Abstract
Objective:
This study aims to determine the ameliorative effect of vitamin E (vit E) on histological features and androgen binding protein (ABP) levels in rats induced by allethrin.
Materials and Methods:
Thirty sexually mature male Wistar rats weighing between 200 and 300 gm, and aging 3 months were taken for this study and were divided into three groups: negative control (NC), positive control (PC), and treatment (T) groups. The PC and T groups were induced by allethrin 12 h per day for 31 days; however, only the T group was given vit E orally at 1 ml/gm body weight (BW) each day for 14 days. The paraffin block method was used to measure tubules’ diameter, thickness of the seminiferous epithelial layer, and Sertoli cell number. The ABP levels were measured by enzyme-linked immunosorbent assay.
Results:
The results showed that vit E gave significant effect (p < 0.05) on tubular diameter at NC 123.67 ± 12.77, PC 147.16 ± 10.64, and T 130.08 ± 10.00; tubular epithelial thickness at NC 33.55 ± 3.21, PC 30.02 ± 1.53, and T 32.96 ± 2.81; Sertoli cells number at NC 55.48 ± 5.9, PC 43.84 ± 3.77, and T 53.44 ± 4.26; and ABP levels at NC 72.35 ± 39.06, PC 38, 48 ± 18.78, and T 86.10 ± 35.77, respectively.
Conclusion:
This study concludes that vit E has an ameliorative effect against the toxic effects of allethrin at testicular histological features and ABP levels.
Keywords: ABP, allethrin, sertoli cells, testicle histology, vit E
Introduction
Inability or difficulty in getting pregnant over a period of at least 12 months even after continuing regular sexual activities without using any safety device (contraception), is defined as infertility [1]. In 2010, the global prevalence for infertility was about 15%–20% consisting of 50–80 million couples, where the male factor contribution was 39% [1]. In 2014, global cases of infertility increased to 121 million couples, in which 50% of cases were caused by male factors [2]. Based on the Central Statistics Agency [3], there were about 10%–15% of infertility cases in Indonesia, which was equivalent to 3.98 million infertile couples, where 15%–25% of them were caused by male factors. In 2013, Indonesia’s health report claimed that 30%–40% infertility cases were caused by male factors [4].
The causal factors of male infertility consist of internal factors (testicular abnormalities, such as hypospadias, cryptorchidism, varicocele, genital tract obstruction, immunological condition, infection, cancer, and systemic disease) and external factors (exposure to environmental chemicals). Exposure to chemicals (such as pesticides/insecticides in mosquito repellent), heavy metals, high temperature, electromagnetic wave radiation, smoking, stress, alcohol, and obesity are able to disrupt the spermatogenesis [5,6]. Indicators of spermatogenesis disruption are observable from sperm quantity, sperm quality, and abnormal testosterone levels, based on the results of the male fertility test [1].
The testicle or testes consists of seminiferous tubules, which is the site where spermatogenesis occurs. The spermatogenesis is influenced by the secretion of Gonadotrophin Releasing Hormone (GnRH) stimulated by the hypothalamus, in order for anterior hypophysis to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). LH binds with the receptor of the Leydig cell, thus begins the testosterone synthesis. FSH acts on the Sertoli cells in the seminiferous tubules to initialize spermatogenesis [7,8].
The exposure of pyrethroid could significantly damage the testicle such as decreasing the testicle weight and seminiferous tubules diameter. If the number of Sertoli cells decreases, the rate of spermatogenesis will also decrease. This disruption of spermatogenesis highly influences both the quality and quantity of spermatozoa [8]. Toxic exposure on rats will greatly affect the androgen binding protein (ABP) concentration [9]. Low concentration of ABP damages the quality and quantity of spermatozoa as well since the ABP has the ability to bond with testosterone to carry them to the seminiferous tubules for spermatogenesis [9].
Allethrin (C19H26O3) is a chemical of the pyrethroid class, which is most commonly used in electric mosquito repellents. The chemical gives a gonadotoxic effect that can potentially form free radicals, thus damaging the organs, specifically, the testicle [5,10]. Free radicals formation can cause lipid peroxidation, protein transformation, DNA damaging, cell destruction, cancer induction, nerve damaging, and reproductive organ damaging. The gonadotoxic effect of allethrin is observable through the integral damage of the sperm membrane, which causes cell destruction, testis damaging, as well as blood-testis barrier [11,12].
Vitamin E (vit E) has known as an antioxidant that enables to prevent oxidative stress due to the imbalance of antioxidants and free radicals. As an antioxidant, vit E is soluble in lipid; therefore, it can protect the unsaturated fatty acids of the phospholipid layer of the testicular cell membrane. Vit E has the ability to suppress the radical oxygen species (ROS) by donating one hydrogen atom (H) to bind the free radicals; therefore, inactivates the lipid peroxidation and prevents cell damage [13]. The intake of vit E has shown to significantly increase the amount of testosterone. It has also shown that it can repair the quality of the sperm cells, as well as the diameter of seminiferous tubules [14,15].
There are many people who still think that using electric mosquito repellent is relatively safe and does not give any negative effect on male fertility. In addition, information on the benefits of vit E on male infertility is still uncommon. To deal with the problem of male infertility, there needs to be an improvement in the identification of cells, tissues, hormones, and androgen binding protein that are involved in spermatogenesis. Therefore, this study was designed to determine the effect of vit E on the improvement of testicular histological structure and ABP levels in rats’ testicle induced or exposed by allethrin.
Materials and Methods
Ethical approval
This study was approved by the Research Ethics Committee, Faculty of Medicine, Andalas University (No.502/KEP/FK/2018).
Sample
This study was carried out using 30 male Wistar rats (Rattus norvegicus), 200–300 gm body weight, 3-months old that obtained from the animal development research unit at Faculty of Pharmacy Andalas University Padang. The rats were randomly divided into the following groups: negative control (NC) group, positive control (PC) group, and treatment (T) group. The PC and T groups were induced by allethrin 12 h per day for 31 days; however, only the T group was given vit E orally at 1 ml/gm BW each day for 14 days. On the 46th day, the rats were anesthetized to collect blood samples from left ventricular intracardial and then they were sacrificed to remove the right testicle.
Histopathological examination
The tissues were processed by the paraffin block method and hematoxylin-eosin staining. Tissues were studied under a light microscope. The analysis of seminiferous tubules diameter was measured from two opposite points on the midline of 15 tubules with 100× magnifications. The thickness of the epithelial layer of seminiferous tubules was measured from the basal membrane to the uppermost layer of spermatids in 15 tubules with 200× magnifications. Sertoli cell numbers in seminiferous tubules were counted at five random field with 200× magnifications.
ABP assay
ABP levels were detected by enzyme-linked immunosorbent assay method. The optical density can be measured with spectrophotometry wavelengths at 450 nm.
Data analysis
Statistical significance was determined by a one-way analysis of variance that followed by post hoc Bonferroni test that was applied to analyze differences between groups. p < 0.05 was considered to be statistically significant.
Results
Effect of vitamin E on testicle histology
The present study showed seminiferous tubules and stromal of control tissue (Fig. 1a). The rats that were exposed to allethrin (PC) experienced a significant increase in the average seminiferous tubules diameter; it is caused by seminiferous tubules dilated, germinal cells partial degeneration, the epithelium is relatively thin compared to the NC group, stroma swelled with blood vessels hyperemia thus increase the diameter seminiferous tubules (Fig. 1b). The rats that were exposed to allethrin that were given vit E intake (T) showed a significant decrease of average seminiferous tubules diameter since vit E can recover the inflammation caused by allethrin exposure (Fig. 1c). There was a significant difference between the NC to PC, as well as the PC to T (p < 0.05; Table 1). There was no significant difference between the NC and T as the vit E intake help to maintain the seminiferous tubule diameter, similar to that of the NC (control).
Figure 1. Representative area of testicular tissue showing seminiferous tubules and stromal of control tissue (a,d,g), allethrin exposure (b,e,h), and allethrin + vitamin E treated animal (c,f,i). Allethrin exposure caused the enlargement of seminiferous tubules, degeneration and thinning of epithelium accompanied by edematous stromal area (b,e). There is hyperemic and inflammatory reaction within stromal. Whereas, vitamin E treated animal showed histomorphological characteristic approaching control condition which appear to recover epithelial structure and thickness (c,f). Allethrin exposure reduced the number of sertoli cells (arrow) within epithelium (h), while vitamin E treatment (i) appear to recover the number of sertoli cells as well as other epithelial within the tubules. Haematoxylin eosin. Bars: 200 μm (a–c), 20 μm (d–f) 10 μm (g–i).
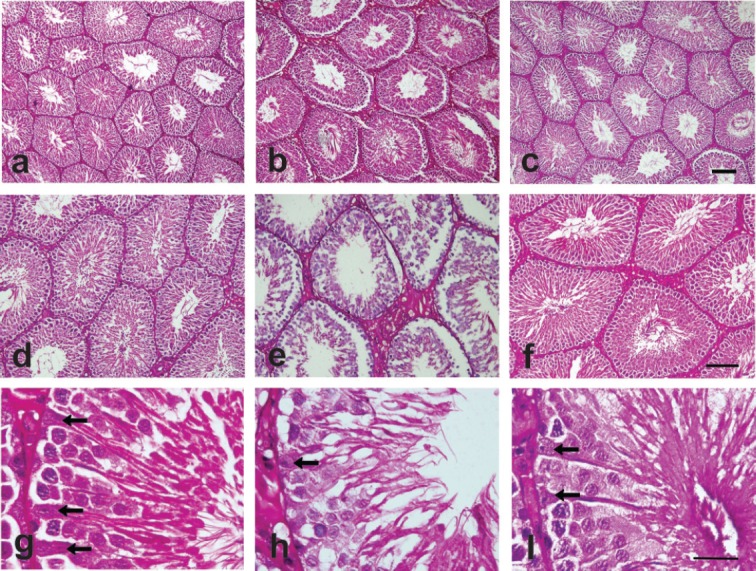
Table 1. Effect of vitamin E administration on histological testicular structure and ABP levels in rats exposed by allethrin.
| Group | Means ± SD | |||
|---|---|---|---|---|
| Tubules Diameter (μm) | Tubules Epithelial Thickness (μm) | Sertoli Cells Number (/5RF) | ABP (pg/ml) | |
| NC | 123.67 ± 12.77 | 33.55 ± 3.21 | 55.48 ± 5.97 | 72,35 ± 39,06 |
| PC | 147.16c ± 10.64 | 30.02b ± 1.53 | 43.84c ± 3.77 | 38,48 a ± 18,78 |
| T | 130.08a ± 10.00 | 32.96a ± 2.81 | 53.44c ± 4.26 | 86,10 b ± 35,77 |
Valued are expressed as mean ± SD. Means with different superscript letters (a, b, and c) between NC – PC and PC – T are significant at p < 0.05. ABP = androgen binding protein, NC = negative control (control), PC = positive control (allethrin), T = treatment (allethrin + vitamin E), 5RF = five random field.
The present study showed comparisons of the thickness of seminiferous epithelium of T and NC group animals (Fig. 1d). The rats that were exposed to allethrin (PC) exhibited a significant decrease in the average seminiferous tubules epithelial thickness (Fig. 1e). On the other hand, the rats that were exposed to allethrin and was given vit E intake (T) showed a significant increase the average of seminiferous tubules epithelial thickness of rats exposed to allethrin (Fig. 1f). There was a significant difference between the NC to PC, as well as the PC to T (p < 0.05; Table 1). There were no significant differences between the NC and T, as the administration of vit E can help maintain the seminiferous tubule epithelial thickness, similar to that of the NC (control).
The present study showed the number of Sertoli cells of seminiferous tubules of T and NC (Fig. 1g). The rats that were exposed to allethrin (PC) exhibited a significant decrease in the average of rat Sertoli cells number (Fig. 1h). The rats that were exposed to allethrin and Vit E administration (T) showed a significant increase in the average of Sertoli cell number of rats exposed to allethrin (Fig. 1i). There was a significant difference between the NC to PC, as well as the PC to T (p < 0.05; Table 1). There were no significant differences between the NC and T, as the administration of vit E can help to increase the number of Sertoli cells that were exposed by allethrin, similar to that of the NC (control).
Effect of vitamin E on androgen binding protein
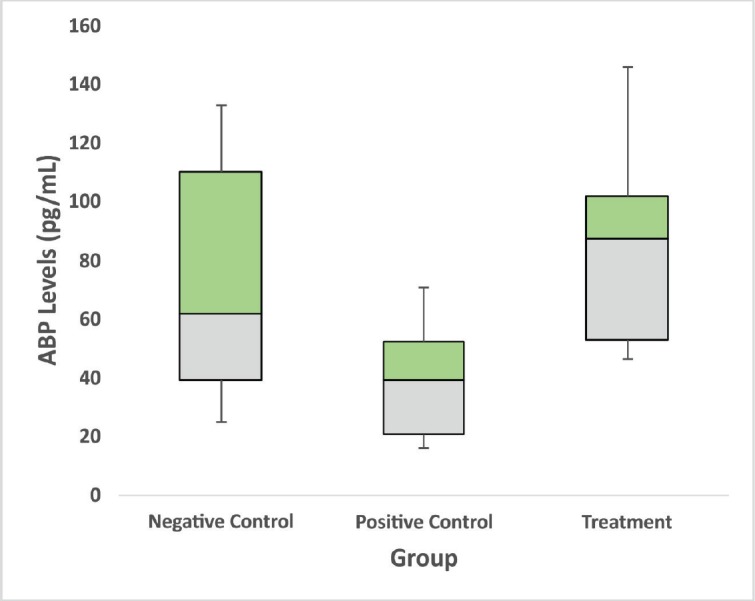
The rats that were exposed to allethrin (PC) experienced a significant decrease in the average of rat ABP levels (Fig. 2). On the other hand, the rats that were exposed to allethrin and were given vit E intake (T) showed a significant increase in the average of ABP levels of rats exposed to allethrin (Fig. 2). Statistically, the result showed a significant difference between NC to PC, as well as PC to T (p < 0.05; Table 1). There were no significant differences between the NC to T, as the administration of vit E can help to elevate ABP levels.
Figure 2. Vitamin E administration effects in ABP levels in male rats exposed by allethrin. Allethrin caused the decreasing of ABP levels, while vitamin E shows amelioration of ABP levels on the allethrin treated animals. Significant difference at p < 0.05. NC = negative control, PC = positive control, and T = treatment.
Discussion
Effect of vitamin E on testicles histology
The accumulation of allethrin in the blood-testis barrier could increase oxidative stress which triggers lipids peroxidation, thus lead to membrane and organelles damage of testicular cells [10]. Present study showed allethrin caused enlargement of seminiferous tubules and edematous stromal reaction (Fig. 1b). Previous studies also revealed that the exposure of pyrethroid such as prallethrin and allethrin exposure has a direct toxic effect to cells, such as toxic effects include infiltration of testicle tissues by inflammatory cells, degeneration, atrophy, hyperemia, necrosis, and edema tubules with the formation of vacuoles and edematous fluid [16,17]. This causes the diameter of the seminiferous tubules to enlarge. Similar results were also reported in other types of pyrethroid studies [18,19].
Vitamin E administration restored the seminiferous tubules diameter size and decreasing tubules diameter (Fig. 1c). In this study, vit E effect can suppress the production of inflammatory mediators in seminiferous tubules. Other study suggested there was a reduction in testicular degenerative changes including degeneration, congestion, and edema caused by lead acetate in rats by vit E administration. Other researchers also report similar results, that vit E decreases the diameter of the seminiferous tubules [19,20,21]. Vit E act as an antioxidant that inhibits the formation of free radicals, protects DNA from oxidative stress, cell damage, and repaired testicular tissue [22,23].
Present study showed allethrin caused degeneration of and thinning of the seminiferous epithelium (Fig. 1e). Other researchers also revealed the loss of spermatogenic cells and interstitial cells due to several mechanisms such as decreased testosterone levels and direct toxic effect of allethrin to cells [24]. Decreased spermatogenic cell population and decreased inflammation cause the thickness of the seminiferous tubular epithelium to decrease.
Oxidative stress can be caused by various agents such as infection, hypoglycemic, and chemical substances. Pyrethroid such as deltamethrin has been shown to induce testicular atrophy, tubular edema, cell degeneration, and spermatogenic cells necrosis [23–25]. Other pyrethroids have also been reported to have toxic potential by reducing the thickness of the seminiferous tubular epithelium [21,26].
The treatment of vit E appears to repair the thickness of seminiferous epithelium within increases epithelial numbers (Fig. 1f). This suggestion was highly supported by other study that showed vit E has a positive effect on testicle weight and testosterone concentration on rat exposed by cypermethrin [27] as well as rabbit [28]. In addition, other researchers also revealed that vit E can increase the thickness of the seminiferous tubular epithelium [19,20,21].
The conducted study showed allethrin exposure reduced the number of Sertoli cells (Fig. 1h). Other researchers revealed that lack of Sertoli cells influenced by several mechanisms, such as a decrease in FSH (in which it stimulates proliferation of Sertoli cells) and testosterone levels as well as a direct toxic effect of allethrin to cells. Sertoli cells directly contribute to spermatogenesis. Sertoli cells undergo degeneration of vacuoles in tubules exposed to pyrethroids [21,29,30] so that the disruption of the function of the Sertoli cells can cause a decrease or even the absence of sperm cells caused by a decrease in ABP levels. Decreased ABP levels also indicate that the supply of testicular androgen (testosterone) for the epididymis (where sperm is mature) can reduce the quality and quantity of sperm [7,8,31].
Treatment with vit E can increase numbers of Sertoli cells in the tubules (Fig. 1i). Other study showed that vit E has the ability to raise protection and antioxidant activity, against toxic effects of pyrethroids such as deltamethrin and cypermethrin in male rats. The study was proven by a significant increase in the Sertoli cell number which improves the quality and quantity of semen [19,20,21]. FSH plays a vital role in the development of spermatozoa. Spermatogenesis is controlled by several mechanisms that are related to FSH and LH [22,32]. Vit E is a fat-soluble antioxidant that plays a role as a protector against oxidative stress and also prevents lipid peroxidation that is toxic for cells, including Sertoli cells [25,27]. This mechanism might responsible for a testicular histological feature that found in our study.
Effect of vitamin E on androgen-binding protein
This study shows allethrin causes decreased levels of ABP (Fig. 2). Allethrin can produce ROS, thus causing oxidative stress. This condition leads to reduce levels of ABP. Oxidative stress triggered lipid peroxidation. Thus, lead to damage of organelles and cell membrane of hypothalamus and testes. The high rate of lipid peroxidation inhibits anterior pituitary gland secreting FSH and also damages the hypothalamus. This condition might impact the steroidogenesis, which suppresses Sertoli cell metabolism to secrete ABP. ABP able to bind with testosterone and brings it to the seminiferous tubules receptors in the germinal cells. This recognition serves as the begins of spermatogenesis [7].
Previous study revealed toxic exposure to testicle can affect the ABP levels and decrease both quantity and quality of the sperm [9]. To be even more specific, allethrin exposure can lead to a significant reduction in number and the motility of sperm cells and also to a decrease of testosterone levels. Pyrethroid exposure also has an effect on the hypothalamus which results in a decrease in ABP production levels [21,33]. This condition leads to infertility in male [34].
Administration of vit E can increase levels of ABP, vit E serves as an antioxidant that is able to break the chains of lipid peroxidation, by donating a hydrogen atom. Vit E prevents oxidative stress, as well as cell and tissue damage in the hypothalamus and testes, increasing FSH thereby also increasing the ABP levels. Previous researchers revealed that an increase in ABP after being given vit E [20,21]. Another study revealed that vit E repairs the damaged of Leydig cells and Sertoli cells, enhances the levels of FSH, and testosterone, as well as prevents sperm abnormalities [22,23].
Conclusion
There is an ameliorative effect of vit E on testicle histology structure such as tubules diameter, epithelial thickness, Sertoli cell number, and ABP levels of male rats induced by allethrin. It is recommended to consume exogenous antioxidants such as vit E to fight free radicals.
Acknowledgments
The authors would like to thank Prof. dr. Rahmatina B. Herman, PhD for valuable comments and suggestions on this manuscript.
Conflict of interests
All authors declared that they have no conflict of interest.
Author’s contribution
Yofa Sukmawati prepared the study design, carried out the research, statistical analysis, and prepared the manuscript under the supervision of Dessy Arisanty and Alimuddin Tofrizal. Arni Amir participated in the preparation of this manuscript. All authors read and approved the final manuscript.
References
- [1].WHO. World Health Organization; Geneva, Switzerland: 2010. World Health Organization—laboratory manual for the examination and processing of human semen. [Google Scholar]
- [2].WHO. World Health Organization; Geneva, Switzerland: 2014. Sexual and Reproductive Health: infertility is a global public health issue. [Google Scholar]
- [3].Central Bureau of Statistics. Central Bureau of Statistics Republic of Indonesia; Jakarta: 2011. BPS Statistics Indonesia 2011. [Google Scholar]
- [4].Agency for Health Research and Development Ministry of the Republic of Indonesia; Jakarta: 2013. Basic Health Research. [Google Scholar]
- [5].Jedrzejowska RW, Wolski JK, Hilczer JS. The role of oxidative stress and antioxidant in male fertility. Cent Eur J Urol. 2013:60–7. doi: 10.5173/ceju.2013.01.art19. https://doi.org/10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics. 2011;66(4):691–700. doi: 10.1590/S1807-59322011000400026. https://doi.org/10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kumar A, Sharma M. Springer Nature; Singapore: 2017. Basics of human andrology: a textbook. https://doi.org/10.1007/978-981-10-3695-8. [Google Scholar]
- [8].Muzakka AT El. Effect of exposure to insect repellent against histopathological picture of Sertoli cells in Sprague Dawley rats. [Mar 12, 2019 ];Media Medika Young J. 2014 Available via https://www.academia.edu/attachments/44343684/download_file?st=MTU2NTEwOTc5NiwxMjUuMTYyLjY5LjE5MA%3D%3D&s=swp-splash-header. [Google Scholar]
- [9].Cui R, Ding Z, Cui L, Yangr U, Xi KY, Cheng X, et al. Influences of fluoride exposure in drinking water on serum androgen binding protein and testosterone of adults males. J Zhengzhou Univ (Med Sci) 2013;48:750–3. http://dx.doi.org/10.3969/j.issn.1671-6825.2013.06.010. [Google Scholar]
- [10].Akuna G, Saalu L, Ogunlade B, Akingbade A, Ogunmodede O. Orient J Sci Res. Lagos State University; Lagos, Nigeria: 2013. [Jan 23, 2019 ]. Pyrethroid-based insecticide induced testicular toxicity via oxidative pathway: study suggest. Available via https://www.researchgate.net/publication/325711369_PyrethroidBased_Insecticide_Induces_Testicular_Toxicity_via_Oxidative_Pathway_Study_Suggest. [Google Scholar]
- [11].Agarwal A, Qiu E, Sharma R. Laboratory assassment of oxidative stress in cement: diagnosis. Arab J Urol. 2018:77–86. doi: 10.1016/j.aju.2017.11.008. https://doi.org/10.1016/j.aju.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Srivastava A, Srivastava P, Al-Khaedhairy A, Musarrat J, Shukla Y. Allethrin-Induced genotoxicity and oxidative stress in swiss albino mice. Mutat Res/Genet Toxicol Environ Mutagenesis. 2012;747(1):22–8. doi: 10.1016/j.mrgentox.2012.03.003. https://doi.org/10.1016/j.mrgentox.2012.03.003. [DOI] [PubMed] [Google Scholar]
- [13].Momeni Hamid R, Mehranjan Malek S, Abnosi MH, Mahmoodi, et al. Effects of vitamin E on sperm parameters and reproductive hormones in developing rats treated with para-nonylphenol. Iran J Reprod Med. 2009;7(3):111–6. [Google Scholar]
- [14].Shittu ST, Oyeyemi WA, Okewumi TA, Salman TM. Role of oxidative stress in therapeutic administration of artesunate on sperm quality and testosterone levels in male albino rats. Afr J Biotechnol. 2013;12(1):70–3. https://doi.org/10.5897/AJB12.315. [Google Scholar]
- [15].Elayapillai SP, Teekaraman D, Paulraj RS. Ameliorative effect of alpha-tocopherol on polychlorinated biphenyl (PCBs) induced testicular sertoli cell dysfunction in F1 Prepuberal rats. Exp Toxicol Pathol. 2017;69:681–94. doi: 10.1016/j.etp.2017.07.001. https://doi.org/10.1016/j. etp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- [16].Akhtar A, Deshmukh A, Raut C, Somkuwar A, Bhagat S. Prallethrin induced serum biochemical changes in Wistar rats. Pestic Biochem Physiol. 2012;102(2):160–8. https://doi.org/10.1016/j. pestbp.2011.12.009. [Google Scholar]
- [17].Naz M, Rehman N, Ansari M, Kamal M, Ganaie M, Awaad A, et al. Comparative study of subchronic toxicities of mosquito repellents (coils, mats, and liquids) on vital organs in Swiss albino mice. Saudi Pharm J. 2019;27(3):348–53. doi: 10.1016/j.jsps.2018.12.002. https://doi.org/10.1016/j. jsps.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dutta H, Meijer H. Sublethal effects of diazinon on the structure of the testis of bluegill, Lepomis macrochirus: a microscopic analysis. Environ Pollut. 2003;125(3):355–60. doi: 10.1016/s0269-7491(03)00123-4. https://doi.org/10.1016/S0269-7491(03)00123-4. [DOI] [PubMed] [Google Scholar]
- [19].Xavier G, Soares P, Junior V, Torres S, Maymone A, Morais R, et al. Effect of dietary selenium and vitamin e supplementation on testicular morphology and serum testosterone concentration in goats following scrotal insulation. Acta Sci Vet. 2016;44(1):1401. https://doi.org/10.22456/1679-9216.81171. [Google Scholar]
- [20].Oda S, El-Maddawy Z. Protective effect of vitamin E and selenium combination on deltamethrin-induced reproductive toxicity in male rats. Exp Toxicol Pathol. 2012;64:7–8. 813–9. doi: 10.1016/j.etp.2011.03.001. https://doi.org/10.1016/j.etp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [21].Desai K, Moid N, Patel P, Highland H. Evaluation of deltamethrin induced reproductive toxicity in male swiss albino mice. Asian Pac J Reprodn. 2016;5(1):24–30. https://doi.org/10.1016/j. apjr.2015.12.004. [Google Scholar]
- [22].Banna H, Kamel G, Galal A, Khattab M, Zakaria A. Reproductive toxicity of deltamethrin in male rats and the protective role of vitamin E. PharmChem J. 2016;3:54–60. [Google Scholar]
- [23].Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99–104. doi: 10.2147/IJGM.S16275. https://doi.org/10.2147/IJGM.S16275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Munaya N, Brahmadhi A, Sakti YBH. Stress effects of fasting on epithelial thickness and diameter of seminiferous tubules Rattus norvegicus. Medika pearls. 2018;18:1–7. https://doi.org/10.18196/mm.180107. [Google Scholar]
- [25].Kumar A, Nagar M. Histomorphometric study of testis in deltamethrin treated albino rats. Rep Toxicol Elsevier. 2014;1:401–10. doi: 10.1016/j.toxrep.2014.07.005. https://doi.org/10.1016/j.toxrep.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schaalan M, Ramadan B, Elwahab A. Ameliorative effect of taurine-chloramine in azathioprine-induced testicular damage; a deeper insight into the mechanism of protection. BMC ComplementAltern Med. 2018;18 doi: 10.1186/s12906-018-2272-z. https://doi.org/10.1186/s12906-018-2272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumar R, Prakash S, Kushwah A, Vijayan V. Effects of vitamin C and E ob PCB (Aroclor 1254) induced oxidative stress, androgen binding protein and lactate in rat Sertoli cells. Reprod Toxicol. 2004;19:201–8. doi: 10.1016/j.reprotox.2004.08.001. https://doi.org/10.1016/j.reprotox.2004.08.001. [DOI] [PubMed] [Google Scholar]
- [28].Yousef MI. Vitamin E modulates reproductive toxicity of pyrethroid lambda-cyhalothrin in male rabbits. Food Chem Toxicol Elsevier. 2010;48(5):1152–9. doi: 10.1016/j.fct.2010.02.002. https://doi.org/10.1016/j.fct.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [29].Nessiem A, Bassily N, Metwally S. Comparative histopathological evaluation of permethrin, pirimiphos methyl and bendiocarb toxicities in testes, liver and kidney of rat. Egypt J Hosp Medicine. 2003;11(1):58–73. http://dx.doi.org/10.12816/EJHM.2003.18719. [Google Scholar]
- [30].Fang L, Chen P, Xia H, Chun X. Effects of cypermethrin on male reproductive system in adult rats. Biomed Environ Sci. 2013;26(3):201–8. doi: 10.3967/0895-3988.2013.03.007. [DOI] [PubMed] [Google Scholar]
- [31].Rebourcet D, Darbey A, Monteiro A, Soffientini U, Tsai YT, Handel I, et al. Sertoli cell number defines and predicts germ and leydig cell population sizes in the adult mouse testis. Endocrinol. 2017;158(9):2955–69. doi: 10.1210/en.2017-00196. https://doi.org/10.1210/en.2017-00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shaikh TM Al, Elfayoumi RI. Protictive effects Of vitamine E against testicular enzymes toxicity induced by cypermethrin in mice. [Jan 29, 2019 ];Int J Pharma Bio Sci. 2013 :942–53. Available via https://ijpbs.net/subscription_renewals.php?articleid=MjMzNw==&subs=UHJpY2U. [Google Scholar]
- [33].Chang J, Xu P, Li W, Li J, Wang H. Enantioselective elimination and gonadal disruption of lambda-cyhalothrin on lizards (Eremias argus) J Agric Food Chem. 2019;67(8):2183–9. doi: 10.1021/acs.jafc.8b05990. https://doi.org/10.1021/acs.jafc.8b05990. [DOI] [PubMed] [Google Scholar]
- [34].Benedict F, Opeyemi A, Oluwatobi A, Wisdom I. Anti-fertility effect of allethrin based liquid electric repellent on the testes and epididymis of adult male sprague-dawley rat. IntJ Res Stud Biosci (IJRSB) 2017;5(2):1–7. https://doi.org/10.20431/2349-0365.0502001. [Google Scholar]


