Abstract
Management of shock in children with severe malnutrition remains controversial. To date, the evidence supporting either benefit or harm of fluid resuscitation or rehydration is weak. This issue, however, is not unique to children with severe malnutrition; pediatric guidelines worldwide have a weak level of evidence and remain unsupported by appropriate clinical studies. In this review we give an overview of the current recommendations in other pediatric populations and appraise the strength of evidence supporting these. We summarize results from the only controlled trial ever undertaken, FEAST (Fluid Expansion As Supportive Therapy), which was conducted in resource-poor hospitals involving 3,141 African children with severe febrile illnesses and shock, including large subgroups with sepsis and malaria but excluding children with severe malnutrition. This high-quality trial provided robust evidence that fluid resuscitation increased the risk of death, leading to an excess mortality of 3 in every 100 children receiving fluid boluses, compared with controls receiving no boluses. These findings may have particular relevance to management of septic shock in children with severe malnutrition. However, they cannot be extrapolated to children with gastroenteritis, since this condition was not included in the trial. Current observational studies under way in East Africa may provide insights into myocardial and hemodynamic function in severe malnutrition, including responses to fluid challenge in those complicated by gastroenteritis. Such studies are an essential step for setting the research agenda regarding fluid management of shock in severe malnutrition.
Keywords: Diarrhea, fluid resuscitation, sepsis, severe malnutrition, shock
Introduction
The mortality rates of severely malnourished pediatric patients admitted to hospitals in sub-Saharan Africa remain unacceptably high, despite implementation of standardized treatment protocols developed by the World Health Organization (WHO) [1–4]. Furthermore, an increasingly higher number of children are now being classified as severely malnourished following the 2006 WHO revisions to the weight and height growth standard and mid-upper-arm circumference (MUAC) references [5].
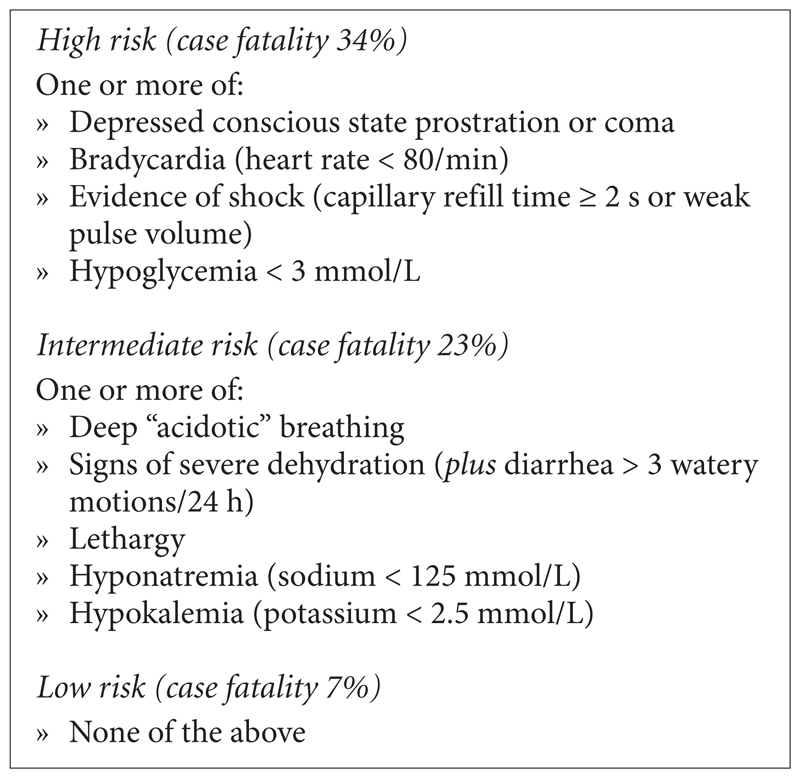
An evaluation of a district hospital in South Africa and a mission hospital in Ghana to investigate the problems, benefits, feasibility, and sustainability of implementation of WHO guidelines on the management of severe malnutrition concluded that implementation of the main principles was feasible, affordable, and sustainable, but that the guidelines could be improved by adaptation to local situations and targeting components that impact mortality [6]. A further study conducted in 2004 in South African rural hospitals examining implementation of the WHO guidelines and their effect on outcome in severe malnutrition came to similar conclusions [1]. These findings are important, as they contrast with previous published reports attributing high mortality among severely malnourished children to factors such as insufficient training of staff and poor compliance with recommended protocol, among others [4, 7, 8]. A mortality rate of over 20% in severely malnourished children is regarded as unacceptable by WHO; however, a good number of countries in sub-Saharan Africa continue to record much higher case fatality rates than this [4, 9, 10]. A prospective study examining prognostic features for poor outcome in 920 Kenyan children with severe malnutrition [4] demonstrated that sepsis, severe dehydration (secondary to diarrhea), and hypovolemic shock were common complications, and that triage features associated with high early mortality included signs of shock and severe dehydration (fig. 1).
Fig.1.
Suggested triage features for identifying high- and intermediate risk groups of children with severe malnutrition at hospital admission
The management of shock in children with severe malnutrition remains very controversial [11]. Current WHO guidelines for the treatment of children with severe malnutrition reserve intravenous fluids for those with decompensated (hypotensive) shock, largely advocating hypo-osmotic solutions and severely limiting fluid volumes due to concerns about the risk of precipitating heart failure in children with severe malnutrition [12]. Furthermore, for both nonmalnourished or severely malnourished children the guidelines do not distinguish the treatment of hypovolemic shock that is secondary to dehydrating diarrhea or septic shock. Alterations in volume and composition of body fluid compartments [13] in severe infantile malnutrition had been reported in 1960, with a risk of hyponatremia and cerebral anoxia from circulatory shock in diarrheal disease [14]. Current guidelines indicate diarrhea to be a benign, self-limiting complication [15] and recommend oral rehydration using low-sodium rehydration solutions. Since sepsis, severe diarrhea, and hypovolemia are important independent determinants of outcome in African children with severe malnutrition, this may strengthen the case for investigating the role of more aggressive fluid resuscitation [16]. Before addressing this issue, we briefly review current guidelines and the strength of evidence for fluid resuscitation in nonmalnourished children treated in both resourcerich and resource-poor settings.
Management of shock
Standard care for the rest of the world
Guidelines from the American College of Critical Care Medicine recommend rapid and early administration of fluid boluses of up to 60ml/kg of isotonic solution or colloids, given over 15 minutes, to correct hemodynamic abnormalities in patients with shock, followed by inotrope therapy, ventilation, and intensive care hemodynamic support for those in whom signs of shock fail to correct [17]. A closer look at the literature, however, reveals a paucity of evidence for the pediatric fluid resuscitation guidelines currently in use. The research informing these guidelines is largely based on two retrospective cohort studies [18, 19]. The first study, reported in 1991, was a 7-year retrospective review of patients admitted to an intensive care unit at a tertiary referral hospital in Pittsburgh who were ventilated and largely inotrope-dependent, examining whether the volume of fluid boluses received in the initial treatment influenced endpoints on the intensive care unit, such as episodes of hypotension, further volume replacement, and mortality. It included 34 children with septic shock and concluded that compared with the two groups receiving either less than 20 mL/kg (n = 11) or between 20 and 40 mL/kg (n = 14), the group receiving more than 40 mL/kg in the first hour (n = 9) had improved survival and decreased recurrence of hypovolemia, without a significant increase in cardiogenic pulmonary edema or adult respiratory distress syndrome [18]. A second study by the same group using a similar design [19] was based on 91 children admitted to the tertiary center intensive care unit over 9 years with septic shock (defined as febrile illness plus any of the following: decreased or altered mental status, capillary refilling time > 2 seconds, diminished pulse, or mottled cool extremities). They found that children (n = 34) in whom shock was reversed after they had received 60 mL/kg of fluid over 15 minutes had a survival rate of 96% and a ninefold increase in the odds of survival compared with those who did not receive this volume of fluid [19]. This recommendation for initial treatment has now become the standard recommendation for most pediatric emergency guidelines internationally. A GRADE evidence review (Delphi Surviving Sepsis Campaign 2008 International Guidelines) concluded that the pediatric guideline was a weak recommendation based on a low level of evidence (level 2C) [20].
Recommendations in resource-poor settings
The WHO handbook and guidelines for Emergency Triage and Treatment (ETAT), which give guidelines for management in hospitals with limited resources [12, 21, 22], also recommend rapid and early administration of fluid boluses in well-nourished children, despite challenges in hemodynamic monitoring and availability of intensive care support. Rapid fluid resuscitation, given as a 20 mL/kg bolus of isotonic crystalloid as fast as possible, is recommended for any child without severe malnutrition who fulfills the WHO definition of shock (capillary refill time of more than 3 seconds, plus a weak and fast pulse and cool peripheries) [12, 21, 22]. The bolus should be repeated twice more if shock fails to correct: i.e., for a total of 60 mL/kg. A GRADE review of the evidence conducted in 2011 considered choice of fluid for resuscitation, but not speed or volume [23]. The current recommendations were considered as a strong recommendation based on low-quality evidence [23]. The evidence reviewed did not include the results of the only large controlled trial of bolus resuscitation—Fluid Expansion As Supportive Treatment (FEAST)—nor a subsequent systematic review addressing this question. For children with severe malnutrition, intravenous fluid resuscitation is only recommended in those with advanced shock, which is defined as impaired consciousness plus the WHO shock definition. The recommendation is to give, over 1 hour, 15 mL/kg of Ringer’s lactate with 5% dextrose, or half-strength Darrow’s solution with 5% dextrose, or 0.45% saline with 5% dextrose, or plain Ringer’s lactate if the preceding solutions are unavailable, and to repeat administration of 15 mL/kg over the next hour for those whose shock improves, but to give whole blood to children whose shock does not improve and are presumed to have septic shock [12, 21].
Physiology of fluid resuscitation in nonmalnourished children: pilot studies and clinical trials
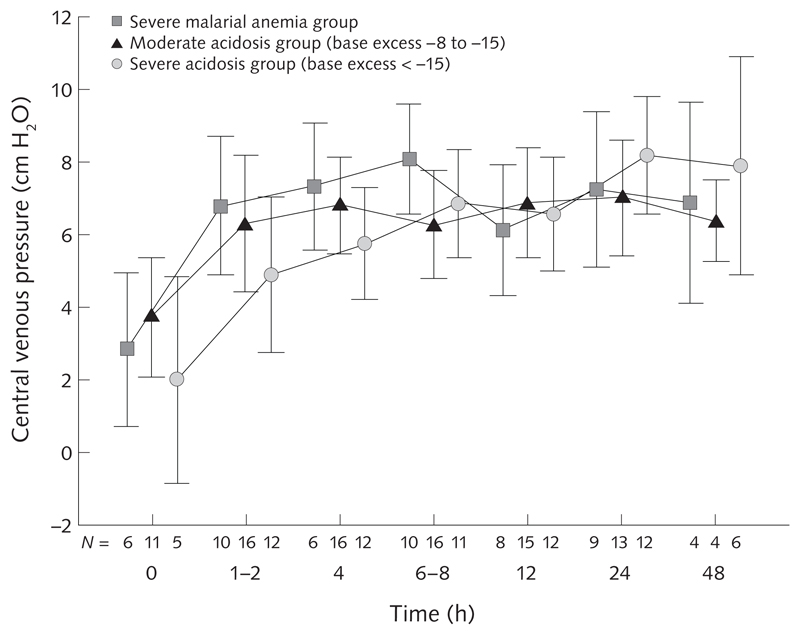
Over the decade prior to FEAST, the group in Kilifi conducted clinical research in children with severe malaria and severe malnutrition. In severe Plasmodium falciparum malaria, metabolic acidosis had emerged as a central feature of severe malaria, and it is the best independent predictor of a fatal outcome. A series of small prospective clinical studies of children with severe malaria demonstrated that alterations of hemodynamic status [24] and cardiac function [25] are similar to those seen in sepsis (hypovolemic shock) and, with the use of full hemodynamic monitoring, that children with acidosis at hospital admission have a low central venous pressure (fig. 2), tachycardia, and a prolonged capillary refill time [24]. Rapid volume expansion with 20 to 40 mL/kg of either saline or human albumin solution given intravenously in the first hour after admission safely corrected the hemodynamic features of volume depletion (tachycardia, tachypnea, and delayed capillary refill time). Further trials examining the optimum fluid for resuscitation compared albumin and saline [24], albumin and gelofusine [26], and 6% Dextran 70 and 6% hydroxyethyl starch [27]. The best outcomes were in the group treated with albumin. No controlled trials were conducted, however, comparing boluses of fluid with maintenance fluids only. A systematic review suggested that albumin appeared to show benefit over other solutions, but that a larger trial was needed to definitively establish this [28].
Fig. 2.
Central venous pressure (CVP) in children with severe malaria following 20- to 40-mL/kg boluses of saline or albumin given postadmission (0 hours). Values shown are mean ± 2 SE.
Fluid resuscitation trials: Malnourished children
A phase II randomized, controlled, safety and efficacy trial of fluid management in malnutrition in Kenya was stopped early before completion [29]. The trial, which compared use of half-strength Darrow’s solution with 5% dextrose (HSD/5%D) with Ringer’s lactate in resuscitation of severely malnourished children with shock, had recruited 20 children with suspected septic shock and 41 children with hypovolemic shock secondary to diarrhea, with an overall 69% of the patients having decompensated hypotensive shock. The trial had high mortality and poor outcomes in both treatment arms, with persistence of oliguria at 8 and 24 hours. Most deaths occurred within 48 hours after hospital admission, without any evidence of fluid overload. Despite evidence of mild improvement in shock and slightly better outcomes with the isotonic Ringer’s lactate compared with the hypotonic HSD/5%D, the evidence was inconclusive, and the investigators recommended a reevaluation of guidelines for treatment of shock in severely malnourished children [29].
In a GRADE review of the effectiveness and safety of intravenous fluids for resuscitation in children with severe malnutrition, clinical trials, observational studies, and case–control studies were included. Four studies met the inclusion criteria [29–32], of which three were before-and-after observational studies and one was a clinical trial [29]. No systematic review was available. Two observational studies compared mortality after the introduction of standardized management with mortality among historical controls when management was not standardized [30, 32]. The two studies involved introduction of a bundle of management, which included restricted use of intravenous fluids. In the Bangladesh study of severely malnourished children with diarrhea, a total of 30 to 40 mL/kg of Ringer’s lactate with 5% dextrose (or normal saline) was given over 2 hours to children aged less than 2 months, and 50% diluted solution with 5% dextrose (supplemented to 20 mmol/L potassium) was given to children aged 2 months or more [30]. The authors reported 17% mortality before and 9% after implementation of the standardized management. In Ugandan children receiving the recommended intravenous infusion and transfusion during the intervention period, either Ringer’s lactate or half-strength Darrow’s solution with 5% dextrose administered as 15 mL/kg over 1 hour was used [32]. The overall case fatality was 23.6% before full implementation and 24.8% following adoption of these recommendations. Two major weaknesses of both of these reports are that the introduction of standardized management included aspects of the bundle, and it was unclear what numbers of children received various fluids.
One further observational study investigated the safety of intravenous infusion of up to 100 mL/kg of isotonic fluid (cholera saline) within 6 hours in malnourished Bangladeshi children with severe dehydration secondary to cholera [31]. Children studied under this observational-cohort aspect of the study were enrolled in a randomized trial of three oral rehydration solution formulations; there was no comparison group, historical or otherwise. No deaths occurred in the study, and pulmonary edema or other signs of fluid overload were not apparent.
The fourth study included in the GRADE review was a randomized clinical trial (reported in full above) [29]. The study was not blinded but had adequate sequence generation and allocation and was prematurely terminated. Termination before obtaining the preset sample size was due to concerns about high mortality in the study and inadequate resolution of shock in both treatment arms, determined by the high prevalence of oliguria. The conclusions of the GRADE review were that in children with severe malnutrition and shock or severe dehydration:
-
»
There is insufficient evidence to support slow fluid resuscitation using low fluid volumes;
-
»
There is no evidence indicating increased harm of rapid fluid expansion using isotonic solutions;
-
»
On the basis of current evidence, there is need for a definitive trial with mortality as an endpoint comparing rapid fluid expansion using isotonic solution to the rates and fluids recommended by WHO.
FEAST: Randomized, controlled trial of fluid resuscitation in nonmalnourished children
The only randomized, controlled trial of fluid resuscitation that has even been undertaken was the FEAST trial [33]. FEAST compared bolus fluid resuscitation with no bolus in well-nourished children over 2 months of age. It was conducted in six African hospitals without intensive care facilities in Kenya, Tanzania, and Uganda, in children with shock and severe febrile illness (including major subgroups of sepsis, malaria, and anemia). Children with gastroenteritis, severe malnutrition, burns, or surgical conditions were excluded from the trial. All children received standard treatments according to their illness, including standard of care maintenance fluids (mainly 5% dextrose/one-fifth saline) at 2.5 to 4 mL/kg/h until they were able to drink and, where indicated, antibiotics, antimalarials, oxygen, and transfusions. FEAST enrolled 3,141 African children who were randomly assigned to receive albumin or normal saline boluses (20–40 mL/kg over 1–2 hours) or no bolus (control group). FEAST was a pragmatic trial including children with malaria (57%), and most of the remaining 43% had presumptive sepsis; 32% had severe anemia, 30% had severe acidosis (lactate > 5 mmol/L), 12% had culture-proven bacteremia, and only 4% were HIV positive. The treatment arms were well matched for clinical severity and malaria status at baseline.
The 48-hour mortality (the primary outcome) was higher in the children receiving the albumin bolus (10.6%) or the saline bolus (10.5%) than in controls receiving no bolus (7.3%) (table 1). The relative risk (RR) for any bolus versus control was 1.45 (95% CI, 1.13 to 1.86; p = .003) (table 2). Similarly, the 4-week mortality and neurological sequelae rates (secondary outcomes) were 12.2%, 12.0%, and 8.7%, respectively (p = .004), and 2.2%, 1.9%, and 2.0% (p = .92). The anticipated adverse effects of fluid boluses (pulmonary edema or raised intracranial pressure) were reported in only 2.6% (albumin), 2.2% (saline), and 1.7% (control) and were not significant for the bolus versus control comparisons (p = .17) [33]. Of note, the WHO/ETAT shock criteria identified only 65 children (2%); however, the adverse effect of fluid boluses was greatest in this subgroup. Mortality in the bolus arms with WHO/ETAT shock criteria was 48%, compared with 20% in the no bolus arm, representing a difference in absolute risk (AR) of 28 percentage points (95% CI, 3.4 to 52.4). Objective measures, such as moderate hypotension, were also associated with increased mortality in the bolus groups (AR difference, 9.4 percentage points; 95% CI, –2.6 to 21.4) [34].
Table 1. 48-hour mortality in the FEAST trial.
| Children | Treatment group |
Total | ||
|---|---|---|---|---|
| Albumin bolus |
Saline bolus |
No bolus |
||
| No. randomized | 1,050 | 1,047 | 1,044 | 3,141 |
| No. died | 111 | 110 | 76 | 297 |
| % died | 10.6 | 10.5 | 7.3 | 9.5 |
Table 2. Pairwise comparisons of 48-hour mortality (primary outcome) in the FEAST trial.
| Comparison group | % died in 1st group | % died in 2nd group | Risk ratio (95% CI) | p |
|---|---|---|---|---|
| Saline bolus vs no bolus | 10.5 | 7.3 | 1.44 (1.09–1.90) | .01 |
| Albumin bolus vs no bolus | 10.6 | 7.3 | 1.45 (1.10–1.91) | .008 |
| Bolus vs no bolus | 10.5 | 7.3 | 1.45 (1.13–1.86) | .003 |
Meta-analysis of all fluid resuscitation trials
A systematic review formally assessing the evidence base for fluid resuscitation for the treatment of children with shock due to sepsis or severe infection was published subsequently [35]. Included were randomized trials, quasi-randomized trials, and controlled before–after studies assessing children with septic shock in which at least one group was treated with bolus fluids. The primary outcome was mortality at 48 hours. Thirteen studies met the inclusion criteria (4 general shock, 4 malaria, 4 dengue, and 1 severe malnutrition). The main result was largely driven by the FEAST trial, indicating that administration of no bolus resulted in a significantly better mortality outcome at 48 hours for children with general septic shock (RR, 0.69; 95% CI, 0.54 to 0.89), and children with malaria (RR, 0.64; 95% CI, 0.46 to 0.91) when compared with administration of any bolus [33]. There are no studies comparing outcomes between those receiving and not receiving bolus fluids among children with dengue fever or severe malnutrition. Colloid and crystalloid boluses were found to have similar effects on mortality across all subgroups (general septic shock, malaria, dengue fever, and severe malnutrition) [35].
The authors concluded that further research, which should extend to children with other common pediatric conditions who were not included in the FEAST trial, is needed to define simple algorithms to support health providers in the triage of patients to determine who could potentially be harmed by the provision of bolus fluids.
Lessons learned from FEAST relevant to malnutrition
Further analysis of the FEAST trial data investigating modes of death indicated that contrary to expectation, circulatory failure, rather than fluid overload, appeared to be the greatest contributor to excess deaths with rapid bolus fluid resuscitation [36]. The new findings indicating that rapid and aggressive volume expansion leads to excess cardiovascular events should prompt a reevaluation of the rate, composition, and volume of resuscitation fluids in children with other common pediatric conditions presenting to hospitals in Africa, who were not included in the trial, i.e., both well-nourished and malnourished children with gastroenteritis. For children with severe malnutrition complicated by presumptive septic shock, where fluid resuscitation remains controversial, it is the authors’ view that the FEAST trial provides overwhelming data to indicate harm in well-nourished children, and it is likely that this harm will extend to children with severe malnutrition; therefore, fluid management should remain conservative.
Severe dehydration due to gastroenteritis
The current WHO recommendation for well-nourished children is to give 100 mL of intravenous fluid per kilogram body weight in two divided portions [12, 21, 22]. The first portion of the intravenous fluid, preferably isotonic Ringer’s lactate (30 mL/kg), is to be given very rapidly, and the remaining 70 mL/kg to complete rehydration is given more slowly over 2.5 to 5 hours, depending on the child’s age. For children who also present with signs of hypovolemic shock, the recommendation for initial management is for fluid boluses of 20 mL/kg, repeated, if shock persists, twice, over 1 hour and to be followed by rehydration recommendations as above; i.e., there is no recommendation to subtract the initial fluid volume given as boluses from the rehydration volume, and thus children may receive up to 160 mL/kg of fluid over 2.5 to 5 hours, equivalent to twice their intravascular volume. From what was learned in the FEAST trial, this volume and speed of correction may have deleterious effects. Whereas the aggressive regimen, although never tested in a clinical trial, may be appropriate for cholera—the only infective cause of secretory diarrhea (leading to excess fluid and electrolyte loss)—this regimen may be less applicable to all other causes of gastroenteritis, which do not involve this mechanism specific to cholera.
For children with severe malnutrition, current recommendations are to rehydrate orally with lowsodium rehydration solution (such as ReSoMal) [37] and to reserve intravenous fluid resuscitation for those with advanced shock (see “Fluid resuscitation trials: Malnourished children” above). This recommendation, however, has been challenged due to the poor level of evidence to support benefit or harm of a more liberal use of intravenous fluids and the high mortality of children who are admitted with severe malnutrition complicated by diarrhea. On the coast of Kenya, where dysentery and cholera are very rare, diarrhea is a complication in 65% of cases of severe acute malnutrition (SAM) in children, with a high mortality (20%) [38]. Gram-negative bacteremia is commonly associated with diarrhea and is the major independent risk factor for death, irrespective of HIV or anthropometric status. Further research is needed to address the safety of a more liberal but cautious intravenous fluid replacement.
Effects of malnutrition on the heart
Because death often occurs suddenly and unexpectedly in severe malnutrition, especially during early realimentation, myocardial dysfunction in the form of either cardiac arrhythmia or heart failure is thought to be the leading cause [39–41]. Reflecting this, international guidelines for children with either edematous malnutrition (kwashiorkor) or nonedematous malnutrition (marasmus) consistently discourage the use of intravenous fluids except in the presence of definitive signs of severe dehydration or decompensated hypotensive shock [42, 43].
Several studies have shown that cardiac structure is modified in severe malnutrition, with reduction in left ventricular mass [44, 45], but that these changes generally occur in proportion to the reduced body size [46, 47]. For example, Öcal and colleagues reported a decrease in cardiac output and left ventricular muscle mass proportional to body size in patients with protein–energy malnutrition, but left ventricular systolic and diastolic functions were preserved even in atrophic hearts [46]. Echocardiographic studies examining cardiac function of children affected by severe malnutrition provide conflicting results and have often only investigated children with kwashiorkor [44, 47, 48]. Most functional studies involve small numbers of patients, with poorly documented contemporaneous data on clinical and hemodynamic status. Therefore it remains unclear whether the observed abnormalities in cardiac function are primary phenomena of malnutrition per se, or are secondary to other abnormalities commonly complicating comorbidities that are common in severe malnutrition, such as sepsis, hypovolemia, acidosis, anemia, and HIV infection.
Observational studies are currently ongoing on the physiologic assessment of cardiac function in severely malnourished Kenyan children, and on myocardial and hemodynamic response to fluid resuscitation for shock due to gastroenteritis in severely malnourished Kenyan and Ugandan children. The results of these observational studies are expected to provide insights into patient responses to fluid resuscitation in accordance with current guidelines, and further set the stage for directing the research agenda on fluid management of shock in severe malnutrition.
Conflicts of interest
The authors declare no conflicts of interest.
Authors’ contributions
Nchafatso Obonyo wrote the first draft of the review; Kathryn Maitland reviewed and edited it. Both authors read and approved the final manuscript.
References
- 1.Ashworth A, Chopra M, McCoy D, Sanders D, Jackson D, Karaolis N, Sogaula N, Schofield C. WHO guidelines for management of severe malnutrition in rural South African hospitals: effect on case fatality and the influence of operational factors. Lancet. 2004;363:1110–5. doi: 10.1016/S0140-6736(04)15894-7. [DOI] [PubMed] [Google Scholar]
- 2.Puoane T, Sanders D, Chopra M, Ashworth A, Strasser S, McCoy D, Zulu B, Matinise N, Mdingazwe N. Evaluating the clinical management of severely malnourished children—a study of two rural district hospitals. S Afr Med J. 2001;91:137–41. [PubMed] [Google Scholar]
- 3.Karaolis N, Jackson D, Ashworth A, Sanders D, Sogaula N, McCoy D, Chopra M, Schofield C. WHO guidelines for severe malnutrition: are they feasible in rural African hospitals? Arch Dis Child. 2007;92:198–204. doi: 10.1136/adc.2005.087346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitland K, Berkley JA, Shebbe M, Peshu N, English M, Newton CR. Children with severe malnutrition: Can those at highest risk of death be identified with the WHO protocol? PLoS Med. 2006;3:e500. doi: 10.1371/journal.pmed.0030500. Epub 2006/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Onis M, Onyango AW, Borghi E, Garza C, Yang H. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO International Growth Reference: Implications for child health programmes. Public Health Nutr. 2006;9:942–7. doi: 10.1017/phn20062005. Epub 2006/12/30. [DOI] [PubMed] [Google Scholar]
- 6.Deen JL, Funk M, Guevara VC, Saloojee H, Doe JY, Palmer A, Weber MW. Implementation of WHO guidelines on management of severe malnutrition in hospitals in Africa. Bull World Health Organ. 2003;81:237–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Manary MJ, Brewster DR. Intensive nursing care of kwashiorkor in Malawi. Acta Paediatr. 2000;89:203–7. doi: 10.1080/080352500750028843. [DOI] [PubMed] [Google Scholar]
- 8.Heikens GT, Bunn J, Amadi B, Manary M, Chhagan M, Berkley JA, Rollins N, Kelly P, Adamczick C, Maitland K, Tomkins A, et al. Case management of HIV-infected severely malnourished children: challenges in the area of highest prevalence. Lancet. 2008;371:1305–7. doi: 10.1016/S0140-6736(08)60565-6. Epub 2008/04/15. [DOI] [PubMed] [Google Scholar]
- 9.Schofield C, Ashworth A. Why have mortality rates for severe malnutrition remained so high? Bull World Health Organ. 1996;74:223–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Bachou H, Tumwine JK, Mwadime RK, Tylleskar T. Risk factors in hospital deaths in severely malnourished children in Kampala, Uganda. BMC Pediatr. 2006;6:7. doi: 10.1186/1471-2431-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster DR. Critical appraisal of the management of severe malnutrition: 3. Complications. J Paediatr Child Health. 2006;42:583–93. doi: 10.1111/j.1440-1754.2006.00933.x. Epub 2006/09/16. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Management of the child with a serious infection or severe malnutrition. Geneva: WHO; 2000. [Google Scholar]
- 13.Kerpel-Fronius E, Romhanyi G, Gati B, Dobak E. Influences of depletion of potassium, of sodium, or of water on function and structure of the kidney. Pediatrics. 1960;26:939–49. Epub 1960/12/01. [PubMed] [Google Scholar]
- 14.Kerpel-Fronius E. Volume and composition of the body fluid compartments in severe infantile malnutrition. J Pediatr. 1960;56:826–33. doi: 10.1016/s0022-3476(60)80321-6. Epub 1960/06/01. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva: WHO; 1999. [Google Scholar]
- 16.Brent B, Obonyo N, Maitland K. Tailoring management of severe and complicated malnutrition: More research is required first. Pathog Glob Health. 2012;106:197–9. doi: 10.1179/2047772412Z.00000000061. Epub 2012/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, Duncan A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. Epub 2009/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–5. [PubMed] [Google Scholar]
- 19.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, Orr RA. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. Epub 2003/10/03. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buison C, Beale R, Calandra T, et al. for the International Surviving Sepsis Campaign Guidelines Committee. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. Epub 2007/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Emergency triage assessment and treatment (ETAT) Geneva: WHO; 2005. [Google Scholar]
- 22.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva: WHO; 2005. [PubMed] [Google Scholar]
- 23.World Health Organization. Recommendations for management of common childhood conditions: Evidence for technical update of pocket book recommendations. Geneva: WHO; 2012. [PubMed] [Google Scholar]
- 24.Maitland K, Pamba A, Newton CR, Levin M. Response to volume resuscitation in children with severe malaria. Pediatr Crit Care Med. 2003;4:426–31. doi: 10.1097/01.PCC.0000090293.32810.4E. Epub 2003/10/04. [DOI] [PubMed] [Google Scholar]
- 25.Yacoub S, Lang HJ, Shebbe M, Timbwa M, Ohuma E, Tulloh R, Maitland K. Cardiac function and hemodynamics in Kenyan children with severe malaria. Crit Care Med. 2010;38:940–5. doi: 10.1097/CCM.0b013e3181cd114a. Epub 2010/01/14. [DOI] [PubMed] [Google Scholar]
- 26.Akech S, Gwer S, Idro R, Fegan G, Eziefula AC, Newton CR, Levin M, Maitland K. Volume expansion with albumin compared to gelofusine in children with severe malaria: Results of a controlled trial. PLoS Clin Trials. 2006;1(5):e21. doi: 10.1371/journal.pctr.0010021. Epub 2006/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akech SO, Jemutai J, Timbwa M, Kivaya E, Boga M, Fegan G, Maitland K. Phase II trial on the use of Dextran 70 or starch for supportive therapy in Kenyan children with severe malaria. Crit Care Med. 2010;38:1630–6. doi: 10.1097/CCM.0b013e3181e81165. Epub 2010/06/08. [DOI] [PubMed] [Google Scholar]
- 28.Akech S, Ledermann H, Maitland K. Choice of fluids for resuscitation in children with severe infection and shock: systematic review. BMJ. 2010;341:c4416. doi: 10.1136/bmj.c4416. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akech SO, Karisa J, Nakamya P, Boga M, Maitland K. Phase II trial of isotonic fluid resuscitation in Kenyan children with severe malnutrition and hypovolaemia. BMC Pediatr. 2010;10:71. doi: 10.1186/1471-2431-10-71. Epub 2010/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed T, Ali M, Ullah MM, Choudhury IA, Haque ME, Salam MA, Rabbani GH, Suskind RM, Fuchs GJ. Mortality in severely malnourished children with diarrhoea and use of a standardised management protocol. Lancet. 1999;353:1919–22. doi: 10.1016/S0140-6736(98)07499-6. [DOI] [PubMed] [Google Scholar]
- 31.Alam NH, Islam S, Sattar S, Monira S, Desjeux JF. Safety of rapid intravenous rehydration and comparative efficacy of 3 oral rehydration solutions in the treatment of severely malnourished children with dehydrating cholera. J Pediatr Gastroenterol Nutr. 2009;48:318–27. doi: 10.1097/mpg.0b013e318180af27. [DOI] [PubMed] [Google Scholar]
- 32.Bachou H, Tumwine JK, Mwadime RKN, Ahmed T, Tylleskar T. Reduction of unnecessary transfusion and intravenous fluids in severely malnourished children is not enough to reduce mortality. Ann Trop Paediatr. 2008;28:23–33. doi: 10.1179/146532808X270644. [DOI] [PubMed] [Google Scholar]
- 33.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. doi: 10.1056/NEJMoa1101549. Epub 2011/05/28. [DOI] [PubMed] [Google Scholar]
- 34.Maitland K, Akech SO, Russell EC. Mortality after fluid bolus in African children with sepsis: Reply. N Engl J Med. 2011;365:1348–53. doi: 10.1056/NEJMc1108712. [DOI] [PubMed] [Google Scholar]
- 35.Ford N, Hargreaves S, Shanks L. Mortality after fluid bolus in children with shock due to sepsis or severe infection: a systematic review and meta-analysis. PLoS One. 2012;7(8):e43953. doi: 10.1371/journal.pone.0043953. Epub 2012/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, Reyburn H, et al. for the FEAST trial group. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. Epub 2013/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Management of the child with a serious infection or severe malnutrition. Geneva: WHO; 2000. [Google Scholar]
- 38.Talbert A, Thuo N, Karisa J, Chesaro C, Ohuma E, Ignas J, Berkley JA, Toromo C, Atkinson S, Maitland K. Diarrhoea complicating severe acute malnutrition in Kenyan children: a prospective descriptive study of risk factors and outcome. PLoS One. 2012;7(6):e38321. doi: 10.1371/journal.pone.0038321. Epub 2012/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dramaix M, Brasseur D, Donnen P, Bahwere P, Porignon D, Tonglet R, Hennat P. Prognostic indices for mortality of hospitalized children in central Africa. Am J Epidemiol. 1996;143:1235–43. doi: 10.1093/oxfordjournals.aje.a008711. [DOI] [PubMed] [Google Scholar]
- 40.Wharton BA, Howells GR, McCance RA. Cardiac failure in kwashiorkor. Lancet. 1967;2:384–7. doi: 10.1016/s0140-6736(67)92006-5. [DOI] [PubMed] [Google Scholar]
- 41.Smythe PM, Swanepoel A, Campbell JA. The heart in kwashiorkor. BMJ. 1962;1:67–73. doi: 10.1136/bmj.1.5271.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashworth A, Khanum S, Jackson A, Schofield C. Guidelines for the inpatient treatment of severely malnourished children. Geneva: WHO; 2003. [Google Scholar]
- 43.Kenya Ministry of Medical Services, and Ministry of Public Health & Sanitation. National guideline for integrated management of acute malnutrition. Nairobi. 2009 [Google Scholar]
- 44.Bergman JW, Human DG, De Moor MM, Schulz JM. Effect of kwashiorkor on the cardiovascular system. Arch Dis Child. 1988;63:1359–62. doi: 10.1136/adc.63.11.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kothari SS, Patel TM, Shetalwad AN, Patel TK. Left ventricular mass and function in children with severe protein energy malnutrition. Int J Cardiol. 1992;35:19–25. doi: 10.1016/0167-5273(92)90050-d. [DOI] [PubMed] [Google Scholar]
- 46.Ocal B, Unal S, Zorlu P, Tezic HT, Oguz D. Echocardiographic evaluation of cardiac functions and left ventricular mass in children with malnutrition. J Paediatr Child Health. 2001;37:14–7. doi: 10.1046/j.1440-1754.2001.00566.x. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 47.Phornphatkul C, Pongprot Y, Suskind R, George V, Fuchs G. Cardiac function in malnourished children. Clin Pediatr. 1994;33:147–54. doi: 10.1177/000992289403300304. [DOI] [PubMed] [Google Scholar]
- 48.Olowonyo MT, Ogunkunle OO, Akinbami FO, Jaiyesimi F. The echocardiographic findings in kwashiorkor. J Trop Pediatr. 1995;41:74–6. doi: 10.1093/tropej/41.2.74. [DOI] [PubMed] [Google Scholar]