according to Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidlines.[9] We conducted a systemic literature search in PubMed, the Cochrane Library, and Embase without language restrictions from inception of each of database to November 18th, 2018. The search was undertaken in these databases using the following medical subject heading (MeSH) terms, keywords, and their combinations: carbetocin; oxytocin; postpartum hemorrhage(“postpartum”, “post partum”,“post-partum”, “hemorrhage” and “haemorrhage”); labor stage third; third stage (“third”, “3rd”). The reference lists of the initially identified articles, previously published systematic reviews and review articles were also manually searched for additional relevant publications. Ethical approval and patient written informed consent were not required, as all data were collected from previous published studies.
2.2. Study selection and data extraction
Randomized controlled trials with outcome measure of blood loss ≥500 ml were eligible if they compared carbetocin with oxytocin to prevent postpartum hemorrhage during the third stage of labor in women undergoing vagianl delivery. Quasi-randomized trials were excluded. Trials published in abstract only were also excluded. Two independent reviewers screened citations at title and abstract level to identify potentially eligible trials. Then the full texts of the relevant citations were retrieved and assessed for inclusion. The data extracted from these trials included the first author, publication year, population characteristics, intervention details, study design, and reported outcomes. Two of the reviewers extracted data from each trial independently. All disagreements between the 2 reviewers were resolved through discussion. The risk of bias in included trials was assessed by the use of criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.[10] Assessments of the risk of bias for each of the included trial were completed according to the 7 domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias). This tool categorizes randomized trials by “low, unclear or high risk of bias” in each domain.
2.3. Outcomes
The primary outcome was blood loss of at least 500 ml after vaginal delivery. The secondary outcomes were blood loss of at least 1000 ml; use of additional uterotonic agents; blood transfusion; uterine massage; flushing; vomiting; abdominal pain; nausea; dizziness; headache; palpitation; itching; shivering.
2.4. Data synthesis
The dichotomous data results after pooling estimates across trials were reported as RR with 95% CIs. Heterogeneity of the results among the trials was assessed with the I2 statistic. If significant heterogeneity was observed, the random-effects model was used. Otherwise, a fixed-effects model was employed. Sensitivity analyses were carried out to assess the effect of risk of bias on the overall results by excluding 1 trial at 1 time. All analyses were performed using Revman statistical software version 5.
3. Results
3.1. search results and characteristics of included studies
Our search strategy identified 211 citations, of which 64 were duplicates, and 142 were excluded based on title and abstract. After assessing full texts of the remaining 5 citations, we included 5 randomized controlled trials comparing carbetocin with oxytocin for prevention of postpartum hemorrhage in this meta-analysis.[11–15] The PRISMA flow diagram summarizing the selection procedure is shown in Figure 1.
Figure 1.
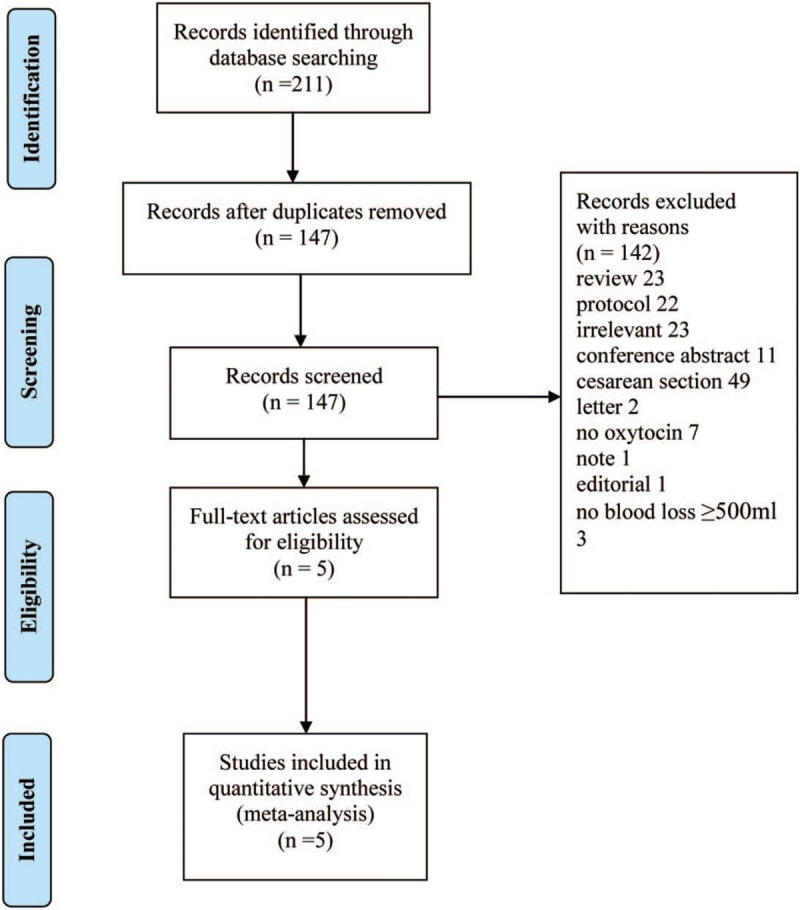
Study selection flow diagram.
Baseline characteristics of the 5 included trials are presented in Table 1.
Table 1.
Characteristics of included trials.
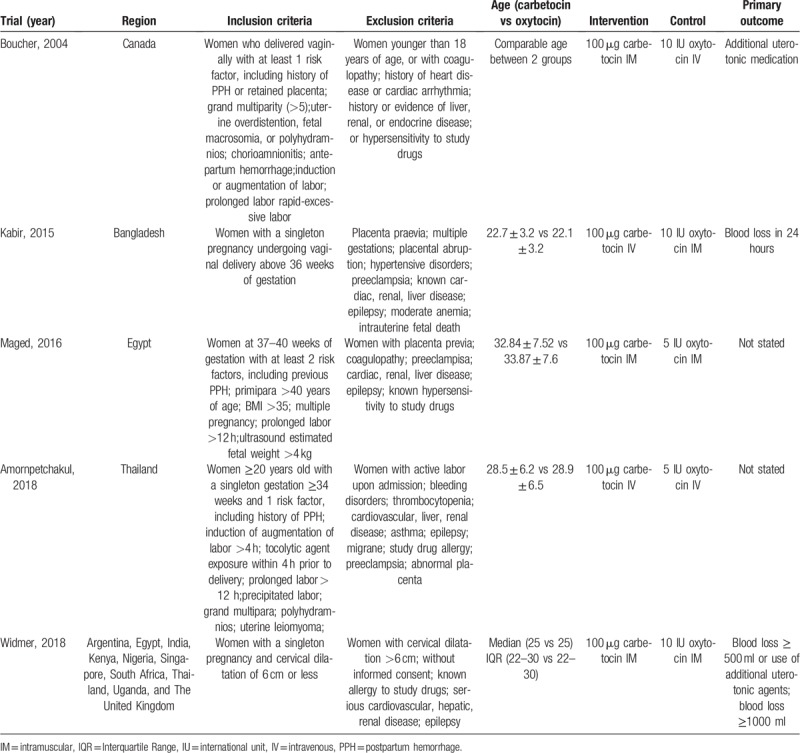
We identified 5 trials that assessed the efficacy and safety of carbetocin vs oxytocin in preventing postpartum hemorrhage in women who delivered vaginally. Differences in baseline characteristics were noticed among these trials. Two trials[12,15] included women with a singleton gestation; 1 trial[14] recruited women with a singleton gestation and 1 risk factor such as prolonged labor >12 h and polyhydramnios; 1 trial[11] enrolled women with at least 1 risk factor including retained placenta and induction of labor; 1 trial[13] recruited women with at least 2 risk factors such as primipara >40 years of age and multiple pregnancy. Women with abnormal conditions such as cardiac, hepatic or renal disease, coagulopathy, and abnormal placenta were excluded. Maternal age was not stated in 1 trial,[11] but was comparable between the carbetocin and oxytocin groups in each trial; Maternal age in 1 trial[12] was much younger than the other 3 trials.[13–15] A standard dose of 100 μg carbetocin was administered across all the trials, while the dose of oxytocin varied from 5 to 10 IU across the trials. The administration route of carbetocin and oxytocin also varied across these trials. Women received a single intramuscular injection of either carbetocin or oxytocin in 2 trials[13,15]; women received a single intravenous injection of either carbetocin or oxytocin in 1 trial[14]; women received a single intramuscular injection of carbetocin or intravenous injection of oxytocin in 1 trial[11]; women received a single intravenous injection of carbetoin or intramuscular injection of oxytocin in the last trial.[12] Primary outcome was not stated in 2 trials,[13,14] and varied across the other 3 trials.[11,12,15] However, these trials all included blood loss at least 500 ml. Assessment of risk of bias is shown in Figure 2. Two trials[14,15] were assessed to be “low risk of bias” in 7 domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias), 1 trial[11] was considered to be “unclear risk of bias” in 1 domain (incomplete outcome data) and “low risk of bias” in the other 6 domains, 1 trial[12] was assessed to be “unclear risk of bias” in 2 domains (allocation concealment, blinding of participants, and personnel) and “low risk of bias” in the other 5 domains, and 1 trial[13] was considered to be “unclear risk of bias” in 1 domain (blinding of participants and personnel) and “low risk of bias” in the other 6 domains.
Figure 2.
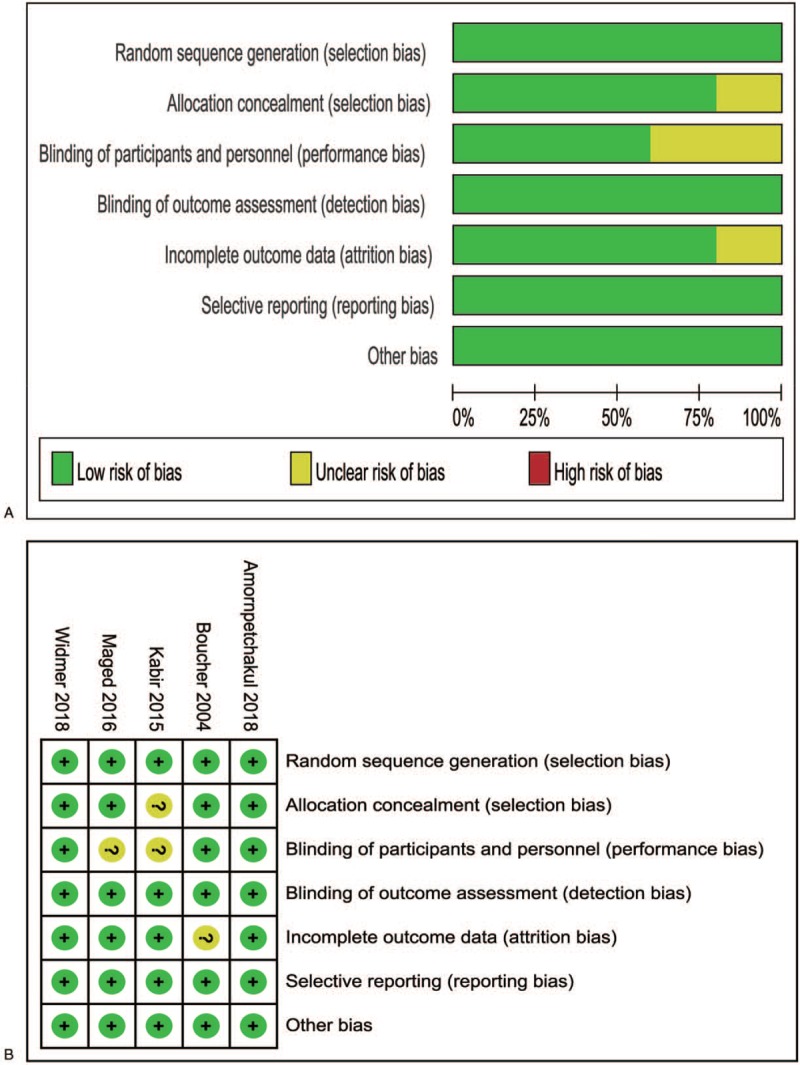
a. Risk of bias graph, b. risk of bias summary (“+” low risk; “?” unclear risk; “-“ high risk).
3.2. Primary outcome: blood loss ≥500 ml
This meta-analysis of the 5 trials (30,314 women) showed there was no significant difference between carbetocin and oxytocin in blood loss ≥500 ml in women undergoing vaginal delivery (RR, 0.52; 95%CI, 0.24–1.15; P = .11; I2 = 69%)(Fig. 3). Due to the high heterogeneity, sensitivity analyses were performed to explore the cause of heterogeneity. The sensitivity analyses by excluding 1 trial at 1 time showed the same results.
Figure 3.
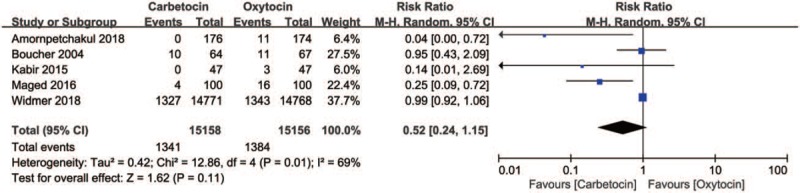
Carbetocin vs oxytocin for blood loss ≥500 ml in women undergoing vaginal delivery.
3.3. Secondary outcome
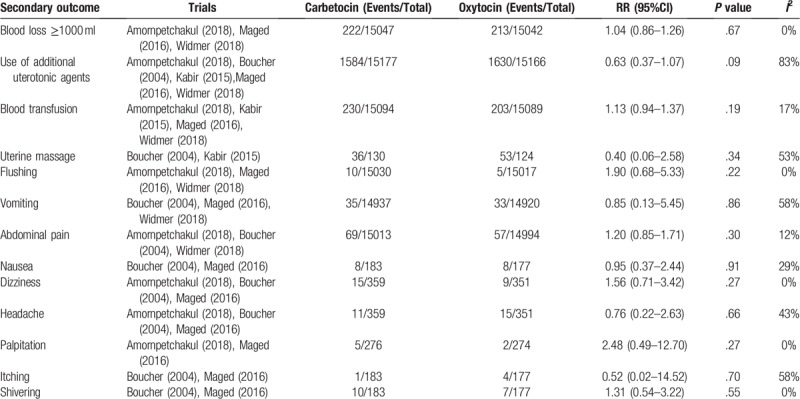
No statistically significant difference was found between the carbetocin and oxytocin groups in blood loss ≥1000 ml (RR, 1.04; 95% CI, 0.86–1.26; P = .67; I2 = 0%), use of additional uterotonic agents (RR, 0.63; 95% CI 0.37–1.07; P = .09; I2 = 83%), blood transfusion (RR, 1.13; 95% CI 0.94–1.37; P = .19; I2 = 17%), uterine massage (RR, 0.40; 95% CI 0.06–2.58; P = .34; I2 = 53%), flushing (RR, 1.90; 95% CI 0.68–5.33; P = .22; I2 = 0%), vomiting (RR, 0.85; 95% CI 0.13–5.45; P = .86; I2 = 58%), abdominal pain (RR, 1.20; 95% CI 0.85–1.71; P = .30; I2 = 12%), nausea (RR, 0.95; 95% CI 0.37–2.44; P = .91; I2 = 29%), dizziness (RR, 1.56; 95% CI 0.71–3.42; P = .27; I2 = 0%), headache (RR, 0.76; 95% CI 0.22–2.63; P = .66; I2 = 43%), palpitation (RR, 2.48; 95% CI 0.49–12.70; P = .27; I2 = 0%), itching (RR, 0.52; 95% CI 0.02–14.52; P = .70; I2 = 58%), and shivering (RR, 1.31; 95% CI 0.54–3.22; P = .55; I2 = 0%)(Table 2).
Table 2.
Carbetocin vs oxytocin for secondary outcomes in women undergoing vaginal delivery.
4. Discussion
This meta-analysis of 5 randomized trials including 30,314 women showed that there was no significant difference between carbetocin and oxytocin in blood loss ≥500 ml in women undergoing vaginal delivery. Sensitivity analyses showed the same results. This meta-analysis also indicated that there were no significant differences between carbetocin and oxytocin in blood loss ≥1000 ml, use of additional uterotonic agents, blood transfusion, uterine massage, and adverse effects.
Access to effective uterotonic agents is the key to prevent atony postpartum hemorrhage. However, quality issues of uterotonic agents are prevalent in low- and middle-income countries.[16] According to latest evidence, due to insufficient amounts of active ingredient, nearly 45.6% to 74.2% of oxytocin samples failed quality tests in these countries.[6,17] Therefore, improving the quality and efficacy of uterotonic agents for prevention of postpartum hemorrhage becomes a critical issue. Carbetocin has advantages over oxytocin for prevention of postpartum hemorrhage. Compared with oxytocin, heat-stable carbetocin, does not need cold-chain transport and storage. Therefore, it is convenient to be stored in facilities at room temperature in low and middle income countries where cold-chain transport and storage are not available. The half-life of carbetocin is 40 minutes, which is 4-10-fold longer than that of oxytocin, and the duration of action is 2 hours after an intramuscular injection,[14] avoiding side effects of intravenous injection. Nevertheless, there is a disparity in price between carbetocin and oxytocin. A vial of carbetocin is 18.20 USD, while a vial of oxytocin is only 0.18 USD, which could be a major barrier for carbetocin to be widely used in low- and middle-income countries. Currently, the WHO does not include a recommendation for carbetocin in preventing postpartum hemorrhage. However, the result of this meta-analysis indicated that there was no significant difference between carbetocin and oxytocin for prevention of postpartum hemorrhage in women undergoing vaginal delivery, and there was a non-significant trend in favor of carbetocin. Although the heterogeneity was high, sensitivity analyses by excluding 1 trial at 1 time showed the same results. No significant differences were found between carbetocin and oxytocin in blood loss ≥1000 ml, use of additional uterotonic agents, blood transfusion, uterine massage, and side effects such as abdominal pain, vomiting, dizziness, and palpitation. Therefore, according to the results of this meta-analysis, it is reasonable that professional organizations might consider to revise their clinical guidelines to clinicians and recommend heat- stable carbetocin as first line treatment for prevention of postpartum hemorrhage in women undergoing vaginal delivery especially in low- and middle-income countries, if carbetocin is proven to be cost-effective.
This meta-analysis has its limitations. First, most of the trials included in this meta-analysis were small, with likely biases, which could hinder the validity of research of carbetocin compared with oxytocin. Second, baseline characteristics of these women in these trials were different from each other. Two trials[12,15] included women with or without risk factors; 1 trial[14] only recruited women with 1 risk factor; 1 trial[11] only enrolled women with at least 2 risk factors; the last trial[13] only included women with at least 2 risk factors. Third, different dosage and administration routes of carbetocin and oxytocin were used in these trials. All the aspects mentioned above might contribute to the inconsistence of trials leading to the high heterogeneity of this meta-analysis. Fourth, we did not conduct an individual patient data (IPD) meta-analysis, which allows for evaluating the efficacy and safety of carbetocin in subpopulation such as women with different maternal age and risk factors. Therefore, we were unable to explore potential sources of heterogeneity and to perform subgroup analyses.
Risk factors of postpartum hemorrhage include history of postpartum hemorrhage, retained placenta, polyhydramnios, multiple pregnancies, prolonged labor, etc.[18] It is not clear which women benefit most from carbetocin for prevention of postpartum hemorrhage. Further trial might focus on certain subpopulations such as women with polyhydramnios, multiple pregnancies, or prolonged labor. Future trials also need to identify the optimal dose, timing, and administration route of carbetocin for these women.
In conclusion, this meta-analysis showed that carbetocin was as effective and safe as oxytocin for prevention of postpartum hemorrhage in women undergoing vaginal delivery, and the choice of carbetocin for routine prophylaxis will depend on cost-effectiveness.
Author contributions
Conceptualization: Xin-Hang Jin.
Data curation: Xin-Hang Jin, Dan Li, Xia Li.
Formal analysis: Xin-Hang Jin, Dan Li.
Investigation: Xin-Hang Jin, Dan Li.
Methodology: Xin-Hang Jin, Dan Li.
Project administration: Xin-Hang Jin, Dan Li, Xia Li.
Resources: Xin-Hang Jin, Dan Li, Xia Li.
Software: Xin-Hang Jin, Dan Li.
Supervision: Xin-Hang Jin, Dan Li.
Validation: Xin-Hang Jin, Dan Li, Xia Li.
Writing – original draft: Xin-Hang Jin, Dan Li.
Writing – review & editing: Xin-Hang Jin.
Footnotes
Abbreviations: CI = confidence interval, IM = intramuscular, IQR = interquartile range, IU = international unit, IV = intravenous, PPH = postpartum hemorrhage, RR = relative risk, USD = United States dollar, WHO = World Health Organization.
How to cite this article: Jin XH, Li D, Li X. Carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery: a meta-analysis. Medicine. 2019;98:47(e17911).
The authors have no conflicts of interest to disclose.
References
- [1].Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- [2].Hogan MC, Foreman KJ, Naghavi M, et al. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet (London, England) 2010;375:1609–23. [DOI] [PubMed] [Google Scholar]
- [3].Heneghan C, Ward A, Perera R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet (London, England) 2012;379:322–34. [DOI] [PubMed] [Google Scholar]
- [4].Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Preg Childbirth 2009;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev 2013;Cd001808. [DOI] [PubMed] [Google Scholar]
- [6].Torloni MR, Gomes Freitas C, Kartoglu UH, et al. Quality of oxytocin available in low- and middle-income countries: a systematic review of the literature. BJOG 2016;123:2076–86. [DOI] [PubMed] [Google Scholar]
- [7].Malm M, Madsen I, Kjellstrom J. Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries. J Peptide Sci 2018;24:e3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database SysReviews (Online) 2012;4:CD005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj-Brit Med J 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boucher M, Nimrod CA, Tawagi GF, et al. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery: a double-blind randomized trial. J Obst Gynaecol Can 2004;26:481–8. [DOI] [PubMed] [Google Scholar]
- [12].Kabir N, Akter D, Daisy TA, et al. Efficacy and safety of carbetocin in comparison to oxytocin in the active management of third stage of labour following vaginal delivery: an open label randomized control trial. Bangladesh J Obst Gynecol 2015;30:3–9. [Google Scholar]
- [13].Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery in high risk women. J Maternal-Fetal Neonat Med 2016;29:532–6. [DOI] [PubMed] [Google Scholar]
- [14].Amornpetchakul P, Lertbunnaphong T, Boriboonhiransarn D, et al. Intravenous carbetocin versus intravenous oxytocin for preventing atonic postpartum hemorrhage after normal vaginal delivery in high-risk singleton pregnancies: a triple-blind randomized controlled trial. Arch Gynecol Obst 2018;298:319–27. [DOI] [PubMed] [Google Scholar]
- [15].Widmer M, Piaggio G, Nguyen TMH, et al. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med 2018;379:743–52. [DOI] [PubMed] [Google Scholar]
- [16].Theunissen FJ, Chinery L, Pujar YV. Current research on carbetocin and implications for prevention of postpartum haemorrhage. Reprod Health 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anyakora C, Oni Y, Ezedinachi U, et al. Quality medicines in maternal health: results of oxytocin, misoprostol, magnesium sulfate and calcium gluconate quality audits. BMC Preg Childbirth 2018;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Combs CA, Murphy EL, Laros RK., Jr Factors associated with postpartum hemorrhage with vaginal birth. Obst Gynecol 1991;77:69–76. [PubMed] [Google Scholar]


