Abstract
G protein-coupled receptors (GPCRs) are the most intensively studied drug targets, largely due to their substantial involvement in human pathophysiology and their pharmacological tractability. Here, we report the first analysis of all GPCR drugs and agents in clinical trials. This reveals the current trends across molecule types, drug targets and therapeutic indications, including showing that 481 drugs (~34% of all drugs approved by the FDA) act at 107 unique GPCR targets. Approximately 320 agents are currently in clinical trials, of which ~36% target 64 potentially novel GPCR targets without an approved drug, and the number of biological drugs, allosteric modulators and biased agonists has grown. The major disease indications for GPCR modulators show a shift towards diabetes, obesity, and Alzheimer’s disease, while other central nervous system disorders remain highly represented. The 227 (57%) non-olfactory GPCRs that are yet to be explored in clinical trials have broad untapped therapeutic potential, particularly in genetic and immune system disorders. Finally, we provide an interactive online resource to analyse and infer trends in GPCR drug discovery.
The majority of drug targets are still in one of five protein families: G protein-coupled receptors (GPCRs), ion channels, kinases, nuclear hormone receptors and proteases1. GPCRs have been of particular long-standing interest as pharmacological targets, as they regulate numerous diverse physiological processes and have druggable sites accessible at the cell surface. Drugs that target GPCRs also account for about 27% of the global market share of therapeutic drugs, with aggregated sales for 2011–2015 of ~US$890 billion2. GPCRs form the largest human membrane protein family, including approximately 800 members, of which about half are 400 olfactory receptors (not included in this analysis). The superfamily has been sub-divided into classes3 and families4 based on evolutionary homology and receptor families with common physiological ligands, which are strikingly diverse spanning ions, small molecular signalling molecules, lipids, peptides and proteins5.
New avenues for GPCR drug discovery have emerged due to recent advances in receptor pharmacology, breakthroughs in structural biology and innovations in biotechnology. Modulation of GPCRs via allosteric sites, which are distinct from that of the binding site for the endogenous ligand, can alter the structure, dynamics and function of the receptor to achieve a potential therapeutic advantage, such as increased spatial and temporal selectivity6. Furthermore, our knowledge of receptor activation has been expanded with the concept of biased agonism7,8 — preferential activation of the desired intracellular signalling pathway while minimizing undesired side effects due to the activation of other pathways. So far, drugs targeting GPCRs have largely been developed without the help of high-resolution structural information, but now the crystal structures of 43 unique receptors and 196 ligand complexes provide a plethora of templates for structure-based drug discovery and design. Of the non-olfactory GPCRs, 130 (33%) are peptide or protein receptors that are potential targets for biological drugs, and many more receptors have become accessible through advances with techniques that exploit natural protein–protein interactions or develop specific antibodies and derivatives. These concepts have led to new opportunities for both established GPCR targets as well as novel GPCR targets.
Recent reviews on GPCRs have described particular drug target families9–11, disease indications12–14, novel tools15,16 or drug discovery mechanisms17–20. Here, we report the first analysis of all GPCR drugs and agents in clinical trials, which reveals the current trends across all molecule types, drug targets and therapeutic indications. General drug discovery trends, such as biologics, allosteric modulators, repurposing, the surge of GPCR structure data and ligands with biased signalling are analysed to investigate if associated agents have reached clinical trials. We also outline the novel targets closest to the market and assess the “dark matter” of pharmaceutically unexplored GPCR targets and unexploited disease associations. Finally, we open up our data for extended analyses by the scientific community through a comprehensive public resource on GPCR drug data (gpcrdb.org/drugs/drugbrowser), analysis (gpcrdb.org/drugs/drugstatistics) and target mapping (gpcrdb.org/drugs/drugmapping); integrated with the GPCR database, GPCRdb21.
GPCR drugs and agents in clinical trials
Numbers of GPCR-targeted agents
All drugs approved in the United States are listed in the Drugs@FDA database. The primary resource for clinical trial registries is the US National Institutes of Health (clinicaltrials.gov), which currently holds information on more than 251,000 clinical trials globally. The current status of agents in clinical trials is available in commercial databases such as CenterWatch’s commercial database, Drugs in Clinical Trials, which covers more than 4,000 such agents in phase I through phase IV trials worldwide or recently discontinued. However, none of these resources is comprehensive with respect to the targets of agents in trials, which can be collected from a combination of public resources, such as Drugbank22, Pharos23 and Open Targets24 (which also has a disease ontology), literature25–27 and company press releases.
By manually curating CenterWatch’s Drugs in Clinical Trials database (data extracted in July 2017) and cross-referencing with public sources, we were able to identify 481 approved drugs that target GPCRs. This accounts for approximately 34% of all FDA-approved drugs, which is similar to previously reported estimates (27-33%) on the number of GPCR-targeting drugs1,25,27. GPCR drug discovery has advanced rapidly. Just in the past five years, 69 new GPCR-targeting drugs have been approved by the FDA (see TABLE 1 for recent new molecular entities (NMEs)). The most recent approval of a GPCR-targeted drug is for abaloparatide28, a parathyroid hormone receptor (PTHR1) agonist used to treat postmenopausal women with osteoporosis. The number of agents currently in trials is 320 (67% of the total number of already approved drugs). Interestingly, 114 (36%) of the agents in trials target potentially novel GPCR targets without an approved drug.
Table 1. New molecular entities acting via GPCRs approved by the FDA since 2014.
| Drug | Substance | Indication(s) | Target(s) | Approval |
|---|---|---|---|---|
| Hycodan | Hydrocodone bitartrate | Narcotic cough | OPRD, OPRM | 2014 |
| Rexulti | Brexpiprazole | Depression | 5HT2A, 5HT1A, DRD2, 5HT7R | 2015 |
| Trulicity | Dulaglutide | Diabetes, Type 2 | GLP1R | 2014 |
| Varubi | Rolapitant | Nausea/vomiting | NK1R | 2015 |
| Akynzeo | Netupitant | Nausea/vomiting | NK1R | 2014 |
| Uptravi | Selexipag | Pulmonary hypertension | PI2R | 2015 |
| Odomzo | Sonidegib | Basal cell carcinoma | SMO | 2015 |
| Tymlos | Abaloparatide | Osteoporosis | PTHR1 | 2017 |
| Nuplazid | Pimavanserin | Parkinson’s disease psychosis | 5HT2A | 2016 |
| Kengreal | Cangrelor | Percutaneous coronary intervention | P2Y12 | 2015 |
| Zontivity | Vorapaxar | Cardiovascular risk reduction | PAR1, PAR3, PAR4 | 2014 |
| Striverdi respimat | Olodaterol | Chronic obstructive pulmonary disease (COPD) | ADRB2 | 2014 |
| Natpara | Parathyroid horomone | Hypoparathyroidism | PTH1R | 2015 |
| Adlyxin | Lixisenatide | Diabetes, Type 2 | GLP1R | 2016 |
| Belsomra | Suvorexant | Insomnia | OX2R, OX1R | 2014 |
| Hetlioz | Tasimelteon | Non-24-hour disorder | MTR1A, MTR1B | 2014 |
| Viberzi | Eluxadoline | Irritable bowel syndrome | OPRM, OPRD | 2015 |
| Movantik | Naloxegol | Constipation | OPRM | 2014 |
| Symproic | Naldemedine | Constipation | OPRM | 2017 |
| Northera | Droxidopa | Orthostatic hypotension | ADRB1-3, ADA2A/B/C, ADA1A/B/D | 2014 |
| Parsabiv | Etelcalcetide | Hyperparathyroidism | CASR | 2017 |
| Aristada | Aripiprazole lauroxil | Schizophrenia | 5HT1A, 5HT2A, DRD2 | 2015 |
| Vraylar | Cariprazine | Schizophrenia | DRD3, DRD2 | 2015 |
| Addyi | Flibanserin | Hypoactive sexual desire disorder | 5HT2A, 5HT1A | 2015 |
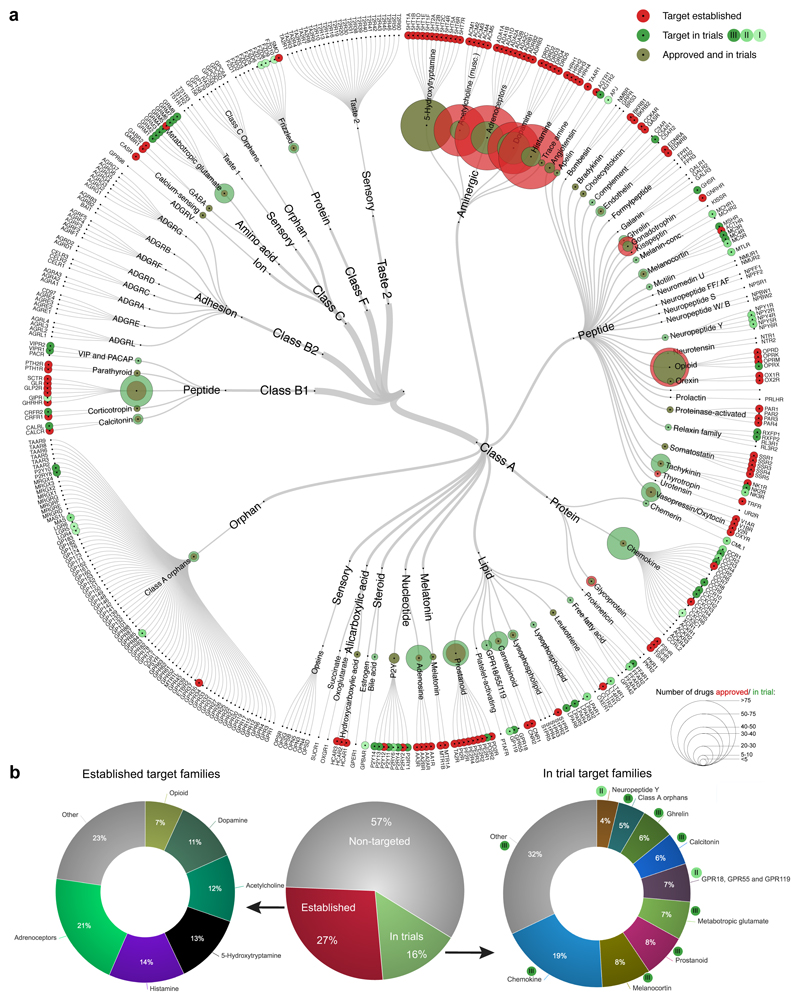
To study the distribution of established and currently investigated drug targets, we mapped approved drugs and clinical trial candidates onto the GPCR superfamily tree (FIG. 1a). The 481 approved drugs mediate their effect via 107 GPCR targets, accounting for 27% of the human non-olfactory GPCRs. Today, all aminergic receptors are established as drug targets, which serve as the targets for 321 of the approved drugs. Currently established GPCR drug targets are utilized by, on average, 10.5 (median = four) distinct approved agents. This indicates a near saturation of the current target space and emphasizes the necessity of expanding to new druggable receptors in order to develop novel medications. Identification and exploitation of new targets is especially warranted for diseases with large unmet medical needs and few current viable targets, such as Alzheimer’s disease.
Figure 1. Established and clinical trial GPCR drug targets.
Established targets have approved drugs as defined in the Drugs@FDA database, and targets of agents in clinical trials were collected from manual annotation of CenterWatch’s Drugs in Clinical Trials database, OpenTargets, Drugbank, Pharos and company press releases. a | Established (red) and phase I-III (shades of green) targets across the GPCR classes, ligand types and receptor families (from center to outer ring). The sizes of the circles represent the number of agents. An interactive tree is available at http://www.gpcrdb.org/drugs/drugmapping. b | There are 398 non-olfactory GPCRs, of which 107 have drugs approved (red), 64 have agents that have reached clinical trials but have not yet been approved (green), and 227 are yet to be targeted by agents in clinical trials (grey). The 107 established GPCR drug targets (left doughnut) are primarily aminergic and opioid receptors, whereas most targets of agents that have at least reached clinical trials are types of peptide receptor (right doughnut).
Success rates of trials for GPCR-targeted agents
The US Food and Drug Administration recently reported 70%, 33% and 25-30% success rates for phases I, II and III, respectively, for all target families. We estimated the success rates of GPCR-targeted agents in 2013–2017 (up to July 2017), by counting successes as agents that successfully made it to at least one subsequent phase, and failures as agents that were reported as discontinued, terminated or withdrawn; or completed before this period but never progressed. This yielded GPCR agent success rates of 78%, 39% and 29% for phases I, II and III, respectively, which is slightly higher than FDAs average for all investigated agents (see Further information). This may reflect the high-level experience in targeting GPCRs. Both our and the FDA’s analyses show a clear trend of more failures in later phases. A recent example is the small-molecule drug fasiglifam, an agonist of the free fatty acid 1 (FFA1) receptor (also known as GPR40). Fasiglifam showed efficacy in a phase III trial for diabetes29, but further development was terminated due to hepatotoxicity.
Table 3. Further information.
| Web site | URL |
|---|---|
| CenterWatch | http://www.centerwatch.com |
| ChEMBL | https://www.ebi.ac.uk/chembl |
| ClinicalTrials | https://clinicaltrials.gov |
| Drugbank | https://www.drugbank.ca |
| Drugs@FDA | https://www.accessdata.fda.gov/scripts/cder/daf |
| FDA Attrition | https://www.fda.gov/forpatients/approvals/drugs/ucm405622.htm |
| GPCRdb | http://www.gpcrdb.org |
| Guide To PHARMACOLOGY | http://www.guidetopharmacology.org |
| Heptares | https://www.heptares.com |
| H. Lundbeck A/S axovant press release | http://www.reuters.com/article/us-health-alzheimers-lundbeck-idUSKBN15N16R |
| Open Targets | https://www.targetvalidation.org |
| PDB | https://www.rcsb.org/pdb/home/home.do |
| Pharos | https://pharos.nih.gov/idg/index |
| PubMed | https://www.ncbi.nlm.nih.gov/pubmed |
| Receptos ozanimod | http://www.msdiscovery.org/research-resources/drug-pipeline/337-ozanimod |
| WIPO | http://worldwide.espacenet.com |
Popularity of peptide/protein receptors
We identified 64 potentially novel GPCR targets in clinical trials; that is, targets that are not yet modulated by approved drugs. Of these, 35 are peptide/protein-activated GPCRs (a selection of which are shown in Table 2, including the calcitonin-gene related peptide (CGRP) receptor for the treatment of migraine, the GPR55 receptor for the treatment of epilepsy and the apelin receptor for the treatment of cardiovascular disorders. Furthermore, the chemokine receptors alone have 22 agents in clinical trials for the treatment of cancer, asthma, rheumatoid arthritis, COPD and HIV. The six members of the glucagon receptor family are also a focus of new approaches for the treatment of type 2 diabetes. Notably, while all recognise natural peptides/protein ligands, these targets span a breadth of receptor families and classes. This suggests that general new approaches, such as increased tractability with biologics, have contributed to the overall increased therapeutic targeting.
Table 2. New GPCR target families and late stage targets for agents currently in clinical trials.
| Receptor family | Target(s) | Indication(s) | Agent(s) |
|---|---|---|---|
| Angiotensin | AGTR2 | Catecholamine resistant hypotension | LJPC-501 |
| Apelin | APJ | Cardiovascular disorders, insulin sensitivity | apelin |
| Bile acid | GPBAR | Liver fibrosis | INT-767 |
| Calcitonin | CALCRL | Migraine | erenumab, ubrogepant |
| Chemerin | CML1 | Dry eye | RX-10045 |
| Chemokine | CCR2, CCR4, CXCR1 | HIV infection, cancer, diabetes type 1 | cenicriviroc, mogamulizumab, reparixin |
| Class A orphans | GPR84, GPR35, MAS | Ulcerative colitis, irritable bowel syndrome, autoimmune diseases, multiple myeloma | GLPG1205, PA101B, TXA127 (angiotensin 1-7) |
| Complement peptide | C5AR1 | Autoimmune diseases | CCX168 |
| Free fatty acid | FFAR2 | Neutrophil-driven inflammation, ulcerative colitis | GLPG0974 |
| GPR18, GPR55 and GPR119 | GPR55 | Spasticity related to multiple sclerosis, epilepsy | VSN16R, cannabidiol (GWP42003), cannabidivarin (GWP42006) |
| Ghrelin | GHSR | Appetite stimulant, antidiabetic, cancer cachexia, gastroparesis, digestive system disease | unacylated ghrelin (AZP-531), anamorelin, macimorelin, ulimorelin |
| Lysophospholipid (LPA) | LPAR1 | Pulmonary fibrosis | AM-152 |
| Melanocortin | MC1R, MC3R, MC4R | Sexual dysfunction, anti-obesity, dermatological | bremelanotide, RM-493, afamelanotide |
| Motilin | MTLR | Gastroparesis | camicinal |
| Prostanoid | PD2R2 (GPR44) | Asthma, allergic rhinitis | fevipiprant, setipiprant |
| Relaxin | RXFP1, RXFP2 | Heart failure | serelaxin |
| VIP and PACAP | VIPR1, VIPR2 | Sexual dysfunction, hypertension | Vasomera (PB1046), vasoactive intestinal peptide |
Orphan receptors enter clinical trials
Interestingly, current clinical trials feature a number of orphan GPCRs, for which endogenous ligands have yet to be discovered. These orphan GPCRs serve as potentially novel targets for treatment of a diverse set of indications, such as GPR119 for treatment of diabetes, leucine-rich repeat-containing G protein-coupled receptors 4 and 5 (LGR4/5) for treatment of gastrointestinal disease, GPR35 for treatment of an allergic inflammatory condition, GPR55 as an antispasmodic target, the proto-oncogene Mas (MAS) for treatment of thrombocytopenia, and GPR84 for treatment of ulcerative colitis. This indicates that the drug discovery process can still take place, and even advance, despite limited knowledge about the endogenous ligand(s) and/or signalling pathway(s). The entry of an agent and target into clinical trials serves as an important qualifier as animal studies, preclinical data and disease relevance look promising enough for involving humans in the following investigations. It can be expected that public information on orphan receptors will increase as candidate drugs progress through clinical trials and/or upon approval.
Trends in agent type and mode of action
The proportion of GPCR-targeted biologics is higher in early-stage clinical trials
Biologics such as monoclonal antibodies (mAbs) are now well-established therapeutic modalities, accounting for 27–33% of the new drugs approved by the FDA in the years 2014 to 201630. Biologics so far have largely targeted proteins such as cytokines and their receptors, with no GPCR-targeted mAbs yet approved by the FDA, although some are close to approval31. With 16 agents currently in clinical trials, targeting GPCRs with mAb-based therapeutics might have a great therapeutic potential in a variety of diseases, including cancer, inflammatory disorders, neurological and metabolic diseases. For instance, targeting the CGRP receptor with mAbs provides a new approach in the treatment of chronic migraine. The CGRP-receptor-specific mAb erenumab prevents the ligand CGRP from binding and has now been submitted to the FDA for approval based on positive data in phase III trials (three other mAbs targeting CGRP rather than its receptor are also in late-stage development).
Several peptide drugs targeting the glucagon-like peptide 1 (GLP1) receptor have been approved for type 2 diabetes, including exenatide, liraglutide, lixisenatide, albiglutide and dulaglutide32, and peptide drugs targeting other GPCRs are in development. For example, bremelanotide is a non-selective peptide melanocortin receptor agonist that has successfully completed two phase III trials for female sexual dysfunction, and a new drug application (NDA) is expected to be submitted to the FDA for approval in 2018. Setmelanotide — another melanocortin receptor agonist that is selective for the MC4 receptor — has shown promising results in phase II trials for the treatment of rare genetic disorders of obesity, and is now in phase III development. Positive phase III data for a synthetic human angiotensin II peptide LJPC-501 in patients with catecholamine-resistant vasodilatory shock has recently been reported and an NDA is expected to be submitted by the end of 201733.
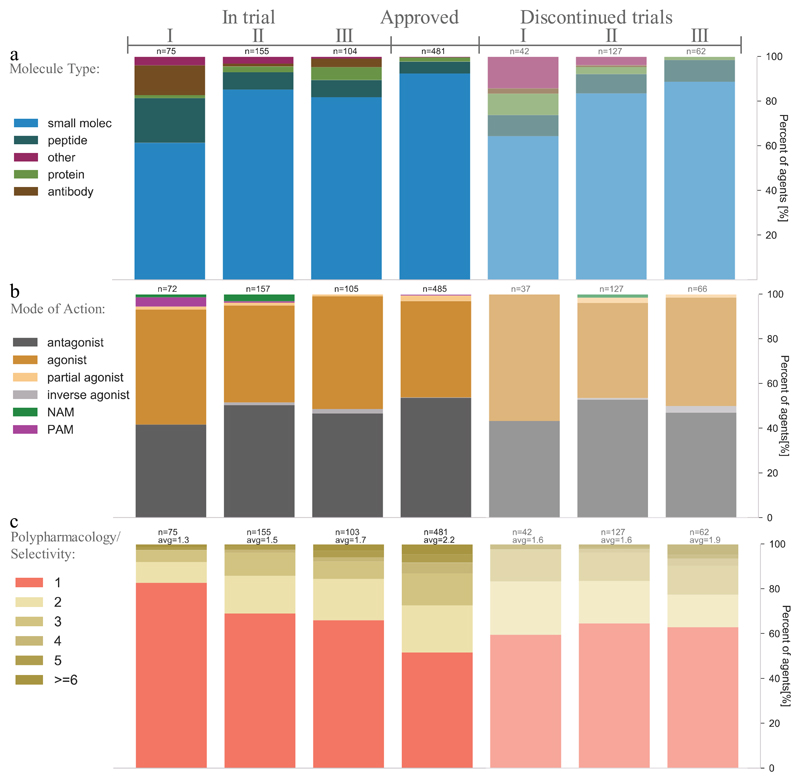
Overall, there are early indications that modalities other than small molecules are becoming more popular for agents targeting GPCRs, as the share of peptides, mAbs and other recombinant proteins31 being studied in phase I trials is higher than in later phases (FIG. 2a).
Figure 2. Trends in agent molecule types and modes of action for GPCR-targeted agents.
a | Most GCPR-targeted agents in clinical trials are still small molecules, but the earlier phases display increasing shares of peptides, monoclonal antibodies, other recombinant proteins and other agent types. Discontinued trials show the same trend, and similar proportions of small molecule versus biologics, suggesting that their overall attrition rates are also similar. b | Modes of action are predominantly agonist and antagonists, but more positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) have entered phases I and II, respectively. Discontinued trials demonstrate similar proportions of agonists and antagonists, suggesting similar rates of attrition. Modes of action had not been reported for 67 agents, which were excluded. c | Target selectivity is increasing (that is, polypharmacology is decreasing) in ongoing clinical trials of GPCR-targeted agents. For panels a, b and c, the data from clinicaltrials.gov was aggregated for agents in trial (completed: 156, recruiting: 91, ongoing: 41, not open yet: 18 and suspended: 3) or discontinued (discontinued: 205, terminated: 14, withdrawn: 4).
More allosteric modulators in early-stage clinical trials
Allosteric modulators offer an attractive novel mechanism: remotely modulating the activity induced at the binding site of the physiological ligand. This often has advantages such as higher selectivity at the target (perhaps by binding to a less well-conserved site across a receptor family with the same endogenous ligand), temporal selectivity (action linked to release of the endogenous ligand) and sometimes also functional selectivity through biased signalling19, as shown for FFA1R34,35. Approved allosteric modulators include cinacalcet, a positive allosteric modulator of the calcium-sensing receptor (CaSR) for the treatment of hyperparathyroidism, and maraviroc, a negative allosteric modulator of the chemokine receptor CCR5 for prevention of cellular entry of HIV-1. Allosteric modulation has also been suggested as a viable option for the peptide hormone class B receptors, which have not been tractable when targeting the orthosteric site18. The allosteric database currently lists 27,769 GPCR-targeting allosteric modulators36, and a number of positive and negative allosteric modulators are in phase I and II trials, respectively (FIG. 2b). There is also an indication that agonists and antagonists may be more and less prevalent, respectively, in phase I trials. Interestingly, several of the allosteric modulators in clinical trials are combination therapies with orthosteric ligands for the treatment of CNS disorders37.
Increasing focus on target selectivity rather than polypharmacology
Many drugs are known to exert their therapeutic effect through multiple targets; that is, polypharmacology [G]. To complement the previous studies by Santos et al.27 and Overington et al.25 assigning 1:1 relationships between approved drugs and their targets, our annotation covered all primary and secondary targets, including for agents in clinical trials. Interestingly, a trend was found for increasing selectivity (decreasing polypharmacology) for earlier trials (FIG. 2c). Furthermore, the currently investigated phase I agents have fewer targets than the discontinued agents, further suggesting an increasing focus on target selectivity rather than polypharmacology.
Polypharmacology could still lead to future opportunities with established targets, as specifically discussed for the dopaminergic system38,39. Drugs exhibiting polypharmacology at dopamine and serotonin receptors include, for example, haloperidol, amoxapine, and asenapine39. Amitryptyline, as an example of a tricyclic antidepressant, also has high affinity at muscarinic and histamine H1 receptors (which can be useful therapeutically). Furthermore, antimuscarinics used in bladder dysfunction are non-selective across the range of five human receptors, although the primary targets appear to be the muscarinic acetylcholine M2 and M3 receptors.
Trends in disease indications
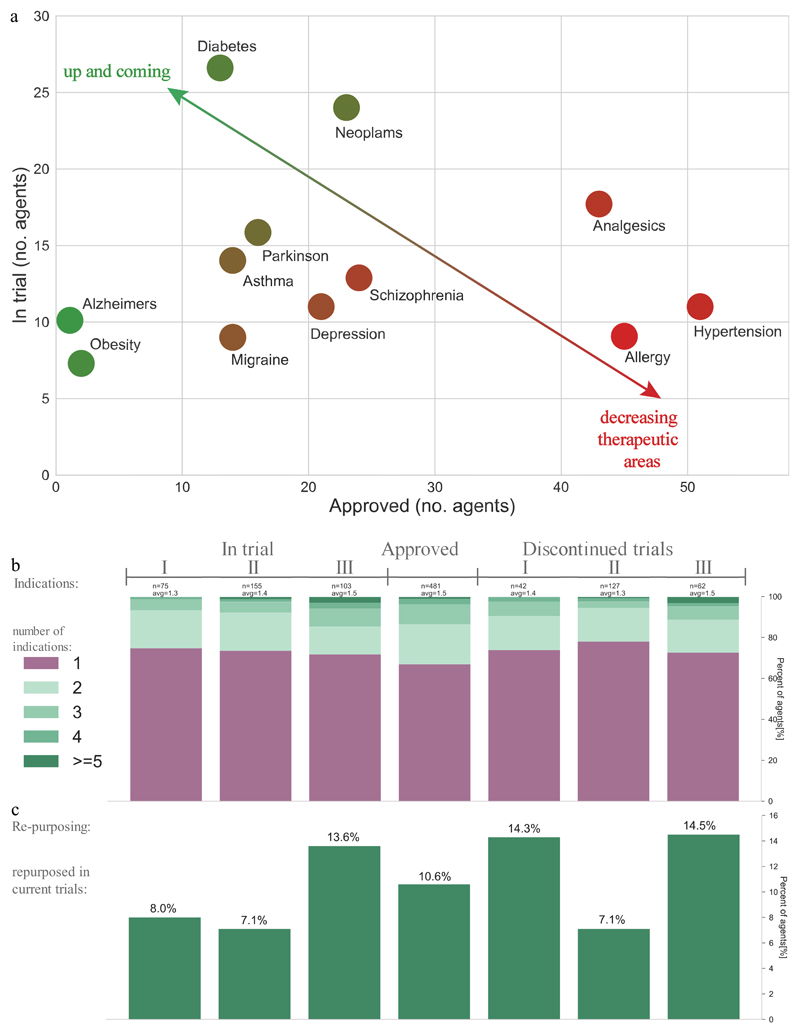
Our data show that the indications for GPCR-targeted agents are expanding from historically popular areas, such as hypertension, allergy, analgesics, schizophrenia and depression, into novel areas such as Alzheimer’s disease and obesity (FIG. 3a). Furthermore, in the past five years GPCRs, have also been targeted for new indications including multiple sclerosis, smoking cessation, short bowel syndrome and hypocalcaemia. Major trends in the indications of GPCR-targeted agents are highlighted below.
Figure 3. Trends in the indications of approved GPCR-targeted drugs and agents in clinical trials.
a | The largest number (>40) of approved agents (x-axis) are seen for analgesics, allergy and hypertension; whereas among agents in trials (y-axis) the highest listings (>20) are diabetes and neoplasms. The diagonal arrow and colour gradient from red to green highlights the most established and novel indications, calculated as the ratio of approved and in-trial agents, respectively. Alzheimer’s disease and obesity are the areas with the highest ratio of in-trial agents to approved agents, followed by asthma, diabetes, Parkinson’s disease and neoplasms. Data was manually annotated from the CenterWatch database and public resources. b | The number of indications is similar for approved drugs and currently investigated agents. c | 51 (11%) of the approved GPCR drugs are currently being repurposed for other indications, and account for 9%, 7% and 14% of the agents in phases I, II and III, respectively. For panels b and c, the data from clinicaltrials.gov was aggregated for agents in trial (completed: 156, recruiting: 91, ongoing: 41, not open yet: 18 and suspended: 3) or discontinued (discontinued: 205, terminated: 14, withdrawn: 4).
Central nervous system disorders remain highly represented among the indications of GPCR-targeted agents
Grouping the indications of approved GPCR-targeted drugs onto higher-level disease terms utilizing the Open Target24 ontology shows that central nervous system (CNS) diseases are the most abundant, accounting for 130 (27%) of all of approved GPCR-targeted drugs. Furthermore, 137 GPCR-targeting agents are currently in clinical trials for CNS indications, demonstrating a continued strong interest. An analysis of receptor baseline expression from the human protein atlas40, and studies on the mouse brain41, show that more than half of all non-olfactory GPCRs are expressed in the cerebral cortex. Malfunctions in GPCR-mediated neurotransmission can lead to multiple neurological and psychiatric disorders, making GPCRs promising targets in such disorders42. Multiple sclerosis, Alzheimer’s disease, Huntington’s disease and fragile X sydrome are highlighted here.
Multiple sclerosis (MS), the most common chronic autoimmune disorder that affects the CNS, is caused by damage to the insulating myelin covers of axons. Data from clinical studies and animal models have uncovered several GPCRs involved in the pathogenesis of MS12, and one GPCR-targeted drug has been approved: fingolimod, a sphingosine 1-phosphate receptor 1 (S1P1) modulator, which reduces relapse rates and the risk of disability progression43. Several other S1P1 receptor modulators — ozanimod, ponesimod and siponimod — are currently in phase II and III trials. These drugs are expected to have advantages over fingolimod, such as higher selectivity for the S1P1 receptor, faster clearance and improved tissue penetration44. Other MS targets include the cannabinoid receptors. A tetrahydrocannabinol and cannabidiol (THC-CBD) oromucosal spray has been tested in phase II and III studies and been shown to reduce spasticity45. A GPR55-selective compound in phase II trials may also potentially be better tolerated than comparable antispasmodics. Taken together, the multitude of agents and targets in late-stage clinical trials indicates that additional MS therapies acting through GPCRs are likely to emerge in the near future.
GPCRs are also involved in several neurotransmitter systems associated with Alzheimer’s disease (AD), with glutamatergic, serotonergic, adrenergic and peptidergic pathways in particular being deregulated in this neurodegenerative disorder46. Targeting these systems might protect against disease progression by modulating the formation of amyloid-β plaques (one of the cardinal disease features) or aberrant signalling following plaque formation46. There is a huge unmet medical need for new therapies for AD, particularly those that might modify disease progression, as the small number of existing therapies (most of which act by increasing levels of acetylcholine by inhibiting its breakdown by acetylcholinesterase) only have limited effectiveness at improving disease symptoms. Leuprolide, a gonadotropin-releasing hormone receptor agonist approved to treat prostate cancer, has been tested in a phase III trial for AD, but it failed to meet the primary or secondary endpoints, although there were signs of an effect on disease progression in patients taking an acetylcholinesterase inhibitor47. An additional nine GPCR-targeting agents are in clinical trials for treatment of AD (FIG. 3). Serotonin receptor modulators are of particular interest, including 5-HT6 receptor antagonists to improve disease symptoms (rather than modify the disease course) by promoting the release of acetylcholine48. However, Lundbeck recently terminated the development of their 5-HT6 receptor antagonist idalopirdine owing to insufficient efficacy in phase III trials. Pfizer also terminated a 5-HT6 receptor antagonist PF-05212377 due to lack of efficacy after a phase II trial in 2016. Phase III trials of another 5-HT6 receptor antagonist, intepirdine, licensed by Axovant from GlaxoSmithKline, are still ongoing.
GPCRs are also potential targets for another neurodegenerative disorder: Huntington’s disease (HD)49, for which current therapies can also only improve some symptoms. HD is caused by numerous repetitions of CAG-triplet repeats within the Huntingtin gene (HTT), which lead to the expression of an abnormal pathogenic huntingtin protein. This leads to cell damage; however, the mechanisms are not fully elucidated. Several GPCR pathways are downregulated in HD patients, and two GPCR-targeted agents are currently in clinical trials for HD: the adenosine A1 receptor antagonist pbf-999 (which is in phase I trials) and the dopamine D2 receptor antagonist pridopidine (which is in phase III trials).
Finally, GPCRs have attracted considerable investment as targets for fragile X syndrome (FXS) — the most common inherited form of intellectual disability and autism —which is caused by alterations in FMR1, the gene coding for FMRP. Studies in Fmr1 knockout mice, which have been widely used as an animal model for FXS, showed that inhibition of the metabotropic glutamate receptor 5 (mGlu5) improved synaptic function in these animals and highlighted the importance of mGlu5 in FXS37. However, mGlu5 negative allosteric modulators such as basimglurant, mavoglurant and STX107, failed in phase II trials, as no improvement over placebo could be demonstrated. Agents targeting the GABAB receptor, which improved function in Fmr1 knockout mice, have also been investigated clinically for FXS. However, despite some indications of efficacy in a phase II trial with the GABAB agonist arbaclofen50, subsequent phase III trials failed due to lack of efficacy compared with placebo.
Diabetes is highly represented among the indications for GPCR-targeted agents currently in clinical trials
The growing market share of metabolic disease medications51 is reflected in the high number of GPCR-targeted agents in clinical trials for diabetes and obesity, with 27 and seven agents targeting GPCRs, respectively (together making ~10% of the total number of agents in clinical trials). 415 million people are estimated to suffer from diabetes mellitus52, with 90% of these having been diagnosed with type 2 diabetes. In contrast to type 1 diabetes, which requires regular insulin injections due to failure of the pancreas to produce enough insulin, type 2 diabetes can be treated with medications that stimulate insulin secretion or increase insulin sensitivity53. The first GPCR-targeted drug for type 2 diabetes — the GLP1 receptor agonist (or incretin mimetic) exenatide — was approved in 2005. As noted above, there are now a number of other approved peptidic GLP1 receptor agonists, including liraglutide, lixisenatide, dulaglutide and albiglutide. They differ in their durations of action, but are all formulated as injectable drugs. However, semaglutide, a once-weekly GLP1 analogue with significantly improved glycemic control54 is now also being tested for oral dosing in phase III trials. Advances in small-molecule screening, including structure-based techniques9, have identified new non-peptide agonists of the glucagon-like peptide (GLP-1) receptor, including the orally bioavailable TTP273, which is currently in phase II trials.
The challenges of treating type 2 diabetes and associated diseases such as diabetic neuropathy and foot ulcers has catalysed investment in further GPCR-targeted agents32,55.At present, eleven GPCRs mediate the therapeutic effects of approved treatments for these conditions, and agents targeting a further 25 GPCRs are under investigation in clinical trials. Among them is MBX-2982, a small-molecule GPR119 agonist currently in phase II trials. MBX-2982 increases both insulin secretion and GLP1 release56,57. Another novel target for which agonists stimulate insulin secretion is the FFA1 receptor (also known as GPR40)34. Despite the recently discontinued development of the small-molecule agonist fasiglifam (TAK-875) due to hepatotoxicity mentioned already above29, the FFA1 receptor remains a viable target, albeit with a need for further characterization of its signalling spectrum, and at least one FFA1 receptor modulator, LY2881835, is in clinical trials. Another novel target for treatment of diabetes is the dopamine D2 receptor. The first dopaminergic agent, bromocriptine, was recently approved for improved glycemic control and glucose tolerance in type 2 diabetes58.
Opportunities emerging for GPCR-targeted agents in oncology
Currently, 21 approved drugs with an antineoplastic indication mediate their effect via 15 distinct GPCRs. Among those are degarelix, a gonadotropin-releasing hormone (GnRH) receptor antagonist that is approved for patients with advanced prostate cancer, and vismodegib, a smoothened (SMO) receptor inhibitor for the treatment of basal-cell carcinoma. The most recent FDA approval for a GPCR-targeted agent in oncology was for another SMO receptor inhibitor, sonidegib in 2015, also for the treatment of basal cell carcinoma.
An additional 23 GPCR-targeted agents for treating cancer — seven of which have potentially novel targets — are in clinical trials. Chemokine receptors and proteins in the Wnt pathway are among the novel GPCR targets being pursued13, often with peptide or mAb therapeutics. For example, CCR2 is the target of the mAb plozalizumab, which is in phase I trials for melanoma, and the small-molecule CCR2 inhibitor CCX872 is in phase I trials for advanced pancreatic cancer. Vantictumab (also known as OMP-18R5) — a mAb specific for the frizzled-7 receptor (FZD7) that has been tested in clinical trials for breast and pancreatic cancer — targets the Wnt signalling pathway, which is dysregulated in many cancers, leading to cancer stem cell activity and tumour growth59. Other biologics in clinical development with targets in the Wnt signalling pathway include ipafricept (a fusion protein targeting FZD8), OTSA-101-DTPA-90Y (a radiolabelled mAb targeting FZD10) and BNC-101 (a mAb targeting LGR5).
Furthermore, several other GPCRs have been suggested as potential cancer targets based on mRNA-expression analyses of tumours60. Besides targeting GPCR-mediated cancer pathways directly, over-expressed receptor homo- and heterodimers that are over-expressed in particular cancers might function as selective markers for cancer treatment61.
Drugs for established GPCR targets in oncology also continue to be investigated, including GnRH antagonists such as relugolix for prostate cancer. GnRH antagonists such as relugolix and elagolix are also being investigated for other hormone-related indications such as endometriosis.
Repurposing of existing GPCR-targeted drugs for new indications
Repurposing of existing drugs for new indications can reduce the time and cost to bring a therapeutic to market62. Of the approved GPCR-targeted drugs, 160 (33%) have more than one indication and the overall average is 1.5 indications (FIG. 2d), demonstrating that many such drugs are already used for several indications. Agents in clinical trials have a similar average number of indications as the approved drugs.
Ongoing clinical trials are evaluating the potential to repurpose 51 (11%) of the approved drugs, which account for ~8%, ~7% and ~14% of the agents in Phases I, II and III, respectively (FIG. 2e). Repurposed agents are as frequent in the ongoing and discontinued trials indicating that they do not necessarily succeed more often than new agents. This suggests that efficacy is typically the limiting factor, rather than safety.
Emerging trends and opportunities
GPCR knowledge in the literature is disproportionately focused
More than half of the human genome-encoded non-olfactory GPCRs (n = 227) remain therapeutically unexploited (FIG. 1a). Drug targets are often first identified and explored in an academic environment. However, due to the long timespan of drug development, there is usually a lag time of many years or even decades before such discoveries are translated into marketed drugs. For this reason, we wanted to investigate how research results in the form of publication output relate to drug discovery efforts, and if this output could give indications of new trends in GPCR-targeted drug discovery.
GPCR research is an area of immense exploration
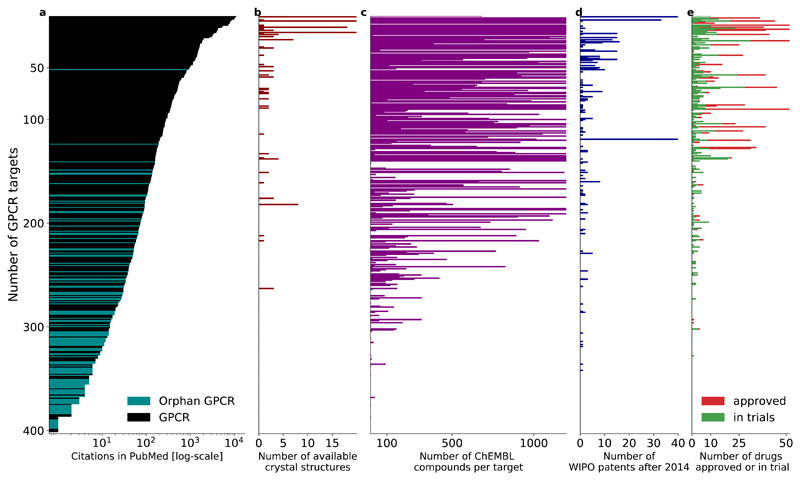
Gene and protein name searches in PubMed abstracts shows that the chemokine receptor type 4 (CXCR4) and the putative adhesion G protein-coupled receptor E4P (AGRE4) have the most publications (11,123) and fewest publications (0, although this may be in part due to new nomenclature for adhesion receptors), respectively (FIG. 4a, note logarithmic scale). GPCRs with approved drugs or agents in clinical trials have on average ~400 publications, whereas this figure is only 20 for non-targeted receptors. Receptors with a crystal structure (FIG. 4b) also have many publications (this probably actually reflects that crystallization efforts in the past decade or so focused initially on therapeutically important and well-characterized GPCRs). Orphan receptors (purple in FIG. 4a), with unknown physiological agonists and functions, typically have very few publications. Nonetheless, some orphan receptors, such as GPR143 and GPR15 have over 100 articles. The disproportionate data foundation underlines that the research efforts on many GPCRs are still in their infancy, and further characterisation is needed to assess their role in (patho)physiology. This is in accordance with a general knowledge deficit and lack of funding for many understudied human proteins, as recently brought to attention by an NIH programme “Illuminating the Druggable Genome”.
Figure 4. GPCR targets from publication to drugs.
For each non-olfactory GPCR (y-axis), the panels show the number of: a | publications (in PubMed), b | crystal structures (in the Protein Data Bank), c | ligands (in ChEMBL), d | patents (filed through the World Intellectual Property Organization after 2014), and e | drugs on the market and in clinical trials. GPCRs are sorted by the number of publications. This comparison reveals a highly disproportionate knowledge landscape for GPCR research and drug discovery, indicating an unutilized expansion potential.
Emerging targets based on disease associations, tool compounds and patents
About 63% of the human GPCRs have at least one ligand reported in ChEMBL (FIG. 4c), including various promising emerging targets for which agents have not yet been explored in clinical trials. For example, the neuropeptide S system, which was described only a few years ago, has been associated with several mental illnesses63, and the relaxin family receptors have recently been examined as potential targets for the treatment of addiction, anxiety, obesity and anorexia64. With a range of specific antagonists at hand, modulation of formyl peptide receptors has been suggested to inhibit tumour angiogenesis in glioblastoma and other cancers65. Bombesin 3 receptor knockout mice develop metabolic disturbances, obesity and hypertension66,67. Disruption of galanin receptor signalling in a number of preclinical studies have indicated that this system is involved in a range of pathologies, including Alzheimer’s disease, epilepsy, depression and cancer68,69. The aforementioned target families are expected to be among the first to enter clinical trials in the coming years due to their promising preclinical findings.
To further evaluate potentially active pharmaceutical investigations on promising new targets, we analysed patents filed between 2014 and October 2016 in medical sciences through the World Intellectual Property Organization (WIPO) (FIG. 4d). Based on those filings, chemokine receptors, with more than 100 filed patents, are currently the most vigorously pursued target family, followed by calcium-sensing receptors (n = 11), glycoprotein hormone receptors (n = 9) and frizzled receptors (n = 9). In addition, 35 patents towards orphan receptors including GPR84, GPR1, GPR17 and LGR5 have also been filed within this timeframe.
Untapped GPCRs as a source for new drug targets in a variety of disease areas
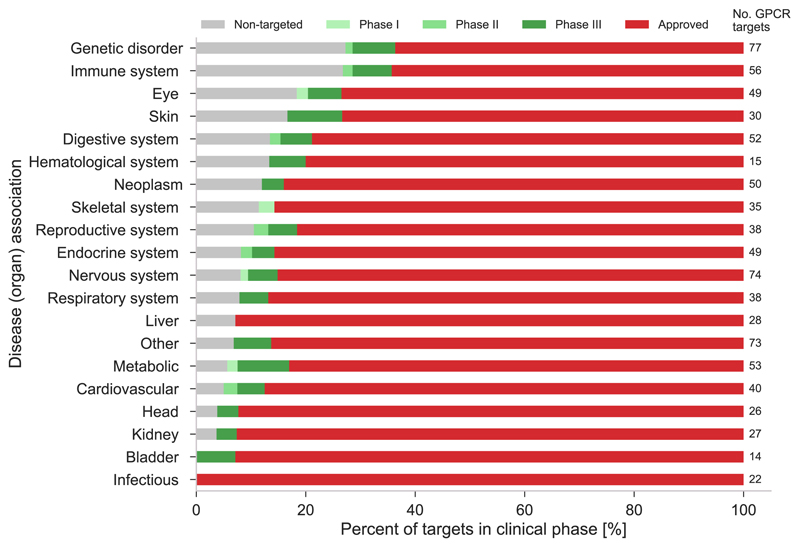
The diverse nature of GPCRs is reflected by our analysis of target disease associations from Open Targets (FIG. 5), which shows that GPCRs cover nearly every aspect of human pathophysiology. Strikingly, emerging targets — those that have not yet been targeted and those for which agents are in trials but none have yet been approved, are found in 18 and 19 of the 20 displayed categories, respectively. Of note, most targets in trials have already reached phase III (dark green) indicating that they may soon transition to established targets. The largest shares of non-targeted receptors are found within genetic disorders and immune system diseases (both 27%), and when including targets in trials, more than one third (37% and 36%, respectively) are non-established targets. Furthermore, eye and skin disorders also show a high prevalence (both 27%) of new and unexploited targets. Notably, immunology is a major growth area with a high demand for new targets. Hence, these disease categories may be where we will see the largest proportions of novel targets in the near to intermediate future. The exploitation of the emerging targets will be facilitated by new avenues in GPCR drug discovery, such as the application of structural data, biased signalling, allosteric modulation, de-orphanization, which are described in the following sections.
Figure 5. Disease associations of all (398) non-olfactory GPCRs.
Disease associations for receptors not yet targeted (grey), with an agent in trial (green) and approved (red) drug. The 227 non-targeted GPCRs are associated with a wide range (18/20) of diseases demonstrating a broad untapped therapeutic potential. Overall, the largest number of GPCR targets (77, numbers to the right) are observed for genetic and nervous system disorders. Disease associations have been agglomerated from the Open Targets platform by combining association scores above a value of 0.5 (at least half of the highest association confidence). The association score summarises the strength of evidence from each data source (genome-wide association studies, genetic variants, expression data and animal models); see Further information. Three ontology terms measurement, phenotype, and biological process, were excluded as they cannot be interpreted as a distinct category. It should be noted that receptors can be associated with multiple systems.
Impact of GPCR structures on drug discovery
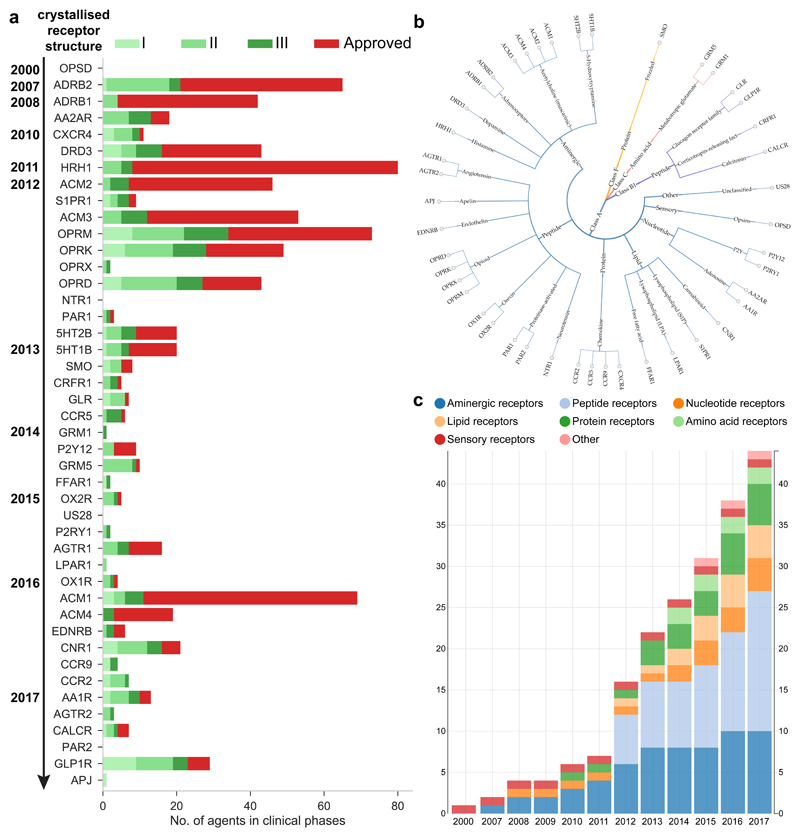
Structure-based drug design has long had a valuable role in drug discovery, particularly for drugs with enzyme targets, such as the HIV protease inhibitor indinavir70, the tyrosine kinase inhibitor imatinib71 and the influenza neuraminidase inhibitor zanamivir72. However, until recently, major challenges in applying X-ray crystallography to GPCRs limited the potential for structure-based drug design for such targets. Now, however, thanks to recent breakthroughs in GPCR crystallography73,74, 44 distinct GPCR structures and 196 ligand–receptor complexes are available across all human GPCR classes A–C and F (FIG. 6)21. This has paved the way for novel lead discovery through virtual screening and better off-target rationalisation75,76. For example, recent docking experiments against the μ opioid receptor structure identified PZM21, a Gi protein-biased agonist with potency and efficacy similar to morphine, but with reduced adverse effects in mice77. In another exciting example, two chemokine receptor structures —for CCR9 and CCR2 — revealed a previously unknown intracellular binding pocket, which might provide a new strategy for drug design78.
Figure 6. Crystallized receptors.
a | No. of agents in each clinical phase for each crystallized receptor sorted by year. b | Classification tree of all crystallised GPCRs. c | Percentage of each receptor type among all crystallised receptors. Figures b-c were collected from GPCRdb (http://gpcrdb.org/structure/statistics).
We investigated if the availability of crystal structures has yet had a measureable impact on the number of agents in clinical trials. FIG. 6a shows that most of the receptors with many approved drugs (red bars) have an early crystal structure published in 2007-2012, with exception of the muscarinic M1 receptor (ACM1) from 2016. It is likely that these receptors were selected for crystallisation based on their high therapeutic relevance, such as many and early validated disease associations. However, these targets also have a significant proportion of agents in phase I and II trials, where the structural templates could have been available long enough to contribute to the lead discovery and/or optimisation processes.
The real impact of structural data may be underestimated due to unpublished proprietary structures. For example, Receptos announced in January 2011 that they were targeting the S1P1 receptor with a proprietary structure, which was not published until the following year. Receptos’ S1P1 agonist ozanimod (which is now being developed by Celgene following their acquisition of Receptos) is expected to be submitted for FDA approval in 2017. Furthermore, Heptares’ pipeline (see Further information) currently lists ten specified, and an even larger number of undisclosed, targets for which agents are in preclinical and clinical trials. Known structure-based candidates that target GPCRs include agents that target the μ opioid receptor for pain77, M1/M4-muscarinic receptors for AD, metabotropic glutamate receptor 5 (mGlu5) for psychiatric disorders, orexin receptor 2 (HCRTR2) for narcolepsy, protease-activated receptor 2 (PAR2) for inflammatory disorders (see Heptares pipeline in Further information) and the adenosine A2A receptor for cancer79.
Biased signalling as a novel mechanism to achieve functional selectivity
Activating the appropriate cellular response through one of the four major Gα families and their intracellular effectors (such as adenylate cyclase and phospholipase C) or by β-arrestin-dependent activation of kinases and others, is critical for a favourable physiological response80–82. Recent discoveries of molecules that preferentially trigger one of these pathways — referred to as biased agonists — offer a new mechanism for reducing side-effects8,20,83,84. This has led to the notion of distinct receptor conformational states that are stabilized by different ligands, and can lead to activation of specific signalling and regulatory proteins85.
Several promising agents with bias through β-arrestin, a G protein or allosteric modulation are being investigated in preclinical studies as well as clinical trials8. Most notable is the µ-opioid receptor ligand oliceridine (TRV130), currently in phase III trials, which was granted ‘Breakthrough Therapy’ designation by the FDA owing to its improved analgesic profile86. Oliceridine, and its current phase I follow-on agent TRV734, do not engage the β-arrestin pathway, which is associated with opioid-induced respiratory depression and constipation87. Conversely, no G protein engagement was observed for the β-arrestin-biased ligand TRV027, which targets the angiotensin II type 1 receptor and is a drug candidate for the treatment of acute heart failure88. However, in May 2016, Trevena announced that TRV027 failed to meet the primary or secondary endpoints in a phase IIb trial.
Therapeutic implications for biased agonists have just started to be elucidated for a number of GPCR systems including adrenergic, angiotensin, opioid, dopamine, serotonin and chemokine receptors7. More knowledge on the signalling repertoire of GPCRs will be required to fully exploit the potential of biased signalling and the fine-tuning of intracellular responses to therapy89. New ways to validate biased signalling in animal models will help development of the tools needed to transition biased ligands towards preclinical development as drug candidates and beyond90.
Outlook for targeting GPCRs
GPCR drug discovery has gained new momentum, as demonstrated by the high number of new drug targets and the scientific impetus in GPCR structural biology, pharmacology and modelling. Although it is to be expected that many of the GPCR-targeted agents currently in clinical trials will not ultimately gain regulatory approval, the demonstrated druggability of the GPCR protein family and the important role of GPCRs in diseases such as diabetes, obesity, AD and psychiatric disorders, provide a strong driving force for continued drug discovery and development efforts in this field.
As the (patho)physiology of GPCRs becomes better characterised, certain groups of receptors may prove intractable, whereas others expand the druggable GPCRome. There is emerging evidence of excreted gut microbiota metabolites that serve as ligands for GPCRs and thereby influence our hormone release91. Additionally, many GPCRs have been identified as nutrient sensors, which could serve as potential targets to treat metabolic dysfunction and inflammatory diseases92. Furthermore, several orphan receptors may have evolved to recognise pathogens and invoke appropriate immune responses93. Characterization of the remaining orphan receptors could reveal new targets for a multitude of indications. Strikingly, the number of orphan receptors could expand significantly, as many of the ~400 olfactory GPCRs are now known to be widely expressed throughout the body and to play important functions beyond the detection of odorants94. Olfactory receptors have also been detected as overexpressed in tumour cells94.
However, some orphan receptors have been shown to not follow the classical paradigm of endogenous activation by an (external) ligand. GPR50 heterodimerizes constitutively and specifically with melatonin receptors MT1 and MT2. The class C orphan receptors GPR156 and GPRC5A-D lack the N-terminus, which contains the orthosteric binding site for the liganded members of this class. Furthermore, the non-orphan protease-activated and adhesion receptors are activated by their own N-terminus upon truncation. These receptors could still be targeted by drugs with a specific mechanism-of-action, such as allosteric modulation, inhibitors interfering with natural protein-protein interactions and antibodies, which are currently used to target e.g. adhesion receptors for antineoplastic treatment95.
Moving forward in GPCR drug discovery depends on solving several critical issues. Suitable tool compounds are required to establish target biological functions and disease relevance96. This could be aided by novel high-throughput ligand identification methods able to probe a larger chemical space, such as DNA-encoded libraries97. Furthermore, improved disease models and genetically engineered systems are needed for unambiguous target validation, which could be aided by gene editing technologies such as CRISPR98. The huge and heterogeneous published and patented chemical and biological data needs to be made available and structured in long-term committing databases such as ChEMBL, Guide to PHARMACOLOGY, GPCRdb and others. These endeavours are too large for any single entity to pursue, and will have to engage the wider interdisciplinary basic and applied GPCR field, calling for a continued investment in public-private pre-competitive partnerships and consortia to accelerate progress.
Glossary terms.
Allosteric site
A site for ligand binding on a receptor that is remote from the binding site of the physiological agonist (known as the orthosteric site).
Biased agonism
A new mechanism for potentially reducing drug side-effects by utilising a surrogate agonist preferentially activating a different intracellular signalling pathway than the physiological agonist.
Established GPCR target
Herein defined as the target of a drug approved by the US Food and Drug Administration (FDA).
Modes of action
Receptor activity defined as ligand stimulation (agonism), blocking (antagonism), inhibition (inverse agonism) or negative or positive allosteric modulation.
Polypharmacology
Ligand binding to multiple targets, all of which contribute to the pharmacological response.
Key points for the online publication.
We report the first analysis of all drugs and agents in clinical trials acting via G protein-coupled receptors (GPCRs) – the most intensively studied drug target family.
481 drugs (~34% of all drugs approved by the FDA) act at 107 unique GPCR targets.
Approximately 320 agents are currently in clinical trials, of which ~35% target 64 potentially novel GPCR targets without an approved drug.
Biological drugs, allosteric modulators and biased agonists are becoming more frequent in clinical trials.
The major disease indications for GPCR modulators show a shift towards diabetes, obesity, and Alzheimer’s disease, while other central nervous system disorders remain highly represented.
The 227 (57%) non-olfactory GPCRs that are yet to be explored in clinical trials have broad untapped therapeutic potential, particularly in genetic and immune system disorders.
Further trends in GPCR drug discovery can be analysed in an interactive resource in the GPCRdb database.
Acknowledgements
We thank A. Hersey, S.M. Soisson, V. Chelliah, L. Jahn, K. Harpsøe and M. Shehata for their valuable comments on this work. D.E.G. has received financial support from the European Research Council (DE-ORPHAN 639125) and the Lundbeck Foundation (R163-2013-16327).
Biographies
Author Biographies
Alexander S. Hauser is a PhD student at the Department of Drug Design and Pharmacology, University of Copenhagen. He applies computational biology methods to gain biological insights of G protein coupled receptors. His main focus is the characterisation of the remaining orphan GPCRs to unravel unknown physiological signalling systems. Alexander has studied biomedicine in Münster, Germany with research projects in India, Sao Paulo, Helsinki and Cambridge, where he focused on computational drug design, mathematical modelling, and pharmacogenomics. He is president of the Danish regional ISCB (International society of computational biology) group.
Misty M. Attwood is a PhD candidate at Uppsala University in the Department of Neuroscience, Functional Pharmacology division. She has studied biology in the United States and bioinformatics at Uppsala University, Sweden. In addition to using bioinformatic tools to research drug discovery and developments, she is also using computational tools to investigate the evolutionary developments of membrane proteins in the central nervous system.
Mathias Rask-Andersen, Ph.D., is a post-doctoral researcher at the dept. of Immunology, Genetics and Pathology, Science for Life laboratory, Uppsala University, Sweden. He received his Ph.D. from the dept. of Neuroscience in 2013 and has received post-doctoral grants from the Swedish Brain Research Foundation, the Medical Faculty at Uppsala University, and is currently the recipient of a grant from the Swedish Society for Medical Reseach (SSMF). Dr. Rask-Andersen’s research interests include population genetics, epigenetics, genetic determinants of body mass and adiposity, the etiology of eating disorders, as well as molecular pharmacology and pharmaco-informatics.
Helgi B. Schiöth received his M.Sc. degree in pharmaceutical science from the University of Iceland, Reykjavík, Iceland, in 1991 and his Ph.D. degree on molecular pharmacology of melanocortin receptors at the department of pharmaceutical biosciences at Uppsala University in 1997. He became an associate professor in 2002 and a professor of pharmacology at the department of neuroscience at the medical faculty at Uppsala University in 2008. He has worked in the pharmaceutical industry at Ferring Pharmaceuticals in Malmö, Sweden, and has served as a director and consultant in the biotechnology and pharmaceutical industries. He had a senior research position that was financed by the Swedish Research Council from 2003–2009 and now leads the unit of functional pharmacology at Uppsala University, which focuses on membrane-bound proteins and the central regulation of food intake.
David E. Gloriam got his PhD from Uppsala University and after two postdocs at EMBL-EBI and GSK moved to the University of Copenhagen in 2008 where he leads a group for computational drug design. In 2013, he took over the stewardship of the community-driven GPCR database, GPCRdb, expanding the services with e.g. reference structure and sequence data and tools for mutagenesis and crystallisation experiments. In 2014, he received both a ERC Starting Grant and a Lundbeck Foundation Fellowship to identify physiological and tool ligands to characterise orphan receptors. His lab is currently setting up receptor crystallography and a biased agonist resource.
Footnotes
Competing interest statement
The authors declare no competing interests.
References
- 1.Rask-Andersen M, Masuram S, Schiöth HB. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9–26. doi: 10.1146/annurev-pharmtox-011613-135943. [DOI] [PubMed] [Google Scholar]
- 2.The IDG Knowledge Management Center. Unexplored opportunities in the druggable human genome. The IDG Knowledge Management Center; 2016. 189205, 189205. [Peer-reviewed poster outlining a major NIH programme to characterise the dark space of major drug target families.] [Google Scholar]
- 3.Kolakowski LF. GCRDb: a G-protein-coupled receptor database. Receptors Channels. 1994;2:1–7. [PubMed] [Google Scholar]
- 4.Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. 2005;142:94–101. doi: 10.1016/j.ygcen.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Southan C, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016;44:D1054–D1068. doi: 10.1093/nar/gkv1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopoulos A, et al. International Union of Basic and Clinical Pharmacology. XC. Multisite Pharmacology: Recommendations for the Nomenclature of Receptor Allosterism and Allosteric Ligands. Pharmacol Rev. 2014;66 doi: 10.1124/pr.114.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–86. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: Promise and progress. Trends Pharmacol Sci. 2014;35:308–316. doi: 10.1016/j.tips.2014.04.007. [Review discussing how biased ligands may deliver safer, better tolerated, and more efficacious drugs, and highlights several biased ligands that are in clinical development.] [DOI] [PubMed] [Google Scholar]
- 9.de Graaf C, et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol Rev. 2016;68:954–1013. doi: 10.1124/pr.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solari R, Pease JE, Begg M. Chemokine receptors as therapeutic targets: Why aren’t there more drugs? Eur J Pharmacol. 2015;746:363–367. doi: 10.1016/j.ejphar.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 11.Nicoletti F, Bruno V, Ngomba RT, Gradini R, Battaglia G. Metabotropic glutamate receptors as drug targets: What’s new? Curr Opin Pharmacol. 2015;20:89–94. doi: 10.1016/j.coph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Du C, Xie X. G protein-coupled receptors as therapeutic targets for multiple sclerosis. Cell Res. 2012;22:1108–28. doi: 10.1038/cr.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Shavit R, et al. G protein-coupled receptors in cancer. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 15.Cromie KD, Van Heeke G, Boutton C. Nanobodies and their Use in GPCR Drug Discovery. Curr Top Med Chem. 2015;15:2543–57. doi: 10.2174/1568026615666150701113549. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Xie X. Tools for GPCR drug discovery. Acta Pharmacol Sin. 2012;33:372–84. doi: 10.1038/aps.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson KA. New paradigms in GPCR drug discovery. Biochemical Pharmacology. 2015;98:541–555. doi: 10.1016/j.bcp.2015.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wootten D, Miller LJ, Koole C, Christopoulos A, Sexton PM. Allostery and Biased Agonism at Class B G Protein-Coupled Receptors. Chem Rev. 2016;117 doi: 10.1021/acs.chemrev.6b00049. acs.chemrev.6b00049. [DOI] [PubMed] [Google Scholar]
- 19.Wootten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov. 2013;12:630–44. doi: 10.1038/nrd4052. [Key review article about the potential therapeutic potential of allosteric ligands.] [DOI] [PubMed] [Google Scholar]
- 20.Pupo AS, et al. Recent updates on GPCR biased agonism. Pharmacol Res. 2016;112:49–57. doi: 10.1016/j.phrs.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Isberg V, et al. GPCRdb: An information system for G protein-coupled receptors. Nucleic Acids Res. 2016;44:D356–D364. doi: 10.1093/nar/gkv1178. [Specialised GPCR database and analysis tool spanning e.g. structures, mutants, crystallisation construct design and drugs and indications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law V, et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids Res. 2014;42:D1091–7. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlgren NA, Ren J, Lu YY, Fuhrman JA, Sun F. Alignment-free d2 oligonucleotide frequency dissimilarity measure improves prediction of hosts from metagenomically-derived viral sequences. Nucleic Acids Res. 2017;45:39–53. doi: 10.1093/nar/gkw1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koscielny G, et al. Open Targets: a platform for therapeutic target identification and validation. Nucleic Acids Res. 2016;45 doi: 10.1093/nar/gkw1055. gkw1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overington JP, Al-Lazikani B, Hopkins aL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 26.Rask-Andersen M, Almén MS, Schiöth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10:579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- 27.Ai L, et al. A comprehensive map of molecular drug targets. Nat Publ Gr. 2016;16:19–34. doi: 10.1038/nrd.2016.230. Jpo@benevolent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller PD, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis. JAMA. 2016;316:722. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 29.Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: A randomized, double-blind, placebo-controlled, phase III trial. Diabetes, Obes Metab. 2015;17:675–681. doi: 10.1111/dom.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullard A. 2016 FDA drug approvals. Nat Rev Drug Discov. 2017;16:73–76. doi: 10.1038/nrd.2017.14. [DOI] [PubMed] [Google Scholar]
- 31.Hutchings CJ, Koglin M, Olson WC, Marshall FH. Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat Rev Drug Discov. 2017 doi: 10.1038/nrd.2017.91. [A review on therapeutic antibodies targeting GPCRs and the current pipeline of agents in clinical trials.] [DOI] [PubMed] [Google Scholar]
- 32.Oh DY, Olefsky JM. G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat Rev Drug Discov. 2016;15:161–172. doi: 10.1038/nrd.2015.4. [DOI] [PubMed] [Google Scholar]
- 33.Khanna A, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017;377:419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 34.Mancini AD, Poitout V. GPR40 agonists for the treatment of type 2 diabetes: Life after ‘TAKing’ a hit. Diabetes, Obes Metab. 2015;17:622–629. doi: 10.1111/dom.12442. [DOI] [PubMed] [Google Scholar]
- 35.Gao ZG, Jacobson KA. Allosteric modulation and functional selectivity of G protein-coupled receptors. Drug Discov Today Technol. 2013;10:e237–43. doi: 10.1016/j.ddtec.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Q, et al. ASD v3.0: Unraveling Allosteric regulation with structural mechanisms and biological networks. Nucleic Acids Res. 2016;44:D527–D535. doi: 10.1093/nar/gkv902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickols HH, Conn JP. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiology of Disease. 2014;61:55–71. doi: 10.1016/j.nbd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anighoro A, Bajorath J, Rastelli G. Polypharmacology: Challenges and opportunities in drug discovery. J Med Chem. 2014;57:7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 39.Butini S, et al. Polypharmacology of dopamine receptor ligands. Prog Neurobiol. 2016;142:68–103. doi: 10.1016/j.pneurobio.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Uhlen M, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419–1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 41.Regard JB, Sato IT, Coughlin SR. Anatomical Profiling of G Protein-Coupled Receptor Expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komatsu H. Novel therapeutic GPCRS for psychiatric disorders. Int J Mol Sci. 2015;16:14109–14121. doi: 10.3390/ijms160614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 44.Radick L, Mehr SR. The latest innovations in the drug pipeline for multiple sclerosis. Am Heal Drug Benefits. 2015;8:448–453. [PMC free article] [PubMed] [Google Scholar]
- 45.Zettl UK, Rommer P, Hipp P, Patejdl R. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther Adv Neurol Disord. 2016;9:9–30. doi: 10.1177/1756285615612659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- 47.Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A clinical study of lupron depot in the treatment of women with Alzheimer’s disease: Preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose lupron over 48 weeks. J Alzheimer’s Dis. 2015;44:549–560. doi: 10.3233/JAD-141626. [DOI] [PubMed] [Google Scholar]
- 48.Ferrero H, Solas M, Francis PT, Ramirez Maria J. Serotonin 5-HT 6 Receptor Antagonists in Alzheimer’s Disease: Therapeutic Rationale and Current Development Status. CNS Drugs. 2017;31:19–32. doi: 10.1007/s40263-016-0399-3. @bullet. [DOI] [PubMed] [Google Scholar]
- 49.Dowie MJ, Scotter EL, Molinari E, Glass M. The therapeutic potential of G-protein coupled receptors in Huntington’s disease. Pharmacol Ther. 2010;128:305–23. doi: 10.1016/j.pharmthera.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Veenstra-Vanderweele J, et al. Arbaclofen in Children and Adolescents with Autism Spectrum Disorder: A Randomized, Controlled, Phase 2 Trial. 2017;42:1390–1398. doi: 10.1038/npp.2016.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014;37:1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 52.IDF. IDF Diabetes Atlas. sixth Ed. 2013. Diabetes Atlas. [DOI] [Google Scholar]
- 53.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27:269–73. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nauck MA, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–241. doi: 10.2337/dc15-0165. [DOI] [PubMed] [Google Scholar]
- 55.Kolar GR, Grote SM, Yosten GLC. Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146. J Intern Med. 2017;281:25–40. doi: 10.1111/joim.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauffer L, Iakoubov R, Brubaker PL. GPR119: ‘Double-dipping’ for better glycemic control. Endocrinology. 2008;149:2035–2037. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- 57.Ritter K, Buning C, Halland N, Pöverlein C, Schwink L. G Protein-Coupled Receptor 119 (GPR119) Agonists for the Treatment of Diabetes: Recent Progress and Prevailing Challenges. J Med Chem. 2016;59:3579–3592. doi: 10.1021/acs.jmedchem.5b01198. [DOI] [PubMed] [Google Scholar]
- 58.Lopez Vicchi F, et al. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res. 2016;109:74–80. doi: 10.1016/j.phrs.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 59.Lynch J, Wang J. G Protein-Coupled Receptor Signaling in Stem Cells and Cancer. Int J Mol Sci. 2016;17:707. doi: 10.3390/ijms17050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 61.Moreno E, et al. Targeting CB2-GPR55 receptor heteromers modulates cancer cell signaling. J Biol Chem. 2014;289:21960–21972. doi: 10.1074/jbc.M114.561761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534:314–316. doi: 10.1038/534314a. [News feature about repurposing old drugs to overcome skyrocketing costs in drug development.] [DOI] [PubMed] [Google Scholar]
- 63.Reinscheid RK. Handbook of Biologically Active Peptides. 2013. Neuropeptide S; pp. 869–874. [DOI] [Google Scholar]
- 64.Halls ML, Bathgate RAD, Sutton SW, Dschietzig TB, Summers RJ. International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides. Pharmacol Rev. 2015;67:389–440. doi: 10.1124/pr.114.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prevete N, Liotti F, Marone G, Melillo RM, De Paulis A. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol Res. 2015;102:184–191. doi: 10.1016/j.phrs.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Ramos-Álvarez I, et al. Insights into bombesin receptors and ligands: Highlighting recent advances. Peptides. 2015;72:128–144. doi: 10.1016/j.peptides.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada K, Wada E, Wada K. Bombesin-like peptides studies on food intake and social behaviour with receptor knock-out mice. Ann Med. 2000;32:519–29. doi: 10.3109/07853890008998831. [DOI] [PubMed] [Google Scholar]
- 68.Lang R, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol Rev. 2015;67:118–75. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- 69.Freimann K, Kurrikoff K, Langel Ü. Galanin receptors as a potential target for neurological disease. Expert Opin Ther Targets. 2015;19:1–12. doi: 10.1517/14728222.2015.1072513. [DOI] [PubMed] [Google Scholar]
- 70.Wlodawer A, Vondrasek J. INHIBITORS OF HIV-1 PROTEASE: A Major Success of Structure-Assisted Drug Design. Annu Rev Biophys Biomol Struct. 1998;27:249–284. doi: 10.1146/annurev.biophys.27.1.249. [DOI] [PubMed] [Google Scholar]
- 71.Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 72.Varghese JN. Development of neuraminidase inhibitors as anti-influenza virus drugs. Drug Dev Res. 1999;46:176–196. [Google Scholar]
- 73.Piscitelli CL, Kean J, de Graaf C, Deupi X. A Molecular Pharmacologist’s Guide to G Protein-Coupled Receptor Crystallography. Mol Pharmacol. 2015;88:536–51. doi: 10.1124/mol.115.099663. [DOI] [PubMed] [Google Scholar]
- 74.Jazayeri A, Dias JM, Marshall FH. From G protein-coupled receptor structure resolution to rational drug design. J Biol Chem. 2015;290:19489–19495. doi: 10.1074/jbc.R115.668251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooke RM, Brown AJH, Marshall FH, Mason JS. Structures of G protein-coupled receptors reveal new opportunities for drug discovery. Drug Discov Today. 2015;20:1355–1364. doi: 10.1016/j.drudis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Tautermann CS, Gloriam DE. Editorial overview: New technologies: GPCR drug design and function—exploiting the current (of) structures. Curr Opin Pharmacol. 2016;30:vii–x. doi: 10.1016/j.coph.2016.07.012. [Special issue with leading academic and industrial groups describing developments in technologies for structure-based drug design.] [DOI] [PubMed] [Google Scholar]
- 77.Manglik A, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:1–6. doi: 10.1038/nature19112. [Computational drug design study to develop a drug that mimics the pain-relieving activity of opioid compounds, but has fewer side effects.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakmar TP, Huber T. Pharmacology: Inside-out receptor inhibition. Nature. 2016;540:344–345. doi: 10.1038/nature20486. [DOI] [PubMed] [Google Scholar]
- 79.Jazayeri A, Andrews SP, Marshall FH. Structurally Enabled Discovery of Adenosine A 2A Receptor Antagonists. Chem Rev. 2017;117:21–37. doi: 10.1021/acs.chemrev.6b00119. [DOI] [PubMed] [Google Scholar]
- 80.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: Emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 81.Neves SR, Ram PT, Iyengar R. G Protein Pathways. Science (80-. ) 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 82.Ludwig A, Belfiore NM, Pitra C, Svirsky V, Jenneckens I. Genome duplication events and functional reduction of ploidy levels in sturgeon (Acipenser, Huso and Scaphirhynchus) Genetics. 2001;158:1203–1215. doi: 10.1093/genetics/158.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Masuho I, et al. Distinct profiles of functional discrimination among G proteins determine the actions of G protein–coupled receptors. Sci Signal. 2015;8 doi: 10.1126/scisignal.aab4068. ra123–ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costa-Neto CM, Parreiras-E-Silva LT, Bouvier M. A pluridimensional view of biased agonism. Mol Pharmacol. 2016;90 doi: 10.1124/mol.116.105940. [DOI] [PubMed] [Google Scholar]
- 85.Nygaard R, et al. The dynamic process of ß2-adrenergic receptor activation. Cell. 2013;152:532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viscusi ER, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 87.Bohn LM. Enhanced Morphine Analgesia in Mice Lacking -Arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 88.Ikeda Y, Kumagai H, Motozawa Y, Suzuki J-I, Komuro I. Biased Agonism of the Angiotensin II Type I Receptor. Int Heart J. 2015;56:485–488. doi: 10.1536/ihj.15-256. [DOI] [PubMed] [Google Scholar]
- 89.Khoury E, Clément S, Laporte SA. Allosteric and biased G protein-coupled receptor signaling regulation: Potentials for new therapeutics. Front Endocrinol (Lausanne) 2014;5:68. doi: 10.3389/fendo.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beaulieu JM. In vivo veritas, the next frontier for functionally selective GPCR ligands. Methods. 2016;92:64–71. doi: 10.1016/j.ymeth.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 91.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest. 2015;125:908–917. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richards JL, Yap YA, McLeod KH, Mackay CR, Marino E. Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunol. 2016;5:e82. doi: 10.1038/cti.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bufe B, et al. Recognition of Bacterial Signal Peptides by Mammalian Formyl Peptide Receptors. J Biol Chem. 2015;290:7369–7387. doi: 10.1074/jbc.M114.626747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foster SR, Roura E, Thomas WG. Extrasensory perception: Odorant and taste receptors beyond the nose and mouth. Pharmacol Ther. 2014;142:41–61. doi: 10.1016/j.pharmthera.2013.11.004. [Review summarising the evidence for expression and function of odorant and taste receptors in tissues beyond the nose and mouth and highlighting their broad potential in (patho)physiology.] [DOI] [PubMed] [Google Scholar]
- 95.Hamann J, et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G Protein-Coupled Receptors. Pharmacol Rev. 2015;67:338–367. doi: 10.1124/pr.114.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wacker D, et al. How Ligands Illuminate GPCR Molecular Pharmacology. Cell. 2017;170:414–427. doi: 10.1016/j.cell.2017.07.009. [Review highlighting the many understudied GPCRs and new methods for identification of tool compounds to elucidate their pharmacology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahn S, et al. Allosteric ‘beta-blocker’ isolated from a DNA-encoded small molecule library. Proc Natl Acad Sci U S A. 2017;114:1708–1713. doi: 10.1073/pnas.1620645114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fellmann C, Gowen BG, Lin P-C, Doudna JA, Corn JE. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16:89–100. doi: 10.1038/nrd.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]